- 1Department of Agricultural Chemistry, Bangladesh Agricultural University, Mymensingh, Bangladesh
- 2Institute of Biotechnology and Genetic Engineering, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
- 3Department of Agricultural Chemistry, Patuakhali Science and Technology University, Patuakhali, Bangladesh
The potentiality of barnyard grass for remediation of arsenic (As)-contaminated soil has been reported in several research works. However, the phytoremediation ability of barnyard grass from industrially polluted multimetal-contaminated soil in comparison to As-amended soil needs to be elucidated. This work investigated the As remediation potentiality of barnyard grass from As-amended and industrially polluted soils, and the fractionation of As was done in soils with plants and without plants grown. The result showed that at the highest As level in the soil, barnyard grass accumulated the highest amount of As in both the root (414.81 mg kg-1) and shoot (114.12 mg kg-1). However, barnyard grass produced the highest amount of biomass in industrially polluted soil that resulted in the highest amount of As uptake. Moreover, barnyard grass also accumulated lead (Pb) and chromium (Cr) from industrially polluted soil. The bioaccumulation factor (BF) of As was >1 in As-amended soil in all the treatments as well as in industrially polluted soil. Fractionation of As in post-harvest soil revealed that compared to soil without plants grown, As in the soil was reduced from residual As (F5); As associated with well-crystallized hydrous oxides of iron (Fe) and aluminum (Al) (F4); As associated with amorphous and poorly crystallized hydrous oxides of Fe and Al (F3), whereas a slight increase was found in non-specifically sorbed As (F1) and specifically sorbed As (F2) due to the plant’s effect. The slight increase in the concentration of As in F1 and F2 fractions contributed to the bioavailable forms of As in the rhizosphere and sustained As concentration for further plant uptake. The maximum plant growth and highest uptake of As in the industrially polluted soil revealed the potentiality of barnyard grass for remediation of multimetal-polluted soil.
Introduction
The availability of arsenic (As) in soil and water and public exposure to As due to food chain contamination have drawn the attention of many researchers, and appropriate remediation technology for those contaminated soil has been attempted. This toxic heavy metal enters the soil and aquatic ecosystem from natural and anthropogenic sources (1, 2), alters the components of the ecosystem, and adversely affects plants, animals, and human beings (3). As contamination in both groundwater and drinking water is a serious public health problem in many countries worldwide (4–6). Chronic exposure to low to moderate levels of As (10−300 μg l-1), especially through drinking water, results in adverse health effects such as skin lesions, cardiovascular disorders, neurological and respiratory complications, and hepatic and renal dysfunctions (7–11). Acute As toxicity could cause organ damage and may lead to death (12). Widespread use of As-laden groundwater for irrigation could be a major source of As buildup in agricultural soils in South Asia and Southeast Asia including Bangladesh (13, 14). Arsenic accumulation in plants, causing phytotoxicity due to increased As content in soil and water and its long-term impact on agricultural yield and subsequent effect on human health due to food chain contamination warrant an appropriate remediation technology for contaminated soils.
Plants are excellent natural resource materials to combat environmental pollution. Plants have the capacity to tolerate adverse environments and soil, water, and environmental pollution and to remediate contaminated soil and water by using environmentally friendly technology. Utilization of this potentiality of plants for remediation of contaminants or toxic substances from the environment is termed phytoremediation (15–22). There is growing interest in the application of this method due to its many advantages, such as sustainability in application, improved cost–benefit, ease of operation, and application in large areas (23, 24). Phytoextraction, a specific phytoremediation approach that uses metal-accumulating plants has been proposed for decreasing the toxic metal concentration of contaminated soils (25–27). The harvested plant parts, in which metals are richly accumulated, can be safely processed by drying, ashing, or composting. Furthermore, some of the metals in the ash, such as nickel, copper, and gold, may be retrievable for future uses if economically feasible (28–30). One approach for phytoextraction is to use hyperaccumulator plants to extract metals from soil (31, 32). Use of hyperaccumulating plants for As phytoremediation has become a research interest since the first application of Chinese brake fern as an As hyperaccumulator (33–35). However, in the case of application of a hyperaccumulator to the contaminated soil, there are generally some problems such as small biomass and a limited adaptation to the growth condition and cultivation. Selection of plants that have strong metal-accumulating ability and are compatible with local weather conditions may give more practical results than selection based solely on high tolerance to toxic metals, and therefore, can maximize the efficiency of phytoextraction (36). In a typical monsoon agricultural field, where the weather is warm and humid, as in the paddy fields of Bangladesh, it is therefore necessary to investigate those plants that are adaptive to the local weather conditions, produce high biomass, and have a high metal-accumulating ability for phytoremediation (37).
As a candidate plant for phytoremediation, weeds have some suitable criteria. Generally, weeds have a high-adaptation capacity in a wide range of environmental conditions and can therefore grow better than crop plants in adverse environments, in problematic soil, in nutrient-deficient soil, and in contaminated soil (37). Several plants have been reported as potential plants for As phytoremediation (38–41). However, the weeds’ potential for As phytoremediation is relatively less explored. Barnyard grass (Echinochloa crus-galli L.) is a common weed growing naturally and abundantly in rice fields and adjacent areas of rice fields in both upland and paddy conditions. Some researchers conducted screening and selection of barnyard grass and other naturally grown weeds as As accumulators from the As-contaminated areas of Bangladesh (42, 43). Later on, the As remediation potentiality of barnyard grass has been tested in hydroponic conditions (44) and in soils with different characteristics (45). Nevertheless, the remediation potentiality of barnyard grass needs to be studied extensively from industrially polluted soil in comparison to As-amended soil for practical use.
The capacity of a plant to absorb heavy metals like As from soil depends on the existing forms of metal in the soil and the availability of metals by plants in soil. Therefore, metal availability is considered a limiting factor for phytoremediation. It was reported that the soluble form of cadmium (Cd) is directly absorbed by plants from the contaminated soil (46–48). In a phytoremediation study using barnyard grass, it was reported that barnyard grass took in As that was available in the soil water of the contaminated soil and it also increased the amount of As in soil water (37). However, the change of As from other soil fractions due to As uptake by barnyard grass has not been investigated yet. Therefore, the present research work was undertaken to investigate the As remediation potentiality of barnyard grass from industrially polluted soil in comparison to arsenic-amended soil. The relationship between the plant uptake of As and the change in As levels in different soil fractions due to phytoremediation were also investigated.
Materials and methods
Chemicals used
A standard inorganic As solution of atomic absorption spectrophotometer (AAS) grade (CAS number-7440382, concentration 1,000 mg l-1) was used for the quantification of As in the samples using an atomic absorption spectrophotometer coupled with a hydride generator (HG-AAS). Appropriate dilution of the As stock solution was done with deionized water to get the working standards of As at various concentrations. Similarly, standard inorganic lead (Pb), chromium (Cr), iron (Fe), and manganese (Mn) of 1,000 mg l-1 were also used for the determination of the respective elements in the sample. All the standards of AAS grade were obtained from Inorganic Ventures, VA, USA. All the other chemicals used were of analytical reagent grade (AR). Arsenate (As(V)) was added to the soil as disodium hydrogen arsenate heptahydrate [Na2HAsO4.7H2O (CAS number 10048-95-0)]. Concentrated nitric acid and 30% H2O2 (AR grade) were used for the extraction of As from soil and plant samples. All the acids and extracting reagents for the sequential extraction of As were obtained from Sigma-Aldrich, MO, USA. Deionized water was used in all standard and sample preparations.
Collection and preparation of soils
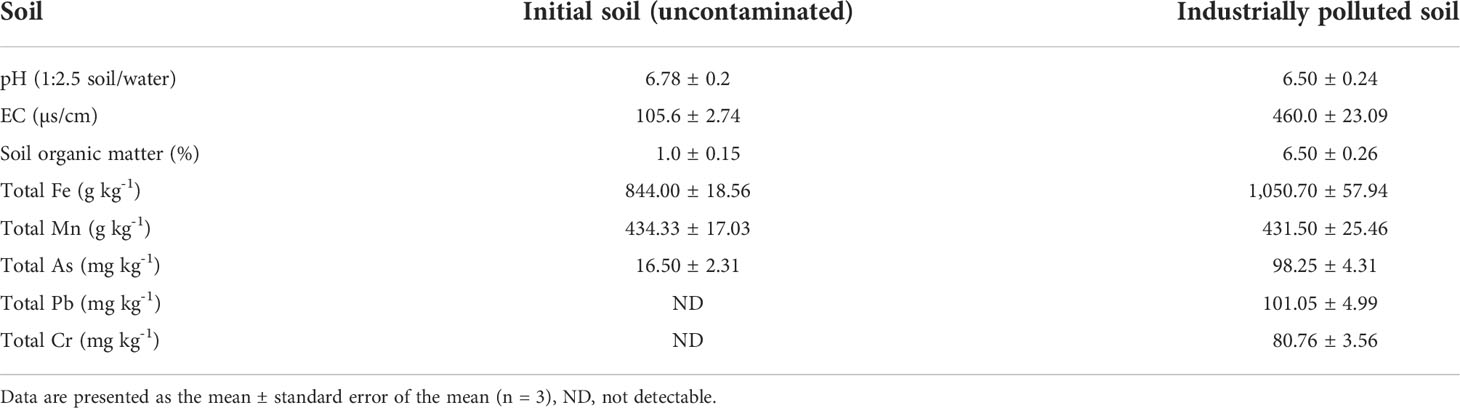
Two types of soil samples were used for the experiment. Fresh/virgin soil was collected from the southwest side of the stadium of Bangladesh Agricultural University (BAU), Mymensingh, at 0–15-cm depth. Industrially polluted heavy metal-contaminated topsoil was collected from the Bhaluka Upazila of the Mymensingh District in Bangladesh. The soil was contaminated mainly by the wastes and effluents of textile, dying, composite, pharmaceutical, ceramic, glass, and battery industries. Unwanted materials like stones, gravels, pebbles, and plant roots were removed from the bulk soil. Then, the soils were air-dried for several days, and the clods were broken and sieved. Soil pH was measured in a 1:2.5 suspension of soil and water on a glass electrode pH meter as described by Jackson (1973) (49). The electrical conductivity (EC) of the soil was determined electrometrically by a 1:5 soil-to-water ratio, with the help of a conductivity meter (50). Then, the soil was analyzed for As and other heavy metals. Total As, Pb, Cr, Fe, and Mn in soil samples were extracted following the procedure described by Tam and Yao (1999) (51). Exactly 1 g of soil was placed in a 250-ml digestion tube, then 10-ml of concentrated HNO3 was added. The content was kept for 16 h at room temperature for pre-digestion and then heated at 105°C for 2 h and 30 min. The content was then cooled, diluted, and filtered, and the final volume was kept at 100 ml with deionized water. All the metals were determined using an atomic absorption spectrophotometer (Model: Shimadzu AA-7000). Total As was determined using the atomic absorption spectrophotometer (AAS) coupled with a hydride generator.
After the initial physical and chemical analyses (Table 1), the soils were used for pot preparation and subsequent plant growth. Pots of 18-cm deep, 23-cm diameter at the top, and 15-cm diameter at the bottom, were used for transplanting the seedling. Each pot contained 3 kg of soil. The fresh soil (non-contaminated soil) was amended with three levels of arsenic, viz., 0 (T0), 50 (T1), and 100 (T2) mg kg-1 arsenic (soil basis) from Na2HAsO4.7H2O (AR grade). Soil was mixed with an appropriate amount of Na2HAsO4.7H2O for each treatment and diluted with deionized water to reach the soil moisture level of approximately 80% of the field capacity. The soil was then kept for 24 h before transplanting the seedlings to reach adsorption of As on soil into the equilibrium as mentioned by Sultana and Kobayashi (2016) (52). The industrially polluted soil was used as another treatment without further addition of As compounds. In all the treatments, a pot was kept without a plant. Urea, triple superphosphate (TSP), muriate of potash (MoP), gypsum, and zinc sulfate were added to each pot at the rates of 135, 100, 70, 60, and 6.5 kg ha-1, respectively.
Plant material
The plant material was barnyard grass (Echinochloa crus-galli L.). Seeds of barnyard grass were collected from the agronomy field of Bangladesh Agricultural University. The seeds were then soaked in deionized water and kept at room temperature for 1 day and germinated in a plastic tray at the same temperature. Then, the germinated seeds were cultured in a modified Kasugai nutrient solution (53) until the three-leaf stage prior to use in the experiment. The pH of the solution was maintained at 5.5–6.2.
Experimental procedure
Six seedlings of barnyard grass were transplanted in each of the pots containing three treatments of As, 0, 50, and 100 ppm. Similarly, six seedlings were also transplanted into the pots of industrially contaminated soil. The experiment was carried out in completely randomized designs (CRDs) with three replications.
The seedlings were irrigated frequently with As-free water to maintain the water level 1.0–1.5 cm above the soil surface in the pot. Undesired weeds were uprooted by hand and mixed thoroughly with the pot soil at the early stage. Plant height (cm) was measured from the ground level to the top of the plants from each pot, and the number of tillers for each pot was recorded. The plants were harvested 45 days after transplanting. Five grams of rhizosphere soil was collected from the root of barnyard grass from each treatment for the analysis of total arsenic in soil and fractionation of arsenic.
Preparation of plant and post-harvest soil samples for analysis
The weeds were washed repeatedly with tap water to remove all the soil particles and mud. Then the samples were washed again with deionized water, air-dried, and oven-dried at 80°C for 48 h. The fresh and dry weights of the root and shoot were recorded appropriately from each treatment and each pot. The samples were then digested for As analysis following the procedure developed by Cai et al. (2000) (54). Briefly, 0.5 g for plant was transferred into dry clean 125-ml conical flasks. Ten milliliters of concentrated nitric acid was added to each of the flasks. The content of the flasks was mixed, and the flasks were left overnight covered with aluminum (Al) foil. The following day, the flasks were placed on a heating block and heated at a temperature slowly raised to 120°C. After heating, the vessels were allowed to cool, and 2 ml of hydrogen peroxide (H2O2) was added. Again, the flasks were heated at 120°C and the volume was reduced to 1–2 ml. The digest was cooled and filtered through a Whatman no. 42 filter paper, and the volume was kept at 100 ml with deionized water and kept in a dry plastic bottle.
For the analysis of total As and other metals from post-harvest soil, extraction and determination were done following the same procedure as described above in the case of initial soil. The concentrations of metals in soil and plant were determined using the atomic absorption spectrophotometer coupled with the hydride generator (Model: Shimadzu AA-7000), with a recovery of 0.2 ppb. The relative standard deviation (RSD) was set to 2% prior to analysis. A reagent blank was used during the determination. The recovery percentage was 95%–105%.
Sequential extraction of arsenic in soil
After harvesting the plants, 1 g of rhizosphere soil from each treatment from the pots with plants grown and from the pots without plants grown was extracted sequentially with 25 ml of (NH4)2SO4 (0.05 M), (NH4)2PO4 (0.05 M), NH4-oxalate (0.2 M), and NH4-oxalate (0.2 M)–ascorbic acid (0.1 M), and finally, with concentrated nitric acid (HNO3) and hydrogen peroxide (H2O2). The five sequential extraction steps were assumed to correspond respectively to non-specifically sorbed As (F1), specifically sorbed As (F2), As associated with amorphous and poorly crystallized hydrous oxides of Fe and Al (F3), As associated with well-crystallized hydrous oxides of Fe and Al (F4), and residual As (F5), as described by Wenzel et al. (2001) (55).
All the extracts were filtered through Whatman no. 42 filters prior to As analysis. All extractions were performed in triplicate, and the amount of As was determined from the control, As-amended soils, and industrially polluted soil with plants grown and without plants grown.
Statistical analysis
The data were statistically analyzed using the Microsoft® Excel program, and the results were expressed as a mean of three replicates with ± standard error (SE). Analysis of variance (ANOVA) was done using Tukey’s test.
Results
Effect of arsenic on the growth and agronomic parameters of the weeds
The effect of As amendments and the presence of As in the industrially polluted soil were investigated in plant height, the number of tillers, and biomass of the weed. As shown in Figure 1, plant height was significantly reduced in response to the added As from treatment T0 to T1. With the increase in the added As level from 0 to 100 mg kg-1, the plant height gradually decreased from 37.25 to 17.75 cm. In the industrially polluted soil, barnyard grass gave an average plant height of 30.75 cm. Similar to the plant height, the number of tillers of barnyard grass also gradually reduced with the increase in the added As in the soil. However, unlike the As-amended soil, the industrially polluted soil barnyard grass showed very good growth and produced the maximum number of tillers (51), although the As level of that soil (98.25 mg kg-1) was very close to the T2 treatment (100 mg kg-1) of added arsenic level.
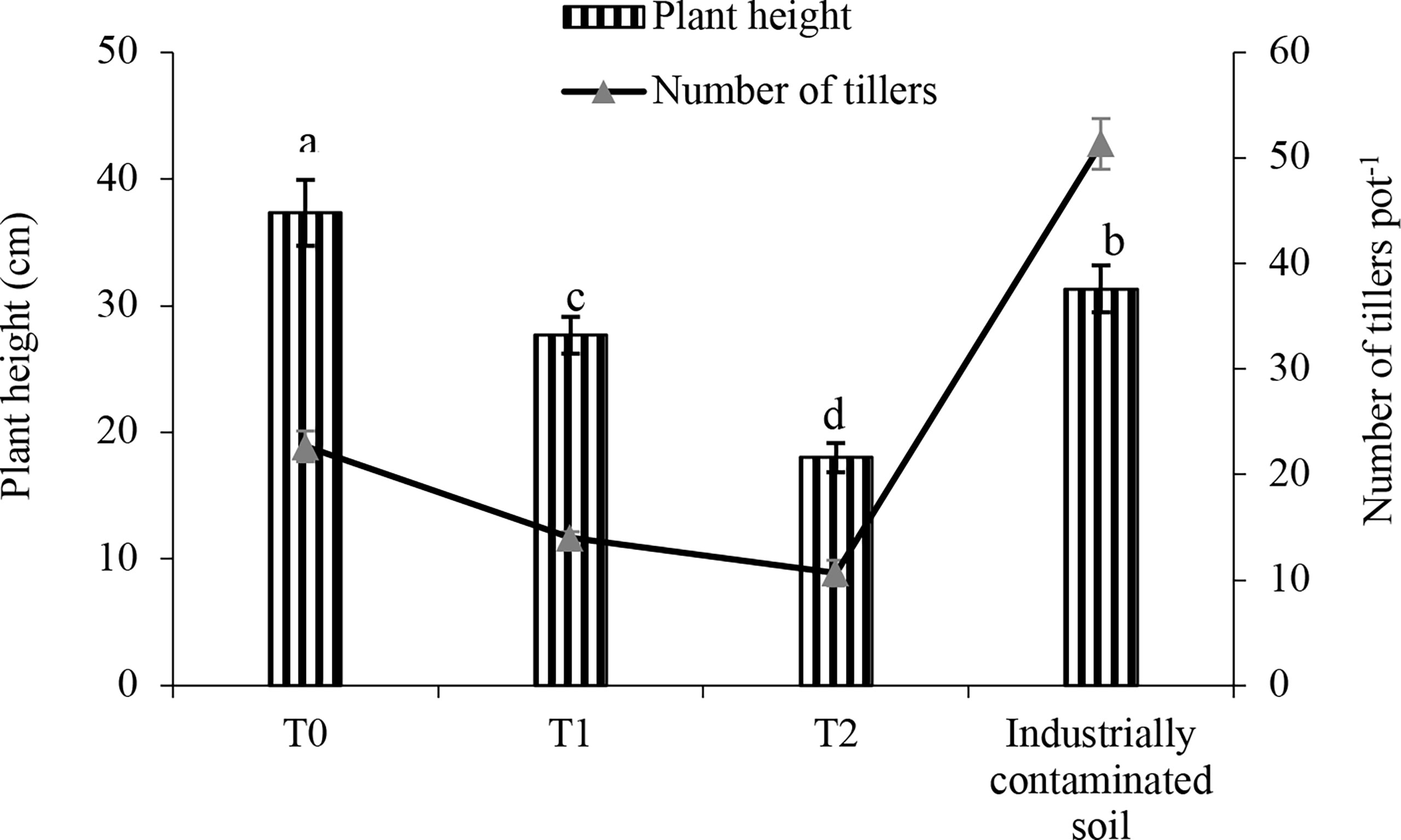
Figure 1 Plant height and the number of tillers of barnyard grass grown in As-amended (T0: 0; T1: 50; T2: 100 mg kg-1 As amendment) and industrially polluted soil. The columns with the same letter are not significantly different at P < 0.05 as determined by Tukey’s test. The number of tillers is shown in the trend line. Bars indicate the ± standard error of the means (n = 3).
Figure 2 shows that plant biomass was also negatively affected by the added As level in the soil from 0 to 100 mg kg-1. In As-amended soil, both fresh and dry biomass were the highest in T0 treatment and gradually decreased with the increase in As level from 0 to 100 mg kg-1 of added arsenic. The highest fresh weight was found in industrially polluted soil in both root and shoot. In industrially polluted soil, barnyard grass produced the root fresh weight very close to the treatment T0 with no added As, while the shoot fresh weight was much higher in that soil than in As-added soil, even significantly higher than in no As-added treatment T0. In the case of dry weight, a sharp decrease was observed with the increase in As level in the soil. The root dry weight was highest in the T0 treatment with no added As followed by industrially polluted soil, and treatments T1 and T2.
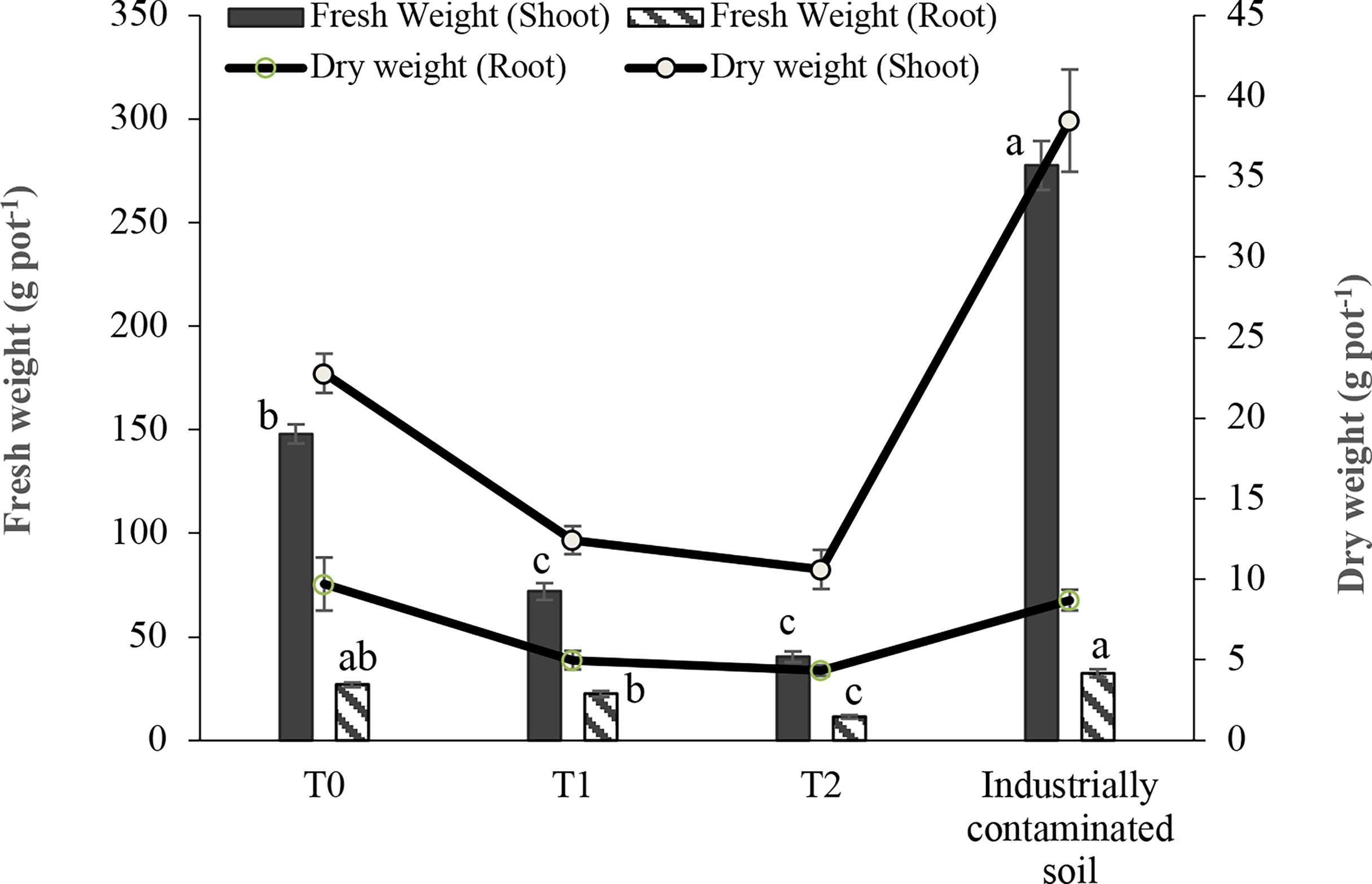
Figure 2 Effect of As on the fresh and dry weights of barnyard grass grown in As-amended (T0: 0; T1: 50; T2: 100 mg kg-1 As amendment) and in industrially polluted soil. The root and shoot fresh biomass in each pot were compared separately among the treatments. The columns with the same letter are not significantly different at P < 0.05 as determined by Tukey’s test. Dry biomass is shown in the trend line. Bars indicate the ± standard error of the means (n = 3).
Concentration of As in barnyard grass and uptake of As by the weed
Accumulation of As was found to be the highest in As-amended soil at the highest As level in the soil and the lowest at the lowest As level in the soil in both the root and shoot (Figure 3). The maximum concentration of As, 414.81 mg kg-1, was found in the root of the T2-treated soil, while in the case of the shoot, the maximum concentration of As, 114.12 mg kg-1, was found in the same treatment T2. The lowest As contents were 58.44 mg kg-1 in the root and 40.55 mg kg-1 in the shoot in 0 mg kg-1 of As-amended soil. In industrially polluted soil, As concentrations were 299.18 mg kg-1 in the root and 101.89 mg kg-1 in the shoot. The concentration of As in the shoot and root of barnyard grass was found to be in accordance with the concentration of As in the soil.
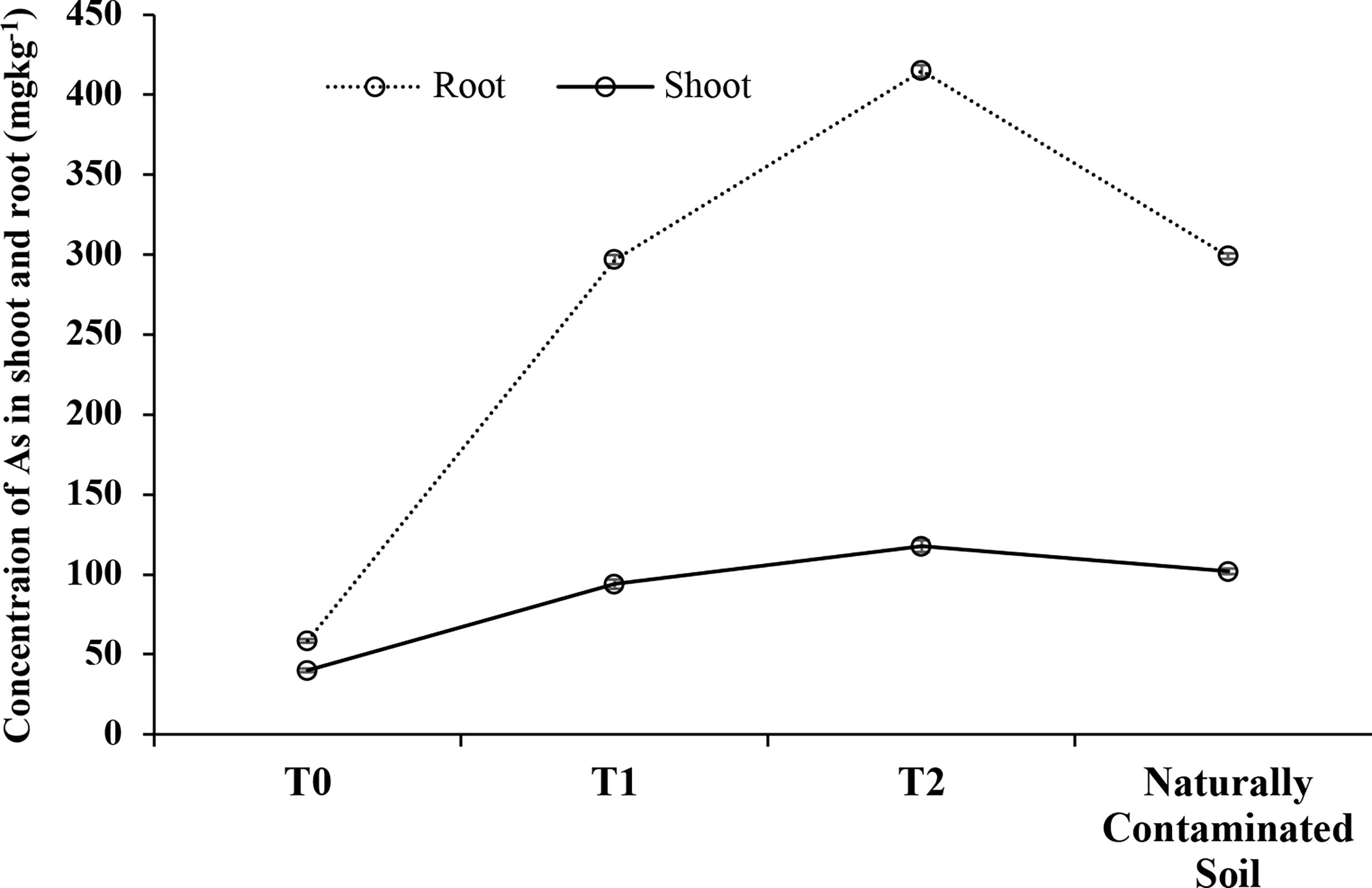
Figure 3 Concentration of As in the root and shoot of barnyard grass grown in As-amended (T0: 0; T1: 50; T2: 100 mg kg-1 As amendment) and in industrially polluted soil. Bars indicate the ± standard error of the means (n = 3).
Unlike the concentration of As in the root and shoot, the uptake of As was not in accordance with the As level in the soil. Figure 4 shows that the maximum amount of As took in by barnyard grass grown in industrially polluted soil and it was 6.35 mg As per pot. Accumulation of As in the shoot was higher than in the root in T0 and in industrially polluted soil, and the highest amount of As was found in the shoot in the industrially polluted soil. Total As accumulation by the grass from each pot was gradually increased from T0 to T2, and the maximum was in industrially polluted soil (Figure 4).
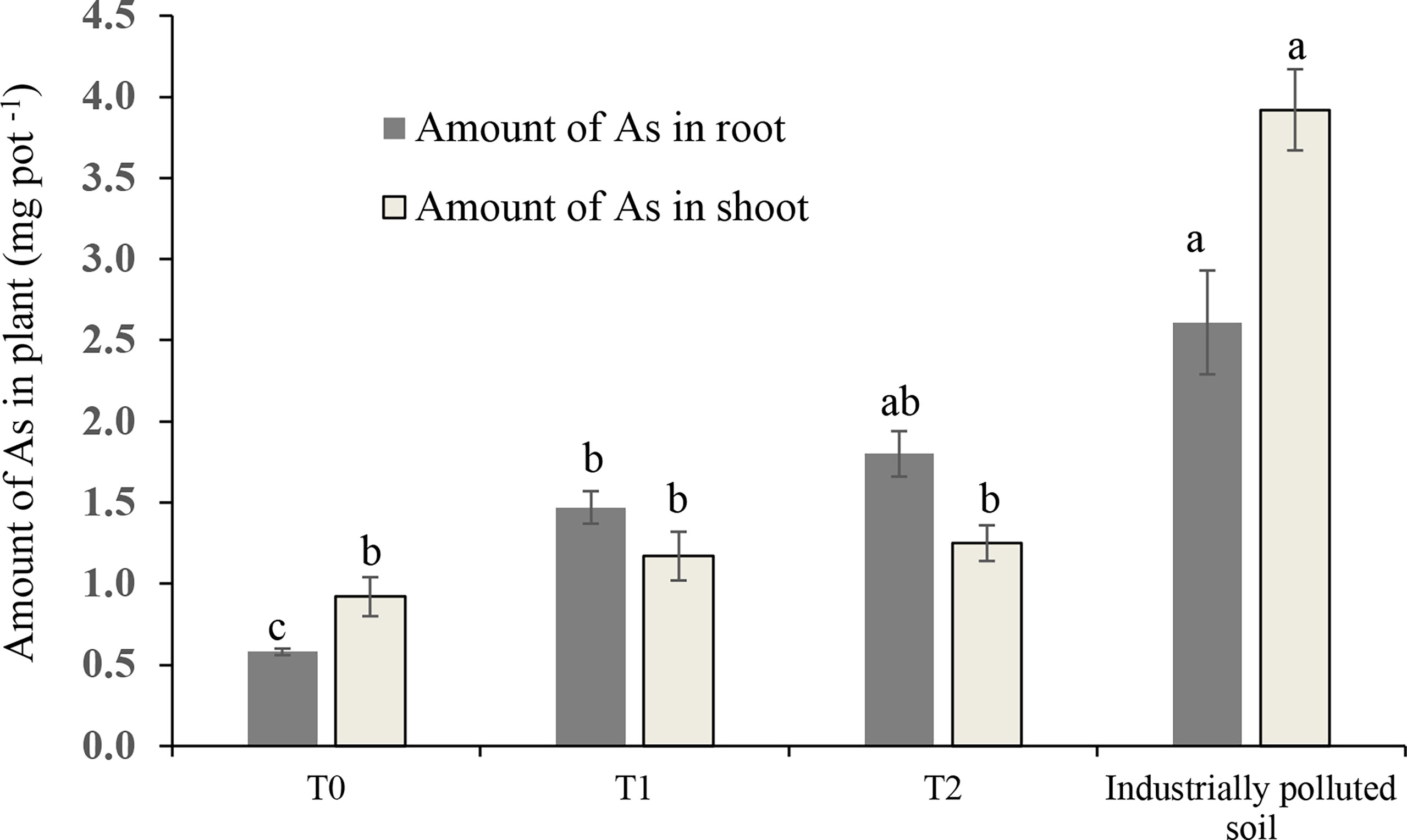
Figure 4 Amount of As in the root and shoot of barnyard grass in each pot in As-amended (T0: 0; T1: 50; T2: 100 mg kg-1 As amendment) and in industrially polluted soil. Data were compared among the shoots and among the roots separately. The columns with the same letter are not significantly different at P < 0.05 as determined by Tukey’s test. Bars indicate the ± standard error of the means (n = 3).
Bioaccumulation factor of As
For an effective phytoremediation process through phytoextraction, metal bioaccumulation is a good indicator. According to Yoon et al. (2006) (56), bioaccumulation factor (BF) is the calculated ratio of the compound accumulated in the aboveground plant parts in relation to the amount of metal in the growth medium. The BF value was also calculated as a bioconcentration factor as a ratio of the concentration of heavy metals in the root (or in total plant biomass) relative to their concentration in the growth media, for phytoremediation (57, 58). In the present research, we considered bioaccumulation factor (BF) as the ratio of As concentration in the shoot relative to the concentration of As in the soil. Table 2 shows that the bioaccumulation factor (BF) of arsenic was found to be >1 in all the treatments, indicating the potentiality of barnyard grass to accumulate As from non-amended soil, As-amended soil, and industrially polluted soil. The highest bioaccumulation factor (2.43) was found in the control treatment where the plant accumulated As from the initial soil. The bioaccumulation factors were 1.43 and 1.01, respectively for 50 mg kg-1 and 100 mg kg-1 of As-amended soil while it was 1.04 in industrially polluted soil (Table 2).
Uptake of Pb and Cr by the weeds in industrially polluted soil
Apart from arsenic, the potentiality of barnyard grass to remediate Pb and Cr from industrially polluted soil was also investigated in this experiment. The results showed that barnyard grass took in both Pb and Cr in its root and shoot (Table 3). However, the concentrations of Pb and Cr in the root and shoot of barnyard grass were significantly lower than the concentration of As in the plant. Although the concentration of Pb in industrially polluted soil was similar to the concentration of As (98.25 mg kg-1 As and 99.03 mg kg-1 Pb), the absorption of As was much higher than the absorption of Pb in both the root and shoot. The result indicated that barnyard grass is preferentially an As accumulator. Cr accumulation was slightly higher than Pb accumulation. Again, the metal concentration in the shoot was slightly higher than in the root for both Pb and Cr. The total uptake of Pb and Cr in the shoot was significantly higher than the metals in the root due to the higher shoot biomass.
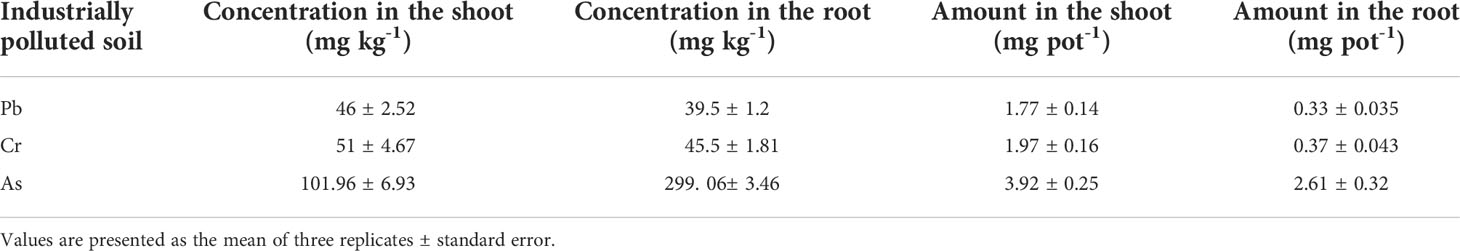
Table 3 Concentration of As, Pb, and Cr in barnyard grass and the amount of metals took in by barnyard grass from industrially polluted soil.
Fractionation of As in post-harvest soil
The post-harvest soils were analyzed for the amount of As that remained in the soil. Fractionation of As in the soil was done in As-amended and industrially polluted post-harvest soils with plants grown and without plants grown. Figure 5 reveals that in both pots with plants and without plants grown, the lion’s share of As remained in the residual fraction of soil designated as F5. However, the amount of residual As (F5) was lower in soils with plants than in soils without plants grown. The second highest fraction of As was in F1, non-specifically sorbed As. Barnyard grass had a significant influence on that fraction. The amount of As in this fraction was slightly increased due to plant growth in all the As-amended soil and industrially polluted soil, except in treatment T0, where no As was added. A similar increase in As in the soil also resulted in a specifically sorbed As (F2) fraction due to plant growth, and the amount of As in the F2 fraction was higher in soils with plants compared to soils without plants. Nevertheless, in both soils with plants and without plants grown, the order of the amount of As was F5 > F1 > F2 > F4 > F3. The amount of As in all the fractions in soil with plants and without plants was compared with the total As to find the recovery of As in the fractionation procedure. The overall recovery of As using the fractionation procedure, as determined by comparing the sum of As obtained in all five fractions with a single total As determination, was found to be within the range of 92.9%–105.9%.

Figure 5 Fractionation of As in the post-harvest soil with plants and without plants grown in As-amended (T0: 0; T1: 50; T2: 100 mg kg-1 As amendment) and industrially polluted soil. Fraction F1 = non-specifically sorbed As; F2 = specifically sorbed As; F3 = As associated with amorphous and poorly crystallized hydrous oxides of Fe and Al; F4 = As associated with well-crystallized hydrous oxides of Fe and Al; and F5 = residual As.
Discussion
The present research aimed to reveal the potentiality of barnyard grass for the remediation of As-contaminated soil from As-amended soil as well as from a multimetal-polluted soil that was polluted by industrial discharge. The results showed that in the As-amended soil, the growth of barnyard grass gradually reduced with the increase in As level. All the growth parameters such as plant height, number of tillers, and root and shoot biomass also had similar gradual decreasing patterns with an increase in As in the soil from 0 to 100 mg kg-1. Similar results have been reported by several researchers where plants tended to suffer from a reduction in root and shoot growth when exposed to excess As in the growth medium (59–62). The excess amount of available As in the soil can interrupt plant metabolism, consequently leading to leaf senescence, reduction in the number of leaves, stunted growth, and reduction in biomass (63–68). At high As concentrations, inhibition of shoot growth could be attributed to a reduction in enzymatic activity (61, 62), while the inhibition of root growth may be associated with reduced mitotic activity in the root meristematic zone or a reduction in cell enlargement in the elongation zone due to decreased turgor of the cell, as reported by other researchers in Brassica juncea, ferns, wheat (Triticum aestivum), rice (Oryza sativa), broad bean (Vicia faba), velvet grass (Holcus lanatus), and Arabidopsis thaliana (61, 62, 66, 69, 70).
The effects of arsenic in the soil on the growth of various plants have been investigated previously by several researchers using different As levels in the soil. Arsenic in the soil significantly reduced the plant height, leaf area, number of leaves, and shoot and root dry weight in Brassica napus and B. juncea at 50 and 75 mg kg-1 As levels in the soil (71). A similar trend of significant decrease in growth parameters was reported by Mehmood et al. (2017) in Zea mays with an increase in As level in the soil from 0 to 120 mg kg-1 (72). In our previous experiment, we found a slight reduction in the growth of barnyard grass when As level in the soil increased from 0 to 100 mg kg-1. In the present experiment, a similar reduction in growth was also found in As-amended soil when the As concentration in the soil increased from 0 to 100 mg kg-1. Nevertheless, the reduction in growth was not observed when barnyard grass was grown in industrially polluted soil with an As level of 98.25 mg kg-1; rather, the number of tillers and the plant shoot biomass were significantly higher than in the control (0 mg kg-1 As amendment). These results clearly demonstrated that not only was the growth of barnyard grass in As-contaminated soil affected by As stress but that soil characteristics are another big determining factor. Although the industrially polluted soil had multimetal load, the organic matter content was much higher in that soil compared to the fresh soil, which might be the reason for the suppression of As-induced stress by the plant and thereby maintenance of better growth in that soil compared to non-contaminated soil.
The results of concentrations of As in the root and shoot showed that barnyard grass absorbed a higher level of As from As-amended soil than from industrially polluted soil. Again, the concentration of As in the root and shoot gradually increased with the increase in As level in the soil. Therefore, it is evident that the concentration of As in the root and shoot of the plant in As-amended soil is linearly related to the As amendment in the soil. In contrast to the As-amended soil, the As concentration in plants was not similar to the industrially polluted soil. The concentration of As in the root of grass in industrially polluted soil was significantly lower compared to the root in As-amended soil of similar As concentration. For instance, the root As concentration was 414.81 mg kg-1 in 100 mg kg-1 of As-amended soil while it was 299.18 mg kg-1 in industrially polluted soil with 98.25 mg kg-1 of As level in the soil. The reason might be the interaction of other metals with As in the industrially polluted soil. Moreover, As, Pb, and Cr absorption was also determined in plants grown in industrially polluted soil. Barnyard grass took in both Pb and Cr from the industrially polluted soil and translocated the metals into the shoot. From a multimetal-polluted soil, uptake of metals depends on the interaction of the metals and the crop preference for the metals. The industrially polluted soil used in this experiment was almost equally loaded by As (98.25 mg kg-1) and Pb (101.05 mg kg-1) and a little less loaded by Cr (80.06 mg kg-1). However, As concentration in the root and shoot was significantly higher than that of Pb, while the concentration of Pb and Cr in the root and shoot was similar. Therefore, it is evident that among the three metals analyzed, barnyard grass preferred As first and then Cr, and Pb last. The BF value was found to decrease slightly with the increase in As load in the soil, and the BF value was similar in As-amended soil at the highest As level and in the industrially polluted soil. It was reported that the bioaccumulation factor decreased with the increase in heavy metal concentrations in the soil (73, 74). In one of our previous studies, the BF values of barnyard grass varieties decreased slightly with the increase in As in the hydroponic solution (33), which is in accordance with the present finding. At high As concentrations, the limited root-to-shoot transport may be the reason for the decrease in the BF value as reported by Pence et al. (2000) (75) in the case of zinc (Zn) accumulation by Thlaspi caerulescens. Plants are categorized as “accumulators” when the BF value is >1 and “excluders” when the BF value is <1 (76). Based on the BF value, it is evident that similar to the soil-free medium, barnyard grass is an As accumulator from soil irrespective of the source of As in the soil, and thus, this plant is useful for phytoremediation of As-contaminated soil from a wide range of soils with different sources of contamination.
For an effective phytoremediation process, especially phytoextraction, a key issue is to enhance the pollutant’s phytoavailability and sustain adequate pollutant concentrations in the soil solution for plant uptake (77). In our previous experiment of As phytoremediation, it was evident that barnyard grass increases As in the soil water while it remediates As from the contaminated soil, thereby, making As available for further phytoremediation (37). In the present research, As in the soil was found to reduce from all the fractions in the pots with plants grown compared to the pots without plants, except for F1 and F2. In fraction F1, non-specifically absorbed fraction, and F2, specifically absorbed fraction, the concentration of As was found to be increased slightly after the plant growth. Fraction 1, designated as F1 has been shown to correlate well with As in field-collected soil solutions and hence, can be used for predicting solute As which can be explained as a readily bioavailable fraction. Fractions 2–4 may provide information on the potential lability of As from different solid phases as a result of soil remediation (55). Therefore, the F1 fraction directly contributes the plant available part of As. It is evident from this research that plant growth increased the readily bioavailable fraction of As and thereby makes it available for further remediation by barnyard grass. With the uptake of As from fraction F1, barnyard grass might create a force that directs As by desorption from residual to gradually labile fractions and thereby increase the As concentration in F2 and F1. This could occur due to the specific microbial association in the barnyard grass rhizosphere or by the secretion of root exudates that makes As bioavailable (37, 78). It is well known that microbial processes play a major role in As cycling in the plant–soil–microbe system (79). Plants secrete root exudates that provide energy and nutrients to microbes, and in return, microbes stimulate the secretion of root exudates by the plants, which enhance the mobility of metals (78), and in the case of barnyard grass, metals mobilize to more bioavailable fractions in the soil. In a contaminated soil environment, barnyard grass might absorb bioavailable As from soil and microbial association and secretion of root exudates in the rhizosphere might be responsible for a continuous supply of As in available form to the root zone of barnyard grass for further uptake. Similar results were also reported by Ultra et al. (2007) (80), where water-soluble As increased in the rhizosphere of sunflower as influenced by arbuscular mycorrhizal fungus (Glomus aggregatum) and increase in water-soluble Zn by rhizosphere bacteria in the rhizosphere of Thlaspi caerulescens (81). Further studies are necessary to confirm the rhizosphere chemistry and microbial association around the root of barnyard grass in As-contaminated soil for effective phytoremediation.
Conclusion
This study showed that barnyard grass (Echinochloa crus-galli L.) was able to grow in non-contaminated soil with As amendment from 0 to 100 mg kg-1 with little reduction in growth attributes as well as in industrially polluted soil with good growth. The plant absorbed and accumulated As in its root and shoot with a bioaccumulation factor of >1 in all the treatments and in both soil types, indicating that barnyard grass is an accumulator of As and it is suitable for phytoextraction of As-contaminated soil. From the fractionation of As in the soil, it was evident that the amount of As was decreased in F5, F4, and F3 fractions, while a slight increase was found in the readily bioavailable fractions F1 and F2. In industrially polluted soil, the higher growth and higher As accumulation indicated that barnyard grass is a potential weed to apply in multimetal-polluted soil for phytoremediation of As and other metals. Although barnyard grass is often used as a forage and grass seeds are consumed by humans in very limited cases, the grass that would be used for phytoremediation should not be used as forage or for any other purposes. The soil after reclamation will be suitable for crop growth. Further study is necessary to investigate the factors influencing the desorption of As from residual and labile fractions that in turn increase As in bioavailable fraction, which is very crucial for the implication of phytoremediation of As using barnyard grass.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RS: Conceptualization, experimental design, resources management, investigation, supervision, data curation and analysis, writing the manuscript, review and editing. TA: Conducting pot experiment, data curation, plant and soil analysis. SI: Experimental design, investigation, statistical analysis of the data, writing the manuscript, review and editing. MU: Experimental design, resources management, investigation, data curation and analysis. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the reviewers for their constructive comments and suggestions that helped to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mandal BK, Suzuki KT. Arsenic round the world: a review. Talanta (2002) 58:201–35. doi: 10.1016/S0039-9140(02)00268-0
2. Smith E, Naidu R, Alston AM. Arsenic in the soil environment: a review. Adv Agron (1998) 64:149–95. doi: 10.1016/S0065-2113(08)60504-0
3. Morgan WE, Dunnette DA. A review of arsenic hazards to plants and animals with emphasis on fishery and wildlife resources In: Nriagu J. O. ed. Arsenic in the Environment, Part 2: Human health and ecosystem effects. New York: Wiley (1994).
4. Dastgiri S, Mosaferi M, Fizi MA, Olfati N, Zolali S, Pouladi N, et al. Arsenic exposure, dermatological lesions, hypertension, and chromosomal abnormalities among people in a rural community of northwest Iran. J Health Popul Nutri (2010) 28:14.
5. Phan K, Sthiannopkao S, Kim KW, Wong MH, Sao V, Hashim JH, et al. Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res (2010) 44:5777–88. doi: 10.1016/j.watres.2010.06.021
6. Freitas-Silva L, Arauíjo TO, Silva LC, Oliveira JA, Araujo JM. Arsenic accumulation in brassicaceae seedlings and its effects on growth and plant anatomy. Ecotox Environ Safe (2016) 124:1–9. doi: 10.1016/j.ecoenv.2015.09.028
7. Ahsan H, Chen Y, Parvez F, Zablotska L, Argos M, Hussain I, et al. Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the health effects of arsenic longitudinal study. Am J Epidemiol (2006) 163:1138–48. doi: 10.1093/aje/kwj154
8. Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, et al. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet (2010) 376:252–8. doi: 10.1016/S0140-6736(10)60481-3
9. Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the health effects of arsenic longitudinal study (HEALS) in Bangladesh. Toxicol Appl Pharmacol (2009) 239:184–92. doi: 10.1016/j.taap.2009.01.010
10. Chen Y, Karagas MR. Arsenic and cardiovascular disease: new evidence from the united states. Ann Int Med (2013) 159:713–4. doi: 10.7326/0003-4819-159-10-201311190-00720
11. Smith AH, Goycolea M, Haque R, Biggs ML. Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol (1998) 147:660–9. doi: 10.1093/oxfordjournals.aje.a009507
12. Abdul KS, Jayasinghe SS, Chandana EP, Jayasumana C, De Silva PM. Arsenic and human health effects: A review. Env Toxicol Pharmacol (2015) 40:828–46. doi: 10.1016/j.etap.2015.09.016
13. Zaman MW. Environmental impacts of groundwater abstraction in barind area. component-b: Water quality and agro-ecology. In: Paper presented at the annual workshop of ARMP contract research. Dhaka, Bangladesh: BARC (2002).
14. Brammer H. Mitigation of arsenic contamination in irrigated paddy soils in south and south- east Asia. Environ Int (2009) 35:856–63. doi: 10.1016/j.envint.2009.02.008
15. Wenzel WW, Jockwer F. Accumulation of heavy metals in plants grown on mineralised soils of the Austrian Alps. Environ pollut (1999) 104:145–55. doi: 10.1016/S0269-7491(98)00139-0
16. Cunningham SD, Lee CR. Phytoremediation: Plant-based remediation of contaminated soils and sediments. Biorem: Sci Appl (1995) 43:145–56. doi: 10.2136/sssaspecpub43.c9
17. Raskin I, Smith RD, Salt DE. Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol (1997) 8:221–6. doi: 10.1016/S0958-1669(97)80106-1
18. McGrath SP, Zhao J, Lombi E. Phytoremediation of metals, metalloids, and radionuclides. Adv Agron (2002) 75:1–56. doi: 10.1016/S0065-2113(02)75002-5
19. Robinson BH, Green S, Mills T, Clothier B, Fung L, Hurst S, et al. Assessment of phytoremediation as best management practice for degraded environments. environmental management using soil-plant systems. In: Currie LD, Stewart RB, Anderson CWN, editors. Occasional report, vol. 16 Palmerston North, New Zealand: HortResearch. (2003). p. 39–49.
20. Behera KK. Phytoremediation, transgenic plants and microbes. Sustain Agric Rev (2014) 13:65–85. doi: 10.1007/978-3-319-00915-5_4
21. Muthusaravanan S, Sivarajasekar N, Vivek JS, Paramasivan T, Naushad M, Prakashmaran J, et al. Phytoremediation of heavy metals: mechanisms, methods and enhancements. Environ Chem Lett (2018) 16:1339–59. doi: 10.1007/s10311-018-0762-3
22. Saha P, Shinde O, Sarkar S. Phytoremediation of industrial mines wastewater using water hyacinth. Int J phytorem (2017) 19:87–96. doi: 10.1080/15226514.2016.1216078
23. Salt DE, Blaylock M, Kumar NP, Dushenkov V, Ensley BD, Chet I, et al. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Bio/Tech (1995) 13:468–74. doi: 10.1038/nbt0595-468
24. Schwitzguebel. Hype or hope: The potential of phytoremediation as an emerging green technology. Remediation: J Env Cleanup Costs Technol Techniq (2001) 11:63–77. doi: 10.1002/rem.1015
25. Ebbs SD. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol (1997) 29:1424–30.
26. Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator thlaspi caerulescens. Plant Physiol (2002) 128:1359–67. doi: 10.1104/pp.010731
27. Peuke H, Rennenberg H. Phytoremediation. EMBO Rep (2005) 6:497–501. doi: 10.1038/sj.embor.7400445
28. Garbisu C, Alkorta I. Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Biores Technol (2001) 77:229–36. doi: 10.1016/S0960-8524(00)00108-5
29. Brooks RR, Chambers MF, Nicks LJ, Robinson BH. Phytomining. Trends Plant Sci (1998) 1:359–62. doi: 10.1016/S1360-1385(98)01283-7
30. Prasad MN. Phytoremediation of metal-polluted ecosystems: hype for commercialization. Russ J Plant Physiol (2003) 50:686–701. doi: 10.1023/A:1025604627496
31. McGrath SP, Sidoli CM, Baker AJ, Reeves RD. The potential for the use of metal-accumulating plants for the in situ decontamination of metal-polluted soils. In: Integrated soil and sediment research: a basis for proper protection. Dordrecht: Springer (1993). p. 673–6.
32. Brown SL, Chaney RL, Angle JS, Baker AJM. Zinc and cadmium uptake of thlaspi caerulescens grown in nutrient solution. Soil Sci Soc Am J (1994) 59:125–33. doi: 10.2136/sssaj1995.03615995005900010020x
33. Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED. A fern that hyperaccumulates arsenic. Nature (2001) 409:579. doi: 10.1038/35054664
34. Tu C, Ma LQ. Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator pteris vittata l. Plant Soil (2003) 249:373–82. doi: 10.1023/A:1022837217092
35. Yang J, Yang SS, Lei M, Yang JX, Wan XM, Chen TB, et al. Comparison among soil additives for enhancing pteris vittata l.: Phytoremediation of as-contaminated soil. Int J Phytorem (2018) 20:1300–6. doi: 10.1080/15226514.2017.1319325
36. Murakami M, Ae N. Potential of phytoextraction of copper, lead and zinc by rice (Oryza sativa l.), soybean (Glycine max l. merr.), and maize (Zea mays l.). J Hazard Mater (2009) 162:1185–92. doi: 10.1016/j.jhazmat.2008.06.003
37. Sultana R, Kobayashi K. Potentiality of barnyard grass and rice for arsenic contaminated soil. Weed Biol Manage (2011) 11:12–7. doi: 10.1111/j.1445-6664.2011.00400.x
38. Rosli RA, Harumain ZA, Zulkalam MF, Hamid AA, Sharif MF, Mohamad MA, et al. Phytoremediation of arsenic in mine wastes by acacia mangium. Remediation J (2021) 31:49–59. doi: 10.1002/rem.21688
39. Visoottiviseth P, Francesconi K, Sridokchan W. The potential of Thai indigenous plant species for the phytoremediation of arsenic contaminated land. Environ pollut (2002) 118:453–61. doi: 10.1016/S0269-7491(01)00293-7
40. Zhang W, Cai Y, Tu C, Ma LQ. Arsenic speciation and distribution in an arsenic hyperaccumulating plant. Sci Tot Environ (2002) 300:167–77. doi: 10.1016/S0048-9697(02)00165-1
41. Rahman MA, Hasegawa H, Ueda K, Maki T, Rahman MM. Influence of phosphate and iron ions in selective uptake of arsenic species by water fern (Salvinia natans l.). Chem Eng J (2008) 145:179–84. doi: 10.1016/j.cej.2008.03.014
42. Zaman MW. Identification of arsenic hyperaccumulating weeds for the remediation of arsenic contaminated soil. In: Proceedings of the 9th international symposium on soil and plant analysis (Cancun, Mexico, 30 January-4 February 2005), vol. Vol. 14. Colegio de Postgraduados (2005).
43. Sultana R, Zaman MW. Phytoremediation potential of some naturally grown weeds for arsenic contaminated soils of Bangladesh. Ann Bd Agric (2008) 12:97–105.
44. Sultana R, Kobayashi K, Kim KH. Comparison of arsenic uptake ability of barnyard grass and rice species for arsenic phytoremediation. Environ Monit Assess (2015) 187:1–9. doi: 10.1007/s10661-014-4101-2
45. Sultana R, Kobayashi K. Arsenic uptake by barnyard grass and rice in two types of soils. Bd J Crop Sci (2011) 21:159–66.
46. Goto S, Hayashi H, Yoneyama T, Chino M. Behavior of cd and zn in rhizosphere of brassica plants grown in an andosoil contaminated with cd and zn. Soil Sci Plant Nutr (2003) 49:735–9. doi: 10.1080/00380768.2003.10410332
47. Ueno D, Zao FJ, Shen R, Ma JF. Cadmium and zinc accumulation by the hyperaccumulator thlaspi caerulescens from soils enriched with insoluble metal compounds. Soil Sci Plant Nutri (2004) 50:511–5. doi: 10.1080/00380768.2004.10408507
48. Liu J, Dong Y, Xu H, Wang D, Xu J. Accumulation of cd, Pb and zn by wetland plant species in constructed wetland. J Hazard Mater (2007) 147:947–53. doi: 10.1016/j.jhazmat.2007.01.125
49. Jackson ML. Soil chemical analysis Vol. 498. . New Delhi, India: pentice hall of India Pvt. Ltd. (1973) p. 151–4.
50. Ghosh AB, Bajaj JC, Hasan R, Singh D. Soil and water testing methods: a laboratory manual. New Delhi, India: IARI (1983) p. 31–6.
51. Tam NF, Yao MW. Three digestion methods to determine concentrations of Cu, zn, cd, Ni, Pb, cr, Mn, and fe in mangrove sediments from sai keng, chek keng, and sha tau kok, Hong Kong. Bullet Environ Contamin Toxicol (1999) 62:708–16. doi: 10.1007/s001289900931
52. Sultana R, Kobayashi K. Adsorption of arsenic on soil under different soil moisture conditions. Pollut (2016) 2:211–20. doi: 10.7508/pj.2016.02.009
53. Yoshiba M. Plant cultivation. In: Experimental methods for plant nutrition (ed. by the editorial committee of experimental methods for plant nutrition). Tokyo: Hakuyusha Ltd (1990). p. 3–4.
54. Cai Y, Georgiadis M, Fourqurean JW. Determination of arsenic in seagrass using inductively coupled plasma mass spectrometry. Spectrochimica Acta Part B: Atomic Spectroscopy (2000) 55:1411–22. doi: 10.1016/S0584-8547(00)00247-0
55. Wenzel WW, Kirchbaumer N, Prohaska T, Stingeder G, Lombic E, Adriano DC. Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta (2001) 436:309–23. doi: 10.1016/S0003-2670(01)00924-2
56. Yoon J, Cao X, Zhou Q, Ma LQ. Accumulation of Pb, Cu, and zn in native plants growing on a contaminated Florida site. Sci Tot Environ (2006) 368:456–64. doi: 10.1016/j.scitotenv.2006.01.016
57. Malik RN, Husain SZ, Nazir I. Heavy metal contamination and accumulation in soil and wild plant species from industrial area of Islamabad, Pakistan. Pak J Bot (2010) 42:291–301.
58. Ali H, Naseer M, Sajad MA. Phytoremediation of heavy metals by trifolium alexandrinum. Int J Environ Sci (2012) 2:1459–69.
59. Cox MS, Bell PF, Kovar JL. Differential tolerance of canola to arsenic when grown hydroponically or in soil. J Plant Nutri (1996) 19:1599–610. doi: 10.1080/01904169609365224
60. Abedin MJ, Meharg AA. Relative toxicity of arsenite and arsenate on germination and early seedling growth of rice (Oryza sativa l.). Plant Soil (2002) 243:57–66. doi: 10.1023/A:1019918100451
61. Gomes MP, Carvalho M, Carvalho GS, Marques T, Garcia QS, Guilherme LRG, et al. Phosphorus improves arsenic phytoremediation by anadenanthera peregrina by alleviating induced oxidative stress. Int J Phytorem (2013) 15:633–46. doi: 10.1080/15226514.2012.723064
62. Ansari MK, Shao HB, Umar S, Ahmad A, Ansari SH, Iqbal M, et al. Screening Indian mustard genotypes for phytoremediating arsenic-contaminated soils. CLEAN–Soil Air Water (2013) 41:195–201. doi: 10.1002/clen.201100752
63. Jiang QQ, Singh BR. Effect of different forms and sources of arsenic in crop yield and arsenic concentration. Water Air Soil pollut (1994) 74:321–43. doi: 10.1007/BF00479798
64. Srivastava S, Akkarakaran JJ, Sounderajan S, Shrivastava M, Suprasanna P. Arsenic toxicity in rice (Oryza sativa l.) is influenced by sulfur supply: Impact on the expression of transporters and thiol metabolism. Geoderma (2016) 270:33–42. doi: 10.1016/j.geoderma.2015.11.006
65. Singh AP, Dixit G, Kumar A, Mishra S, Singh PK, Dwivedi S, et al. Nitric oxide alleviated arsenic toxicity by modulation of antioxidants and thiol metabolism in rice (Oryza sativa l.). Fron Plant Sci (2016) 6:1272. doi: 10.3389/fpls.2015.01272
66. Lewinska K, Karczewska A. Influence of soil properties and phosphate addition on arsenic uptake from polluted soils by velvetgrass (Holcus lanatus). Int J Phytorem (2013) 15:91–104. doi: 10.1080/15226514.2012.683205
67. Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, et al. Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. I J Env Res Public Health (2018) 15:59. doi: 10.3390/ijerph15010059
68. Niazi NK, Bibi I, Shahid M, Ok YS, Shaheen SM, Rinklebe J, et al. Arsenic removal by Japanese oak wood biochar in aqueous solutions and well water: Investigating arsenic fate using integrated spectroscopic and microscopic techniques. Sci Tot Environ (2018) 621:1642–51. doi: 10.1016/j.scitotenv.2017.10.063
69. Codling EE. Phosphorus and arsenic uptake by corn, wheat and soybean from broiler lietter ash and egg layer manure ash. J Plant Nutri (2013) 36:1083–101. doi: 10.1080/01904167.2013.776079
70. Zhao FJ, Dunham SJ, McGrath SP. Arsenic hyperaccumulation by different fern species. New Phytol (2002) 156:27–31. doi: 10.1046/j.1469-8137.2002.00493.x
71. Niazi NK, Bibi I, Fatimah A, Shahid M, Javed MT, Wang H, et al. Phosphate-assisted phytoremediation of arsenic by brassica napus and brassica juncea: morphological and physiological response. Int J Phytorem (2017) 19:670–8. doi: 10.1080/15226514.2016.1278427
72. Mehmood T, Bibi I, Shahid M, Niazi NK, Murtaza B, Wang H, et al. Effect of compost addition on arsenic uptake, morphological and physiological attributes of maize plants grown in contrasting soils. J Geochem Explor (2017) 178:83–91. doi: 10.1016/j.gexplo.2017.03.018
73. Efroymson RA, Sample BE, Suter GW. Uptake of inorganic chemicals from soil by plant leaves: regressions of field data. Environ Toxicol Chem: Int J (2001) 20:2561–71. doi: 10.1002/etc.5620201123
74. Zhao FJ, Lombi E, McGrath SP. Assessing the potential for zinc and cadmium phytoremediation with the hyperaccumulator thlaspi caerulescens. Plant Soil (2003) 249:37–43. doi: 10.1023/A:1022530217289
75. Pence NS, Larsen PB, Ebbs SD, Letham DL, Lasat MM, Garvin DF, et al. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator thlaspi caerulescens. Proc Natl Acad Sci (2000) 97:4956–6490. doi: 10.1073/pnas.97.9.4956
76. Baker AJM. Accumulators and excluders-strategies in the response of plants to heavy metals. J Plant Nutri (1981) 3:1–4. doi: 10.1080/01904168109362867
77. Lombi E, Zhao FJ, Dunham SJ, Mcgrath SP. Phytoremediation of heavy metal contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J Environ Qual (2001) 30:1919–26. doi: 10.2134/jeq2001.1919
78. Kim S, Lim H, Lee I. Enhanced heavy metal phytoextraction by echinochloa crus-galli using root exudates. J Biosci Bioeng (2010) 109:47–50. doi: 10.1016/j.jbiosc.2009.06.018
79. Lampis S, Santi C, Ciurli A, Andreolli M, Vallini G. Promotion of arsenic phytoextraction efficiency in the fern pteris vittata by the inoculation of as-resistant bacteria: a soil bioremediation perspective. Front Plant Sci (2015) . 6:1–12. doi: 10.3389/fpls.2015.00080
80. Ultra VU Jr., Tanaka S, Sakurai K, Iwasaki K. Arbuscular mycorrhizal fungus (Glomus aggregatum) influences biotransformation of arsenic in the rhizosphere of sunflower (Helianthus annuus l.). Soil Sci Plant Nuti (2007) 53:499–508. doi: 10.1111/j.1747-0765.2007.00143.x
Keywords: arsenic, phytoremediation, barnyard grass, fractionation, industrially polluted soil
Citation: Sultana R, Ahmed T, Islam SMN and Uddin MN (2022) Barnyard grass (Echinochloa crus-galli L.) as a candidate plant for phytoremediation of arsenic from arsenic-amended and industrially polluted soils. Front. Soil Sci. 2:927589. doi: 10.3389/fsoil.2022.927589
Received: 24 April 2022; Accepted: 06 July 2022;
Published: 02 August 2022.
Edited by:
Khalid Rehman Hakeem, King Abdulaziz University, Saudi ArabiaReviewed by:
Alaa El-Dein Omara, Agricultural Research Center, EgyptAbhiroop Chowdhury, O.P. Jindal Global University, India
Copyright © 2022 Sultana, Ahmed, Islam and Uddin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Razia Sultana, cmF6c0BiYXUuZWR1LmJk
 Razia Sultana
Razia Sultana Tamim Ahmed1
Tamim Ahmed1 Shah Mohammad Naimul Islam
Shah Mohammad Naimul Islam