- Soil Ecosystem Dynamics Lab, School of Environment, Resources, and Sustainability, University of Waterloo, Waterloo, ON, Canada
African countries are urbanizing at a rapid rate. Research on urban agriculture may be key to ensuring urban food and soil security. This study aimed to evaluate pathways for integrated soil fertility management using a mixed methods approach to consider both social perspectives and soil quality in the city of Mwanza, Tanzania. The social component of urban agriculture was explored using semi-structured interviews with urban farmers (n=34), through judgement and snowball sampling. Qualitative analyses showed that urban farmers range in age and gender, as well as in experiences and cultivation practices, though all use hand tools. Farmers reported reliance on rainy seasons for cultivating. However, farmers also raised concerns about a changing climate and unpredictability of rain, which impacts crop productivity. Most interviewed farmers (82%) would like to improve their soils, and many use manure as an amendment stating that animal manure is the best way to improve soil. Additionally, most urban farmers (62%) have not tried any form of food waste compost but responded positively to try it if they had access and were taught how to use it. For the second aspect of this study a field trial was conducted to evaluate and compare the effects of organic and inorganic amendments on soil quality and crop productivity over the short-term. The results from the field trial determined that organic amendments (poultry manure and food waste compost) improved soil water holding capacity by 14 to 19% and enhanced microbial biomass 1.7 to 4 times compared to treatments with inorganic nitrogen fertilizer. Crop productivity with organic amendments was comparable to that in treatments with nitrogen fertilizer. We conclude that urban agriculture is an integral aspect of Mwanza City, and the application of organic amendments improves urban soil quality compared to the application of inorganic fertilizer, which has implications for urban soil security, land use planning, and food sovereignty in developing countries.
1 Introduction
It is expected that by 2030 almost 5 billion people will be living in urban areas (1). The global food demand in 2050 is expected to be driven by population and income growth, as well as rapid urbanization (2). Currently, Africa is urbanizing at 3.4% per year from both natural population growth and rural-to-urban migration, which is faster than any other continent (3). Agriculture, including subsistence farming, is a significant source of income for a majority of the African population (4), and a substantial share of income for the urban poor (5). Historically, food production required the conversion of natural landscapes to agricultural use, a change that results in the release of atmospheric carbon and depletion of soil organic carbon (SOC) (6). Additionally, land-use conversion and increased intensity of land-use, due to population pressure, is strongly linked to soil degradation, including soil erosion and nutrient depletion (7). Since most soils in Africa are inherently low in fertility due to their age and lack of volcanic rejuvenation (8), they are highly erodible and strongly weathered with low water holding capacity and nutrient availability (9). Therefore, crop productivity in many parts of Africa is primarily limited by nutrients (10).
Moreover, nutrients in African soils continue to be depleted annually through cultivation (11). For example, nitrogen (N) losses in sub-Saharan Africa (SSA) occur at a rate of 22 kg N ha-1 per year (12), which is equivalent to US$4 billion worth of fertilizer (13). However, the average fertilizer use in SSA is only about 8 to 9 kg ha-1 per year, which is insufficient to maintain crop productivity (8, 14). Barriers to fertilizer use in SSA include cost, absence of economic incentives, and lack of information on how to use fertilizers appropriately (13, 15, 16). Nonetheless, many African governments argue that the most practical solution to replenishing nutrients is increasing the use of inorganic fertilizers (13), which can be advantageous for promoting crop yield due to the high solubility that helps plants take up nutrients (17). However, inorganic nitrogen fertilization may not always improve the negative nutrient balance in soil if nutrients are continually lost via leaching and crop uptake (18). Additionally, the use of fertilizers in agricultural systems effectively bypasses the biological processes that help sustain long-term crop productivity (19).
While agriculture in Africa is generally considered a rural activity, it also has a long-standing indigenous tradition in urban areas (20). De Bon et al. (21) found that urban agriculture in SSA is a risk-sharing strategy and is also of cultural and traditional significance. In Dar es Salaam, Tanzania, Sawio (22) found that urban agriculture plays a key role in urban household survival in all social groups, including the socially marginal, because it supplements daily food expenses. Further, Zezza and Tasciotti (5) determined a positive correlation between active engagement in urban agriculture and greater dietary diversity for urban households.
Urban agriculture in SSA encounters similar constraints as rural agriculture, which includes a high cost or lack of fertilizers (4) and water availability in rain-fed environments (10). Low moisture availability in urban agricultural soil also contributes to lower fertility (8), which is prevalent during phases of drought (23). However, urban agriculture can also augment environmental problems. For example, in Mwanza, Tanzania, policy makers recognized the contribution of urban agriculture to land degradation through vegetation clearing and runoff from fertilizer and animal waste leading to lake pollution (24).
Enhancing agricultural productivity while reducing the environmental impact of agriculture in urban SSA will require proactive and informed urban planning policies (20, 21, 25, 26). However, there is a lack of critical information about urban areas and the urbanization process (27), especially urban agriculture (28). White and Hamm (29) argue that urban agriculture needs to be understood as an integrated dimension of urban food systems, that incorporates socio-ecological processes. Additionally, since over 50% of the generated municipal solid waste in developing countries is organic in nature (30–33), there is a potential to initiate urban nutrient cycling by transferring nutrients and carbon stored within organic food wastes into the soil for crop cultivation. With rapid urbanization in SSA, this is particularly relevant for urban agriculture since urban waste management is a key factor that influences the sustainable development of urban areas (34). However, agricultural management practices implemented by urban farmers ultimately affect soil fertility and impact the surrounding natural and built environments. Therefore, the forces driving urban agriculture in developing countries, including SSA, must be evaluated from the point of view of urban farmers. Further research is also required on how organic waste produced in urban centers can maintain or enhance soil fertility for urban crop productivity.
While urban agriculture is practiced by 800 million people worldwide, including 29 million households in Africa (34), urban agriculture received little attention in the literature until 2008 (28) despite its significance in developing countries (35). Our goal was to understand the role of urban agriculture as it pertains to urban farmers and amendment application for urban soil management and crop productivity in Mwanza, Tanzania, a rapidly growing city in east Africa. To do this, we evaluated the driving and constraining factors of urban farmers to determine the current state of urban agricultural management practices and the perceived risks of urban farming. We also evaluated the impact of different soil amendment types on soil quality and crop productivity in an urban context. Our aim was to compare advantages and disadvantages between amendments from organic wastes and fertilizers on urban farms. Our research contributes to a current knowledge gap on urban agriculture in developing countries and it enhances our understanding of the challenges and opportunities of urban agriculture and its contribution to soil security and food sovereignty.
2 Material and Methods
2.1 Study Site
The research was conducted in Mwanza City (Figure 1) located within the Nyamagana district of Mwanza Region on the southern shore of Lake Victoria in Northwest Tanzania (36). Mwanza region experiences a bimodal rainfall pattern. The short rainy season (kivuli) between October and December, averages 500 to 800 mm rainfall (36, 37) and the long rainy season (masika) between March and May, averages 1000 to 1400 mm rainfall (36–38). The mean temperatures range from 15.4°C to 18.6°C in the cool (rainy) seasons and 25.7°C to 30.2°C in the hot (dry) seasons (36).
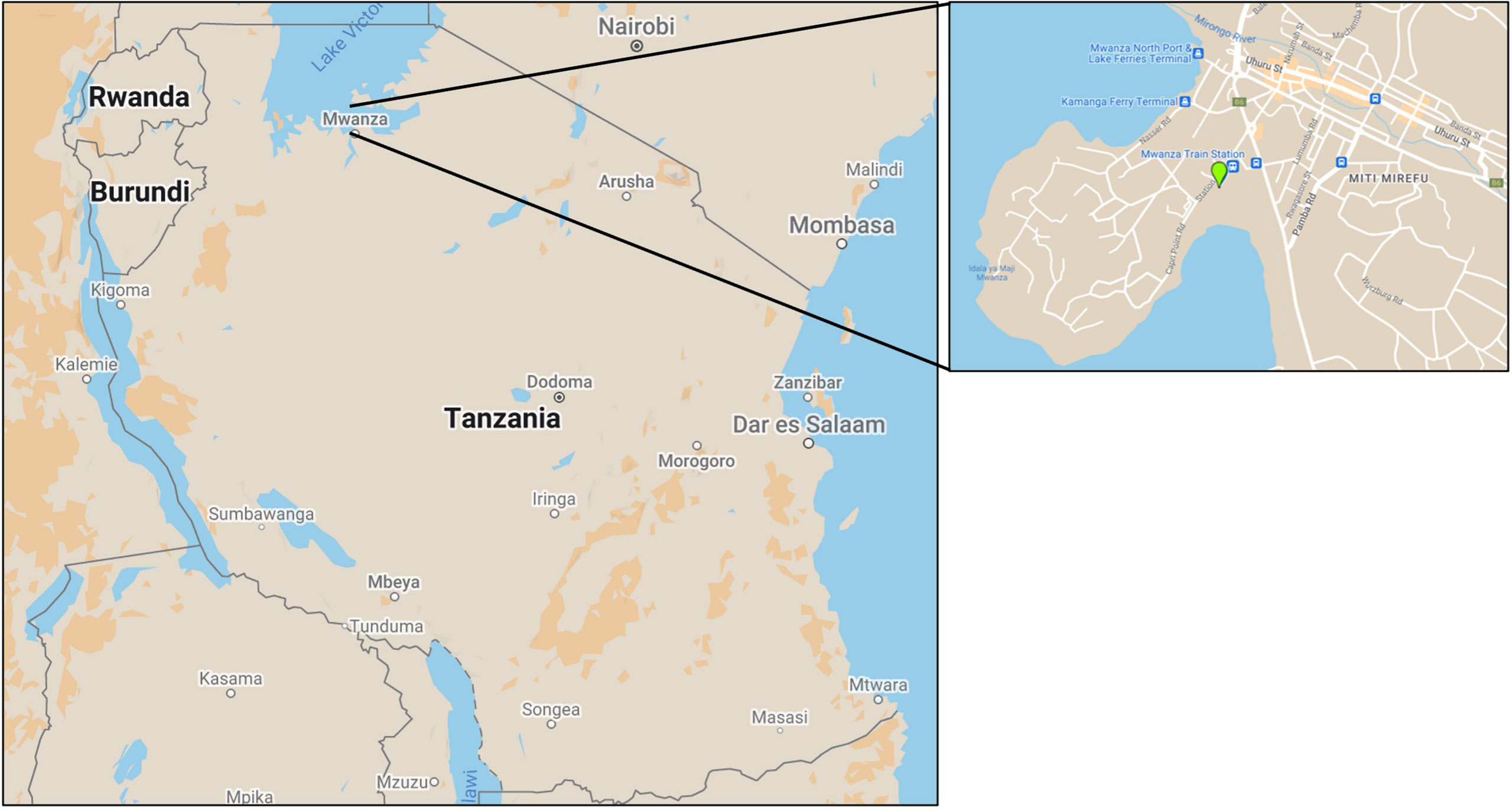
Figure 1 Map depicting the country of Tanzania where Mwanza is located at the shores of Lake Victoria. The enlarged section to the right shows Mwanza, which was the focus of this study, including the location of the field site [2°31’24.71”S, 32°53’51.46”E] (Map data ©2022 Google).
Mwanza is located 1140 m above sea level and is characterized by well-drained sandy loam soil generated from coarse grained cretaceous rock (39). Over 390,000 households engage in agriculture in Mwanza region, averaging 16 agricultural households per square km, with over 55% of households only cultivating crops (37). Nyamagana district was excluded from the last completed agriculture census for Mwanza Region published in 2012 due to the urban nature of the district (37). The Nyamagana district is composed of six rural and twelve urban wards (36), this study focused on seven of the twelve urban wards.
Fruits and vegetables grown in the region include tomatoes (Solanum lycopersicum L.), okra (Abelmoschus esculentus L.), onions (Allium cepa L.), amaranths (Amaranthus var.), cabbage (Brassica oleracea var. capitata L.), spinach (Spinacia oleracea L.), and chillies (Capsicum annuum L.) (37). While there is no distinct difference in the crops grown in the short versus the long rainy season, larger areas are planted, and more households engage in cultivation during the short rainy season than the long rainy season (37).
2.2 Interviews With Urban Farmers
In-depth, semi-structured interviews were conducted with urban farmers in Mwanza’s city center from January to February 2018 using a combination of judgement sampling and snowball sampling; methods used by Flynn (24) in a similar study in Mwanza, Tanzania. We used judgment sampling rather than probability sampling because 1) a sampling framework (e.g., list of urban dwellers practicing urban agriculture) was not available; and 2) to deliberately select participants based on criteria best suited to answering the research question which pertained to urban farmers. These criteria were 1) geographical location to ensure inclusion of the various wards within Mwanza City (Table 1); and 2) indication of urban cultivation.
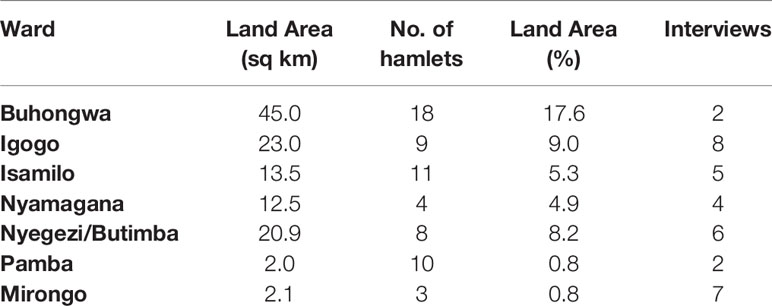
Table 1 Mwanza City ward locations with corresponding information on the land area and the number of hamlets in each ward (Mwanza City Council, 2017; Mwanza City Council, 2008), and the number of interviews conducted in each ward.
The study received guidance and approval from the University of Waterloo Office of Research Ethics. In accordance with ethics requirements, the prospective respondents were provided an information letter with a brief overview of the research, and a participation form to request their consent before the interviews were conducted or recorded. The recorded interviews did not bear any identifying information to ensure anonymity of participants. A total of 34 respondents were interviewed in-person in Kiswahili, Tanzania’s national language. The duration of the interviews ranged from 20 to 50 minutes, depending on the length of responses. The interview guide (c.f., Supplementary Material) consisted of short questions to gather quantitative demographic information (henceforth referred to as descriptors) as well as open-ended questions about cultivation practices, soil fertility, and amendment use.
The interview transcriptions were analysed using Dedoose software (39). This software enables both quantitative analyses for respondent descriptors, and qualitative thematic coding for interview content. The emerging themes were divided into six categories: Personal, Cultivation, Amendments, Soil, Water, and Pests/Disease. Where relevant, excerpts from interviews are presented in Kiswahili (original interview language) followed by an English translation.
2.3 Amendment Field Trial
2.3.1 Agricultural Field Site
A 5 m x 5 m plot located within the boundary of Mwanza City [2°31’24.71”S, 32°53’51.46”E] (Figure 1) was used for our field study. Before our study, the site was used for tomato cultivation. Most recently the site was used to smolder wood for charcoal production causing the entire area to be covered with ash. For our study, the plot was tilled manually to a 15 cm depth using a hand hoe. Debris, including large stones and large pieces of charcoal were removed from the plot. Cultivation techniques and debris removal occurred in accordance with local urban agricultural practices (40). The 5 m x 5 m plot was divided into 16 subplots of 1 m x 1 m. The subplots were designated treatments following a complete randomized design. Treatments were assigned randomly, and each treatment was replicated three times (Figure 2). The treatments included poultry manure (PM), inorganic fertilizer (INF), two types of food waste composts (FWC-1 and FWC-2) and a control (CTRL).
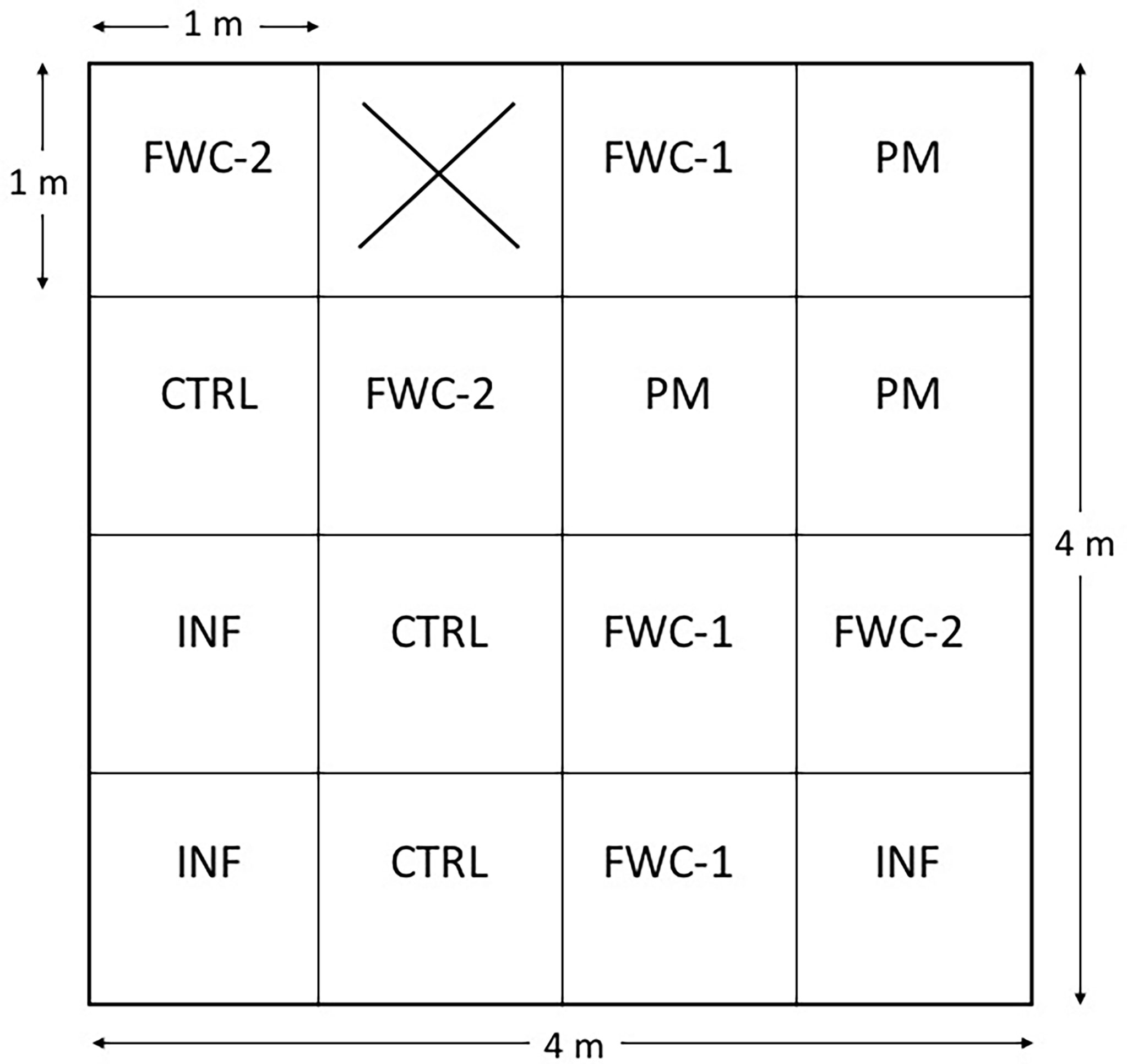
Figure 2 Complete randomized design arrangement of four treatments (PM = poultry manure, FWC-1 = commercial marketplace compost from Dar es Salaam, FWC-2 = food waste compost generated in Mwanza, INF = inorganic nitrogen fertilizer, and CTRL = control). The treatment assignments were randomly generated. The X on the schematic represents a subplot that was not part of the experiment (i.e., no treatment).
Food waste compost was obtained from two different sources including Guavay Company Ltd. in Dar Es Salaam (41). Guavay produces compost from market waste using windrow composting. The company retails this compost commercially (FWC-1). The second type of compost (FWC-2) was generated by the researcher from urban organic (food) wastes in Mwanza using a rapid composting method. The composts were analyzed (c.f., Supplementary Material) at the University of Waterloo to determine the organic carbon (OC), total nitrogen (TN), and phosphorus (P) content (Table 2). The poultry manure was acquired locally from a horticulturalist and the inorganic fertilizer was purchased from an agricultural supplier in Mwanza.
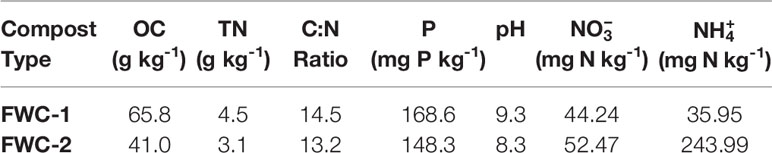
Table 2 Comparison of chemical characteristics of the two types of food waste composts used in this study: compost sourced from Dar es Salaam (FWC-1) and compost generated in Mwanza (FWC-2).
2.3.1.1 Rapid Composting Method
The composting experiment was conducted in December 2017 on a rented plot of land within Mwanza City, which is situated at 2°31’24.71”S 32°53’51.46”E (Figure 1). The chosen area was cleared of debris and a blue tarpaulin was laid on the ground, covering a total area of 1.22 m by 3.05 m. The tarpaulin was secured with a border of flat stones. Each pile had square base dimensions of 0.91 m by 0.91 m, and a height of 38.1 cm to start.
Food wastes were collected from marketplaces, street restaurants, and street fruit vendors within the urban centre over three days. The feedstock (raw materials) comprised of general fruit and vegetable wastes, such as pineapple peels (Ananas comosus L.), watermelon shells (Citrullus lanatus), potato peels (Solanum tuberosum L.), plantain skins (Musa paradisiaca L.), tomatoes (Solanum lycopersicum var.), spinach (Spinacia oleracea L.), amaranth greens (Amaranthus var.), and corn husks (Zea mays L.).
The food wastes were coarsely chopped to 2 cm to 4 cm using a machete, which helps to expedite the composting process (42). Some components of the fibrous food wastes were difficult to chop manually, such as dried corn husks and sugar cane residue, and were left to be greater than 4 cm. On the third day of collection a sufficient volume was collected for pile construction, and the food wastes were distributed evenly into three piles. The wet weight of the feedstock (food waste) in each pile was measured using a locally available body scale and recorded when the piles were constructed. The starting weights of the piles were: 44.3 kg in Pile 1; 43.8 kg in Pile 2; and 44.3 kg in Pile 3. While the collected wastes may have experienced preliminary decomposition on the first and second day of waste collection, the equal distribution between the three piles mitigated the influence of the effects. The day when the compost piles were constructed was labelled as Day 1.
The composting experiment was conducted during the rainy season in Mwanza city. To prevent the piles from getting wet, the piles were covered with a single, woven polyethylene tarp. The tarp exterior was blue and water resistant, and the interior was white. The tarp was propped on stakes set at the corners of the composting area and secured with stones on the front end to keep rainwater away from the piles and to prevent the tarp from flying away in heavy winds. Ventilation, an important component of the composting process (41, 43–45), was provided by creating space for airflow between the tarp and the enclosing net on either side of the area and at the back of the piles, which was 15 cm away from the white concrete wall. The piles were always covered with the tarp except when measuring compost characteristics once per day for 31 days. The compost piles were exposed to additional ventilation during pile turning (41, 44).
The compost piles were monitored for three parameters daily: temperature, pH, and height. Pile height was measured at the centre of each pile from the bottom to the peak of the pile. Before the piles were constructed a small, flat stone, 1 cm in height, was placed at the centre of the square to pinpoint the location once the square was covered with food waste. At each measurement for pile height, a long stick was put through the peak of the pile until it met the stone. A marker was used to mark the depth, which was then measured using a measuring tape.
The temperature dictated the turning of the piles. When the temperature dropped to 43.3°C (which was the threshold on the compost thermometer) or below in the first two weeks, the piles were turned. The piles were turned manually using rubber gloves such that the content that was in the middle of the piles was moved to the outside and the material that was on the outside of the piles was shifted into the centre of the pile. The temperature, pH, moisture, and height of the piles were measured again after the piles were turned. The base dimensions were also measured after the piles were turned. After the second week, the piles were turned when there was a decrease in temperature after already having increased until the temperatures dropped below 26.5°C. At this point the piles were turned one more time and left to mature for one week. The piles were monitored and turned again at the end of the week to ensure no changes had occurred and were left to continue to mature.
At the end of the process, the resulting compost from each pile was weighed and sampled. The samples were sieved <2 mm, air-dried, and sealed in plastic bags in accordance with importing procedures for transport to Canada. The remainder of the compost was utilized in the field trial.
2.3.1.2 Amendment Application and Crop Planting
Soil amendments FWC-1 and PM were applied at a rate of 2.5 kg m-2, whereas FWC-2 was applied at a rate of 1.5 kg m-2. The application rate for FWC-2 was limited by the amount of compost that was generated. The organic amendments were spread out evenly on their designated replicates and mixed into the top 10 cm of the soil with a hand-hoe one week before the first soil sampling, which in turn was one week before the first planting. Amendments were not reapplied during the field trial.
The two crops selected for the field trial were cabbage (Brassica oleracea var. Capitata L.) and amaranth greens (Amaranthus gangeticus L.), both commonly cultivated leafy green vegetables in Mwanza. Amaranth greens (also called mchicha in Kiswahili) are especially cultivated for their three-week maturation period, whereas cabbages can take anywhere from two to five months before harvest. The seeds for both crops were purchased from an agricultural supplier in Mwanza. The crops were co-planted from mid-December 2017 to early January 2018 (first growing period) and from early January to mid-February 2018 (second growing period). Fertilizer was applied either at the time of planting or after seedling emergence. Fertilizer application rate, method and frequency was based on consultation with the agricultural supplier where the fertilizer was purchased and from information provided by the Ukiriguru Agriculture Research Institute (ARI-Ukiriguru) in Mwanza. During the first planting, fertilizer was placed in the hole with the amaranth seeds but was applied around the cabbage seedlings after germination. In the second planting, fertilizer was placed in the hole with cabbage seeds but was applied around the amaranth seedlings after germination. The application of the fertilizer with the seed during planting is indicated by INF*.
Our intention was to primarily use rainfall for crop growth with limited supplementary irrigation on days with no rain. However, the city did not experience a strong rainy period in December 2017, instead the city experienced brief, heavy rains in January 2018.
2.3.1.3 Soil Sampling and Crop Growth
Soil samples were taken from two depths: 0 - 10 cm (D1) and 10 - 20 cm (D2) and at two points in time. Composite soil samples were collected using a soil auger (5 cm diameter) from three individual points randomly selected in a ‘V’ shaped design for each treatment replicate. Edge effects were minimized with a 10 cm border within the edge of each subplot designated to be no-planting and no-sampling zones. The first soil samples were collected one week before planting in early December 2017 (T1), which was one week after organic amendments (PM, FWC-1, and FWC-2) were applied. The second set of soil samples were collected mid-harvest in late January 2018 (T2). Bulk density sampling was conducted by a soil technician from ARI-Ukiriguru at only one point in time, halfway between T1 soil sampling and T2 soil sampling. The soil samples were transported to the soil laboratory at ARI-Ukiriguru where they were analysed for soil texture, bulk density, pH, soil organic carbon (SOC), total nitrogen (TN), available phosphorus (P), electrical conductivity (EC), and cation exchange capacity (CEC). Due to the limited testing capabilities at ARI-Ukiriguru, soils were analyzed for water holding capacity (WHC), ammonium , nitrate , and soil microbial biomass carbon (SMB-C) at the University of Waterloo (c.f. Supplementary Material). During the field trial, heights (cm) of each plant were measured from soil level to the base of the highest leaf using a measuring tape every day for one week after germination. Subsequent measurements were taken every other day until harvest.
2.4 Statistical Analysis
Soil physical, chemical, and biological parameters were analysed for statistical significance (p<0.05) using a three-way factorial (time, treatment, and depth) analysis of variance (ANOVA) in SPSS software (46). The exceptions were soil texture, which was analyzed with a two-way ANOVA, and bulk density with a one-way ANOVA. The data were checked for normality using the Shapiro-Wilk test, though the ANOVA model has been found to be robust against normality assumption violations (47). The data were also checked for homogeneity using Levene’s Test and in cases where the homogeneity assumption was not met, the data were transformed using either a natural logarithm or inverse square root function to satisfy the homogeneity requirement. Statistical significances with respect to treatment were further investigated using post hoc tests (Tukey HSD and LSD) to compare estimated marginal means between treatment groups.
Plant heights were compiled and analyzed for statistical significance in crop growth with respect to time and treatment using repeated measures ANOVA in SPSS software (46). The main assumption for repeated measures ANOVA is sphericity (homogeneity-of-variance-of-differences), which is determined using Mauchly’s test. Where the sphericity assumption was not met, the Lower-bound test and associated adjusted degrees of freedom were used. Homogeneity of data sets were confirmed using Levene’s Test and estimated marginal means were compared with Tukey HSD post hoc tests.
3 Results
3.1 Urban Farmers
Of the 34 interviewed respondents, 19 identified as female (56%) and 15 as male (44%). The ages ranged from 20 to 73 years old, with a relatively uniform distribution across age ranges (Figure 3). Respondents’ education level ranged from no formal education to completion of university (Figure 3), with highest peaks at Standard 7 (41% respondents) and Form 4 (29% respondents). In the Tanzanian education system, Standard 1 - 7 is primary school, with national exams set for passing Standards 4 and 7. Forms 1 - 4 make up the first phase of secondary school, which leads to the Forms 5 and 6 (i.e., the last phase of secondary school), upon completion of the Form 4 examination. More males reported completing Standard 7 as the highest level of formal education, whereas more females reported completing Form 4.
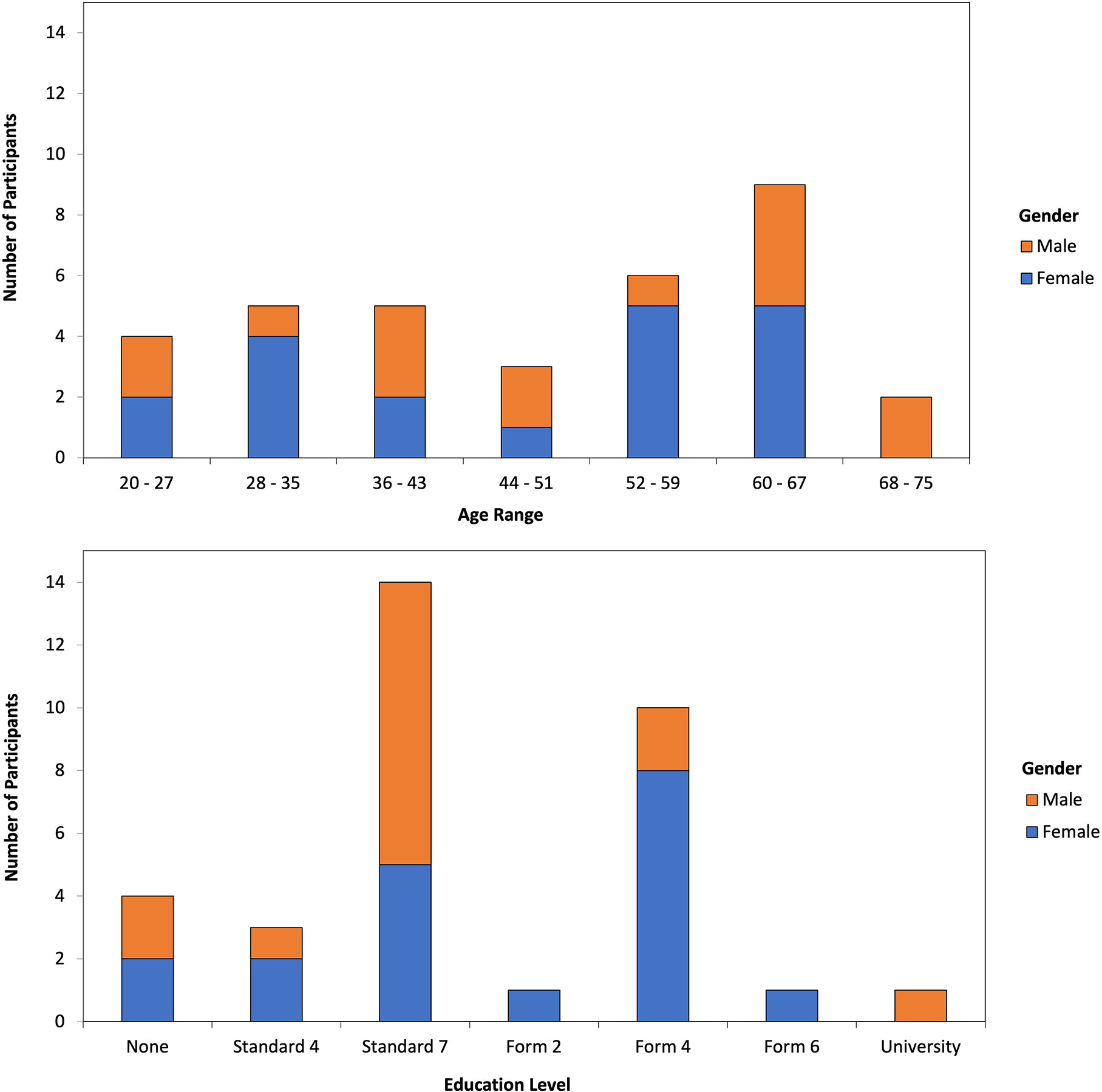
Figure 3 Distribution of urban farmers’ age (top) and education level (bottom) corresponding to their gender. Standard 7 (primary school) completion was reported by more males than females, but Form 4 (secondary school) completion was reported by more females than males.
The education level of interviewed respondents was compared to upbringing locations, which was reported either as village (rural area) or city (urban area). Of the 34 respondents, 8 reported growing up in the city whereas 26 reported having been brought up in village districts (rural areas). Growing up in villages did not mean complete lack of education, as 46% of respondents from rural areas reported completing primary education and 27% completed the first phase of secondary school (Figure 4). There were also several respondents from both city and village upbringings that received little to no formal schooling.
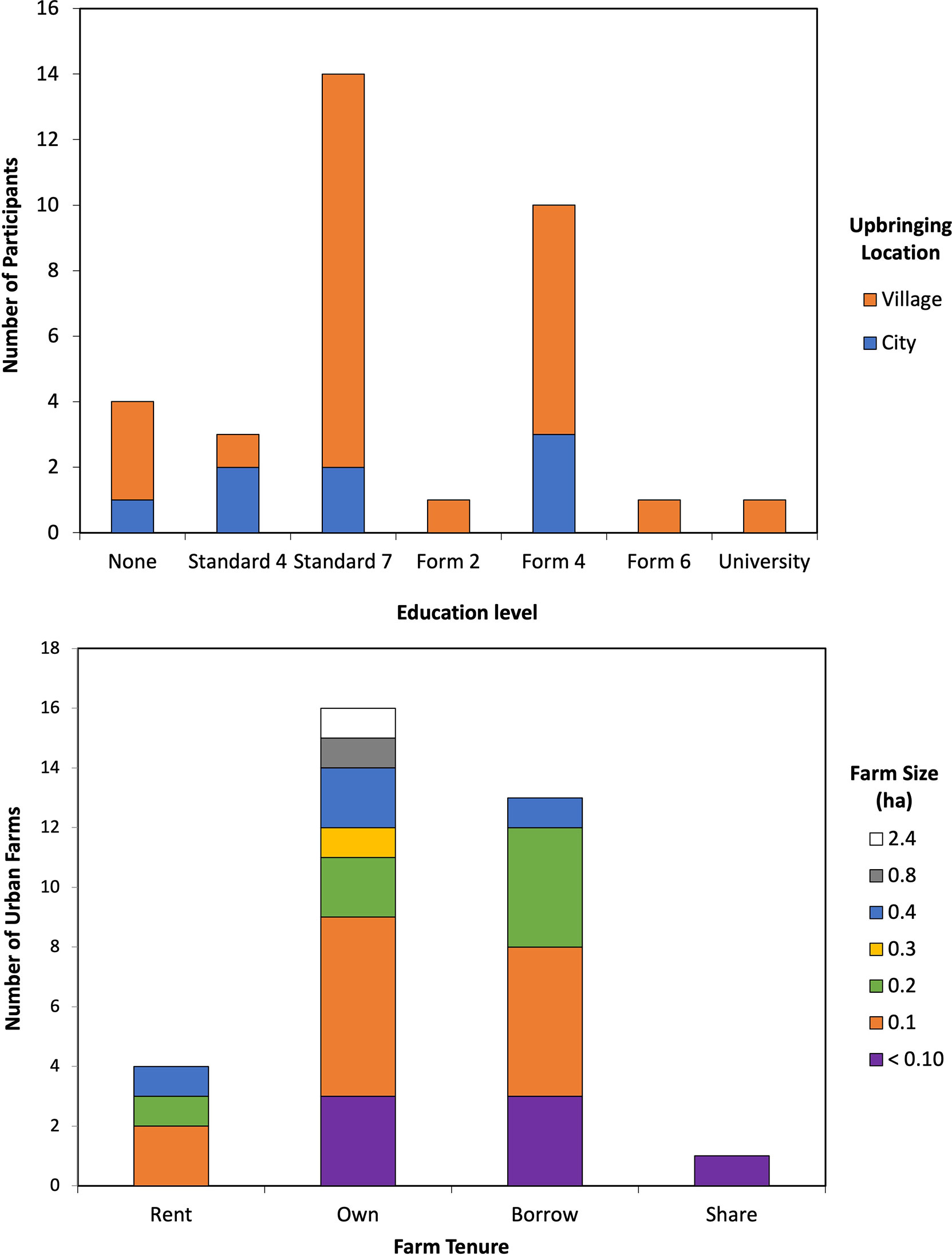
Figure 4 (Top) Distribution of urban farmers’ education level in relation to their upbringing location. More respondents reported upbringing in the village districts than city upbringing and a majority reported completing some form of formal education. (Bottom) Distribution of urban farm tenure corresponding to the urban farm size. Most urban farms are owned, though many urban farms are also borrowed. Farm sizes vary with ownership, though most farms are 0.2 ha or less for both owned and borrowed farms.
Many interview respondents (59%) said they began growing crops in the city over 10 years ago, 58% of whom reported learning to grow crops as children with their families. Many respondents (64%) reported growing up in families of farmers and were taught by their parents or other members of their family. Fewer females (47%) than males (87%) reported coming from families of farmers, and most of these females were over 45 years old. Approximately 41% of respondents began growing crops in the city less than ten years ago, 64% of whom began growing crops in the last five years from the time of this study (2018), of which a majority (89%) were female. The respondents who had not grown up in families of farmers reported learning due to circumstances or by observation of other farms either in the city or in rural areas (Table 3).
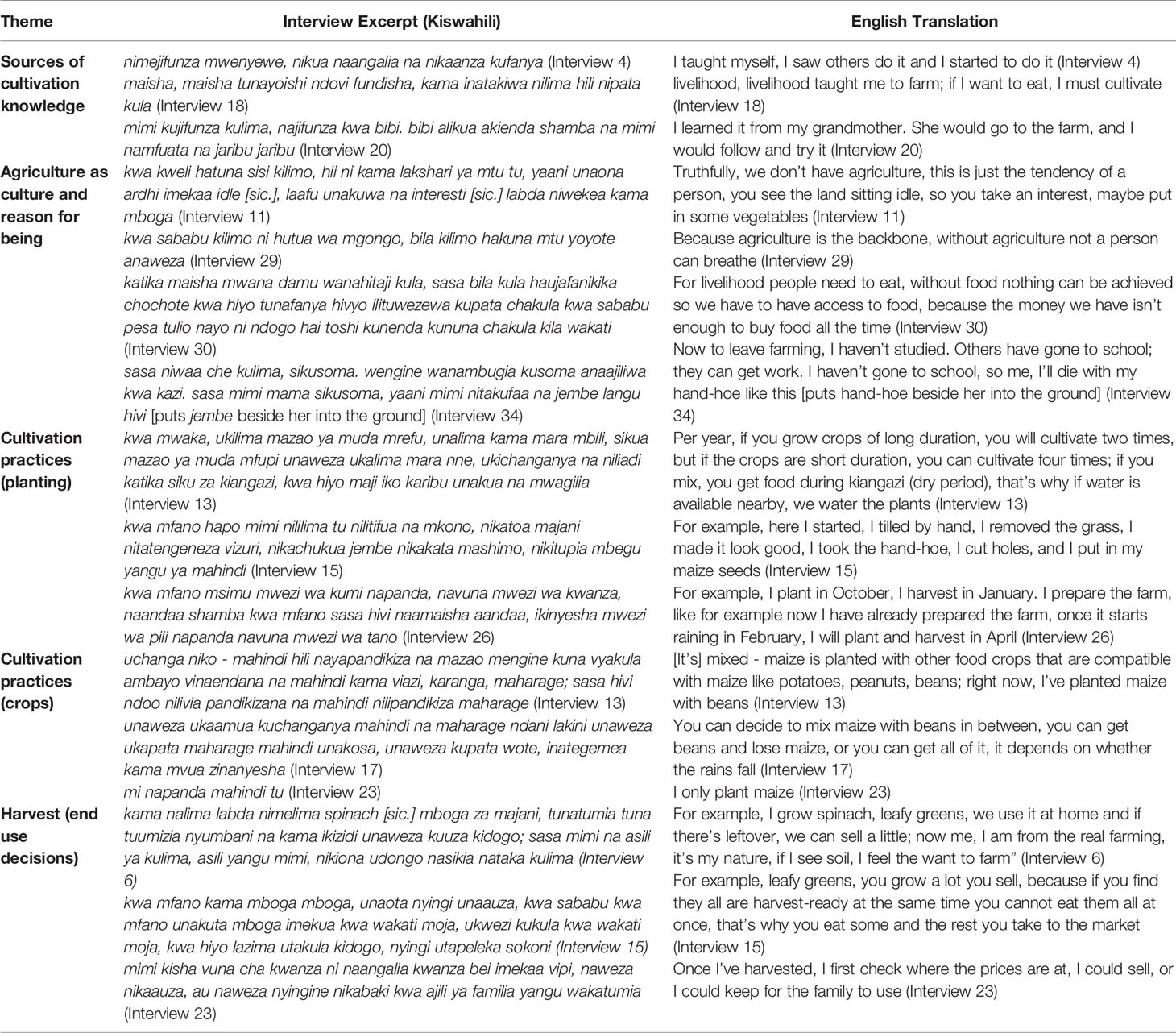
Table 3 Excerpts from interviews that express urban farmers’ motivations, constraints, and cultivation practices with respect to urban agriculture in Mwanza.
3.1.1 Motivations and Constraints
Reasons for growing crops varied and some farmers expressed more than one reason, which included food for subsistence (44%), food to consume and sell, especially that which is surplus (29%), strictly to sell (15%), to help with children’s schooling (24%), for tradition (21%), and for enjoyment/exercise (18%). While many respondents reported producing crops either alone or with one other person, most of them reported having 4 to 6 members in their households. For 65% of respondents, farming was not their only source of income. Either they or their spouse had a primary occupation and urban farming was a supplementary source of livelihood. However, for 35% of the respondents, farming was their only source of income and livelihood.
One of the major constraints in urban areas is land availability. While all interviewed participants had urban farms, 15 respondents reported having more than one farm, 8 of whom said the other farm was in a rural vicinity. The sizes and tenure of the urban farms varied (Figure 4). The farm sizes varied from less than 0.10 ha to 2.43 ha, though most of the farm sizes were at 0.10 ha (41%), less than 0.1 ha (24%), and 0.2 ha (21%). The tenure of the cultivated lands also varied; 47% respondents reported owning the land, 12% reported renting, while the other 41% reported borrowing the land, of which 43% borrowed land belonging to the government. While respondents did not regard land availability and land tenure as a challenge for urban agriculture, they did consider it a restriction to increasing crop production. Goals for their farm also varied among respondents, though most were related to land acquisition, such as wanting to own land (19%), wanting more land area (22%), and being able to grow more crops (28%). Other goals included making money (6%), continuing to provide food for the family (6%), owning farm animals (6%), improving the farm (3%), using store-bought fertilizer (3%), and leaving other work (3%). There were also respondents who reported having no goals (31%), of whom 20% stated the space was not their own to have goals.
In addition to crop cultivation, 22% of respondents reported practicing animal husbandry, predominantly with chickens and ducks, although two respondents also reported keeping goats and pigs. While challenges related to urban agriculture varied among respondents, three major recurring themes were pests (31%), lack of soil amendments or pesticides (22%), and water availability (including dependence on rain) (31%). Other challenges included manual labour (13%), market price variability (9%), lack of money (6%), and lack of support (6%). Some respondents (9%) reported no challenges, stating that it was work to which they had become accustomed.
3.1.2 Cultivation Practices
3.1.2.1 Tools, Frequency, and Weather Reliance for Cultivation
Regarding tools, all (100%) of the respondents reported the use of hand tools, such as a jembe (hand-hoe), panga (machete knife), and rake, of which jembe is the primary tool for cultivation and preparing the soil manually. The jembe is also a symbol of the farmers’ identity. One of the respondents stated that she could not leave farming and would die alongside her jembe when the time comes (Table 3).
Frequency of growing crops was dependent on water sources available to, and accessible by, each urban farmer. There was generally an even divide between respondents cultivating continuously throughout the year (53%) and respondents cultivating only with the rainfall seasons, twice a year (47%). Respondents categorize cultivation in Mwanza as ‘kilimo cha kufuata msimu’ (rain-dependent agriculture) and ‘kilimo mwagiliaji’ (irrigated agriculture). Similarly, cultivated crops are also categorized either as ‘mazao wa msimu’ (seasonal crops), which are primarily grown during the rain seasons, and ‘mazao mwagiliaji’ (irrigated crops) or ‘mazao bila msimu’ (crops with no seasons).
Many respondents (66%) reported relying on weather predictability for cultivation. Most (68%) also reported reliance on rainwater as the primary source of irrigation for growing seasonal crops. A small portion (26%) of these respondents reported having access to another source of water to supplement lack of rain or enable growing crops during the dry ‘kiangazi’ seasons, such as spring water (9%) and municipal water supply (6%). Respondents that did not rely on rain seasons at all relied instead on nearby water sources such as municipal water (9%), rivers (9%), the lake (6%), and wells (6%).
3.1.2.2 Crops Grown, Source of Seeds, and Fate of Harvest
Maize (Zea mays L.) is the primary crop grown during the rainfall seasons, and the main source of maize seeds is from agricultural suppliers in the city. While some respondents reported growing solely maize, other respondents reported co-planting maize with other crops such as potatoes (Solanum tuberosum L.), beans (Phaseolus vulgaris L.), or peanuts (Arachis hypogaea L.) where the crops would alternate in each cultivated row. Rice (Oryza glaberrima S.) is also grown in rainy seasons. Respondents reported growing cassava (Manihot esculenta C.), taro (Colocasia esculenta L.), sugar cane (Saccharum officinarum L.), okra (Abelmoschus esculentus L.), and onions (Allium cepa L.), which do not fall in either seasonal or irrigated category of crops, most likely because they are perennial tropical plants.
Short duration crops include greens, also called ‘mboga mboga’, such as spinach (Spinacia oleracea L.), amaranth greens (Amaranthus gangeticus L.), collard greens (Brassica oleracea var. Viridis), cabbage (Brassica oleracea var. Capitata L.), Chinese cabbage (Brassica rapa L.), and tomatoes (Solanum lycopersicum L.). These crops need to be watered regularly, which is why they are also referred to as ‘mazao mwagiliaji’ and they consist mostly of greens that are ready for harvest within a short period, which are referred to as ‘mazao wa muda mfupi’. Some respondents (14%) who produced crops via continuous cultivation, also reported rotating crops after harvest.
When asked about sources of seeds, agricultural suppliers in the city of Mwanza were reported to be the main resource since they offer a variety of seeds, including hybrid maize seeds. Most urban farmers lacked awareness of the type of seed cultivar (e.g., hybrid seeds), while other urban farmers preferred to obtain seeds from rural farms, whose seeds can be used for future plantings (e.g., non-hybrid seeds). Many of the respondents also reported that management for pests and diseases was necessary especially due to a recent fall armyworm, Spodoptera frugiperda, infestation affecting maize crops across Tanzania (48). While respondents discussed the use of pesticides purchased at agricultural supply shops, some respondents also reported using home-based remedies, such as the use of ash, though the effectiveness of ash was contested among the respondents. One respondent reported using a tobacco-based remedy passed on in his family.
With regards to the harvest, 35% of respondents reported using the harvest for food only, 15% reported growing to sell only, and 50% reported using the harvest for food and to sell, of which 41% reported selling only a little or that which was in surplus. One respondent reported checking market prices first before making the decision of either selling the crops or using them for food (Table 3). The respondents that grew maize and cassava also reported making flour out of the crops for better storage and continued food availability for the family beyond the harvest.
3.1.3 Perspectives on Soil Fertility and Soil Amendments
While perceptions of cultivated soil varied, with some respondents stating that the soil is good and some saying it is not doing so well, most of the respondents (82%) would like to improve their soils. Respondents recognized soil depletion through anthropomorphic statements, such as ‘imechoka udongo’, which means that the soil is tired or ‘ache ipumzikwe’, which means to let it rest, or ‘amechakachaka’, which means it has worn out. Two different ways to gauge soil fertility were reported by respondents: look (colour) and feel (texture) of the soil (38%), and plant growth (44%). One respondent reported having her soil tested and was awaiting results. Some respondents (16%) did not report any means of gauging soil fertility. Respondents reported noticing changes in soil over time as is expressed in the excerpt in Table 4.

Table 4 Excerpts from interviews that express the perspectives of soil fertility and use of amendments among urban farmers in Mwanza, Tanzania.
The choices regarding amendments varied; some use a variety of amendments, some stand firm with their preferred choice, and some even use none. Across all the urban farmers that were interviewed, the use of manure was a common practice, with cow manure as the most popular choice used by 53% of respondents, followed by poultry manure (38%), goat manure (9%), rabbit manure (3%), and pig manure (3%). Other inputs used by urban farmers included inorganic fertilizers bought from agricultural suppliers (38%), crop residue (29%), and waste compost (9%). Respondents who used a variety of amendments demonstrated an understanding of the role the differing amendments can play, with some working to establish crops while others helping crops grow and fruit. Some respondents explained the manure equivalency to store-bought fertilizer (excerpts in Table 4). For manure source, some respondents reported using manure from the animals they kept, some asked neighbours or others in the city that keep animals, and others reported travelling to rural vicinities to get the manure. The frequency of amendment use (manures or inorganic fertilizers), varied among respondents. However, it appeared that manure was generally applied in anticipation of the rainy season. The type and frequency of amendment use also varied year to year with the situation of each respondent.
Respondents recognized the need to improve soil fertility, and several (32%) stated animal manure as the best way to do so. However, some respondents stated that the soil needed fertilizer (9%) or more water (9%). When asked about their opinion of using composted food waste as a soil amendment, 77% of respondents responded positively even though 63% of them had not yet tried any form of food waste-based compost. Some respondents (23%) expressed scepticism regarding compost from food waste, whereas some respondents already made their own composts from kitchen/food wastes. Respondents who reported using leaves on their farm did not initially offer that information when asked about amendment use. The practice of using crop residue, such as leaves of maize and cassava crops, appears to be a habitual aspect of farm preparation, and is considered to work as a fertilizer for the soil. It is also used as a strategy for intercropped plants where residues of harvested crops, such as maize, are returned to the soil for crops still growing, such as cassava (excerpt in Table 4).
3.2 Field Trial
3.2.1 Soil Characteristics
The soil texture of all samples, regardless of depth, was sandy loam, averaging at 72% sand, 14% silt, and 13% clay. There were no significant differences in bulk density among treatments. The mean bulk density was 1.48 g cm-3.
There were no combined interaction effects between any of the factors (time*treatment, time*depth, treatment*depth, or time*treatment*depth) for any of the soil physical, chemical, and biological parameters, though there were significant changes in parameters with respect to time, depth, and treatment (Table 5). From time T1 (Dec 2017) to time T2 (Jan 2018), there were significant increases in SOC (p=0.001), C:N ratio (p=0.009), and pH (p<0.001), i.e., the soil became more alkaline with time (Table 7). There was also a significant increase in CEC (p=0.024) between T1 (9.32 cmol kg-1) and T2 (11.40 cmol kg-1). With respect to depth, WHC (p=0.028), SOC (p=0.014), TN (p=0.001), available P (p<0.001), (p<0.001), and SMB-C (p=0.014) were significantly greater within the 0-10 cm depth than the 10-20 cm depth.
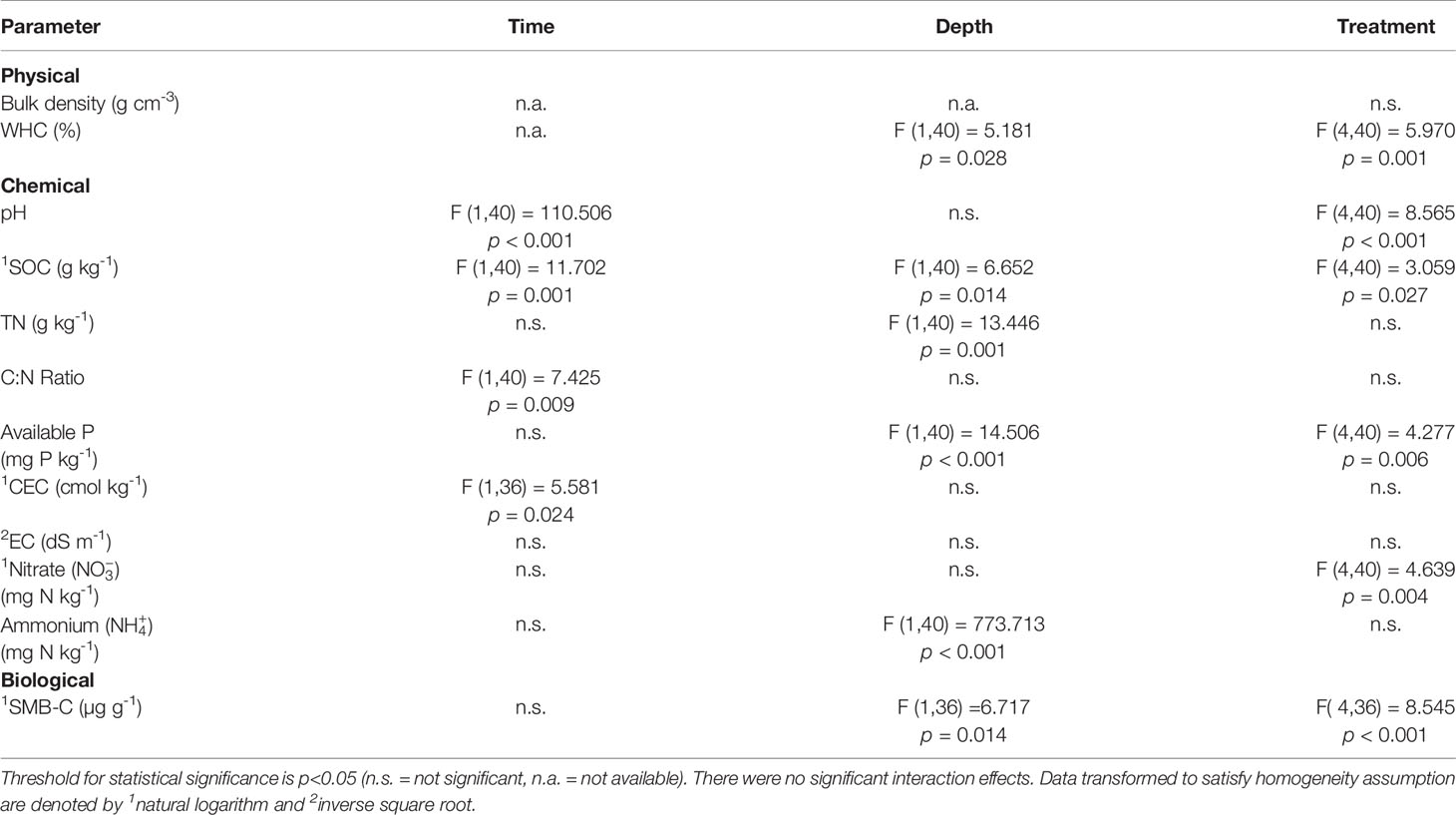
Table 5 Summary of statistical significance of soil physical, chemical, and biological parameters using factorial analysis of variance (ANOVA) for time, treatment, and depth factors.
There were statistically significant differences with respect to treatment in WHC, pH, SOC, available P,, and SMB-C. The WHC was significantly greater in FWC-1 (p=0.002), FWC-2 (p=0.026), and PM (p=0.001) treatments compared to the INF treatments (Table 6). We found that soils with PM were significantly more acidic (p<0.001) compared to the other treatments, including the control, by a value of 0.3 and that SOC in FWC-1 and PM treatments were significantly greater (p<0.05) compared to INF and the control (Table 7). Available soil P was greatest (p=0.006) in the treatment with PM and lowest in the treatment with INF. However, available P was not significantly different between the organic amendments and the control (Table 7). Soil nitrate was significantly lower in the INF-treated soils than in FWC-2 (p=0.004) and PM (p=0.032) treatments (Table 8). Soil microbial biomass C was significantly greater in PM (p<0.001) and FWC-2 (p=0.005) compared to INF and control treatments (Table 8).

Table 6 Physical properties of the soils – soil texture (sand-silt-clay), mean bulk density, and water holding capacity (WHC) – with respect to amendment treatment (food waste composts FWC-1 and FWC-2), poultry manure (PM), inorganic nitrogen fertilizer (INF), and control (CTRL).
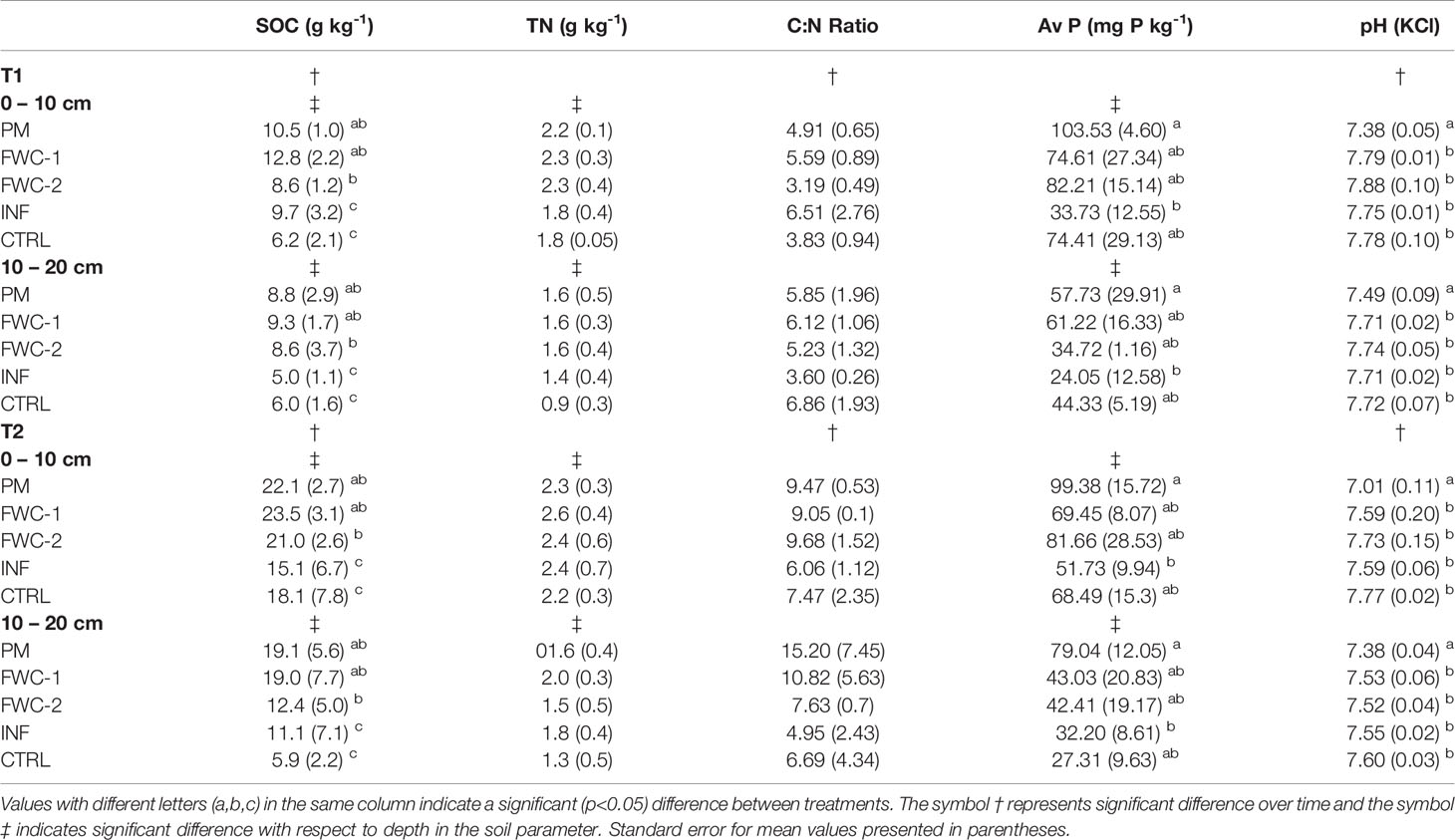
Table 7 Mean values of soil organic carbon, total nitrogen, carbon-to-nitrogen ratio, available phosphorus, and pH at time T1 (Dec 2017) and time T2 (Jan 2018) in the upper (0-10 cm) and lower (10-20 cm) depths for all treatments (food waste composts FWC-1 and FWC-2), poultry manure (PM), inorganic nitrogen fertilizer (INF), and control (CTRL).
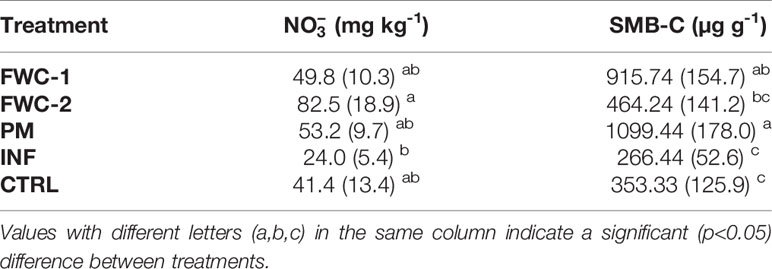
Table 8 Mean values for soil nitrate and microbial biomass-carbon at 0 - 20 cm depth for all treatments (food waste composts FWC-1 and FWC-2), poultry manure (PM), inorganic nitrogen fertilizer (INF), and control (CTRL). Values with different letters (a,b,c) in the same column indicate a significant (p<0.05) difference between treatments.
3.2.2 Crop Growth
In the first growing period, amaranth seeds germinated two days after planting for all treatments, except INF*, where fertilizer was applied with the seeds at time of planting. Germination in INF* treatments was delayed to 6 - 8 days after planting. The amaranth experienced defoliation, due to high wind speeds (≥20 km/h) prevalent at 3, 6, and 7 days after planting. The plants regained their growth and grew significantly (p=0.047) from 8 to 16 days after planting and were harvested 22 days after planting when growth had stagnated. Growth under control and FWC-2 treatments exceeded (p<0.05) that in PM, FWC-1, and INF* treatments (Figure 5). Cabbage seeds germinated in all treatments 7 days after planting in the first growing period, and fertilizer was applied around the cabbage seedlings in the INF treatment replicates 14 days after planting. Cabbages were harvested 55 days after planting; cabbage heights from 51 to 55 days after planting were significantly greater (p<0.05) than 15 to 43 days after planting. Growth under FWC-1 and INF treatments was significantly greater (p<0.05) than growth under PM treatments but was comparable to growth under FWC-2 and control (Figure 5).
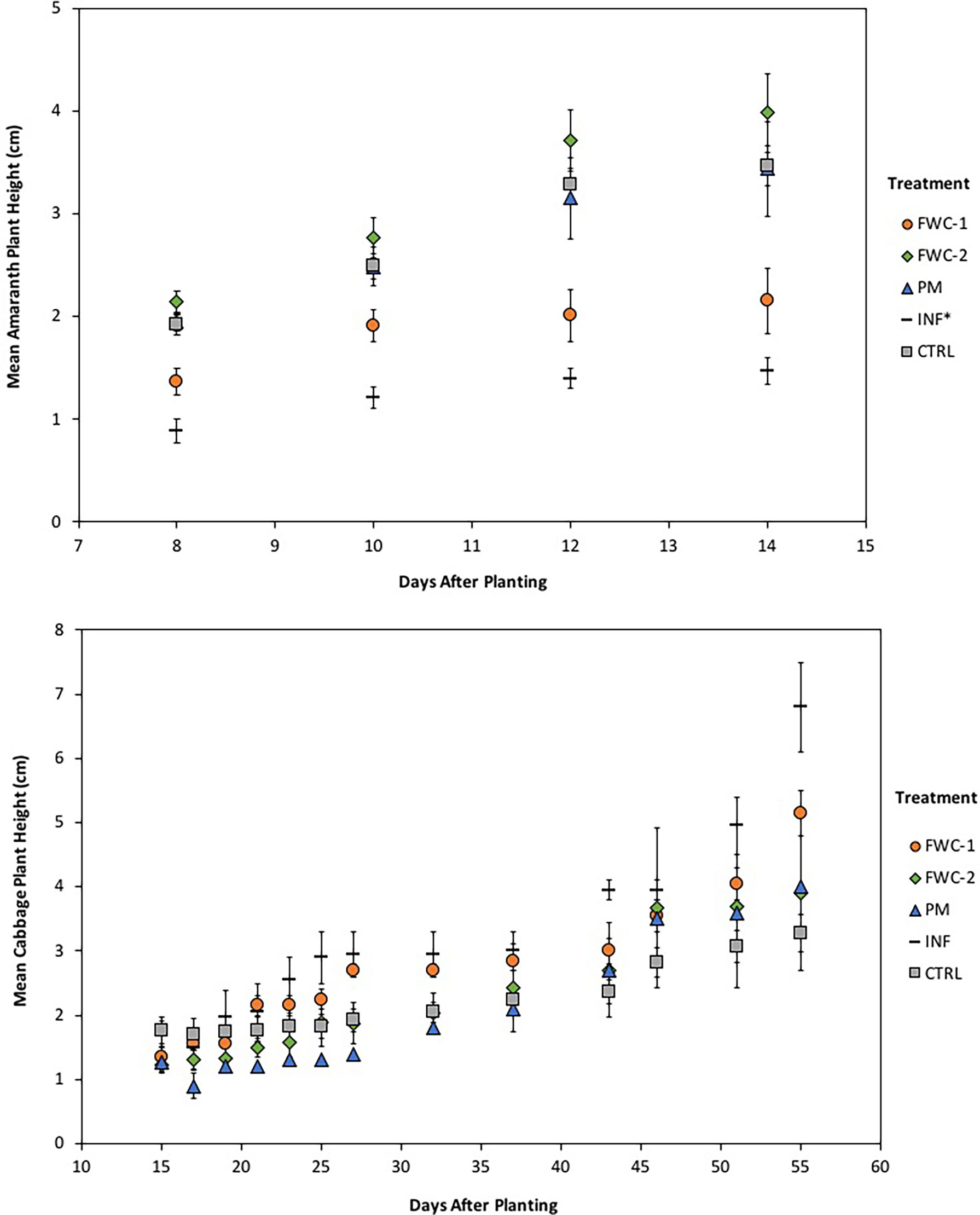
Figure 5 (Top) Growth of amaranth greens for the first planting is shown from 8 to 14 days after planting. The growth in FWC-2 treatments exceeds that of growth in PM and control treatments, which in turn exceed the growth in FWC-1 and INF* treatments. (Bottom) Growth of cabbage for the first growing period is shown from 15 to 55 days after planting. The growth in INF treatments is followed closely by growth in FWC-1 treatments, until 43 days after planting at which point growth in INF treatments exceeds that of other treatments. Cabbage growth in FWC-2 and PM treatments follows similar trends and exceed growth in CTRL treatments.
In the second growing period, amaranth seeds germinated 3 days after planting for all treatments. The amaranth growth was significantly rapid (p<0.05) from 18 to 32 days after planting at which point the plants were harvested. Amaranth growth under PM treatments significantly exceeded (p<0.05) other treatments. While amaranth growth under FWC-1, INF, and control treatments was comparable, they significantly exceeded (p<0.05) growth under FWC-2 treatments (Figure 6). Cabbage seeds in the second growing period germinated in all treatments at 5 days after planting, except INF* treatments where only one cabbage seed germinated at 13 days after planting, out of all three replicates. Growth in INF* treatment, therefore, was excluded from the statistical analyses. Cabbage heights differed significantly (p<0.05) from 21 to 32 days after planting. Cabbage growth under FWC-1, PM, and control treatments was comparable (no significant differences), though growth under PM treatments significantly exceeded (p<0.05) that in FWC-2 treatments (Figure 6). The weather conditions (rain days and daily temperature ranges) as well as the effects of treatment on amaranth and cabbage growth are summarized in Table 9.
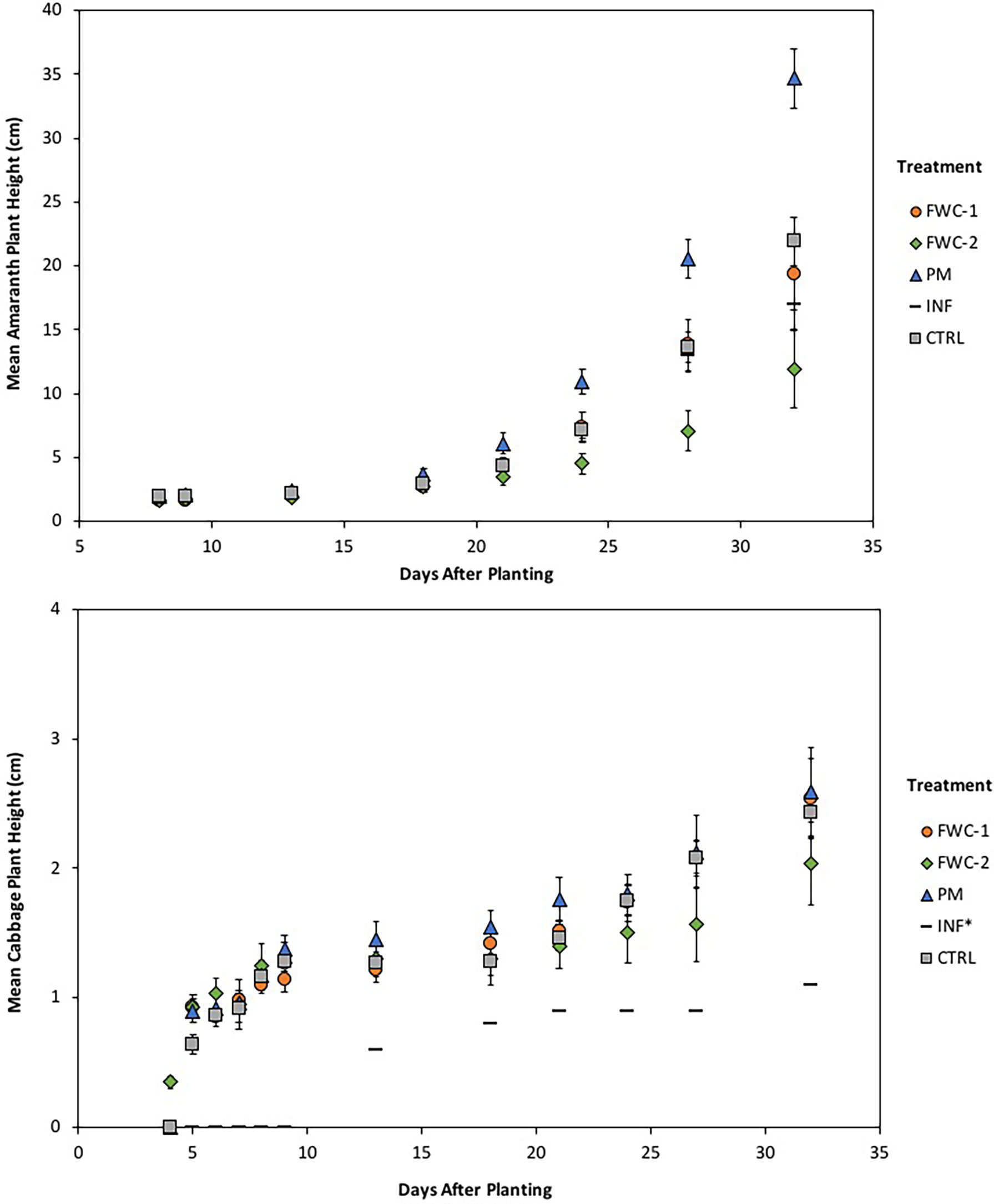
Figure 6 (Top) Growth of amaranth greens for the second planting from 8 to 32 days after planting. From 24 to 32 days after planting, growth in PM treatments exceeds other treatments, growth in FWC-1, CTRL, and INF are similar and exceed growth in FWC-2 treatment. (Bottom) Growth of cabbage for the second planting is shown from 7 to 27 days after planting. The growth in all treatments follows closely with one another until 21 days after planting at which point growth in PM, FWC-1, and CTRL treatments exceeds that of FWC-2 treatments. Cabbage growth INF* treatments is shown without standard error bars because only one seed germinated (excluded from statistical analysis).
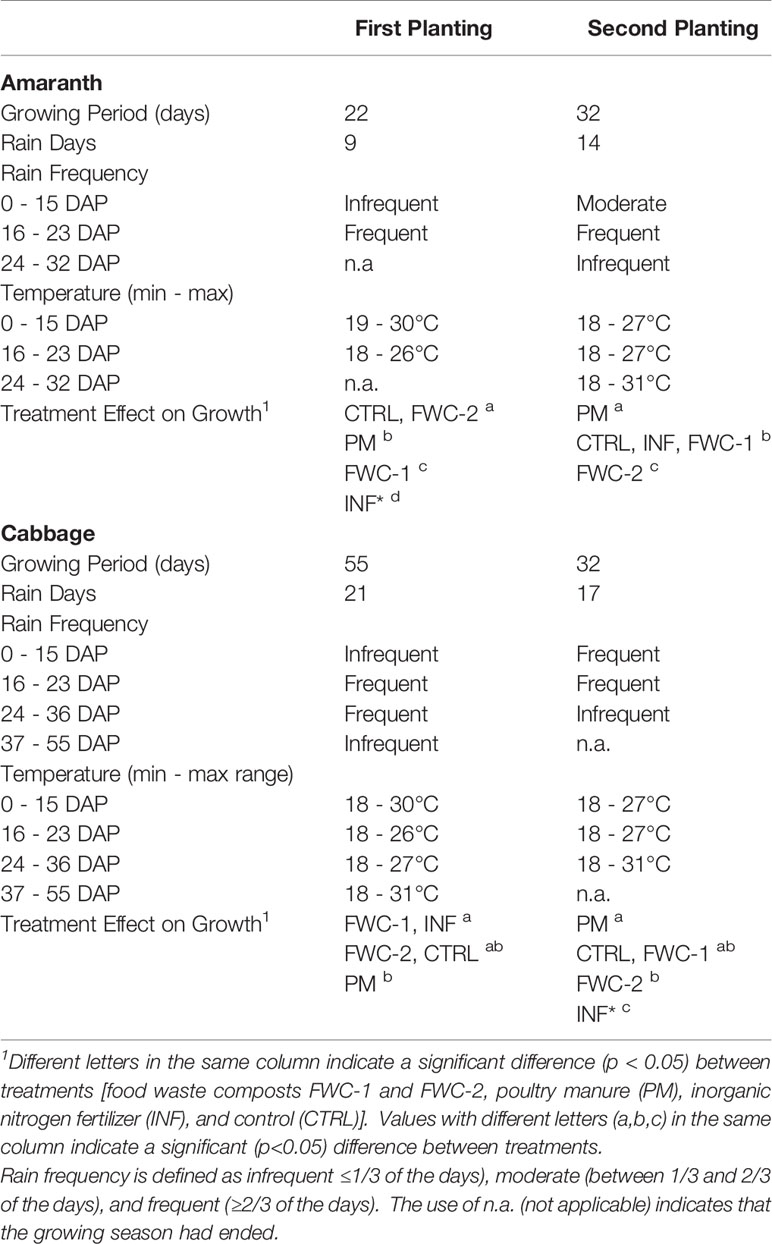
Table 9 Summary of the weather conditions and treatment effects within the two growing periods for amaranth and cabbage plants over the number of days after planting.
4 Discussion
4.1 Urban Farmers in Mwanza: Sources of Knowledge and Motivations
The interviews with farmers illustrated that urban agriculture is a core activity in Mwanza, Tanzania and does not show signs of declining, which reflects the notion that urban agriculture has a long-standing indigenous tradition in Africa (20).
Most of the interviewed participants were engaged in urban agriculture for over ten years, which is similar to other studies that showed agriculture is practiced by established urban residents and not limited to recent rural migrants (22, 24, 49). Most who engaged in urban agriculture learned their farming practices from a young age through knowledge transfer from family members, consistent with observations by Prain et al. (50) in Kampala where farmers involved children as early as age 8. Furthermore, Sawio (22) found that most urban farmers within Dar es Salaam were born in agriculturally rich regions bordering the city. However, Prain et al. (50) and Sawio (22) did not evaluate how and when knowledge of cultivation was passed on to the respondents who now practice urban agriculture. We found that urban farmers predominantly acquired cultivation knowledge from family members, but some also learned through observation and trial and error. We did not find evidence of formal education received by urban farmers, directly or indirectly, for agricultural practices, though there is transfer of knowledge from agricultural suppliers, where seeds, fertilizers, and pesticides can be bought. Therefore, our study contributed to the literature by further illuminating sources of urban farmers’ cultivation knowledge and presents the groundwork to further explore networks of knowledge transfer.
Several interviewed residents were brought up in the city of Mwanza and began practicing urban agriculture within the last ten years. Our study determined that those who began agriculture in the last five years were female. According to Sawio (22), women tend to practice urban agriculture more frequently than men in Dar es Salaam. Other studies also support that mostly women are responsible for cultivating urban agriculture plots (51, 52). Bryld (53) posits that women dominate urban agriculture because the closeness of cultivated plots to the home allows for agricultural activities to fit easily into women’s daily work routines, and that men generally regard agriculture as a marginal activity rather than a serious occupation (53). However, our study found that urban agriculture in Mwanza was not limited to a specific gender or age group, with both males and females amply represented among the respondents, with ages ranging from 20 to 73 years old. While the proximity of the plot to home may enable women to engage more easily in urban agriculture, not all women cultivated plots that were close to home. Several women in this study indicated having more than one farm plot, at least one of which was in a rural vicinity. Additionally, our results do not concur with the argument that men regard urban agriculture as a marginal activity. Both men and women interviewed in this study regarded urban agriculture as a means for livelihood and a way to give life to idle land. This research does support that cultivation is not the only source of livelihood for most households and is used to supplement household income as is the case for much of the Global South (54). Therefore, gender variance in urban agriculture may be the result of economic well-being of individual households, and the city overall, rather than the proximity of land or the attitudes toward urban agriculture.
The reasons for engaging in urban agriculture varied among urban farmers, though sustenance was the main thread. De Bon et al. (21) determined that urban agriculture is a source of food for urban dwellers, primarily for self-consumption. However, with increasing land pressure, selling crops has become the preferred alternative. We found that home consumption was preferred over selling, although some of the respondents also reported cultivating solely to sell. As Howorth (55) concluded, the role of urban agriculture for food production is gaining importance, especially since urban agriculture is a means of employment for a significant proportion of the urban population. As urban growth and pressures on land continue, the fate of urban harvest can be expected to tend strongly toward market options (21).
4.2 Cultivation Practices and Constraints
Urban farming in Mwanza was practiced using manual labour and hand tools, also observed by Prain et al. (50) in Uganda. Hand tools are also commonly used in rural agriculture (56, 57). The hand hoe, specifically, is also a multipurpose tool in Sudanese (57) and Ghanaian (58) agriculture. Makki et al. (57) found that women often use ill-suited hand tools designed for men, but they would rather stop farming than spend resources on tools whose durability they doubt.
One of the major constraints in urban areas is the availability of land for cultivation. There are different terms in the literature for the different agricultural land spaces in urban environments. Flynn (24) described urban cultivated plots as either a kitchen garden, garden, squatter garden, or farm. This is consistent with some of the comments by the respondents who insisted that what they had was a bustani, garden, not a shamba, a farm. This is most likely due to the smaller size of agriculture operations in urban areas. The urban farm sizes in Mwanza were predominantly 0.2 ha or less, with a majority equal to or less than 0.1 ha, which is consistent with the findings of Prain et al. (50) in urban Bukesa, a district in Kampala, Uganda, where plots of more than 0.1 ha were scarce. However, the major goal of interviewed urban farmers was to increase crop production, ideally through more land space. Land constraints, therefore, intensify the need to improve soil fertility for continued food production.
4.3 Soil Perspectives and Improvement
We found that most interviewed farmers want to improve their soil fertility, and most believed manure application was the best way to do so. A similar philosophy was observed by Solomon et al. (59) in Ghana and Liberia where smallholder farmers transformed highly weathered tropical soils to fertile soil that was high in organic matter by intentionally adding organic amendments.
We also found that urban farmers in Mwanza recognize the ability of organic wastes to increase soil fertility, from the use of crop residues to generating compost from kitchen/food wastes. This is in addition to the animal manure, which are also organic wastes. According to Kimaro (60), investments in organic amendments take a long time before paying back, and so may be impractical for farmers who lack clear land tenure systems. However, this study demonstrated in a short-term field trial that organic amendments are comparable to, and in some cases outperform, nitrogen fertilizer use in urban agriculture.
Mwanza Region, and most of the Western Zone of Tanzania, are characterised by sandy loam soils known to have low WHC (38). The typical bulk density of sandy loam soils is 1.51 g cm-3 (61), which is close to the average bulk density of 1.48 g cm-3 at our study site. The addition of organic amendments at the study site improved soil water retention, increased SOC, and enhanced microbial abundance in the sandy loam urban soils of Mwanza City. The WHC was higher by 14 - 19% in soils treated with organic amendments than with nitrogen fertilizer. The increase in WHC is attributed to the presence of soil organic matter content (60, 62). In other studies, the addition of organic amendments such as compost reportedly increased soil water capacity by as little as 14% and as much as 35% (63, 64).
Soils treated with organic amendments in our study had SOC values similar to those reported for Tanzanian soil, whereas SOC of the control soil (9.1 g kg-1) was lower than that reported in the literature (65–67). For example, the research by Sugihara (66) reported 12.4 g kg-1 SOC for Tanzanian soils without any amendments, which is similar to the SOC in the soil amended with FWC-1 at time T1 in this study, and the research by Ndakidemi (65) reported values around 22 g kg-1 SOC, which is similar to the SOC in the soil amended by PM, FWC-1, and FWC-2 at time T2 in this study (Table 7). This indicates that Mwanza’s urban soils are inherently lower in SOC, due to their sandy texture but that can be remedied with organic amendments. Long-term studies found that the addition of organic amendments increased SOC with time (63, 68–70), and that the sustained increase of SOC in soil was dependent on the nature of the organic amendment (69). In this study we did not see a significant difference between treatments, likely due to the short-term (1.5 months) nature of our study.
Total N, CEC, EC, and values from our study are also comparable to other soil studies in Tanzania (65–67). For example, Ndakidemi (65) reported total N values between 0.7 to 2.7 g kg-1 in Tanzanian soils and the results in this study ranged from 0.9 g kg-1 (CTRL) to 2.6 g kg-1 (FWC-1). Our results were also similar to those reported by El-Sharkawi (71) on a loamy soil amended with urea fertilizer (1.9 g kg-1) and organic treatments (2.1 g kg-1 for rice straw-manure compost and 2.2 g kg-1 for sludge treatment) over a 2-year study period. Notably, our results demonstrated increased TN within 1.5 months compared to 2 years (71). Values of soil in our study were greater compared to those reported in other studies on Tanzanian soils (66, 72), which is attributed to increased N-mineralization caused by greater soil microbial activity and microbial biomass in our study (73).
The pH of the non-amended control soils at the field site was also greater than that reported for Tanzanian soils (65). For example, pH ranged from 4.9 to 7.5 among three different studies on Tanzanian soils (60, 65, 66) whereas the soil this study had a pH of 7.7 (CTRL). The higher pH levels at the site can be explained by the presence of wood ash prior to the field trial from the previous use of wood smouldering on the land. Ashes are strongly alkaline (64) and composts produced with wood ash have a higher liming potential (74), similar to the liming potential of biochar, which has been shown to increase the pH of highly weathered tropical soils (73). Soil pH in PM-amended soils was significantly lower because manure is generally more acidic than food or green waste composts (43, 75).
The soils around Mwanza region are of volcanic origin leading to a high soil P content, but low P availability due to the inherently low soil pH (28). The higher available P observed in all treatments in our study was the result of a higher pH in the soil (64). Due to their alkalinity, ash and charcoal (biochar) increase soil pH which enhances plant available P (76, 77).
According to the literature, the input of organic amendments to agricultural soil increases soil microbial activity and the soil microbial biomass SMB-C (66, 68, 69, 72, 74, 78). Our study additionally showed that organic amendment treatments had a significantly greater SMB-C than the fertilizer treatments, suggesting that urban soils of Mwanza benefited from the addition of organic amendments. Furthermore, soil SMB-C content in our study was greater compared to that of other studies (66, 68). For example, values of SMB-C reported for sandy loam soils in India ranged from 120.5 µg C g-1 in control soil to 167.2 µg C g-1 in soils treated with leaves and compost (68). However, the values for SMB-C in our study are comparable to studies where soil and compost had been mixed with wood ash. Gay-des Combes et al. (64) reported 1000 µg C g-1 in compost-treated sandy loam soils and 1200 µg C g-1 in soils treated with a combination of compost and ash. While compost and ash treatments respectively increased SMB-C in sandy clay loam soil, compost-plus-ash treatments had lower SMB-C (769 µg C g-1) than compost-only treatments (929 µg C g-1) (74). Furthermore, the soil microbial biomass was likely influenced by the presence of charcoal and ash in our study. For example, Jien et al. (73) found that the soil microbial community increased when charcoal (biochar) was added to the soil, due to an increase in soil pH which enhanced the activity of the microbial community and the decomposition of organic matter. Therefore, organic amendments, such as composts from food waste, improve soil quality similarly to poultry manure and enhance soil fertility more than inorganic fertilizer application.
4.4 Factors Affecting Crop Growth: Water Availability and Amendment Application
Water availability was the driving factor for interviewed farmers in deciding cultivation frequency, which we divided into three categories: 1) reliance of rain for cultivation, corresponding to bimodal rainy seasons and seasonal (twice-a-year) cultivation of rice or maize (monocrop or intercropped with cassava, beans, or peanuts); 2) production of seasonal crops during the rainy season combined with irrigation from an accessible water source during the dry season to grow short duration vegetables (mboga mboga); and 3) continuous water access resulting in continuous cultivation and allowing for year-round fruits and vegetable production. The variety of crops chosen and planted by the urban farmers in the latter two cultivation choices constitute mixed farming systems (76).
We found that urban farmers in Mwanza determined soil fertility primarily through the ability of plants to grow in the soil, though some also used soil colour and texture as indicators. Additionally, while most urban farmers relied primarily on manure to amend their soil, some used inorganic fertilizers to increase crop growth. Poultry manure is often favoured as a type of manure in tropical countries because of its high P content (17).
For amaranth crops, growth under PM treatment was comparable to control and food waste compost in the first growing period but outperformed all other treatments in the second growing period. Akanbi and Togun (77) found higher amaranth yields with compost made from maize stover and poultry manure compared to fertilizer. Studies on the impact of food waste (compost) amendments on amaranth growth are limited. For example, Aynehband et al. (79) found no significant differences between amaranth grown under only fertilizer or fertilizer combined with compost treatment, but found significant differences in amaranth height and stem diameter under combined fertilizer and vermicompost treatment compared to non-amended soil (79). In our study, amaranth growth under food waste compost was comparable to inorganic fertilizer treatments.
Cabbage growth under INF treatment did significantly better in the first growing period whereas growth under PM treatment was better in the second growing period. However, cabbage growth under FWC-1 was comparable to both inorganic fertilizer in the first growing period and to PM amendment in the second growing period, which demonstrates the stability and consistency of food waste composts in enabling plant growth. Shrestha et al. (80) found that cabbage yields under rain and supplementary irrigation were 26% greater in soils amended with fertilizer, manure, or municipal composts than soils without any amendments. In our study, we advance the recommendation to favour food waste composts over fertilizer or poultry manure as amendments for cabbage growth.
One of the barriers to the use of fertilizer in SSA is the lack of information on how to use fertilizers appropriately (16). Our study supported this statement as the conflicting instructions received for fertilizer application negatively impacted crop germination and crop growth. Applying urea fertilizer with the seeds during planting proved to be the incorrect method as is indicated by the delayed and lack of germination in both amaranth (first planting) and cabbages (second planting). Incorrect fertilizer application had a greater effect on cabbage than amaranth seed germination. Nitrogen fertilizer is highly soluble (17), and the lack of rain at the beginning of the first growing period compared to the abundance of rain at the beginning of the second growing period may have contributed to the suppressed cabbage seed germination. The inorganic N released from the fertilizer in the soil would result in acidic conditions, which are known to hinder cabbage germination and growth (81).
Crop growth under food waste compost was comparable to fertilizer treatment. According to Orsini et al. (76) fruit and vegetable crops have a much higher yield in African countries compared to other food crops, such as grains. However, shifting to fruit and vegetable production from predominantly seasonal cultivation of maize or rice will require access to water for irrigation. Based on our study, compost application will increase soil water holding capacity. The addition of organic amendments will increase crop yield due to improved soil water retention (78).
Admittedly, growth in one of the food waste composts (FWC-2) treatments was consistently lower in the second growing period for both crops, which is attributed to the lower initial application rate (1.5 kg m-2). According to Bass et al. (78), compost is generally not stable over medium to long-term timescales, and so regular reapplication is required over extended periods for significant SOC improvements. Additionally, most African countries do not have standards for the application of manure or compost to land, which makes it difficult to monitor nutrient addition and provide guidelines for organic matter (30). Based on the performance of FWC-1 in our study, the application rate of 2.5 kg m-2 for food waste compost is deemed sufficient for amaranth and cabbage growth and was shown to be stable for a short-term (1.5 month) duration. We recognize, though, that further research is needed to evaluate efficacy of application rates without the influence of wood ash, under differing cropping systems, durations, and watering regimens (e.g., rain-fed versus irrigation).
Uncertainties are increasingly growing concerns for smallholder farmers, such as changing weather patterns that affect predictability of rainy seasons. Nonetheless, our study advances that the use of organic amendments for urban agriculture over the use of inorganic fertilizers may be one of the pathways toward increasing soil security, decreasing vulnerability related to water availability, and increasing crop productivity.
4.5 Study Limitations and Recommendations for Further Research
The soil in this study had wood ash present due to previous land use, which should be taken into consideration for future uses of this study. The presence of wood ash at the site prior to the field experiment had a pronounced effect on the pH of the soil, as well as the available P and SMB-C contents of the soil. Gay-des Combes (64) found that compost and ashes were complementary fertilizing pathways that promote soil fertility through positive effects on soil moisture, pH, organic matter, and microbial activity. Therefore, future studies could also explore the potential for organic amendments blended with wood ash for improving crop productivity in tropical acidic soils.
There are some limitations to wider applicability of this research. This research is based on one study location and the results may be dependent on the characteristics of the study area. More robust sampling from different regions to gather regional perspectives and a large sample size is needed to validate findings at a national level. Additionally, a more comprehensive understanding of urban agriculture is needed from perspectives of other relevant stakeholders, such as the Ministry of Agriculture and city planners. Future research should also examine the complex dynamics of urban agriculture, including the interface between urban/rural agriculture, market opportunities and networks for urban agricultural producers, as well as intrinsic and extrinsic factors of agricultural production within urban environments. Further assessments of other urban soil types are also required for the soil parameters presented in this study as well as additional soil parameters, such as soil porosity and available/total potassium.
Future studies should also examine the nutritional elements and contaminants in the crops grown in urban agriculture, specifically in cabbage and amaranth greens under differing amendment applications. Links between urban agriculture and heavy metal content in food have been documented in the literature as has the potential for soil-borne pathogens due to contact with contaminated water or animal manure (82). Therefore, more in-depth public health assessments are also recommended to evaluate the impacts of organic amendment, specifically manure, application in urbanized areas.
5 Conclusions
This study adds to the currently limited literature on urban agriculture in developing countries and enhances our understanding of the challenges and opportunities presented by urban agriculture. Agriculture in Mwanza engages urban farmers of all skill levels, from adept experts with inherited and tested knowledge of cultivation to recent novices learning by trial and error. Constraints to cultivation include constricted space for crop growth, reliance on rainy seasons for crop irrigation, and declining soil fertility without adequate access to, and availability of, amendments to meet cultivation needs. Urban farmers recognize the need for improving soil fertility to increase crop productivity. Urban farmers tend toward using organic amendments such as manure or crop residue to replenish cultivated soil. This presents an opportunity for rapidly urbanizing cities to create nutrient cycles between growing urban organic (food) wastes and urban agriculture. The outcomes of the study demonstrate that urban agriculture is a strong presence in Mwanza City, which is an indicator of urban agriculture in rapidly urbanizing cities in sub-Saharan Africa in general. The presence of urban agriculture is expected to continue as people who cultivate urban land do so not only for subsistence and income, but also for enjoyment and tradition. To further identify the benefits of urban agriculture to food security and to prepare for mitigation of environmental impacts from cultivation practices, urban agriculture should be given legal consideration and be integrated in urban land use and zoning policies locally and nationally.
The assessments of the effects of varying soil treatments (organic and inorganic) on urban soil quality and crop growth demonstrated that the application of organic amendments improves urban soil quality compared to the application of fertilizer. Soils amended with poultry manure and composts had higher soil water holding capacity and increased soil microbial biomass (carbon), which can be used as indicators for short duration field trials. Additionally, crop growth under compost treatments was comparable to crop growth in fertilized soils and soils without amendments. Crop growth performed best with poultry manure treatments under frequent rain conditions. It was also found that improper use of fertilizer can significantly delay germination and crop growth. Therefore, the study concludes that the use of only fertilizers to improve crop yield should be discouraged, particularly in urban agriculture. Instead, use of organic amendments, such as composts from food waste or manure, should be encouraged. However, future research is required to evaluate standards for compost and manure application rates on urban agricultural soils in Tanzania, especially pertaining to soil quality, crop nutrition, and human health in urbanized areas.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the University of Waterloo Office of Research Ethics. The participants provided their written informed consent to participate in this study.
Author Contributions
First author (SE) is grateful for the opportunity to work with second author (MO) in the fields of soil science and sustainable agriculture. Author MO has an established Soil Ecosystem Dynamics research group with international experience, focusing on sustainable agriculture and the impacts of climate change on plant and soil ecosystems. Author SE was born in Tanzania and came into this research with an environmental engineering background and a specific interest in waste reuse, though soon found a passion for soil security and urban agriculture. Through this research, MO and SE fostered international relationships and gained a deeper understanding of regulations concerning soil management and analyses. It is not often that studies lend themselves to exploring interdisciplinary connections between social sciences and natural sciences. Through this research, the authors broadened their scope as women in science by combining social research methods, like interviews, with quantitative scientific inquiry, such as field experiments. The authors found the interdisciplinary approach provided a more holistic understanding of the influence of urban agriculture on soil security. As women in STEM, the authors’ vision is to collaborate locally and internationally and to generate quality data that inform sound soil management decisions and policies, especially with respect to sustainable agriculture, which can help combat the impacts of climate change on soil security. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge funding and support from the Dean’s Doctoral Initiative (DDI) in the Faculty of Environment at the University of Waterloo as well as the LeDrew International Experience Award and the Faculty Professional Expense Reimbursement Plan provided by the University of Waterloo.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge approval received from Tanzania Commission for Science and Technology (COSTECH) to conduct this research in Tanzania. The authors would like to thank the School of Environment, Resources, and Sustainability at the University of Waterloo and the Soil Ecosystem Dynamics lab group. The authors would also like to thank partners in Tanzania: Ahad from Guavay for the contribution of compost sample and creating the compost market in Dar es Salaam; Anthony Kimaro for providing critical logistical support; Michael Yhdego, pioneering researcher in Tanzania on compost-to-soil input; Makarius Victor Mdemu from Ardhi University; Highness Msuya, Cevin Tibihenda, and Elmens Kaboni from ARI-Ukiriguru; Raheel Kanji for assisting with Kiswahili translations, and especially Shailina Ratansi for providing local accommodation, transportation, and support. This work was largely from the doctoral dissertation of author S.E. (83), who is grateful for the guidance and feedback from committee members, including Andrew Trant, Dawn Bazely, Christine Barbeau, and Chris Opio.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsoil.2022.905664/full#supplementary-material
References
2. FAO. The State of Food and Agriculture. Food and Agriculture Organization of the United Nations (2016). p. 166. Available at: http://books.google.com/books?id=V1qrPwAACAAJ&dq=intitle:Livestock+in+the+balance&hl=&cd=15&source=gbs_api%5Cnpapers2://publication/uuid/F6B30CC3-5968-4026-8CAD-676C21F4EDBC. Rome, Italy.
3. Anderson PML, Okereke C, Rudd A, Parnell S. Regional Assessment of Africa. In: In: Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities: A Global Assessment. Bonn, Germany: SpringerLink (2013). p. 453–9.
4. Sotamenou J, Parrot L. Sustainable Urban Agriculture and the Adoption of Composts in Cameroon. Int J Agric Sustain (2013) 11(3):282–95. doi: 10.1080/14735903.2013.811858
5. Zezza A, Tasciotti L. Urban Agriculture, Poverty, and Food Security: Empirical Evidence From a Sample of Developing Countries. Food Policy [Internet]. (2010) 35(4):265–73. doi: 10.1016/j.foodpol.2010.04.007
6. Lal R. Managing Soils and Ecosystems for Mitigating Anthropogenic Carbon Emissions and Advancing Global Food Security. Bioscience (2010) 60(9):708–21. doi: 10.1525/bio.2010.60.9.8
7. Drechsel P, Kunze D, Penning de Vries F. Soil Nutrient Depletion and Population Growth in Sub-Saharan Africa : A Malthusian Nexus? Popul. Environ (2014) 22(4):411–23.
8. Bationo A. Constraints and New Opportunities for Achieving a Green Revolution in Sub-Saharan Africa Through Integrated Soil Fertility Management. In: Proc Int Plant Nutr Colloq XVI (2009). Available at: https://escholarship.org/uc/item/7hr282j2. Department of Plant Sciences.
9. European Commission. Soil Atlas of Africa. In: Soil Atlas of Africa (2013). EU Publications Office, Luxembourg, Luxembourg. Available at: http://eusoils.jrc.ec.europa.eu/library/maps/africa_atlas/.
10. Tittonell P, Giller KE. When Yield Gaps are Poverty Traps: The Paradigm of Ecological Intensification in African Smallholder Agriculture. F. Crop Res [Internet]. (2013) 143:76–90. doi: 10.1016/j.fcr.2012.10.007
11. Sanchez PA, Shepherd KD, Soule MJ, Place FM, Buresh RJ, Izac A-MN, et al. Soil Fertility Replenishment in Africa: An Investment in Natural Resource Capital. In: Buresh RJ, Sanchez PA, Calhoun F, editors. Replenishing Soil Fertility in Africa, Volume 51. SSSA Speci. Soil Science Society of America, Inc. Madison, WI: American Society of Agronomy, Inc (1997). p. 1–46.
12. Smaling EMA, Nandwa SM, Janssen BH. Soil Fertility in Africa Is at Stake. In: Buresh RJ, Sanchez PA, Calhoun F, editors. Replenishing Soil Fertility in Africa, vol. 51 . Madison, WI: SSSA Speci. Soil Science Society of America, Inc. American Society of Agronomy, Inc (1997). p. 47–61.
13. Gilbert N. Dirt Poor: The Key to Tackling Hunger in Africa is Enriching its Soil. The Big Debate is About How to do it. Nature (2012) 483(7391):525. doi: 10.1038/483525a
14. Wanzala M, Groot R. Fertiliser Market Development in Sub-Saharan Africa. In.: Int Fertil. Soc. (2013) 731:1–33.
15. Wichelns D. Policy Recommendations to Enhance Farm-Level Use of Fertilizer and Irrigation Water in Sub-Saharan Africa. J Sust. Agric (2003) 23(2):53–77. doi: 10.1300/J064v23n02_06
16. McIntire JM. Transforming African Agriculture. Glob. J Emerg Mark. Econ. (2014) 6(2):145–79. doi: 10.1177/0974910114525697
17. Thangarajan R, Bolan NS, Tian G, Naidu R, Kunhikrishnan A. Role of Organic Amendment Application on Greenhouse Gas Emission From Soil. Sci Tot. Environ (2013) 465:72–96. doi: 10.1016/j.scitotenv.2013.01.031
18. Drechsel P, Gyiele L, Kunze D, Cofie O. Population Density, Soil Nutrient Depletion, and Economic Growth in Sub-Saharan Africa. Ecol Econ (2001) 38(2):251–8. doi: 10.1016/S0921-8009(01)00167-7
19. Baillie IC, Anderson JM, Ingram JSI. Tropical Soil Biology and Fertility: A Handbook of Methods. J Ecol 2nd. Ed (1990) 78(2):547. doi: 10.2307/2261129
20. Drechsel P, Dongus S. Dynamics and Sustainability of Urban Agriculture: Examples From Sub-Saharan Africa. Sust Sci (2010) 5(1):69–78. doi: 10.1007/s11625-009-0097-x
21. De Bon H, Parrot L, Moustier P. Sustainable Urban Agriculture in Developing Countries. A. Rev Agron Sust Dev (2010) 30:21–32. doi: 10.1051/agro:2008062
22. Sawio CJ. Who Are the Farmers of Dar Es Salaam? In: Cities Feeding People. Ottawa.: Int Dev Res Centre; (1994) p:23–44.
23. Malley ZJU, Taeb M, Matsumoto T, Takeya H. Linking Perceived Land and Water Resources Degradation, Scarcity and Livelihood Conflicts in Southwestern Tanzania: Implications for Sustainable Rural Livelihood. Environ Dev Sustain (2008) 10(3):349–72. doi: 10.1007/s10668-006-9069-9
24. Flynn KC. Urban Agriculture in Mwanza, Tanzania. Africa (Lond) [Internet] (2001). Available at: http://www.scopus.com/inward/record.url?eid=2-s2.0-0039149616&partnerID=40&md5=6d7e37f7696944b2c644ee3d8f2ac401.
25. Game I, Primus R. GSDR 2015 Brief: Urban Agriculture [Internet] (2015). Available at: https://sustainabledevelopment.un.org/content/documents/5764Urban%20Agriculture.pdf.
26. Ayambire RA, Amponsah O, Peprah C, Takyi SA. A Review of Practices for Sustaining Urban and Peri-Urban Agriculture: Implications for Land Use Planning in Rapidly Urbanising Ghanaian Cities. Land. Use Policy [Internet] (2019) 84(February):260–77. doi: 10.1016/j.landusepol.2019.03.004
27. Seto KC, Parnell S, Elmqvist T, Güneralp B, Marcotullio P J., McDonald R I.. A Global Outlook on Urbanization. In: Elmqvist T, Fragkias M, Goodness J, Güneralp B, McDonald RI, Marcotullio PJ, et al, editors. Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities. Springer (2013). p. 1–12. Dordrecht & Heidelberg & New York & London
28. Graefe S, Buerkert A, Schlecht E. Trends and Gaps in Scholarly Literature on Urban and Peri-Urban Agriculture. Nutr Cycl. Agroecosys. [Internet]. (2019) 115(2):143–58. doi: 10.1007/s10705-019-10018-z
29. White SA, Hamm MW. A View From the South: Bringing Critical Planning Theory to Urban Agriculture. In: WinklerPrins A, editor. Global Urban Agriculture. Boston, Massachusetts: CABI Publishing (2017). p. 12–23.
30. Couth R, Trois C. Cost Effective Waste Management Through Composting in Africa. Waste. Manag. [Internet] (2012) 32(12):2518–25. doi: 10.1016/j.wasman.2012.05.042
31. Narayana T. Municipal Solid Waste Management in India: From Waste Disposal to Recovery of Resources? Waste. Manag. [Internet] (2009) 29(3):1163–6. doi: 10.1016/j.wasman.2008.06.038
32. Sujauddin M, Huda SMS, Hoque ATMR. Household Solid Waste Characteristics and Management in Chittagong, Bangladesh. Waste. Manage (2008) 28(9):1688–95. doi: 10.1016/j.wasman.2007.06.013
33. Parizeau K, Maclaren V, Chanthy L. Budget Sheets and Buy-in: Financing Community-Based Waste Management in Siem Reap, Cambodia. Environ Urban (2008) 20(2):445–63. doi: 10.1177/0956247808096122
34. Lorenz K. Organic Urban Agriculture. Soil Sci (2015) 180(4–5):146–53. doi: 10.1097/SS.0000000000000129
35. Hamilton AJ, Burry K, Mok HF, Barker SF, Grove JR, Williamson VG. Give Peas a Chance? Urban Agriculture in Developing Countries. A. Rev. Agron Sust. Dev (2014) 34(1):45–73. doi: 10.1007/s13593-013-0155-8
36. Mwanza City Council. Mwanza City Council Socio-Economic Profile 2016. Mwanza, Tanzania: National Bureau of Statistics & Mwanza City Council (2017). Available at: http://www.mwanzacc.go.tz.
37. National Bureau of Statistics. National Sample Census of Agriculture 2007/2008. In: Regional Report - Mwanza Region, vol. V. Mwanza, Tanzania: Ministry of Agriculture & National Bureau of Statistics (2012).
38. National Bureau of Statistics. Environment Statistics 2014. Environment Statistics in Tanzania Mainland In: Dar es Salaam: National Bureau of Statistics (2015).
39. Dedoose Version 7.0.23. Web Application for Managing, Analyzing, and Presenting Qualitative and Mixed Method Research Data. Los Angeles, CA: SocioCultural Research Consultants. LLC (2019). Available at: www.dedoose.com.
40. Mwanza City Council. Mwanza City Profile. Mwanza, Tanzania: National Bureau of Statistics & Mwanza City Council (2008).
41. Mussari FP, Smith JE, Midlane D, Finch R, Norris M. Accelerated Composting Methods and Equipment. Proc Water Environ Fed (2013) 5:15–26. doi: 10.2175/193864713813536967
42. Oberlin AS, Szanto GL, Szántó GL. Community Level Composting in a Developing Country: Case Study of KIWODET, Tanzania. Waste. Manag. Res [Internet]. (2011) 29(10):1071–7. doi: 10.1177/0734242X11402871
43. Fernandes L, Zhan W, Patni NK, Jui PY. Temperature Distribution and Variation in Passively Aerated Static Compost Piles. Bioresour Technol (1994) 48(3):257–63. doi: 10.1016/0960-8524(94)90155-4
44. Guo R, Li G, Jiang T, Schuchardt F, Chen T, Zhao Y, et al. Effect of Aeration Rate, C/N Ratio and Moisture Content on the Stability and Maturity of Compost. Bioresour. Technol [Internet]. (2012) 112:171–8. doi: 10.1016/j.biortech.2012.02.099
45. Abdoli MA, Omrani G, Safa M, Samavat S. Comparison Between Aerated Static Piles and Vermicomposting in Producing Co-Compost From Rural Organic Wastes and Cow Manure. Int J Environ Sci Technol (2019) 16(3):1551–62. doi: 10.1007/s13762-017-1607-5
46. Page MC, Braver SL, MacKinnon DP. Levine’s Guide to SPSS for Analysis of Variance. 2nd Editio. Mahwah, New Jersey: Lawrence Erlbaum Associates, Inc (2003).
47. Schmider E, Ziegler M, Danay E, Beyer L, Bühner M. Is It Really Robust?: Reinvestigating the Robustness of ANOVA Against Violations of the Normal Distribution Assumption. Methodology (2010) 6(4):147–51. doi: 10.1027/1614-2241/a000016
48. Sisay B, Simiyu J, Mendesil E, Likhayo P, Ayalew G, Mohamed S, et al. Fall Armyworm, Spodoptera Frugiperda Infestations in East Africa: Assessment of Damage and Parasitism. Insects (2019) 10(7):1–10. doi: 10.3390/insects10070195
49. Schmidt S. Getting the Policy Right: Urban Agriculture in Dar Es Salaam, Tanzania. Int Dev Plan Rev (2012) 34(2):129–45. doi: 10.3828/idpr.2012.9
50. Prain G, Karanja N, Lee-Smith D. African Urban Harvest. Springer: International Development Research Centre (2010).
51. Maxwell DG. Alternative Food Security Strategy: A Household Analysis of Urban Agriculture in Kampala. World Dev (1995) 23(10):1669–81. doi: 10.1016/0305-750X(95)00073-L
52. Mbiba B. Urban Agriculture in Harare: Between Suspicion and Repression. In: Bakker M, Dubbeling S, Guendel U, Koschella S, de Zeeuw H, editors. Growing Cities, Growing Food: Urban Agriculture on the Policy Agenda. Feldafing, Germany: DSE (2000). p. 285–302.
53. Bryld E. Potentials, Problems, and Policy Implications for Urban Agriculture in Developing Countries. Agric Hum Val. (2003) 20(1):79–86. doi: 10.1023/A:1022464607153
54. WinklerPrins AMGA. Global Urban Agriculture. Global Urban Agriculture. Boston, Massachusetts: CABI Publishing (2017) p. 1–255.
55. Howorth C, Convery I, O’Keefe P. Gardening to Reduce Hazard: Urban Agriculture in Tanzania. L. Degrad. Dev (2001) 12(3):285–91. doi: 10.1002/ldr.441
56. Doraiswamy PC, McCarty GW, Hunt ER, Yost RS, Doumbia M, Franzluebbers AJ. Modeling Soil Carbon Sequestration in Agricultural Lands of Mali. Agric Syst (2007) 94(1):63–74. doi: 10.1016/j.agsy.2005.09.011
57. Makki EK, Lodala MJ, Diko B. Women Farmers and Agricultural Hand Tools in Terekaka, Southern Sudan. Ahfad. J (2009) 26(2):47–58.
58. Naab JB, Mahama GY, Yahaya I, Prasad PVV. Conservation Agriculture Improves Soil Quality, Crop Yield, and Incomes of Smallholder Farmers in North Western Ghana. Front Plant Sci (2017) 8(June):1–15. doi: 10.3389/fpls.2017.00996
59. Solomon D, Lehmann J, Fraser JA, Leach M, Amanor K, Frausin V, et al. Indigenous African Soil Enrichment as a Climate-Smart Sustainable Agriculture Alternative. Front Ecol Environ (2016) 14(2):71–6. doi: 10.1002/fee.1226
60. Kimaro J. A Review on Managing Agroecosystems for Improved Water Use Efficiency in the Face of Changing Climate in Tanzania. Adv Meteorol (2019) 2019:1–12. doi: 10.1155/2019/9178136
61. Gardiner DT, Miller RW. Soil Fertility Management. In: Soils in Our Environment. Eleven. Columbus, Ohio: Pearson Prentice Hall (2008).
62. Evanylo G, Sherony C, Spargo J, Starner D, Brosius M, Haering K. Soil and Water Environmental Effects of Fertilizer-, Manure-, and Compost-Based Fertility Practices in an Organic Vegetable Cropping System. Agric Ecosyst Environ (2008) 127(1–2):50–8. doi: 10.1016/j.agee.2008.02.014
63. Agegnehu G, Bass AM, Nelson PN, Muirhead B, Wright G, Bird MI. Biochar and Biochar-Compost as Soil Amendments: Effects on Peanut Yield, Soil Properties and Greenhouse Gas Emissions in Tropical North Queensland, Australia. Agric Ecosyst Environ [Internet] (2015) 213:72–85. doi: 10.1016/j.agee.2015.07.027
64. Gay-des-Combes JM, Sanz Carrillo C, Robroek BJM, Jassey VEJ, Mills RTE, Arif MS, et al. Tropical Soils Degraded by Slash-and-Burn Cultivation can be Recultivated When Amended With Ashes and Compost. Ecol Evol (2017) 7(14):5378–88. doi: 10.1002/ece3.3104
65. Ndakidemi PA, Semoka JMR. Soil Fertility Survey in Western Usambara Mountains, Northern Tanzania. Pedosphere (2006) 16(2):237–44. doi: 10.1016/S1002-0160(06)60049-0
66. Sugihara S, Funakawa S, Kilasara M, Kosaki T. Dynamics of Microbial Biomass Nitrogen in Relation to Plant Nitrogen Uptake During the Crop Growth Period in a Dry Tropical Cropland in Tanzania. Soil Sci Plant Nutr (2010) 56(1):105–14. doi: 10.1111/j.1747-0765.2009.00428.x
67. Kamiri H, Kreye C, Becker M. Dynamics of Agricultural Use Differentially Affect Soil Properties and Crop Response in East African Wetlands. Wetl. Ecol Manage (2013) 21(6):417–31. doi: 10.1007/s11273-013-9315-5
68. Sharma KL, Grace JL, Mandal UK, Gajbhiye PN, Srinivas K, Korwar GR, et al. Evaluation of Long-Term Soil Management Practices Using Key Indicators and Soil Quality Indices in a Semi-Arid Tropical Alfisol. Aust J Soil Res (2008) 46:368–77. doi: 10.1071/SR07184
69. Ngo PT, Rumpel C, Doan TT, Jouquet P. The Effect of Earthworms on Carbon Storage and Soil Organic Matter Composition in Tropical Soil Amended With Compost and Vermicompost. Soil Biol Biochem [Internet]. (2012) 50:214–20. doi: 10.1016/j.soilbio.2012.02.037
70. Doan TT, Henry-Des-Tureaux T, Rumpel C, Janeau JL, Jouquet P. Impact of Compost, Vermicompost and Biochar on Soil Fertility, Maize Yield and Soil Erosion in Northern Vietnam: A Three Year Mesocosm Experiment. Sci Tot. Environ [Internet]. (2015) 514:147–54. doi: 10.1016/j.scitotenv.2015.02.005
71. El-Sharkawi HM. Effect of Nitrogen Sources on Microbial Biomass Nitrogen Under Different Soil Types. ISRN. Soil Sci (2012) 2012:1–7. doi: 10.5402/2012/310727
72. Srivastava P, Singh R, Bhadouria R, Tripathi S, Singh P, Singh H, et al. Organic Amendment Impact on SOC Dynamics in Dry Tropics: A Possible Role of Relative Availability of Inorganic-N Pools. Agric Ecosyst Environ [Internet]. (2016) 235:38–50. doi: 10.1016/j.agee.2016.09.036
73. Jien SH, Kuo YL, Liao CS, Wu YT, Igalavithana AD, Tsang DCW, et al. Effects of Field Scale in Situ Biochar Incorporation on Soil Environment in a Tropical Highly Weathered Soil. Environ Pollut [Internet]. (2021) 272:116009. doi: 10.1016/j.envpol.2020.116009
74. Bougnom BP, Knapp BA, Elhottová D, Koubová A, Etoa FX, Insam H. Designer Compost With Biomass Ashes for Ameliorating Acid Tropical Soils: Effects on the Soil Microbiota. Appl Soil Ecol [Internet]. (2010) 45(3):319–24. doi: 10.1016/j.apsoil.2010.05.009
75. Bolan NS, Kunhikrishnan A, Choppala GK, Thangarajan R, Chung JW. Stabilization of Carbon in Composts and Biochars in Relation to Carbon Sequestration and Soil Fertility. Sci Tot. Environ [Internet] (2012) 424:264–70. doi: 10.1016/j.scitotenv.2012.02.061
76. Orsini F, Kahane R, Nono-Womdim R, Gianquinto G. Urban Agriculture in the Developing World: A Review. Agron Sust. Dev (2013) 33(4):695–720. doi: 10.1007/s13593-013-0143-z
77. Akanbi WB, Togun AO. The Influence of Maize-Stover Compost and Nitrogen Fertilizer on Growth, Yield and Nutrient Uptake of Amaranth. Sci Hortic (Amsterdam). (2002) 93(1):1–8. doi: 10.1016/S0304-4238(01)00305-3
78. Bass AM, Bird MI, Kay G, Muirhead B. Soil Properties, Greenhouse Gas Emissions and Crop Yield Under Compost, Biochar and Co-Composted Biochar in Two Tropical Agronomic Systems. Sci Tot. Environ [Internet]. (2016) 550:459–70. doi: 10.1016/j.scitotenv.2016.01.143
79. Aynehband A, Tudeshki MM, Fateh E. Fodder Production of Two Amaranth (Amaranthus Spp. L.) Cultivars Affected by Organic and Chemical Fertilizer Management. Būm/shināsī-i kishāvarzī (2017) 9(3):675–88.
80. Shrestha P, Small GE, Kay A. Quantifying Nutrient Recovery Efficiency and Loss From Compost-Based Urban Agriculture. PloS One [Internet]. (2020) 15(4):1–15. doi: 10.1371/journal.pone.0230996
81. Mitchell E, Frisbie SH. Emergence and Growth of Cabbage Seedlings in Plastic, Peat, Paper, and Newspaper Containers. Cogent. Food Agric [Internet]. (2017) 3(1):1–10. doi: 10.1080/23311932.2017.1326444
82. Brevik EC, Slaughter L, Singh BR, Steffan JJ, Collier D, Barnhart P, et al. Soil and Human Health: Current Status and Future Needs. Air. Soil Water Res (2020) 13(2):1–23. doi: 10.1177/1178622120934441
Keywords: urban farmers, sustainable agriculture, organic amendments, nutrient management, soil quality, soil management
Citation: Esmail S and Oelbermann M (2022) Investigating Farmer Perspectives and Compost Application for Soil Management in Urban Agriculture in Mwanza, Tanzania. Front. Soil Sci. 2:905664. doi: 10.3389/fsoil.2022.905664
Received: 27 March 2022; Accepted: 30 May 2022;
Published: 30 June 2022.
Edited by:
Sabine Grunwald, University of Florida, United StatesReviewed by:
Heba Elbasiouny, Al-Azhar University, EgyptFathy Elbehiry, Kafrelsheikh University, Egypt
Copyright © 2022 Esmail and Oelbermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shefaza Esmail, c2hlZmF6YS5lc21haWxAdXdhdGVybG9vLmNh
 Shefaza Esmail
Shefaza Esmail Maren Oelbermann
Maren Oelbermann