
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Soft Matter , 20 February 2023
Sec. Granular Matter
Volume 3 - 2023 | https://doi.org/10.3389/frsfm.2023.1143958
This article is part of the Research Topic Editors’ Showcase 2023: Granular Matter View all 5 articles
 Antonius Armanious1
Antonius Armanious1 Heyun Wang2†
Heyun Wang2† Peter A. Alpert3†
Peter A. Alpert3† Chiara Medaglia4
Chiara Medaglia4 Mohammad Peydayesh1
Mohammad Peydayesh1 Arnaud Charles-Antoine Zwygart4
Arnaud Charles-Antoine Zwygart4 Christian Gübeli1
Christian Gübeli1 Stephan Handschin1
Stephan Handschin1 Sreenath Bolisetty5
Sreenath Bolisetty5 Markus Ammann3
Markus Ammann3 Caroline Tapparel4
Caroline Tapparel4 Francesco Stellacci2,6,7
Francesco Stellacci2,6,7 Raffaele Mezzenga1,8*
Raffaele Mezzenga1,8*The ongoing COVID-19 pandemic has revealed that developing effective therapeutics against viruses might be outpaced by emerging variants, waning immunity, vaccine skepticism/hesitancy, lack of resources, and the time needed to develop virus-specific therapeutics, emphasizing the importance of non-pharmaceutical interventions as the first line of defense against virus outbreaks and pandemics. However, fighting the spread of airborne viruses has proven extremely challenging, much more if this needs to be achieved on a global scale and in an environmentally-friendly manner. Here, we introduce an aerosol filter media made of granular material based on whey protein nanofibrils and iron oxyhydroxides nanoparticles. The material is environmentally-friendly, biodegradable, and composed mainly of a dairy industry byproduct. It features filtration efficiencies between 95.91% and 99.99% for both enveloped and non-enveloped viruses, including SARS-CoV-2, the influenza A virus strain H1N1, enterovirus 71, bacteriophage Φ6, and bacteriophage MS2. While the filtration efficiencies were relatively high, they came at the cost of high pressure drop (≈0.03 bar). We believe that the methods and results presented here will contribute to advancing our understanding of granular-based aerosol filters, hopefully helping the design of highly-efficient granular media with low-pressure drops.
The development of vaccines and other therapeutics is an essential component of our panoply to fight viral pandemics. Therapeutics alone, however, might not be sufficient to end a pandemic at the desired speed, leading to potentially avoidable fatalities (Adam, 2022; Collaborators et al., 2022) and adverse socioeconomic consequences (Josephson et al., 2021). The emergence of new variants that can evade the immune response of vaccinated and convalescent patients (Pulliam et al., 1979; Liu et al., 2021; Colson et al., 2022; Mannar et al., 2022; Servellita et al., 2022), waning immunity (Evans et al., 2022; Thompson et al., 2022022; Gupta and Topol, 2021; Levine-tiefenbrun et al., 2022), vaccine skepticism/hesitancy (de Figueiredo and Larson, 2021; Kerr et al., 2021; Solís Arce et al., 2021), and lack of resources for production and administration of vaccines on a global scale within a short time period (Khamsi, 2020; Krammer, 2020; Liu et al., 2020; Forni et al., 2021; Sparrow et al., 2021; Wouters et al., 2021) are all factors that might compromise efforts to end a pandemic through therapeutics. Therefore, a key tool in this fight is preventing the transmission of viruses through non-pharmaceutical interventions (Hatchett et al., 2007; Flaxman et al., 2020; Perkins and España, 2020; Li et al., 2021; Morris et al., 2021). It has long been thought that the transmission of airborne viruses is mainly driven by droplets; (Wang et al., 1979; Samet et al., 2021); a growing body of evidence reveals that aerosols substantially contribute to airborne viral transmission, particularly in indoor spaces (Wang et al., 1979; Stadnytskyi et al., 2020; Samet et al., 2021; Lewis, 2022; Port et al., 2022). Mask mandates, social distancing, increased ventilation, and the use of air filters are measures used to combat airborne virus transmission in indoor spaces. Introducing air filters offers several advantages over other measures, i.e., they could contribute to maintaining indoor space capacities, offer a cost-effective solution for mechanically-ventilated spaces, and would be less sensitive to personal choices and/or behavioral discipline. High-efficiency particulate air (HEPA) filters constitute the gold standard for the filtration of aerosols. Producing HEPA filters requires relatively advanced fabrication technologies (Henning et al., 2021), with a large proportion made of either glass fibers or plastics, the former of which requires an energy-intensive fabrication process, and the latter of which relies heavily on the petrochemical industries (First, 1998; Henning et al., 2021). In addition, over time, a filter cake builds up on the fibers of the filter, resulting in increased resistance to airflow and the imminent need to replace the whole filter, with very limited options for cleaning and/or reuse (First, 1998). Indeed, using HEPA filters on a global scale to combat the transmission of airborne viruses in indoor spaces incurs prohibitive environmental and financial costs. Trying to combat a global pandemic using HEPA filters and other fiber-based filters is, in its essence, the outcome of being locked in a historical trap. Historically HEPA filters were developed to protect against airborne radioactive contaminants associated with the production of nuclear energy. For this purpose, it was necessary to achieve filtration efficiencies ≥99.97% of aerosols in the size range between 0.3 and 1.0 µm. None of these criteria is relevant to the filtration of airborne viruses, where the relevant efficiency is that of the reduction in the number of infectious viruses that go through the filter. Reduction in the number of infectious viruses can happen through trapping of the virus-containing aerosols and/or inactivation of the viruses during the filtration process. Different viruses are expected to have different inactivation efficiencies and are also likely to have different concentrations in aerosols with different sizes. Therefore, assessing the filtration efficiency based only on aerosol entrapment will likely result in an underestimation of the filtration efficiency for viruses, thus wrongly classifying filtration media as inadequate for virus filtration. This comes in crisis time, i.e., a pandemic, where such margins can make an immense difference in fighting the pandemic on a global scale.
In this work, we prepare a granular material composed of amyloid nanofibrils (AF) and iron (Fe) oxyhydroxides nanoparticles, i.e., AF-Fe. The material is environmentally friendly, biodegradable, and mainly composed of a diary industry byproduct (Palika et al., 2021). AF and Fe nanoparticles have been previously used as the building blocks of membrane filters for waterborne viruses, showing high efficiency in trapping viruses, including SARS-CoV-2, in bulk water (Palika et al., 2021). The rationale of this work was to use the same chemical building blocks as those used for the membrane filters to bring an additional advantage when filtering virus-loaded aerosols. Once the aerosols are trapped by the granular AF-Fe, the AF-Fe has the potential to inactivate and/or irreversibly attach the viruses in the trapped aerosols. The granular form of the material enables its application for aerosol filtration while increasing its mechanical stability, workability, and simplicity of handling. To assess the filtration efficiency of virus-loaded aerosols, we have designed and built a compact experimental setup that can be housed in laminar flow hoods of BSL2 and BSL3 laboratories. That setup was built to enable the direct assessment of the infectious viruses that pass through the filtration media. It complies with all biosafety regulations, enabling so far the first and only aerosol filtration study of SARS-CoV-2.
Using a facile fabrication process, we prepared a granular filtration material composed of amyloid nanofibrils and iron oxyhydroxides nanoparticles (AF-Fe), as shown in Figure 1A. The AF is prepared from whey protein extract, a by-product of the dairy industry, by lowering the pH to 2 and cooking at 90°C for ≈5 h. Afterward, Fe nanoparticles are precipitated on the fibrils by adding FeCl3·6H20 and raising the pH to 7. A full characterization of the AF-Fe before converting it to the granular form has been reported earlier (Palika et al., 2021). The material is converted to a granular form by decanting the water from the material and baking it at 80°C-90°C. The material is then passed through a 12 US mesh sieve (pore size ≈1.7 mm) to remove larger pieces. However, larger aggregates of AF-Fe might form during packaging and storage (Figure 1B). The material, thus, has a broad size distribution with 50% of its mass smaller than 3 mm, as determined using sieve analysis (Figure 1C). Figure 1D shows scanning electron microscopy (SEM) micrographs of the material at various magnification scales where the iron oxyhydroxides nanoparticles can be visualized at the highest magnifications. The chemical composition of the material was further verified using Fourier transform infrared spectroscopy (FTIR; Figure 1E), which shows the three peaks for amide groups, representative of the amyloid fibrils, and one of the Fe-O-H group, representative of the iron oxyhydroxides nanoparticles. The material has a surface area of 44.1 m2 g−1 (Figure 1F), i.e., the 50% surface coverage capacity of 1 g of the material is ≈7 × 1014 and ≈3 × 1013 for 30 or 150 nm virus particles, respectively. Its specific density, ρs, is 2.1 g cm−3 and bulk densities are 1.7 and 1.4 g cm−3 of air-equilibrated and oven-dried samples, respectively, showing a relatively high intra-particle porosity of 36% with 30% volumetric water content. The intra-particle pore-size distribution was further investigated using mercury intrusion porosimetry, revealing pores in size ranges of tens and thousands of nanometers (Figure 1G; Supplementary Figure S1). The size range of these pores has been previously suggested to serve as trapping cavities for viruses, preventing their release once they are attached to the surface of the AF-Fe (Canh et al., 2021). The material has a filling density of 0.99–1.04 g cm−3, resulting in a relatively high inter-particle porosity of 39%–42%. Collectively, the properties of the granular AF-Fe make it a highly promising candidate for filtering virus-loaded aerosols, with the potential of inactivating and/or irreversibly attaching viruses that come in contact with the material (Canh et al., 2021; Palika et al., 2021).
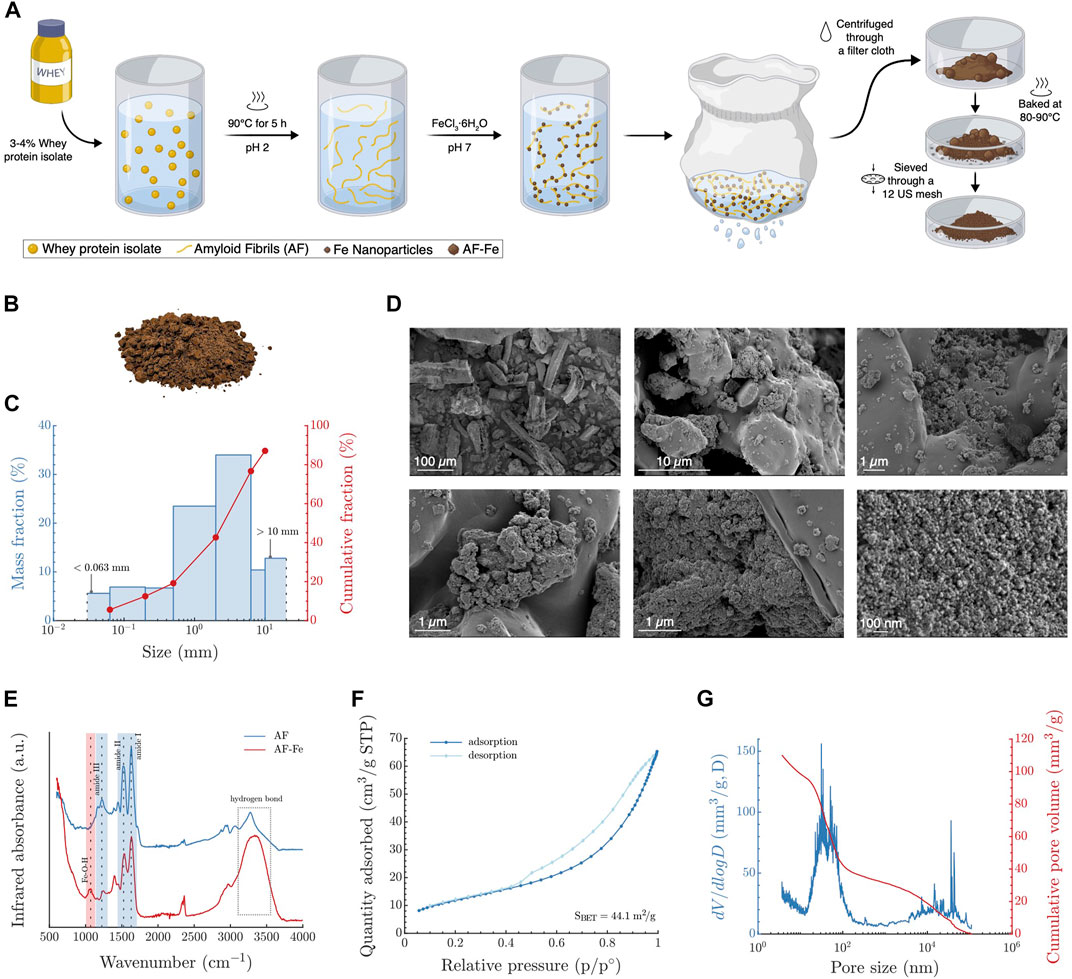
FIGURE 1. Fabrication and characterization of the granular AF-Fe. (A) A schematic showing the fabrication process of the AF-Fe. (B) A vectorized photo of the material after removing the background. (C) Size distribution of the AF-Fe determined using sieve analysis. (D) SEM micrographs of the material at varying magnifications. (E) FTIR analysis of both AF and AF-Fe. (F) N2 adsorption analysis of the AF-Fe to determine its surface area. (G) Intra-particle pore size distribution of the AF-Fe determined using mercury intrusion porosimetry.
To test the filtration efficiency of the material, we designed and built a compact experimental setup (Figure 2; Supplementary Figure S2) in which virus-loaded aerosols are generated, passed through the AF-Fe at a flowrate of 7.5 L min−1 (equivalent to face velocity of 7.2 cm s−1), and then collected on a gelatine membrane that traps ≥99% of viruses passing through while maintaining their infectivity, as independently verified and detailed in Supplementary Figure S3. These gelatine membranes are water-soluble, facilitating virus extraction and subsequent infectivity and genome count assessment. The filtration efficiency of AF-Fe was determined by comparing the infectious viruses trapped on the gelatine membranes in the presence of AF-Fe versus in its absence. The setup is compact, allowing for complete operation inside of laminar flow hoods (Supplementary Figure S2). Additionally, all of its connections are tightly sealed, and its outlet tube is supplemented with an impinger and a HEPA vent (cytiva, Whatman, United Kingdom) to ensure safe operation, particularly when used to assess human viruses. To the best of our knowledge and by fulfilling all the biosafety requirements, this study is the first and so far only study that assesses the filtration of SARS-CoV-2-loaded aerosols. A detailed account of the setup, and the experimental procedure is presented in the Materials and Methods section.
FIGURE 2. Schematic of the experimental setup and procedure. The experimental setup, left, is composed of: a filter holder with a nebulizer fitted on its top and three hoses connected to three compressed air tanks; the granular AF-Fe placed on a gelatine membrane, which traps the virus-loaded aerosols that pass through the AF-Fe media; an impinger serving as an additional trapping mechanism for any aerosols that go through the gelatine membrane; a HEPA filter as a third safety measure to avoid releasing any virus-loaded aerosols into the surrounding air. For both experiments with (bottom right) and without AF-Fe (top right), the gelatine membrane was dissolved in a buffer to determine the concentration of infectious viruses and the genome count.
The filtration efficiencies of the AF-Fe against H1N1 (the influenza virus strain responsible for the flu pandemic in 2009) and SARS-CoV-2 were, on average,
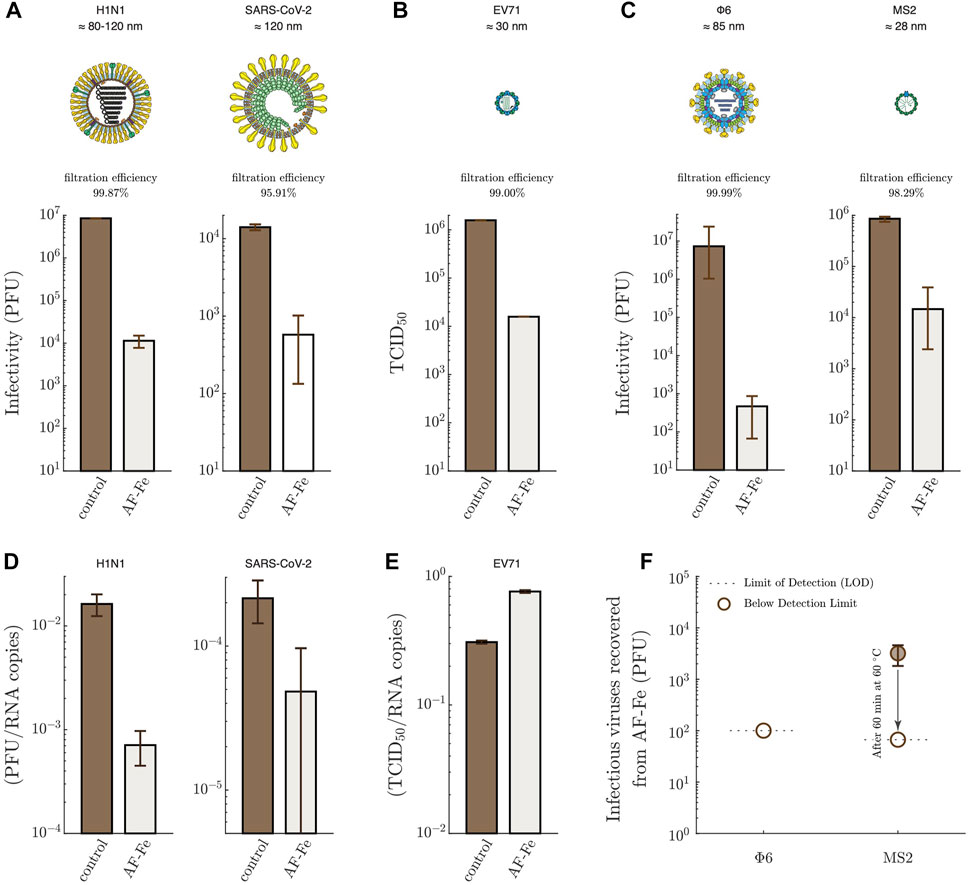
FIGURE 3. Filtration of virus-loaded aerosols using AF-Fe. Infectious viruses trapped on the gelatine membranes in the absence (control) and the presence of AF-Fe for H1N1 and SARS-CoV-2 (A); EV71 (B); Φ6 and MS2 (C). The plotted values for H1N1 (control and AF-Fe), SARS-CoV-2 (control and AF-Fe), EV71 (control and AF-Fe), and Φ6 (AF-Fe) represent the average from two replicas, with the error bars representing the range. For Φ6 (control) and MS2 (control), the plotted values represent the average from four replicas, with the error bars representing the range. For MS2 (AF-Fe), the plotted value represents the average from three replicas, with the error bar representing the range. For H1N1, SARS-CoV-2, Φ6, and MS2, the infectivity was determined using plaque-forming units (PFU) assays. The infectivity of EV71 was determined using median tissue culture infectious dose (TCID50). The ratio of infectious viruses to RNA copies, determined using RT-qPCR, is shown for H1N1 and SARS-CoV-2 (D), and EV71 (E). (F) Infectious viruses recovered after incubating the AF-Fe in PBS buffer for ≈1 h for both Φ6 and MS2. Representations of virions were reproduced with permission (Le Mercier, 2023)
Work on filtration of virus-loaded aerosols using granular material is very scarce, even less the work that involves human viruses. By screening the literature, we could identify only one recent and relevant study, which investigated the filtration of MS2-loaded aerosols using zero-valent nanosilver/titania-chitosan granules (Wang et al., 2021). When using the same thickness of the material we used, i.e., 2 cm, Wang et al. (2021) (Wang et al., 2021) achieved a filtration efficiency of ≈60%, compared to ≈98% for AF-Fe (Figure 3C). The higher filtration efficiency of AF-Fe is additionally complemented by the non-toxic nature of AF-Fe compared to the silver-based material. It is important to keep in mind that differences in the experimental conditions, such as the size distribution of aerosols, air flow rate, geometry of the filtration setup, and the capturing approach for the aerosol downstream of the filter, can also potentially affect the filtration efficiency across different studies. There are, however, more studies on the filtration of aerosols alone, i.e., virus-free aerosols, using granular media; some of these media reached an efficiency of 99.99% at face velocities comparable to our study, albeit using less sustainable materials and processes (Henning et al., 2021). These efficiencies are expected to be even higher if tested using virus-loaded aerosols due to the potential inactivation of viruses during the filtration process.
With no straightforward way to measure the pressure drop across the AF-Fe alone, i.e., without the filter support and the gelatin membrane, we opted for the approach detailed in the Supporting Information in which the pressure in the upper chamber of the filtration setup was recorded using a digital manometer in the absence and presence of the AF-Fe. To put this in context with other filtration media, we also assessed the pressure drop across a HEPA membrane extracted from a HEPA vent (cytiva, Whatman, United Kingdom) using the same experimental approach. The results, as shown in Supplementary Figure S4, show that the pressure drop across the AF-Fe (≈0.03 bar) is close to that of the HEPA membrane (≈0.009 bar). We further tested the effect of reducing the amount of AF-Fe, and thus the pressure drop, on the filtration efficiencies. Using as low as two-thirds, 32 g, of the material, instead of 48 g, equivalent to a thickness of ≈2 cm, used in the reported experiments had little to no effect on filtration efficiencies (Supplementary Figures S5, S6) while reducing the pressure drop to two-thirds of its initial value (≈0.02 bar; Supplementary Figure S4). Therefore, the quality factor (QF) of the AF-Fe ranges between ≈0.001 and 0.005 Pa−1, which is comparable to other granular-based materials but 1 to 2 orders of magnitude lower than fibrous and cellular media (Henning et al., 2021). Yet, these granular materials tend to come at a higher environmental footprint.
Figure 3D shows that the ratio of infectious H1N1 and SARS-CoV-2 to total genome count decreased after passing the AF-Fe, indicating that the viruses are not just trapped, but are also partially inactivated. A direct interaction between the viruses and AF-Fe would be needed for inactivation to occur. Part of the trapped aerosols is likely to be re-aerosolized again by the airflow shear forces. In this transient period of being attached to the AF-Fe, the viruses are inactivated. However, it also remains possible that the mechanical stress of re-aerosolization contributes to the observed inactivation. Such inactivation, however, was not observed for EV71 (Figure 3E), confirming that non-enveloped viruses are more robust and resistant to mechanical stresses due to the interactions with AF-Fe and the re-aerosolization process.
To assess the safe handling of the material, we incubated the AF-Fe for ≈1 h in phosphate-buffered saline (PBS) buffer after filtering aerosols loaded with Φ6 or MS2. No infectious viruses were recovered in the case of Φ6 (Figure 3F), indicating that the AF-Fe completely inactivated the virus and/or trapped it irreversibly. In the case of the non-enveloped MS2,
The aerosol trapping mechanism of the AF-Fe is likely to have contributions from several sources, among which are the size, shape, roughness, charge, and hydrophobicity of the AF-Fe. Systematic experimental assessment of all these parameters is practically impossible, as there is no straightforward way to selectively vary one parameter while keeping the others constant. Despite its own limitations, which are discussed later, we restored to theoretical modeling to gain deeper insights into the contributions of the different aerosol trapping mechanisms. For this end, we modeled four key aerosol entrapment processes (Figure 4A): 1) diffusion, which is driven by Brownian motion of the aerosol droplets; 2) interception, which occurs when the airflow line comes within one aerosol radius distance from the grains of the filter; 3) gravitational settling, which is driven by gravitational forces acting on the aerosol particles; and 4) impaction, which is driven by the inertia of the aerosol particles. All of these processes depend on both the size of the AF-Fe grains,
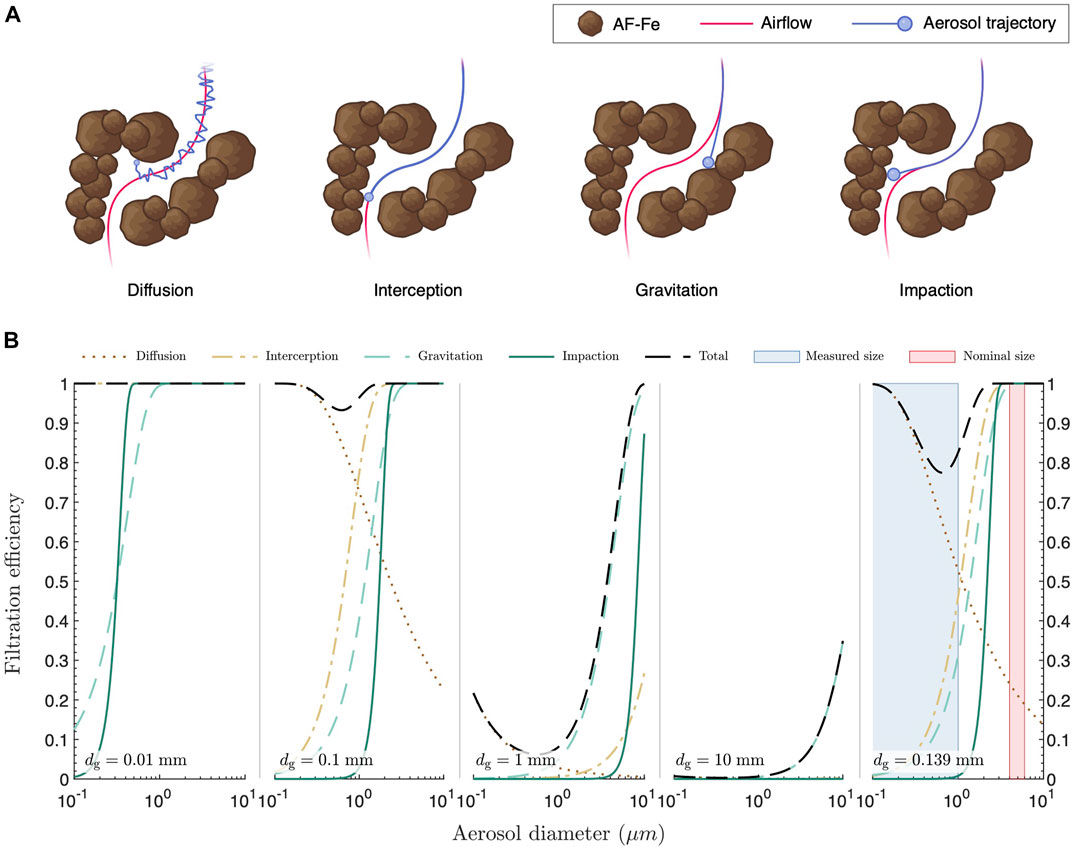
FIGURE 4. Mechanisms of aerosol entrapment. (A) Schematics depicting different processes involved in aerosol entrapment. (B) Filtration efficiencies due to diffusion, interception, gravitational settling, and impaction through grain bed filters with monodispersed grain size,
Altogether, our results demonstrate that AF-Fe can filter virus-loaded aerosols with very good efficiencies. There is, however, a need for substantial improvement in the pressure drop across the AF-Fe for the material to be comparable to existing filtration media and achieve economic and commercial feasibility. We foresee further developments in the design of granular-based material for aerosol filtration applications to simultaneously achieve high efficiencies and low pressure drops, while benefiting from the several advantages offered by granular material: e.g., broad availability in nature, ease of fabrication, safety of handling, and various options of recycling and reuse. The use of granular material for aerosol filtration in this study is also expected to inspire the search for new, local, environmentally-friendly materials that could also be used as the main building block for aerosol filters. Such research activities will be facilitated by the experimental setup used in this work, which fulfills all the biosafety requirements for studying emerging viruses in the aerosol phase while using affordable and easily accessible components.
A full detailed version of the materials and methods is provided in the Supplementary Information. A summary of the most relevant sections is presented here.
AF-Fe. The granular AF-Fe material was provided by BluAct (Switzerland) and prepared as detailed later.
Viruses. SARS-CoV-2 virus hCoV-19/Switzerland/un-2012212272/2020 was a generous gift from Prof. Isabella Eckerle (University Hospital in Geneva, Geneva, Switzerland). The virus was replicated twice in Vero-E6 cells prior to the experiments. Human H1N1 virus A/Netherlands/602/2009 was a generous gift from Prof. Mirco Schmolke (Department of Microbiology and Molecular Medicine, University of Geneva, Geneva, Switzerland) and was propagated in embryonated chicken eggs (Riegger et al., 2015). Enterovirus 71 (EV71) was isolated from a clinical specimen in the University Hospital of Geneva in RD cells and propagated in Vero cells (Tseligka et al., 2018). Φ6 (21518 DSMZ) and MS2 (13767 DSMZ) bacteriophages were purchased from DSMZ culture collection (Germany) and propagated in their host bacterial cells.
Preparation of the AF-Fe granular material. Amyloid fibrils were prepared from whey protein isolate (BiPro, Agropur, United States) by lowering the pH to 2.0 and heating at 90°C for 5 h (Jung et al., 2008). Then, the amyloid fibrils were coated with iron nanoparticles by mixing FeCl3·6H2O and adjusting the pH to 7.0 using NaOH (Palika et al., 2021). The solution was then centrifuged through a filter cloth to decant the water. Afterward, the retentate was dried at 80°C-90°C and sieved through a 12 US mesh (pore size of ≈1700 µm) to remove large particles. It is important to note that some of the material formed larger aggregates during storage and packaging. The material is patented and produced by BluAct (Switzerland) and used as received without further treatment.
Assessment of aerosol filtration. The filtration efficiency of AF-Fe against virus-loaded aerosols was assessed using a compact experimental setup composed mainly of a polycarbonate filtration holder (Sartorius, Germany) connected to three 1.5 L compressed air tanks (PanGas, Switzerland) and an Aeroneb® Lab nebulizer unit (Kent Scientific, U.S.A.). Virus-containing solutions were prepared and added to the nebulizer: MS2 and Φ6 were prepared in artificial saliva/mucin solution, H1N1 in allantoid fluid, SARS-CoV-2 in high glucose Dulbecco’s Modified Eagle Medium (DMEM, GlutaMAX™) supplemented with 2.5% fetal bovine serum (FBS), and EV71 in 2.5% serum DMEM. For each virus, 100 µL of the virus-containing solution was nebulized, generating aerosols with an average diameter of 4–6 μm, as indicated by the manufacturer, in the upper compartment of the filter holder. The generated aerosols were carried through the filtration media with the airflow from the compressed air tanks at 7.5 L/min (3 × 2.5 L/min). The aerosols passing through the AF-Fe media were then trapped using gelatine membranes (Sartorius, Germany). The membranes are water-soluble and designed to trap virus-loaded aerosols while retaining their infectivity. After disassembling the setup, the membranes were dissolved in 10 ml of PBS buffer for downstream analysis of infectivity and genome count of viruses. The efficiency of the AF-Fe media for the filtration of each virus was determined by comparing the infective viruses in the gelatine membrane in the presence versus the absence of the AF-Fe media. All experiments were conducted in a laminar flow hood.
Additional methods. Experimental details on the propagation of Φ6, MS2, H1N1, SARS-CoV-2, and EV71 viruses, infectivity assays, and RT-qPCR assays are given in full in the Supporting Information along with details on the materials and solutions used, experimental setup, and procedure for aerosol filtration, determination of AF-Fe size distribution, FTIR, SEM, N2 adsorption, mercury intrusion porosimetry, water content and density determination, pressure drop measurements, determination of aerosol size distribution, and modeling aerosol entrapment mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AA designed the experimental setup for aerosol experiments; conducted all the aerosol experiments with Φ6, MS2, H1N1, and EV71; compiled the figures; and wrote the manuscript. HW (under the supervision of FS) conducted the aerosol experiments with SARS-CoV-2 and evaluated its infectivity. PA (under the supervision of MA) conducted the aerodynamics modeling and calculations; and performed the aerosol size distribution measurements. CM and AC-AZ (under the supervision of CT) propagated H1N1 and EV71, and evaluated their infectivity and genome count; evaluated the genome count of SARS-CoV-2. MP performed the FTIR and N2 adsorption experiments and analyzed their data. CG propagated Φ6 and MS2, and evaluated their infectivity for all aerosol experiments. SH carried out the SEM imaging. SB developed, synthesized, and provided the AF-Fe material. AA and RM wrote the manuscript. RM designed and directed the study; acquired funds; analyzed data; and wrote the manuscript. All authors edited and approved the final manuscript.
The authors gratefully acknowledge funding from the Swiss National Science Foundation project N° 31CA30_196217. Open access funding provided by ETH Zurich.
Martin Loessner (ETH Zurich) is deeply acknowledged for allowing access to his laboratory facilities; Isabella Eckerle, Manel Essaidi-Laziosi, and Meriem Bekliz (University Hospital of Geneva, Switzerland) for providing SARS-CoV-2 strains; Daniel Kiechl, Peter Bigler, Carmen Saez Garcia Wanzenried, and Rasha Aziz (ETH Zurich) for providing excellent technical support; Michael Plötze from the ClayLab (ETH Zurich) for conducting the size distribution, density, and porosity determination experiments; Terttaliisa Lind (PSI) for valuable discussions; Sonia Monti for the design of scientific illustrations; Eleonora Simeoni, Vivianne Padrun, and Anna Maria Novello (EPF Lausanne) for biosafety support with SARS-CoV-2 experiments. The authors also gratefully acknowledge the support of the Scientific Center for Optical and Electron Microscopy (ScopeM) of the ETH Zurich.
RM and SB are the inventors of a filed patent application related to the work presented here. SB is employed by BluAct Technologies GmbH, Zurich, Switzerland. BluAct Technologies kindly donated the antiviral granular material studied in this work. All other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsfm.2023.1143958/full#supplementary-material
Adam, D. (2022). 15 million people have died in the pandemic, WHO says. Nature 605, 206. doi:10.1038/d41586-022-01245-6
Canh, V. D., Tabata, S., Yamanoi, S., Onaka, Y., Yokoi, T., Furumai, H., et al. (2021). Evaluation of porous carbon adsorbents made from rice husks for virus removal in water. Water (Basel) 13 (9), 1280. doi:10.3390/w13091280
Collaborators, C.-19 E. M., Paulson, K. R., Pease, S. A., Watson, S., Comfort, H., Zheng, P., et al. (2022). Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020 – 21. Lancet 6736 (21), 1513–1536. doi:10.1016/S0140-6736(21)02796-3
Colson, P., Fournier, P.-E., Delerce, J., Million, M., Bedotto, M., Houhamdi, L., et al. (2022). Culture and identification of a “Deltamicron” SARS-CoV-2 in a three cases cluster in southern France. J. Med. Virol. 94, 3739–3749. No. March. doi:10.1002/jmv.27789
de Figueiredo, A., and Larson, H. J. (2021). Exploratory study of the global intent to accept COVID-19 vaccinations. Commun. Med. 1 (1), 30–10. doi:10.1038/s43856-021-00027-x
Evans, J. P., Med, S. T., Evans, J. P., Zeng, C., Carlin, C., Lozanski, G., et al. (2022). Neutralizing antibody responses elicited by SARS-CoV-2 MRNA vaccination wane over time and are boosted by breakthrough infection. Sci. Transl. Med. 14, eabn8057. doi:10.1126/scitranslmed.abn8057
First, M. W. (1998). Hepa filters. J. Am. Biol. Saf. Assoc. 3 (1), 33–42. doi:10.1177/109135059800300111
Flaxman, S., Mishra, S., Gandy, A., Unwin, H. J. T., Mellan, T. A., Coupland, H., et al. (2020). Estimating the effects of non-pharmaceutical interventions on COVID-19 in europe. Nature 584, 257–261. doi:10.1038/s41586-020-2405-7
Forni, G., Mantovani, A., Forni, G., Mantovani, A., Moretta, L., Rappuoli, R., et al. (2021). COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 28 (2), 626–639. doi:10.1038/s41418-020-00720-9
Gupta, R. K., and Topol, E. J. (2021). COVID-19 vaccine breakthrough infections. Science 374 (6575), 3741561–3741562. doi:10.1126/science.abl8487
Hatchett, R. J., Mecher, C. E., and Lipsitch, M. (2007). Public health interventions and epidemic intensity during the 1918 influenza pandemic. Proc. Natl. Acad. Sci. 104 (18), 7582–7587. doi:10.1073/pnas.0610941104
Henning, L. M., Abdullayev, A., Vakifahmetoglu, C., Simon, U., Bensalah, H., Gurlo, A., et al. (2021). Review on polymeric, inorganic, and composite materials for air filters: From processing to properties. Adv. Energy Sustain. Res. 2 (5), 2100005. doi:10.1002/aesr.202100005
Josephson, A., Kilic, T., and Michler, J. D. (2021). Socioeconomic impacts of COVID-19 in low-income countries. Nat. Hum. Behav. 5 (5), 557–565. doi:10.1038/s41562-021-01096-7
Jung, J. M., Savin, G., Pouzot, M., Schmitt, C., and Mezzenga, R. (2008). Structure of heat-induced β-lactoglobulin aggregates and their complexes with sodium-dodecyl sulfate. Biomacromolecules 9 (9), 2477–2486. doi:10.1021/bm800502j
Kerr, J. R., Schneider, C. R., Recchia, G., Dryhurst, S., Sahlin, U., Dufouil, C., et al. (2021). Correlates of intended COVID-19 vaccine acceptance across time and countries: Results from a series of cross-sectional surveys. BMJ Open 11 (8), 0480255–e48111. doi:10.1136/bmjopen-2020-048025
Khamsi, R. (2020). Can the world make enough coronavirus vaccine? Nature 580 (7805), 578–580. doi:10.1038/d41586-020-01063-8
Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature 586 (7830), 516–527. doi:10.1038/s41586-020-2798-3
Levine-tiefenbrun, M., Yelin, I., Alapi, H., Herzel, E., Kuint, J., Chodick, G., et al. (2022). Waning of SARS-CoV-2 booster viral-load reduction effectiveness. Nat. Commun. 13 (1237), 1237–1244. doi:10.1038/s41467-022-28936-y
Lewis, D. (2022). Why the WHO took two years to say COVID is airborne. Nature 604 (7904), 26–31. doi:10.1038/d41586-022-00925-7
Li, Y., Campbell, H., Kulkarni, D., Harpur, A., Nundy, M., Wang, X., et al. (2021). The temporal association of introducing and lifting non-pharmaceutical interventions with the time-varying reproduction number (R) of SARS-CoV-2: A modelling study across 131 countries. Lancet Infect. Dis. 21 (2), 193–202. doi:10.1016/S1473-3099(20)30785-4
Liu, L., Iketani, S., Guo, Y., Chan, J. F.-W., Wang, M., Liu, L., et al. (2021). Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 602, 676–681. doi:10.1038/s41586-021-04388-0
Liu, Y., Salwi, S., and Drolet, B. C. (2020). Multivalue ethical framework for fair global allocation of a COVID-19 vaccine. J. Med. Ethics 46 (8), 499–501. doi:10.1136/medethics-2020-106516
Mannar, D., Saville, J. W., Zhu, X., Srivastava, S. S., Berezuk, A. M., Tuttle, K. S., et al. (2022). SARS-CoV-2 omicron variant: Antibody evasion and cryo-EM structure of spike protein–ACE2 complex. Science 375 (6582), 760–764. doi:10.1126/science.abn7760
Morris, D. H., Rossine, F. W., Plotkin, J. B., and Levin, S. A. (2021). Optimal, near-optimal, and robust epidemic control. Commun. Phys. 4 (78), 78–8. doi:10.1038/s42005-021-00570-y
Palika, A., Armanious, A., Rahimi, A., Medaglia, C., Gasbarri, M., Handschin, S., et al. (2021). An antiviral trap made of protein nanofibrils and iron oxyhydroxide nanoparticles. Nat. Nanotechnol. 16 (8), 918–925. doi:10.1038/s41565-021-00920-5
Perkins, T. A., and España, G. (2020). Optimal control of the COVID-19 pandemic with non-pharmaceutical interventions. Bull. Math. Biol. 82 (118), 118–124. doi:10.1007/s11538-020-00795-y
Port, J. R., Yinda, C. K., Avanzato, V. A., Schulz, J. E., Holbrook, M. G., van Doremalen, N., et al. (2022). Increased small particle aerosol transmission of B.1.1.7 compared with SARS-CoV-2 lineage A in vivo. Nat. Microbiol. 7 (2), 213–223. doi:10.1038/s41564-021-01047-y
Pulliam, J. R. C., van Schalkwyk, C., Govender, N., von Gottberg, A., Cohen, C., Groome, M. J., et al. (1979). Increased risk of SARS-CoV-2 reinfection associated with emergence of omicron in south Africa. Science 2022, 4947. doi:10.1126/science.abn4947
Riegger, D., Hai, R., Dornfeld, D., Mänz, B., Leyva-Grado, V., Sánchez-Aparicio, M. T., et al. (2015). The nucleoprotein of newly emerged H7N9 influenza A virus harbors a unique motif conferring resistance to antiviral human MxA. J. Virol. 89 (4), 2241–2252. doi:10.1128/jvi.02406-14
Samet, J. M., Burke, T. A., Lakdawala, S. S., Lowe, J. J., Marr, L. C., Prather, K. A., et al. (2021). SARS-CoV-2 indoor air transmission is a threat that can Be addressed with science. Proc. Natl. Acad. Sci. 118 (45), 21161551188–e2116155125. doi:10.1073/pnas.2116155118
Servellita, V., Morris, M. K., Sotomayor-Gonzalez, A., Gliwa, A. S., Torres, E., Brazer, N., et al. (2022). Predominance of antibody-resistant SARS-CoV-2 variants in vaccine breakthrough cases from the san francisco bay area, California. Nat. Microbiol. 7 (2), 277–288. doi:10.1038/s41564-021-01041-4
Solís Arce, J. S., Warren, S. S., Meriggi, N. F., Scacco, A., McMurry, N., Voors, M., et al. (2021). COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 27 (8), 1385–1394. doi:10.1038/s41591-021-01454-y
Sparrow, E., Wood, J. G., Chadwick, C., Newall, A. T., Torvaldsen, S., Moen, A., et al. (2021). Global production capacity of seasonal and pandemic influenza vaccines in. Vaccine 39 (3), 512–520. doi:10.1016/j.vaccine.2020.12.018
Stadnytskyi, V., Bax, C. E., Bax, A., and Anfinrud, P. (2020). The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. U. S. A. 117 (22), 11875–11877. doi:10.1073/pnas.2006874117
Thompson, M. G., Natarajan, K., Irving, S. A., and Rowley, E. A. (2022). Effectiveness of a third dose of MRNA vaccines against COVID-19 – associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance — VISION network, 10 States. Morb. Mortal. Wkly. Rep. 71, 255–263.
Tseligka, E. D., Sobo, K., Stoppini, L., Cagno, V., Abdul, F., Piuz, I., et al. (2018). A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism. PLoS Pathog. 14 (8), 10071900–e1007225. doi:10.1371/journal.ppat.1007190
Turgeon, N., Toulouse, M. J., Martel, B., Moineau, S., and Duchaine, C. (2014). Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 80 (14), 4242–4250. doi:10.1128/AEM.00767-14
Wang, C. C., Prather, K. A., Sznitman, J., Jimenez, J. L., Lakdawala, S. S., Tufekci, Z., et al. (1979). Airborne transmission of respiratory viruses. Science 2021 (6558), 373. doi:10.1126/science.abd9149
Wang, I. J., Chen, Y. C., Su, C., Tsai, M. H., Shen, W. T., Bai, C. H., et al. (2021). Effectiveness of the nanosilver/TiO2-chitosan antiviral filter on the removal of viral aerosols. J. Aerosol Med. Pulm. Drug Deliv. 34 (5), 293–302. doi:10.1089/jamp.2020.1607
Keywords: SARS-CoV-2, aerosol, filtration, amyloids, nanoparticles, environmentally friendly, protein-based materials, waste-valorization
Citation: Armanious A, Wang H, Alpert PA, Medaglia C, Peydayesh M, Zwygart AC-A, Gübeli C, Handschin S, Bolisetty S, Ammann M, Tapparel C, Stellacci F and Mezzenga R (2023) Trapping virus-loaded aerosols using granular material composed of protein nanofibrils and iron oxyhydroxides nanoparticles. Front. Soft. Matter 3:1143958. doi: 10.3389/frsfm.2023.1143958
Received: 13 January 2023; Accepted: 07 February 2023;
Published: 20 February 2023.
Edited by:
Lou Kondic, New Jersey Institute of Technology, United StatesReviewed by:
Yaoyao Chen, E Ink Company, United StatesCopyright © 2023 Armanious, Wang, Alpert, Medaglia, Peydayesh, Zwygart, Gübeli, Handschin, Bolisetty, Ammann, Tapparel, Stellacci and Mezzenga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raffaele Mezzenga, cmFmZmFlbGUubWV6emVuZ2FAaGVzdC5ldGh6LmNo
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.