
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Sleep , 04 December 2024
Sec. Insomnia
Volume 3 - 2024 | https://doi.org/10.3389/frsle.2024.1405398
This article is part of the Research Topic Women in Insomnia View all 8 articles
 Hatty Lara1*†
Hatty Lara1*† Melissa Nevarez-Brewster2†
Melissa Nevarez-Brewster2† Cori Manning3
Cori Manning3 Matthew J. Reid4
Matthew J. Reid4 Stephanie H. Parade5,6
Stephanie H. Parade5,6 Gina M. Mason5,7*‡
Gina M. Mason5,7*‡ Darlynn M. Rojo-Wissar5,6,7‡
Darlynn M. Rojo-Wissar5,6,7‡Sleep disturbances are posited to play a key role in the development of poor mental and physical health outcomes related to early life adversity (ELA), in part through effects on brain development. Language development is critically important for health and developmental outcomes across the lifespan, including academic achievement and emotion regulation. Yet, very little research has focused on the dynamic contributions of ELA, sleep, and brain development on language outcomes. In this mini review, we summarize the current pediatric literature independently connecting ELA and sleep to language development, as well as the effects of ELA and sleep on language-relevant aspects of brain structure and function. We then propose a framework suggesting that sleep disturbances and subsequent effects on brain structure and function may act as key mechanisms linking ELA and language development. Future research investigating the associations among ELA, sleep, brain, and language development will refine our proposed framework and identify whether sleep should be included as an intervention target to mitigate the effects of early life adversity on language development.
Early life adversity (ELA) is a major risk factor for numerous mental and physical health issues from childhood to adulthood (Oh et al., 2018; Zee and Turek, 2006; Duffy et al., 2018). Experiences of ELA include “subjectively perceived threats to the safety or security of the child's bodily integrity, family, or social structures” (Suglia et al., 2018) prior to the age of 18, and can also involve the absence of expected stimuli in cases of deprivation and neglect (Wade et al., 2022). Sleep disturbances are theorized to be a central mechanism linking ELA to poor health outcomes, in part due to their profound effects on multiple neurobiological systems that are also affected by ELA (Fuligni et al., 2021). While a variety of health outcomes have been integrated in these theoretical frameworks, including mood disorders, cardiovascular disease, and diabetes, language development has remained largely overlooked. Here, we summarize evidence in support of language development as another important, but underexamined consequence of ELA that may be partially mediated by sleep disturbances. We first review the literature on language development in the context of ELA and sleep, followed by shared effects of ELA and sleep disturbances on language-related brain structures and functions. We then discuss the plausibility of sleep as a mechanism linking ELA to language-related outcomes during childhood and suggest areas for future research. Much of the research on language development focuses on early childhood, as many fundamental aspects of language development occur within the first 6 years of age (Visser-Bochane et al., 2020; Fenson et al., 1994). Thus, we primarily focus on early childhood, but also broadly cover middle childhood to adolescence.
Language learning is a prominent aspect of development made up of various constructs (Table 1). It is critical for navigating our social environments and forming social bonds (Carouso-Peck et al., 2021; Fitch, 2005; Locke, 2001), scaffolding our own thoughts and shared concepts (Carruthers, 2002; Lupyan et al., 2007), and for later academic achievement and emotion regulation (Eisenberg et al., 2005). While some language-related abilities such as vowel and voice recognition emerge in-utero (Decasper and Fifer, 1980; Moon et al., 2013), language learning is a continuous process, with infants successively identifying and consolidating new words and grammar through exposures in their ambient social environment (e.g., caregivers speaking and pointing) (Visser-Bochane et al., 2020). At school age, language learning priorities shift to reading, grammatical rules (e.g., sentence structure), and writing, which are linked to later occupational and social outcomes (Gaab and Petscher, 2022; Graham, 2022). Although there is a lack of empirical research examining ELA, sleep, and language development together, both ELA and sleep have been independently linked to language development.
ELA has been consistently associated with poorer child language outcomes, with younger children being particularly vulnerable (Sylvestre et al., 2016; Lum et al., 2015; Matte-Landry and Collin-Vézina, 2020). The most studied aspect of ELA relative to language development is child maltreatment, encompassing sexual, physical, and emotional abuse and neglect (Hildyard and Wolfe, 2002). Generally, abuse and neglect tend to demonstrate comparable effects on language outcomes (Sylvestre et al., 2016; Culp et al., 1991; Allen and Oliver, 1982). However, in early childhood, neglect has specifically been related to poorer pragmatic skills (Hyter, 2021). Overall, child maltreatment in early childhood is associated with multiple dimensions of language development, including lower vocabulary skills, poorer grammar (Coster et al., 1989; Eigsti and Cicchetti, 2004), and impaired auditory comprehension and verbal abilities (Lum et al., 2015; Coster et al., 1989; Eigsti and Cicchetti, 2004; Pickett, 2020; Fox et al., 1988). Work extending beyond early childhood has also linked maternal intimate partner violence, in addition to child maltreatment, to poorer receptive, expressive, and social skills (in 5–12-year-olds) (Lum et al., 2017), and pragmatic and reading skills (in 10-year-olds) (Di Sante et al., 2019; Conway et al., 2021). Several reviews support the above, demonstrating links between ELA and communication, reading abilities, and academic performance and achievement in children of all ages (Hyter, 2021; Mills et al., 2011; Ferrara et al., 2023; Palazón-Carrión and Sala-Roca, 2020; Romano et al., 2015; Snow, 2020).
More recent studies have also examined the effects of cumulative exposure to adverse childhood experiences (ACEs), including household dysfunction and child maltreatment, on language development. One study found a graded relation between number of ACEs and poorer vocabulary scores (1–3-year-olds) (McKelvey et al., 2017), and another found that those with ≥3 ACEs had below-average language and literacy skills (2–5-year-olds) (Jimenez et al., 2016). In contrast, in a study of children (Mean age = 6.9) with any of 55 traumatic event types, those who developed specific language impairments were 1.46 × more likely to have been exposed to physical trauma specifically, but were not more likely to have experienced a greater number of ELA types (Selin et al., 2022). In summary, both maltreatment and number of ACEs confer risk for compromised language development across different domains (Stewart-Tufescu et al., 2022; Qu et al., 2024), but effects may differ based on characteristics of the ELA (e.g., type, intensity) and language outcome being assessed. Further, while most research on ELA and language has been conducted in early childhood, studies in middle childhood and adolescence suggest that ELA can affect language outcomes beyond early childhood (Mills et al., 2011; Selin et al., 2022).
Sleep is also implicated in language development (Dionne et al., 2011; Mohammed et al., 2021). In this mini review, we focus on aspects of sleep that have been most robustly linked with ELA (Brown et al., 2022; Schønning et al., 2022) specifically, sleep disturbances related to insomnia (Mindell et al., 1999). Sleep disturbances related to pediatric insomnia can include trouble falling or staying asleep, and related difficulties with sleep consolidation (i.e., consolidating sleep into one continuous overnight interval), short sleep duration, and poor sleep quality (Brown and Malow, 2016; Owens and Mindell, 2011). Children with shorter overnight sleep or less consolidated sleep also tend to have longer daytime naps in early childhood (Jones and Ball, 2014; Ordway et al., 2020). Given that several fundamental language milestones occur when infants and toddlers are still napping (Visser-Bochane et al., 2020), most studies in these age groups have focused on the benefits of naps for language (Simon et al., 2017; Horváth et al., 2016; Hupbach et al., 2009; Gómez et al., 2006; Sweeney et al., 2023; Sandoval et al., 2017; Friedrich et al., 2017); however, the effects of naps on immediate and enduring sleep disturbances are unclear. Studies on sleep consolidation in infancy demonstrate that more consolidated overnight sleep and better parent-led sleep hygiene are associated with better vocabulary, receptive language, and overall communicative development in toddlerhood (Qu et al., 2024; Gliga et al., 2023; Knowland et al., 2022; Hernandez-Reif and Gungordu, 2022). Short sleep duration across the first 3 years of life, and among preschool and school-aged children, has consistently been linked with poorer cognitive scores (Smithson et al., 2018), and worse vocabulary and academic performance overall (Friedrich et al., 2017; Gliga et al., 2023; Knowland et al., 2022; Hernandez-Reif and Gungordu, 2022; Smithson et al., 2018). With regard to sleep disturbances more broadly, studies from infancy to adolescence have shown that multiple types of sleep disturbances (e.g., difficulty falling asleep or staying asleep) are independently associated with poorer language skills and lower verbal intelligence quotients (McGregor and Alper, 2015). For example, disturbances in sleep onset, duration, and nighttime-awakenings predicted decreased expressive, receptive, and social-linguistic skills in 3–18-year-olds (Botting and Baraka, 2018). Together, these studies emphasize that early sleep insufficiency, as well as disrupted sleep, may have an enduring negative impact on language abilities.
Few studies have examined associations between sleep measured with polysomnography (PSG) and language development. One study found that greater neonatal electroencephalogram (EEG) connectivity during non-rapid-eye-movement (NREM) sleep was associated with more advanced vocabulary scores at 18 months (Shellhaas et al., 2022). In another study of 8-year-olds, mean spindle frequency was negatively linked to executive functions, including planning ability and working memory (Chatburn et al., 2013). A study of 10-year-olds with dyslexia showed that greater spindle density during NREM sleep was positively correlated with reading abilities (Bruni et al., 2009). Finally, developmental increases in frontal slow spindle density (11–13 Hz) from ages 8–11 to 14–18 years were positively associated with general cognitive abilities at 14–18 years, including vocabulary skills (Hahn et al., 2019). Additional studies are needed to elucidate how PSG measures of sleep are linked to ELA-related subjective sleep disturbances, and in turn language development.
Brain development in early human life is characterized by abundant dendritic arborization, synaptogenesis and connectivity, as well as reorganization and overproduction of cortical circuits (Kostović and Jovanov-Milošević, 2006; Ouyang et al., 2019; Stiles and Jernigan, 2010). Synaptic density in white and gray matter within the frontal cortex peaks by the second year of life (Kwan et al., 2012). Increased axonal density and myelination in white matter connections also occur (Matsuzawa et al., 2001). Inevitably, this orchestrated growth culminates in an adult-like brain structure (Pfefferbaum et al., 1994) and promotes environment-directed learning, language acquisition, and emotion processing later in life (Keunen et al., 2017).
Early language learning is, arguably, a whole-brain endeavor. While some theories—largely based on adult findings—have proposed that specialized neocortical areas are responsible for specific language abilities (Friederici, 2006), more recent perspectives elucidate the importance of more domain-general brain networks in language, including social-motivational limbic brain systems (Syal and Finlay, 2011), hippocampal and temporal memory-related networks (Goldstein et al., 2010), and broader sensory and motor pathways (Mason et al., 2019; Paterson et al., 2006).
In new word learning, typically-developing infants must map auditory input they receive from caregivers with co-occurring visual and/or tactile sensations across multiple experiences (Mason et al., 2019). Since language learning is an experience-dependent process, caregivers facilitate infants' learning by providing exaggerated (“infant-directed”) and/or simplified speech that is reliable and appropriately timed (Elmlinger et al., 2023, 2019; Fernald and Simon, 1984). Even so, infants must be able to attend to and detect predictable patterns in caregivers' feedback (e.g., how frequently/promptly/reliably caregivers respond to infant behaviors) (Elmlinger et al., 2019; Tamis-LeMonda and Bornstein, 1989; Venditti et al., 2023), and find social exchanges rewarding to continue engaging and learning from others (Syal and Finlay, 2011; Venditti et al., 2023). Given this multifaceted pathway to language learning, it is reasonable that multiple brain networks, including those involved in sensory processing, memory, and reward, would be implicated in language.
At later ages, when language becomes more of an academic undertaking in formal educational settings, brain areas and networks subserving attention, memory consolidation, and cognitive flexibility may be most at play for promoting academic success in language-related skills (e.g., writing composition and grammar). Such areas include the dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) (Skeide and Friederici, 2016; Opitz and Friederici, 2003; Hertrich et al., 2021; Piai et al., 2013). Broca's and Wernicke's areas are also well-known for language comprehension and production (Rosselli et al., 2014).
During adolescence, sustained attention, working memory capacity, and strategic memory processes continue to refine due to changes in the prefrontal cortex (PFC) and hippocampus, enabling adolescents to engage in complex cognitive demands in academic settings (Calabro et al., 2020). Effective memory processes enable students to encode, store, and retrieve information necessary for academic success, including factual knowledge, problem-solving strategies, and conceptual understanding (Swanson and Alloway, 2011). In high schoolers, higher density of gray matter in the left DLPFC is associated with higher academic achievement (Wang et al., 2017). From infancy to adolescence, these brain changes reflect the progressive development of language abilities and cognitive skills essential for effective communication, literacy, and academic achievement.
Across species, ELA is associated with alterations in neurodevelopment (Berman et al., 2022; Callaghan and Tottenham, 2016; Luby et al., 2020; Short and Baram, 2019), which may contribute to subsequent impairments in language learning. In rodents, poverty as an ELA can be simulated by limiting bedding and nesting materials, and inconsistent parenting can be represented by unpredictable behaviors from the rodent mother (i.e., dam). In controlled experiments, these forms of ELA in rodents are linked to alterations in corticolimbic and frontolimbic white matter (e.g., uncinate fasciculus), reductions in hippocampal volume and intra-hemispheric hippocampal white matter, accelerated development of whole-cortex connectivity, and changes to neural circuitry in the amygdala and ventral and dorsal striatal regions (Short and Baram, 2019; Molet et al., 2016; Birnie et al., 2020; Chan et al., 2024). Behaviorally, such changes in brain structure and function are related to anxiety-like behaviors and emotion processing, executive and cognitive functions, memory consolidation and recall, and reward sensitivity (Birnie et al., 2020; Van Petten, 2004).
Randomized controlled trials and observational studies in school-aged children (8–11-years-old) indicate that ELA in the form of institutionalized rearing relates to greater amygdala white matter volume (Tottenham et al., 2010) and accelerated structural development of amygdala-medial PFC connectivity (Gee et al., 2013), both of which are implicated in emotion processing and abnormally heightened threat detection (Tottenham et al., 2010; Ribeiro et al., 2023). On a conceptual level, such alterations in emotion processing could influence language learning by altering the interpretation and processing of non-verbal cues that aid language development. Heightened threat detection could also inhibit language learning by limiting attention toward language-promoting stimuli, and consequently their processing and encoding into memory. Both child maltreatment and unpredictable maternal signals have been linked to greater brain myelination in 9–11-year-old children, and less myelination in 18–22-year-olds, in a major frontolimbic tract (uncinate fasciculus) (Hanson et al., 2015; Granger et al., 2021). Opposing findings regarding the magnitude of myelination in these two studies may be partially due to age and developmental maturity of the brain at assessment (Gee et al., 2013; Ribeiro et al., 2023; Hanson et al., 2015). Nevertheless, both greater and less myelination have been related to compromised emotion processing, suggesting that adequate myelination within sensitive windows of development is required for optimal language function (Banihashemi et al., 2020; Mincic, 2015; Modi et al., 2013). Overall, the link between ELA and brain structure and function is undisputed, and there is now a push to identify intermediary pathways linking them (Shackman and Gee, 2023), including sleep-related variables such as insomnia (Fuligni et al., 2021; Luby et al., 2020).
Recent findings highlight the critical role of pediatric sleep in neurodevelopment, with implications for language learning (Mason et al., 2021; Alrousan et al., 2022; Galván, 2020). Developmental experiments in nonhuman animals show that adequate sleep duration promotes molecular and neural network adaptation (Bridi et al., 2015), while insufficient or disturbed sleep gives way to maladaptive growth and development, such as exacerbating the effects of visual deprivation on the development of visual-cortical systems (Frank et al., 2001). Further, sleep in earlier stages of childhood may be more robustly associated with brain structure and function (Lokhandwala and Spencer, 2022). For example, in a sample of 200 children (4–8-years-old), longer 24-hr sleep duration predicted larger hippocampal subfield volumes, but only in the younger cohort (4–6 vs. 6–8 years) (Riggins and Spencer, 2020). The accumulation of findings has enabled scholars to identify putative neurobiological pathways in the association between early life sleep disturbances and compromised cognitive development (Alrousan et al., 2022; Jan et al., 2010; Mason and Spencer, 2022).
Changes in sleep-related brain activity (e.g., sleep spindles and slow oscillations) relevant for brain structure and dendritic density are some mechanisms through which sleep disturbances in early life (Lokhandwala and Spencer, 2022; Kurth et al., 2010; Pittner et al., 2023) may lead to impaired emotion processing, cognition, and memory (Kurth et al., 2015; Lopez et al., 2010), which may in turn impact language. Illustrating the relations between early sleep disturbance and brain development, one study reported that insufficient sleep in 6–9-month-olds prospectively predicted smaller white matter volume at 1-year (Pittner et al., 2023). This finding suggests that sleep disturbances in infancy may affect myelination (Frank et al., 2001) and communication across brain regions that facilitate complex cognitive processes, such as executive functioning and emotion processing (Lokhandwala and Spencer, 2022). Similarly, in a longitudinal cohort study of 720 six-year-old children, pediatric sleep disturbances, such as trouble falling asleep and resisting going to bed, were associated with decreased gray matter volume (Kocevska et al., 2017), which may be associated with later psychopathology (Wise et al., 2017). On a functional level, a study conducted in 5–9-year-olds found that shorter sleep duration on weekdays was associated with lower amygdala-ACC resting state functional connectivity (Hansen et al., 2024), which is also related to emotional processing (Etkin et al., 2015). These findings extend to school-age children and adolescents, as summarized by a review of this work demonstrating associations between a variety of sleep disturbances (e.g., in quality, duration, and variability) and brain connectivity in areas related to memory, attention, and reward processing (Dutil et al., 2018). Notably, some findings link changes in sleep from infancy to early childhood to white and gray matter (Pittner et al., 2023; Kocevska et al., 2017), suggesting that developmental sleep trajectories may be an important and understudied predictor of neurodevelopment that may subsequently affect language.
Due to overlap in the negative neurobiological and mental and physical health consequences of both ELA and sleep disturbances, a previous theoretical model suggests that sleep disturbances are a key mechanism linking ELA to poor health outcomes across the lifespan (Fuligni et al., 2021). We extend this theoretical model to include language development as an outcome, with a focus on childhood (see Figure 1 for a schematic representation of our framework). Thus far, we have summarized the effects of ELA and sleep disturbances on both brain structure and function, and language-related outcomes. Additional support of this model comes from the accumulating literature on associations between ELA and sleep disturbances.
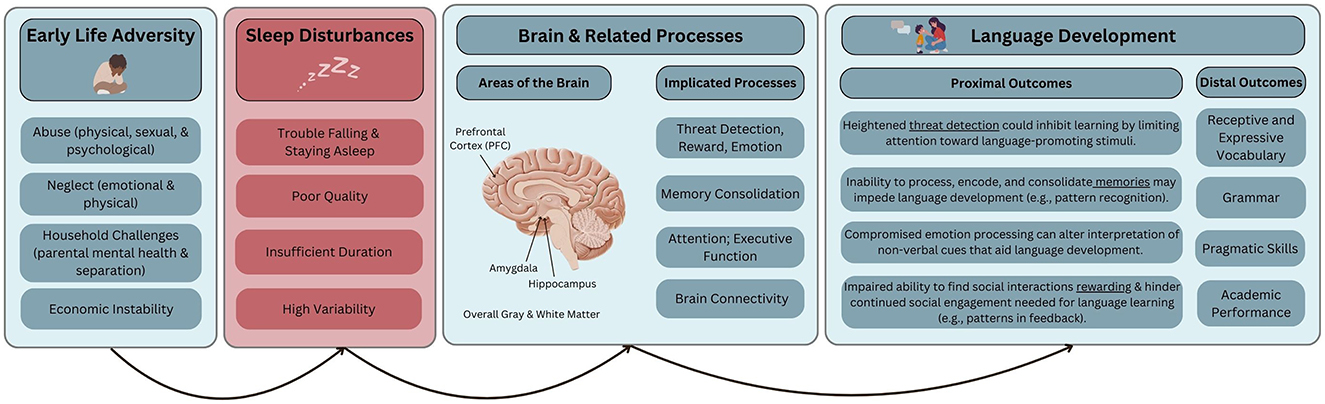
Figure 1. Conceptual model of sleep's potential role as a mechanism linking early life adversity to impaired language development via alterations to brain structure and function. We acknowledge that associations among these constructs are complex and often bidirectional. However, the arrows represent the theoretical model focused on in this review.
ELA has been linked with several behavioral sleep disturbances during childhood and adolescence (Brown et al., 2022; Schønning et al., 2022). The most robust associations have been with insomnia and insomnia-related symptoms, including difficulty falling and staying asleep (Brown et al., 2022; Schønning et al., 2022). A recent meta-analysis showed that child maltreatment is associated with four times greater odds of insomnia symptoms among children 5–18 years of age (Schønning et al., 2022). In a nationally representative study of children born to unwed parents, number of caregiver-reported ACEs through age 9 was associated with trouble falling asleep ≥3 times per week at age 15 (Rojo-Wissar et al., 2021). A similar epidemiologic study showed that adolescents with ≥1 ACE were 1.93 times more likely to develop an insomnia disorder compared to those with no ACEs, and those with ≥5 ACEs were at 3 times greater risk (Wang et al., 2016). Evidence has also demonstrated links of ELA with shorter sleep duration, lower sleep efficiency, and nightmares in children (Brown et al., 2022; Schønning et al., 2022; Sadikova and Mazurek, 2024). In particular, 6–36-month-olds from socioeconomically disadvantaged homes have shown shorter than recommended sleep duration for their age (Ordway et al., 2020; Mindell and Williamson, 2018).
Very few studies have examined associations between ELA (in the form of abuse or institutionalized rearing) and sleep measured via actigraphy in children. Most of these studies show associations with worse sleep efficiency but are somewhat mixed with regard to other sleep parameters (Glod et al., 1997; Tininenko et al., 2010; Sadeh et al., 1995). There are no known studies examining the relationship between ELA and sleep measured by PSG in children. However, there are a few studies in adults that suggest ELA, such as perceived lack of safety or exposure to traumatic events (e.g., abuse), may be associated with greater fragmentation in rapid-eye-movement (REM) sleep and less slow spindle density (Insana et al., 2012; Nielsen et al., 2019).
Strong evidence links ELA with the development of sleep disturbances, and sleep disturbances with language-relevant alterations in brain structure and function; nonetheless, limited research focuses on ELA, brain structure and function, sleep disturbances, and language outcomes altogether. However, a few studies provide initial evidence for the potential role of sleep as a mechanism linking ELA to impaired language development via alterations to brain structure and function. One study among young adults (18–19-years) found that sleep efficiency partially mediated the association between retrospectively reported child maltreatment and reductions in gray matter hippocampal volume (Teicher et al., 2017), which has been linked to later language outcomes (Deniz Can et al., 2013; Bellander et al., 2016). Similarly, another study found that sleep quality in middle and high schoolers partially mediated the relationship between ELA and academic achievement (e.g., English proficiency) (Qu et al., 2024). There is also evidence that maternal sleep disturbances may mediate or moderate associations between maternal ELA and offspring emotion processing (Ciciolla et al., 2022) and epigenetic aging (Sosnowski et al., 2024), supporting theoretical work positing that sleep during sensitive developmental periods (e.g., infancy and childhood) may act as a conduit mitigating or exacerbating the effects of ELA on health and language outcomes (Fuligni et al., 2021). Given robust theoretical evidence but scant empirical evidence focusing on the dynamic relations among ELA, sleep disturbances, brain structure and function, and language, it is critical for future studies to examine these developmental factors simultaneously.
The present literature has allowed scholars to understand the effects of ELA on brain structure and function, sleep disturbances, and language development separately; however, there are limitations in the current body of knowledge. First, though some work has begun to ascertain how ELA types may differentially affect neurodevelopmental outcomes (McLaughlin et al., 2014; Gee, 2021), relatively little research has considered important characteristics of ELA above and beyond exposure, including ELA timing, type(s), chronicity, severity, and cumulative burden (but see Selin et al., 2022; Bethell et al., 2017; Berman et al., 2022). Future studies are needed to determine the relative importance of these ELA characteristics, and how they interact to affect sleep and language development. Second, although some interventions such as home visiting programs have been shown to prevent child maltreatment (Han and Oh, 2022), promote healthy sleep (Schwichtenberg et al., 2019; Kuhn and Elliott, 2003; Fangupo et al., 2021), and improve language development (Peacock et al., 2013; Henwood et al., 2020; Pentimonti et al., 2022) they have not been examined all together in the same study. Additionally, studies focusing on specific clinical populations, including children with neurodevelopmental delays such as autism spectrum disorder, are needed to further elucidate the full effects of ELA on language outcomes (Sadikova and Mazurek, 2024). Future studies examining the effects of pediatric sleep interventions following ELA on language development in both general and clinical populations are warranted.
In addition to brain development, the Hypothalamic-Pituitary-Adrenal (HPA) axis and immune systems are pathways that have been implicated as mechanisms linking ELA and sleep disturbances to poorer mental and physical health outcomes (Fuligni et al., 2021). Here we focused on reviewing the sleep and brain-related pathways which are likely the most relevant to language development specifically, but future research should also explore the potential roles of the HPA-axis and immune system. Finally, previous evidence demonstrates that racial/ethnic disparities exist in ELA exposure (Suglia et al., 2020; O'Connor et al., 2020) and poor sleep outcomes (El-Sheikh et al., 2022; Billings et al., 2021; Jean-Louis and Grandner, 2016), and the accumulation of disparities across these systems may result in disparities in language development (Zuckerman et al., 2014; Justice et al., 2020). Future studies seeking to understand the multilevel disparities in place that affect ELA, sleep disturbances, brain structure and function, and language outcomes are crucial for effectively promoting language development in children from all families and backgrounds. Overall, longitudinal studies assessing ELA, sleep disturbances, and their combined effects on brain and language development are needed to further develop and elucidate the theoretical framework that sleep disturbances are an important pathway to consider in the associations between ELA and brain and language development.
The intricate connections among ELA, sleep disturbances, and brain structure and function underscore the multifaceted nature of overall development and its long-term implications for health and language outcomes specifically. Language development, from infancy through adolescence, engages an interplay of brain regions and networks, encompassing sensory processing, memory, reward, attention, and executive function. ELA, historically characterized as child maltreatment and ACEs, is consistently linked to compromised language skills across various developmental stages (Sylvestre et al., 2016; Lum et al., 2015), affecting vocabulary, grammar, pragmatic skills, and academic achievement. Likewise, sleep disturbances, spanning from infancy to adolescence, have shown enduring effects on language abilities, with insufficient or disrupted sleep predicting lower cognitive scores (Cheng et al., 2021), and worse performance on vocabulary measures (McGregor and Alper, 2015; St. Laurent et al., 2023), and academically overall (Ravid et al., 2009; Williamson et al., 2020). Our proposed framework considers both subjective and objective measures of sleep. However, given the lack of research using actigraphy and polysomnography, future studies should incorporate objective sleep assessments to evaluate the impact of measurement methodology and advance this framework.
Understanding the intricate and dynamic associations among ELA, sleep disturbances, and brain structure and function is imperative for promoting healthy language development in children of all backgrounds. Brain structures that play critical roles in emotion processing, memory, and cognitive functions are notably affected by both ELA (Luby et al., 2020; Gee et al., 2013; Dutil et al., 2018) and sleep disturbances (Mason and Spencer, 2022; Pittner et al., 2023; Kurth et al., 2015), suggesting converging neurobiological pathways that may mediate subsequent effects on language development. However, there is a scarcity of studies examining all of these together, and those that do, haven't considered language as an outcome. Moving forward, future research addressing these limitations by exploring mechanistic models and elucidating the role of sleep interventions to address language deficits associated with ELA, is needed. Additionally, considering disparities in access to healthcare and resources, particularly among underserved populations, is paramount for developing targeted interventions that mitigate the adverse effects of ELA and sleep disturbances on language development.
HL: Conceptualization, Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, Software. MN-B: Conceptualization, Data curation, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. CM: Writing – review & editing, Writing – original draft. MR: Conceptualization, Data curation, Supervision, Writing – original draft, Writing – review & editing. SP: Conceptualization, Writing – review & editing. GM: Writing – review & editing, Writing – original draft, Visualization, Supervision, Investigation, Data curation, Conceptualization. DR-W: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Investigation, Data curation, Conceptualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. DR-W was supported by NHLBI 1K01HL169495 (PI: Rojo-Wissar, Darlynn) and DR-W and SP were supported by NIGMS P20GM139767 (PI: Stroud, Laura). SP was additionally supported by R01HD095837 (PI: Parade, Stephanie). MN-B was supported by diversity supplement under parent grant R01HL155744-01S1 (PI: Davis, Elysia). GM was supported by NICHD R01HD103655 (PI: Saletin, Jared).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allen, R. E., and Oliver, J. M. (1982). Effects of child maltreatment on language development. Child Abuse Negl. 6, 299–305. doi: 10.1016/0145-2134(82)90033-3
Alrousan, G., Hassan, A., Pillai, A. A., Atrooz, F., and Salim, S. (2022). Early life sleep deprivation and brain development: insights from human and animal studies. Front. Neurosci. 16:833786. doi: 10.3389/fnins.2022.833786
Banihashemi, L., Bertocci, M. A., Alkhars, H. M., Versace, A., Northrup, J. B., Lee, V. K., et al. (2020). Limbic white matter structural integrity at 3 months prospectively predicts negative emotionality in 9-month-old infants: a preliminary study. J. Affect. Disord. 273, 538–541. doi: 10.1016/j.jad.2020.04.029
Bellander, M., Berggren, R., Mårtensson, J., Brehmer, Y., Wenger, E., Li, T. Q., et al. (2016). Behavioral correlates of changes in hippocampal gray matter structure during acquisition of foreign vocabulary. Neuroimage 131, 205–213. doi: 10.1016/j.neuroimage.2015.10.020
Berman, I. S., McLaughlin, K. A., Tottenham, N., Godfrey, K., Seeman, T., Loucks, E., et al. (2022). Measuring early life adversity: a dimensional approach. Dev. Psychopathol. 34, 499–511. doi: 10.1017/S0954579421001826
Bethell, C. D., Carle, A., Hudziak, J., Gombojav, N., Powers, K., Wade, R., et al. (2017). Methods to assess adverse childhood experiences of children and families: toward approaches to promote child well-being in policy and practice. Acad Pediatr. 17(7 Suppl.), S51–S69. doi: 10.1016/j.acap.2017.04.161
Billings, M. E., Cohen, R. T., Baldwin, C. M., Johnson, D. A., Palen, B. N., Parthasarathy, S., et al. (2021). Disparities in sleep health and potential intervention models: a focused review. Chest 159, 1232–1240. doi: 10.1016/j.chest.2020.09.249
Birnie, M. T., Kooiker, C. L., Short, A. K., Bolton, J. L., Chen, Y., Baram, T. Z., et al. (2020). Plasticity of the reward circuitry after early-life adversity: mechanisms and significance. Biol. Psychiatry 87, 875–884. doi: 10.1016/j.biopsych.2019.12.018
Botting, N., and Baraka, N. (2018). Sleep behaviour relates to language skills in children with and without communication disorders. Int. J. Dev. Disabil. 64, 225–230. doi: 10.1080/20473869.2017.1283766
Bridi, M. C. D., Aton, S. J., Seibt, J., Renouard, L., Coleman, T., Frank, M. G., et al. (2015). Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv. 1:1500105. doi: 10.1126/sciadv.1500105
Brown, K. M., and Malow, B. A. (2016). Pediatric insomnia. Chest 149, 1332–1339. doi: 10.1378/chest.15-0605
Brown, S. M., Rodriguez, K. E., Smith, A. D., Ricker, A., and Williamson, A. (2022). Associations between childhood maltreatment and behavioral sleep disturbances across the lifespan: a systematic review. Sleep Med. Rev. 64:101621. doi: 10.1016/j.smrv.2022.101621
Bruni, O., Ferri, R., Novelli, L., Terribili, M., Troianiello, M., Finotti, E., et al. (2009). Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep 32, 1333–1340. doi: 10.1093/sleep/32.10.1333
Calabro, F. J., Murty, V. P., Jalbrzikowski, M., Tervo-Clemmens, B., and Luna, B. (2020). Development of hippocampal-prefrontal cortex interactions through adolescence. Cereb. Cortex 30, 1548–1558. doi: 10.1093/cercor/bhz186
Callaghan, B. L., and Tottenham, N. (2016). The neuro-environmental loop of plasticity: a cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology 41, 163–176. doi: 10.1038/npp.2015.204
Carouso-Peck, S., Goldstein, M. H., and Fitch, W. T. (2021). The many functions of vocal learning. Philos. Transact. R. Soc. B 376:235. doi: 10.1098/rstb.2020.0235
Carruthers, P. (2002). The cognitive functions of language. Behav. Brain Sci. 25, 657–726. doi: 10.1017/S0140525X02000122
Chan, S. Y., Ngoh, Z. M., Ong, Z. Y., Teh, A. L., Kee, M. Z. L., Zhou, J. H., et al. (2024). The influence of early-life adversity on the coupling of structural and functional brain connectivity across childhood. Nat. Mental Health 2, 52–62. doi: 10.1038/s44220-023-00162-5
Chatburn, A., Coussens, S., Lushington, K., Kennedy, D., Baumert, M., Kohler, M., et al. (2013). Sleep spindle activity and cognitive performance in healthy children. Sleep 36, 237–243. doi: 10.5665/sleep.2380
Cheng, W., Rolls, E., Gong, W., Du, J., Zhang, J., Zhang, X. Y., et al. (2021). Sleep duration, brain structure, and psychiatric and cognitive problems in children. Mol. Psychiatry 26, 3992–4003. doi: 10.1038/s41380-020-0663-2
Ciciolla, L., Addante, S., Quigley, A., Erato, G., and Fields, K. (2022). Infant sleep and negative reactivity: the role of maternal adversity and perinatal sleep. Infant Behav. Dev. 66:101664. doi: 10.1016/j.infbeh.2021.101664
Conway, L. J., Cook, F., Cahir, P., Brown, S., Reilly, S., Gartland, D., et al. (2021). Children's language abilities at age 10 and exposure to intimate partner violence in early childhood: Results of an Australian prospective pregnancy cohort study. Child Abuse Negl. 111:104794. doi: 10.1016/j.chiabu.2020.104794
Coster, W. J., Gersten, M. S., Beeghly, M., and Cicchetti, D. (1989). Communicative functioning in maltreated toddlers. Dev. Psychol. 25, 1020–1029. doi: 10.1037/0012-1649.25.6.1020
Culp, R. E., Watkins, R. V., Lawrence, H., Letts, D., Kelly, D. J., Rice, M. L., et al. (1991). Maltreated children's language and speech development: abused, neglected, and abused and neglected. First Lang. 11, 377–389. doi: 10.1177/014272379101103305
Decasper, A. J., and Fifer, W. P. (1980). Of human bonding: newborns prefer their mothers' voices. Science 208, 1174–1176. doi: 10.1126/science.7375928
Deniz Can, D., Richards, T., and Kuhl, P. K. (2013). Early gray-matter and white-matter concentration in infancy predict later language skills: a whole brain voxel-based morphometry study. Brain Lang. 124, 34–44. doi: 10.1016/j.bandl.2012.10.007
Di Sante, M., Sylvestre, A., Bouchard, C., and Leblond, J. (2019). The pragmatic language skills of severely neglected 42-month-old children: results of the ELLAN study. Child Maltreat. 24, 244–253. doi: 10.1177/1077559519828838
Dionne, G., Touchette, E., Forget-Dubois, N., Petit, D., Tremblay, R. E., Montplaisir, J. Y., et al. (2011). Associations between sleep-wake consolidation and language development in early childhood: a longitudinal twin study. Sleep 34, 987–995. doi: 10.5665/SLEEP.1148
Duffy, K. A., McLaughlin, K. A., and Green, P. A. (2018). Early life adversity and health-risk behaviors: proposed psychological and neural mechanisms. Ann. N. Y. Acad. Sci. 1428:151. doi: 10.1111/nyas.13928
Dutil, C., Walsh, J. J., Featherstone, R. B., Gunnell, K. E., Tremblay, M. S., Gruber, R., et al. (2018). Influence of sleep on developing brain functions and structures in children and adolescents: a systematic review. Sleep Med. Rev. 42, 184–201. doi: 10.1016/j.smrv.2018.08.003
Eigsti, I. M., and Cicchetti, D. (2004). The impact of child maltreatment on expressive syntax at 60 months. Dev. Sci. 7, 88–102. doi: 10.1111/j.1467-7687.2004.00325.x
Eisenberg, N., Sadovsky, A., and Spinrad, T. L. (2005). Associations of emotion-related regulation with language skills, emotion knowledge, and academic outcomes. New Dir. Child Adolesc. Dev. 1, 109–118. doi: 10.1002/cd.143
Elmlinger, S. L., Goldstein, M. H., and Casillas, M. (2023). Immature vocalizations simplify the speech of Tseltal Mayan and U.S. Caregivers. Top Cogn Sci. 15, 315–328. doi: 10.1111/tops.12632
Elmlinger, S. L., Schwade, J. A., and Goldstein, M. H. (2019). The ecology of prelinguistic vocal learning: parents simplify the structure of their speech in response to babbling. J. Child Lang. 46, 998–1011. doi: 10.1017/S0305000919000291
El-Sheikh, M., Gillis, B. T., Saini, E. K., Erath, S. A., and Buckhalt, J. A. (2022). Sleep and disparities in child and adolescent development. Child Dev. Perspect. 16, 200–207. doi: 10.1111/cdep.12465
Etkin, A., Büchel, C., and Gross, J. J. (2015). The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693–700. doi: 10.1038/nrn4044
Fangupo, L. J., Haszard, J. J., Reynolds, A. N., Lucas, A. W., McIntosh, D. R., Richards, R., et al. (2021). Do sleep interventions change sleep duration in children aged 0–5 years? A systematic review and meta-analysis of randomised controlled trials. Sleep Med. Rev. 59:101498. doi: 10.1016/j.smrv.2021.101498
Fenson, L., Dale, P. S. P., Reznick, J. S., Bates, E., Thal, D. J., Pethick, S. J., et al. (1994). Variability in early communicative development. Monogr. Soc. Res. Child Dev. 59, 1–189. doi: 10.2307/1166093
Fernald, A., and Simon, T. (1984). Expanded intonation contours in mothers' speech to newborns. Dev. Psychol. 20, 104–113. doi: 10.1037/0012-1649.20.1.104
Ferrara, A. M., Mullins, C. A., Ellner, S., and Van Meter, P. (2023). Early child maltreatment and reading processes, abilities, and achievement: a systematic review. Child Abuse Negl. 142:105857. doi: 10.1016/j.chiabu.2022.105857
Fitch, W. T. (2005). The evolution of language: a comparative review. Biol. Philos. 20, 193–203. doi: 10.1007/s10539-005-5597-1
Fox, L., Long, S. H., and Langlois, A. (1988). Patterns of language comprehension deficit in abused and neglected children. J. Speech Hear. Disord. 53, 239–244. doi: 10.1044/jshd.5303.239
Frank, M. G., Issa, N. P., and Stryker, M. P. (2001). Sleep enhances plasticity in the developing visual cortex. Neuron 30, 275–287. doi: 10.1016/S0896-6273(01)00279-3
Friederici, A. D. (2006). The neural basis of language development and its impairment. Neuron 52, 941–952. doi: 10.1016/j.neuron.2006.12.002
Friedrich, M., Wilhelm, I., Mölle, M., Born, J., and Friederici, A. D. (2017). The sleeping infant brain anticipates development. Curr. Biol. 27, 2374–2380.e3. doi: 10.1016/j.cub.2017.06.070
Fuligni, A. J., Chiang, J. J., and Tottenham, N. (2021). Sleep disturbance and the long-term impact of early adversity. Neurosci. Biobehav. Rev. 126, 304–313. doi: 10.1016/j.neubiorev.2021.03.021
Gaab, N., and Petscher, Y. (2022). Screening for early literacy milestones and reading disabilities: the why, when, whom, how, and where. Perspect. Lang. Lit. 11–7.
Galván, A. (2020). The need for sleep in the adolescent brain. Trends Cogn. Sci. 24, 79–89. doi: 10.1016/j.tics.2019.11.002
Gee, D. G. (2021). Early adversity and development: parsing heterogeneity and identifying pathways of risk and resilience. Am. J. Psychiatry 178, 998–1013. doi: 10.1176/appi.ajp.2021.21090944
Gee, D. G., Gabard-Durnam, L. J., Flannery, J., Goff, B., Humphreys, K. L., Telzer, E. H., et al. (2013). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 110, 15638–15643. doi: 10.1073/pnas.1307893110
Gliga, T., Hendry, A., Kong, S. P., Ewing, B., Davies, C., McGillion, M., et al. (2023). More frequent naps are associated with lower cognitive development in a cohort of 8–38-month-old children, during the Covid-19 pandemic. JCPP Adv. 3:12190. doi: 10.1002/jcv2.12190
Glod, C. A., Teicher, M. H., Hartman, C. R., Harakal, T., and McGreenery, C. E. (1997). Enduring effects of early abuse on locomotor activity, sleep, and circadian rhythms. Ann. N. Y. Acad. Sci. 821, 465–467. doi: 10.1111/j.1749-6632.1997.tb48306.x
Goldstein, M. H., Waterfall, H. R., Lotem, A., Halpern, J. Y., Schwade, J. A., Onnis, L., et al. (2010). General cognitive principles for learning structure in time and space. Trends Cogn. Sci. 14, 249–258. doi: 10.1016/j.tics.2010.02.004
Gómez, R. L., Bootzin, R. R., and Nadel, L. (2006). Naps promote abstraction in language-learning infants. Psychol. Sci. 17, 670–674. doi: 10.1111/j.1467-9280.2006.01764.x
Graham, S. (2022). A walk through the landscape of writing: Insights from a program of writing research. Educ. Psychol. 57, 55–72. doi: 10.1080/00461520.2021.1951734
Granger, S. J., Glynn, L. M., Sandman, C. A., Small, S. L., Obenaus, A., Keator, D. B., et al. (2021). Aberrant maturation of the uncinate fasciculus follows exposure to unpredictable patterns of maternal signals. J. Neurosci. 41, 1242–1250. doi: 10.1523/JNEUROSCI.0374-20.2020
Hahn, M., Joechner, A. K., Roell, J., Schabus, M., Heib, D. P. J., Gruber, G., et al. (2019). Developmental changes of sleep spindles and their impact on sleep-dependent memory consolidation and general cognitive abilities: a longitudinal approach. Dev. Sci. 22:e12706. doi: 10.1111/desc.12706
Han, K., and Oh, S. (2022). The effectiveness of home visiting programs for the prevention of child maltreatment recurrence at home: a systematic review and meta-analysis. Child Health Nurs. Res. 28:41. doi: 10.4094/chnr.2022.28.1.41
Hansen, M., Simon, K. R., He, X., Steele, N., Thomas, M. L., Noble, K. G., et al. (2024). Socioeconomic factors, sleep timing and duration, and amygdala resting-state functional connectivity in children. Front. Psychiatry. 15:1373546. doi: 10.3389/fpsyt.2024.1373546
Hanson, J. L., Knodt, A. R., Brigidi, B. D., and Hariri, A. R. (2015). Lower structural integrity of the uncinate fasciculus is associated with a history of child maltreatment and future psychological vulnerability to stress. Dev. Psychopathol. 27, 1611–1619. doi: 10.1017/S0954579415000978
Henwood, T., Channon, S., Penny, H., Robling, M., and Waters, C. S. (2020). Do home visiting programmes improve children's language development? A systematic review. Int. J. Nurs. Stud. 109:103610. doi: 10.1016/j.ijnurstu.2020.103610
Hernandez-Reif, M., and Gungordu, N. (2022). Infant sleep behaviors relate to their later cognitive and language abilities and morning cortisol stress hormone levels. Infant Behav. Dev. 67:101700. doi: 10.1016/j.infbeh.2022.101700
Hertrich, I., Dietrich, S., Blum, C., and Ackermann, H. (2021). The role of the dorsolateral prefrontal cortex for speech and language processing. Front. Hum. Neurosci. 15:645209. doi: 10.3389/fnhum.2021.645209
Hildyard, K. L., and Wolfe, D. A. (2002). Child neglect: developmental issues and outcomes. Child Abuse Negl. 26, 679–695. doi: 10.1016/S0145-2134(02)00341-1
Horváth, K., Liu, S., and Plunkett, K. (2016). A daytime nap facilitates generalization of word meanings in young toddlers. Sleep 39, 203–207. doi: 10.5665/sleep.5348
Hupbach, A., Gomez, R. L., Bootzin, R. R., and Nadel, L. (2009). Nap-dependent learning in infants. Dev. Sci. 12, 1007–1012. doi: 10.1111/j.1467-7687.2009.00837.x
Hyter, Y. D. (2021). Childhood maltreatment consequences on social pragmatic communication: a systematic review of the literature. Perspect. ASHA Spec. Int. Groups 6, 262–287. doi: 10.1044/2021_PERSP-20-00222
Insana, S. P., Kolko, D. J., and Germain, A. (2012). Early-life trauma is associated with rapid eye movement sleep fragmentation among military veterans. Biol. Psychol. 89, 570–579. doi: 10.1016/j.biopsycho.2012.01.001
Jan, J. E., Reiter, R. J., Bax, M. C. O., Ribary, U., Freeman, R. D., Wasdell, M. B., et al. (2010). Long-term sleep disturbances in children: a cause of neuronal loss. Eur. J. Paediatr. Neurol. 14, 380–390. doi: 10.1016/j.ejpn.2010.05.001
Jean-Louis, G., and Grandner, M. (2016). Importance of recognizing sleep health disparities and implementing innovative interventions to reduce these disparities. Sleep Med. 18, 1–2. doi: 10.1016/j.sleep.2015.08.001
Jimenez, M. E., Wade, R., Lin, Y., Morrow, L. M., and Reichman, N. E. (2016). Adverse experiences in early childhood and kindergarten outcomes. Pediatrics 137:1839. doi: 10.1542/peds.2015-1839
Jones, C. H. D., and Ball, H. (2014). Exploring socioeconomic differences in bedtime behaviours and sleep duration in english preschool children. Infant Child Dev. 23, 518–531. doi: 10.1002/icd.1848
Justice, L. M., Jiang, H., Bates, R., and Koury, A. (2020). Language disparities related to maternal education emerge by two years in a low-income sample. Matern. Child Health J. 24, 1419–1427. doi: 10.1007/s10995-020-02973-9
Keunen, K., Counsell, S. J., and Benders, M. J. N. L. (2017). The emergence of functional architecture during early brain development. Neuroimage 160, 2–14. doi: 10.1016/j.neuroimage.2017.01.047
Knowland, V. C. P., Berens, S., Gaskell, M. G., Walker, S. A., and Henderson, L. M. (2022). Does the maturation of early sleep patterns predict language ability at school entry? A Born in Bradford study. J. Child Lang. 49, 1–23. doi: 10.1017/S0305000920000677
Kocevska, D., Muetzel, R. L., Luik, A. I., Luijk, M. P. C. M., Jaddoe, V. W., Verhulst, F. C., et al. (2017). The developmental course of sleep disturbances across childhood relates to brain morphology at age 7: the generation R study. Sleep. 40:zsw022. doi: 10.1093/sleep/zsw022
Kostović, I., and Jovanov-Milošević, N. (2006). The development of cerebral connections during the first 20-45 weeks' gestation. Semin. Fetal Neonatal Med. 11, 415–422. doi: 10.1016/j.siny.2006.07.001
Kuhn, B. R., and Elliott, A. J. (2003). Treatment efficacy in behavioral pediatric sleep medicine. J. Psychosom. Res. 54, 587–597. doi: 10.1016/S0022-3999(03)00061-8
Kurth, S., Olini, N., Huber, R., and LeBourgeois, M. (2015). Sleep and early cortical development. Curr Sleep Med Rep. 1, 64–73. doi: 10.1007/s40675-014-0002-8
Kurth, S., Ringli, M., Geiger, A., LeBourgeois, M., Jenni, O. G., and Huber, R. (2010). Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. J. Neurosci. 30, 13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010
Kwan, K. Y., Šestan, N., and Anton, E. S. (2012). Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 139, 1535–1546. doi: 10.1242/dev.069963
Locke, J. L. (2001). First communion : the emergence of vocal relationships. Soc. Dev. 10, 294–308. doi: 10.1111/1467-9507.00167
Lokhandwala, S., and Spencer, R. M. C. (2022). Relations between sleep patterns early in life and brain development: a review. Dev. Cogn. Neurosci. 56:101130. doi: 10.1016/j.dcn.2022.101130
Lopez, J., Hoffmann, R., and Armitage, R. (2010). Reduced sleep spindle activity in early-onset and elevated risk for depression. J. Am. Acad. Child Adolesc. Psychiatry 49, 934–943. doi: 10.1016/j.jaac.2010.05.014
Luby, J. L., Baram, T. Z., Rogers, C. E., and Barch, D. M. (2020). Neurodevelopmental optimization after early-life adversity: cross-species studies to elucidate sensitive periods and brain mechanisms to inform early intervention. Trends Neurosci. 43, 744–751. doi: 10.1016/j.tins.2020.08.001
Lum, J. A. G., Powell, M., and Snow, P. C. (2017). The influence of maltreatment history and out-of-home-care on children's language and social skills. Child Abuse Negl. 76, 65–74. doi: 10.1016/j.chiabu.2017.10.008
Lum, J. A. G., Powell, M., Timms, L., and Snow, P. (2015). A meta-analysis of cross sectional studies investigating language in maltreated children. J. Speech Lang. Hear. Res. 58, 961–976. doi: 10.1044/2015_JSLHR-L-14-0056
Lupyan, G., Rakison, D. H., and McClelland, J. L. (2007). Language is not just for talking: redundant labels facilitate learning of novel categories. Psychol. Sci. 18, 1077–1083. doi: 10.1111/j.1467-9280.2007.02028.x
Mason, G. M., Goldstein, M. H., and Schwade, J. A. (2019). The role of multisensory development in early language learning. J. Exp. Child Psychol. 183, 48–64. doi: 10.1016/j.jecp.2018.12.011
Mason, G. M., Lokhandwala, S., Riggins, T., and Spencer, R. M. C. (2021). Sleep and human cognitive development. Sleep Med. Rev. 57:101472. doi: 10.1016/j.smrv.2021.101472
Mason, G. M., and Spencer, R. M. C. (2022). Sleep and memory in infancy and childhood. Annu. Rev. Dev. Psychol. 4, 89–108. doi: 10.1146/annurev-devpsych-121020-033411
Matsuzawa, J.. Matsui, M., Konishi, T., Noguchi, K., Gur, R. C., and Bilker, W. (2001). Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb. Cortex 19:335. doi: 10.1093/cercor/11.4.335
Matte-Landry, A., and Collin-Vézina, D. (2020). Cognitive outcomes of children who have experienced complex trauma: a systematic review protocol. JBI Evid. Synth. 18, 543–552. doi: 10.11124/JBISRIR-D-19-00036
McGregor, K. K., and Alper, R. M. (2015). Sleep disorders as a risk to language learning and use. EBP Briefs 10, 1–21.
McKelvey, L. M., Selig, J. P., and Whiteside-Mansell, L. (2017). Foundations for screening adverse childhood experiences: exploring patterns of exposure through infancy and toddlerhood. Child Abuse Negl. 70, 112–121. doi: 10.1016/j.chiabu.2017.06.002
McLaughlin, K. A., Sheridan, M. A., and Lambert, H. K. (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 47, 578–591. doi: 10.1016/j.neubiorev.2014.10.012
Mills, R., Alati, R., O'Callaghan, M., Najman, J. M., Williams, G. M., Bor, W., et al. (2011). Child abuse and neglect and cognitive function at 14 years of age: findings from a birth cohort. Pediatrics 127, 4–10. doi: 10.1542/peds.2009-3479
Mincic, A. M. (2015). Neuroanatomical correlates of negative emotionality-related traits: a systematic review and meta-analysis. Neuropsychologia. 77, 97–118. doi: 10.1016/j.neuropsychologia.2015.08.007
Mindell, J. A., Owens, J. A., and Carskadon, M. A. (1999). Developmental features of sleep. Child Adolesc. Psychiatr. Clin. N. Am. 8, 695–725. doi: 10.1016/S1056-4993(18)30149-4
Mindell, J. A., and Williamson, A. A. (2018). Benefits of a bedtime routine in young children: sleep, development, and beyond. Sleep Med. Rev. 40, 93–108. doi: 10.1016/j.smrv.2017.10.007
Modi, S., Trivedi, R., Singh, K., Kumar, P., Rathore, R. K. S., Tripathi, R. P., et al. (2013). Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behav. Brain Res. 238, 188–192. doi: 10.1016/j.bbr.2012.10.007
Mohammed, D., Park, V., Bogaardt, H., and Docking, K. (2021). The impact of childhood obstructive sleep apnea on speech and oral language development: a systematic review. Sleep Med. 81, 144–153. doi: 10.1016/j.sleep.2021.02.015
Molet, J., Maras, P. M., Kinney-Lang, E., Harris, N. G., Rashid, F., Ivy, A. S., et al. (2016). MRI uncovers disrupted hippocampal microstructure that underlies memory impairments after early-life adversity. Hippocampus 26, 1618–1632. doi: 10.1002/hipo.22661
Moon, C. M., Lagercrantz, H., and Kuhl, P. K. (2013). Language experienced in utero affects vowel perception after birth: a two-country study. Acta Paediatr. 102, 156–160. doi: 10.1111/apa.12098
Nielsen, T., Carr, M., Picard-Deland, C., Marquis, L. P., Saint-Onge, K., Blanchette-Carrière, C., et al. (2019). Early childhood adversity associations with nightmare severity and sleep spindles. Sleep Med. 56, 57–65. doi: 10.1016/j.sleep.2019.03.004
O'Connor, M., Slopen, N., Becares, L., Burgner, D., Williams, D. R., Priest, N., et al. (2020). Inequalities in the distribution of childhood adversity from birth to 11 years. Acad. Pediatr. 20, 609–618. doi: 10.1016/j.acap.2019.12.004
Oh, D. L., Jerman, P., Silvério Marques, S., Koita, K., Purewal Boparai, S. K., Burke Harris, N., et al. (2018). Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatr. 18:83. doi: 10.1186/s12887-018-1037-7
Opitz, B., and Friederici, A. D. (2003). Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. Neuroimage 19, 1730–1737. doi: 10.1016/S1053-8119(03)00170-8
Ordway, M. R., Sadler, L. S., Jeon, S., O'Connell, M., Banasiak, N., Fenick, A. M., et al. (2020). Sleep health in young children living with socioeconomic adversity. Res. Nurs. Health. 43, 329–340. doi: 10.1002/nur.22023
Ouyang, M., Dubois, J., Yu, Q., Mukherjee, P., and Huang, H. (2019). Delineation of early brain development from fetuses to infants with diffusion MRI and beyond. Neuroimage 185, 836–850. doi: 10.1016/j.neuroimage.2018.04.017
Owens, J. A., and Mindell, J. A. (2011). Pediatric insomnia. Pediatr. Clin. North Am. 58, 555–569. doi: 10.1016/j.pcl.2011.03.011
Palazón-Carrión, E., and Sala-Roca, J. (2020). Communication and language in abused and institutionalized minors. A scoping review. Child. Youth Serv. Rev. 112:104904. doi: 10.1016/j.childyouth.2020.104904
Paterson, S. J., Heim, S., Thomas Friedman, J., Choudhury, N., and Benasich, A. A. (2006). Development of structure and function in the infant brain: Implications for cognition, language and social behaviour. Neurosci. Biobehav. Rev. 30, 1087–1105. doi: 10.1016/j.neubiorev.2006.05.001
Peacock, S., Konrad, S., Watson, E., Nickel, D., and Muhajarine, N. (2013). Effectiveness of home visiting programs on child outcomes: a systematic review. BMC Public Health 13:17. doi: 10.1186/1471-2458-13-17
Pentimonti, J., Danielle, S. A., Little, M. H., Holod, A., Buysse, V., Walker, D., et al. (2022). Impacts of a parent-implemented language intervention on children's language development within home visiting. Infants Young Child. 35, 285–302. doi: 10.1097/IYC.0000000000000224
Pfefferbaum, A., Mathalon, D. H., Sullivan, E. V., Rawles, J. M., Zipursky, R. B., Lim, K. O. A., et al. (1994). Quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch. Neurol. 51, 874–887. doi: 10.1001/archneur.1994.00540210046012
Piai, V., Roelofs, A., Acheson, D. J., and Takashima, A. (2013). Attention for speaking: domain-general control from the anterior cingulate cortex in spoken word production. Front. Hum. Neurosci. 7:832. doi: 10.3389/fnhum.2013.00832
Pickett, J. (2020). Infant language development: the consequences of trauma. Undergrad. J. Psychol. 15, 79–94.
Pittner, K., Rasmussen, J., Lim, M. M., Gilmore, J. H., Styner, M., Entringer, S., et al. (2023). Sleep across the first year of life is prospectively associated with brain volume in 12-months old infants. Neurobiol. Sleep Circ. Rhythms 14:100091. doi: 10.1016/j.nbscr.2023.100091
Qu, G., Liu, H., Han, T., Zhang, H., Ma, S. Liang, S., et al. (2024). Association between adverse childhood experiences and sleep quality, emotional and behavioral problems and academic achievement of children and adolescents. Eur. Child Adolesc. Psychiatry 1:3. doi: 10.1007/s00787-023-02185-w
Ravid, S., Afek, I., Suraiya, S., Shahar, E., and Pillar, G. (2009). Sleep disturbances are associated with reduced school achievements in first-grade pupils. Dev. Neuropsychol. 34, 574–587. doi: 10.1080/87565640903133533
Ribeiro, M., Yordanova, Y. N., Noblet, V., Herbet, G., and Ricard, D. (2023). White matter tracts and executive functions: a review of causal and correlation evidence. Brain 147, 352–371. doi: 10.1093/brain/awad308
Riggins, T., and Spencer, R. M. C. (2020). Habitual sleep is associated with both source memory and hippocampal subfield volume during early childhood. Sci. Rep. 10:15304. doi: 10.1038/s41598-020-72231-z
Rojo-Wissar, D. M., Sosnowski, D. W., Ingram, M. M., Jackson, C. L., Maher, B. S., Alfano, C. A., et al. (2021). Associations of adverse childhood experiences with adolescent total sleep time, social jetlag, and insomnia symptoms. Sleep Med. 88, 104–115. doi: 10.1016/j.sleep.2021.10.019
Romano, E., Babchishin, L., Marquis, R., and Fréchette, S. (2015). Childhood maltreatment and educational outcomes. Trauma Violence Abuse. 16, 418–437. doi: 10.1177/1524838014537908
Rosselli, M., Ardila, A., Matute, E., and Vélez-Uribe, I. (2014). Language development across the life span: a neuropsychological/neuroimaging perspective. Neurosci. J. 2014:585237. doi: 10.1155/2014/585237
Sadeh, A., Hauri, P. J., Kripke, D. F., and Lavie, P. (1995). The role of actigraphy in the evaluation of sleep disorders. Sleep 18, 288–302. doi: 10.1093/sleep/18.4.288
Sadikova, E., and Mazurek, M. O. (2024). The association between adverse childhood experiences and sleep in children with autism spectrum disorder. J. Autism Dev. Disord. doi: 10.1007/s10803-024-06321-6
Sandoval, M., Leclerc, J. A., and Gómez, R. L. (2017). Words to sleep on: naps facilitate verb generalization in habitually and nonhabitually napping preschoolers. Child Dev. 88, 1615–1628. doi: 10.1111/cdev.12723
Schønning, V., Sivertsen, B., Hysing, M., Dovran, A., and Askeland, K. G. (2022). Childhood maltreatment and sleep in children and adolescents: a systematic review and meta-analysis. Sleep Med. Rev. 63:101617. doi: 10.1016/j.smrv.2022.101617
Schwichtenberg, A. J., Abel, E. A., Keys, E., and Honaker, S. M. (2019). Diversity in pediatric behavioral sleep intervention studies. Sleep Med. Rev. 47, 103–111. doi: 10.1016/j.smrv.2019.07.004
Selin, C., Rice, M. L., and Jackson, Y. (2022). Adversity exposure, syntax, and specific language impairment: an exploratory study. J. Speech Lang. Hear. Res. 65:3471. doi: 10.1044/2022_JSLHR-21-00578
Shackman, A. J., and Gee, D. G. (2023). Maternal perinatal stress associated with offspring negative emotionality, but the underlying mechanisms remain elusive. Am. J. Psychiatry 180, 708–711. doi: 10.1176/appi.ajp.20230630
Shellhaas, R. A., Chervin, R. D., Barks, J. D. E., Hassan, F., Carlson, M. D., Burns, J. W., et al. (2022). Lateralized neonatal EEG coherence during sleep predicts language outcome. Pediatr. Res. 91, 962–969. doi: 10.1038/s41390-021-01554-y
Short, A. K., and Baram, T. Z. (2019). Early-life adversity and neurological disease: age-old questions and novel answers. Nat. Rev. Neurol. 15, 657–669. doi: 10.1038/s41582-019-0246-5
Simon, K. N. S., Werchan, D., Goldstein, M. R., Sweeney, L., Bootzin, R. R., Nadel, L., et al. (2017). Sleep confers a benefit for retention of statistical language learning in 6.5 month old infants. Brain Lang. 167, 3–12. doi: 10.1016/j.bandl.2016.05.002
Skeide, M. A., and Friederici, A. D. (2016). The ontogeny of the cortical language network. Nat. Rev. Neurosci. 17, 323–332. doi: 10.1038/nrn.2016.23
Smithson, L., Baird, T., Tamana, S. K., Lau, A., Mariasine, J., Chikuma, J., et al. (2018). Shorter sleep duration is associated with reduced cognitive development at two years of age. Sleep Med. 48, 131–139. doi: 10.1016/j.sleep.2018.04.005
Snow, P. C. (2020). Psychosocial adversity in early childhood and language and literacy skills in adolescence: the role of speech-language pathology in prevention, policy, and practice. Perspect ASHA Spec Interest Groups. 6, 253–261. doi: 10.1044/2020_PERSP-20-00120
Sosnowski, D. W., Rojo-Wissar, D. M., Peng, G., Parade, S. H., Sharkey, K., Hoyo, C., et al. (2024). Maternal childhood adversity and infant epigenetic aging: Moderation by restless sleep during pregnancy. Dev. Psychobiol. 66:22464. doi: 10.1002/dev.22464
Stewart-Tufescu, A., Struck, S., Taillieu, T., Salmon, S., Fortier, J., Brownell, M., et al. (2022). Adverse childhood experiences and education outcomes among adolescents: linking survey and administrative data. Int. J. Environ. Res. Public Health 19:11564. doi: 10.3390/ijerph191811564
Stiles, J., and Jernigan, T. L. (2010). The basics of brain development. Neuropsychol. Rev. 20, 327–348. doi: 10.1007/s11065-010-9148-4
St. Laurent, C. W., Rasmussen, C. L., Holmes, J. F., Cremone-Caira, A., Kurdziel, L. B. F., Desrochers, P. C., et al. (2023). Associations of activity, sedentary, and sleep behaviors with cognitive and social-emotional health in early childhood. J. Act. Sedent. Sleep Behav. 2L7. doi: 10.1186/s44167-023-00016-6
Suglia, S. F., Campo, R. A., Brown, A. G. M., Stoney, C., Boyce, C. A., Appleton, A. A., et al. (2020). Social determinants of cardiovascular health: early life adversity as a contributor to disparities in cardiovascular diseases. J. Pediatr. 219, 267–273. doi: 10.1016/j.jpeds.2019.12.063
Suglia, S. F., Koenen, K. C., Boynton-Jarrett, R., Chan, P. S., Clark, C. J., Danese, A., et al. (2018). Childhood and adolescent adversity and cardiometabolic outcomes: a scientific statement from the American Heart Association. Circulation 137, e15–28. doi: 10.1161/CIR.0000000000000536
Swanson, H. L., and Alloway, T. P. (2011). Working memory, learning, and academic achievement. APA Educ. Psychol. Handb. 1, 327–66. doi: 10.1037/13273-012
Sweeney, L. M., Lara, H., and Gómez, R. L. (2023). Developmental changes in retention and generalization of nonadjacent dependencies over a period containing sleep in 18-mo-old infants. Learn. Mem. 30, 212–220. doi: 10.1101/lm.053772.123
Syal, S., and Finlay, B. L. (2011). Thinking outside the cortex: social motivation in the evolution and development of language. Dev. Sci. 14, 417–430. doi: 10.1111/j.1467-7687.2010.00997.x
Sylvestre, A., Bussières, È. L., and Bouchard, C. (2016). Language problems among abused and neglected children: a meta-analytic review. Child Maltreat. 21, 47–58. doi: 10.1177/1077559515616703
Tamis-LeMonda, C. S., and Bornstein, M. H. (1989). Habituation and maternal encouragement of attention in infancy as predictors of toddler language, play, and representational competence. Child Dev. 60, 738–751. doi: 10.1111/j.1467-8624.1989.tb02754.x
Teicher, M. H., Ohashi, K., Khan, A., Garcia, L. C. H., Klengel, T., Anderson, C. M., et al. (2017). Does sleep disruption mediate the effects of childhood maltreatment on brain structure? Eur. J. Psychotraumatol. 8:1450594. doi: 10.1080/20008198.2018.1450594
Tininenko, J. R., Fisher, P. A., Bruce, J., and Pears, K. C. (2010). Sleep disruption in young foster children. Child Psychiatry Hum. Dev. 41, 409–424. doi: 10.1007/s10578-010-0177-2
Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., et al. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61. doi: 10.1111/j.1467-7687.2009.00852.x
Van Petten, C. (2004). Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 42, 1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006
Venditti, J. A., Murrugarra, E., McLean, C. R., and Goldstein, M. H. (2023). Curiosity constructs communicative competence through social feedback loops. Adv. Child Dev. Behav. 65, 99–134. doi: 10.1016/bs.acdb.2023.05.007
Visser-Bochane, M. I., Reijneveld, S. A., Krijnen, W. P., van der Schans, C. P., and Luinge, M. R. (2020). Identifying milestones in language development for young children ages 1 to 6 years. Acad. Pediatr. 20, 421–429. doi: 10.1016/j.acap.2019.07.003
Wade, M., Wright, L., and Finegold, K. E. (2022). The effects of early life adversity on children's mental health and cognitive functioning. Transl. Psychiatry 12:244. doi: 10.1038/s41398-022-02001-0
Wang, S., Zhou, M., Chen, T., Yang, X., Chen, G., Wang, M., et al. (2017). Examining gray matter structure associated with academic performance in a large sample of Chinese high school students. Sci. Rep. 7:893. doi: 10.1038/s41598-017-00677-9
Wang, Y., Raffeld, M. R., Slopen, N., Hale, L., and Dunn, E. C. (2016). Childhood adversity and insomnia in adolescence. Sleep Med. 21, 12–18. doi: 10.1016/j.sleep.2016.01.011
Williamson, A. A., Mindell, J. A., Hiscock, H., and Quach, J. (2020). Longitudinal sleep problem trajectories are associated with multiple impairments in child well-being. J. Child Psychol. Psychiatry 61, 1092–1103. doi: 10.1111/jcpp.13303
Wise, T., Radua, J., Via, E., Cardoner, N., Abe, O., Adams, T. M., et al. (2017). Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: evidence from voxel-based meta-analysis. Mol. Psychiatry 22, 1455–1463. doi: 10.1038/mp.2016.72
Zee, P. C., and Turek, F. W. (2006). Sleep and health: everywhere and in both directions. Arch. Intern. Med. 166, 1686–1688. doi: 10.1001/archinte.166.16.1686
Keywords: early life adversity (ELA), sleep disturbances (SD), childhood, brain development, language development
Citation: Lara H, Nevarez-Brewster M, Manning C, Reid MJ, Parade SH, Mason GM and Rojo-Wissar DM (2024) The role of sleep disturbances in associations between early life adversity and subsequent brain and language development during childhood. Front. Sleep 3:1405398. doi: 10.3389/frsle.2024.1405398
Received: 22 March 2024; Accepted: 13 November 2024;
Published: 04 December 2024.
Edited by:
Sushil K. Jha, Jawaharlal Nehru University, IndiaReviewed by:
Nataliia Kozhemiako, Brigham and Women's Hospital and Harvard Medical School, United StatesCopyright © 2024 Lara, Nevarez-Brewster, Manning, Reid, Parade, Mason and Rojo-Wissar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hatty Lara, aGF0dHlsYXJhQGFyaXpvbmEuZWR1; Gina M. Mason, Z2luYV9tYXNvbkBicm93bi5lZHU=
†These authors share lead authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.