
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Sleep , 18 January 2024
Sec. Pediatric and Adolescent Sleep
Volume 2 - 2023 | https://doi.org/10.3389/frsle.2023.1302509
Background/objective: There is a paucity of literature regarding “flagging” abnormal sleep studies for expedited review. This single-center retrospective analysis (n = 266) of flagged polysomnography studies from 2019 to 2022 aimed to investigate flagging and its impact on the clinical timeline.
Methods: Two hundred sixty-six flagged polysomnography studies from 2019 to 2022 were retrospectively reviewed.
Results: Flagged study etiologies included repetitive brief oxygen desaturations (46.6%), sustained desaturations (32.3%), sustained hypercapnia (5.6%), or other concerning events (15.5%). The median time between a flagged study and scoring report finalization, medical intervention, and surgical intervention were 0 (2) days, 2 (3) days, 5 (11.25) days, and 44 (73) days, respectively. Patients with apnea–hypopnea index >30 had less time between a flagged study and surgical intervention (65.3 ± 96.7 days vs. 112 ± 119 days, p = 0.044).
Conclusion: As anticipated, the time to surgical intervention was longer than to medical intervention. Patients with a higher disease severity experienced quicker scoring, report finalization, and surgical intervention.
Untreated pediatric obstructive sleep apnea (OSA) syndrome has been well documented to have long-term sequelae involving multiorgan system dysfunction (Schechter, 2002; Kheirandish-Gozal and Gozal, 2017). Manifestations include autonomic and endothelial dysfunction, cardiac remodeling (Ingram et al., 2017; Baker-Smith et al., 2021), insulin resistance, dyslipidemia, metabolic derangements (Blechner and Williamson, 2016), negative associations with longitudinal growth (Park et al., 2018), impaired neurocognition, cortical thinning, and impaired attention (Thomas et al., 2022). However, these sequelae may not be permanent.
Recent research has found that early recognition and appropriate surgical treatment to improve respiration during sleep can lower the risk of consequences such as pulmonary hypertension, cor pulmonale (Baker-Smith et al., 2021), and insulin resistance (Bhatt et al., 2021). Other studies have documented improvements in neuropsychological testing in children with OSA syndrome postsurgical interventions such as tonsillectomy and adenoidectomy (Yu et al., 2017). Earlier detection may present the opportunity for intervention for overall improved health quality and health outcomes in pediatric patients, but future research is necessary.
In the presence of significant sleep-related physiologic abnormalities or other concerning events encountered during a polysomnography, accredited sleep laboratories and performing technologists utilize a standardized protocol with the involvement of an on-call supervising physician for flagging a study for expedited scoring and review. While there is literature pertaining to sleep emergencies (Thomas and Chang-Ho, 2011; Collop, 2021), no studies report specific criteria for the expedited scoring and interpretation of pediatric polysomnographies or their potential association with treatment timing.
Despite the growing evidence supporting earlier interventions for pediatric OSA management (Marcus et al., 2012, 2013), there is a dearth of research discerning a standard timeline for definitive management. Without exploring these timelines, the optimal management of sleep-related respiratory disturbances in children cannot be adequately assessed.
The present study aimed to explore pediatric OSA syndrome by providing data on the impact of polysomnography flagging and the associated timelines on clinical management.
This was a single-center retrospective chart review study conducted at Nemours Children's Hospital in Delaware, a pediatric tertiary care center, with a defined study period from 2019 through 2022. All data collection and protocols were approved by the Nemours institutional review board. All pediatric patients who had a polysomnography flagged for expedited review during the study period were included. Studies were flagged based on established laboratory protocols and at the discretion of the on-call physician. All data including basic demographic, clinical, and polysomnography parameters were obtained from the electronic medical record. The baseline characteristics included age, race, ethnicity, sex at birth, body mass index, and medical comorbidities.
The selected polysomnography parameters included total sleep time (TST), sleep efficiency, apnea–hypopnea index (AHI), OSA index, arousal index, and minimum oxygen saturation (SpO2), as well as the presence of hypoventilation (end-tidal carbon dioxide [EtCO2] > 50 torr for at least 25% of TST) and hypoxia (SpO2 < 90% for at least 5% of TST). The reasons for polysomnography flagging were obtained from the sleep laboratory database by an experienced sleep medicine technologist.
The dates of the polysomnography encounter, report flagging, scoring, clinical physician encounter note finalization, and any subsequent medical or surgical intervention were recorded. Medical intervention was defined as the ordering of nocturnal supplemental oxygen therapy or positive airway pressure with either continuous positive airway pressure (CPAP) or bilevel positive airway pressure. Surgical intervention included adenoidectomy, tonsillectomy, and adenotonsillectomy. Patients who had received prior airway surgery were not coded in the surgical group unless they received a new or revised surgical procedure in response to the flagged polysomnography study. Notation was made of patients admitted overnight after surgery due to new oxygen or CPAP requirements in the immediate postoperative period.
Statistical analyses were conducted using Student's t-test for continuous variables and both the chi-square and Fisher's exact tests for categorical variables. Further tests included the Mann–Whitney U-test to assess for impact of skew on non-parametric data. Statistical significance was defined as p < 0.05. Statistical analyses were performed using SPSS Statistics version 27 (IBM Corp., Armonk, NY) and Microsoft Excel version 16.60 (Redmond, WA).
The demographic, clinical, and polysomnography data of the pediatric patients with flagged polysomnographies are illustrated in Table 1. Most patients were white non-Hispanic males. The most common pulmonary, cardiac, genetic, and neuromuscular comorbidities were asthma (20.7%), congenital heart disease (22.9%), syndromic chromosomal abnormalities (14.3%), and cerebral palsy (7.9%), respectively. Notably, 30.1% of patients had a history of airway surgery prior to their flagged polysomnography, with 70% of those surgeries being adenotonsillectomy. Patients older than 8 years had higher rates of prior airway surgery compared with those younger than 8 years (43.7% vs. 21.5%, p < 0.0001).
The mean TST, sleep efficiency, and AHI were 372 ± 86.9 minutes, 79.3 ± 14.3%, and 47.2 ± 36.1 events per hour, respectively. The mean percentage of TST with SpO2 < 90% and EtCO2 > 50 torr were 8.60 ± 15.5% and 25.1 ± 30.6%, respectively.
Polysomnographies were flagged most frequently secondary to repetitive, brief desaturations with SpO2 < 80% (46.6%), sustained desaturations with SpO2 < 85% (32.3%), and other concerning events (15.5%), as well as sustained EtCO2 > 60 torr (5.6%). The prevalence of sleep studies that met diagnostic criteria for nocturnal hypoxemia (35.0%) and hypoventilation (36.8%) were similar.
The mean times between a flagged study and scoring, report finalization, medical intervention, and surgical intervention were 1.18 ± 2.09 days, 4.29 ± 6.01 days, 26.6 ± 93.0 days, and 78.2 ± 105 days, respectively, as shown in Figure 1. Subgroup analysis revealed that patients with a greater disease severity characterized by AHI > 30 experienced shorter mean intervals between study flagging and scoring (0.970 vs. 1.49 days, p = 0.048), report finalization (3.42 vs. 5.61 days, p = 0.005), and surgical intervention (65.3 vs. 112 days, p = 0.044).
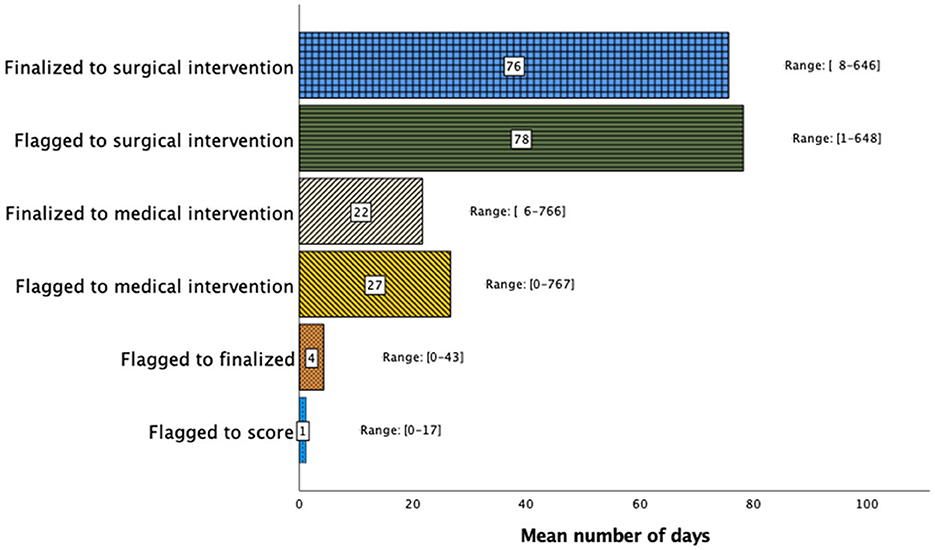
Figure 1. Sleep study clinical timeline demonstrating the timing interval (days) between study flagging, scoring, report finalization, medical intervention, and surgical intervention. Although the time between flagging and report finalization was short, the time to medical or surgical treatment varied widely.
The median time between a flagged study and scoring, report finalization, medical intervention, and surgical intervention were 0 (2) days, 2 (3) days, 5 (11.25) days, and 44 (73) days, respectively. The median time and interquartile ranges for timelines are depicted in Table 2 and Figure 2. Subgroup analysis using the Mann–Whitney U-test of patients with greater disease severity characterized by AHI > 30 experienced shorter median intervals between study flagging and scoring (p = 0.033) and surgical intervention (p = 0.001). This analysis was not statistically significant for the time between study flagging and report finalization (p = 0.215) and medical intervention (p = 0.992).
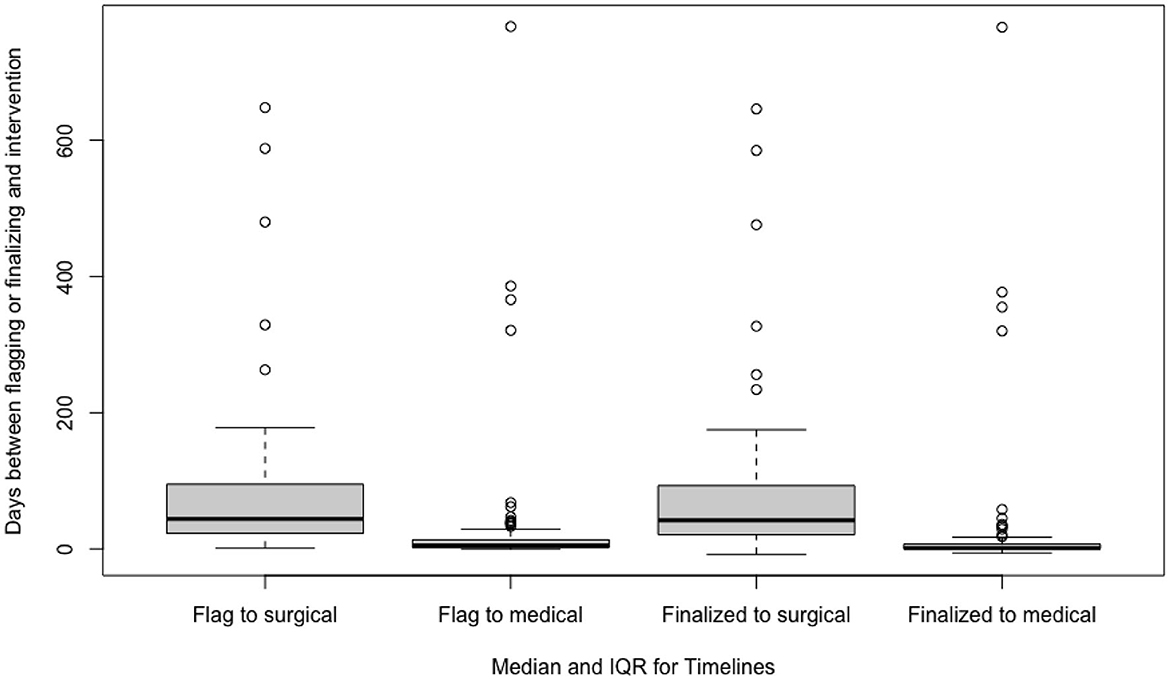
Figure 2. Median time and interquartile ranges between a flagged study to scoring, report finalization, medical intervention, and surgical intervention.
There were similar proportions of surgical (37.9%) and medical (40.6%) treatment interventions following flagged polysomnographies. Of those who underwent surgery, with adenotonsillectomy (82.1%) being the most common, 36.6% required an overnight admission due to a new CPAP or oxygen requirement.
The most frequent reason for flagging polysomnographies was repetitive brief oxygen desaturations. The frequency of surgical interventions and medical treatment interventions following a flagged study were similar, although, on average, medical treatment occurred more quickly than surgical intervention. Adenotonsillectomy was the most frequently employed surgical treatment method compared with adenoidectomy and tonsillectomy alone.
Patients with higher disease severity experienced quicker scoring, report finalization, and surgical intervention, which may prevent or attenuate consequent sequelae. It is important to note that there may have been skew in these averages as the analyses of the median did not provide statistical significance for report finalization and medical intervention. This study is novel in its contribution of a 3-year management timeline after flagging a polysomnography, thereby creating a foundation for assessing future interventions to expedite treating pediatric OSA.
There are several limitations to this study. Namely, this study examines only a single center's approach, and there was no control group depicting the timelines of the studies that were not flagged. The authors did not further explore the ramifications between earlier interventions vs. later interventions regarding flagged polysomnographies. These weaknesses should inform future research on the management of polysomnographies. Future directions should include the establishment of protocols to facilitate streamlined care between the sleep laboratory and both the surgical and medical teams. Investigations into the efficacy of these established algorithms also warrant future examination. Pandemic staffing shortages, with operating theaters being shut for days, and recent positive airway pressure machine recalls may have impacted the timeline in both interventions. Therefore, more studies that include a comparison group for timeline differences in the post-pandemic period may provide additional insights.
In conclusion, the most frequent reason for flagging polysomnographies was repetitive brief oxygen desaturations. The mean AHI of the cohort was in the severe OSA range. Adenotonsillectomy was the most frequently performed surgery. As hypothesized in our review of flagged polysomnography studies, the time to surgical intervention was longer than medical intervention. Patients with higher disease severity experienced quicker scoring, report finalization, and surgical interventions.
The raw data supporting the conclusions of this article will be made available by the authors upon request.
The studies involving humans were approved by Nemours Institutional Review Board, Nemours Children's Health. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this study was a retrospective chart review and all data has been de-identified.
SR: Conceptualization, Data curation, Investigation, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. JS: Data curation, Formal analysis, Methodology, Software, Writing—original draft, Writing—review & editing. KC: Software, Writing—original draft, Writing—review & editing. AK: Data curation, Formal analysis, Methodology, Resources, Software, Writing—review & editing. VG: Resources, Software, Writing—review & editing. AS: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing—review & editing. AC: Conceptualization, Resources, Supervision, Validation, Visualization, Writing—review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to thank the Nemours Sleep Medicine Team: Elaine Stevenson CPNP, APRN; Genevieve Walsh, RN, BSN, CPN; and Jennifer A. Marriner, MSN, CPNP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Baker-Smith, C. M., Isaiah, A., Melendres, M. C., Mahgerefteh, J., Lasso-Pirot, A., Mayo, S., et al. (2021). Sleep-disordered breathing and cardiovascular disease in children and adolescents: a scientific statement from the American Heart Association. J. Am. Heart Assoc. 10, e022427. doi: 10.1161/JAHA.121.022427
Bhatt, S. P., Guleria, R., and Kabra, S. K. (2021). Metabolic alterations and systemic inflammation in overweight/obese children with obstructive sleep apnea. PLoS ONE 16, e0252353. doi: 10.1371/journal.pone.0252353
Blechner, M., and Williamson, A. A. (2016). Consequences of obstructive sleep apnea in children. Curr. Probl. Pediatr. Adolesc. Health Care 46, 19–26. doi: 10.1016/j.cppeds.2015.10.007
Collop, N. A. (2021). Sleep lab emergencies: better to be prepared than be scared. J. Clin. Sleep Med. 17, 1335–1336. doi: 10.5664/jcsm.9336
Ingram, D. G., Singh, A. V., Ehsan, Z., and Birnbaum, B. F. (2017). Obstructive sleep apnea and pulmonary hypertension in children. Paediatr. Respir. Rev. 23, 33–39. doi: 10.1016/j.prrv.2017.01.001
Kheirandish-Gozal, L., and Gozal, D. (2017). Pediatric OSA syndrome morbidity biomarkers: the hunt is finally on! Chest 151, 500–506. doi: 10.1016/j.chest.2016.09.026
Marcus, C. L., Brooks, L. J., Ward, S. D., Draper, K. A., Gozal, D., Halbower, A. C., et al. (2012). Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 130, e714–e755. doi: 10.1542/peds.2012-1672
Marcus, C. L., Moore, R. H., Rosen, C. L., Giordani, B., Garetz, S. L., Taylor, H. G., et al. (2013). A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 368, 2366–2376. doi: 10.1056/NEJMoa1215881
Park, D. Y., Choi, J. H., Kang, S. Y., Han, J., Park, H. Y., Hwang, J. S., et al. (2018). Correlations between pediatric obstructive sleep apnea and longitudinal growth. Int. J. Pediatr. Otorhinolaryngol. 106, 41–45. doi: 10.1016/j.ijporl.2018.01.001
Schechter, M. S. (2002). Section on Pediatric Pulmonology, and Subcommittee on Obstructive Sleep Apnea Syndrome: Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 109, e69. doi: 10.1542/peds.109.4.e69
Thomas, R. J., and Chang-Ho, Y. (2011). “Emergencies during and due to polysomnography,” in Acute and Emergent Events in Sleep Disorders, eds. S. Chokroverty, and P. Sahota (Oxford, NY: Oxford Academic), 484–98. doi: 10.1093/med/9780195377835.003.0028
Thomas, S., Patel, S., Gummalla, P., Tablizo, M. A., and Kier, C. (2022). You cannot hit snooze on OSA: sequelae of pediatric obstructive sleep apnea. Children (Basel) 9, 261. doi: 10.3390/children9020261
Keywords: polysomnography, abnormal sleep studies, expedited review, pediatrics, obstructive sleep apnea
Citation: Rani S, Schanz J, Chauhan K, Kolb A, Gatta V, Strang A and Chidekel AC (2024) Pediatric polysomnography-flagging etiologies and impact on the clinical timeline. Front. Sleep 2:1302509. doi: 10.3389/frsle.2023.1302509
Received: 26 September 2023; Accepted: 31 December 2023;
Published: 18 January 2024.
Edited by:
David Ingram, Children's Mercy Kansas City, United StatesReviewed by:
Daniel Combs, University of Arizona, United StatesCopyright © 2024 Rani, Schanz, Chauhan, Kolb, Gatta, Strang and Chidekel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seema Rani, c2VlbWEucmFuaUBuZW1vdXJzLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.