- 1Department of Psychiatry, University of Oxford, Oxford, United Kingdom
- 2Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom
- 3Fulbourn Hospital, Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge, United Kingdom
- 4Sleep Unit, Neurological Department, Centre Hospitalier Universitaire Montpellier, Montpellier, France
- 5L'Institut de Neurosciences de Montpellier, Inserm, Université de Montpellier, Montpellier, France
- 6Edinburgh Dementia Prevention, Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 7Scottish Brain Sciences, Edinburgh, United Kingdom
- 8Global Brain Health Institute, Trinity College Dublin, Dublin, Ireland
- 9Trinity College Institute of Neuroscience, Trinity College Dublin, Dublin, Ireland
- 10Imperial College London and the University of Surrey, UK Dementia Research Institute Care Research and Technology Centre, London, United Kingdom
- 11Brain Sciences, Imperial College London, London, United Kingdom
- 12Imperial College Healthcare NHS Trust, Clinical Neurosciences, Charing Cross Hospital, London, United Kingdom
Introduction: As the global population ages, the economic, societal, and personal burdens associated with worsening cognition and dementia onset are growing. It is therefore becoming ever more critical to understand the factors associated with cognitive decline. One such factor is sleep. Adequate sleep has been shown to maintain cognitive function and protect against the onset of chronic disease, whereas sleep deprivation has been linked to cognitive impairment and the onset of depression and dementia.
Objectives: Here, we aim to identify and explore mechanistic links between several sleep parameters, depressive symptoms and cognition in a cohort of middle-aged adults.
Methods: We investigated data from the PREVENT dementia programme via structural equation modeling to illustrate links between predictor variables, moderator variables, and two cognitive constructs (i.e., Executive Function and Memory).
Results: Our model demonstrated that sleep quality, and total hours of sleep were related to participants' depressive symptoms, and that, participant apathy was related to higher scores on the Epworth Sleepiness and Lausanne NoSAS Scales. Subsequently, depressive symptoms, but not sleep or apathy ratings, were associated with Executive Function.
Conclusions: We provide evidence for an indirect relationship between sleep and cognition mediated by depressive symptoms in a middle-aged population. Our results provide a base from which cognition, dementia onset, and potential points of intervention, may be better understood.
1. Introduction
As the global population grows older (United Nations, 2020) it has become increasingly important to understand the factors which contribute to healthy aging. The normal aging process impacts all physical and behavioral functions, including cognition (Harada et al., 2013). Broadly, cognition encompasses the crucial day-to-day abilities necessary to correctly respond to one's environment and includes components such as stimulus processing, memory (Harada et al., 2013), and higher-order executive functions (see Diamond, 2013). The trajectory of cognitive performance over time follows an inverted U. That is, cognition develops in childhood and adolescence, peaks during adulthood, and most domains begin to steadily decline after middle-age (Krivanek et al., 2021). Protecting cognition has garnered increased attention due to the massive economic and social burdens associated with its unhealthy decline and the onset of dementia (Alzheimer's Association, 2021). A myriad of factors may influence the rate at which cognition declines and the likelihood of developing dementia, including sleep patterns (Scullin and Bliwise, 2015).
Adequate sleep is vital for the maintenance of cognitive performance (Dzierzewski et al., 2018; Matricciani et al., 2019) and appears to be critical for the clearance of beta-amyloid (Aβ) protein (see Wang and Holtzman, 2020). Regarding the latter, self-reported poor sleep quality has been shown to be related to higher Aβ concentrations in middle-aged and older adults (Spira et al., 2013; Sprecher et al., 2015; but see Gabelle et al., 20191) and chronic sleep deprivation has resulted in greater accumulation of this neurotoxic protein (Tabuchi et al., 2015). More recently, Shokri-Kojori et al. (2018) demonstrated that acute sleep deprivation (i.e., one night) yielded increased Aβ concentrations in the hippocampus and thalamus of individuals aged between 22 and 72 years. These results may be related to decreased clearance of Aβ contingent upon adequate glymphatic function (e.g., Xie et al., 2013) and/or γ-oscillations during rapid eye-movement sleep (Aron and Yankner, 2016). Alternatively, results may reflect increased synthesis of Aβ in response to the lack of sleep (Castellano et al., 2011). These are important results as they demonstrate a potential link between acute and chronic sleep disruptions and the development of Alzheimer's disease pathologies (Ju et al., 2013, 2014; Wang and Holtzman, 2020). Moreover, studies of sleep deprivation demonstrate a general worsening of cognition (for review see Killgore, 2010). For example, Lo et al. (2016) found that acute partial (i.e., 5 h sleep for seven nights) and total (i.e., one night) sleep deprivation contributes to the formation of false memories, and Gevers et al. (2015) demonstrate a general slowing of Stroop task reaction times (RT). Sleep deprivation is thought to impair the ability of the brain to consolidate memories (Yoo et al., 2007) and reduce the availability of the resources necessary for adequate stimulus processing and top-down executive control (e.g., Botvinick et al., 2001). Similarly, too much sleep (e.g., 10 or more hours) has been found to be a risk factor for the development of global cognitive decline (Ma et al., 2020) and dementia onset (Cavaillès et al., 2022b). The mechanisms underlying the relationship between cognitive decline and sleep durations are not completely clear, but biological factors including elevated inflammation (Patel et al., 2009) and thinning in executive brain regions (Spira et al., 2016) have been proposed. Optimal health is likely supported by ~7 h of sleep per night (Watson et al., 2015); however, overall sleep quality (i.e., related to factors such as sleep disturbances, trouble falling asleep, long waketime after sleep onset) is also related to global cognition. In a recent systematic review, Casagrande et al. (2022) identified that a greater frequency of sleep disturbance is associated with impaired cognition. Moreover, individuals who regularly sleep poorly are at a higher risk for developing symptoms of depression (Riemann et al., 2020) and dementia (Sabia et al., 2021). The mechanism(s) by which sleep influences the onset of depression are unclear. However, evidence indicates that poorer sleep quality may disrupt neural plasticity and synaptic health within the brain's emotion processing regions (Disner et al., 2011; Riemann et al., 2020).
It is important to note that the relationship between sleep disruption and depressive symptoms is bi-directional. Symptoms of depression include poorer sleep quality, as well as increased fatigue, diminished concentration, decreased ability to make decisions, low mood, and apathy (Blazer, 2003). Importantly, and perhaps in conjunction with poorer sleep quality (Jaussent et al., 2011), depressive symptoms impair cognition (Varghese et al., 2022) and foster a greater likelihood for dementia development (Kessing, 2012; Cavaillès et al., 2022a). For example, Lindert et al. (2021) demonstrated that longitudinally, higher scores on the Center for Epidemiologic Studies Depression (CES-D) scale were positively related to worse scores on measures of episodic memory and executive function. This relationship has been demonstrated across the lifespan (see also Dotson et al., 2020) and has been attributed to elevated cortisol concentrations (Sapolsky, 2000) and increased neurotoxicity due to increased inflammation (Furtado and Katzman, 2015). Taken together, the current research landscape provides evidence for the links between sleep, depression and cognition. Importantly, however, the mechanistic nature of these associations remains poorly understood, especially in a mid-age population. Here, the PREVENT cohort—composed of individuals between the ages of 40 and 59—was used to examine the relationship between sleep, depression symptomology and cognition prior to any dementia diagnosis. We hypothesized that measures of sleep quality and quantity would be related to cognitive performance and it may be that this association is explained via an indirect association with depression or its symptoms.
2. Materials and methods
2.1. Participants
Seven hundred individuals (age range 40–59) from the PREVENT dementia programme (Ritchie et al., 2013) were included in this investigation. All participants self-reported being cognitively healthy at the time of collection. We note that a minority of participants reported a current diagnosis of depression (n = 23), sleep disorder (n = 93), anxiety disorder (n = 60), mood disorder (n = 24), psychotic disorder (n = 2), alcoholism (n = 3) and drug misuse (n = 2). We note that the data used in these analyses is secondary data where ethical approval has been obtained by the source cohort (i.e., PREVENT). All participants provided informed written consent.
2.2. Cognitive assessments
Participants' cognitive function was assessed via the COGNITO battery and tests included measures of executive function (i.e., Stroop color, word, and interference tasks), and memory (COGNITO Tasks 8—Articulation and Immediate Recall—and 17—Delayed Recall of Names). These tasks were selected due to their sensitivity to cognitive decline/disruption over the lifespan (Levy et al., 2002; Guarino et al., 2019; Taconnat et al., 2022).
2.3. Sleep assessments
Participants' sleep health was assessed via the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), the Epworth Sleepiness Scale (Johns, 1991) and the Lausanne NoSAS (Marti-Soler et al., 2016). The latter two scales were not included in baseline assessments and therefore constitute less of the dataset than the PSQI (see Table 1). Higher scores on the latter scales indicate increased daytime sleepiness and an increased risk of sleep-disordered breathing. Due to the low predictive validity of a total PSQI score (Landry et al., 2015; Parsey et al., 2015), we chose to use several of its components: hours of sleep per night, waking in the night or early morning, and self-reported overall sleep quality. Note that higher ratings of the latter three PSQI components (scored 0–3) correspond to worse sleep.
Finally, participants self-estimate of sleep duration (SleepGroup) was also assessed and reported in three groups: short- (i.e., <6 h), medium- (i.e., 6–7.99 h), and long-sleepers (i.e., >8 h).
2.4. Depression symptomology
The degree to which participants suffered from depressive symptoms was determined via the CES-D scale (Radloff, 1977). The CES-D is a 20-item depression symptom assessment with each item being scored on a scale 0–3 (i.e., total score from 0–60), and includes questions regarding participants' feelings of loneliness and the degree to which they enjoy life. Higher scores indicate more symptoms.
2.5. Apathy scores
Participants' apathy was assessed via a three-item apathy scale wherein participants reported whether they experienced “emotional blunting,” a “lack of initiative,” and/or a “lack of interest.” The frequency with which these symptoms occurred are summed to create a total score. Higher total scores are indicative of higher ratings of apathy. We chose to include a measure of apathy here because lack of interest/apathy has been defined as a core symptom of clinical depression (Blazer, 2003). Hence, apathy may indirectly mediate the association between depression symptoms and cognitive function (see Fishman et al., 2019).
2.6. Statistical analyses
2.6.1. Pre-processing
All data processing and subsequent analyses were performed in Stata SE 16.1. Prior to modeling, we assessed and processed responses to cued, free, immediate and list recall tasks; Stroop color, word and interference RTs; sleep, and depression and apathy scores. Where appropriate, skewed (i.e., g1 > 1.0) data were log-transformed for normalization. We note that apathy scores remained skewed following log-transformation and were subsequently z-transformed to minimize their lack of normality. All cognitive variables of interest and sleep scores were z-transformed to normalize scaling (see Figure 1).
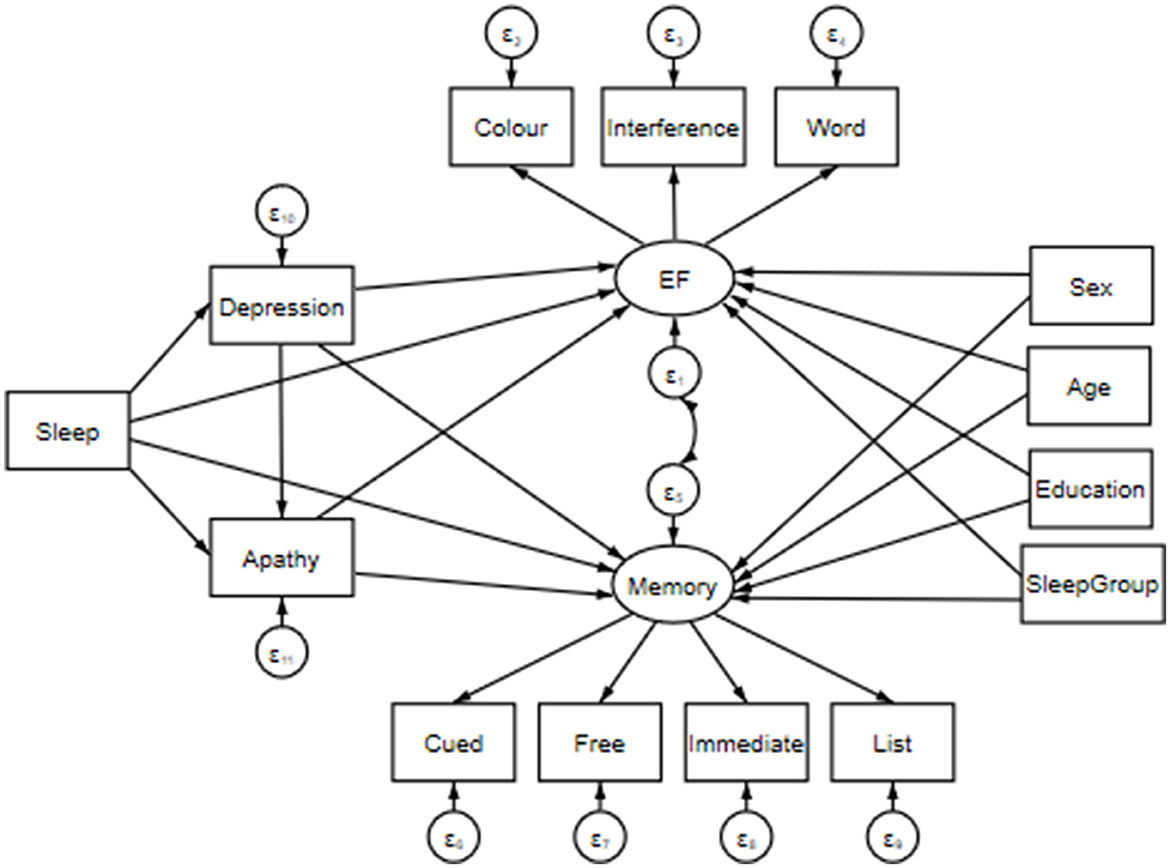
Figure 1. Structural equation model including predictor variables: Sleep, Depression (i.e., CES-D total score), and Apathy (i.e., apathy scale total score); mediator variables: Sex (i.e., male v. female), Age (in years), Education (i.e., total years of education), and SleepGroup (i.e., <6 h = Short; 6–7.99 h = Medium; >8 h = Long). Note that for data visualization purposes, sleep has been inserted into the model as a single measurement; however, it is composed of separate measures of hours of sleep per night, waking in the night or early morning, self-reported overall sleep quality, the Epworth Sleepiness Scale, and the Lausanne NoSAS. Paths extend from these variables to two latent constructs: Executive Function (EF; i.e., Stroop Color, Word and Interference task reaction times) and Memory (i.e., correct responses in Cued, Free, Immediate and List recall tasks). The latent constructs are joined via a covariance link.
2.6.2. Pairwise correlations
Pairwise correlations were employed to explore any associations between participant age, sex, years of education, sleep (i.e., hours of sleep per night, waking in the night or early morning, self-reported overall sleep quality, Epworth Sleepiness Scale, Lausanne NoSAS), depression and anxiety scores, Stroop color, word and interference task RTs, and correct responses to cued, free, immediate and list recall tasks. Correlations were Bonferroni corrected and associations were considered significant if p < 0.05.
2.6.3. Structural equation model
We employed a structural equation model (SEM) to assess direct and indirect effects between sleep, depression, apathy and cognitive function. That is, we aimed to create a single model to assess a mechanistic pathway by which our predictor variables may influence cognition. Prior to creating our model, simple regressions of the variables of interest were performed to better inform direct and indirect model paths. The presented model was estimated using a maximum likelihood with missing values (MLMV) test. We report standardized coefficients and beta values. The MLMV method assumes joint normality and, if present, randomly occurring missing values. The resulting model contains the following variables.
Cognition was assessed via two latent constructs. First, Executive Function (EF) was composed of Stroop color, word and interference task RTs (Periáñez et al., 2021). We then collated performance on the COGNITO tasks 8 and 17 into a Memory construct consisting of cued, free, immediate and list recall responses. A covariance link was applied between these constructs. Predictor variables included various indicators of sleep quality: TotalSleep (i.e., hours of sleep per night), WNEM (i.e., waking in the night or early morning), Quality (i.e., self-reported overall sleep quality), Epworth (i.e., the Epworth Sleepiness Scale), and NoSAS (i.e., Lausanne NoSAS). Depression and Apathy were included in our SEM as the total CES-D score, and total apathy score, respectively. Finally, Age and Education (i.e., years; continuous variables), SleepGroup (i.e., <6 h = 0, Short; 6–7.99 h = 1, Medium; >8 h = 2, Long), as well as Sex (i.e., females = 1, males = 2) were entered into our SEM as covariates to control for any confounds. Effects were deemed significant when p < 0.05.
3. Results
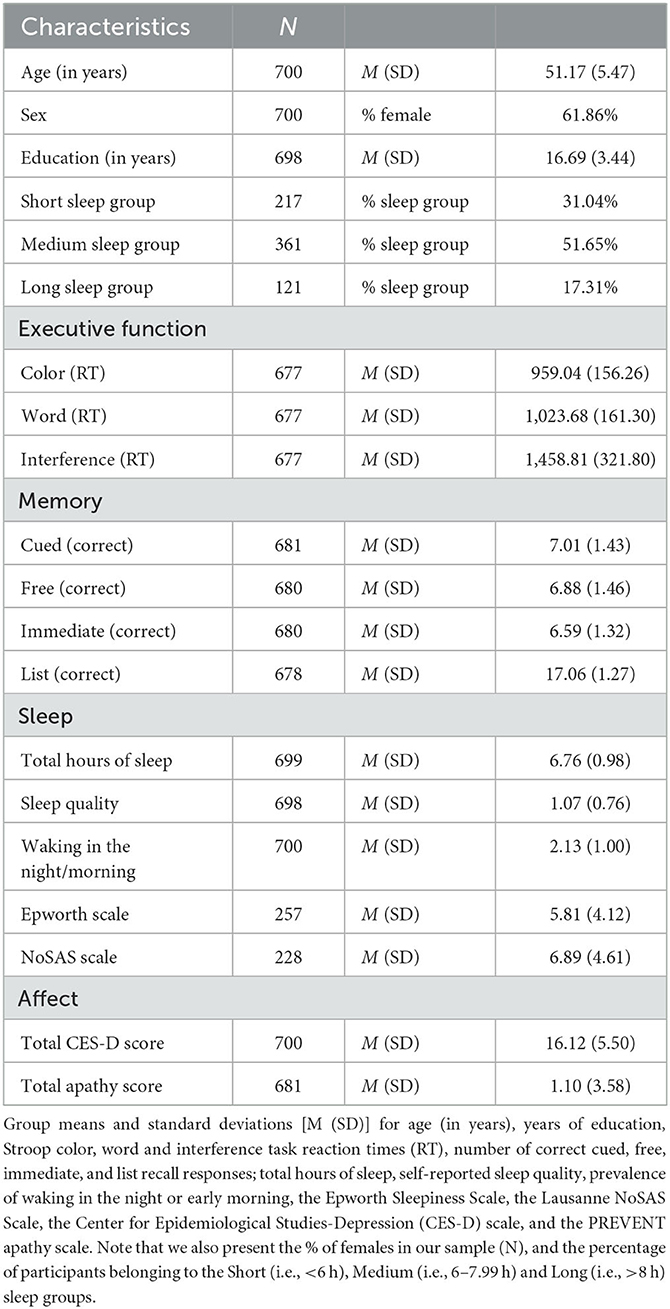
On average, the included sample was 51.17 years old (SD = 5.47), comprised of mostly females (i.e., 62%), had completed 16.69 (SD = 3.44) years of education, and most slept between 6 and 8 h a night (i.e., 52%; see Table 1).
3.1. Pairwise correlations
Initial pairwise correlations show no associations between predictor variables (i.e., indices of sleep, Apathy, Depression) and our chosen cognitive variables (rs <−0.002, ps > 0.99). We note, however, that Depression was related to TotalSleep, Quality, and WNEM (rs = −0.25, 0.36, 0.22, ps <0.001), whereas Apathy was only related to Quality (r = 0.14, p = 0.047); Depression and Apathy were also related to each other (r = 0.23, p < 0.001). In addition, the PSQI measures used here were correlated (rs > −0.27, ps <0.001) as well Stroop color, word and interference task RTs (rs > 0.51, ps <0.001), and the number of correct cued, free, immediate, and list recall responses (rs > 0.44, p < 0.001). Scores on the Epworth and NoSAS scales were not related to each other (r = 0.14, p > 0.99).
3.2. Structural equation model
3.2.1. Regression paths
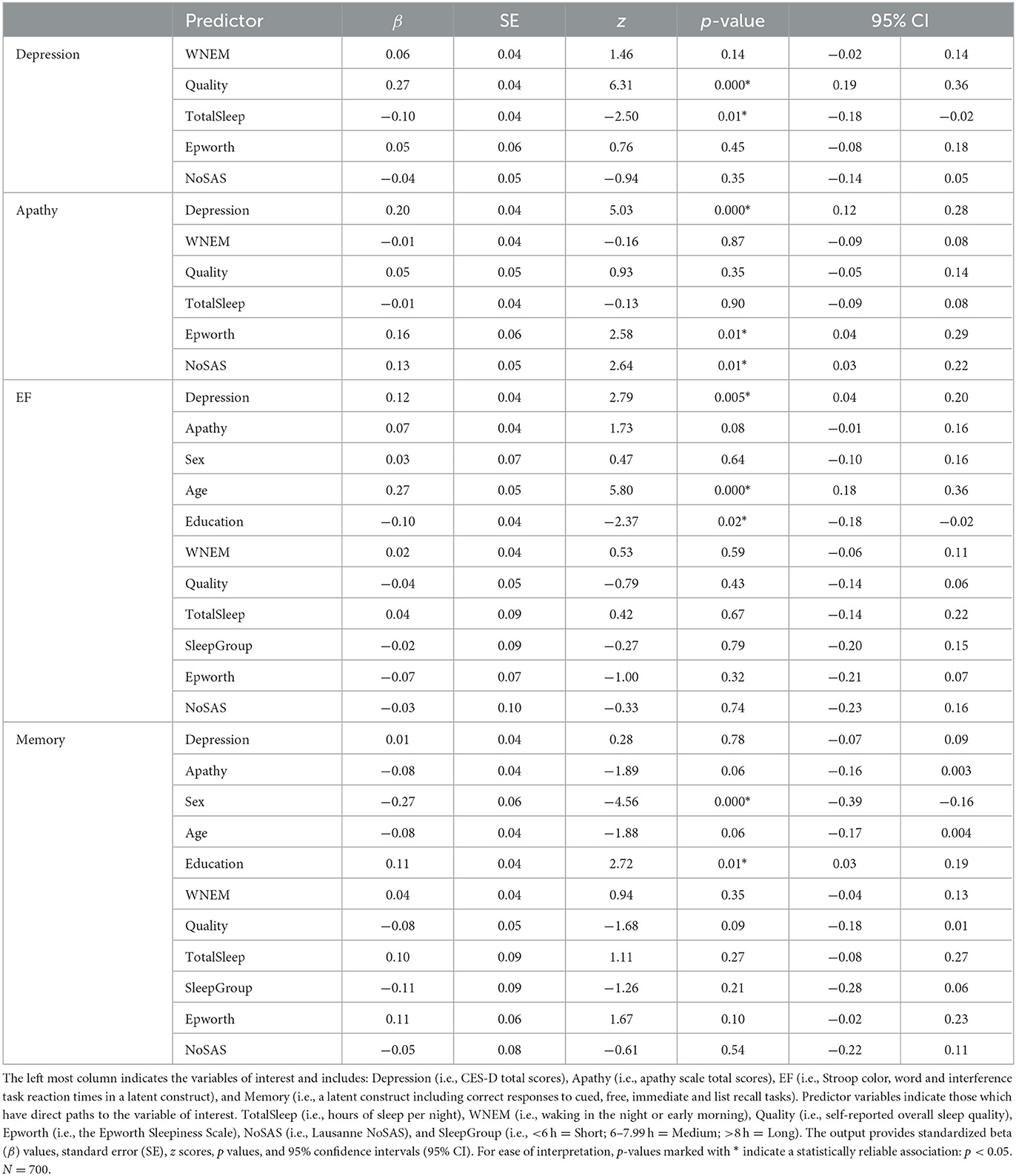
As demonstrated in Figure 1, a direct path was extended from each predictor (i.e., Sleep including separate measures of TotalSleep, WNEM, Quality, Epworth, NoSAS, Depression, Apathy) and mediator (i.e., Sex, Age, Education, SleepGroup) variable to both cognitive latent constructs (i.e., EF and Memory). Links were also included between all sleep indices and Depression and Apathy, as well as between Depression and Apathy to assess the mediation of any relationship between our predictors and cognition.
3.2.2. Estimation and fit
Our model fit was deemed good according to accepted standards (e.g., Kline, 2016). Our model possesses a root mean squared error of approximation (RMSEA: differences between predicted and observed outcomes) = 0.025; the Tucker Lewis index (i.e., relative reduction in misfit per degree of freedom) = 0.982; the comparative fit index (CFI: metric of the model's improvement from baseline to proposed iterations) = 0.988.
Table 2 contains the SEM output for our model and demonstrates that worse sleep quality and fewer hours of sleep were associated with more depression symptoms (βs = 0.27, −0.10, ps <0.01). Moreover, higher scores on the Epworth and NoSAS scales were associated with more apathy symptoms (βs = 0.16, 0.13, ps <0.01) and depression and apathy symptoms were positively related (β = 0.20, p < 0.001). Neither Depression nor Apathy, nor any of the indices of sleep quality were related to memory performance (βs <−0.08, ps > 0.06); however, higher depression symptoms were found to be linked to worse executive function (i.e., longer RTs; β = 0.12, p = 0.005; see also Figure 2). Results demonstrated mediation of the effect of sleep on cognitive performance by depression symptoms. Indeed, β values regarding the relationship between sleep indices and executive function (β <−0.07) were attenuated by the effect of depression symptoms (β = 0.12). In terms of our covariates, we note that neither EF nor Memory were related to the sleep group to which participants belonged (i.e., Short: <6 h, Medium: 6–7.99 h, Long: >8 h; βs = −0.02, −0.11, ps > 0.21). Older age and less education were related to poorer executive function (βs = 0.27, −0.10, ps <0.02), whereas being more highly educated and female were related to better memory performance (βs = 0.11, −0.27, ps <0.01). The relationship between memory and participant age approached, but did not achieve conventional levels of statistical significance (β = −0.08, p = 0.06).
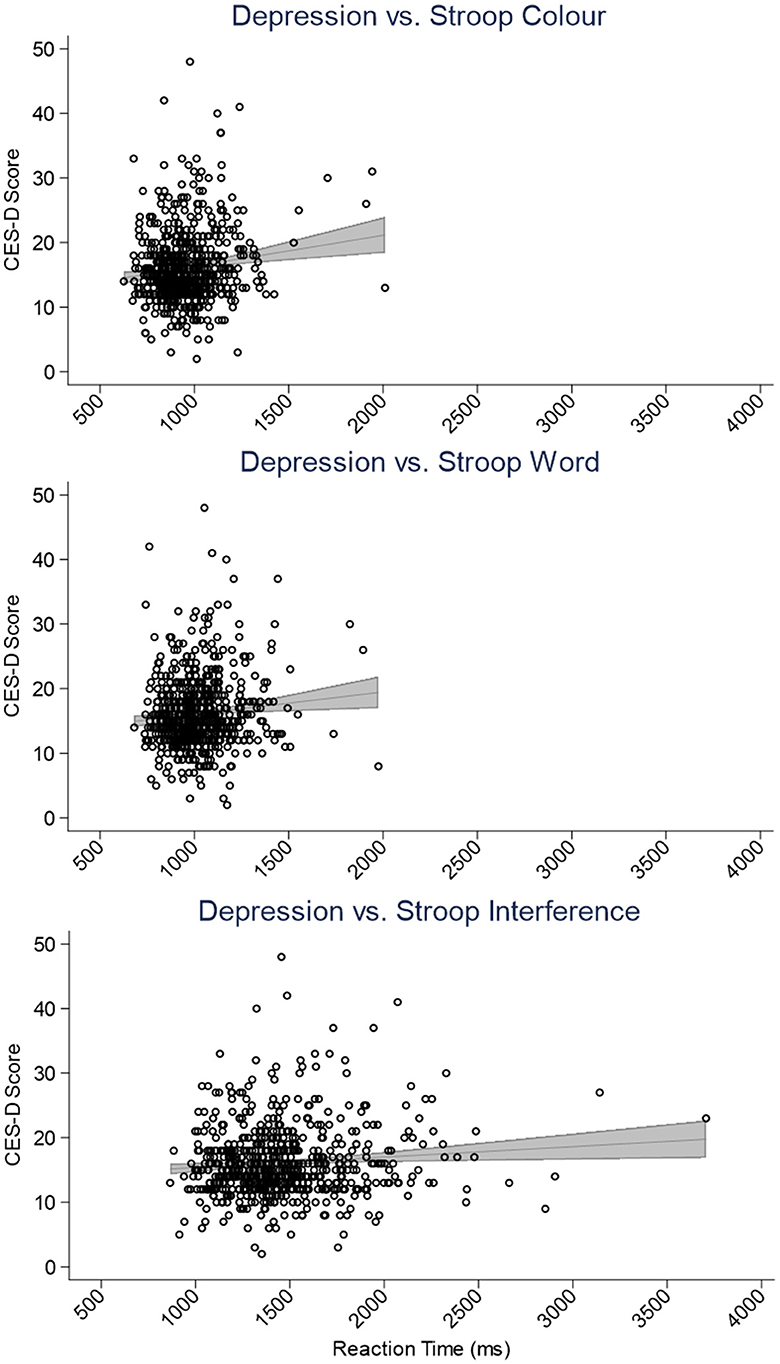
Figure 2. Scatter plots depicting the relationship between the Center for Epidemiological Studies-Depression (CES-D) scale scores and Stroop color (top), word (center), and interference (bottom) task reaction times (ms). The panels include simple linear regressions accompanied by gray 95% confidence interval bands.
4. Discussion
Our investigation sought to explore the association between sleep, depressive symptoms and cognition in healthy middle-aged adults. Below, we discuss the links between four confounding variables and the assessed cognitive constructs prior to explaining a model which demonstrates a direct link between depression symptoms and executive function.
4.1. Age, sex and years of education: mediators of memory and executive function
Our model showed no association between the sleep group to which individuals belonged and cognition. This is contrary to recent results presented by Ma et al. (2020) demonstrating an inverted-U relationship between sleep duration and the likelihood of global cognitive decline. We note that the authors had a considerably larger sample size (N = 20,065) and this is a likely explanation as to why this result is absent from our model. Moreover, we demonstrated that sex and education were related to memory and executive function. That is, being male (e.g., Voyer et al., 2021) and less educated (e.g., Murayama et al., 2013) was associated with fewer correct responses to memory tasks, whereas more education was related to improved executive function. Our memory composite comprised verbal memory subtasks tapping episodic memory, a domain typically demonstrating a performance advantage for women relative to men (Asperholm et al., 2019), although inherent hormonal differences (i.e., estrogen concentrations) may modulate memory performance for females over time (Duarte-Guterman et al., 2015). Similarly, Staekenborg et al. (2020) and Han et al. (2023) indicate that increased cognitive reserve gained from more years of education may support memory and executive function in later life, while Joannette et al. (2020) show that educational attainment moderates the relationship between episodic memory and amyloid load. In contrast, being older was associated with worse performance on executive function. This is unsurprising as work has consistently demonstrated an association between increasing age and slowed RTs on executive functiontasks (Krivanek et al., 2021). What is more, detriments to global cognition related to age have been attributed to cortical thinning, demyelination, and brain volume loss (Blinkouskaya et al., 2021), as well as inefficient preparation of responses to stimuli (Williams et al., 2007; Hardwick et al., 2022). Accordingly, three of the confounding variables used here were related to cognition in a way that is aligned with the current corpus of literature.
4.2. Sleep may indirectly predict cognition via depression symptoms
Better sleep has been linked to improved mental health (Sadler et al., 2018), and has been shown to support cognition (Dzierzewski et al., 2018; Matricciani et al., 2019) and aide in the clearance of harmful Aβ protein (Xie et al., 2013; Tabuchi et al., 2015; Shokri-Kojori et al., 2018). In our model, however, none of the sleep measures investigated here were related to either cognitive construct. This may be an unexpected result given that cognitive dysfunction and insomnia share common neural mechanisms such as impaired functional connectivity and structural abnormalities within the amygdala, prefrontal cortex, anterior cingulate cortex and insula (e.g., Bagherzadeh-Azbari et al., 2019). Our null findings may be explained by the fact that the PREVENT cohort is composed of middle-, rather than old-aged, adults. This is notable because it is during this time in the lifespan where cognition is comparatively less vulnerable to insult (Diamond, 2013; Krivanek et al., 2021). However, even studies involving middle-aged individuals have demonstrated an association between sleep and impaired cognition (Ma et al., 2020), as well as Aβ concentrations (Sprecher et al., 2015). An alternative explanation may be that sleep is associated with cognition via some mediator(s) or that the relationship between sleep and poor cognitive outcome demonstrated in the literature may not always reflect cognitive deterioration toward organic dementia. Instead, this may be related to the compromised emotion-regulated processes underlying pseudo-dementia (e.g., Leonhardi et al., 2022). Although, depressive pseudo-dementia has shown to predict organic dementia longitudinally, at least in the elderly (Sáez-Fonseca et al., 2007; Diniz et al., 2013).
Our model found that fewer hours of sleep and lower self-reported sleep quality were related to more symptoms of depression, and that higher depression symptoms and more daytime sleepiness and sleep apnoea likelihood (i.e., Epworth Sleepiness and NoSAS Scales) were related to participants' total apathy score. We will address these results in turn. First, literature has previously demonstrated a link between sleep and depression (Scott et al., 2021; Joo et al., 2022). For example, a recent meta-analysis by Scott et al. (2021) provides evidence for a small-to-medium association between improved sleep quality and reduced symptoms of anxiety, stress, psychosis, and depression. Their analyses were conducted on studies from various countries and offers insight to the generalisability of this association across populations. In contrast, when Joo et al. (2022) assessed the relationship between each component of the PSQI and symptoms of depression, they found a dose-response relationship for each component of the index, except sleep duration. The link between sleep and depression has been explained biologically via increased activity in the amygdala (Yoo et al., 2007) and reduced functional connectivity between the amygdala and the prefrontal cortex (Motomura et al., 2013). What is more, one night of sleep deprivation has been linked with elevated sympathetic nervous system activity, increased heart rate variability and a subsequently diminished capacity to respond to emotional challenges (Zhong et al., 2005; Appelhans and Luecken, 2006; Sauvet et al., 2010; for review see Goldstein and Walker, 2014). Hence, that various measures of sleep were related to depression symptoms in our model was to be expected. Second, apathy affects various neurological outcomes and is common in individuals who present with symptoms of depression (Steffens et al., 2022); however, it is nosologically and neurobiologically distinct from depression (Tagariello et al., 2009). It is for this reason that we chose to include apathy in our model. We demonstrated that depression symptoms were unsurprisingly related to apathy scores and that apathy ratings were related to daytime sleepiness. Indeed, evidence has demonstrated that individuals with higher ratings of depression are less willing or likely to respond to rewards (Le Heron et al., 2018) and that resulting apathy symptoms are associated with alterations to frontoparietal executive networks (e.g., pre-frontal cortex and the anterior cingulate cortex; for review see Steffens et al., 2022). Previous work has shown that excessive sleepiness and sleep apnea disrupt normal activity within the pre-frontal cortex (e.g., Durning et al., 2014) and induce intermittent states of hypoxia (e.g., Bucks et al., 2013). These disruptions to neural activity and metabolism may be the mechanism(s) underlying how individuals develop/manifest feelings of apathy. When taken together, our results support literature demonstrating the links between less sleep, more depression symptoms and higher apathy present here.
Individuals with higher depressive and/or apathy symptoms often perform poorly on tests of executive function (e.g., McPherson et al., 2002; Funes et al., 2018). Here, our model demonstrated that depression, but not apathy, was associated with executive function. To understand these results, we considered the construction of our executive function latent construct. Rather than treating the Stroop color, word, and interference tasks strictly as measures of stimulus processing and inhibition, respectively, we grouped them into one latent construct. This is because recent work from Periáñez et al. (2021) has demonstrated that performance on the Stroop color, word, and interference tasks reflect visual search speed; Stroop color and interference performance are indicative of working memory; and execution of the Stroop interference task is related to conflict monitoring. Accordingly, the ability to complete the various iterations of the Stroop task is dependent on a combination of several higher-order executive functions. Despite some evidence affirming the link between apathy and executive function, the literature is mixed. Tests of executive function and global cognition have yielded no reliable association with symptoms of apathy (Marin et al., 2003; Brodaty et al., 2010) and this can be explained by the different pathways by which apathy and executive function are mediated in the brain (see Gonçalves et al., 2020). On the other hand, depression has been shown to negatively alter performance on tasks which require top-down control. A recent investigation identified that symptoms of depression are related to higher cortical noise which negatively impacts executive performance (Yao et al., 2022). Although not directly assessed here, it is therefore possible that higher and increasingly inefficient frontoparietal brain activity associated with less sleep and higher depression symptoms (e.g., Steffens et al., 2022) underlies the detrimental relationship between depression and cognition.
Last, our model demonstrated neither depression symptoms nor apathy scores were related to Memory. The literature regarding these associations is mixed. For example, Fishman et al. (2019) demonstrated that stroke patients with elevated apathy ratings performed worse on free recall tasks, whereas depression symptoms did not elicit a similar result. Conversely, Szymkowicz et al. (2018) found that Parkinson's disease patients with higher ratings of depression, but not apathy, performed worse on memory tasks. Our results do not seem to support these findings. However, it is worth noting that the relationships described above were found in individuals with psychiatric and/or physical co-morbidity, and in individuals who have been diagnosed with clinical depression and/or apathy disorders. Indeed, our results were obtained by modeling mostly cognitively and psychiatrically healthy individuals, and it may be the lack of relevant co-morbidity which spares any association between depression and/or apathy symptoms and memory performance.
4.3. Limitations, future directions, and conclusions
We are aware that our study presents several limitations. First, our sample is middle-aged, cognitively healthy, well-educated and ethnically homogenous. Therefore, it is unlikely that the present model can be generalized outside of this demographic. Future investigations should aim to explore a more diverse cohort in terms of age, socio-economic and cognitive status. Similarly, the presented model may serve as a launch point for investigations regarding the quantification of dementia likelihood via follow-up of this cohort, or further exploration of the biological mechanisms of the associations we present here; for example, via investigations of neurodegeneration and brain volume. It is possible our data show the effects of depression on both sleep and executive function which may be related to functional rather than organic mental disorder. Longitudinal data will be important to investigate this. Second, the CES-D and apathy scales used here are multi-component assessments of their respective constructs. That is, whether a specific sub-component of each scale drives the above interactions is not yet known. Moreover, as we incorporated self-report measures of sleep, future studies might benefit from incorporating objective measures to assess sleep duration/quality (e.g., actigraphy or polysomnography); this is especially relevant given data questioning the PSQI's predictive validity for objective sleep duration and quality (Landry et al., 2015; Parsey et al., 2015). As well, Evangelista et al. (2021) have demonstrated the importance for objective measures of sleep. The authors found discrepancies related to objective (i.e., polysomnography, multiple sleep latency test) and subjective (i.e., Epworth Sleepiness Scale) reports of sleep. Third, we note that the amount of data available for the Epworth and NoSAS scales was much less than other sleep measures due to their late introduction into this protocol. It may be that the related results observed here were due to this discrepancy in Ns. Finally, although the observed link between executive function and depressive symptoms intimates a possible deleterious neurological/physiological cascade across frontoparietal regions, the data necessary to confirm or quantify this relationship are unavailable. Regardless of the aforementioned limitations, our study provides evidence for an association between sleep and cognitive function mediated by depression symptoms in a middle-aged population. These results are pertinent in so much as they will encourage further investigation of the indirect relationship between sleep and cognition and the relevance of this relationship for the development of dementia. Furthermore, this work may serve as a basis to further explore the potential nature and timing of any treatments to prevent or ameliorate the development of dementia in later life.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://community.addi.ad-datainitiative.org/datasets/f/prevent-dementia-research-programme and/or https://portal.dementiasplatform.uk/.
Ethics statement
The studies involving humans were approved by the UK London-Camberwell St Giles National Health Service (NHS) Research Ethics Committee (REC reference: 12/LO/1023, IRAS project ID: 88938), which operates according to the Helsinki Declaration of 1975 (and as revised in 1983). Separate ethical applications for the Dublin site were submitted and given favorable opinions by the Trinity College School of Psychology Research Ethics Committee (SPREC022021-010) and the St James Hospital/Tallaght University Hospital Joint Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
BT and IK wrote the first draft of the manuscript and analyzed the data. CR, KR, and IK are investigators for the PREVENT Dementia Program. CR, JO'B, BL, PM, and IK supervised the data collection. All authors edited and commented on subsequent versions of the manuscript and approved the final version of the manuscript.
Funding
The PREVENT study was funded by the Alzheimer's Society (grant numbers 178 and 264), the Alzheimer's Association (grant number TriBEKa-17-519007), and the Global Brain Health Institute and philanthropic donations. BT and VR would like to acknowledge funding for salary via the Medical Research Council (Dementias Platform UK). IK declares funding for this work through Medical Research Council (Dementias Platform UK) and NIHR Oxford Health Biomedical Research Centre and NIHR personal awards. AL acknowledges salary funding from NIHR. TW would like to acknowledge a NIHR and Alzheimer's Society Dementia Fellowship for salary support. BU's post is part-funded by a donation from Gnodde Goldman Sachs Giving. This funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication. GMT acknowledges the support of the Osteopathic Heritage Foundation through funding for the Osteopathic Heritage Foundation Ralph S. Licklider, D.O., Research Endowment in the Heritage College of Osteopathic Medicine. JO'B was supported by the NIHR Cambridge Biomedical Research Centre.
Acknowledgments
The authors would like to thank and acknowledge the PREVENT dementia programme study participants. The data collection was also supported by research facilities at West London NHS Trust, NHS Lothian, Oxford Health NHS Foundation trust and Cambridgeshire and Peterborough NHS Foundation trust and St James's hospital, Dublin. This work has been previously shared as a pre-print on medRxiv.org (Tari et al., 2023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Results from this paper identified no relationship between amyloid burden and sleep quality in 143 elderly (i.e., 70–85 years of age) individuals.
References
Alzheimer's Association (2021). 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 17, 327–406. doi: 10.1002/alz.12328
Appelhans, B. M., and Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 10, 229–240. doi: 10.1037/1089-2680.10.3.229
Aron, L., and Yankner, B. A. (2016). Neurodegenerative disorders: neural synchronization in Alzheimer's disease. Nature 540, 207–208. doi: 10.1038/540207a
Asperholm, M., Högman, N., Rafi, J., and Herlitz, A. (2019). What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychol. Bull. 145, 785–821. doi: 10.1037/bul0000197
Bagherzadeh-Azbari, S., Khazaie, H., Zarei, M., Spiegelhalder, K., Walter, M., Leerssen, J., et al. (2019). Neuroimaging insights into the link between depression and insomnia: a systematic review. J. Affect. Disord. 258, 133–143. doi: 10.1016/j.jad.2019.07.089
Blazer, D. G. (2003). Depression in late life: review and commentary. J. Gerontol. A Biol. Sci. Med. Sci. 58, 249–265. doi: 10.1093/gerona/58.3.M249
Blinkouskaya, Y., Caçoilo, A., Gollamudi, T., Jalalian, S., and Weickenmeier, J. (2021). Brain aging mechanisms with mechanical manifestations. Mech. Ageing Dev. 200, 111575. doi: 10.1016/j.mad.2021.111575
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652. doi: 10.1037/0033-295X.108.3.624
Brodaty, H., Altendorf, A., Withall, A., and Sachdev, P. (2010). Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. Int. Psychogeriatr. 22, 426–436. doi: 10.1017/S1041610209991335
Bucks, R. S., Olaithe, M., and Eastwood, P. (2013). Neurocognitive function in obstructive sleep apnoea: a meta-review. Respirology 18, 61–70. doi: 10.1111/j.1440-1843.2012.02255.x
Buysse, D. J., Reynolds, C. F. III, Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Casagrande, M., Forte, G., Favieri, F., and Corbo, I. (2022). Sleep quality and aging: a systematic review on healthy older people, mild cognitive impairment and Alzheimer's disease. Int. J. Environ. Res. Public Health 19, 8457. doi: 10.3390/ijerph19148457
Castellano, J. M., Kim, J., Stewart, F. R., Jiang, H., DeMattos, R. B., Patterson, B. W., et al. (2011). Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci. Transl. Med. 3, 89ra57. doi: 10.1126/scitranslmed.3002156
Cavaillès, C., Berr, C., Helmer, C., Gabelle, A., Jaussent, I., and Dauvilliers, Y. (2022a). Complaints of daytime sleepiness, insomnia, hypnotic use, and risk of dementia: a prospective cohort study in the elderly. Alzheimers. Res. Ther. 14, 12. doi: 10.1186/s13195-021-00952-y
Cavaillès, C., Carrière, I., Wagner, M., Dartigues, J. F., Berr, C., Dauvilliers, Y., et al. (2022b). Trajectories of sleep duration and timing before dementia: a 14-year follow-up study. Age Ageing 51, afac186. doi: 10.1016/j.sleep.2022.05.279
Diamond, A. (2013). Executive functions. Annu. Rev. Psychol. 64, 135–168. doi: 10.1146/annurev-psych-113011-143750
Diniz, B. S., Butters, M. A., Albert, S. M., Dew, M. A., and Reynolds, C. F. III (2013). Late-life depression and risk of vascular dementia and Alzheimer's disease: systematic review and meta-analysis of community-based cohort studies. Br. J. Psychiatry 202, 329–335. doi: 10.1192/bjp.bp.112.118307
Disner, S. G., Beevers, C. G., Haigh, E. A., and Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467–477. doi: 10.1038/nrn3027
Dotson, V. M., McClintock, S. M., Verhaeghen, P., Kim, J. U., Draheim, A. A., Syzmkowicz, S. M., et al. (2020). Depression and cognitive control across the lifespan: a systematic review and meta-analysis. Neuropsychol. Rev. 30, 461–476. doi: 10.1007/s11065-020-09436-6
Duarte-Guterman, P., Yagi, S., Chow, C., and Galea, L. A. (2015). Hippocampal learning, memory, and neurogenesis: effects of sex and estrogens across the lifespan in adults. Horm. Behav. 74, 37–52. doi: 10.1016/j.yhbeh.2015.05.024
Durning, S. J., Capaldi, V. F. 2nd, Artino, A. R. Jr, Graner, J., van der Vleuten, C., Beckman, T. J., et al. (2014). A pilot study exploring the relationship between internists' self-reported sleepiness, performance on multiple-choice exam items and prefrontal cortex activity. Med. Teach. 36, 434–440. doi: 10.3109/0142159X.2014.888408
Dzierzewski, J. M., Dautovich, N., and Ravyts, S. (2018). Sleep and cognition in older adults. Sleep Med. Clin. 13, 93–106. doi: 10.1016/j.jsmc.2017.09.009
Evangelista, E., Rassu, A. L., Barateau, L., Lopez, R., Chenini, S., Jaussent, I., et al. (2021). Characteristics associated with hypersomnia and excessive daytime sleepiness identified by extended polysomnography recording. Sleep 44, zsaa264. doi: 10.1093/sleep/zsaa264
Fishman, K. N., Ashbaugh, A. R., Lanctôt, K. L., Cayley, M. L., Herrmann, N., Murray, B., et al. (2019). The role of apathy and depression on verbal learning and memory performance after stroke. Arch. Clin. Neuropsychol. 34, 327–336. doi: 10.1093/arclin/acy044
Funes, C. M., Lavretsky, H., Ercoli, L., St Cyr, N., and Siddarth, P. (2018). Apathy mediates cognitive difficulties in geriatric depression. Am. J. Geriatr. Psychiatry 26, 100–106. doi: 10.1016/j.jagp.2017.06.012
Furtado, M., and Katzman, M. A. (2015). Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 229, 37–48. doi: 10.1016/j.psychres.2015.05.036
Gabelle, A., Gutierrez, L. A., Jaussent, I., Ben Bouallegue, F., De Verbizier, D., Navucet, S., et al. (2019). Absence of relationship between self-reported sleep measures and amyloid load in elderly subjects. Front. Neurol. 10, 989. doi: 10.3389/fneur.2019.00989
Gevers, W., Deliens, G., Hoffmann, S., Notebaert, W., and Peigneux, P. (2015). Sleep deprivation selectively disrupts top-down adaptation to cognitive conflict in the Stroop test. J. Sleep Res. 24, 666–672. doi: 10.1111/jsr.12320
Goldstein, A. N., and Walker, M. P. (2014). The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 10, 679–708. doi: 10.1146/annurev-clinpsy-032813-153716
Gonçalves, S. A. B., Caramelli, P., Mariano, L. I., Guimarães, H. C., Gambogi, L. B., Resende, E. P. F., et al. (2020). Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. 1737, 146799. doi: 10.1016/j.brainres.2020.146799
Guarino, A., Favieri, F., Boncompagni, I., Agostini, F., Cantone, M., and Casagrande, M. (2019). Executive functions in Alzheimer disease: a systematic review. Front. Aging Neurosci. 10, 437. doi: 10.3389/fnagi.2018.00437
Han, S., Lee, J. Y., Cho, S. I., Oh, D. J., and Yoon, D. H. (2023). Risk factors for various cognitive function decline trajectories in adults over 40 years of age: a retrospective cohort study. Psychiatry Investig. 20, 293–300. doi: 10.30773/pi.2022.0188
Harada, C. N., Natelson Love, M. C., and Triebel, K. L. (2013). Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752. doi: 10.1016/j.cger.2013.07.002
Hardwick, R. M., Forrence, A. D., Costello, M. G., Zackowski, K., and Haith, A. M. (2022). Age-related increases in reaction time result from slower preparation, not delayed initiation. J. Neurophysiol. 128, 582–592. doi: 10.1152/jn.00072.2022
Jaussent, I., Bouyer, J., Ancelin, M. L., Akbaraly, T., Pérès, K., Ritchie, K., et al. (2011). Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep 34, 1103–1110. doi: 10.5665/SLEEP.1170
Joannette, M., Bocti, C., Dupont, P. S., Lavallée, M. M., Nikelski, J., Vallet, G. T., et al. (2020). Education as a moderator of the relationship between episodic memory and amyloid load in normal aging. J. Gerontol. A Biol. Sci. Med. Sci. 75, 1820–1826. doi: 10.1093/gerona/glz235
Johns, M. W. (1991). A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545. doi: 10.1093/sleep/14.6.540
Joo, H. J., Kwon, K. A., Shin, J., Park, S., and Jang, S. I. (2022). Association between sleep quality and depressive symptoms. J. Affect. Disord. 310, 258–265. doi: 10.1016/j.jad.2022.05.004
Ju, Y. E., Lucey, B. P., and Holtzman, D. M. (2014). Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat. Rev. Neurol. 10, 115–119. doi: 10.1038/nrneurol.2013.269
Ju, Y. E., McLeland, J. S., Toedebusch, C. D., Xiong, C., Fagan, A. M., Duntley, S. P., et al. (2013). Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 70, 587–593. doi: 10.1001/jamaneurol.2013.2334
Kessing, L. V. (2012). Depression and the risk for dementia. Curr. Opin. Psychiatry 25, 457–461. doi: 10.1097/YCO.0b013e328356c368
Killgore, W. D. (2010). Effects of sleep deprivation on cognition. Prog. Brain Res. 185, 105–129. doi: 10.1016/B978-0-444-53702-7.00007-5
Kline, R. B. (2016). Principles and practice of structural equation modeling, 4th ed. New York, NY: Guilford Press.
Krivanek, T. J., Gale, S. A., McFeeley, B. M., Nicastri, C. M., and Daffner, K. R. (2021). Promoting successful cognitive aging: a ten-year update. J. Alzheimers. Dis. 81, 871–920. doi: 10.3233/JAD-201462
Landry, G. J., Best, J. R., and Liu-Ambrose, T. (2015). Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front. Aging Neurosci. 7, 166. doi: 10.3389/fnagi.2015.00166
Le Heron, C., Plant, O., Manohar, S., Ang, Y. S., Jackson, M., Lennox, G., et al. (2018). Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson's disease. Brain 141, 1455–1469. doi: 10.1093/brain/awy110
Leonhardi, J., Barthel, H., Speerforck, S., Dietzel, J., Schroeter, M. L., Saur, D., et al. (2022). Differential diagnosis between Alzheimer's disease-related depression and pseudo-dementia in depression: a new indication for amyloid-β imaging? J. Alzheimers. Dis. 88, 1029–1035. doi: 10.3233/JAD-215619
Levy, G., Jacobs, D. M., Tang, M. X., Côté, L. J., Louis, E. D., Alfaro, B., et al. (2002). Memory and executive function impairment predict dementia in Parkinson's disease. Mov. Disord. 17, 1221–1226. doi: 10.1002/mds.10280
Lindert, J., Paul, K. C., Lachman, M. E., Ritz, B., and Seeman, T. E. (2021). Depression-, anxiety-, and anger and cognitive functions: findings from a longitudinal prospective study. Front. Psychiatry 12, 665742. doi: 10.3389/fpsyt.2021.665742
Lo, J. C., Chong, P. L., Ganesan, S., Leong, R. L., and Chee, M. W. (2016). Sleep deprivation increases formation of false memory. J. Sleep Res. 25, 673–682. doi: 10.1111/jsr.12436
Ma, Y., Liang, L., Zheng, F., Shi, L., Zhong, B., and Xie, W. (2020). Association between sleep duration and cognitive decline. JAMA Netw. Open 3, e2013573. doi: 10.1001/jamanetworkopen.2020.13573
Marin, R. S., Butters, M. A., Mulsant, B. H., Pollock, B. G., and Reynolds III, C. F. (2003). Apathy and executive function in depressed elderly. J. Geriatr. Psychiatry Neurol. 16, 112–116. doi: 10.1177/0891988703016002009
Marti-Soler, H., Hirotsu, C., Marques-Vidal, P., Vollenweider, P., Waeber, G., Preisig, M., et al. (2016). The NoSAS score for screening of sleep-disordered breathing: a derivation and validation study. Lancet Respir. Med. 4, 742–748. doi: 10.1016/S2213-2600(16)30075-3
Matricciani, L., Paquet, C., Galland, B., Short, M., and Olds, T. (2019). Children's sleep and health: a meta-review. Sleep Med. Rev. 46, 136–150. doi: 10.1016/j.smrv.2019.04.011
McPherson, S., Fairbanks, L., Tiken, S., Cummings, J. L., and Back-Madruga, C. (2002). Apathy and executive function in Alzheimer's disease. J. Int. Neuropsychol. Soc. 8, 373–381. doi: 10.1017/S1355617702813182
Motomura, Y., Kitamura, S., Oba, K., Terasawa, Y., Enomoto, M., Katayose, Y., et al. (2013). Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS ONE 8, e56578. doi: 10.1371/journal.pone.0056578
Murayama, N., Iseki, E., Tagaya, H., Ota, K., Kasanuki, K., Fujishiro, H., et al. (2013). Intelligence or years of education: which is better correlated with memory function in normal elderly Japanese subjects?. Psychogeriatrics 13, 9–16. doi: 10.1111/j.1479-8301.2012.00408.x
Parsey, C. M., Schmitter-Edgecombe, M., and Belenky, G. (2015). Sleep and everyday functioning in older adulthood. J. Appl. Gerontol. 34, 48–72. doi: 10.1177/0733464812458364
Patel, S. R., Zhu, X., Storfer-Isser, A., Mehra, R., Jenny, N. S., Tracy, R., et al. (2009). Sleep duration and biomarkers of inflammation. Sleep 32, 200–204. doi: 10.1093/sleep/32.2.200
Periáñez, J. A., Lubrini, G., García-Gutiérrez, A., and Ríos-Lago, M. (2021). Construct validity of the Stroop color-word test: influence of speed of visual search, verbal fluency, working memory, cognitive flexibility, and conflict monitoring. Arch. Clin. Neuropsychol. 36, 99–111. doi: 10.1093/arclin/acaa034
Radloff, L. S. (1977). The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. doi: 10.1177/014662167700100306
Riemann, D., Krone, L. B., Wulff, K., and Nissen, C. (2020). Sleep, insomnia, and depression. Neuropsychopharmacology 45, 74–89. doi: 10.1038/s41386-019-0411-y
Ritchie, C. W., Wells, K., and Ritchie, K. (2013). The PREVENT research programme–a novel research programme to identify and manage midlife risk for dementia: the conceptual framework. Int. Rev. Psych. 25, 748–754. doi: 10.3109/09540261.2013.869195
Sabia, S., Fayosse, A., Dumurgier, J., van Hees, V. T., Paquet, C., Sommerlad, A., et al. (2021). Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 12, 2289. doi: 10.1038/s41467-021-22354-2
Sadler, P., McLaren, S., Klein, B., Harvey, J., and Jenkins, M. (2018). Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep 41, zsy104. doi: 10.1093/sleep/zsy104
Sáez-Fonseca, J. A., Lee, L., and Walker, Z. (2007). Long-term outcome of depressive pseudodementia in the elderly. J. Affect. Disord. 101, 123–129. doi: 10.1016/j.jad.2006.11.004
Sapolsky, R. M. (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925–935. doi: 10.1001/archpsyc.57.10.925
Sauvet, F., Leftheriotis, G., Gomez-Merino, D., Langrume, C., Drogou, C., Van Beers, P., et al. (2010). Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 108, 68–75. doi: 10.1152/japplphysiol.00851.2009
Scott, A. J., Webb, T. L., Martyn-St James, M., Rowse, G., and Weich, S. (2021). Improving sleep quality leads to better mental health: a meta-analysis of randomised controlled trials. Sleep Med. Rev. 60, 101556. doi: 10.1016/j.smrv.2021.101556
Scullin, M. K., and Bliwise, D. L. (2015). Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. 10, 97–137. doi: 10.1177/1745691614556680
Shokri-Kojori, E., Wang, G. J., Wiers, C. E., Demiral, S. B., Guo, M., Kim, S. W., et al. (2018). β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA. 115, 4483–4488. doi: 10.1073/pnas.1721694115
Spira, A. P., Gamaldo, A. A., An, Y., Wu, M. N., Simonsick, E. M., Bilgel, M., et al. (2013). Self-reported sleep and β-amyloid deposition in community-dwelling older adults. JAMA Neurol. 70, 1537–1543. doi: 10.1001/jamaneurol.2013.4258
Spira, A. P., Gonzalez, C. E., Venkatraman, V. K., Wu, M. N., Pacheco, J., Simonsick, E. M., et al. (2016). Sleep duration and subsequent cortical thinning in cognitively normal older adults. Sleep 39, 1121–1128. doi: 10.5665/sleep.5768
Sprecher, K. E., Bendlin, B. B., Racine, A. M., Okonkwo, O. C., Christian, B. T., Koscik, R., et al. (2015). Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol. Aging 36, 2568–2576. doi: 10.1016/j.neurobiolaging.2015.05.004
Staekenborg, S. S., Kelly, N., Schuur, J., Koster, P., Scherder, E., Tielkes, C. E. M., et al. (2020). Education as proxy for cognitive reserve in a large elderly memory clinic: ‘Window of Benefit'. J. Alzheimers. Dis. 76, 671–679. doi: 10.3233/JAD-191332
Steffens, D. C., Fahed, M., Manning, K. J., and Wang, L. (2022). The neurobiology of apathy in depression and neurocognitive impairment in older adults: a review of epidemiological, clinical, neuropsychological and biological research. Transl. Psychiatry 12, 525. doi: 10.1038/s41398-022-02292-3
Szymkowicz, S. M., Dotson, V. M., Jones, J. D., Okun, M. S., and Bowers, D. (2018). Symptom dimensions of depression and apathy and their relationship with cognition in Parkinson's disease. J. Int. Neuropsychol. Soc. 24, 269–282. doi: 10.1017/S1355617717001011
Tabuchi, M., Lone, S. R., Liu, S., Liu, Q., Zhang, J., Spira, A. P., et al. (2015). Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr. Biol. 25, 702–712. doi: 10.1016/j.cub.2015.01.016
Taconnat, L., Bouazzaoui, B., Bouquet, C., Larigauderie, P., Witt, A., and Blaye, A. (2022). Cognitive mechanisms underlying free recall in episodic memory performance across the lifespan: testing the control/representation model. Psychol. Res. 87, 1370–1388. doi: 10.1007/s00426-022-01736-1
Tagariello, P., Girardi, P., and Amore, M. (2009). Depression and apathy in dementia: same syndrome or different constructs? A critical review. Arch. Gerontol. Geriatr. 49, 246–249. doi: 10.1016/j.archger.2008.09.002
Tari, B., Ben Yehuda, M., Laurell, A. A. S., Ritchie, K., Dauvilliers, Y., Ritchie, C., et al. (2023). The indirect relationship between sleep and cognition in the PREVENT cohort: identifying targets for intervention. medRxiv. [preprint]. doi: 10.1101/2023.05.18.23290160
United Nations (2020). Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2015 Revision: Key Findings and Advance Tables. United Nations Working Paper ESA/P/WP.241. Washington, DC: United Nations.
Varghese, S., Frey, B. N., Schneider, M. A., Kapczinski, F., and de Azevedo Cardoso, T. (2022). Functional and cognitive impairment in the first episode of depression: a systematic review. Acta Psychiatr. Scand. 145, 156–185. doi: 10.1111/acps.13385
Voyer, D., Saint Aubin, J., Altman, K., and Gallant, G. (2021). Sex differences in verbal working memory: a systematic review and meta-analysis. Psychol. Bull. 147, 352–398. doi: 10.1037/bul0000320
Wang, C., and Holtzman, D. M. (2020). Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120. doi: 10.1038/s41386-019-0478-5
Watson, N. F., Badr, M. S., Belenky, G., Bliwise, D. L., Buxton, O. M., Buysse, D., et al. (2015). Recommended amount of sleep for a healthy adult: a joint consensus statement of the American academy of sleep medicine and sleep research society. Sleep 38, 843–844. doi: 10.5665/sleep.4716
Williams, B. R., Strauss, E. H., Hultsch, D. F., and Hunter, M. A. (2007). Reaction time inconsistency in a spatial Stroop task: age-related differences through childhood and adulthood. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 14, 417–439. doi: 10.1080/13825580600584590
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. doi: 10.1126/science.1241224
Yao, H. K., Guet-McCreight, A., Mazza, F., Moradi Chameh, H., Prevot, T. D., Griffiths, J. D., et al. (2022). Reduced inhibition in depression impairs stimulus processing in human cortical microcircuits. Cell Rep. 38, 110232. doi: 10.1016/j.celrep.2021.110232
Yoo, S. S., Hu, P. T., Gujar, N., Jolesz, F. A., and Walker, M. P. (2007). A deficit in the ability to form new human memories without sleep. Nat. Neurosci. 10, 385–392. doi: 10.1038/nn1851
Keywords: sleep, depression, apathy, dementia, cognition, structural equation modeling
Citation: Tari B, Ben Yehuda M, Laurell AAS, Ritchie K, Dauvilliers Y, Ritchie CW, Lawlor B, Naci L, Muniz Terrera G, Malhotra P, Watermeyer T, Dudas R, Underwood BR, O'Brien JT, Raymont V and Koychev I (2023) The indirect relationship between sleep and cognition in the PREVENT cohort: identifying targets for intervention. Front. Sleep 2:1226824. doi: 10.3389/frsle.2023.1226824
Received: 22 May 2023; Accepted: 18 October 2023;
Published: 15 November 2023.
Edited by:
Satyajit Kundu, North South University, BangladeshReviewed by:
Enrico Giora, Vita-Salute San Raffaele University, ItalyAbdulbari Bener, Istanbul Medipol University, Türkiye
Copyright © 2023 Tari, Ben Yehuda, Laurell, Ritchie, Dauvilliers, Ritchie, Lawlor, Naci, Muniz Terrera, Malhotra, Watermeyer, Dudas, Underwood, O'Brien, Raymont and Koychev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Tari, YmVuamFtaW4udGFyaUBwc3ljaC5veC5hYy51aw==
 Benjamin Tari
Benjamin Tari Michael Ben Yehuda1
Michael Ben Yehuda1 Yves Dauvilliers
Yves Dauvilliers Graciela Muniz Terrera
Graciela Muniz Terrera Paresh Malhotra
Paresh Malhotra Ivan Koychev
Ivan Koychev