
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Sleep, 28 November 2023
Sec. Pediatric and Adolescent Sleep
Volume 2 - 2023 | https://doi.org/10.3389/frsle.2023.1177878
This article is part of the Research TopicWomen in Pediatric and Adolescent SleepView all 6 articles
Introduction: Aspects of circadian sleep health including circadian alignment, circadian phase, or chronotype may be related to mental health outcomes in adolescents. Using novel and robust data collection methods, this study explored the relationship between adolescents' circadian sleep health and traits related to depression, anxiety, stress, and emotional regulation.
Methods: Fifty-two healthy 14–18-year-olds (58% female; 94% European American) participated in this study. Across a 10-day period, participants completed wrist-worn actigraphy. Next, participants completed a dim-light melatonin onset (DLMO) protocol where 12 saliva samples were collected over a 6-h period to measure circadian phase. Circadian phase was calculated as the duration of time between DMLO to average sleep onset time across the monitoring period. Social jetlag was measured as the discrepancy between sleep times from weekday to weekend. Participants completed the Depression Anxiety Stress Scales (DASS-21), Emotion Regulation Questionnaire (ERQ), and the Morningness-Eveningness Questionnaire for Adolescents (MEQ). Following dichotomizing sleep outcomes into clinically relevant groups (late vs. early circadian phase, aligned vs. misaligned circadian rhythms, minimal social jetlag vs. presence of social jetlag, intermediate to morningness vs. eveningness chronotype), we conducted general linear models to determine circadian group differences in mental health outcomes (depression, anxiety, stress, expressive suppression, and cognitive reappraisal) while controlling for gender and pubertal development.
Results: Circadian phase had a large effect on depression symptoms in adolescents, with adolescents with later DLMO having significantly higher depression scores than those with earlier DLMO (p = 0.031). Chronotype had a medium but non-significant effect on anxiety and stress symptoms in adolescents, with adolescents with eveningness-tendencies having higher anxiety and stress symptoms than those with intermediate to morningness-tendencies (p's = 0.140 and 0.111, respectively).
Conclusions: In the first ever study using gold-standard methodologies to examine the relationship between mental health and circadian sleep health in healthy adolescents, we observed that adolescents with later circadian phase had increased depressive symptoms compared to earlier circadian phase. Furthermore, adolescents who endorsed behaviors that suggest eveningness tendencies may have heightened stress/anxiety. These conclusions encourage future experimental research regarding this topic and may help inform interventions aimed to decrease depression, anxiety, and stress in adolescents.
Global estimates suggest that 10–20% of children and adolescents will experience a mental health condition before adulthood [Evaluation I of HM and Global Health Data Exchange (GHDx), 2021]. The United States' Center for Disease Control and Prevention found a 33% increase in youth endorsing feelings of persistent hopelessness between 2008 and 2021 (Centers for Disease Control and Prevention, 2023). Such symptoms can have profound effects on adolescents' daily functioning and quality of life (Jaycox et al., 2009; Geng et al., 2020; Petito et al., 2020). Tragically, in 2021, 22% of adolescents reported seriously considering suicide (Centers for Disease Control and Prevention, 2023). Between 2008 and 2015, hospital visits for active suicidality nearly doubled, with adolescent girls being most strongly represented in this shift (Plemmons et al., 2018). Fortunately, previous studies have found that early interventions are associated with improved symptom prognosis not only within the adolescent period, but also across the lifespan (Das et al., 2016; McGorry and Mei, 2018; Petito et al., 2020). Therefore, understanding the factors that can increase or decrease rates of mental illness is vital to youth's current and future wellbeing.
Given its strong association with psychological and physical health across previous studies, sleep may be an especially salient yet clinically and culturally overlooked influencing factor of adolescent mental wellness (Campbell et al., 2012; Tarokh et al., 2016; Galván, 2020; Kuhlman et al., 2020; Lucien et al., 2021). Previous research suggests that sleep may be an early indicator of mental health difficulties, as well as serve as an accessible treatment target for individual therapies and public health initiatives (Blake et al., 2018; Petito et al., 2020). Sleep may help prevent some symptoms of mental distress due to its proposed impact on emotional regulation and the HPA axis (Palmer and Alfano, 2017; Blake et al., 2018; Nicolaides et al., 2020). Despite strong evidence suggesting that adequate sleep is also protective factor against depressive, anxious, and suicidal symptoms (Pasch et al., 2010; Lovato and Gradisar, 2014; Zhai et al., 2015; Ojio et al., 2016; Robillard et al., 2018), it is estimated that <30% of adolescents are receiving the 8–10 h of sleep of sleep recommended for their age group (Paruthi et al., 2016; Wheaton, 2018), with most adolescents only sleeping an average of 7.4 h per night (Galland et al., 2018) and more than 40% of US adolescents sleeping <7 h per night (Twenge et al., 2017). Disturbances in an adolescent's sleep pattern can impact the development of psychiatric conditions during this period as well as later in life (Tesler et al., 2013). To complicate matters further, these increases in mental health disorders have the potential to negatively impact sleep (Gregory and Sadeh, 2012; Baron and Reid, 2014; Lovato and Gradisar, 2014). This suggests that earlier improvements in sleep may lead to greater improvements in mental health outcomes over time.
Adolescents are at the highest risk of obtaining short and ill-timed sleep compared to any other developmental group (Crowley et al., 2018). This increased susceptibility can be due to social pressures placed on school-aged adolescents, early school start times, extracurricular activities being scheduled in the early morning hours (selected by administrators within older age groups with different sleep needs) (Dunster et al., 2018), and biological changes in intrinsic circadian rhythms and homeostatic sleep processes (Crowley et al., 2015; Logan and McClung, 2019). Some adolescents may also use sleep to exert their autonomy, delaying sleep to spend more with friends, catch up on homework, or browse social media (Power et al., 2017). Together, these factors can lead to reduced sleep duration.
Short sleep has long been associated with poorer mental health. While the majority of research demonstrating the negative impact of short sleep on mental health has been cross-sectional in nature (Short et al., 2019), a large two-wave study found that short sleep predicted anxiety disorders at 1 year follow-up, but baseline anxiety did not predict short sleep (Roberts and Duong, 2017), suggesting that short sleep may be a modifiable causal factor of anxious symptoms, rather than an unavoidable side effect of their presence. Beyond anxiety, a review of 73 studies in adolescents also found that reduced sleep was associated with a 55% increase in likelihood of mood difficulties (Short et al., 2020). Indeed, pooled data from over 500,000 adolescents demonstrates a curvilinear relationship between short sleep and suicidality, with 8–9 h of sleep per night being associated with the lowest risks of suicidal ideation or attempts in this age group (Chiu et al., 2018).
In addition to impacting sleep duration, adolescents' social demands can also clash with their sleep chronotype, or the preference a person has for morningness (sleeping early and waking early) or eveningness (sleeping late and waking late) behavioral patterns. Preference for eveningness typically peaks in adolescence and declines as individuals reach young adulthood, with males typically experiencing a later peak than females (Fischer et al., 2017; Randler et al., 2017a). Due to its association with daily behaviors, chronotype is most frequently measured via self-report measures (Levandovski et al., 2013). Self-reported eveningness has been associated with increased symptoms of depression across the lifespan (Hasler et al., 2010; Kivelä et al., 2018; Bauducco et al., 2020) as well as increased prevalence of seasonal affective disorder in adults (Tonetti et al., 2012). In adolescents, eveningness has been linked with lower self-regulation (Owens et al., 2016) and broad depressive symptoms (Chan et al., 2020; Chen et al., 2021) but less consistently with anxiety or suicidality (Alvaro et al., 2014; Díaz-Morales, 2016; Haraden et al., 2019; Chan et al., 2020; Chen et al., 2021; Kuula et al., 2022), despite previous studies identifying such associations in adults (Gaspar-Barba et al., 2009; Rumble et al., 2020; Bradford et al., 2021; Mokros et al., 2021). There is debate as to why mood may be negatively impacted by evening chronotype. It is thought that eveningness may be more associated with negative mental health outcomes due to pleiotropic impacts of genes associated with both sleep disorders and changes in neuronal development (Veatch et al., 2017). It is also possible that individuals with more evening chronotypes are at greater risk of short sleep due to circadian misalignment, or the mismatch between circadian phase and current sleep patterns. As societal demands tend to favor earlier chronotypes, adolescents with later chronotypes may be especially susceptible to effects of circadian misalignment on mental health, on top of the sleep debt risks already inherent to their age group.
Physiological measures of circadian timing can also provide insights into endogenous patterns of sleep and wakefulness, known as circadian phase. The suprachiasmatic nucleus (SCN) of the brain mediates the entrainment of internal circadian patterns with external light cues. Dim Light Melatonin Onset (DLMO) protocols, which aim to identify the onset of melatonin secretion, are considered the gold standard for assessing human circadian phase (Lewy, 1999). Existing studies in youth have used DLMO to identify associations between later circadian phase and higher negative and lower positive affect in a general sample (Dolsen and Harvey, 2018) as well as shorter melatonin secretion periods and worsened mood in adolescents with high BMIs (Simon et al., 2020). These findings suggest that adolescent preference for eveningness, as well as later endogenous circadian phases, increase risk for mood disorders, potentially due to circadian misalignment and sleep debt that are inherent in this developmental group.
Indeed, circadian misalignment may be the driving force behind the relationship between later chronotype and circadian phase and mental health. Several studies have demonstrated the relationship of circadian misalignment with mental health outcomes (Pasch et al., 2010; Winsler et al., 2015; Bauducco et al., 2020), though none (to our knowledge) have utilized gold-standard methods of assessing circadian misalignment to address this gap in the literature in community samples of this age group. In adolescents with diagnosed mood disorders, greater DLMO-assessed circadian misalignment was associated with greater depressive symptoms (Robillard et al., 2018). Circadian misalignment has also been linked with increased depressive symptoms in adults with major depressive disorder (Emens et al., 2009; Coleman et al., 2019), increased atypical symptoms of depression such as changes in appetite in pre- and peri-menopausal women (Meliska et al., 2011), and depression ratings in adults with Seasonal Affective Disorder (Lewy et al., 2006). These findings suggest that similar patterns may also be evident in general population samples of adolescents with subclinical symptom profiles.
One specific type of circadian misalignment that has received a deal of attention in its relationship to mental health in youth is “social jetlag” - the discrepancy between sleep timing on work/school days vs. free days. In the context of the adolescent population, social jetlag generally presents as students sleeping very little on weekdays due to later circadian phase and early school start times and then attempting to catch up on missed sleep on the weekends via falling asleep late and sleeping late into the morning (when school does not necessitate early wake times) (Baron and Reid, 2014). Social jetlag has historically been measured using self-report methods where the difference of midsleep between work/school days and “free days” are calculated (Jankowski, 2017). Using this calculation, greater self-reported social jetlag has been linked with increased depressive and anxious symptoms in adolescents (Mathew et al., 2019); however, social jetlag has yet to be robustly assessed alongside physiological measures of circadian misalignment in the context of anxiety and mood-related symptoms (Henderson et al., 2019).
Taken together, these findings suggest the potential associations between circadian sleep health (involving chronotype, circadian phase, and circadian alignment) and mental health in adolescents, though more studies examining these relationships simultaneously are needed. Methodologies are important to consider since subjective report of sleep behaviors has been linked to increased bias (Biddle et al., 2015; Cespedes et al., 2016) and ±1.5 h differences in circadian phase estimates (Lovato et al., 2016). However, physiological methods of assessing circadian phase can be costly and time intensive. If more affordable means of measuring circadian sleep health are sufficient to detect risk of mental health difficulties in this age group, they may be well suited to more widespread clinical use in diagnosis and treatment monitoring, even outside of research settings. However, if widely utilized circadian-related surveys are not as sensitive to increased risk of mental health difficulties as physiological measures (i.e., DLMO), this may suggest a need for improved self-report measures or increased referral and insurance support for formal DLMO procedures.
In response to the need for greater understanding of sleep as a risk factor for negative mental health outcomes in adolescence and to identify affordable and accessible methodologies to assess circadian alignment in this cohort, we used multi-modal methods of calculating circadian sleep health (i.e., DLMO, actigraphy, and self-report) to investigate how various aspects of adolescent sleep correspond with mental health symptoms. We compared relationships between physiological measures of circadian phase to those of self-report measures of chronotype and actigraphy-assessed social jetlag (weekday/weekend-specific changes in sleep timing). We hypothesized that increased self-reported levels of depression, stress, and anxiety, as well as decreased self-reported levels of emotional regulation ability, would be predicted by:
1. Later circadian phase (assessed through DLMO)
2. Evening chronotype (assessed through the morning-eveningness questionnaire; MEQ)
3. Greater levels of social jetlag (assessed through actigraphy)
4. More extreme circadian misalignment (assessed as whether the time elapsed between DLMO and average sleep onset falls outside of the expected range)
Findings from this study may inform future assessments for mental health disorders in this age group and provide insight into aspects of circadian sleep health which are most relevant to treatment of particular mental health symptom clusters.
This analysis was conducted as part of a larger sleep study using actigraphy, DLMO, and MRI data to explore the effects of adolescent circadian misalignment on executive function and obesity risk. All procedures in this study were approved by the Brigham Young University Institutional Review Board (IRB2021–306).
Healthy adolescents ages 14–18 years old were contacted through lunchroom recruitment booths at high schools near the sponsoring organization, printed flyers posted on university and high school bulletin boards, and digital flyers in schools' email newsletters. Interested adolescents completed an online survey to determine eligibility based on age and current enrollment in school. Due to an MRI protocol employed during other phases of this project, adolescents with metal implants, significant claustrophobia, or history of traumatic brain injury were excluded. As this study was also part of a larger examination of obesity-related factors, individuals whose BMIs were in the obese range (BMI%iles > 95%ile) as well as those who took medication known to interfere with diet or sleep behaviors (i.e., antidepressants, sleep medications) were also excluded.
All baseline appointments were held on a Tuesday or a Wednesday. During baseline appointments, parental consent and participant assent were obtained and participants were given a sleep diary to track their time spent in bed as well as their perceived sleep and wake times over a 10-day monitoring period. Participants were also taught how to wear and care for a wrist-worn accelerometer (ActiWatch 2) for nighttime use (time entering bed with intention to sleep until leaving the bed in the morning). Participants then completed a measure about their preferred sleeping timing, known as sleep habits including the Morningness-Eveningness Questionnaire (MEQ). The PDS (Pubertal Development Scale) was also administered during this phase to account for differences in circadian alignment associated with biological maturation.
For the first 7 days of the monitoring period, adolescents were instructed to sleep as they normally would; for nights 8–10, adolescents were asked to self-select a “typical” bedtime and waketime (a time that they felt their body would naturally want to fall asleep and wake up at) that they would adhere to across the three nights. This method was selected to stabilize sleep in a developmental population that faces highly variable sleep (Crowley et al., 2018) to enhance the likelihood of obtaining a valid DLMO estimate. Between the baseline and the assessment appointments, participants received daily text reminders to wear the accelerometers and to complete the sleep diary. Participants were also reminded of their assessment appointment time and of the sleep/wake times for nights 8–10 that were determined during the baseline appointment.
Following the 10-day monitoring period, participants attended the assessment appointment, where they returned their completed sleep diary and accelerometer. After completing an MRI scan for a different segment of the project, they spent 6 h in a dimly lit sleep lab for DLMO procedures where they were required to provide a saliva sample every 30 min. Throughout the 6-h session, participants filled out measures of emotional wellbeing, including the Emotion Regulation Questionnaire (ERQ) and the Depression, Anxiety, and Stress Scale – 21 Items (DASS-21). Due to the low-light environment, these measures were administered via paper and pencil and in large-print font. Between completing surveys and providing saliva samples, participants were free to participate in pleasant, low-light activities, including playing board games or solving puzzles, or playing video games or watching shows/movies on a dimly lit television. Applicable questions within measures were used to screen for current suicidality; any indicative responses were addressed with a suicide assessment conducted by a trained clinician or supervised graduate student. At the conclusion of the DLMO appointment, participants were compensated according to their adherence to and participation in study procedures.
To account for covariates that may relate to affect or sleep behaviors, age, sex, race, ethnicity, height, weight, medication use, date of last menstrual period, and familial income were voluntarily collected via an online survey at the baseline appointment.
We assessed for level of pubertal maturation using the Pubertal Development Scale (PDS) (Robertson et al., 1992). Given that most adolescents undergo a shift toward later circadian alignment (evening chronotype) following puberty, with chronotype generally not returning to prepubertal levels until youths' mid-to late- twenties (Crowley et al., 2018), it is important for studies of adolescent sleep to assess whether each subject has undergone puberty and is therefore subject to greater endogenous circadian misalignment. The PDS has been shown to be a reliable and valid measure of physical development (ICC = 81–0.92, Cronbach's Alpha = 0.91–0.96) (Koopman-Verhoeff et al., 2020). PDS is thus comparable measure to physical assessments performed by healthcare professionals such as Tanner staging, but its self-report format is more comfortable for participants and more conducive to research settings (Koopman-Verhoeff et al., 2020). Scores were included as a covariate. Internal consistency for this sample was adequate at 0.70.
We used the Emotional Regulation questionnaire (ERQ) for Children and Adolescents to conceptualize our primary outcome of emotional regulation (Gross and John, 2003; Gullone and Taffe, 2012). This 10-item survey looks at two facets of emotional regulation: (1) cognitive reappraisal, or how an individual's definition of a stressor can change its emotional impact, and (2) expressive suppression, or how much someone inhibits emotionally expressive behaviors over time. In a general community sample of over 1,000 Australian adults, the ERQ has been found to have strong internal consistency reliability (Cronbach's Alpha = 0.89–0.90 for cognitive appraisal, = 0.76–0.80 for expressive suppression) (Preece et al., 2020). The revised version for children and adolescents also displayed strong internal consistency (Cronbach's Alpha = 0.82–0.86) and moderate 12-month stability (ICC = 0.37–0.47 for cognitive reappraisal, = 0.40–0.63 for expressive suppression) (Gullone and Taffe, 2012). Within our sample, our Cronbach's Alpha for the cognitive reappraisal facet was good at 0.82 and the expressive suppression facet was fair at 0.60.
Depressive, anxious, and stress-associated primary outcomes were measured using an abbreviated version of the Depression Anxiety and Stress Scale (DASS-21) (Lovibond and Lovibond, 1995). These subscales have been found to be reliable in adolescent populations with Cronbach's Alpha measuring 0.87 for depression, 0.79 for anxiety, and 0.83 for tension/stress (Szabó, 2010); within our sample, our Cronbach's Alpha was 0.83.
Chronotype was measured with the Morningness-Eveningness Questionnaire (Horne and Östberg, 1976). This 19-item, multiple choice, self-report survey was used to determine participant chronotype, which we analyzed as a dichotomous predictor of mental health outcomes. The MEQ asks subjects to identify when they most prefer to wake up and go to bed, as well as their peak times of functioning. Total scores range from 16 to 86, with scores below 41 suggesting an evening chronotype and scores above 59 suggesting a morning chronotype. Although validation studies of the MEQ have not specifically looked at the adolescent population, several studies that include young adults showed strong internal consistency [Cronbach's α = 0.81–0.86 (Thun et al., 2012; Inomata et al., 2014; Treven Pišljar et al., 2019); within our study sample, the Cronbach's alpha was adequate at 0.70]. To facilitate interpretation, we dichotomized the sample into two groups: eveningness-tendencies (MEQ total score ≤ 41) and intermediate to morningness-tendencies (MEQ total score ≥42).
Social Jetlag was measured using sleep estimates gathered by actigraphy. Actigraphy was used with the knowledge that adolescents are notoriously poor at estimating their sleep habits (Bauer and Blunden, 2008). Further, there is good evidence of actigraphy serving as a strong estimate of social jetlag in pervious literature (Malone et al., 2016; Crowley et al., 2018; Cespedes Feliciano et al., 2019; Lucas-Thompson et al., 2021). Specifically, a wrist-worn accelerometer (ActiWatch 2) was initialized to collect data in 30 s epochs. Participants were instructed to wear the accelerometer at night, beginning when they entered bed with the intention of falling asleep. Participants also completed sleep diaries, where they self-reported the times that they entered their bed with the intention to sleep, fell asleep, woke up, and stepped out of bed. Information recorded in sleep diaries was used to verify sleep and wake onset times and to guide scoring in the actigraphy data. Data from wrist-worn actigraphs has been shown to correlate with polysomnography (r =0.81–0.83); however, specificity for wake times is weaker (Meltzer et al., 2012; Quante et al., 2018). The Sadeh algorithm (Sadeh et al., 1994) was used to calculate sleep onset, sleep offset, and sleep midpoint, and sleep duration.
We used an updated social jetlag calculation that accounts for the degree of weekend oversleep to produce a social jetlag estimate independent of sleep debt. Specifically, we used the following equation to calculate social jetlag: SJLsc = |sleep onset on free days – sleep onset on weekend days| (Jankowski, 2017). Using this estimate of SJL, we then dichotomized the sample into two groups: minimal social jetlag (≤1 hour) or presence of social jet lag (>1 h). This cutoff was selected as a shift in as little as 1 h in sleep patterns (as in the case of daylight savings time) can have a negative impact on youth health (Johnson and Malow, 2023).
Circadian phase was assessed through salivary Dim Light Melatonin Onset (DLMO) (Benloucif et al., 2008) during a six-hour assessment appointment that occurred on a Friday or Saturday evening. This assessment was scheduled to begin 5 h before and end 1 h beyond their predetermined typical bedtime that was selected at the baseline appointment. We used this self-selected typical bedtime to inform the assessment window as previous research has demonstrated that lack of personalization of this assessment window can lead to high missingness of DLMO (Keijzer et al., 2011). The assessment took place in a dimly-lit room (<5 lux at eye-level, confirmed every 30 min via hand-held luxometer) where DLMO salivary samples were collected. Specifically, each 30 min, participants were instructed to soak a cotton swab with saliva for ~2 min, or until the cotton swab was saturated, for a total of 12 saliva samples across the 6 h period. After each collection, the sample was weighed; samples that did not meet processing requirements (<2 mL) were disposed of and participants immediately provided a replacement sample. Samples >2mL were time-stamped, centrifuged at 3000 g for 5 min to remove the salvia from the cotton swab, and frozen for analysis. Frozen samples were then shipped to and processed by SolidPhase Inc., an external lab that uses Novolytix RIA kits to measure salivary melatonin concentrations. In order to minimize potential contamination, during the 10 min prior to each sample collection, adolescents refrained from eating or drinking and ensured that their mouths were clean by brushing their teeth with a soft bristle toothbrush while relaxing in a reclined position.
Our lab used a threshold of 4 pg/mL to determine saliva melatonin concentration values, a threshold that has been previously validated in adolescent populations (Carskadon et al., 1997). Adolescents were categorized into two circadian phase groups: early timing (DLMO prior to 10:00 pm) or late timing (DLMO at 10:00 pm or later). This cut point was determined based on previously established mean DLMO times in later adolescents (Crowley et al., 2014) and because having a DLMO time of 10:00 pm or later would suggest a physiologically appropriate bedtime of midnight or later (based on mean phase angle reported in later adolescents) (Crowley et al., 2014) that may increase the likelihood of sleep restriction due to early school start times. Circadian alignment (also referred to as phase angle) was calculated as the difference in time between DLMO and the average sleep onset time that was recorded via actigraphy over the 10-day monitoring period. Following determine the phase angle of adolescents, we categorized the sample into two groups: “aligned,” representing cases where there was a phase angle of ≥ 2 h between DLMO and the average sleep onset time (reflecting the anticipated delay between DLMO and sleep initiation in low-light conditions) (Pandi-Perumal et al., 2007; Crowley et al., 2014) and “misaligned,” signifying instances where the a time gap of <2 h (indicating adolescents initiating sleep onset before their physiological readiness). No adolescent in our study had a sleep onset phase angle gap of >4 h, which would have also been considered “misaligned” (indicating adolescents falling asleep later than their body's natural readiness).
ActiWatch software (Actiware 6.3) was used to download wrist-worn accelerometer data in 30 s poch intervals. Sleep period onset and offset data was cross verified between actigraphy measurements and sleep diaries. In the event that sleep onset or waketime was not noted in a participant's sleep diary (N = 2) or, in cases of noticeable discrepancies between the sleep diary and the ActiWatch log (i.e., significant physical activity after diary suggested they had fallen asleep or significant inactivity before the diary suggested they had fallen asleep (N = 2), two reviewers jointly estimated in-bed and out-of-bed times. In these 4 instances, in-bed time was defined as the beginning of the last downward trend of physical activity prior to sleep, and out-of-bed time was defined as the beginning of the first prolonged period of activity following sleep-associated inactivity. We used the ActiWatch software to clean the data and the Sadeh et al. (1994) algorithm was used to generate a report of sleep onset and offset and sleep duration. Midsleep was calculated as the halfway point between sleep offset and sleep onset.
We conducted an a priori power analysis using G*Power 3.1.9.7 and found that to conduct a linear multiple regression (random model) to test three sleep predictors (i.e., chronotype, social jetlag, and circadian alignment) as well as three covariates (to be determined at time of analyses), we needed a sample size of 48 to have 0.80 power to detect a large effect with a 0.05 alpha error probability. Under the assumption that we would have at least four instances of equipment failure (i.e., accelerometers not recording sleep, DLMO sample loss), we aimed to recruit 52 adolescents.
Data were analyzed using SPSS (Version 28, IBM Inc., Chicago, IL). First, sample characteristics were generated using both means (SD) and frequencies. Meaningful covariates included in our models included sex assigned at birth (given frequently observed sex differences in anxiety and mood-related mental illnesses) (Yoon et al., 2023), pubertal status (given that transition through puberty is often associated with increased mental illness) (Pfeifer and Allen, 2021), and income (as this may be a factor related to social determinants of sleep health in adolescents) (Hawkins and Takeuchi, 2016). We then conducted a series of general linear models that tested whether mental health outcomes (anxiety, stress, depression, or emotional regulation facets) differed across four aspects of circadian health, including chronotype group (morningness vs. eveningness), timing (early vs. late DLMO), circadian alignment (aligned vs. misaligned), and social jetlag (minimal social jet lack, presence of social jet lag). We determined that the assumptions for these models were met prior to analyses. To determine statistical significance, a two-tailed p-value of 0.05 was selected. We utilized partial eta squared () as an estimate of effect size; specifically, partial eta squared informs how large of an effect the independent variable has on the dependent variable (with of 0.01, 0.06, and 0.14 indicating small, medium, and large effect, respectively). Due to our smaller sample size, we interpreted research findings if the estimated was medium (0.06) or larger.
A total of 52 adolescents completed the experimental procedures [mean age 15.54, SD = 1.37; 58% female (sex assigned at birth); 94% European-American; see Table 1 for detailed demographic characteristics]. Of these 52 adolescent participants, 43 had valid accelerometry data for estimation of circadian misalignment and social jetlag (there were 5 instances of accelerometer non-compliance and 4 instances of equipment malfunction). Additionally, 47 participants had valid DLMO estimates for estimating circadian misalignment (3 instances where saliva melatonin concentrations did not exceed 4 pg/ml and 2 instances where there no instances where concentrations fell below 4 pg/ml). All 52 participants were able to complete the required measures.
As summarized in Table 1, participants presented with (on average) mild levels of depression (mean = 11.15, SD = 5.88) and anxiety (mean = 9.86, SD = 7.76) and normal levels of stress (mean = 13.70, SD = 5.80). Based on established DASS-21 cut-offs, 13 participants had moderate levels of depression, 2 participants had severe levels of depression, and 1 had extremely severe levels of depression. Furthermore, 10 participants had moderate anxiety, 1 had severe anxiety, and 9 had extremely severe anxiety. Finally, 10 participants had moderate levels of stress, and 1 had severe levels of stress. Normal distributions were observed in circadian alignment (phase angle; ranging from 0:09 to 3:52), DLMO (ranging from 19:00 to 24:30), MEQ total score (ranging from 35 to 68), and social jetlag (0:05–3:34).
Circadian phase had a large effect on depression symptoms in adolescents, with adolescents with later DLMO having significantly higher depression scores (M = 14.89, SD = 7.62) than those with earlier DLMO (M = 9.24, SD = 5.14; p = 0.031; = 0.13; see Table 2 and Figure 1). Differences in anxiety, stress, or emotional regulation facets across circadian phase groups were small ( ≤ 0.02) and non-significant (p's > 0.432).
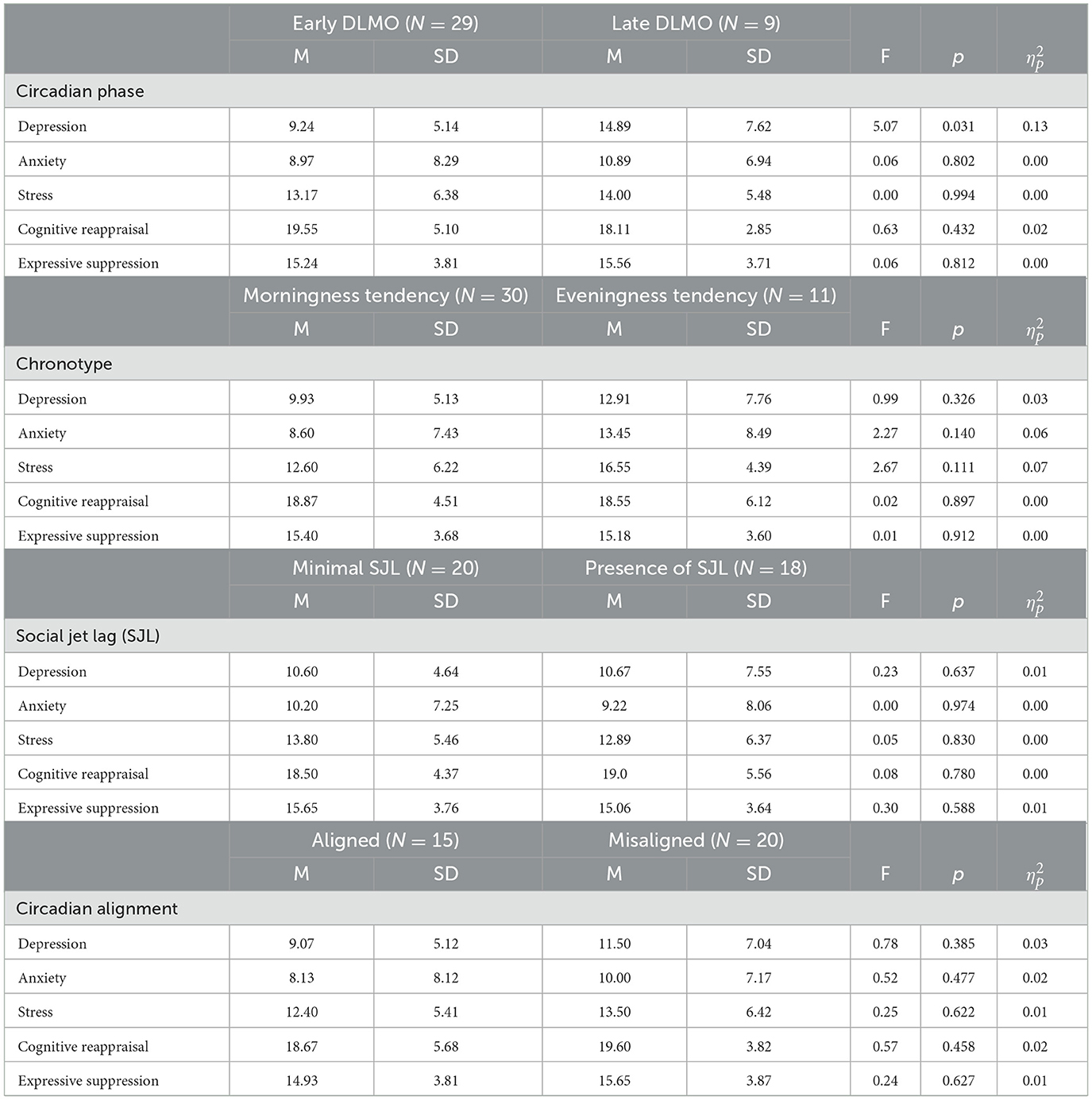
Table 2. Group differences in obesity-related outcomes amongst early circadian phase vs. late circadian phase.
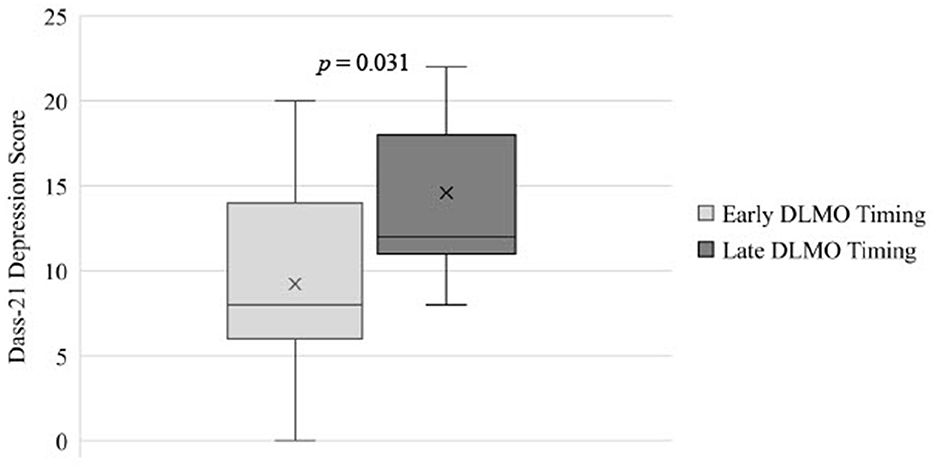
Figure 1. Differences in depression scores for adolescents with earlier DLMO vs. Later DLNIO timing. DLMO, Dim Light Melatonin Onset; DASS-21, The Depression, Anxiety, and Stress Scale — 21 Items.
Chronotype had a medium but non-significant effect on anxiety symptoms in adolescents, with adolescents with eveningness-tendencies having higher anxiety scores (M = 13.45, SD = 8.49) than those with intermediate to morningness-tendencies (M = 8.60, SD = 7.43; p = 0.140; = 0.059; see Table 2 and Figure 2). Additionally, adolescents with eveningness-tendencies trended toward having higher levels of stress (M = 16.55, SD = 4.39) than those with intermediate to morningness-tendencies (M = 12.60; SD = 6.22; p = 0.111, = 0.069; see Table 2 and Figure 2). Differences in depression or emotional regulation facets across chronotype groups were small ( ≤ 0.027) and non-significant (p's > 0.326).
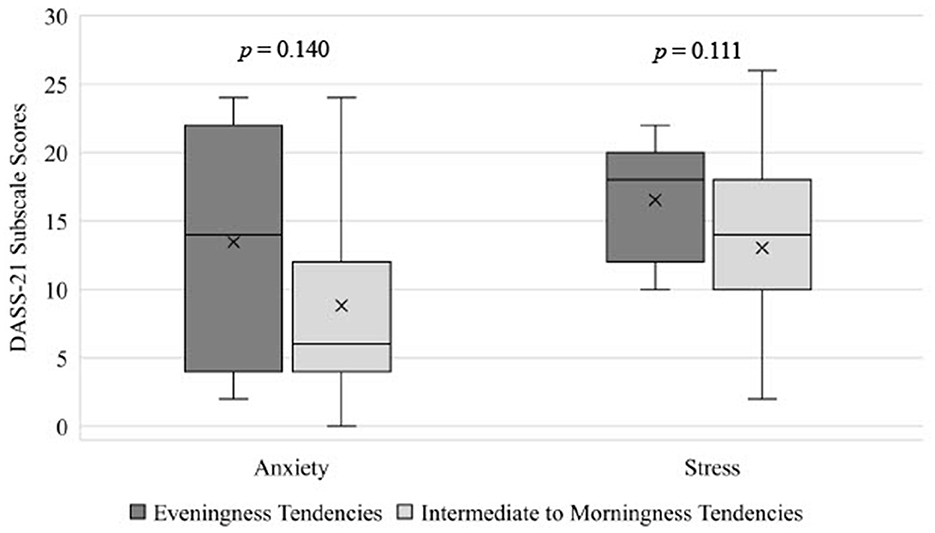
Figure 2. Differences in anxiety and stress in adolescents mith eveningness tendencies and intermediate to morningness tendencies, estimated from the MEQ. DASS-21, The Depression, Anxiety, and Stress Scale — 21 Items; MEQ, Momingness/Eveningness Questionnaire.
No differences in depression, anxiety, stress, or emotional regulation facets across SJL groups were noted ( ≤ 0.009; p's > 0.588).
No differences in depression, anxiety, stress, or emotional regulation facets across circadian alignment groups were noted ( ≤ 0.025; p's > 0.385).
Given the imperative role sleep plays in the development and maintenance of mental health pathology in adolescents, research examining more granular areas of sleep may be vital to early intervention, prevention, and effective treatment (Blake et al., 2018; Petito et al., 2020). This study aimed to examine the relationship between aspects of sleep circadian health and mental health symptomology in adolescents. While considering carefully considering unique nature of circadian phase, chronotype, and circadian alignment, we employed multi-modal and gold-standard physiological measurement of circadian phase (actigraphy and DLMO) along with self-report measures of chronotype and psychological wellbeing to thoroughly investigate potential connections between circadian health and mental health.
We observed that even after accounting for sex and pubertal status, adolescents with later circadian phase (as measured by DMLO) had increased depressive symptoms. This corroborates previous research findings that indicate that later circadian phase is associated with higher negative affect in youth (Alvaro et al., 2014). Similarly, recent research has examined the effectiveness of a behavioral intervention aimed to advance bedtime earlier into the evening (the Transdiagnostic Sleep and Circadian Intervention; TranS-C) on sleep and depression outcomes in adolescents. Specifically, one pilot trial in adolescents with ADHD (Becker et al., 2022) and a randomized control trial in adolescents with comorbid evening circadian preference and depression (Asarnow et al., 2023) demonstrated that TranS-C improved adolescent depression symptomatology following the intervention, and improved circadian alignment may be the mechanism that reduces depressive symptoms (Asarnow et al., 2023). These findings, paired with adult recommendations on how to control light levels across the 24 h day to help align circadian rhythms (Brown et al., 2022), provide compelling evidence that incorporating interventions that aim to advance sleep timing and promote circadian alignment in adolescents may serve as a powerful target for reducing depression.
Alternatively, our combined models did not replicate previous associations between adolescent circadian misalignment (Robillard et al., 2018), social jetlag (Mathew et al., 2019), or chronotype (Tonetti et al., 2012; Owens et al., 2016; Chan et al., 2020) and mental health outcomes; however, our findings neared significance for chronotype on anxiety and stress, where adolescents with identified eveningness-tendencies had greater symptoms of both anxiety and stress, compared to adolescents with identified morningness-tendencies. We hesitate to interpret non-significant findings but acknowledge that despite lacking statistical significance (which may be due to our smaller sample size), the differences in anxiety and stress had a medium effect size. Should our research findings be replicated using larger sample sizes (Watts and Norbury, 2017), these findings would corroborate fMRI research that demonstrates that later chronotype is associated with reduced connectivity between the amygdala and the cingulate cortex (Horne and Norbury, 2018), a circuit previously associated with high neuroticism (Cremers et al., 2010). This may suggest a neural basis for associations between genetic or temperamental sleep preferences and emotional responsiveness. However, given the mixed cross-sectional and longitudinal findings on chronotype and anxiety in adolescents (Power et al., 2017; Haraden et al., 2019), future research on this topic is warranted.
It is worth noting that genetic traits related to circadian rhythm development and maintenance also influence neural development or connectivity involved in mental health (Veatch et al., 2017). Such individual genetic variance may explain why some adolescents may be especially susceptible to negative effects of short or misaligned sleep. The hypothesis that different trends may emerge for different genetic or behavioral circadian profiles would be in alignment with previous findings on adults with delayed sleep phase disorder, which point to significant heterogeneity in impacts of circadian factors on sleep and mental health symptoms (Murray et al., 2017). This may suggest that circadian phase and misalignment may be more impactful on mental health in some groups than others, even when focusing on those who already demonstrate significant clinical sleep concerns.
Another significant area of heterogeneity and individual variance in associations between sleep and mental health is personality. Many traits such as extraversion, openness, agreeableness and conscientiousness have been associated with morning chronotype (Randler et al., 2017b). Of particular relevance to this context, neuroticism has also been associated with evening chronotype (Randler et al., 2017b). This is in alignment with our findings that adolescents with later chronotype trended toward having higher levels of anxiety and stress. Eveningness, lower levels of conscientiousness, and higher levels of neuroticism have also been associated with greater symptoms of depression (Gorgol et al., 2022). Future studies could examine personality as a moderating factor in this age group.
To our knowledge, this is the first study to use DLMO to examine circadian phase and circadian misalignment in conjunction with mental health outcomes in a population of adolescents from the general community. A key strength of this study is its naturalistic observation of sleep behaviors during the weekend minimal manipulation of participants' typical bedtime routines (excluding the three-night stabilization period prior to DLMO assessment). Additionally, our multimodal assessment of circadian health allows for a comprehensive view of sleep timings' potential influence on affect. Our focus on physiological measures may explain some differences between our results and those of past investigations focusing on self-report.
A primary limitation of this study is our small sample size, which reduced power of all analyses. This small sample size was chosen based on MRI outcomes being included in the primary analyses of the larger project wherein this data was collected. Future investigations would benefit from increased sample sizes which may facilitate increased sensitivity. This may be especially important to consider in regard to our lack of observed effects in the combined models as well as our lack of observed relationships between any circadian-related measurement and adolescents' stress or anxiety levels. Due to our focus on multiple methods of measurement as well as multiple mental health symptom clusters, we conducted multiple analyses, which increases our likelihood of Type I error. Our study design was also cross-sectional, meaning that even correlations identified as statistically significant should not be used to infer causality. The same can be said for studies using DLMO to study circadian alignment at large, although relevant longitudinal studies have been published (Haraden et al., 2019; Chen et al., 2023). Future studies could also use manipulation protocols to further confidence in suggested trends.
Additionally, actigraphy measurement only spanned a seven-day period, increasing the likelihood of altered SJL estimates; future studies should consider implementing a longer collection period. Further, in our attempt to stabilize sleep to increase the likelihood of obtaining a valid DLMO sample, we may have inadvertently altered the naturalistic sleep patterns of adolescents and the DLMO estimate; future research should consider broadening the DLMO assessment window to allow adolescents the flexibility of sleeping naturalistically leading up to the appointment while still maximizing odds of obtaining valid DLMO samples. Finally, while our participants were recruited from the general community, there was limited ethnic, geographic, and socioeconomic variability in our sample that limits the generalizability of our findings. Further, the sample included healthy adolescents which likely limited the severity of mental health difficulties within our sample which may have limited our ability to detect effects that may be present in more clinical cohorts. Future studies should investigate whether these findings generalize to more representative adolescent populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Brigham Young University Institutional Review Board (IRB2021–306). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
KD served as the principle investigator on this project, oversaw data collection, analyzed statistics, and contributed to manuscript preparation. SK helped prepare the data and significantly contributed to manuscript preparation. IW participated in data collection, helped prepare the data, analyzed statistics, and contributed to writing the manuscript. KR helped with data collection and contributed to the manuscript. JM and MA with preparing the data and contributed in writing the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded through start-up funds provided by the Brigham Young University's Psychology Department. Open access publication fees were funded by Brigham Young University College of Family Home and Social Sciences.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alvaro, P. K., Roberts, R. M., and Harris, J. K. (2014). The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. 15, 934–941. doi: 10.1016/j.sleep.2014.03.019
Asarnow, L. D., Soehner, A., Dolsen, E., Dong, L., and Harvey, A. G. (2023). Report from a randomized control trial: improved alignment between circadian biology and sleep–wake behavior as a mechanism of depression symptom improvement in evening-type adolescents with depressive symptoms. J. Child Psychol. Psychiatr. doi: 10.1111/jcpp.13880. [Epub ahead of print].
Baron, K. G., and Reid, K. J. (2014). Circadian misalignment and health. Int. Rev. Psychiatr. 26, 139–154. doi: 10.3109/09540261.2014.911149
Bauducco, S., Richardson, C., and Gradisar, M. (2020). Chronotype, circadian rhythms and mood. Curr. Opin. Psychol. 34, 77–83. doi: 10.1016/j.copsyc.2019.09.002
Bauer, K. M., and Blunden, S. (2008). How accurate is subjective reporting of childhood sleep patterns? A review of the literature and implications for practice. Curr. Pediatr. Rev. 4, 132–142. doi: 10.2174/157339608784462025
Becker, S. P., Duraccio, K. M., Sidol, C. A., Fershtman, C. E. M., Byars, K. C., Harvey, A. G., et al. (2022). Impact of a behavioral sleep intervention in adolescents with ADHD: feasibility, acceptability, and preliminary effectiveness from a pilot open trial. J. Atten. Disord. 26, 1051–1066. doi: 10.1177/10870547211056965
Benloucif, S., Burgess, H. J., Klerman, E. B., Lewy, A. J., Middleton, B., Murphy, P. J., et al. (2008). Measuring melatonin in humans. J. Clin. Sleep Med. 04, 66–69. doi: 10.5664/jcsm.27083
Biddle, D. J., Robillard, R., Hermens, D. F., Hickie, I. B., and Glozier, N. (2015). Accuracy of self-reported sleep parameters compared with actigraphy in young people with mental ill-health. Sleep Health 1, 214–220. doi: 10.1016/j.sleh.2015.07.006
Blake, M. J., Trinder, J. A., and Allen, N. B. (2018). Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: implications for behavioral sleep interventions. Clin. Psychol. Rev. 63, 25–40. doi: 10.1016/j.cpr.2018.05.006
Bradford, D. R. R., Biello, S. M., and Russell, K. (2021). Insomnia symptoms mediate the association between eveningness and suicidal ideation, defeat, entrapment, and psychological distress in students. Chronobiol. Int. 38, 1397–1408. doi: 10.1080/07420528.2021.1931274
Brown, T. M., Brainard, G. C., Cajochen, C., Czeisler, C. A., Hanifin, J. P., Lockley, S. W., et al. (2022). Recommendations for daytime, evening, and nighttime indoor light exposure to best support physiology, sleep, and wakefulness in healthy adults. PLOS Biol. 20, e3001571. doi: 10.1371/journal.pbio.3001571
Campbell, I. G., Grimm, K. J., de Bie, E., and Feinberg, I. (2012). Sex, puberty, and the timing of sleep EEG measured adolescent brain maturation. Proc. Nat. Acad. Sci. 109, 5740–5743. doi: 10.1073/pnas.1120860109
Carskadon, M. A., Acebo, C., Richardson, G. S., Tate, B. A., and Seifer, R. (1997). An approach to studying circadian rhythms of adolescent humans. J. Biol. Rhyth. 12, 278–289. doi: 10.1177/074873049701200309
Centers for Disease Control and Prevention (2023). Youth Risk Behavior Surveillance System: Data Summary & Trends Report 2023. Available online at: https://www.cdc.gov/healthyyouth/data/yrbs/pdf/yrbs_data-summary-trends_report2023_508.pdf
Cespedes Feliciano, E. M., Rifas-Shiman, S. L., Quante, M., Redline, S., Oken, E., Taveras, E. M., et al. (2019). Chronotype, social jet lag, and cardiometabolic risk factors in early adolescence. JAMA Pediatr. 173, 1049–1057. doi: 10.1001/jamapediatrics.2019.3089
Cespedes, E. M., Hu, F. B., Redline, S., Rosner, B., Alcantara, C., Cai, J., et al. (2016). Comparison of self-reported sleep duration with actigraphy: results from the hispanic community health study/study of latinos sueño ancillary study. Am. J. Epidemiol. 183, 561–573. doi: 10.1093/aje/kwv251
Chan, N. Y., Zhang, J., Tsang, C. C., Li, A. M., Chan, J. W. Y., Wing, Y. K., et al. (2020). The associations of insomnia symptoms and chronotype with daytime sleepiness, mood symptoms and suicide risk in adolescents. Sleep Med. 74, 124–131. doi: 10.1016/j.sleep.2020.05.035
Chen, P., Chen, Y., Jin, S., and Lu, P. A. (2023). cross-sectional and longitudinal study of how two intervention methods affect the anxiety, sleep quality, and physical activity of junior high school students under quarantine. Front. Psychol. 14, 1099732. doi: 10.3389/fpsyg.2023.1099732
Chen, S. J., Zhang, J. H., Li, S. X., Tsang, C. C., Chan, K. C. C., Au, C. T., et al. (2021). The trajectories and associations of eveningness and insomnia with daytime sleepiness, depression and suicidal ideation in adolescents: a 3-year longitudinal study. J. Aff. Dis. 294, 533–542. doi: 10.1016/j.jad.2021.07.033
Chiu, H. Y., Lee, H. C., Chen, P. Y., Lai, Y. F., and Tu, Y. K. (2018). Associations between sleep duration and suicidality in adolescents: a systematic review and dose–response meta-analysis. Sleep Med. Rev. 42, 119–126. doi: 10.1016/j.smrv.2018.07.003
Coleman, M. Y., McGlashan, E. M., Vidafar, P., Phillips, A. J. K., and Cain, S. W. (2019). Advanced melatonin onset relative to sleep in women with unmedicated major depressive disorder. Chronobiol. Int. 36, 1373–1383. doi: 10.1080/07420528.2019.1644652
Cremers, H. R., Demenescu, L. R., Aleman, A., Renken, R., van Tol, M. J., van der Wee, N. J. A., et al. (2010). Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage 49, 963–970. doi: 10.1016/j.neuroimage.2009.08.023
Crowley, S. J., Cain, S. W., Burns, A. C., Acebo, C., and Carskadon, M. A. (2015). Increased sensitivity of the circadian system to light in early/mid-puberty. J. Clin. Endocrinol. Metab. 100, 4067–4073. doi: 10.1210/jc.2015-2775
Crowley, S. J., Van Reen, E., LeBourgeois, M. K., Acebo, C., Tarokh, L., Seifer, R., et al. (2014). A longitudinal assessment of sleep timing, circadian phase, and phase angle of entrainment across human adolescence. PloS ONE 9, e112199. doi: 10.1371/journal.pone.0112199
Crowley, S. J., Wolfson, A. R., Tarokh, L., and Carskadon, M. A. (2018). An update on adolescent sleep: new evidence informing the perfect storm model. J. Adol. 67, 55–65. doi: 10.1016/j.adolescence.2018.06.001
Das, J. K., Salam, R. A., Lassi, Z. S., Khan, M. N., Mahmood, W., Patel, V., et al. (2016). Interventions for adolescent mental health: an overview of systematic reviews. J. Adol. Health 59, S49–60. doi: 10.1016/j.jadohealth.2016.06.020
Díaz-Morales, J. F. (2016). Anxiety during adolescence: considering morningness–eveningness as a risk factor. Sleep Biol. Rhythms 14, 141–147. doi: 10.1007/s41105-015-0032-8
Dolsen, E. A., and Harvey, A. G. (2018). Dim light melatonin onset and affect in adolescents with an evening circadian preference. J. Adol. Health 62, 94–99. doi: 10.1016/j.jadohealth.2017.07.019
Dunster, G. P., de la Iglesia, L., Ben-Hamo, M., Nave, C., Fleischer, J. G., Panda, S., and de la Iglesia, H. O. (2018). Sleepmore in Seattle: later school start times are associated with more sleep and better performance in high school students. Sci. Adv. 4, eaau6200. doi: 10.1126/sciadv.aau6200
Emens, J., Lewy, A., Kinzie, J. M., Arntz, D., and Rough, J. (2009). Circadian misalignment in major depressive disorder. Psychiatr. Res. 168, 259–261. doi: 10.1016/j.psychres.2009.04.009
Evaluation I of HM and Global Health Data Exchange (GHDx) (2021). Institute of Health Metrics and Evaluation, Seattle, WA.
Fischer, D., Lombardi, D. A., Marucci-Wellman, H., and Roenneberg, T. (2017). Chronotypes in the US – Influence of age and sex. PLoS ONE 12, e0178782. doi: 10.1371/journal.pone.0178782
Galland, B. C., Short, M. A., Terrill, P., Rigney, G., Haszard, J. J., Coussens, S., et al. (2018). Establishing normal values for pediatric nighttime sleep measured by actigraphy: a systematic review and meta-analysis. Sleep 41, zsy017. doi: 10.1093/sleep/zsy017
Galván, A. (2020). The need for sleep in the adolescent brain. Trends Cognit. Sci. 24, 79–89. doi: 10.1016/j.tics.2019.11.002
Gaspar-Barba, E., Calati, R., Cruz-Fuentes, C. S., Ontiveros-Uribe, M. P., Natale, V., Ronchi, D., et al. (2009). Depressive symptomatology is influenced by chronotypes. J. Aff. Dis. 119, 100–106. doi: 10.1016/j.jad.2009.02.021
Geng, Y., Gu, J., Zhu, X., Yang, M., Shi, D., Shang, J., et al. (2020). Negative emotions and quality of life among adolescents: a moderated mediation model. Int. J. Clin. Health Psychol. 20, 118–125. doi: 10.1016/j.ijchp.2020.02.001
Gorgol, J., Waleriańczyk, W., and Stolarski, M. (2022). The moderating role of personality traits in the relationship between chronotype and depressive symptoms. Chronobiol. Int. 39, 106–116. doi: 10.1080/07420528.2021.1979995
Gregory, A. M., and Sadeh, A. (2012). Sleep, emotional and behavioral difficulties in children and adolescents. Sleep Med. Rev. 16, 129–136. doi: 10.1016/j.smrv.2011.03.007
Gross, J. J., and John, O. P. (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 85, 348–362. doi: 10.1037/0022-3514.85.2.348
Gullone, E., and Taffe, J. (2012). The emotion regulation questionnaire for children and adolescents (ERQ–CA): a psychometric evaluation. Psychol. Assess. 24, 409–417. doi: 10.1037/a0025777
Haraden, D. A., Mullin, B. C., and Hankin, B. L. (2019). Internalizing symptoms and chronotype in youth: a longitudinal assessment of anxiety, depression and tripartite model. Psychiatr. Res. 272, 797–805. doi: 10.1016/j.psychres.2018.12.117
Hasler, B. P., Allen, J. J. B., Sbarra, D. A., Bootzin, R. R., and Bernert, R. A. (2010). Morningness–eveningness and depression: preliminary evidence for the role of the behavioral activation system and positive affect. Psychiatr. Res. 176, 166–173. doi: 10.1016/j.psychres.2009.06.006
Hawkins, S. S., and Takeuchi, D. T. (2016). Social determinants of inadequate sleep in US children and adolescents. Pub. Health 138, 119–126. doi: 10.1016/j.puhe.2016.03.036
Henderson, S. E. M., Brady, E. M., and Robertson, N. (2019). Associations between social jetlag and mental health in young people: a systematic review. Chronobiol. Int. 36, 1316–1333. doi: 10.1080/07420528.2019.1636813
Horne, C. M., and Norbury, R. (2018). Late chronotype is associated with enhanced amygdala reactivity and reduced fronto-limbic functional connectivity to fearful versus happy facial expressions. NeuroImage 171, 355–363. doi: 10.1016/j.neuroimage.2018.01.025
Horne, J. A., and Östberg, O. (1976). Morningness-eveningness questionnaire. J. Appl. Psychol. 4, 97–110. doi: 10.1037/t02254-000
Inomata, Y., Echizenya, M., Takeshima, M., Shimizu, K., and Shimizu, T. (2014). Validity and reliability of the Japanese version of the morningness-eveningness questionnaire evaluated from actigraphy. Sleep Biol. Rhythms 12, 289–296. doi: 10.1111/sbr.12073
Jankowski, K. S. (2017). Social jet lag: Sleep-corrected formula. Chronobiol. Int. 34, 531–535. doi: 10.1080/07420528.2017.1299162
Jaycox, L. H., Stein, B. D., Paddock, S., Miles, J. N. V., Chandra, A., Meredith, L. S., et al. (2009). Impact of teen depression on academic, social, and physical functioning. Pediatrics 124, e596–605. doi: 10.1542/peds.2008-3348
Johnson, K. G., and Malow, B. A. (2023). Implications of sleep health policy: daylight saving and school start times. Continuum: Lifelong Learn. Neurol. 29, 1253. doi: 10.1212/CON.0000000000001331
Keijzer, H., Smits, M. G., Peeters, T., Looman, C. W. N., Endenburg, S. C., Gunnewiek, J. M. T. K., et al. (2011). Evaluation of salivary melatonin measurements for dim light melatonin onset calculations in patients with possible sleep–wake rhythm disorders. Clin. Chim. Acta. 412, 1616–1620. doi: 10.1016/j.cca.2011.05.014
Kivelä, L., Papadopoulos, M. R., and Antypa, N. (2018). Chronotype and psychiatric disorders. Curr. Sleep Med. Rep. 4, 94–103. doi: 10.1007/s40675-018-0113-8
Koopman-Verhoeff, M. E., Gredvig-Ardito, C., Barker, D. H., Saletin, J. M., and Carskadon, M. A. (2020). Classifying pubertal development using child and parent report: comparing the pubertal development scales to tanner staging. J. Adol. Health. 66, 597–602. doi: 10.1016/j.jadohealth.2019.11.308
Kuhlman, K. R., Chiang, J. J., Bower, J. E., Irwin, M. R., Seeman, T. E., McCreath, H. E., et al. (2020). Sleep problems in adolescence are prospectively linked to later depressive symptoms via the cortisol awakening response. Dev. Psychopathol. 32, 997–1006. doi: 10.1017/S0954579419000762
Kuula, L., Halonen, R., Lipsanen, J., and Pesonen, A. K. (2022). Adolescent circadian patterns link with psychiatric problems: a multimodal approach. J. Psychiatr. Res. 150, 219–226. doi: 10.1016/j.jpsychires.2022.03.056
Levandovski, R., Sasso, E., and Hidalgo, M. P. (2013). Chronotype: a review of the advances, limits and applicability of the main instruments used in the literature to assess human phenotype. Trends Psychiatr. Psychother. 35, 3–11. doi: 10.1590/S2237-60892013000100002
Lewy, A. (1999). The dim light melatonin onset, melatonin assays and biological rhythm research in humans. Neurosignal. 8, 79–83. doi: 10.1159/000014573
Lewy, A. J., Lefler, B. J., Emens, J. S., and Bauer, V. K. (2006). The circadian basis of winter depression. Proc. Nat. Acad. Sci. 103, 7414–7419. doi: 10.1073/pnas.0602425103
Logan, R. W., and McClung, C. A. (2019). Rhythms of life: circadian disruption and brain disorders across the lifespan. Nat. Rev. Neurosci. 20, 49–65. doi: 10.1038/s41583-018-0088-y
Lovato, N., and Gradisar, M. (2014). A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med. Rev. 18, 521–529. doi: 10.1016/j.smrv.2014.03.006
Lovato, N., Micic, G., Gradisar, M., Ferguson, S. A., Burgess, H. J., Kennaway, D. J., et al. (2016). Can the circadian phase be estimated from self-reported sleep timing in patients with delayed sleep wake phase disorder to guide timing of chronobiologic treatment? Chronobiol. Int. 33, 1376–1390. doi: 10.1080/07420528.2016.1220386
Lovibond, S. H., and Lovibond, P. F. (1995). Manual for the Depression Anxiety Stress Scales, 2nd Edn. Sydney: Psychology Foundation.
Lucas-Thompson, R. G., Crain, T. L., and Brossoit, R. M. (2021). Measuring sleep duration in adolescence: comparing subjective and objective daily methods. Sleep Health 7, 79–82. doi: 10.1016/j.sleh.2020.06.005
Lucien, J. N., Ortega, M. T., and Shaw, N. D. (2021). Sleep and puberty. Curr. Opin. Endocr. Metab. Res. 17, 1–7. doi: 10.1016/j.coemr.2020.09.009
Malone, S. K., Zemel, B., Compher, C., Souders, M., Chittams, J., Thompson, A. L., et al. (2016). Social jet lag, chronotype and body mass index in 14–17-year-old adolescents. Chronobiol. Int. 33, 1255–1266. doi: 10.1080/07420528.2016.1196697
Mathew, G. M., Li, X., Hale, L., and Chang, A. M. (2019). Sleep duration and social jetlag are independently associated with anxious symptoms in adolescents. Chronobiol. Int. 36, 461–469. doi: 10.1080/07420528.2018.1509079
McGorry, P. D., and Mei, C. (2018). Early intervention in youth mental health: progress and future directions. BMJ Ment. Health 21, 182–184. doi: 10.1136/ebmental-2018-300060
Meliska, C. J., Martínez, L. F., López, A. M., Sorenson, D. L., Nowakowski, S., Parry, B. L., et al. (2011). Relationship of morningness–eveningness questionnaire score to melatonin and sleep timing, body mass index and atypical depressive symptoms in peri- and post-menopausal women. Psychiatry Res. 188, 88–95. doi: 10.1016/j.psychres.2010.12.010
Meltzer, L. J., Montgomery-Downs, H. E., Insana, S. P., and Walsh, C. M. (2012). Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 16, 463–475. doi: 10.1016/j.smrv.2011.10.002
Mokros, Ł., Nowakowska-Domagała, K., Koprowicz, J., Witusik, A., and Pietras, T. (2021). The association between chronotype and suicidality among students of the medicine and psychology faculties – the mediating role of general mental health indices. Chronobiol. Int. 38, 509–517. doi: 10.1080/07420528.2020.1865393
Murray, J. M., Sletten, T. L., Magee, M., Gordon, C., Lovato, N., Bartlett, D. J., et al. (2017). Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep 40, zsw002. doi: 10.1093/sleep/zsw002
Nicolaides, N. C., Vgontzas, A. N., Kritikou, I., and Chrousos, G. (2020). “HPA axis and sleep,” in Endotext, eds K. R. Feingold, B. Anawalt, M. R. Blackman, et al. (South Dartmouth, MA: MDText.com, Inc.). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK279071/
Ojio, Y., Nishida, A., Shimodera, S., Togo, F., and Sasaki, T. (2016). Sleep duration associated with the lowest risk of depression/anxiety in adolescents. Sleep 39, 1555–1562. doi: 10.5665/sleep.6020
Owens, J. A., Dearth-Wesley, T., Lewin, D., Gioia, G., and Whitaker, R. C. (2016). Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics 138, e20161406. doi: 10.1542/peds.2016-1406
Palmer, C. A., and Alfano, C. A. (2017). Sleep and emotion regulation: an organizing, integrative review. Sleep Med. Rev. 31, 6–16. doi: 10.1016/j.smrv.2015.12.006
Pandi-Perumal, S. R., Smits, M., Spence, W., Srinivasan, V., Cardinali, D. P., Lowe, A. D., et al. (2007). Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog. Neur. Psychopharmacol. Biol. Psychiatr. 31, 1–11. doi: 10.1016/j.pnpbp.2006.06.020
Paruthi, S., Brooks, L. J., D'Ambrosio, C., Hall, W. A., Kotagal, S., Lloyd, R. M., et al. (2016). Recommended amount of sleep for pediatric populations: a consensus statement of the American academy of sleep medicine. J. Clin. Sleep Med. 12, 785–786. doi: 10.5664/jcsm.5866
Pasch, K. E., Laska, M. N., Lytle, L. A., and Moe, S. G. (2010). Adolescent sleep, risk behaviors, and depressive symptoms: are they linked? Am. J. Health Behav. 34, 237–248. doi: 10.5993/AJHB.34.2.11
Petito, A., Pop, T. L., Namazova-Baranova, L., Mestrovic, J., Nigri, L., Vural, M., et al. (2020). The burden of depression in adolescents and the importance of early recognition. The J. Pediatr. 218, 265–267. doi: 10.1016/j.jpeds.2019.12.003
Pfeifer, J. H., and Allen, N. B. (2021). Puberty initiates cascading relationships between neurodevelopmental, social, and internalizing processes across adolescence. Biol. Psychiatr. 89, 99–108. doi: 10.1016/j.biopsych.2020.09.002
Plemmons, G., Hall, M., Doupnik, S., Gay, J., Brown, C., Browning, W., et al. (2018). Hospitalization for suicide ideation or attempt: 2008–2015. Pediatrics 141, e20172426. doi: 10.1542/peds.2017-2426
Power, S., Taylor, C., and Horton, K. (2017). Sleepless in school? The social dimensions of young people's bedtime rest and routines. J. Youth Stu. 20, 945–958. doi: 10.1080/13676261.2016.1273522
Preece, D. A., Becerra, R., Robinson, K., and Gross, J. J. (2020). The emotion regulation questionnaire: psychometric properties in general community samples. J. Pers. Assess. 102, 348–356. doi: 10.1080/00223891.2018.1564319
Quante, M., Kaplan, E. R., Cailler, M., Rueschman, M., Wang, R., Weng, J., et al. (2018). Actigraphy-based sleep estimation in adolescents and adults: a comparison with polysomnography using two scoring algorithms. NSS 10, 13–20. doi: 10.2147/NSS.S151085
Randler, C., Faßl, C., and Kalb, N. (2017a). From Lark to Owl: developmental changes in morningness-eveningness from new-borns to early adulthood. Sci. Rep. 7, 45874. doi: 10.1038/srep45874
Randler, C., Schredl, M., and Göritz, A. S. (2017b). Chronotype, Sleep Behavior, and the Big Five Personality Factors. SAGE Open. 7, 2158244017728321. doi: 10.1177/2158244017728321
Roberts, R. E., and Duong, H. T. (2017). Is there an association between short sleep duration and adolescent anxiety disorders? Sleep Med. 30, 82–87. doi: 10.1016/j.sleep.2016.02.007
Robertson, E. B., Skinner, M. L., Love, M. M., Elder Jr, G. H., Conger, R. D., Dubas, J. S., et al. (1992). The pubertal development scale: a rural and suburban comparisON. The J. Early Adol. 12, 174–186. doi: 10.1177/0272431692012002003
Robillard, R., Carpenter, J. S., Rogers, N. L., Fares, S., Grierson, A. B., Hermens, D. F., et al. (2018). Circadian rhythms and psychiatric profiles in young adults with unipolar depressive disorders. Transl. Psychiatr. 8, 1–8. doi: 10.1038/s41398-018-0255-y
Rumble, M. E., McCall, W. V., Dickson, D. A., Krystal, A. D., Rosenquist, P. B., Benca, R. M., et al. (2020). An exploratory analysis of the association of circadian rhythm dysregulation and insomnia with suicidal ideation over the course of treatment in individuals with depression, insomnia, and suicidal ideation. J. Clin. Sleep Med. 16, 1311–1319. doi: 10.5664/jcsm.8508
Sadeh, A., Sharkey, M., and Carskadon, M. A. (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep 17, 201–207. doi: 10.1093/sleep/17.3.201
Short, M. A., Bartel, K., and Carskadon, M. A. (2019). “Chapter 32 - sleep and mental health in children and adolescents,” in Sleep and Health, ed. M. A. Grandne (London: Academic Press), 435–445.
Short, M. A., Booth, S. A., Omar, O., Ostlundh, L., and Arora, T. (2020). The relationship between sleep duration and mood in adolescents: a systematic review and meta-analysis. Sleep Med Rev. 52, 101311. doi: 10.1016/j.smrv.2020.101311
Simon, S. L., Diniz Behn, C., Laikin, A., Kaar, J. L., Rahat, H., Cree-Green, M., et al. (2020). Sleep and circadian health are associated with mood and behavior in adolescents with overweight/obesity. Behav. Sleep Med. 18, 550–559. doi: 10.1080/15402002.2019.1629444
Szabó, M. (2010). The short version of the depression anxiety stress scales (DASS-21): factor structure in a young adolescent sample. J. Adol 33, 1–8. doi: 10.1016/j.adolescence.2009.05.014
Tarokh, L., Saletin, J. M., and Carskadon, M. A. (2016). Sleep in adolescence: physiology, cognition and mental health. Neurosci. Biobehav. Rev. 70, 182–188. doi: 10.1016/j.neubiorev.2016.08.008
Tesler, N., Gerstenberg, M., and Huber, R. (2013). Developmental changes in sleep and their relationships to psychiatric illnesses. Curr. Opin. Psychiatr. 26, 572–579. doi: 10.1097/YCO.0b013e328365a335
Thun, E., Bjorvatn, B., Osland, T., Steen, V. M., Sivertsen, B., Johansen, T., et al. (2012). An actigraphic validation study of seven morningness-eveningness inventories. Eur. Psychol. 17, 222–230. doi: 10.1027/1016-9040/a000097
Tonetti, L., Fabbri, M., Martoni, M., and Natale, V. (2012). Circadian type and mood seasonality in adolescents. Psychiatr. Clin. Neurosci. 66, 157–159. doi: 10.1111/j.1440-1819.2011.02303.x
Treven Pišljar, N., Štukovnik, V., Zager Kocjan, G., and Dolenc-Groselj, L. (2019). Validity and reliability of the slovene version of the morningness-eveningness questionnaire. Chronobiol. Int. 36, 1409–1417. doi: 10.1080/07420528.2019.1651326
Twenge, J. M., Krizan, Z., and Hisler, G. (2017). Decreases in self-reported sleep duration among U.S. adolescents 2009–2015 and association with new media screen time. Sleep Med. 39, 47–53. doi: 10.1016/j.sleep.2017.08.013
Veatch, O. J., Keenan, B. T., Gehrman, P. R., Malow, B. A., and Pack, A. I. (2017). Pleiotropic genetic effects influencing sleep and neurological disorders. The Lancet Neurol. 16, 158–170. doi: 10.1016/S1474-4422(16)30339-8
Watts, A. L., and Norbury, R. (2017). Reduced effective emotion regulation in night owls. J. Biol. Rhyth. 32, 369–375. doi: 10.1177/0748730417709111
Wheaton, A. G. (2018). Short sleep duration among middle school and high school students — United States, 2015. MMWR 67, 1–10. doi: 10.15585/mmwr.mm6703a1
Winsler, A., Deutsch, A., Vorona, R. D., Payne, P. A., and Szklo-Coxe, M. (2015). Sleepless in fairfax: the difference one more hour of sleep can make for teen hopelessness, suicidal ideation, and substance use. J. Youth Adol. 44, 362–378. doi: 10.1007/s10964-014-0170-3
Yoon, Y., Eisenstadt, M., Lereya, S. T., and Deighton, J. (2023). Gender difference in the change of adolescents' mental health and subjective wellbeing trajectories. Eur. Child Adolesc. Psychiatr. 32, 1569–1578. doi: 10.1007/s00787-022-01961-4
Keywords: circadian alignment, DLMO, actigraphy, chronotype, mental health, depression, adolescence
Citation: Duraccio KM, Kamhout S, Wright ID, Rugh KE, Miskin J and Amdal M (2023) Multimodal assessment of circadian sleep health in predicting mental health outcomes in adolescents. Front. Sleep 2:1177878. doi: 10.3389/frsle.2023.1177878
Received: 02 March 2023; Accepted: 18 October 2023;
Published: 28 November 2023.
Edited by:
Helen S. Heussler, Children's Health Queensland, AustraliaReviewed by:
Brant Hasler, University of Pittsburgh, United StatesCopyright © 2023 Duraccio, Kamhout, Wright, Rugh, Miskin and Amdal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kara McRae Duraccio, a2FyYV9kdXJhY2Npb0BieXUuZWR1
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.