- Signal Analysis Research Group, Department of Electrical, Computer, and Biomedical Engineering, Ryerson University, Toronto, ON, Canada
Single-lead wearable electrocardiographic (ECG) devices for remote monitoring are emerging as critical components of the viability of long-term continuous health and wellness monitoring applications. These sensors make it simple to monitor chronically ill patients and the elderly in long-term care homes, as well as empower users focused on fitness and wellbeing with timely health and lifestyle information and metrics. This article addresses the future developments in single-lead electrocardiogram (ECG) wearables, their design concepts, signal processing, machine learning (ML), and emerging healthcare applications. A literature review of multiple wearable ECG remote monitoring devices is first performed; Apple Watch, Kardia, Zio, BioHarness, Bittium Faros and Carnation Ambulatory Monitor. Zio showed the longest wear time with patients wearing the patch for 14 days maximum but required users to mail the device to a processing center for analysis. While the Apple Watch and Kardia showed good quality acquisition of raw ECG but are not continuous monitoring devices. The design considerations for single-lead ECG wearable devices could be classified as follows: power needs, computational complexity, signal quality, and human factors. These dimensions shadow hardware and software characteristics of ECG wearables and can act as a checklist for future single-lead ECG wearable designs. Trends in ECG de-noising, signal processing, feature extraction, compressive sensing (CS), and remote monitoring applications are later followed to show the emerging opportunities and recent innovations in single-lead ECG wearables.
1 Introduction
As of 2016, the number of wearables in medical application markets was approximately 55 devices (Athavale and Krishnan 2017). While the lifestyle niche market had many wearables of around 200 devices (Athavale and Krishnan 2017). Both market segments are expanding at a faster rate bringing in the notion of “prevention is better than cure” through monitoring an individual’s vital signs and predicting whether their health is impacted (Borysiewicz 2009). The above ideology is what lead to the development of long-term unobtrusive remote monitoring devices. These devices can collect many sorts of physiological signals including; ECG (Lobodzinski and Laks 2012), Photoplethysmography (PPG) (Elgendi et al., 2019), blood pressure (Park et al., 2014), respiration rate (Se Dong et al., 2010) and body core temperature (Blank and Sinclair 2011). Long-term monitoring utilizes a longitudinal study approach where an individual’s signals are collected for a long period of time amounting to months or even years to determine risk factors and the chances of disease development (Caruana et al., 2015). This concept is the ultimate goal of long-term remote monitoring technology. Long-term remote monitoring may signify that single-lead ECG devices, such as the Zio Patch, can continually record unobtrusively or do frequent recordings, such as the Kardia and Apple Watch. The primary reason for remote monitoring is to acquire ECG signals without involving professionals or technicians in the placement of the sensors on the human body. By predicting/measuring illness risk variables, preventive interventions can be done to reduce deaths and disabilities. Additionally, as compared to curative healthcare, which focuses on treating patients in hospitals and incurs substantial expenditures for patients and governmental institutions, the role of preventive healthcare in an economy and society results in individuals having a higher income and health stock (Wang, 2018). For example, long-term ECG monitoring of up to 30 days has shown to be cost-effective with monitoring cryptogenic strokes of patients with stroke recurrence risk (Yong et al., 2016). With the advances in wearables, it is cheaper to implement preventive healthcare by monitoring people remotely leading to lower costs and burden on the healthcare system (Pallinet al., 2014; Selvaraj 2014).
One of the most significant physiological signals that health-tech wearables focus on acquiring is PPG. PPG is used to measure the blood flow of the heart by measuring the difference between blood volume changes on the skin through non-invasive light optical sensors. A pulse-oximeter is an example of a PPG device that obtains blood circulation information by measuring blood volumes changes at the tip of your fingers (Jubran 2015). This technology is used in many consumer applications for obtaining heart rate (HR) and monitoring overall health of individuals. Health-consumer based PPG devices include; iHeart, Apple Watch (Falter et al., 2019), Fitbit (Benedetto et al., 2018) and smartphones such as Samsung’s Galaxy smartphone with a dedicated PPG sensor (Askarian et al., 2019). Wearable technology companies focus on PPG for activity tracking and HR measurements due to its low cost (Blasco and Peris-Lopez 2018), mediocre accuracy (Castaneda et al., 2018), and high user-comfortability (Kuncoro et al., 2020). However, PPG lacks consistency and accuracy when compared to acquisition of other physiological signals such as ECG that offer HR, heart rate variability (HRV), and achieve long-term monitoring goals. PPG potential inaccuracies include; motion artifacts, diverse skin tones, and signal crossover (Bent et al., 2020). It is evident that even a 30% increase in human activity from its resting stage can cause probable inaccuracies in a PPG signal (Bent et al., 2020). Some of these inaccuracies can be mitigated through appropriate pre-processing and post-processing software techniques with a significant computational cost if performed through a small wearable device such as using Discrete Wavelet Transform (DWT) or complex demodulation algorithms for motion artifact cancellation (Bashar et al., 2018; Pollreisz and TaheriNejad 2019).
On the other hand, using ECG instead of PPG may alleviate some pain points revolving around accuracy and its suitability for long-term remote monitoring. ECG measures the electrical activity of the heart, defining it as the gold-standard for obtaining HR and using its information for medical decision making. Some pre-processing and post-processing techniques for ECG are computationally inexpensive which can be implemented on small wearable devices. A good example of a HR detection algorithm that uses ECG and has a high accuracy, robustness, and reliability in the clinical domain is the Pan-Tompkins algorithm (Liu et al., 2018). The Pan-Tompkins algorithm can be implemented through hardware making it an efficient algorithm for long-term remote monitoring that may need low-power device requirements (Pavlatos et al., 2003). However, other issues with ECG that cannot be mitigated easily such as the lack of user-comfortability and the presence of skin rash caused by using wet-electrodes for extended periods of time. Currently, there are dry electrode solutions for this problem and can overcome it easily without hampering signal quality (Meziane et al., 2013; Chlaihawi et al., 2018; Arquilla et al. 2020). ECG has a significant potential to be applied for long-term remote monitoring solutions (Lobodzinski 2013; Guo et al., 2016; Health Quality Ontario 2017). From using heart rate variability (HRV) metrics to predict mortality of patients after heart attacks, to detecting arrythmias post-surgery for stroke and elder patients, ECG can be a viable tool for preventive medicine through remote monitoring. Also, ECG can be used through wearable technology to accurately obtain HR and monitor overall health of health-oriented consumers to assist them in lifestyle-based decisions (Wilson and Laing 2018).
Single-Lead ECG
ECG measurements are well established and needs minimal training for first-time users. The electrical waveform propagates from one end of the heart to the other. This signal can be measured using electrical conductive electrodes that are placed in appropriate positions on the skin. The electrical current that travels throughout the heart can be detected on the skin non-invasively because the human body contains fluids with ions that allow for electrical conduction. The heart’s electrical signal may show small amplitude values in the range of microVolts. The graphical representation waveform of ECG is produced by computing the electrical potential difference between two electrodes. Therefore, electrode placement is an important factor in measuring ECG where different placements can yield different waveforms and shapes of the same signal. For example, a 3-lead system yields 3 different waveforms, identified as channels, representing the same electrical activity of the heart. Lead-I measures from the top right side of the body to the left, horizontally. Lead-II measures from the right side, at the same point as Lead-I, diagonally to the left side abdomen of the body. While Lead-III measures vertically between the upper left side downwards to the lower left side of the body. Each lead produces a different waveform containing variable electrophysiological information in each channel. Lead-II and III offer information pertaining to the inferior surface of the heart. While Lead-I offers lateral information (Meek and Morris 2002). The above described 3-lead system is identified as Einthoven’s triangle, named after William Einthoven, a physiologist and physicist who developed the first ECG machine. This work led to future improvements in detecting the electrical activity of the body.
Single-lead ECG systems use two electrodes to detect a single ECG signal. The placement of the electrodes is still important to determine the type of information that will be obtained from the system. For example, a single-lead, Lead-I, placed between the right and left arms of the body horizontally will show a different waveform shape of ECG signal when compared to a Lead-II ECG signal. Lead-II shows a higher R-peak than Lead-I making it more suitable for R-R interval detection and HR calculation. But both Lead I and II offer an upright P-wave that can be used in detection of certain cardiac illnesses (Meek and Morris 2002). On the other hand, signal amplitude can help assess other cardiac functions because it is related to myocardial mass, and the varying properties of the cardiac tissues (Meek and Morris 2002). The main advantages of single-lead ECGs are low-cost and increased user comfortability. A single-lead uses less hardware components than a 12-lead system. While the patient has an increase in comfort and motion because a single-lead system does not require many cumbersome attachments to the body. The patient will be able to move and function normally without the hassle of wires when a wireless patch of a single-lead ECG system is used. However, the significant disadvantage of single-lead ECGs is the lack of wholesome information in the medical diagnoses of heart illnesses. Some heart diseases cannot be detected by a single-lead, requiring more information from multiple angles of the heart which is offered in a 12-lead system. However, research has shown that the single-lead systems have great potential in becoming medically relevant with the advancements in microelectronics, biosensors, and optimized software algorithms (Samol et al., 2019b; Steinberg et al., 2019). Himmelreich et al. concluded that a smartphone-operated 1-lead ECG device, Kardia, had excellent accuracies in the detection of atrial fibrillation (AF) and moderate diagnostic accuracies for other arrhythmias in primary care populations, as shown in their work (Himmelreich et al., 2019).
The rest of the paper is structured as follows. Section 2 provides an in-depth look into some single-lead ECG wearables, Apple Watch, Zio by iRythm, Kardia by AliveCor and BioHarness by Zephyr. While Section 3 briefly offers the desirable characteristics of single-lead ECG devices for long-term remote monitoring. As for Section 4 the different single-lead ECG design consideration themes is discussed in-depth. Section 5 discusses the different trends in ECG denoising and filtering techniques in research and industry. Section 6 discusses the current and future progress in compressive sensing for single-lead ECG. While Section 7 goes in-depth in the current and future trends in single-lead ECG feature extraction techniques. Section 8 briefly show the future of machine learning algorithms in cardiac disorders classification applications. Sections 9 and 10 discuss the future trends and opportunities of remote single-lead ECG devices in clinical and wellness applications, respectively. In Section 11 the conclusion is presented. The goal of this paper is to take the reader through a journey from the device level in single-lead ECG to data processing and analysis that are used in decision making whether in clinical or wellness applications. The current shortcomings and the future trends are discussed in-depth to allow for a full picture of this research field.
2 Single-Lead Electrocardiographic Wearables
There are many devices in the single-lead ECG category, the following subsections cover the state-of-the-art wearables and their intended applications. It is important to note that these devices are not an exhaustive list of all wearables that are currently researched and developed but a shortlist of those that have already shown success and feasibility in real-world contexts as shown in Table 1. Other devices not included ECG247, QuardiaCore, Max-ECG Monitor and Frontier X.
Apple Watch
The Apple Watch series 4 contains stainless steel electrodes placed at the bottom and side of the watch. These metal points act as the 2 electrodes used in single-lead ECGs. Samol et al. in their work explore the use of an Apple Watch as a viable diagnostic tool used by clinicians in medical decision making (Samol et al., 2019b). The study shows that the quality of a single-lead ECG from a smartwatch can be medically relevant, making the Apple Watch a suitable device for patients to self-measure their ECGs remotely. Their results revealed the robustness of the Apple Watch’s ECG software in removing noise, such as EMG and power-line interference, and amplifying the ECG signal for clear detection and recording.
However, the Apple Watch can only record up to 30 s of ECG and can send the data wirelessly to the physician using WiFi. This short recording period is not suitable for post-surgery long term (<24 h) ECG monitoring. Other single-lead ECGs uptake this role of post-operation remote monitoring by offering long-term continuous ECG recordings lasting more than 24 h such as Holter monitors, and Zio, the wireless ECG patch (Barrett et al., 2014, 24). The apple-watch may also require patient pre-training to be used appropriately in placing the electrodes in the optimum positions for single-lead detection as specified by Samol et al. for Wilson-like leads (Samol et al., 2019b).
The Apple Watch has a small form factor and its ECG software is currently FDA-cleared as a class II medical device for the detection of AF for persons above the age of 22 (Kruger 2018). The Apple Watch’s ECG sampling frequency and bit resolution information are not readily available acting as a limitation to this study review. The device also cannot be used for the detection of a recurrent AF in patients, it can only be used in the recognition of the first AF of a person (Kruger 2018).
AliveCor Kardia
AliveCor’s Kardia mobile application and hardware single-lead ECG, with stainless steel electrodes, brought significant attention to the viability of single-lead ECGs in telemedicine. It requires the person to place his left- and right-hand fingers on the conductive pads and wait for 30 s. This process measures Lead-I ECGs. The hardware must be placed close to a Bluetooth-enabled device and the recording is initiated through the Kardia app. The signal can be seen in real-time. After the recording is complete, the app analyzes the 30 s ECG recording for AF and provides the filtered signal, HR in BPM, and the overall results to the individual. Lua et al. in their work uses the Kardia device and app to detect silent AFs remotely in patients with a previous history of strokes in the Netherlands (Lau et al., 2013). Kardia became part of a community screening program for stroke prevention by early detection of AF (Selder et al., 2019). The researchers conclude that a single-lead ECG placed with a smart phone is suitable for community screenings remotely due to the abundance and widespread of smartphones. This research exemplifies the significant potential of Kardia to be used by the medical community for the detection of AFs using Lead-I ECG measurements.
Another journal article by Marinucci et al. examines the quality of Kardia-based ECG recordings and its feasibility in the implementation of an artificial neural network (ANN) for AF detection (Marinucci et al., 2020). Their results are significant because an ANN can be seen a potential tool for reliable AF detection from short-duration ECG recordings acquired by Kardia. This work shows the promising future of Kardia as a reliable single-lead ECG data acquisition module with high SNR ECG signals that can be used by medical artificial intelligence and in clinical diagnosis.
Kardia is an FDA-cleared small hand-held device that can fit in a pocket. The device features a battery life for 200 h of operational time, a lightweight of 18 g, 16-bit ECG resolution and a 300 Hz sampling rate for data acquisition. While the software utilizes edge computing, which analyses the patient’s 30 s ECG data using the smartphone’s processor. Edge computing is described as computations performed on an access point rather than in the cloud (Qureshi and Krishnan 2018). The results of the ECG recordings are transmitted to a secure server on the cloud for physicians to access. Kardia’s disadvantage include the short recording time of ECGs of 30 s which is not suitable for long-term continuous monitoring, and the usage of a separate hardware module. Also, Kardia requires the user to relax and record their ECG signals in a steady and seated position. This process is unsuitable for active individuals who want to monitor their HR during and after physical activities. Nevertheless, Kardia can be used by laypersons to monitor their heart health. It is evident that the intended purpose of this device is focused on patients with heart problems that can be detected using a single-lead ECG such as AF. It is important to note that Kardia also offers a 6-lead hardware, KardiaMobile 6L, that has 3 electrodes and obtains Lead I,II,III, aVF, aVR, and aVL. The third electrode can be found at the bottom of the module where it is required to be placed on your left leg, acting as a reference point similar to Einthoven’s triangle. The details of this device is beyond the scope of this paper.
iRhythm’s Zio Patch
Lastly, Zio by iRhythm, a company specializing in ambulatory cardiac monitoring, is a single-lead ECG wearable patch that is attached to the body for continuous unobtrusive ECG monitoring lasting up to 14 days. The Zio patch measures Lead- II ECGs (Vosslers 2017). Zio is an FDA-approved Class-II medical device that is prescription based. The device features 10-bit ECG resolution, 200 Hz sample rate for data acquisition, a symptom trigger button, water-resistance, and 2 lithium coin cell batteries for a battery life of 14 days (Fung et al., 2015). After 14 days, the patient mails the Zio patch to a processing center where iRhythm uses proprietary machine-learning algorithms to analyze ECG recordings for heart symptoms including AF, and irregular heartbeats. Turakhia et al. also showed how Zio leads to higher diagnostic yields of detecting arrhythmia types (Turakhia et al., 2013). The extended continuous ECG monitoring using Zio offered more medically relevant information to physicians for medical decision making such as AF management and treatments than Holter monitors.
iRhythm’s Zio processing service uses a deep neural network (DNN) for the classification and detection of arrythmia’s in ambulatory ECGs. Hannun et al. explore Zio’s DNN in arrhythmia classification (Hannun et al., 2019). The authors analyzed 91,232 single-lead ECGs from 53,549 patient who used Zio’s single-lead ECG device (Hannun et al., 2019). Their algorithm outperformed cardiologists in classifying AF and other arrhythmias. For AF, the DNN algorithm had a sensitivity of 86.1% compared to the cardiologists’ 71.0%. iRhythm’s DNN algorithm is FDA-cleared to be used with the Zio patch (Hannun et al., 2019). The company developed Zio AT, a service product that uses the DNN algorithm in arrhythmia classifications, offering faster results than the normal Zio patch service mentioned above.
Zephyr’s BioHarness
Zephyr’s BioHarness is worth mentioning to provide a holistic image of upcoming single-lead ECG technology present in multi-purpose wearables. In sports, Zephyr’s BioHarness is used to monitor activity performance and the physiological features of athletes during intense workouts. This purpose is also intended in defense where the system is used in monitoring overall stress levels of military personnel in training. On the other hand, this device is used in ensuring the health and safety of first responders such as firemen and personnel in coal mine rescue operations. Zephyr’s BioHarness is a multi-module sensor system that acquires HR and HRV through ECG, breathing rate through respiratory signal and posture, activity, peak acceleration, and impact through accelerometer data. The ECG sampling rate is 250 Hz with a minimum and maximum bit resolution of 10 and 12 bits respectively (BioHarness 3.0 User Manual, 2012). The device is attached through a chest strap and has an active battery life between 2 and 24 h between charges based on whether the raw signals are transmitted through Bluetooth to a PC/smartphone or stored internally (BioHarness 3 Medical Data Sheet, 2012).
Zephyr’s BioHarness has shown its reliability in monitoring healthy individuals in intense physical activity. Nazari et al. examined the BioHarness and Fitbit charge’s reliability in obtaining approximate HR values during rest, a fitness test and recovery (Nazari et al., 2019). The authors concluded that Zephyr’s BioHarness and Fitbit Charge are reliable wearables in obtaining consistent and stable HR measurements that can be cross referenced over a number of sessions in sports monitoring applications (Nazari et al., 2019). However, in clinical applications the BioHarness showed disadvantages. Nepi et al. showed that Zephyr’s BioHarness may not be suitable for clinical applications due to its unreliable HRV measurements (Nepi et al., 2016). For sports applications, the authors concluded that mean HR calculated for each subject is highly correlated to the HR signal obtained through the BioHarness. While HRV measurements recorded by BioHarness were found to be unreliable for clinical applications. Nepi et al. concluded that the BioHarness may not be suitable for general population cardiac-risk evaluation but may be complementary to other clinical ECG acquisition modules in the detection of sudden cardiac death in high-risk individuals (Nepi et al., 2016). Both studies show how Zephyr’s BioHarness is suitable for sport applications, and consumer-like fields rather than clinical applications involving HRV measurements.
Bittium Faros 360
Bittium Faros is a reusable ECG monitor that can operate as a single-lead or a multi-lead system. The device comprises of a hardware unit and detachable electrodes. There are 3 types of hardware units 90, 180, and 360 eMotion Faros. For this study, the 360 eMotion Faros device will be examined because it contains all features present in the other two devices and is the highest grade that Bittium offers for cardiac monitoring. It can operate as a single-lead or a 3 lead multielectrode configuration depending on the physician’s recommendations for patients. The device is waterproof, wireless through Bluetooth and can record up to 180 days in memory. However, the system has a battery life of up to 7 days and requires a 1-h recharging time. ECG sampling rate can be varied and can be adjusted up to 1,000 Hz with a resolution of 24 bits analog to digital converter (ADC). Also, it features a 3-axis accelerometer that can have a sampling rate of up to 100 Hz. It is important to note that Bittium Faros is an FDA-cleared class-II medical device that is prescription based.
Bittium Faros has been used in many studies ranging different research fields. In some recent studies, it is used as the criterion standard because of its ability to produce high quality ECG signals. A review study examined the potential of using the device as a wearable for military field action operators due to its high resolution and ability to transmit and store HRV data for later analysis (Hinde et al., 2021). The authors concluded that when compared to other ECG and PPG based wearables, Bittium Faros is a likely candidate for remote monitoring of military personnel (Hinde et al. 2021). While another study examined the use of Bittium Faros and other wearables to monitor patients remotely (Godkin et al., 2021). The study performed in Sunnybrook Health Sciences Centre, Toronto showed that subject adherence was high with Bittium Faros and is achievable in a multi-sensor system for patient remote monitoring (Godkin et al., 2021).
Bradydx’s Carnation Ambulatory Monitor
CAM is a continuous single-lead ECG wearable patch that is placed directly on the sternum. The patch can be worn up to 14 days like Zio. This class-II FDA cleared device features an event recorder button to be pressed when a patient undergoes any cardiac symptoms. CAM is a water-resistant monitor and should not be submerged under water. The device is prescribed for patients with known cardiac problems and is used to monitor their heart activity remotely. CAM uses a sampling rate of 171 Hz and examines a filtered signal ranging from 0.67 to 25 Hz. It uses two electrodes to record the augmented aVF single lead ECG behavior.
The main difference between CAM and other devices presented in this study is its P-wave centric approach in detecting cardiac arrythmias. Generally, P-wave cannot be characterized clearly in ECG recordings using single-lead ECG patches due to the short inter-electrode distance between 2 electrodes. This problem is mitigated in CAM by increasing the inter-electrode distance to 8.89 cm placed across the sternum. In a clinical investigation the authors compared the diagnostic capabilities of CAM to a standard 3-lead Holter monitor (Smith et al., 2017). CAM showed a high positive correlation of PR, QRS and QT wave intervals with Holter intervals with coefficients of 0.93, 0.86, and 0.94, respectively. The 50 patient study concluded that CAM yielded more diagnostic quality ECG, was easy to use, and comfortable to wear, where 86% of subjects preferred CAM over Holter (Smith et al., 2017). The diagnostic yield showed that CAM identified arrythmias in 46% of patients that altered their care management compared to 12% with Holter (Smith et al., 2017). Some studies compared CAM to other single-lead ECG patches such as Zio XT to show the benefits of P-wave and its role in improving signal quality (Rho et al., 2018). The study explored the difference in ECG signal clarity and showed CAM to detect more distinct arrythmias than Zio XT. However this study was limited to the small sample size of 30 patients with a mean age of 73 years old (Rho et al., 2018). Also, it is important to note that P-wave based arrythmia detection still requires further research due to the limiting small sample sizes present in many P-wave indices studies and the lack of a standardized protocol in P-wave signal acquisition and analysis (Magnani et al., 2009).
3 Single-Lead ECG Design Factors
A 4-axis concept is proposed here that explores the specific design factors of single-lead ECG wearables for long term remote monitoring. These factors include wireless capabilities, power consumption, real-time factor, weight, recording time and form factor of the device under the umbrella of 4 dimensions, power requirements, quality of signal, computational complexity, and human factors, as shown in Table 2 summarizing the main points behind the proposed design factors. Each dimension takes into consideration the overall single-lead ECG system and the detailed components used in its design. The power requirements dimension is important in single-lead ECG designs for very long-term remote monitoring because of the reliance on light-weight batteries for all the device’s operational needs. While computational complexity dimension mainly focuses on the real-time capabilities of the device. This dimension is crucial for single-lead ECG wearable designs because it determines the speed and accuracy of algorithms used to detect ECG abnormalities that can underly cardiac diseases and cause sudden death. Also, the quality of the ECG signal is an important dimension. A good quality signal will offer a clear ECG waveform that can assist doctors in their diagnosis when compared to a low-quality signal (Kligfield et al., 2007; Satija et al. 2017). A clear noise-free signal would also improve output accuracies of HR and Arrythmia detection algorithms. The last dimension, human factors explore the comfortability of a device in use. Highly comfortable ECG wearable devices have a high user compliance rate which is important in long-term remote monitoring. A high compliance will yield more accurate results for medical professionals to analyze (Tai Wong et al., 2019).
4 Single-Lead ECG Design Considerations
In power requirements, the single-lead wearable should not require high power consumption, uses low-power density batteries, and does not operate on power intensive hardware. These factors are important and desired for continuous long-term remote monitoring. Low power consumption saves on battery power which increases the longevity of the device in operation. For example, Zio achieves this factor as evident by its continuous operation for up to 14 days. On the other hand, low energy density batteries help in achieving the small form factor and lightweight features of the wearable. Higher density batteries are heavier which can add up to the wearable’s weight causing its unbearableness during usage but offer more power that can be used in computations. This factor relates to both power requirements, human factors, and computational complexity. Low-density batteries may not be suitable for processing power hungry complex algorithms. However, low power density power sources are a desired factor to limit the not so considered excessive power consumption of hardware modules and help designers focus on developing low-power, low-complex algorithms and choosing power-efficient hardware components. Lastly, the oscillating frequency of microcontroller units (MCUs) is considered in power requirements, and computational complexity to show how power consumption and digital algorithms are related to one another through MCUs and their hardware limitations.
Computational complexity factors are highly related to power requirements. Highly complex algorithms require significant computational resources such as high oscillating frequencies of MCUs, which in turn increases power consumption. Therefore, by limiting frequency oscillations to a few MHz, the designer is limited to developing low-complex algorithms that take into consideration the Big O Notation, the order of mathematical equations. For example, if the Big O Notation is limited to constant-time algorithms,
For signal quality, it is desirable to get signals that are noise-free and can be used in clinical and consumer settings alike. 10 consecutive QRS complexes is chosen as a good indicator of clinical grade ECG quality is due to previous research in the field that reproduced this factor. Samol et al. were able to use this indicator consistently, by reproducing it in different studies (Samol et al., 2019a; Samol et al., 2019b). This process has shown its reliability as an indicator of good ECG signal quality of wearable based ECG systems that can be used in clinical diagnosis. While a minimum of 12-bit resolution ECG show great amplitude changes because it offers in-depth amplitude information that has shown promise as presented in multiple studies working with ECG (Narayanaswamy, 2002). For example, Narayanaswamy can obtain clinically significant health information using a 12-bit ADC and a sampling frequency of 1,000 Hz (Narayanaswamy 2002). While the choice of providing a minimum of 125 Hz for sampling frequency is based on the work where authors showed that algorithms that functioned with at least 125 Hz ECG signal yielded less inaccuracies (Ajdaraga and Gusev, 2017). Higher sampling frequencies of more than 125 Hz is recommended for clinical applications, especially for the detection of arrythmias which may require a minimum of 1,000 Hz sampling rate (Abboud and Barnea 1995). Some frequency ranges that are used significantly in clinical literature include 250 Hz, 500 and 1,000 Hz. While the last factor that impacts signal quality is motion artifacts. Motion artifacts are an unavoidable element that creates difficulties for researchers and developers alike when acquiring ECG signals. Reduced motion artifacts during signal capture play a significant role in the total preprocessing, analysis, and postprocessing stages required in an ECG system, which can lead to reduced power consumption and improved signal quality. Therefore, it is important for single-lead ECG wearable developers to consider the fixation of wearables on users as to limit motion artifacts while also maintaining good quality ECG signals.
Human factors are a subjective measure that can be further improved upon by examining the presence of rash or severe skin marks caused by the single-lead ECG wearables. The human factors dimension is highly user subjective which may require developers to approach this factor through prototyping, field testing and pilot studies. However, it is still an important dimension to consider in any single-lead ECG design because compliance rate can impact user wear time which in turn relates to the amount and duration of signals that can be analyzed for clinical diagnosis. After having looked into the design considerations for single-lead ECG signals, in the next sections, data consideration and algorithmic challenges and opportunities will be discussed in detail.
5 Trends in Filtering and Denoising of Long-Term ECG Data
ECG like other electrophysiological signals encounter noise that can ruin the quality and in turn the diagnostic value of the recordings. Signal processing and noise filtering techniques become a paramount step in any ECG analysis algorithm that helps to extract a clear PQRST waveform that can be used in all sorts of applications including HRV estimation, HR, arrythmia detection, and general wellbeing monitoring. The most common noise that can be found with an ECG signal include baseline wander, motion artifacts, electromagnetic interference (EMI), electrode contact, muscle contraction and other unwanted electrophysiological noise. Baseline wandering is a low frequency noise mainly caused by respiration. Its frequency can range from 0.15 Hz to over a few Hz (Kher. 2019). While motion artifacts are caused by body movements. Motion artifacts can be of low and/or of high frequency nature depending on the physical activity of the subject. Up to this date, a robust solution for eliminating motion artifacts is still elusive. On the other hand, EMI is one of the first noise sources that are eliminated in a signal processing technique. EMI can be power-line interference noise characterized by the 50/60 Hz produced from power sources such as electric outlets. Also, EMI include electromagnetic waves produced by electronic devices such as cellphones that interfere with the highly sensitive ECG processing circuitry. Muscle contractions create EMG signals that interferes with ECG in the same frequency bands. EMG are high frequency signals. Lastly, electrode contact noise is caused by the movement of electrodes relative to the skin contact surface. The noise is apparent with high amplitudes in an ECG that look like R-peaks but larger (Kher, 2019). This type of noise is a main contributor to the false detection of R-peaks for HR detection.
Signal filters can come in many types such as digital, analog, mechanical and optical. The focus for this work will be digital and analog filters that are highly applicable in long-term ECG signal processing. Digital filters are approaches performed in software, and they are designed and implemented in general purpose computers or in specialized hardware. Some of the most prominent digital filters used for ECG and other electrophysiological signals include Butterworth, Chebyshev, Bessel, Elliptic, and Gaussian filters. While analog filters for ECG manipulate the electrical signal of the heart directly. Analog filters can be designed using resistors, capacitors, and general-purpose electronics. ECG signal filtering is becoming a saturated field of research. Since early 1980s, research have examined different approaches and filtering techniques that up to this date are considered optimal for application use. A good example is the Pan-Tompkins algorithm, developed in 1985. The algorithm features a band-pass filter, by cascading low and high pass filters, that eliminates most EMI noise and is considered the golden standard for HR detection (Pan and Tompkins 1985). Since then, researchers have examined different filter configuration, focusing on filter design, and optimizing its transfer function. The recent progress in ECG denoising currently revolves two aspects: hardware and software techniques. Hardware techniques can be attributed to hardware accelerators and their role in denoising by offering low-power circuitry that can eliminate EMI noise effectively without compromising signal quality and achieving a low-power consumption compared to software-based approaches (George et al., 2020). As for software denoising techniques, the goal is to improve on the signal using adaptive approaches that are highly accurate and can be used in numerous diagnoses of arrythmias and early detection of cardiac disease precursors in ECG. For example, Finite Impulse Response (FIR) filters are robust in eliminating most unwanted ECG noise that are actively used in many ECG analysis algorithms. But some researchers are examining the feasibility of simple filtering and data driven techniques in real-time denoising for long-term remote monitoring applications (Mukhopadhyay and Krishnan 2020).
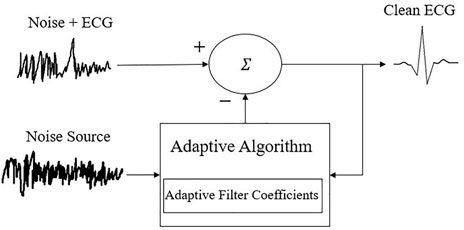
Many researchers examined the role of denoising and offered full comparisons between different techniques for ECG (Malghan and Kumar Hota, 2020). It is important to note that software approaches can also be performed on the cloud or through on-edge computing in access point devices such as smartphones. Band-pass filtering is the most prominent and simple method used to eliminate unwanted noise outside a certain frequency range. Useful information in ECG can be found in the range of a few Hz to around 100 Hz. However, noise can still exist in this frequency band. To eliminate the unwanted noise within the signal, notch filters are used to suppress a certain frequency. For example, a 50 Hz notch filter is used to eliminate power line interference noise. Other techniques include adaptive filters, DWT, and empirical mode decomposition (EMD). Adaptive filters are denoising approaches that improve by taking into consideration the error produced from the output signal and feeding that information back into the digital filter in developing more optimal filter coefficients for separating overlapping signal and noise components, as shown in Figure 1.
On the other hand, DWT is used in ECG filtering by separating the input signal into different frequency bands with unique resolutions. Multistage wavelet filtering involves wavelet decomposition of the input signal by dividing the signal into ‘N’ number of bands with different frequency resolutions (Malghan and Kumar Hota., 2020). Based on the noise level at hand, the N number of decomposed levels is determined. Also, wavelet can be used with soft and hard thresholding. He et al. developed an adaptive wavelet thresholding method (AWT) that enhances ECG signals (He and Tan 2018). Their algorithm produces automatically the most appropriate base wavelet for a signal by looking at cross correlation coefficients and the energy to entropy ratio. While Oliveria et al. used wavelets to eliminate power line interference without thresholding (Oliveira et al., 2018). Their algorithm was more robust and presented better results compared to notch filtering and other threshold approaches.
Wavelet requires a pre-selected basic function that is used in the initial breakdown of the signal. This issue is what lead to the rise of EMD based denoising algorithms that do not require any function initial configurations. EMD decomposes signals into M number of signals known as intrinsic mode functions (IMFs). Jain et al. developed an EMD based approach that separated the input signal into different IMFs (Jain, Bajaj, and Kumar 2018). The IMFs with the highest low-pass and high pass frequency noise are determined using recognition algorithms and underwent complex filtering. The de-noised IMFs, with the dominant IMFs, are later used in the reconstruction of the ECG signal (Jain et al., 2018). EMD, DWT and some adaptive filter techniques are not optimal for long-term remote monitoring due to the high complexity and computational cost.
Future trends in filtering techniques for single-lead long-term remote monitoring ECG should focus on power and some hardware considerations. In essence, simple arithmetic-based filtering such as low-order low-complexity filtering is optimal for these applications. Filters with a computational complexity of
6 Trends in Compressive Sensing for Long-Term ECG Data Collection
Compression helps in power constrained and bandwidth limited applications. Classical compression techniques are seen as a post-processing tool, where the compression happens after the signal has been captured. In the realm of compressive sensing (CS), the compression happens at the acquisition phase itself. Classical compression algorithms can be either lossy or lossless approaches. Lossy algorithms lose information during encoding which cannot be retrieved later. To mitigate this issue with lossy algorithms, innovation in low-power cost signal reconstruction algorithms is examined due to its vital importance in extracting all the information needed appropriately. While lossless compression algorithms do not lose any information during encoding and allow for full information recovered at signal reconstruction. Lempel-Ziv Welch and Huffman coding are two lossless algorithms that show good CR without losing any of the ECG cardiac information but are not optimum compared to lossy methods. Some researchers examined a hybrid of both types to achieve high CR, with minimum information loss and increase the battery life of the device.
Arrythmia detection is an important area for long-term remote monitoring applications. Accurate automatic detection helps physicians identify arrythmias and allow for large amounts of recorded ambulatory ECG to be scanned quicker and easier for an efficient timely prognosis. The long-term nature of ECG remote monitoring requires large amounts of storage and wirelessly transmitting it for cloud computing. This concept takes a significant amount of battery power which impacts the device’s overall longevity. To alleviate this issue, researchers undertook the approach of compressing ECG data into small sets that can be stored and processed directly on the device. CS are algorithms that help decrease the size of the data while maintaining its integrity to be decoded later. This process decreases the total amount of data points being transmitted wirelessly which allows for an increase in battery longevity, as shown in Figure 2. A good example of classical compression scheme is the DWT that is being used to compress images.
The current widely accepted approach in ECG compression is digital wavelet transform based algorithms (DWT). These algorithms are split into embedded zerotree wavelet (EZW), set partitioning in hierarchical trees (SPIHT), and thresholding-based approaches (Hilton 1997; Lu et al., 2000). EZW is a lossy compression algorithm that is mainly used for images but has seen its role in other applications such as biomedical signal compressions. While SPIHT examines the fundamental similarities present between the different frequency ranges in a wavelet decomposition. Lastly, the thresholding-based algorithms uses wavelet decomposition and focuses on iterating the resultant coefficients through a threshold until a fixed number target of wavelet coefficients are zeroed. All three approaches require DWT that is complex in nature which takes up a lot of memory and computational resources. These approaches can be found in hospital ECG machines. For long-term remote monitoring, DWT is not feasible if battery efficiency and memory are prioritized.
To eliminate this issue, researchers examined different CS algorithms dependent on the ECG periodical form to eliminate redundant information to be stored efficiently on the device (Mamaghanian et al., 2011). The sparsity of the signal is highly relevant to most CS approaches including DWT based algorithms. Sparsity in simple terms can be defined as a signal that has an S amount of non-zero elements is said to be S-sparse. The idea revolves around that if a signal is represented is an S-sparse form such as wavelet coefficients, the signal can be reconstructed without taking into consideration the Nyquist sampling theorem of the signal. This process in turn is the founding concept behind CS and helps in lowering the computational costs of performing operations on the signal. An approach developed by Mamaghanian et al. utilizes sparse binary sensing matrices to execute the matrix multiplications of CS. The algorithm is as follows; linear transformation by implementing a sub-gaussian random matrices using sparse binary sensing, removing interpacket redundancy defined as removing the redundant information presented in packets before encoding, and Huffman encoding to develop the codebook to be transmitted wirelessly or be stored using less memory on the device (Mamaghanian et al., 2011). The authors later performed a comparative analysis of both their CS and the accepted DWT algorithm and found out that DWT outperforms the developed CS, 90% compression ratio (CR) vs. 71%, respectively for good reconstruction quality ECG. However, code execution time in milliseconds (ms) dropped from 580 ms for DWT to 25 ms, appropriate for real-time compression. Overall, the device’s battery longevity increased by 37.1% amounting to 146.8 h compared to DWT’s life time of 107.05 h using 280 mAh at 3.7 V (Mamaghanian et al., 2011).
Other researchers examined ECG sparsity differently. Pant and Krishnan extracted the second-order difference of sparsity and with using dictionary learning developed a reconstruction algorithm that is highly accurate (Pant and Krishnan 2014). The algorithm is compared to other highly accepted approaches such as 1d-lp regularized least squares and some Bayesian learning methods. Average SNR values for their proposed algorithms were higher compared to reconstructed signals from state of the art. As for CPU runtime, the dictionary learning approach took 1,244 s–3,824 s depending on the size of the original and reconstructed signals (Pant and Krishnan 2014). The approach was developed on a PC however the authors argue that due to the simple arithmetic calculations of this algorithm, the method can be implemented on specialized hardware architectures such as Field Programmable Gate Arrays (FPGAs).
It is important to note that the future for single-lead ECG devices will prioritize battery life, small form-factor and automated arrythmia detection. CS is becoming an important cornerstone to lower the sampling rate, memory size, and wireless transmission power costs. The trend in ECG compression revolves around CS and using the sparsity of the signal. Signal sparsity detection and implementation is the main tendency to mitigate the classical Nyquist theorem sampling rate. In turn, the hope revolves around collecting the ECG signal using a low sample rate, below the conventional Nyquist rate that the theorem states, saving on computational resources and thereby prolonging battery life.
7 Trends in Feature Extraction From ECG Data
An input signal can contain many features that make it unique and can be used in classification, and analysis easily. The main goal of many signal analysis algorithms is defining a pattern or a set of values that can help the user to determine the signal or part of a signal at hand. Feature extraction is the process of identifying these values that are hidden within the acquired ECG that can have significant use to both analysis and ML applications. Krishnan et al. offers a detailed overview of biomedical signal feature extraction techniques showing the most common and vital processes used by researchers (Krishnan and Athavale 2018). Feature types are split into 4 different domains defined in its signal processing development over the past 70 years: time domain, frequency domain, joint time-frequency, signal decomposition and sparse domain features. Time domain features are defined as characteristics relating to the change of the signal over time. Signal statistical features such as mean, variance and standard deviation are time domain based. Time domain features yield general temporal information about the signal which may not be optimal for specific analysis applications such as personalized automatic arrhythmia detection. Frequency domain features are aspects that relate to the rate of change of the signal’s values. These features can be computed using frequency transformation calculations, where the signal is converted from time (real) domain to a frequency domain representation in most cases using Fast Fourier transform (FFT). This approach provides highly relevant frequency information but is limited in giving off accurate temporal data. On the other hand, joint time-frequency (TF) domain features try to mitigate each domain’s separate disadvantage by giving off good temporal and frequency information all together. A good example for this process is Short-Time Fourier Transform (STFT) that uses windowing to localize the TF representation of parts of the signal. The above three approaches use the classical Nyquist sampling rate and requires the full signal for feature extraction.
As mentioned before, Nyquist theorem is a major factor in device power consumption, ideally lowering the sampling rate will increase the longevity of the device’s battery life. To this end, researchers have examined a new theory revolving around sparsity and CS to decrease the sampling rate while maintaining an accurate representation of the signal for reconstruction and analysis. The same sparsity techniques mentioned in Section 5 for ECG denoising can be used for feature extraction. With sparsity comes dictionary learning. Dictionary learning is used for the reconstruction of the signal. It is a major aspect in sparse signal processing where a dictionary is created using basis functions that can be combined to form the input signal. A few disadvantages of sparsity techniques include that the reconstructed signal cannot be 100% reconstructed accurately (Krishnan and Athavale 2018). Another disadvantage includes the storage of a dictionary/cookbook in memory which is not optimal for small form factor devices. However, the periodic nature of ECG allows for dictionary creation that is suitable for long-term remote monitoring where repetitive patterns can be encoded using the same code. The goal of sparsity techniques in feature extractions is to use smaller amount of data in the form of distinctive patterns to better classify signals.
Common features that are extracted for ECG are PQRST time-domain, morphological, wavelet, statistical and non-linear features (Saini and Gupta 2021). While the extraction methods for time-domain features are Autoregressive Modelling (AR), linear predictive coding (LPC). Both are parametric approaches where coefficient parameters are estimated using previous samples. In AR, the signal is represented by a linear combination of past and current values. In LPC, the signal sample is estimated from previous values and the weighted sum of these values. On the other hand, frequency domain extraction methods for ECG have usually relied on FFT and Discrete Cosine Transform, and discrete orthogonal stockwell transform (DOST) (Raj and Ray 2017). DOST is used to extract the morphological features in an ECG. While time-frequency extraction methods include STFT, and DWT. DWT is used to decompose the signal into low and high frequency components. The low frequency approximate coefficients (cAi) and high frequency detailed coefficients (cDi) are determined for feature extraction and denoising (Saini and Gupta 2021).
In long-term consumer single-lead ECG wearables, the current feature extraction methodologies are time-domain features due to their low computational complexity and easy implementation in C programming, the main language for most microcontrollers. Some features that are commonly extracted in time-domain analysis include R-R intervals, HRV, HR, QRS interval, signal kurtosis, skewness, standard deviation, root-mean square, and variance. In consumer ECG wearables, time-domain features allow for real-time analysis and detection of certain cardiac information that is important for their intended applications. It is important to note that low-order wavelet transform can be performed on hardware through a cascade of band-pass filters separating different frequency components in each level. Liu et al. developed such an algorithm for long-term remote ECG monitoring (Liu et al., 2011). For on-edge and cloud computing applications, the more complex algorithms can be performed such as EMD. It is important to note that most single-lead ECG wearables rely on transmission the data across to a more capable device for ECG signal analysis. Up to this date, there is a lack of a robust single-lead wearable signal analysis approaches that can perform complex feature extraction and denoising techniques. The current trend in this research area focuses on the use of sparsity and CS to lower the overall sample rate, and the size of the wireless data packet transmission to the cloud. However, sparsity and CS still requires further work in terms of device feasibility and use. There are currently no sparse based algorithms for denoising and/or feature extraction of 1D biomedical signals including single-lead ECGs performed on small embedded system microcontrollers due to the lack of the computational tools that can be found in MATLAB and other software packages.
8 Machine Learning Considerations for Long-Term ECG Data
Arrythmias have many different types and classes. These classes are normal beats, ventricular ectopic beats (VEB), supraventricular ectopic beats (SVEB), mixed normal, VEB and unknown beats (Saini and Gupta 2021). It is crucial for physicians to be able to diagnose these arrythmias quickly and accurately which requires significant domain knowledge and time. Automatic heartbeat detection can alleviate the current constraints by identifying and classifying the different types with ease and high diagnostic value. ML has a role in the goal of automatic classification of arrythmias without any human labelling. The process is believed to help detect severe immediate attention type of arrythmias such as ventricular fibrillation and tachycardia. Also, it is assumed to predict future arrythmias for cardiac prevention methods. In the field of ECG, ML is used for the classification of heart beats and different types of arrythmias as shown in Figure 3. The current artificial intelligence methods used in this field include; Support Vector Machines (SVMs), Artificial Neural Networks (ANNs), Linear Discriminants (LDs), Convolutional and Probabilistic Neural Networks (CNNs, PNNs), and Fuzzy systems (Saini and Gupta, 2021). We will not delve into details behind each ML algorithm for ECG but it is important to note this does not cover all ML algorithms in the literature.
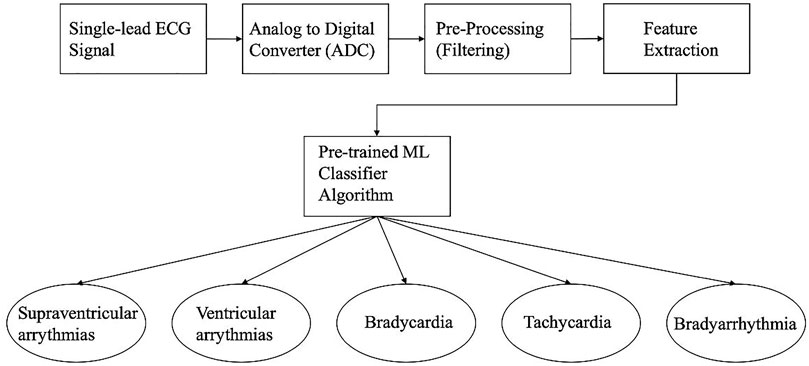
FIGURE 3. Block diagram of Single-lead ECG with feature extraction and a Machine Learning Classifier that classifies the 5 different types of cardiac arrythmias.
Implementation of predictive models do not constitute high computational cost on the device if they are small prediction models with a limited number of weighted coefficients. Also, other classification algorithms that are based on hand-crafted features are not mentioned due to the limitation in its use for specific arrythmia classification compared to the above-mentioned models that cover many arrythmia types and classes.
For long-term remote single-lead ECG monitoring, it is recommended to perform the learning phase of AI algorithms offline. Once the model is developed, it can be employed into embedded systems. This approach is optimal for power and computing consumption. Future trends in long-term ECG monitoring still aims in developing ML algorithms to be performed through On-Edge and cloud computing platforms. However, some progress has been made for the development of reinforcement learning (RL) methods that allows for automatic classification in the hopes of developing a solution for user-specific arrythmia detection (Ebrahimi et al., 2020). Currently there is a lack of RL for long-term remote ECG monitoring performed on device and/or through on-edge computing. However, TinyML day by day is becoming a reality where some progress show promise as discussed in (Sepahvand and Abdali-Mohammadi, 2022). The authors examined the use of teacher-student knowledge distillation approaches, highly used in power efficient classification in ECG. Their work showed promise where arrythmia classification accuracy was not impacted significantly by decreasing the number of ECG leads from 12 to 1 (Sepahvand and Abdali-Mohammadi, 2022). It is imperative to know that this field of research is growing at a fast pace with many emerging ML techniques published for general biomedical data.
9 Emerging Single-Lead ECG Applications and Opportunities for Healthcare
From this review, it is evident there is a lack of a wearable, wireless, low power, real-time, low-cost, lightweight, continuous single-lead ECG device that can perform very long-term remote ECG monitoring of more than 14 days for wellness and medical applications using dry electrodes or novel biosensors. These limitations in wearable remote ECG monitoring devices can be alleviated with continuous research and technology progression in wearable designs. Optimizing signal processing techniques to work on low-power hardware architectures can assist in the realization of more than 14 days of continuous ECG monitoring. For example, the implementation of low-complexity ECG compression algorithms in ECG remote monitoring applications can assist in reducing the energy consumption of battery-powered ECG devices while maintaining high quality and accurate reconstructed ECG signals. These advancements can increase the number of applications of long-term remote ECG monitoring. For example, 30 days of continuous ECG monitoring can be performed for post-operation patient home monitoring (Kernan et al., 2014).
Some recommended applications for a very-long term continuous ECG monitoring device include managing care for patients with long-term chronic diseases such as coronary heart disease.
Wearable Single-Lead ECG for Health Applications Include
1) Early prediction of cardiac abnormalities and determining risk factors using long-term longitudinal ECG data
2) Monitoring post-op patients remotely
3) Using long-term wearable ECG in monitoring elders in retirement homes and long-term care centers during pandemic scenarios such as COVID-19 pandemic.
4) Astronaut vital ECG monitoring in space for long durations of time.
5) Utilizing long-term wearables for telehealth and telemedicine applications in remote locations that do not have access to critical vital sign diagnostic technology or medical facilities
6) Medical Mental health monitoring
A plethora of single-lead ECG medical wearables are being tested and validated for clinical use. Some already are used in the medical field such as Zio Patch, CAM, BF, and ECG247. Most of the single-lead ECG patches can record up to 14 days continuously but cannot transmit cardiac information wirelessly. While BioIntelliSense can monitor patients up to 30 days of continuous ECG monitoring. The main application for these patches is monitoring cardiac patients post-op to ensure operation success. Also, they are prescribed by physicians to monitor the cardiac activity during medication and/or treatment. Clinicians rely on accurate data from the patches which is why they are all FDA cleared for use. As for Kardia, Apple Watch and other consumer single-lead wearable devices, they are currently being used to detect and monitor certain cardiac conditions as discussed earlier. Kardia, and Apple Watch monitor the presence of AF in patients with chronic cardiac illnesses. On the other hand, there is a lack of single-lead ECG wearables for long-term elder care, and telehealth in remote location applications. These applications rely more on PPG based cardiac information which is not entirely suitable for arrythmia detection due to the signal’s behavior and low quality. The current progress for these applications relies on portable single-lead ECG devices such as Kardia to record non-continuous ECG over short periods of time. It is evident there is a lack of single-lead ECG patches or wearables for continuous monitoring for elder care and telemedicine in remote communities due to constraints in developing such a device and the wide acceptance of the current status quo of PPG based technology.
On the other hand, ECG monitoring for space applications have shown great promise and progress. Zypher’s BioHarness is currently used by NASA as part of their Mars simulation to monitor astronaut’s health. Another promising textile wearable for space is Skiin, a textile that is currently being tested for long-term remote applications especially for early detection of CVD. Currently the wearable can monitor different vital signs and is collaborating with Mayo Clinic.
Also, ECG has shown its importance in psychiatry. Hage et al. used ECG, and HRV to differentiate bipolar disorder from major depression patients (Hage et al., 2019). The authors used 15 min of ECG to be able to create this differentiation. HRV features such as low and high frequency components of the signal are used. The authors hope their findings can assist physicians in differential diagnosis and managing therapeutics for their patients (Hage et al., 2019).
All devices in health applications require to undergo significant testing and validation before use. It is obvious that development requires a few years to produce single-lead ECG wearables for health. However, most if not all single-lead ECG wearables undergo a class-II FDA clearance due to their similar purpose and technologies when compared to Holter monitors and other multi-lead ECG devices. Most of the current trend in innovation is miniaturization and remote monitoring of the raw ECG signals. Up to this date, a continuous single lead ECG wearable that can perform automatic arrythmia detection, provide insights into signal features and allow for sparse CS is lacking.
10 Emerging Single-Lead ECG Applications and Opportunities for Wellness Applications
Some of the above-mentioned wearables can be used for wellness applications including;
1) HRV and HR for wellbeing
2) Improving wellbeing practices such as meditating and maintaining a healthy lifestyle
3) Daily stress analysis
4) Monitoring daily sleep circadian rhythm (Ankitha et al., 2021)
5) Monitoring workforce wellbeing in high risk, high intensity workplace such as Military, and first responders.
ECG monitoring for wellbeing applications is not a new field of study. Currently there is some lack of use for single-lead ECG for consumer wellbeing applications when compared to PPG based monitoring. However, as single-lead ECG development cost decreases with the progress of hardware friendly signal processing, and power-efficient sensors, the technology is becoming comparable to PPG. For wellness, most applications do not require ECG but its surrogate signals HR, and HRV in its analysis. Some single-lead ECG wearables for wellness include Apple Watch, Kardia, BioHarness, DuoEK and Amazfit Smart Watch 2. Although Kardia and Apple Watch are being used for AF, it can also be used to monitor general cardiac activity for wellness. Apple Watch’s ECG can be used to determine HR and determine the overall stress level of individuals by identifying periods of high HR vs. low HR.
While Ishaque et Al. used HRV to analyze stress levels of individuals participating in virtual reality video games. The authors later used different types of ML models to clearly classify HRV data and identify stressed VR situations vs. relaxed times (Ishaque et al., 2020). Their work is a promising precursor to the use of HRV obtained from single-lead ECG in the analysis of individual behavior and ultimately their mental wellbeing during daily activities.
Lastly, the current progress for workforce wellbeing can be seen through the BioHarness’s role in monitoring military and first responders through their band. The device is currently used intensively to monitor their stress levels and identify pitfalls of human performance. Also, they are using this technology to determine fatigue levels in people and how physical activity related to cognitive behavior. It is evident that with continuous progress and research in single-lead ECG, the goal of implementing more commercial grade single-lead ECG wearables for wellness and clinical applications is realizable. However, there are important factors that should be considered as mentioned earlier. Using low computational and power efficient signal processing and analysis algorithms allow for real-time computing. While device power requirements for long-term applications should be examined in the design stage itself. Moving forward, researchers should focus on developing appropriate algorithms that are power efficient and highly applicable for the common wellness use cases of HR, and HRV detection. While they should also focus on the in-depth complex algorithms used for common cardiac problems and wellness tracking applications. ECG signal quality and information content can be enhanced by using appropriate signal processing techniques and this would form as the foundational step in data-driven machine learning approaches (Krishnan, 2021). As wearables continually collect more ECG data, AI algorithms can be developed for the different wellness and clinical applications. Also, it is important to note that user comfortability is another important factor in the use of single-lead ECG. With the advancements in dry electrodes, a dry sensor could be the ideal candidate that allows for high user compliance and high-quality ECG acquisition.
11 Conclusion
The article covered important aspects of single-lead ECG wearable design and data analysis considerations for long-term, continuous, and remote monitoring applications. A quick overview of different prominent devices, Apple Watch, Kardia, Zio, BioHarness, Bittium Faros 360 and CAM in their wide use in medical and consumer applications is discussed. Zio and CAM can detect many arrythmias while Kardia is used in community screening for AF. On the other hand, Apple Watch is used for both AF detection, and wellbeing roles. BioHarness is suitable for emergency services, and in tracking elite athletes. While Bittium Faros can be applicable for military personnel. Guidelines for efficient wearable design using a novel four parametric representation domain has been proposed. Hardware-efficient software algorithmic design considerations become of importance due to power constraints. With the current progress in microelectronics, hardware accelerators, low-complexity algorithms, and sensors, single-lead ECG is becoming a necessary diagnostic modality for a plethora of healthcare and wellness applications.
Author Contributions
Conceptualization: AA and SK; methodology, AA and SK; formal analysis, AA; investigation, AA; resources, AA and SK; data curation, AA; writing–original draft preparation, AA; writing–review and editing, SK; visualization, AA and SK; supervision, SK; project administration, SK; funding acquisition, SK. Both authors have read and agreed to the published version of the manuscript.
Funding
Natural Sciences and Engineering Research Council (NSERC), Canada and Ryerson University, Canada.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully acknowledge the advice provided by our clinical and international collaborators in the area of wearables design and computing.
References
Abboud, S., and Barnea, O. (1995). “Errors Due to Sampling Frequency of the Electrocardiogram in Spectral Analysis of Heart Rate Signals with Low Variability,” in Computers in Cardiology. Vienna, Austria: IEEE, 461–463.
Abdou, A., and Krishnan, S. (2021). “ECG Dry-Electrode 3D Printing and Signal Quality Considerations,” in 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) (Mexico: IEEE), 6855–6858. doi:10.1109/EMBC46164.2021.9630599
Ajdaraga, E., and Gusev, M. (2017). “Analysis of Sampling Frequency and Resolution in ECG Signals,” in 2017 25th Telecommunication Forum (TELFOR). Belgrade, Serbia: IEEE, 1–4. doi:10.1109/TELFOR.2017.8249438
Ankitha, V., Manimegalai, P., Jose, P. S. H., and Raji, P. (2021). Literature Review on Sleep APNEA Analysis by Machine Learning Algorithms Using ECG Signals. J. Phys. Conf. Ser. 1937 (1), 012054. doi:10.1088/1742-6596/1937/1/012054
Arquilla, K., Webb, A., and Anderson, A. (2020). Textile Electrocardiogram (ECG) Electrodes for Wearable Health Monitoring. Sensors 20 (4), 1013. doi:10.3390/s20041013
Askarian, B., Jung, K., and Chong, J. W. (2019). Monitoring of Heart Rate from Photoplethysmographic Signals Using a Samsung Galaxy Note8 in Underwater Environments. Sensors 19 (13), 2846. doi:10.3390/s19132846
Athavale, Y., and Krishnan, S. (2017). Biosignal Monitoring Using Wearables: Observations and Opportunities. Biomed. Signal Process. Control. 38 (September), 22–33. doi:10.1016/j.bspc.2017.03.011
Barrett, P. M., Komatireddy, R., Haaser, S., Topol, S., Sheard, J., Encinas, J., et al. (2014). Comparison of 24-Hour Holter Monitoring with 14-Day Novel Adhesive Patch Electrocardiographic Monitoring. Am. J. Med. 127 (1), 95.e11–7. doi:10.1016/j.amjmed.2013.10.003
Bashar, S. K., Han, D., Soni, A., McManus, D. D., and Chon, K. H. (2018). “Developing a Novel Noise Artifact Detection Algorithm for Smartphone PPG Signals: Preliminary Results,” in 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI) (Las Vegas, NV, USA: IEEE), 79–82. doi:10.1109/BHI.2018.8333374
Benedetto, S., Caldato, C., Bazzan, E., Greenwood, D. C., Pensabene, V., and Actis, P. (2018). Assessment of the Fitbit Charge 2 for Monitoring Heart Rate. PLoS One 13 (2), e0192691. doi:10.1371/journal.pone.0192691
Bent, B., Goldstein, B. A., Kibbe, W. A., and Dunn, J. P. (2020). Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. Npj Digit. Med. 3 (1), 18. doi:10.1038/s41746-020-0226-6
Bio Harness 3 Medical Data Sheet (2012). Zephyr Technology. Available at: https://pdf.medicalexpo.com/pdf/zephyr/zephyr-bioharness/83995-97427-_4.html.
Bio Harness 3.0 User Manual (2012). Zephyr Technology. Available at: https://www.zephyranywhere.com/media/download/bioharness3-user-manual.pdf.
Blank, M., and Sinclair, M. (2011). “Non-Invasive and Long-Term Core Temperature Measurement,” in Proceedings of the First ACM Workshop on Mobile Systems, Applications, and Services for Healthcare - MHealthSys 11 (Seattle, Washington: ACM Press), 1. doi:10.1145/2064942.2064957
Blasco, J., and Peris-Lopez, P. (2018). On the Feasibility of Low-Cost Wearable Sensors for Multi-Modal Biometric Verification. Sensors 18 (9), 2782. doi:10.3390/s18092782
Borysiewicz, L. (2009). Prevention Is Better Than Cure. Clin. Med. 9 (6), 572–583. doi:10.7861/clinmedicine.9-6-572
Caruana, E. J., Roman, M., Hernández-Sánchez, J., and Solli, P. (2015). Longitudinal Studies. J. Thorac. Dis. 7 (11), E537–E540. doi:10.3978/j.issn.2072-1439.2015.10.63
Chlaihawi, A. A., Narakathu, B. B., Emamian, S., Bazuin, B. J., and Atashbar, M. Z. (2018). Development of Printed and Flexible Dry ECG Electrodes. Sensing Bio-Sensing Res. 20 (September), 9–15. doi:10.1016/j.sbsr.2018.05.001
Ebrahimi, Z., Loni, M., Daneshtalab, M., and Gharehbaghi, A. (2020). A Review on Deep Learning Methods for ECG Arrhythmia Classification. Expert Syst. Appl. X 7 (September), 100033. doi:10.1016/j.eswax.2020.100033
Elgendi, M., Eskofier, B., Dokos, S., and Abbott, D. (2014). Revisiting QRS Detection Methodologies for Portable, Wearable, Battery-Operated, and Wireless ECG Systems. PLoS ONE 9 (1), e84018. doi:10.1371/journal.pone.0084018
Elgendi, M., Fletcher, R., Liang, Y., Howard, N., Lovell, N. H., Abbott, D., et al. (2019). The Use of Photoplethysmography for Assessing Hypertension. Npj Digit. Med. 2 (1), 60. doi:10.1038/s41746-019-0136-7
Falter, M., Budts, W., Goetschalckx, K., Cornelissen, V., and Buys, R. (2019). Accuracy of Apple Watch Measurements for Heart Rate and Energy Expenditure in Patients with Cardiovascular Disease: Cross-Sectional Study. JMIR Mhealth Uhealth 7 (3), e11889. doi:10.2196/11889
Fung, E., Jã¤rvelin, M.-R., Doshi, R. N., Shinbane, J. S., Carlson, S. K., Grazette, L. P., et al. (2015). Electrocardiographic Patch Devices and Contemporary Wireless Cardiac Monitoring. Front. Physiol. 6 (May). doi:10.3389/fphys.2015.00149
George, G. C., Moitra, A., Caculo, S., Prince, A. A., Buch, J. J. U., and Pathak, S. K. (2020). A Novel and Efficient Hardware Accelerator Architecture for Signal Normalization. Circuits Syst. Signal. Process. 39 (5), 2425–2441. doi:10.1007/s00034-019-01262-3
Ghamari, M., Esparza, A., Ghamari, M., Soltanpur, C., and Nazeran, H. (2018). A Review on Wearable Photoplethysmography Sensors and Their Potential Future Applications in Health Care. Ijbsbe 4 (4), 195–202. doi:10.15406/ijbsbe.2018.04.00125
Godkin, F. E., Turner, E., Demnati, Y., Vert, A., Roberts, A., Swartz, R. H., et al. (2021). Feasibility of a Continuous, Multi-Sensor Remote Health Monitoring Approach in Persons Living with Neurodegenerative Disease. J. Neurol., 1–14. doi:10.1007/s00415-021-10831-z
Guo, S. L., Han, L. N., Liu, H. W., Si, Q. J., Kong, D. F., and Guo, F. S. (2016). The Future of Remote ECG Monitoring Systems. J. Geriatr. Cardiol. 13 (6), 528–530. doi:10.11909/j.issn.1671-5411.2016.06.015
Hage, B., Britton, B., Daniels, D., Heilman, K., Porges, S. W., and Halaris, A. (2019). Low Cardiac Vagal Tone Index by Heart Rate Variability Differentiates Bipolar from Major Depression. World J. Biol. Psychiatry 20 (5), 359–367. doi:10.1080/15622975.2017.1376113
Hannun, A. Y., Rajpurkar, P., Haghpanahi, M., Tison, G. H., Bourn, C., Turakhia, M. P., et al. (2019). Cardiologist-Level Arrhythmia Detection and Classification in Ambulatory Electrocardiograms Using a Deep Neural Network. Nat. Med. 25 (1), 65–69. doi:10.1038/s41591-018-0268-3
He, H., and Tan, Y. (2018). A Novel Adaptive Wavelet Thresholding with Identical Correlation Shrinkage Function for ECG Noise Removal. Chin. J. Electron. 27 (3), 507–513. doi:10.1049/cje.2018.02.006
Health Quality Ontario (2017). Long-Term Continuous Ambulatory ECG Monitors and External Cardiac Loop Recorders for Cardiac Arrhythmia: A Health Technology Assessment. Ont Health Technol. Assess. Ser. 17 (1), 1–56.Provide the doi for “Health Quality Ontario, 2017, Narayanaswamy, 2002”.
Hilton, M. L. (1997). Wavelet and Wavelet Packet Compression of Electrocardiograms. IEEE Trans. Biomed. Eng. 44 (5), 394–402. doi:10.1109/10.568915
Himmelreich, J. C. L., Karregat, E. P. M., Wim, A. M. L., van Weert, H. C. P. M., de Groot, J. R., Handoko, M. L., et al. (2019). Diagnostic Accuracy of a Smartphone-Operated, Single-Lead Electrocardiography Device for Detection of Rhythm and Conduction Abnormalities in Primary Care. Ann. Fam. Med. 17 (5), 403–411. doi:10.1370/afm.2438
Hinde, K., White, G., and Armstrong, N. (2021). Wearable Devices Suitable for Monitoring Twenty Four Hour Heart Rate Variability in Military Populations. Sensors 21 (4), 1061. doi:10.3390/s21041061
Imtiaz, S. A., Mardell, J., Saremi–Yarahmadi, S., and Rodriguez–Villegas, E. (2016). ECG Artefact Identification and Removal in MHealth Systems for Continuous Patient Monitoring. Healthc. Techn. Lett. 3 (3), 171–176. doi:10.1049/htl.2016.0020
Ishaque, S., Rueda, A., Nguyen, B., Khan, N., and Krishnan, S. (2020). “Physiological Signal Analysis and Classification of Stress from Virtual Reality Video Game,” in 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) (Montreal, QC, Canada: IEEE), 867–870. doi:10.1109/EMBC44109.2020.9176110
Jain, S., Bajaj, V., and Kumar, A. (2018). Effective De‐noising of ECG by Optimised Adaptive Thresholding on Noisy Modes. IET Sci. Meas. Techn. 12 (5), 640–644. doi:10.1049/iet-smt.2017.0203
Kernan, W. N., Ovbiagele, B., Black, H. R., Bravata, D. M., Chimowitz, M. I., Ezekowitz, M. D., et al. (2014). Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: a Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 45 (7), 2160–2236. doi:10.1161/STR.0000000000000024
Kher, R. (2019). Signal Processing Techniques for Removing Noise from ECG Signals. J. Biomed. Eng. Res. 1, 1–9. doi:10.17303/jber.2019.3.101
Kligfield, P., Gettes, L. S., Bailey, J. J., Childers, R., Deal, B. J., Hancock, E. W., et al. (2007). Recommendations for the Standardization and Interpretation of the Electrocardiogram. Circulation 115 (10), 1306–1324. doi:10.1161/CIRCULATIONAHA.106.180200
Krishnan, S. (2021). Biomedical Signal Analysis for Connected Healthcare. London, United Kingdom: Elsevier.
Krishnan, S., and Athavale, Y. (2018). Trends in Biomedical Signal Feature Extraction. Biomed. Signal Process. Control. 43 (May), 41–63. doi:10.1016/j.bspc.2018.02.008
Kruger, A. (2018). Electrocardiograph Software for Over-the-counter Use. U.S Food & Drug Administration (FDA) https://www.accessdata.fda.gov/cdrh_docs/pdf18/DEN180044.pdf.
Kuncoro, C. B. D., Luo, W.-J., and Kuan, Y.-D. (2020). Wireless Photoplethysmography Sensor for Continuous Blood Pressure Biosignal Shape Acquisition. J. Sensors 2020 (February), 1–9. doi:10.1155/2020/7192015
Lau, J. K., Lowres, N., Neubeck, L., Brieger, D. B., Sy, R. W., Galloway, C. D., et al. (2013). IPhone ECG Application for Community Screening to Detect Silent Atrial Fibrillation: A Novel Technology to Prevent Stroke. Int. J. Cardiol. 165 (1), 193–194. doi:10.1016/j.ijcard.2013.01.220
Liu, F., Liu, C., Jiang, X., Zhang, Z., Zhang, Y., Li, J., et al. (2018). Performance Analysis of Ten Common QRS Detectors on Different ECG Application Cases. J. Healthc. Eng. 2018, 1–8. doi:10.1155/2018/9050812
Liu, X., Zheng, Y., Phyu, M. W., Zhao, B., Je, M., and Yuan, X. (2011). Multiple Functional ECG Signal Is Processing for Wearable Applications of Long-Term Cardiac Monitoring. IEEE Trans. Biomed. Eng. 58 (2), 380–389. doi:10.1109/TBME.2010.2061230
Lobodzinski, S. S. (2013). ECG Patch Monitors for Assessment of Cardiac Rhythm Abnormalities. Prog. Cardiovasc. Dis. 56 (2), 224–229. doi:10.1016/j.pcad.2013.08.006
Lobodzinski, S. S., and Laks, M. M. (2012). New Devices for Very Long-Term ECG Monitoring. Cardiol. J. 19 (2), 210–214. doi:10.5603/cj.2012.0039
Lu, Z., Kim, D. Y., and Pearlman, W. A. (2000). Wavelet Compression of ECG Signals by the Set Partitioning in Hierarchical Trees Algorithm. IEEE Trans. Biomed. Eng. 47 (7), 849–856. doi:10.1109/10.846678
Magnani, J. W., Williamson, M. A., Ellinor, P. T., Monahan, K. M., and Benjamin, E. J. (2009). P Wave Indices. Circ. Arrhythmia Electrophysiol. 2 (1), 72–79. doi:10.1161/CIRCEP.108.806828
Malghan, P. G., and Kumar Hota, M. (2020). A Review on ECG Filtering Techniques for Rhythm Analysis. Res. Biomed. Eng. 36 (2), 171–186. doi:10.1007/s42600-020-00057-9
Mamaghanian, H., Khaled, N., Atienza, D., and Vandergheynst, P. (2011). Compressed Sensing for Real-Time Energy-Efficient ECG Compression on Wireless Body Sensor Nodes. IEEE Trans. Biomed. Eng. 58 (9), 2456–2466. doi:10.1109/TBME.2011.2156795
Marinucci, D., Sbrollini, A., Marcantoni, I., Morettini, M., Swenne, C. A., and Burattini, L. (2020). Artificial Neural Network for Atrial Fibrillation Identification in Portable Devices. Sensors 20 (12), 3570. doi:10.3390/s20123570
Meek, S., and Morris, Francis. (2002). ABC of Clinical Electrocardiography: Introduction. I---Leads, rate, rhythm, and cardiac axis. BMJ (Clinical Research Ed.) 324 (7334), 415–418. doi:10.1136/bmj.324.7334.415
Meziane, N., Webster, J. G., Attari, M., and Nimunkar, A. J. (2013). Dry Electrodes for Electrocardiography. Physiol. Meas. 34 (9), R47–R69. doi:10.1088/0967-3334/34/9/r47
Mukhopadhyay, S. K., and Krishnan, S. (2020). A Singular Spectrum Analysis-Based Model-Free Electrocardiogram Denoising Technique. Comput. Methods Programs Biomed. 188 (May), 105304. doi:10.1016/j.cmpb.2019.105304
Narayanaswamy, S. (2002). High Resolution Electrocardiography. Indian Pacing Electrophysiol. J. 2 (2), 50–56.
Nazari, G., MacDermid, J. C., Sinden, K. E., Richardson, J., and Tang, A. (2019). Reliability of Zephyr Bioharness and Fitbit Charge Measures of Heart Rate and Activity at Rest, During the Modified Canadian Aerobic Fitness Test, and Recovery. J. Strength Conditioning Res. 33 (2), 559–571. doi:10.1519/JSC.0000000000001842
Nepi, D., Sbrollini, A., Agostinelli, A., Maranesi, E., Di Nardo, F., Fioretti, S., et al. (2016). “Validation of the Heart:Rate Signal Provided by the Zephyr BioHarness 3.0,” in 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11-14 Sept. 2016. doi:10.22489/CinC.2016.106-358
Noh, S., Yoon, C., Hyun, E., Yoon, H. N., Chung, T. J., Park, K. S., et al. (2014). Ferroelectret film‐based patch‐type sensor for continuous blood pressure monitoring. Electron. Lett. 50 (3), 143–144. doi:10.1049/el.2013.3715
Oliveira, B. R. d., Duarte, M. A. Q., Abreu, C. C. E. d., and Vieira Filho, J. (2018). A Wavelet-Based Method for Power-Line Interference Removal in ECG Signals. Res. Biomed. Eng. 34 (1), 73–86. doi:10.1590/2446-4740.01817
Pallin, D. J., Espinola, J. A., and Camargo, C. A. (2014). US Population Aging and Demand for Inpatient Services. J. Hosp. Med. 9 (3), 193–196. doi:10.1002/jhm.2145
Pan, J., and Tompkins, W. J. (1985). A Real-Time QRS Detection Algorithm. IEEE Trans. Biomed. Eng. 32 (3), 230–236. doi:10.1109/TBME.1985.325532
Pant, J. K., and Krishnan., S. (2014). Compressive Sensing of Electrocardiogram Signals by Promoting Sparsity on the Second-Order Difference and by Using Dictionary Learning. IEEE Trans. Biomed. Circuits Syst. 8 (2), 293–302. doi:10.1109/TBCAS.2013.2263459
Pavlatos, C., Dimopoulos, A. C., George, M., and Papakonstantinou, G. K. (2003). Hardware Implementation Of Pan & Tompkins Qrs Detection Algorithm 1.
Pollreisz, D., and TaheriNejad, N. (2019). Detection and Removal of Motion Artifacts in PPG Signals. Mobile Netw. Appl.. doi:10.1007/s11036-019-01323-6
Qureshi, F., and Krishnan, S. (2018). Wearable Hardware Design for the Internet of Medical Things (IoMT). Sensors 18 (11), 3812. doi:10.3390/s18113812
Raj, S., and Ray, K. C. (2017). ECG Signal Analysis Using DCT-Based DOST and PSO Optimized SVM. IEEE Trans. Instrum. Meas. 66 (3), 470–478. doi:10.1109/TIM.2016.2642758
Rho, R., Vossler, M., Blancher, S., and Poole, J. E. (2018). Comparison of 2 Ambulatory Patch ECG Monitors: The Benefit of the P-Wave and Signal Clarity. Am. Heart J. 203 (September), 109–117. doi:10.1016/j.ahj.2018.03.022
Saini, S. K., and Gupta, R. (2021). Artificial Intelligence Methods for Analysis of Electrocardiogram Signals for Cardiac Abnormalities: State-of-the-Art and Future Challenges. Artif. Intell. Rev. 55, 1519–1565. doi:10.1007/s10462-021-09999-7
Samol, A., Bischof, K., Luani, B., Pascut, D., Wiemer, M., and Kaese, S. (2019b). Single-Lead ECG Recordings Including Einthoven and Wilson Leads by a Smartwatch: A New Era of Patient Directed Early ECG Differential Diagnosis of Cardiac Diseases? Sensors 19 (20), 4377. doi:10.3390/s19204377
Samol, A., Bischoff, K., Luani, B., Pascut, D., Wiemer, M., and Kaese, S. (2019a). Recording of Bipolar Multichannel ECGs by a Smartwatch: Modern ECG Diagnostic 100 Years after Einthoven. Sensors 19 (13), 2894. doi:10.3390/s19132894
Satija, U., Ramkumar, B., and Sabarimalai Manikandan, M. (2017). Real-Time Signal Quality-Aware ECG Telemetry System for IoT-Based Health Care Monitoring. IEEE Internet Things J. 4 (3), 815–823. doi:10.1109/JIOT.2017.2670022
Se Dong, M., Jin Kwon, K., Hang Sik, S., Yong Hyeon, Y., Chung Keun, L., and Myoungho, L. (2010). Noncontact Respiration Rate Measurement System Using an Ultrasonic Proximity Sensor. IEEE Sensors J. 10 (11), 1732–1739. doi:10.1109/JSEN.2010.2044239
Selder, J. L., Breukel, L., Blok, S., van Rossum, A. C., Tulevski, I. I., and Allaart, C. P. (2019). A Mobile One-Lead ECG Device Incorporated in a Symptom-Driven Remote Arrhythmia Monitoring Program. The First 5,982 Hartwacht ECGs. Neth. Heart J. 27 (1), 38–45. doi:10.1007/s12471-018-1203-4
Selvaraj, N. (2014). “Long-Term Remote Monitoring of Vital Signs Using a Wireless Patch Sensor,” in 2014 IEEE Healthcare Innovation Conference (HIC) (Seattle, WA, USA: IEEE), 83–86. doi:10.1109/HIC.2014.7038880
Sepahvand, M., and Abdali-Mohammadi, F. (2022). A novel method for reducing arrhythmia classification from 12-lead ECG signals to single-lead ECG with minimal loss of accuracy through teacher-student knowledge distillation. Inf. Sci. 593–77. doi:10.1016/j.ins.2022.01.030
Smith, W. M., Riddell, F., Madon, M., and Gleva, M. J. (2017). Comparison of Diagnostic Value Using a Small, Single Channel, P-Wave Centric Sternal ECG Monitoring Patch with a Standard 3-Lead Holter System over 24 hours. Am. Heart J. 185 (March), 67–73. doi:10.1016/j.ahj.2016.11.006
Steinberg, C., Philippon, F., Sanchez, M., Fortier-Poisson, P., O’Hara, G., Molin, F., et al. (2019). A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 9 (1), 17. doi:10.3390/bios9010017
Tai Wong, D. L., Muthu Kumaran Sathappan, S., Yu, J., Heng, C. H., Kojodjojo, P., and Feng, M. (2019). “Continuous ECG Monitoring Trial for Outpatient - Patient Receptiveness and Signal Accuracy,” in 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (Berlin, Germany: IEEE), 1144–1148. doi:10.1109/EMBC.2019.8857368
Turakhia, M. P., Hoang, D. D., Zimetbaum, P., Miller, J. D., Froelicher, V. F., Kumar, U. N., et al. (2013). Diagnostic Utility of a Novel Leadless Arrhythmia Monitoring Device. Am. J. Cardiol. 112 (4), 520–524. doi:10.1016/j.amjcard.2013.04.017
Vosslers, M. (2017). Comparison of Continuous Sternal ECG Patch Monitors (Carnation and Zio) Trial. Washington, United States: EvergreenHealth. Clinical Trial NCT02952781 https://clinicaltrials.gov/ct2/show/study/NCT02952781.
Wang, F. (2018). “The Roles of Preventive and Curative Health Care in Economic Development. PloS One 13 (11), e0206808. doi:10.1371/journal.pone.0206808
Wilson, S., and Laing, R. (2018). “Wearable Technology: Present and Future,” in 91st World Conference of The Textile Institute. Leeds, United Kingdom. 23-26 July, 2018
Keywords: ECG, wearables, telemedicine, remote monitoring, long-term care
Citation: Abdou A and Krishnan S (2022) Horizons in Single-Lead ECG Analysis From Devices to Data. Front. Sig. Proc. 2:866047. doi: 10.3389/frsip.2022.866047
Received: 30 January 2022; Accepted: 22 March 2022;
Published: 11 April 2022.
Edited by:
Mohamed Elgendi, ETH Zürich, SwitzerlandReviewed by:
Eros Gian Alessandro Pasero, Politecnico di Torino, ItalyColin K. Drummond, Case Western Reserve University, United States
Copyright © 2022 Abdou and Krishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdelrahman Abdou, YWJkZWxyYWhtYW4uYWJkb3VAcnllcnNvbi5jYQ==
 Abdelrahman Abdou
Abdelrahman Abdou Sridhar Krishnan
Sridhar Krishnan