
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Sens., 21 February 2025
Sec. Sensor Devices
Volume 6 - 2025 | https://doi.org/10.3389/fsens.2025.1513701
This article is part of the Research TopicNanomaterials for Affordable Biomedical Devices, Environmental and Energy ApplicationsView all 6 articles
 Shelby Defeo1*
Shelby Defeo1* Samuel Erickson2
Samuel Erickson2 Maria F. Perez Mendoza2
Maria F. Perez Mendoza2 Alexia Cooper1
Alexia Cooper1 Bruce Barrios2
Bruce Barrios2 Zachary Malone1
Zachary Malone1 Ryan D. Baxter3
Ryan D. Baxter3 Sayantani Ghosh2
Sayantani Ghosh2 Thomas C. Harmon1
Thomas C. Harmon1Water quality monitoring is essential for identifying risks to environmental and human health. Nitrate monitoring is of particular importance, as its anthropogenic point and nonpoint sources are common globally and have deleterious effects on water quality and usability as well as aquatic ecosystem health. Standard methods for assessing nitrate concentrations in water generally involve laboratory techniques, as methods available for field testing face significant tradeoffs between cost, precision, and portability. Given its relatively ubiquitous nature and the widespread regulation of nitrate pollution, it is a prime target for sensor development. The growing field of nanomaterials (e.g., nanoparticles, nanotubes, and 2-dimensional materials) offers the potential to eliminate these tradeoffs through a new generation of field-ready nitrate sensors. However, transitioning nano-sensors from the lab to the field remains challenging. In this perspective we examine the challenges of lab-to-field transition of nano-sensors for nitrate, highlighting the importance of a user-centered design approach under the framework of FOCUS (form factor, operational robustness, cost, user interface, and sensitivity).
Nitrate (NO3−) pollution is a global concern because of its ubiquitous nature and negative effects on human health and ecosystem function. Elevated anthropogenic nitrate releases overwhelm crops, soil microbes, and aquatic ecosystems, leading to incomplete nitrogen assimilation (Zhang et al., 2015). Elevated concentrations can generally be traced to three sources: point discharges of treated and raw wastewater from municipal systems (Choudhary et al., 2022), releases from confined livestock operations (Lockhart et al., 2013), and land application of agricultural fertilizers resulting in diffuse or nonpoint source pollution (Singh and Craswell, 2021). While all these sources can be problematic, the nonpoint sources are the most challenging to delineate and control because of their connections with a variety of locations and activities (Drevno, 2016). The consumption of nitrate in drinking water is associated with deleterious health effects for pregnant people and infants, and there is growing evidence of negative health outcomes in other populations as well (Temkin et al., 2019; Ward et al., 2018). The United States Environmental Protection Agency established the maximum contaminant level (MCL) of 10 mg per liter (mg/L) nitrate-as-nitrogen or NO3−-N in public drinking water supplies in the United States at (US EPA, 2019), which is consistent with global limits set for the contaminant. Nitrate pollution does not affect all communities equally; agricultural areas, as well as rural and Hispanic communities, have been found to be at significantly higher risk for nitrate pollution exposure within the United States (Schaider et al., 2019). Given its diffuse distribution in surface water and groundwater, low cost and user-friendly nitrate sensing technologies would be highly desirable. For example, such sensors would enable rural water consumers to monitor their own tap water and empower citizen science groups to test their local rivers, lakes, and wetlands.
The current methodologies of measuring nitrate concentration involve huge trade-offs among prevailing methodologies with regard to precision, cost, and field usability. Although laboratory-based techniques can be highly accurate, they are unrealistic for many stakeholders due to the use of expensive equipment and personnel training. On the other side, simplistic field methods such as colorimetric test strips are imprecise, while the existing portable sensors face serious challenges on interference, calibration drift, and operational lifetime. The field of nanomaterials, currently under fast development, opens new perspectives in the overcoming of such limitations through tunable properties, high surface-to-volume ratio, and for selective detection potentially.
Successfully transitioning nanomaterial-based sensors from laboratory demonstrations to practical field applications requires careful consideration of multiple design factors. A systematic approach considering both technical performance and user needs is essential for developing sensors that will be adopted and used effectively in real-world settings. This work offers perspectives on leveraging the growing field of nanomaterials (e.g., nanoparticles, nanotubes, and 2-dimensional materials) to advance more quickly to a new generation of field-ready nitrate sensors. In this perspective we examine the challenges of lab-to-field transition of nano-sensors, highlighting the importance of a user-centered design approach under a framework we refer to as FOCUS (form factor, operational robustness, cost, user interface, and sensitivity).
Observing aqueous nitrate concentrations in situ and in near-real time is valuable to water quality managers and stakeholders. It enables the mapping and understanding and analysis of nitrate distributions and dynamics in natural and engineered water systems. The field detection methods outlined here (colorimetric test strips, electrochemical sensors, and spectroscopic sensors) are not intended to be exhaustive. Instead, the methods and devices discussed are intended to highlight the common challenges associated with currently available field techniques, including lack of precision, robustness, and relatively high unit costs. It is worth noting that nitrate measurements can be expressed in various ways (ppb (parts per billion), ppm (parts per million), ppm-NO3—N (parts per million nitrate-as-nitrogen), mg/L (milligrams per liter), molarity, etc.). In this paper, we use ppm and ppb as nitrate for consistency and a broader audience (42 ppm nitrate is the corresponding US EPA MCL).
Colorimetric nitrate test strips for predetermined ranges (0–500 ppm) can provide nitrate concentration assessments in less than 1 min (Brockhage et al., 2022). The test colorimetric strips work through the reduction of nitrate to nitrite which can be visually quantified (or standardized for phone cameras). While such test strips are relatively inexpensive and easy to use, they are designed to quantify wide ranges and their readings may deviate from comparative laboratory results (Brockhage et al., 2022; Loperfido et al., 2010). However, test strips may be sufficient for uses such as rapid sample screening (e.g., prior to more precise lab analyses) and for community science projects, also known as citizen science projects, exploring nitrate presence/absence or identifying trends.
Electrochemical or spectroscopic nitrate sensing devices are also commercially available. Ion selective electrodes (ISE) operate potentiometrically and exist for a variety of environmental analytes of interest (Crespo, 2017). For a nitrate ISE, the working electrode material is coated with a membrane doped with an ionic carrier (e.g., quaternary ammonium ions, as noted in Singh et al. (2022)), which renders it selective for nitrate ions. The affinity of the nitrate ion for the surface of the working electrode alters the chemical potential (voltage) in a log-linear relationship with nitrate concentration. While nitrate ISEs can work well in laboratory and under carefully controlled field conditions, they are thus far unsuitable for autonomous field deployment. Hindrances to deployment include their lack of adequate sensitivity for some applications (often precision ±10% with detection limit of 0.5 ppm in commercially available ISEs), need for frequent calibration, sensitivity to interfering ions, and need for frequent cleaning to prevent biofouling of the membrane surface (Crespo, 2017).
Spectroscopic devices for measuring nitrate concentrations operate in the ultraviolet (UV) range and can have limit of detections down to the sub ppb (Mahmud et al., 2020). Nitrate absorbs at specific wavelengths, generally within the range of 190–250 nm (nm), though multiple wavelengths may be necessary due to interfering absorbance of other compounds also occurring in this range (Singh et al., 2019). Robust UV probes that limit interferences and are field-ready can be expensive ($10,000+), bulky (on the order of a meter long), and complicated to operate, therefore presenting problems with implementation for a wide variety of aquatic research.
Laboratory techniques remain the standard for nitrate determination when high precision and accuracy are needed for scientific or regulatory purposes. Flow injection analysis is among the commonly used laboratory techniques for nitrate determination and generally utilizes a cadmium column to facilitate the reduction of nitrate to nitrite for analysis (Kazemzadeh and Ali, 2001). While this method provides results across a wide range of concentrations from the sub ppb to ppm level, there is debate regarding variation in results due to column preparation and influences of pH and dissolved oxygen (Gal et al., 2004).
Colorimetric methods for the detection of nitrate are predicated on the development of a visible color using reagents, in some cases through the development of color using Greiss reagents and the reduction of nitrate to nitrite (Michalski and Kurzyca, 2006). This method is relatively simple in application but requires the use of prefabricated reagent packets appropriate for a finite range of concentrations and therefore may require sample dilution for higher concentrations. In addition, these reagent packets are relatively expensive consumables and produce hazardous waste in the lab. Colorimetric methods are generally able to quantify nitrate concentrations between 0.05 ppm and 22 ppm, but high sensitivity versus broad sensitivity versions of the method require different procedures.
Ion chromatography is another common method for the determination of nitrate (Michalski and Kurzyca, 2006). Ions are separated by their interactions with a resin and progress through the system at differentiating rates. Chromatography provides highly accurate results (±5%) with low detection limits in the ppb level, but the high cost of the instrument, time and cost associated with sample collection and transport, need for skilled technicians, and maintenance limit its use outside of a university, industry, or research laboratory. Raman spectroscopy is an optical-signal-based laboratory method capable of determining nitrate in water samples. The method utilizes light to quantify energy shifts originating from the vibrational modes of the chemical bonds of the molecule and is comparable to other laboratory techniques in accuracy. While similar in accuracy, the method is hindered by a higher detection limit of 0.5 ppm (Gajaraj et al., 2013).
Laboratory techniques of a wide variety are available for the detection of nitrate and serve a valuable purpose in scientific discovery, however they provide only partially the needs of nitrate determination due to their restrictive nature. While both laboratory and field methods have contributed to the current understanding of nitrates, gaps remain between available technology and the practical needs of users. Laboratory techniques are limited by their need for a skilled technician, high investment cost and cost of continuing maintenance, sample preparation, and delay in data analysis. Current field methods are restricted by tradeoffs between precision, cost, robustness, ease of use, and portability. The next-generation of nitrate sensors will need to bridge the associated gaps between lab and field detection, potentially through new technologies.
The past decade has featured rapid growth in nanomaterial-based detectors for nitrate dissolved in water. While these lab devices are not yet commercialized or produced at scale, they present excellent detection limits as low as 0.045 ppm and as high as 6,000 ppm (Hassan et al., 2019; Essousi et al., 2019). Likewise, the hardware needed to make use of emergent nanomaterials is often compact and conducive to use in field sensors. Finally, many nanoscale sensors have few interfering ions, though nitrite (NO2−) is the most common (Stortini et al., 2015; Liang et al., 2016; Tang et al., 2016). Supplementary Table S-1 summarizes the parameters of nanomaterials based sensors highlighted in this perspective. This section will focus on promising electrochemical, spectroscopic, biological, and electrical nanosensors and their function (Figure 1). The advantages and disadvantages of each will be discussed briefly, as will comparisons between devices to better understand their roles in future nitrate detection. Herein, electrochemical sensors will include voltametric and potentiometric devices, the latter using ion selective electrodes (ISEs). Similarly, electrical sensors will include chemiresistors, capacitors, and field effect transistors (FETs).
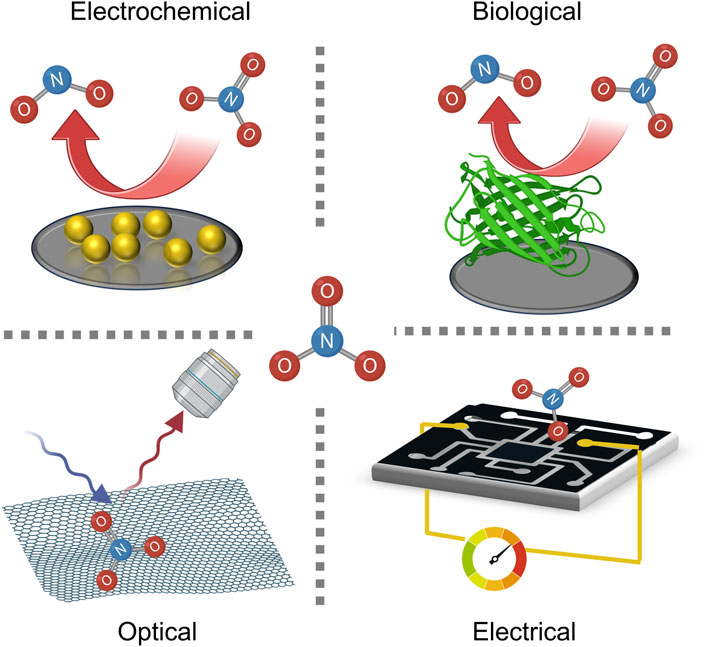
Figure 1. Schematic representation of typical nitrate sensing platforms. Both electrochemical and biological sensors rely on nitrate reduction to generate electrical responses, with the latter utilizing a biological molecule as the active medium. Optical techniques are non-invasive, leveraging changes in spectral absorption or emission properties of a substrate in response to nitrate adsorption. Electrical sensing devices use the same approach of molecular adsorption, but the detection method is a change of electrical conductivity of the sensor.
Popular electrochemical nanosensors for nitrate detection include metal/metal oxide nanoparticles (NPs), graphene, carbon nanotubes (CNTs), electropolymerized films, and combinations of these. Most materials in this category act as electrode modifiers, as bulk copper (Cu) and other common conductors are poor nitrate detectors near neutral pH. These contacts also tend to degrade and experience interference with other molecules and ions, especially nitrite (NO2−) and chloride (Cl−), without proper modifiers (Li et al., 2019). Copper nanoparticles (CuNPs) and nanowires in combination with various substates have nitrate detection ranges in the sub ppb (Essousi et al., 2019; Stortini et al., 2015). CuNPs deposited on graphene catalyzed nitrate reduction to ammonia with analysis by differential pulse voltammetry have been shown to have a detection limit of 0.49 ppm (Wang et al., 2018). More recently, Cu nanowires were grown by galvanic deposition to detect nitrate by linear sweep voltammetry. This cost effective and stable growth technique enabled measurement of nitrate concentrations as low as 0.56 ppm and performed well in natural water samples (Patella et al., 2021). An undesirable characteristic of these sensors is that they require a pH between two and three for proper electrocatalytic reduction of nitrate. Silver (Ag) nanostructures have low limits of detection (as low as 24 ppb) near neutral pH and experience few interferents but are limited to concentrations under 62 ppm (Chen Legrand et al., 2017; Hu et al., 2013). Potentiometric sensors utilize ISEs including multiwalled carbon nanotubes (MWCNTs), graphene, and polypyrrole (Cuartero et al., 2018; Schwarz et al., 2018; Pięk et al., 2016; Gil et al., 2024). Work with organic nanotubes has also shown low detection limits of 0.02 ppm (Kundu, 2023). Most ISEs are based on three nitrate ionophores: quaternary ammonium, nitrate ionophore V and VI, and tridodecylmethylammonium nitrate (TDMAN). Very low nitrate concentrations between 10−7–10−2 ppm have been detected by measuring the potential difference between the ISE and a reference electrode (Singh et al., 2022; Liu et al., 2020). Electrochemical sensors can be complicated in their construction, but recent work has highlighted alternative constructions with linear ranges between 10 and 100 ppm with a detection limit of approximately 2 ppm (Concepcion et al., 2024) while other work has highlighted possibilities for environmental sustainability with linear ranges of 1–100 ppm (Sarwar Inam et al., 2023).
Spectroscopic nitrate detection is the most popular method in the lab due to the highly precise nature of spectrometers and other optical instruments. By performing a nitrate to nitrite reduction via a Griess assay, nitrate concentrations as low as 10−5 ppm can be measured with high resolution fluorescence spectroscopy (Yang et al., 2015). Vanadium (III) chloride (VCl3) in hydrochloric acid (HCl) has also been used with limits of detection as low as 0.006 ppm, though sensing times range from 3–60 min (Garcia-Robledo et al., 2014; Wang et al., 2016). CNTs and CuNPs and been utilized in tandem with optical fibers for both surface plasmon resonance and ultraviolet-visible (UV-Vis) absorption detection techniques (Zhang et al., 2019; Parveen et al., 2017; Moo et al., 2016). Surface-enhanced Raman spectroscopy also offers large linear detection ranges spanning multiple orders of magnitude and low limits of detection of ppm or sub ppm (Gajaraj et al., 2013; Li et al., 2024). While these measurements take only milliseconds, have very low detection limits, and are highly reproducible, they require expensive optical systems and calibration training.
Biosensors based on nitrate reductase (NR) enzymes can detect nitrate with high specificity and sensitivity at neutral pH through adsorption onto electrodes. The primary drawbacks of NR sensors are their high cost and the low temperature required for storage (Singh et al., 2022). Some methods for improving the sensitivity and stability of NR sensors through biological enhancements include incorporating NR from plants and fungi (Kalimuthu et al., 2015; Kalimuthu et al., 2021), combining biosensing elements with nanomaterials such as carbon nanotubes and zinc oxide (ZnO) nanostructures (Can et al., 2012; Ahmad et al., 2017), and utilizing whole-cell organisms (Machado et al., 2022). While nanomaterials can improve the capabilities of biological nanosensors, enzymatic sensors are still susceptible to degradation under environmental conditions (Singh et al., 2022). To overcome the limitations of enzymatic sensors, there is a need for more research focused on developing novel designs and synthesis methods that can minimize degradation under environmental conditions.
While potentiometric and optical sensors have very low detection limits, they require sample preparation including pH balance and control calibration. New chemiresistors, capacitors, and FETs avoid these problems, showing great aptitude as nitrate detectors largely due to their ‘lab-on-a-chip’ design. In one recent study, graphene nanowire was created by melting high density polyethylene (HDPE) between two bulk Cu contacts. By measuring current-voltage response, nitrate concentrations between 50–5,000 ppm were successfully determined (Ahmadi et al., 2021). FETs in particular can provide extremely low detection limits (45 ppb) with no interfering species (Minami et al., 2016). By replacing the gate metal on a standard FET with a nitrate sensing material, researchers created chemically sensitive FETs (CHEMFETs) in the early 1970s (Janata, 2022). The amount of nitrate present modulates the electric field in the gate, changing current flow across the device. As in all transistors, various forms of CHEMFETs allow minute field changes in the gate to produce large currents through the device. This sensitivity allowed for ppb detection in some ion selective FETs (ISFETs) and organic FETs (OFETs) over the past decade (Minami et al., 2016; Kim et al., 2020a). Other ISFETs have used nitrate selective membranes on chemical vapor deposited graphene and nitrone coated polyvinyl chloride for detection with impressive results (Kim et al., 2020b; Chaisriratanakul et al., 2020). These electrical sensors can easily be integrated into ‘internet of things’ devices and are likely to see largescale field deployment with wireless data communication (Agir et al., 2021; Alahi et al., 2018).
Contemporary laboratory sensors address some of the challenges with existing field methods, but there are additional considerations with their transition from the lab to field. Most novel sensors in the scientific literature remain at the proof-of-concept stage, with emphasis on unique materials or interesting transduction mechanisms. Integrating the transduction, signal acquisition/processing/conditioning, and power supply is a secondary step that takes time, money, and effort that is not typically rewarded in the academic world. Packaging the integrated sensor system to make it useable and resilient in the environment is a tertiary step which can sometimes involve innovation (e.g., creative geometry, filters, or other features for specific environmental sampling challenges). An additional hurdle to field implementation is that the end user of the device is important to consider ensuring the need and relevancy of a device. This translates to more time and effort for researchers to consult with users early in the development period. While each category of sensor has specific strengths and weaknesses, researchers need to consider form factor, operational robustness, cost, user interface, and sensitivity (FOCUS) during development if the gap between the lab and field is to be bridged.
Different user groups will have different requirements relating to form factor (i.e., size, shape, and other physical considerations). Limnologists or oceanographers will likely find a device that falls into the probe or sonde category, as something 2-liter bottle sized, amenable to their needs, while a community member or farmer interested in well water testing will likely be interested in something less bulky. The nanomaterial-based sensors described previously in this paper all contain small components. However, the sensors integrated system can exist across a wide range of device sizes. The addition of extra components and detectors can hamper portability, and the devices best suited for small form factors would be those that have integrated readout electronics (and display) on the sensor, and do not rely on bulky external components to read results. The intended audience and uses of device, along with the necessary system components, will play a large part in shaping the appropriate form factor of the device.
Device robustness is important to consider during the development as this will affect the appropriate uses and audience. The robustness of a sensor is important for technical applications like wastewater testing and extended field sampling events by scientists and water managers. Factors such as the length of time until failure, number of measurements until required calibration or maintenance, structural suitability for long term deployment, and accuracy, precision, and sensitivity with time all compose the idea of robustness. These factors will need to be investigated in different capacities depending on the intended application. Sensors based on nanomaterials, such as nanotubes and nanoparticles deposited on electrodes, show promise with respect to resistance to structural damage and chemical degradation, as such devices could still function if a certain portion of the nanomaterial is damaged or degraded. Other users of nitrate sensing devices may not require the same level of robustness, and sensors that excel in alternative categories may be more appropriate. Electrical FET sensors based on a single sheet of nanomaterials are among the least robust with respect to structural damage and chemical degradation, as damage of the nanomaterial can easily render the device unusable. In general, the anticipated device users need to be consulted regarding electrical FET design and packaging, as these devices are among the most sensitive.
Cost is an important consideration in the development of any device, although there is a market for nitrate sensing devices that span a broad price spectrum. Scientists and wastewater managers may need relatively expensive sensors, driven by requirements for low detection limits and high precision. In contrast, community-based science projects or water monitoring will likely require more affordable sensor options with detection limits and precision being less of an integral issue. Spatiotemporal coverage issues will also play a role in dictating the appropriate price point. If a scientific or regulatory question required occasional sampling or sampling in only one place, then devices that are expensive but reliable and rugged would be appropriate. Other applications may be willing to sacrifice accuracy and precision for smallness and inexpensiveness because of the need to deploy many sensors at one time. While it can be difficult to discern the exact cost of a device still in a proof-of-concept stage, the overall cost can be estimated with the given components. Sensors containing integrated electronic readout circuitry, which can be mass-produced, would end up being more cost effective than those requiring additional equipment for every single measurement. Additionally, sensors requiring materials that are easy to procure in scalable quantities or are commercially available would lend themselves to a lower final cost. A variety of devices across a price continuum are needed to meet the unique needs of users related to nitrate sensing.
Much of the literature surrounding the development of nitrate sensors neglects the user interface, but the usability and interfacing of a device is important for its transition to the field. Although some work does consider the user interface (Agir et al., 2021; Alahi et al., 2018), the majority of current devices fail to mention the user experience or the collection of data by a new user in the field. The difficulty and complexity of interface should reflect the intended audience a. The interface for scientists could include more data options and even statistics, while an interface for the general public should be simple, possibly even including easy to interpret colors and icons to be usable across literacy and languages. A major expense associated with sensors installed in the environment is associated with operation and maintenance (e.g., checking, resetting, and recalibrating) in the field because of the travel time and human hours expended. Thus, technicians, interns, and students often fulfill these duties, and the user interface needs to consider the appropriate complexity for people at these career and educational stages. In order to complete a transition from a laboratory tested device to a field utilized product, data and results must be available to the users of the sensors and therefore user interface must be considered.
The necessary sensitivity of a device is directly related to the purpose of the sensing effort. Falling within the category of sensitivity are also important considerations of accuracy, precision, and reproducibility. Sensitivity is important for ecological applications in less impacted areas, especially in cases like alpine lake ecology monitoring, or in the low nitrate concentrations of the open ocean. Accuracy and reproducibility are critical in regulatory situations, which are typically associated with human-impacted areas and therefore less likely to need low detection limits. Instead, they need reliable measurements which can meet regulatory standards and legally binding agreements. Less precise sensors can also still be relevant to community science projects or personal home monitoring, such as a sensor that simply indicates the presence or absence of nitrate to the user. Electrical devices that are 2-dimensional material-based FET sensors may be among the sensors that can provide the best sensitivity in the low ppb range, however the consumer and intended use are important to consider when developing highly sensitive devices. Future work will need to consider the tradeoffs between sensitivity, accuracy, and robustness and would benefit from a comprehensive evaluation of the device and user.
To link prospective nitrate sensors to users, we identified four broad user groups: wastewater managers, farmers, scientists, and community members. For each user, we assigned numerical scores to each FOCUS parameter on the scale of 1–5, where one is least important to the user and five the most, and plotted these in Figure 2A. Wastewater managers prioritize operational robustness (O = 5) the most because monitoring systems must function reliably in harsh environments with varying conditions. Sensitivity (S = 4) follows next as accurate measurements are needed to ensure regulatory compliance and early detection of relevant issues. Cost (C = 3) takes middle priority as while budgets matter, reliable equipment justifies higher expenses. User interface (U = 2) is less critical since staff are trained in complex systems, and form factor (F = 1) ranks lowest as treatment plants have adequate installation space. Scientists place S highest because research demands precise, reliable data for experimental validity. U is also crucial for detailed control over measurements and comprehensive data access. O ranks third as scientists work in a mix of controlled and uncontrolled environments, while F is less important as lab setups are adaptable to research needs and end goals. C ranks lowest because most scientists value data quality data over quantity, justifying higher equipment expenses. This contrasts with farmers, whose priorities center on practical and economic factors, with C ranking highest. O follows next as their equipment must withstand outdoor conditions and physical impacts. F and U share lower priority. While portability and ease of use matter, they are secondary to cost and robustness. S ranks lowest since basic accuracy typically meets agricultural monitoring needs. Community members prioritize C as the main barrier to adoption in voluntary monitoring situations. U ranks second as systems must be accessible without technical training, while F takes middle priority to as portability and power needs are often important for community engaged sampling. O ranks lower as short-term durability often suffices for citizen groups, and S is least important since community monitoring typically emphasizes large numbers of less precise data.
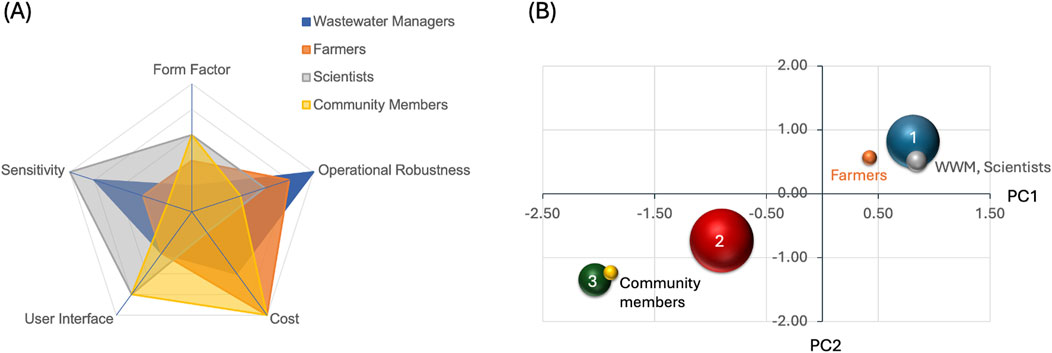
Figure 2. The concept of FOCUS. (A) The five FOCUS metrics with allocated numerical values for four likely groups of users highlight their varied needs. (B) Principal Component Analysis of the relevant citations as a bubble plot showing three distinct clusters (1–3). The data are mapped onto two principal components (PC1 and PC2), with bubble sizes proportional to cluster membership. The specific works that aligned with each end user group are indicated in the vicinity of their clusters by the citation numbers.
We applied the FOCUS analysis to all relevant sensors cited in this perspective (Supplementary Figure S-2). The analysis of the entire dataset of 37 entries indicates that U and S show a clear and consistent negative correlation (Supplementary Figure S-3). This reflects a fundamental design challenge: Highly sensitive sensors require more complex controls, calibration options, and detailed readouts, which lead to less user-friendly interfaces. The relationship demonstrates the inherent challenge of balancing sophisticated measurement capabilities with user friendliness. We performed a cluster analysis on the FOCUS ratings and, as shown in Figure 2B, it reveals three clusters. We plot the results as functions of two principal components (PC1 and PC2), which is a dimensionality reduction technique to reduce the number of variables while preserving as much variance as possible in the data. PC1 is measuring a trade-off between sensitivity/operational robustness versus user interface/form factor, while PC2 compares cost/form factor versus operational robustness. Cluster 1, with positive PC1 and PC2 values, is a collection of sensors with high sensitivity, good operational robustness, strong form factors, but more complicated user interface and higher cost. Cluster two includes sensors that have moderate scores across the FOCUS board, while cluster three comprises sensors with strong operational robustness at lower cost.
Next, we performed a similarity analysis to find which of the 37 entries align best with preferences of each of the end users, based on the profiles for them in Figure 2A. Our results implementing a recommendation system based on cosine similarity reveals the best matches for each user type and are indicated in Figure 2B relative to the three main clusters. Gajaraj et al. (2013) describes a SERS-based nitrate detection system using commercially available gold nano substrates. This scores highest for both wastewater management and scientific research, offering precision equivalent to ion chromatography, suitable detection range (1–100 mg/L), and reliability when faced with interfering compounds. Its quick analysis time, minimal preparation needs, and non-destructive approach support high-throughput monitoring and research requirements. Despite initial equipment costs, the lower per-test expenses and reduced preprocessing make it cost-effective for both routine wastewater testing and scientific studies.
However, while technically sophisticated, the SERS-based approach is less suitable for farmers and community members primarily due to its complexity and operational requirements. It demands complex sample preparation and advanced Raman spectroscopy instrumentation. While highly sensitive, its technical requirements added to high equipment costs make it impractical for non-technical users. Instead, we find the sensor described in Ahmad, et al. (2017) to be ideal for farmers, featuring durable zinc oxide nanorods for field conditions, rapid response time, and minimal sample preparation. The reported detection range suits agricultural needs, covering trace to excess nitrate levels. With proven reliability in real samples, interference resistance, and month-long stability, it enables quick on-site testing for timely fertilizer and irrigation decisions. And finally, Machado et al. (2022) describes the best sensor for community users, with affordable materials and simple construction, while also maintaining good sensitivity. Its cartridge-based design, minimal sample preparation, and Arduino-based system make it user-friendly for citizen scientists. With reliable reproducibility and real-time measurements, it enables effective community water monitoring without requiring technical expertise.
Nanomaterials are part of an exciting new era in sensor technology research and development, and there is a great opportunity for new field devices for sensing nitrate. A variety of sensors exist at the proof-of-concept stage, and different types of sensors excel and struggle under various categories within the FOCUS (form factor, operational robustness, cost, user interface, and sensitivity) framework. With the many possible applications of nitrate sensors, there is no single ideal sensor, instead the ideal is found in successfully meeting the needs of the intended application and user. Electrochemical sensors that are easy to use, low power, and inexpensive could be adequate for monitoring agricultural watersheds, where the limited range of detection overlaps typical field observations (e.g., Patella et al. (2021)). However, to our knowledge, the electrochemical nitrate sensing literature has yet to deeply explore critical FOCUS aspects like operational longevity. Similarly, the impressive detection ranges offered by spectroscopic approaches (e.g., Li et al. (2024)) begs for effort dedicated to lowering the cost of high precision optical components. Integrated lab-on-a-chip sensors can likely overcome these (and other) challenges by enabling self-calibration to extend the lifecycle and autonomy of electrochemical sensors or component miniaturization to reduce material costs. Such approaches are ripe for further developments in nano-enabled materials. As mentioned above, moving beyond the proof-of-concept stage requires attention to all FOCUS aspects, including system integration, power supply optimization, followed by environmental packaging and user interface design. These are steps that are often not rewarded or supported in the academic realm. It would prove beneficial to the field of sensing if funding bodies began investing in the connection of academia and industry to embrace the secondary and tertiary steps of full development, hardening, and environmental packaging. This cross-boundary collaboration, coupled with a new starting lens in research considering the target user first, could prove to be the push the field needs to revolutionize nitrate (and other) sensing technology.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SD: Writing–original draft, Writing–review and editing. SE: Writing–original draft, Writing–review and editing. MP: Writing–original draft, Writing–review and editing. AC: Writing–original draft, Writing–review and editing. BB: Writing–original draft, Writing–review and editing. ZM: Writing–original draft, Writing–review and editing. RB: Writing–original draft, Writing–review and editing. SG: Writing–original draft, Writing–review and editing. TH: Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by grant no. DGE-2125510 from the National Science Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsens.2025.1513701/full#supplementary-material
Agir, I., Yildirim, R., Nigde, M., and Isildak, I. (2021). Internet of things implementation of nitrate and ammonium sensors for online water monitoring. Anal. Sci. 37, 971–976. doi:10.2116/analsci.20P396*
Ahmad, R., Bhat, K. S., Ahn, M. S., and Hahn, Y. B. (2017). Fabrication of a robust and highly sensitive nitrate biosensor based on directly grown zinc oxide nanorods on a silver electrode. New J. Chem. 41 (19), 10992–10997. doi:10.1039/C7NJ02526B
Ahmadi, M. T., Bodaghzadeh, M., Rahimian Koloor, S. S., and Petrů, M. (2021). Graphene nanoparticle-based, nitrate ion sensor characteristics. Nanomaterials 11 (1), 150. doi:10.3390/nano11010150
Alahi, Md E. E., Pereira-Ishak, N., Mukhopadhyay, S. C., and Burkitt, L. (2018). An internet-of-things enabled smart sensing system for nitrate monitoring. IEEE Internet Things J. 5 (6), 4409–4417. doi:10.1109/JIOT.2018.2809669
Brockhage, F., Lüsse, M., Klasmeier, J., Pietzner, V., and Beeken, M. (2022). Citizen science as an innovative approach to analyze spatial and temporal influences on nitrate pollution of water bodies: results of a participatory research project in Germany. Sustain. Basel, Switz. 14 (15), 9516. doi:10.3390/su14159516
Can, F., Korkut Ozoner, S., Ergenekon, P., and Erhan, E. (2012). Amperometric nitrate biosensor based on carbon nanotube/polypyrrole/nitrate reductase biofilm electrode. Mater. Sci. and Eng. C 32 (1), 18–23. doi:10.1016/j.msec.2011.09.004
Chaisriratanakul, W., Bunjongpru, W., Pankiew, A., Srisuwan, A., Jeamsaksiri, W., Chaowicharat, E., et al. (2020). Modification of polyvinyl chloride ion-selective membrane for nitrate ISFET sensors. Appl. Surf. Sci. 512, 145664. doi:10.1016/j.apsusc.2020.145664
Chen Legrand, D., Barus, C., and Garçon, V. (2017). Square wave voltammetry measurements of low concentrations of nitrate using Au/AgNPs electrode in chloride solutions. Electroanalysis 29 (12), 2882–2887. doi:10.1002/elan.201700447
Choudhary, M., Muduli, M., and Ray, S. (2022). A comprehensive review on nitrate pollution and its remediation: conventional and recent approaches. Sustain. Water Resour. Manag. 8 (4), 113. doi:10.1007/s40899-022-00708-y
Concepcion, R., Duarte, B., Gemel Palconit, M., Jahara Baun, J., Bandala, A., Rhay Vicerra, R., et al. (2024). Screen-printed graphite electrode on polyvinyl chloride and parchment strips integrated with genetic programming for in situ nitrate sensing of aquaponic pond water. Inf. Process. Agric. 11, 187–201. doi:10.1016/j.inpa.2023.02.002
Crespo, G. A. (2017). Recent advances in ion-selective membrane electrodes for in situ environmental water analysis. Electrochimica Acta 245, 1023–1034. doi:10.1016/j.electacta.2017.05.159
Cuartero, M., Crespo, G., Cherubini, T., Pankratova, N., Confalonieri, F., Massa, F., et al. (2018). In situ detection of macronutrients and chloride in seawater by submersible electrochemical sensors. Am. Chem. Soc. (ACS) 90, 4702–4710. doi:10.1021/acs.analchem.7b05299
Drevno, A. (2016). Policy tools for agricultural nonpoint source water pollution control in the U.S. And E.U. Manag. Environ. Qual. Int. J. 27 (2), 106–123. doi:10.1108/MEQ-12-2014-0177
Essousi, H., Barhoumi, H., Bibani, M., Ktari, N., Wendler, F., Al-Hamry, A., et al. (2019). Ion-imprinted electrochemical sensor based on copper nanoparticles-polyaniline matrix for nitrate detection. J. Sensors, 2019, 1–14. doi:10.1155/2019/4257125
Gajaraj, S., Fan, C., and Hu, Z. (2013). Quantitative detection of nitrate in water and wastewater by surface-enhanced Raman spectroscopy. Environ. Monit. Assess. 185 (7), 5673–5681. doi:10.1007/s10661-012-2975-4
Gal, C., Frenzel, W., and Moller, J. (2004). Re-examination of the cadmium reduction method and optimisation of conditions for the determination of nitrate by flow injection analysis. Mikrochim. Acta 146 (2), 155–164. doi:10.1007/s00604-004-0193-7
Garcia-Robledo, E., Alfonso, C., and Sokratis, P. (2004). A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes. Mar. Chem. 162, 30–36. doi:10.1016/j.marchem.2014.03.002
Gil, R., Rodriguez-Lorenzo, L., Espiña, B., and Queirós, R. B. (2024). All-solid-state potentiometric sensor based on graphene oxide as ion-to-electron transducer for nitrate detection in water samples. Chemosensors 12, 86. doi:10.3390/chemosensors12060086
Hassan, S., Eldin, A. G., Amr, A. E. G. E., Al-Omar, M. A., Kamel, A. H., and Khalifa, N. M. (2019). Improved solid-contact nitrate ion selective electrodes based on multi-walled carbon nanotubes (MWCNTs) as an ion-to-electron transducer, Sensors (Basel). doi:10.3390/s19183891
Hu, J., Sun, J., Bian, C., Tong, J., and Shanhong, X. (2013). 3D dendritic nanostructure of silver-array: preparation, growth mechanism and application in nitrate sensor. Electroanalysis 25 (2), 546–556. doi:10.1002/elan.201200465
Janata, J. (2022). Chemically sensitive field-effect transistors, past, present and future. ChemElectroChem 9 (23). doi:10.1002/celc.202200784
Kalimuthu, P., Fischer-Schrader, K., Schwarz, G., and Bernhardt, P. V. (2015). A sensitive and stable amperometric nitrate biosensor employing arabidopsis thaliana nitrate reductase. J. Biol. Inorg. Chem. 20 (2), 385–393. doi:10.1007/s00775-014-1171-0
Kalimuthu, P., Kruse, T., and Bernhardt, P. V. (2021). A highly sensitive and stable electrochemical nitrate biosensor. Electrochimica Acta 386, 138480. doi:10.1016/j.electacta.2021.138480
Kazemzadeh, A., and Ali, A. E. (2001). Simultaneous determination of nitrite and nitrate in various samples using flow-injection spectrophotometric detection. Simultaneous Determ. Nitrite Nitrate Var. Samples using Flow-Injection Spectrophotometric Detect., 69, 61–68. doi:10.1016/s0026-265x(01)00072-8
Kim, J., Liu, Q., and Cui, T. (2020a). Graphene-based ion sensitive-FET sensor with porous anodic aluminum oxide substrate for nitrate detection. J. Microelectromechanical Syst. 29 (5), 966–971. doi:10.1109/JMEMS.2020.3008048
Kim, J., Liu, Q., and Cui, T. (2020b). Solution-gated nitrate sensitive field effect transistor with hybrid film: CVD graphene/polymer selective membrane. Org. Electron. 78, 105551. doi:10.1016/j.orgel.2019.105551
Kundu, M., Krishnan, P., and Vashit, A. (2023). Development of electrochemical impedance biosensor using organic nanotubes deposited on screen printed electrodes. J. Agric. Phys. 23.
Li, Y., Han, H., Pan, D., and Zhang, P. (2019). Fabrication of a micro-needle sensor based on copper microspheres and polyaniline film for nitrate determination in coastal river waters. J. Electrochem. Soc. 166 (12), B1038–B1043. doi:10.1149/2.1281912jes
Li, Z., Hu, Y., Wang, L., Liu, H., Ren, T., Wang, C., et al. (2024). Selective and accurate detection of nitrate in aquaculture water with surface-enhanced Raman scattering (SERS) using gold nanoparticles decorated with β-cyclodextrins. Sensors 24, 1093. doi:10.3390/s24041093
Liang, J., Zheng, Y., and Liu, Z. (2016). Nanowire-based Cu electrode as electrochemical sensor for detection of nitrate in water. Sensors Actuators. B, Chem. 232, 336–344. doi:10.1016/j.snb.2016.03.145
Liu, Y., Liu, Y., Meng, Z., Qin, Y., Jiang, D., Xi, K., et al. (2020). Thiol-functionalized reduced graphene oxide as self-assembled ion-to-electron transducer for durable solid-contact ion-selective electrodes. Talanta Oxf. 208, 120374. doi:10.1016/j.talanta.2019.120374
Lockhart, K. M., King, A. M., and Harter, T. (2013). Identifying sources of groundwater nitrate contamination in a large alluvial groundwater basin with highly diversified intensive agricultural production. J. Contam. Hydrology 151, 140–154. doi:10.1016/j.jconhyd.2013.05.008
Loperfido, J. V., Beyer, P., Just, C. L., and Schnoor, J. L. (2010). Uses and biases of volunteer water quality data. Environ. Sci. Technol. 44 (19), 7193–7199. doi:10.1021/es100164c
Machado, M. C., Vimbela, G. V., and Tripathi, A. (2022). Creation of a low cost, low light bioluminescence sensor for real time biological nitrate sensing in marine environments. Environ. Technol. 43, (25), 4002–4009. doi:10.1080/09593330.2021.1939792
Mahmud, M. A. P., Ejeian, F., Azadi, S., Myers, M., Pejcic, B., Abbassi, R., et al. (2020). Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere Oxf. 259, 127492. doi:10.1016/j.chemosphere.2020.127492
Michalski, R., and Kurzyca, I. (2006). Determination of nitrogen species (Nitrate, Nitrite and Ammonia Ions) in environmental samples by ion chromatography. Pol. J. Environ. Stud. 15 (1), 5–18.
Minami, T., Sasaki, Y., Minamiki, T., Wakida, S. i., Kurita, R., Niwa, O., et al. (2016). Selective nitrate detection by an enzymatic sensor based on an extended-gate type organic field-effect transistor. Biosens. and Bioelectron. 81, 87–91. doi:10.1016/j.bios.2016.02.036
Moo, Y. C., Matjafri, M., Lim, H., and Tan, C. (2016). New development of optical fibre sensor for determination of nitrate and nitrite in water. Opt. Stuttg. 127 (3). 1312–1319. doi:10.1016/j.ijleo.2015.09.072
Parveen, S., Pathak, A., and Gupta, B. D. (2017). Fiber optic SPR nanosensor based on synergistic effects of CNT/Cu-nanoparticles composite for ultratrace sensing of nitrate. Sensors Actuators. B, Chem. 246, 910–919. doi:10.1016/j.snb.2017.02.170
Patella, B., Russo, R., O'Riordan, A., Aiello, G., Sunseri, C., and Inguanta, R. (2021). Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta Oxf. 221, 121643. doi:10.1016/j.talanta.2020.121643
Pięk, M., Piech, R., and Paczosa-Bator, B. (2016). All-solid-state nitrate selective electrode with graphene/tetrathiafulvalene nanocomposite as high redox and double layer capacitance solid contact. Electrochimica Acta 210, 407–414. doi:10.1016/j.electacta.2016.05.170
Sarwar Inam, A. K. M., Islam, M. N., Riam, S. Z., Perez, F., Delhom, C., Abidi, N., et al. (2023). Circular sensing of nitrate levels in water with flexible screen-printed sensors on biodegradable cellulose substrate. IEEE Sensors Lett. 7, 1–4. doi:10.1109/LSENS.2023.3301834
Schaider, L. A., Swetschinski, L., Campbell, C., and Rudel, R. A. (2019). Environmental justice and drinking water quality: are there socioeconomic disparities in nitrate levels in U.S. Drinking water? Environ. Health 18 (1), 3. doi:10.1186/s12940-018-0442-6
Schwarz, J., Trommer, K., and Mertig, M. (2018). Solid-contact ion-selective electrodes based on graphite paste for potentiometric nitrate and ammonium determinations. Am. J. Anal. Chem. 9 (12), 591–601. doi:10.4236/ajac.2018.912043
Singh, B., and Craswell, E. (2021). Fertilizers and nitrate pollution of surface and ground water: an increasingly pervasive global problem. SN Appl. Sci. 3 (4), 518–524. doi:10.1007/s42452-021-04521-8
Singh, P., Singh, M. K., Beg, Y. R., and Nishad, G. R. (2019). A review on spectroscopic methods for determination of nitrite and nitrate in environmental samples. Talanta Oxf. 191, 364–381. doi:10.1016/j.talanta.2018.08.028
Singh, S., Anil, A. G., Kumar, V., Kapoor, D., Subramanian, S., Singh, J., et al. (2022). Nitrates in the environment: a critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere Oxf. 287, 131996. doi:10.1016/j.chemosphere.2021.131996
Stortini, A. M., Moretto, L., Mardegan, A., Ongaro, M., and Ugo, P. (2015). Arrays of copper nanowire electrodes: preparation, characterization and application as nitrate sensor. Sensors Actuators. B, Chem. 207, 186–192. doi:10.1016/j.snb.2014.09.109
Tang, I. H., Sundari, R., Lintang, H. O., and Yuliati, L. (2016). Detection of nitrite and nitrate ions in water by graphene oxide as a potential fluorescence sensor. IOP Conf. Ser. Mater. Sci. Eng. 107 (1), 012027–012032. doi:10.1088/1757-899X/107/1/012027
Temkin, A., Evans, S., Manidis, T., Campbell, C., and Naidenko, O. V. (2019). Exposure-based assessment and economic valuation of adverse birth outcomes and cancer risk due to nitrate in United States drinking water. Environ. Res. 176, 108442. doi:10.1016/j.envres.2019.04.009
US EPA (2019). Regulated drinking water contaminants: inorganic chemicals. Available at: https://www.epa.gov/ground-water-and-drinking-water/table-regulated-drinking-water-contaminants (accessed on April 15, 2023).
Wang, Li, Kim, J., and Cui, T. (2018). Self-assembled graphene and copper nanoparticles composite sensor for nitrate determination. Microsyst. Technol. Sensors, Actuators, Syst. Integr. 24 (9), 3623–3630. doi:10.1007/s00542-018-3792-7
Wang, S., Lin, K., Chen, N., Yuan, D., and Ma, J. (2016). Automated determination of nitrate plus nitrite in aqueous samples with flow injection analysis using vanadium (III) chloride as reductant. Talanta Oxf. 146, 744–748. doi:10.1016/j.talanta.2015.06.031
Ward, M. H., Jones, R., Brender, J., De Kok, T., Weyer, P., Nolan, B., et al. (2018). Drinking water nitrate and human health: an updated review. Int. J. Environ. Res. Public Health 15 (7), 1557. doi:10.3390/ijerph15071557
Yang, Y., Chen, S., and Ni, X.-L. (2015). Anion recognition triggered nanoribbon-like self-assembly: a fluorescent chemosensor for nitrate in acidic aqueous solution and living cells. Anal. Chem. 87 (14), 7461–7466. doi:10.1021/acs.analchem.5b01774
Zhang, X., Davidson, E. A., Mauzerall, D. L., Searchinger, T. D., Dumas, P., and Shen, Y. (2015). Managing nitrogen for sustainable development. Manag. Nitrogen Sustain. Dev. 528 51–59. doi:10.1038/nature15743
Keywords: monitoring, nutrient, pollution, contamination, user, technology, device, field
Citation: Defeo S, Erickson S, Perez Mendoza MF, Cooper A, Barrios B, Malone Z, Baxter RD, Ghosh S and Harmon TC (2025) Making nanomaterial-enabled nitrate sensors useful for real water systems: user-centric design perspectives. Front. Sens. 6:1513701. doi: 10.3389/fsens.2025.1513701
Received: 18 October 2024; Accepted: 05 February 2025;
Published: 21 February 2025.
Edited by:
Sandeep Kumar, Punjab engineering college (Deemed to be University), IndiaReviewed by:
Jaroslav Filip, Tomas Bata University in Zlín, CzechiaCopyright © 2025 Defeo, Erickson, Perez Mendoza, Cooper, Barrios, Malone, Baxter, Ghosh and Harmon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shelby Defeo, c2RlZmVvQHVjbWVyY2VkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.