- 1School of Chemistry, The University of Sydney, Sydney, NSW, Australia
- 2Department of Rheumatology, Westmead Hospital, Westmead, NSW, Australia
- 3Department of Rheumatology, Faculty of Medicine and Health, University of Sydney, Westmead, NSW, Australia
- 4The University of Sydney Nano Institute (Sydney Nano), The University of Sydney, Sydney, NSW, Australia
- 5Australian Research Council Centre of Excellence for Innovations in Peptide and Protein Science, The University of Sydney, Sydney, NSW, Australia
Inflammatory rheumatological disorders are often characterised by altered levels of matrix metalloproteinase levels in joint fluid and joint membranes. Therefore, monitoring of MMP activity in synovial fluid is essential to enable timely diagnosis, prognosis, and effective treatment. Here we report a novel fluorescence assay to measure protease activity in aspirated synovial fluid. Our fluorescent reporters contain cleavable linkers based on the peptide sequences of protease substrates, showing a ratiometric output upon cleavage. We have validated our reporters in patient-derived synovial fluid, and demonstrated their ability to characterise disease type.
Introduction
Erosions as often seen in rheumatoid arthritis, psoriatic arthritis, and gout are amongst the leading cause of disability that result of joint destruction and progressive limitation of joint function. Such inflammatory joint conditions are associated with the gradual erosion of bone and cartilage, caused by the destruction of structural and functional proteins such as collagen and aggrecan (Saha and Kohles, 2012). If left unchecked, the action of proteases leads to bone damage and deformities in late stages of disease. Consequently, the close monitoring of disease activity is essential in order to combat the progression of erosive disease to more severe forms which may require more aggressive therapy (Heidari, 2011; Chu et al., 2012).
Several diagnostic and prognostic methods are routinely used in clinical practice to monitor erosive joint conditions, and each has specific advantages and drawbacks. These include imaging methods (e.g. X-ray and magnetic resonance imaging), and biochemical methods to assess the levels of inflammatory markers [e.g. the C-reactive protein (CRP), erythrocyte sedimentation rate (ESR)] (Crockson et al., 1977). While diagnostic imaging can provide specific structural information about the level of damage to bone and cartilage, these structural deformities often progress slowly over time and take a long time to develop (Alasaarela et al., 1998; Døhn et al., 2006). Short-term fluctuations in disease activity can be monitored by measuring inflammatory markers, which are typically of high sensitivity. However, many of these markers, such as CRP and ESR, are non-specific and lack identifiable disease detail, since they report only the presence of systemic inflammation observed in many diseases (Bray et al., 2016). Furthermore, anti-inflammatory medications can directly affect the results of biochemical tests such as CRP (Orr et al., 2018).
The identification of reliable disease biomarkers is a necessary step towards improving the accuracy of diagnosis, monitoring of disease, progression, and prognosis. Towards this end, proteinases have attracted much research interest, as they play a major role in the destruction of structural proteins that line synovial joints. These include collagen, the major organic structural component of bone and cartilage, and proteoglycans, which form a hydrated gel that protects cartilage from wear and tear. The matrix-metalloproteinases (MMPs) and adamalysins are also considered to be major mediators of cartilage destruction (Rengel et al., 2007). Due to their similarities in function and form, many MMPs have been investigated as potential biomarkers of disease: for example, levels of MMP-3, MMP-1 and MMP-13, are known to be elevated in arthritis (Burrage et al., 2006).
Overall proteolytic activity at the site of disease can be an effective method of measuring disease activity, since continued proteolysis underpins the gradual erosion and re-modelling of bone and cartilage. Protease levels have been traditionally measured by antibody-based techniques such as Western blot and enzyme-linked immunosorbent assay (ELISA), but these techniques require multiple steps and reagents, and must be specific to quantities of an individual protease, rather than to general protease activity. An alternative approach is to assess protease activity directly using artificial substrates, through which the products of an enzyme-catalysed reaction are detected optically (Bisswanger, 2014; Robinson, 2015). Given that the protease acts as a catalyst, the optical product can build up over time and subsequent amplification of the signal allows enzymes to be detected at extremely low levels, improving assay sensitivity. Amongst optical readouts, fluorescence has particularly high sensitivity.
We report here the design and application of three novel artificial substrate-based probes to assess protease activity in synovial fluid. We have shown that the results of this new assay positively correlates with disease activity measured by the current gold standard biochemical tests such as CRP and ESR. Linear discriminant analysis (LDA) has shown that our assay provides valuable diagnostic insight as an adjunctive disease marker.
Methods
Synthesis
The 4-amino-1,8-naphthalimide and hemicyanine dyes were prepared by standard synthetic methods as described in Supplementary Material. Peptide linkers were synthesized by solid phase peptide synthesis on Wang resin (Supplementary Material). Experimental details are provided in the Supplementary Material.
Clinical studies
Ethics approval was granted by the Western Sydney Local Health District Human Research Ethics Committee. All patients who presented to Westmead or Royal Prince Alfred Hospitals between May 2017 and September 2018 with a joint effusion for which a synovial fluid joint aspiration was deemed necessary for diagnostic or therapeutic purposes were considered for inclusion in this study. Each synovial fluid joint aspiration was undertaken using an aseptic technique. Specimens were stored at −80°C within 15 min of collection. The synovial fluid specimens were thawed and pipetted into sterile 96-well black clear-bottomed plates with 0.2% w/v sodium azide as a preservative. They were stored at −20°C for no more than 14 days prior to analysis. Clinical and laboratory parameters were collected as described in Supplementary Material.
Spectroscopic studies
Excitation and emission spectra were collected on a PerkinElmer Enspire Multimode Plate Reader in flat-bottomed clear and black 96-well polypropylene or polystyrene plates, or a Varian Cary Eclipse fluorometer in 1 cm pathlength quartz cuvettes. Details of synovial fluid assays are provided in Supplementary Material.
Data was analysed using the SPSS statistics package, which was used to calculate Spearman’s rho correlation coefficient and to perform linear discriminant analysis (LDA) as described in Supplementary Material.
Results and discussion
Probe design, synthesis and fluorescence
To monitor protease activity, we designed a set of ratiometric substrate-based probes. Overall, the designed probes have three functional units: 1) two fluorophores that provide ratiometric fluorescence readout; 2) a cleavable peptide linker that is recognised by MMPs; and 3) a charged group that increases water solubility (Figure 1).
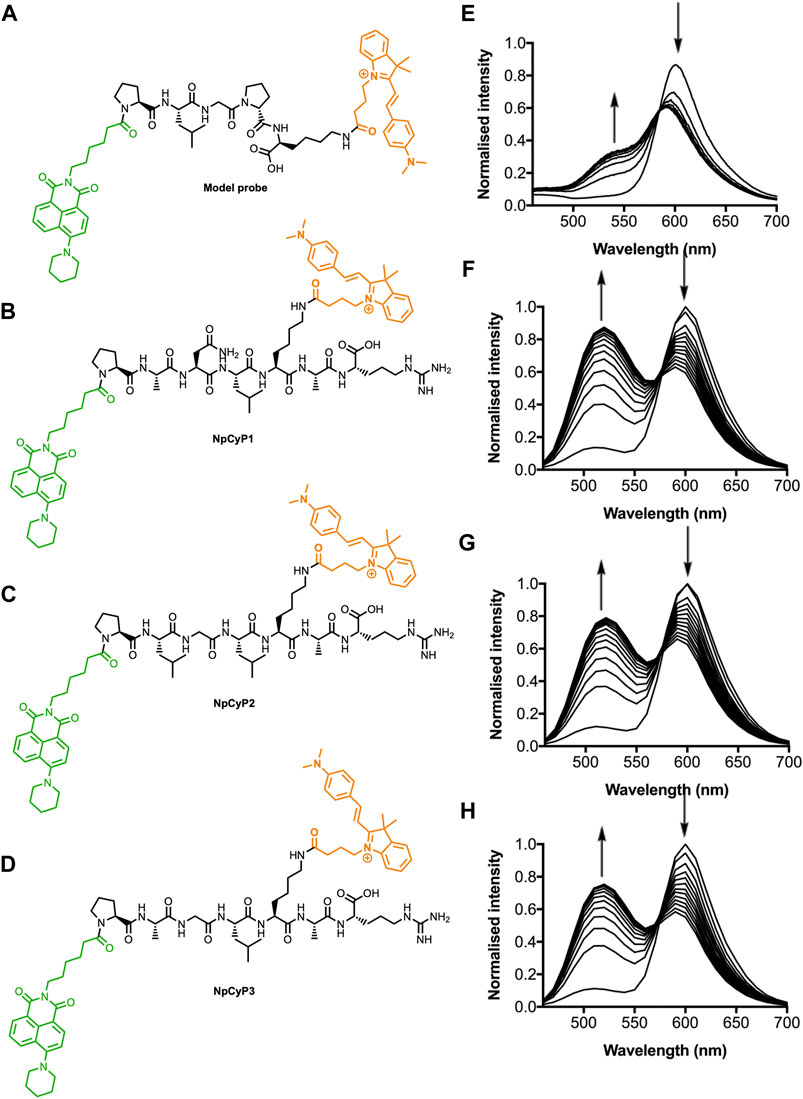
FIGURE 1. Structure and normalised fluorescence spectra of probes during digestion with proteases. Structures of the NpCyC (A), NpCyP1 (B), NpCyP2 (C), and NpCyP3 (D). Normalised fluorescence emission spectra of NpCyC during digestion with type IV collagenase (E). The reaction mixture contained probe (50 μM) and type IV collagenase (3.3 μg/ml) in Krebs-Ringer buffer (pH 7.4) and was monitored at 25°C taking 1 scan every 2 min using 405 nm excitation. Normalised fluorescence emission spectra of NpCyP1 (F), NpCyP2 (G), and NpCyP3 (H) in patient-derived synovial fluid presenting with inflammatory arthritis. Spectra represent changes to fluorescence in 10 min intervals over 2 h. Arrows indicate changes with increasing incubation time.
The use of two fluorophores at each end of the probe was designed to provide a ratiometric fluorescence output that minimises background effects that would normally affect overall intensity such as fluctuations in probe concentration, varying intensity of the excitation source and turbidity (New, 2016). In this case, we selected a green-emitting 4-amino-1,8-naphthalimide and an orange-emitting hemicyanine to ensure two distinct emission peaks with minimal spectral overlap (Supplementary Figure S1). Furthermore, this fluorophore pair has previously been shown to be a suitable donor-acceptor pair for Förster resonance energy transfer (FRET), a mechanism commonly employed for the preparation of ratiometric systems (Shen and Qian, 2019, 2020). Throughout this work, excitation wavelengths between 405 nm and 460 nm were used because this is the spectral region of the donor fluorophore excitation (Supplementary Figure S1).
The peptide linker was chosen to maximise the sensitivity of MMP detection. Eight optimal recognition sequences were selected from those previously reported to be recognised by MMP activity (Eckhard et al., 2016). From the eight optimal recognition sequences published by Eckhard et al. (2016), we identified the three most distinct sequences. We anticipated that these substrates, when used together, should be capable of detecting activity from MMPs within a wide range of MMP classes (Eckhard et al., 2016). Each peptide also included a C-terminal arginine residue so that the zwitterionic character would increase water-solubility. The synthesis of the probe involved initial preparation of fluorophores functionalised with a carboxylic acid for subsequent peptide coupling, followed by assembly of the probe on Wang resin via solid phase peptide synthesis (SPPS) (Supplementary Material).
This method was used to synthesise four probes with different enzyme recognition sequences (Figure 1): three bearing the MMP recognition peptides described above (NpCyP1, NpCyP2 and NpCyP3), and a model probe, NpCyC. Since MMPs could not be purchased commercially, NpCyC was designed containing the recognition sequence of commercially available type IV collagenase (PLGP). Upon addition of the collagenase IV to a solution of the probe, an emission change from orange to green was observed, which is consistent with cleavage of the peptide linker and disruption of FRET. In the unreacted form we expect FRET from the napthalimide donor to the hemicyanine acceptor. Upon reaction, the cleavage creates physical distance between the donor and the acceptor, so only naphthalimide emission is observed upon excitation of the naphthalimide.
Following promising studies of NpCyC with isolated enzyme, we investigated whether NpCyP1, NpCyP2 and NpCyP3 could detect protease activity in synovial fluid. Sinovial fluid aspirates were collected from the joints of patients presenting with a range of erosive conditions (Supplementary Table S1). As we observed for the collagenase IV and NpCyC system, addition of synovial fluid caused a change from orange to green fluorescence emission in the MMP probes, consistent with cleavage of the linker (Figure 1). In order to confirm that this colour change was caused by enzyme activity, control experiments were performed in which highly active synovial fluid specimens were chosen and a portion was denatured using heat and sodium dodecyl sulfate (SDS). In these denatured samples, no change in the fluorescence ratio was observed (Supplementary Figure S2). Overall, these experiments indicate that MMP probes, NpCyP1, NpCyP2 and NpCyP3, have sufficient sensitivity to detect protease activity at biologically relevant concentrations in synovial fluid, while the control experiments demonstrate that fully folded proteins are needed to catalyse this fluorescence response.
Patient data
Having demonstrated the sensitivity of our set of sensors, we applied it to synovial fluid that was collected from patients presenting with a range of rheumatic conditions. From the 58 patients included in this study, 66 synovial fluid samples were collected. The biochemical and haematological markers and the observations of synovial fluid microscopy and culture were also recorded (Supplementary Table S1).
The protease activity varied greatly amongst the different synovial fluid specimens, which is represented by the varied reaction profiles (Figure 2). Overall, these reaction profiles differed mainly in the initial rate of reaction over the first 15 min and the final fluorescence ratio [green (535 nm): orange (610 nm)] recorded after 120 min. Because NpCyP1, NpCyP2 and NpCyP3 could successfully discriminate between the synovial fluid specimens, we aimed to perform high throughput assays to obtain data for correlative studies that investigate the relationship between protease activity and clinical biomarkers of disease activity.

FIGURE 2. Fluorescence of MMP substrates (NpCyP1, NpCyP2, NpCyP3) over time in synovial fluid- Graphs represent the fluorescence ratio of NpCyP1 (blue), NpCyP2 (grey), and NpCyP3 (red) over time in synovial fluid. Four representative graphs show varied fluorescence response in four different synovial fluid samples. Patients presented with a tibial fracture (A), osteoarthritis (B), rheumatoid arthritis (C) and gout (D). Error bars show standard error of the mean from values obtained when the assay was repeated 3 times, and error bars are too small to be seen in panel (A). Table shows Spearman’s correlation coefficient (ρ), two-tailed significance (P) and sample size (N) (E). Variables with significant correlations (p < 0.05) are represented in bold. Asterisks mark significant correlations (* p < 0.05, **p < 0.01). The cells are colour coded such that the intensity of the blue colour correlates to weak (0.2–0.4), moderate (0.4–0.6), strong (0.6–0.8) or very strong (0.8–1) correlation.
Both the initial rate and the final ratio were used in subsequent correlative analysis. Overall, we found that the initial rate could more finely discriminate between the synovial fluid specimens, showing greater dynamic ranges for all three probes than the final fluorescence ratio (Supplementary Table S2). These differences in the dynamic range are likely because the initial rate can better discriminate between weakly and highly active specimens, whereas the final ratio at the end point of the reaction is similar (Figures 2C,D).
Overall, the result gained from the individual probes were highly correlated (Supplementary Table S3). NpCyP1 and NpCyP3 were the most closely related, consistent with their reported high reactivity towards MMP-3 (Eckhard et al., 2016), which is most commonly investigated as a biomarker of arthritis (So et al., 1999; Eckhard et al., 2016; Lerner et al., 2018).
In order to determine whether the probes could distinguish between mild and severe forms of erosive disease, we examined the correlations between our probe and the gold-standard biomarkers of disease activity. Predominantly strong correlations were observed between probe reactivity and local markers of inflammation, while predominantly moderate correlations were observed between probe reactivity and the local markers of inflammation (Figure 2D). NpCyP1 and NpCyP3 showed the stronger correlation to the inflammatory biomarkers compared to NpCyP2, consistent with their high reactivity to MMP-3, a well-researched biomarker of arthritis (So et al., 1999; Eckhard et al., 2016; Lerner et al., 2018).
Ultimately, the assay results were significantly correlated to key biomarkers of disease activity including the synovial leukocyte and polymorph count (ρ = 0.433–0.716), and CRP (ρ = 0.545, 0.449, 0.524 for NpCyP1, 2, and 3 respectively). Interestingly, there were only weak to moderate correlations between the biochemical markers of inflammation themselves (Supplementary Tables S4, S5), confirming our speculation that a reliance on inflammation markers alone for diagnosis may not always prove accurate, and justifying the need to explore alternative biomarkers. We employed linear discriminant analysis (LDA) on our clinical patient data to determine whether adding the data from our MMP probes could enhance the accuracy of diagnosis and prognosis.
LDA is a multivariate statistical method that can be used to find linear combinations to characterise or separate data classes: in this case, rheumatological condition diagnoses. A smaller subset of the original 66 specimens was used in LDA, as these cases contained complete clinical datasets. Of these 33 specimens, 13 had diagnoses of a crystal arthritis condition (including gout and pseudogout), 8 had a diagnosis of inflammatory arthritis (including rheumatoid arthritis, psoriatic arthritis, reactive arthritis and inflammatory arthritis of an unknown aetiology), 3 had a diagnosis of osteoarthritis and 9 specimens had a combination of diagnoses or an uncertain diagnosis. The first application of LDA aimed to discriminate between crystal arthritis, inflammatory arthritis and osteoarthritis cases (combination and uncertain diagnoses were not used in the discriminant analysis) using just clinical blood test data. This gave 79.2% accuracy, which dropped to 62.5% in the leave-one-out cross validation test (Supplementary Table S6).
In our next LDA test, clinical synovial fluid data (leukocytes, erythrocytes, polymorphs, mononuclear cells, uric acid and calcium pyrophosphate deposition (CPPD)) was used as input data, instead of blood test results such as CRP. This set of input data yielded a very similar diagnosis accuracy of 79.2% in the initial classification and 75.0% in the leave-one-one cross-validation test (Figure 3B, Supplementary Table S7). Encouragingly, when we added the rates and ratios obtained from our MMP probe assay to the synovial fluid clinical test dataset, the classification accuracy rose to 91.7% (70.8% cross-validated) (Supplementary Table S8). Furthermore, visualisation of LDA scores against the first three discriminant functions shows superior clustering compared to the tests performed using blood tests or clinical synovial fluid tests alone (Figure 3).

FIGURE 3. LDA score plots to discriminate between specimens with diagnoses of crystal arthritis (n = 13), inflammatory arthritis (n = 8) and osteoarthritis (n = 3) s using blood test clinical data only (A), synovial fluid clinical data only (B) and synovial fluid clinical data and MMP probe assay data (C).
In general, cross-validation accuracies that we achieved from LDA are consistent with those observed for sample sets of this size. LDA performance increases with sample size, so a larger clinical trial in the future would be expected to give initial and cross-validation accuracies closer to 100% (Mitchell et al., 2021). The 91.7% accuracy using a combination of synovial fluid clinical test and MMP probe assay results surpasses the original accuracy using CRP and other blood test results. Therefore, protein proxy measurements, such as our MMP probe assay, are useful tools to increase diagnosis accuracy in synovial fluid alone, precluding the need to collect a second body fluid such as blood.
Conclusion
We have established and demonstrated the design, synthesis, and application of novel ratiometric fluorescent probes to measure and investigate MMP activity in synovial fluid. There is potential for the reported assay to be applied as an adjunctive marker of disease activity, as it was able to distinguish between mild and severe cases of arthritis through its significant correlation to traditional biomarkers of disease activity (CRP, ESR, WCC, and synovial polymorphonuclear cells). Additionally, LDA was used to show that our assay could provide valuable diagnostic information, especially in cases where standard markers such as CRP are absent. The probes were designed with the goal of maximising sensitivity, which was achieved through reaction-based amplification of the fluorescence signal as one enzyme can convert many substrates over time. Furthermore, quantitative comparison between samples was made possible, as the florescence ratio provides internal standardisation to control for variables that can alter the fluorescence intensity at a single wavelength such as sample turbidity, probe concentration and instrumental variation. Hence ratiometricity and reaction-based signal amplification are desirable properties, and the application of such probes may enable the development of fast, high throughput and convenient assays that have the potential to be applied in a clinical diagnostic setting. Since MMP activity is a feature of other conditions such as cancer (Vihinen and Kähäri, 2002), glaucoma (Sahay et al., 2017), multiple sclerosis (Matrix metalloproteinase 9 as a marker of disease activity in multiple sclerosis, 2006), and alcoholic liver disease (Prystupa et al., 2015), it is possible to envisage the use of these or derivative probes for a wide range of other clinical applications.
As developments in functional proteomics advance, there is greater potential to discover a wider range of enzyme-based disease biomarkers, presenting opportunities for the subsequent rational design of diagnostic tools. While this paper presents an example where newly developed probes are applied to sense protease activity in the context of erosive joint disease, there is future potential to further this research and build ratiometric substrates for other enzyme biomarkers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Western Sydney Local Health District Human Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KY, NM, and EN conceptualised the study. KY synthesised the probes, and carried out spectroscopic studies. KP collected patient samples, including collection of clinical and laboratory parameters. EN performed correlation analysis, and AB performed LDA. KY and KP wrote the manuscript. AB, NM, and EN edited the manuscript.
Acknowledgments
The authors would like to acknowledge the Westpac Scholars Trust for a Research Fellowship (EN), the University of Sydney for a SOAR Fellowship (EN), the University of Sydney for a Commercial Development and Industry Partnership Development Grant (EN and NM), the Australian government for Research Training Program Scholarships (KY and AB), and the University of Sydney Nano Institute Postgraduate Supplementary Scholarship (KY), Bruce Veness Chandler Research Support Scholarship in Food Chemistry (KY), Vice Chancellors Research Scholarship (KY), and the Henry Bertie and Florence Mabel Gritton Medallist Award (AB). The authors acknowledge the facilities and the scientific and technical assistance of Sydney Analytical, a core research facility at The University of Sydney.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsens.2022.970921/full#supplementary-material
Reference
Alasaarela, E., Suramo, I., Tervonen, O., Lahde, S., Taklo, R., Hakala, M., et al. (1998). Evaluation of humeral head erosions in rheumatoid arthritis: A comparison of ultrasonography, magnetic resonance imaging, computed tomography and plain radiography’, British journal of rheumatology. Br. J. Rheumatol. 37 (11), 1152–1156. doi:10.1093/Rheumatology/37.11.1152
Bisswanger, H. (2014). Enzyme assays’, Perspectives in science. Perspect. Sci. (Neth). 1 (1–6), 41–55. doi:10.1016/J.PISC.2014.02.005
Bray, C., Bell, L. N., Liang, H., Haykal, R., Kaiksow, F., Mazza, J. J., et al. (2016). Erythrocyte sedimentation rate and C-reactive protein measurements and their relevance in clinical medicine. Wis. Med. J. 115 (6), 317–321.
Burrage, P. S., Mix, K. S., and Brinckerhoff, C. E. (2006). Matrix metalloproteinases: Role in arthritis. Front. Biosci. 11, 529–543. doi:10.2741/1817
Chu, C. R., Williams, A. A., Coyle, C. H., and Bowers, M. E. (2012). Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis Res. Ther. 14 (3), 212. doi:10.1186/ar3845
Crockson, R. A., Crockson, A. P., and McConkey, B. (1977). Rheumatoid arthritis: Relation of serum C-reactive protein and erythrocyte sedimentation rates to radiographic changes. Br Med J. Br. Med. J. Publ. Group 1 (6055), 195–197. doi:10.1136/BMJ.1.6055.195
Døhn, U. M., Ejbjerg, B. J., Court-Payen, M., Hasselquist, M., Narvestad, E., Szkudlarek, M., et al. (2006). Are bone erosions detected by magnetic resonance imaging and ultrasonography true erosions? A comparison with computed tomography in rheumatoid arthritis metacarpophalangeal joints. Arthritis Res. Ther. 8 (4), R110. doi:10.1186/ar1995
Eckhard, U., Huesgen, P. F., Schilling, O., Bellac, C. L., Butler, G. S., Cox, J. H., et al. (2016). Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 49, 37–60. doi:10.1016/j.matbio.2015.09.003
Heidari, B. (2011). Rheumatoid arthritis: Early diagnosis and treatment outcomes. Casp. J. Intern. Med. 2 (1), 161–170. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24024009.
Lerner, A., Neidhofer, S., Reuter, S., and Matthias, T. (2018). MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis’, Best Practice & Research Clinical Rheumatology. Best. Pract. Res. Clin. Rheumatol. 32 (4), 550–562. doi:10.1016/J.BERH.2019.01.006
Matrix metalloproteinase 9 as a marker of disease activity in multiple sclerosis (2006). Nature clinical practice neurology. Nat. Publ. Group. 2, 464. doi:10.1038/ncpneuro0231
Mitchell, L., Shen, C., Timmins, H. C., Park, S. B., and New, J. E. (2021). A versatile fluorescent sensor array for platinum anticancer drug detection in biological fluids. ACS Sensors. Am. Chem. Soc. 6 (3), 1261–1269. doi:10.1021/ACSSENSORS.0C02553/SUPPL_FILE/SE0C02553_SI_001.PDF
New, E. J. (2016). Harnessing the potential of small molecule intracellular fluorescent sensors. ACS Sens. 1 (4), 328–333. doi:10.1021/acssensors.6b00148
Orr, C. K., Young, F., McGarry, T., Binickea, M., Fearon, U., Veale, D. J., et al. (2018). The utility and limitations of CRP, ESR and DAS28-CRP in appraising disease activity in rheumatoid arthritis. Front. Med. 5 (JUN), 185. doi:10.3389/FMED.2018.00185/BIBTEX
Prystupa, A., Boguszewska-Czubara, A., Bojarska-Junak, A., and Toruń-Jurkowska, A. (2015). Activity of MMP-2, MMP-8 and MMP-9 in serum as a marker of progression of alcoholic liver disease in people from Lublin Region, eastern Poland. Ann. Agric. Environ. Med. 22 (2), 325–328. doi:10.5604/12321966.1152088
Rengel, Y., Ospelt, C., and Gay, S. (2007). Proteinases in the joint: Clinical relevance of proteinases in joint destruction’, arthritis research & therapy. Arthritis Res. Ther. 9 (5), 221. doi:10.1186/AR2304
Robinson, P. K. (2015). Enzymes: Principles and biotechnological applications. Essays Biochem. 59 (0), 1–41. doi:10.1042/bse0590001
Saha, A. K., and Kohles, S. S. (2012). A cell-matrix model of anabolic and catabolic dynamics during cartilage biomolecule regulation’, International Journal of Computers in Healthcare. Int. J. Comput. Healthc. 1 (3), 214. doi:10.1504/ijcih.2012.046995
Sahay, P., Rao, A., Padhy, D., Sarangi, S., Das, G., Reddy, M. M., et al. (2017). Functional activity of matrix metalloproteinases 2 and 9 in tears of patients with glaucoma’, investigative ophthalmology & visual science. Invest. Ophthalmol. Vis. Sci. 58 (6), BIO106–BIO113. doi:10.1167/IOVS.17-21723
Shen, R., and Qian, Y. (2019). A mitochondria-oriented fluorescent probe for ultrafast and ratiometric detection of HSO3− based on naphthalimide–hemicyanine’, New Journal of Chemistry. New J. Chem. 43 (20), 7606–7612. doi:10.1039/C9NJ01467E
Shen, R., and Qian, Y. (2020). A novel ratiometric fluorescent probe for specific detection of HSO3- at nanomolar level through 1, 4-Michael addition. J. Photochem. Photobiol. A Chem. 387, 112110. doi:10.1016/J.JPHOTOCHEM.2019.112110
So, A., Chamot, A. M., Peclat, V., and Gerster, J. C. (1999). Serum MMP-3 in rheumatoid arthritis: Correlation with systemic inflammation but not with erosive status. Rheumatology 38 (5), 407–410. doi:10.1093/rheumatology/38.5.407
Keywords: ratiometric fluorescent sensor, artificial substrate, diagnostic array, matrix metalloproteinase, rheumatological conditions
Citation: Yang K, Pavic K, Bowyer AA, Manolios N and New EJ (2022) Supplementing clinical diagnostics of erosive joint diseases with bio-inspired ratiometric sensors. Front. Sens. 3:970921. doi: 10.3389/fsens.2022.970921
Received: 17 June 2022; Accepted: 29 July 2022;
Published: 23 August 2022.
Edited by:
Junjie Zhu, Nanjing University, ChinaCopyright © 2022 Yang, Pavic, Bowyer, Manolios and New. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elizabeth J. New, RWxpemFiZXRoLm5ld0BzeWRuZXkuZWR1LmF1
 Kylie Yang
Kylie Yang Katrina Pavic
Katrina Pavic Amy A. Bowyer
Amy A. Bowyer Nicholas Manolios2,3
Nicholas Manolios2,3 Elizabeth J. New
Elizabeth J. New