
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Sens. , 10 May 2022
Sec. Electrochemical Sensors
Volume 3 - 2022 | https://doi.org/10.3389/fsens.2022.901628
 R. Rejithamol*†
R. Rejithamol*† S. Beena†
S. Beena†The relevance of the carbon-paste electrodes in the field of neurotransmitter electrochemical sensing is focused on in this review. The significance of biomolecules especially neurotransmitters in treatments related to different diseases has tremendously expanded the scope of analytical detection of these biomolecules. The detection of them from biological fluids and pharmaceutical dosages is highly recommendable because the normal functioning of a human body is very much related to the exact concentrations of these biomolecules. Therefore, electroanalytical techniques can be employed for the quantification of these molecules as these techniques take over the advantage of fast response time, are easy to handle, and possess highly sensitive results. Due to the cost-effectiveness and vague electron transfer kinetics, many carbon-paste electrode-based electrochemical sensors have been developed for various biomolecules, environmental pollutants, food additives, and pharmaceuticals. This review gives an intuition on different materials used for the quantification of neurotransmitters using carbon-paste electrode modified electrochemical methods. The electrochemical analysis of neurochemicals by probing the various analytical utilities of carbon-paste electrodes can enlighten the upcoming research on these molecules.
To date, numerous electrochemical methods have been fabricated by many research groups for analytical applications in the fields of biology, food, and pharmaceuticals (Cheraghi et al., 2017; Chandra et al., 2017; Saifeldin, 2020). Many analytical techniques have been developed in these fields, including chromatography, spectrophotometers, etc. (Gupte and Luthra, 2017; Shi et al., 2020), but electrochemical techniques are used to measure electroactive analytes at low cost and with high sensitivity. It has a lot of uses because it provides an easy way. The salient features of electrochemical technology, such as simple equipment, fast response times, high sensitivity, and easy miniaturization, have led researchers to explore this field extensively (Deepa et al., 2016; David et al., 2017; Purushothama et al., 2018). This review focuses on updating the advances in electrochemical sensors in the field of biology with carbon paste electrodes (CPE) that take neurotransmitters into account. Scientists have recently focused on exploring numerous electrochemical methods based on a novel approach for detecting neurotransmitters. It highlights the properties of carbon paste-based electrochemical sensors for analytical studies of neurotransmitters in biological samples (Sasya et al., 2020; Santhy et al., 2021) for the past 10 years (Figure 1). Numerous modified CPE has also been identified to improve electrochemical detection of neurochemicals over bare electrodes.
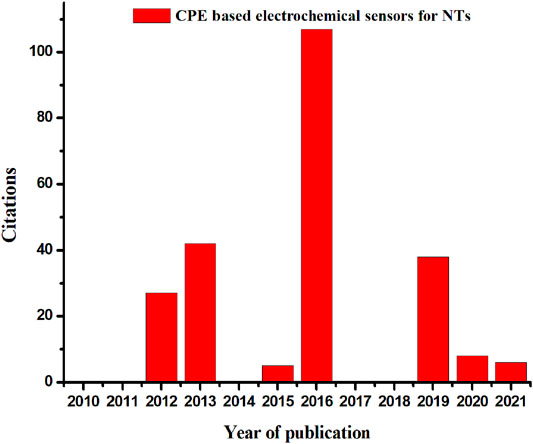
FIGURE 1. A graph showing the evolution of published work and citations in the field of CPE based electrochemical sensors for NTs.
The determination of neurochemicals such as dopamine, serotonin, epinephrine, norepinephrine, gamma-aminobutyric acid, glutamate, endorphins, adenosine, adenosine triphosphate, and acetylcholine from biological samples is highly requisite according to the clinical point of view. These molecules are very important in boosting and balancing nerve signals and help in the normal functioning of the brain. Therefore, proper analysis of these molecules is necessary, and sophisticated analytical devices could also serve as a tool for the determination of these neurochemicals from real-life samples. The widely available analytical techniques comprising conductometry (Sartori et al., 2011), colorimetry (Idowu et al., 2004), spectrophotometry (El-Ries et al., 2000; Gowda et al., 2002; Salem, 2002; Gölcü, 2008), chromatography (Rapado-Martínez et al., 1997; El-Saharty, 2003), fluorescent spectroscopy (Muñoz de la Peña et al., 1991; Pérez Ruiz et al., 1998; Ramesh et al., 2003; Tabrizi, 2007), potentiometry (Hassan et al., 2003) and voltammetry (El-Ries et al., 2002; Sartori et al., 2010; Lourencao et al., 2014) were reported for the detection of neurotransmitters. Among these analytical techniques, voltammetric methods are a dynamic electroanalytical technique used to study the electrochemistry and mechanism of the redox reactions of various organic and inorganic molecules (Heinze, 1984). The instrumentation involves a potentiostat, an electrochemical cell, and a three-electrode system with a working electrode, a counter electrode, and a reference electrode. A range of potential is applied between the working and the reference electrode and the obtained current due to the electrooxidation or reduction of the analyte is measured between the counter and working electrode (Li et al., 2012). The electrochemical sensors are devices that use the working electrode as the sensing element, which consists of two parts, a recognition element for analytes and a transducer (Bakker and Telting-Diaz, 2002). The molecular identification constituent produces an electrochemical response during the redox reaction of the analyte and the corresponding message was transformed into a suitable signal by a transducer. The signal was then detected by advanced electrical expedients (Sehit and Altintas, 2020).
The development of a precise and dependable technique for the quantitative detection of analytes in biological samples has sparked a lot of interest, especially in early diagnosis and clinical research. The advancement of a new class of modified electrodes known as biosensors has been aided by research into biological recognition components and their capabilities (Salem, 2002). Non-enzymatic biosensors are less specific and superior to enzyme-catalyzed biosensors (El-Saharty, 2003). Enzymatic biosensors, on the other hand, have some drawbacks, such as maintaining a low temperature, electrolyte pH, clean ambient variables, high cost, and protein immobilization on the electrode surface (Rapado-Martínez et al., 1997). As a result, non-enzymatic biosensors have sparked far more studies than enzymatic biosensors. Biosensors are analytical tools that can be expanded to include neurotransmitters and come in a variety of sorts and methodologies. According to several research, neurotransmitters are usually produced at the same time, therefore a physiological sample is likely to contain many neurotransmitters. Simultaneous measurement and identification of neurotransmitters are quite useful in these situations and this review introduces a variety of developed biosensors that can monitor numerous neurotransmitters at the same time. With respect to the simultaneous diagnosis of various neurotransmitters, a variety of multifunctional biosensors have been introduced.
The voltammetric methods are the most suitable method for the fabrication of point-of-care electrochemical sensing devices due to their sensitive detection of analytes with lower detection limits and the amenability for miniaturization (Sartori et al., 2011). Also, multiple analyte detection was possible with easy sample preparation in a cost-effective way via voltammetric approaches than other methods mentioned above (Mathew et al., 2018). So, this review gives a comprehensive study on the CPE for the electrochemical determination of various neurochemicals from biological and real samples. Most of the electrochemical sensors for neurotransmitters employed are modified and bare CPEs for the electrochemical analysis. Unmodified CPEs have a low sensitivity and low limits of detection (LODs), which makes them unsuitable for regular analyses but on the other hand, the modified electrode possesses enhanced properties like high selectivity, and electrochemical response of the analyte with increased electrocatalytic activity than the bare electrodes (Wring and Hart, 1992). A multitude of techniques like electropolymerization, coating of the electrode with nanoparticles or nanocomposites by drop-casting, self-assembled monolayers, electrodeposition techniques, and molecular imprinting were selected by researchers for the fabrication of various electrochemical sensors (Figure 2) (Madhusudhana et al., 2020; Mandler and Kraus-Ophir, 2011; Rao et al., 2017; Vadivaambigai et al., 2015; Vedhi et al., 2009; Willander et al., 2008). Also, a wide range of nanomaterials like carbon nanotubes (CNTs), graphene, metal nanoparticles, metal oxide nanomaterials, MXenes, etc. was exploited for developing numerous electrochemical sensors so far (Figure 3A) (Ramakrishnan et al., 2015; Sehit and Altintas, 2020; Szuplewska et al., 2020). Among these carbon-based nanomaterials, CNTs and graphene possessed several astonishing behaviors such as electrical and optical properties (Zhu et al., 2012; Martín et al., 2015). Moreover, the carbon nanomaterials are more suitable for the modification of the electrodes due to their sp2 hybridization of carbon and the capability to form charge transfer complexes (Giuliani et al., 2016). The following section provides detailed information regarding the electrochemical investigation of different neurotransmitters from real samples with several modified and bare CPEs.
Biomolecules are the fundamental building blocks of living cells that provide the foundation of life. The exact measurement of the concentration of a biomolecule in a living system is very important both from the medicinal and clinical points of view. Electrochemical methods are suited for the qualitative and quantitative determination of biomolecules, as these methods offer high selectivity and sensitivity in clinical diagnostics. Neurotransmitters (NTs) are important chemicals in the human system that play important roles in physical and physiological health. Among the various NTs, biogenic amines, including dopamine, serotonin, epinephrine, norepinephrine, and especially amino acids such as tyrosine and acetylcholine, are potentially important (Figure 3B). NT mediates important functions of the nervous system such as behavior and cognitive function. They affect and regulate muscle tension, heart rate, sleep, learning, consciousness, memory, appetite, and mood. The irregular levels of neurochemicals are associated with physical, psychotic, and neurodegenerative disorders such as Alzheimer’s disease, Parkinson’s disease, dementia, addiction, depression, and schizophrenia (Table 1) (Sasya et al., 2020). Neurotransmitters are found in very low concentrations in the nervous system, and they are combined with a variety of other biological compounds and minerals, making their detection and quantification challenging. Even though several strategies have been proposed in the literature, neurotransmitter monitoring in the brain remains a problem and a focus of current research. Several parameters are important to consider when evaluating the sensor’s performance: the sensor’s sensitivity and limit of detection (LOD) must be enough for the target neurotransmitter’s concentration in serum. In addition, the sensor’s selectivity must be high enough because the real sample may contain a lot of interference. For a robust study, reproducibility is also essential. The electrodes may foul because of protein sticking or adsorption in real samples (Sartori et al., 2010). Fouling can have a significant impact on sensor response, hence it is important to build bio-fouling-resistant electrode surfaces (Heinze, 1984). Multiple neurotransmitters must be detected at the same time, which is a tough process because many neurotransmitters have similar molecular structures and physicochemical properties, making it impossible to distinguish one from the other. The detection and monitoring of neurochemicals in the brain remain difficult due to the inadequacy of the existing methods and therefore new approaches continue to receive great attention in this field. To monitor the complex intercommunication of NTs, neurotechnology is highly desirable because it provides high throughput, rapid and selective determination of targeted analytes in the brain without adversely affecting the transplanted area (Giuliani et al., 2016). Therefore, it is important to develop sensitive and reliable clinical evaluation methods to observe and coordinate these molecules and detect diseases related to them. The highlight of this review is a summary of present-day electrochemical analytical methods that enable the real-time detection of neurotransmitters with high time resolution.
CPEs has been used for the electrochemical determination of different neurotransmitters by adopting various electrochemical strategies. CPE electrode was developed by Nada et al. to measure paracetamol (ACOP) and neurotransmitters such as dopamine (DA), epinephrine (EP), noradrenaline (NP), levodopa (L-DOPA), and serotonin (5HT). Differential pulse voltammetry (DPV) and cyclic voltammetric (CV) studies have revealed that the analytes undergo oxidation on the electrode surface via adsorption controlled reversible two-electron step. The overlapped anode peaks of these NTs were sensitively and effectively resolved by mixing CPE-Au nano with Nafion. They investigated the interference of other biomolecules mainly ascorbic acid (AA) and uric acid (UA) with the current response of paracetamol, namely the destructive effects of Nafion have been shown to increase the sensitivity of the sensor’s current signal to the oxidation of the compounds under study. The applicability of the method developed for measuring drugs in human urine samples has also been demonstrated (Nada et al., 2011). The same research group has been developed a promising electrochemical sensor using CPEs incorporated with gold nanoparticles and Nafion as the linker. This method is sensitive to the measurement of catecholamine compounds: DA, EP, NP, LDOPA, and 5-HT in the presence of interfering molecules such as UA and AA. DPV, CV, and electrochemical impedance spectroscopy were used to examine the behavior of the compound under study at the body’s physiological pH. Simultaneous measurements of DA with 5-HT and L-DOPA with acetaminophen (ACOP) gave very good peak separation. The L-DOPA linear response ranges from 2.0 × 10−7 to 2.0 × 10−5 mol L−1, with a correlation coefficient of 0.9990 and a second range from 5.0 × 10−5 to 3.0 × 10−3 MolL−1 obtained with a correlation coefficient of 0.9985. The LODs were found to be 1.45 × 10−9 mol L−1 and 2.84 × 10−6 mol L−1, respectively. The usefulness of this modified electrode has been demonstrated to measure L-DOPA in human urine (Nada, 2012; Atta et al., 2020).
Wang et al. reported the incorporation of Zeolite on CPEs show preferential uptake of the neurotransmitters DA and EP while rejecting anionic ascorbic acid species. The ion exchange properties of zeolite particles significantly improved the selectivity resulting a sensitive and easy method for the quantification of these neurochemicals. The quantification was demonstrated using cyclic voltammetry, discontinuous amperometry, and flow injection voltammetry experiments. Experimental parameters that affect dopamine uptake, including solution pH and ionic strength, zeolite loading, are the parameters optimized. The attractive properties of zeolite-modified electrodes may be useful for electrochemistry of the brain in vivo (Joseph and Alain, 1996). The preparation of the acrylamide modified CPE and the electrocatalytic oxidation to DA in 0.2 M phosphate buffer (PBS) at pH 7.4 were described by Kumara Swamy et al. Several parameters such as DA concentration, scanning rate variability, and pH were studied using CV. The peak current was proportional to the DA concentration and the detection limit was 3.5 × 10−7 M. The concentration effect of the modifier on the CPEs is that changing the electrode interface can increase the amount of positively charged dopamine on the surface of the electrode. The stability and reproducibility of the electrodes for DA measurement were also investigated (Sharath Shankar et al., 2010). A poly (carmagite) film was electropolymerized on the surface of the CPEs based on an electrochemical method by Umesh et al. The polymer film coated electrodes exhibit excellent electrode catalytic activity for detecting DA at a pH of 7. The effect of scan rate study was found to be the adsorption drive electrode process and the concentration effect of DA was also investigated. The DA redox peak potential was observed to be pH dependent, and the electrodes coated with this polymer film were excellent for simultaneous detection of DA in the presence of high concentrations of AA and UA. The proposed method was applied to detect DA in injection samples (Umesh et al., 2010).
Mohammad et al. developed a CPE modified with ZrO2 nanoparticles to study the electrochemical oxidation of NE, AC, folic acid (FA) and mixtures thereof by electrochemical techniques. DPV was used to investigate the selective and simultaneous measurements of NE, AC, and FA on modified electrodes. Peak currents for NE, AC, and FA were observed to be linearly increased with concentration. The detection limits for NE, AC and FA were 8.95 × 10−8, 9.12 × 10−7, 9, 86 × 10−6 M, respectively. It demonstrated a powerful ability to resolve the overlapping voltammetric responses of NE, AC, and FA into three well-defined voltammetric peaks (Mohammad et al., 2010). Acetylcholine is a neurotransmitter found at the intersections of nerves and muscles in the lymph nodes of the internal organs, the musculoskeletal system, and various parts of the central nervous system. A decrease in acetylcholine in the brain is associated with Alzheimer’s disease. Therefore, it is an important active ingredient in this disease. Nidhi et al. fabricated, a dienzyme biosensor system using acetylcholinesterase and choline oxidase using a CPEs modified with carbon nanodots (3-aminopropyl) triethoxysilane (CDsAPTES), and the amount of acetylcholine was measured. Acetylcholinesterase and choline oxidase enzymes were immobilized on modified CPEs by cross-linking with glutaraldehyde. Acetylcholine was determined by oxidizing enzymatically produced H2O2 to Ag/AgCl at 0.4 V. The effects of temperature, pH, and substrate concentration on the acetylcholine response of the biosensors produced were studied by the group. In addition, they investigated the optimal number of CD-APTES, the linear operating range of the biosensor, and the interference effect (Bodur et al., 2021).
Tetradecyltrimethylammonium bromide (TTAB) immobilized CPE (TTABMCPE) of EP and 5-HT in the presence of AA by voltammetry in phosphoric acid was reported by Sharath et al. The method proposed for simultaneous study and measurement of these neurotransmitters in buffer solution (PBS) with pH 7.4. Anode peaks for EP, 5HT, and AA were observed at scanning rates of 50 mVs−1 at 198 mV, 363 mV, and 17 mV, respectively. In TTABMCPE, the peak currents of all electroactive molecules increased. It was found that EP and 5-HT can be measured simultaneously with good sensitivity even in the presence of high concentrations of AA. Interference studies have shown that modified electrodes have excellent selectivity for measuring EP in the presence of large excesses of AA and 5HT. The difference in oxidation peak potential between EP-AA and EP-5-HT was about 215 and 165 mV, respectively. Voltammetric resolution is large enough to determine AA, EP, and 5-HT individually. The detected electrode detection limit for the modified electrode was 0.12 μM with differential pulse voltammetry technology. The developed method was applied to the measurement of EP in synthetic samples with satisfactory results (Sharath Shankar and Kumara Swamy, 2014). CPEs coated with poly (malachite green) film were prepared by Umesh et al. for sensitive and selective measurement of DA in pH 7 phosphate buffer. A fully enhanced redox peak was observed with a detection limit of 2.5 × 107 M by cyclic voltammetry. Further studies have shown that DA oxidation shifts negatively by increasing pH. The effects of concentration and scan rate resulted in a linear response and the entire electrode process was diffusion controlled. The fabricated CPE acts as suitable and sensitive electrode for measuring DA. Simultaneous studies have shown excellent potential differences between DA and other neurotransmitters using both DPV and CV techniques (Umesh et al., 2013).
Manjunatha et al. reported a biopolymer modified chemical sensor by electrolytically polymerizing arginine on a CPE to quantify DA in the presence of UA and AA. The developed electrode exhibits excellent electrochemical catalytic activity towards DA oxidation. CV studies have shown that oxidation of DA on the surface of polyarginine modified carbon nanotube paste electrodes is a two-electron quasi reversible process, a diffusion rate determining process, with improved current response compared to bare carbon nanotube paste electrodes. It showed that, under optimized conditions, a concentration range of 8 × 106–5 × 105 M and 6 × 105–2 × 104 M and a detection limit of 10 × 107 M were observed. The sensor has also been proven to break down DA in real samples with sufficient recovery (Raril and Manjunatha, 2018).
Galal et al. evaluated the out turn of the ionic liquid type of carbon paste composites (ILC) on the electrode-catalyzed oxidation of L-dopa. The best electrochemical responds were obtained with the ILC in comparison to other different types of ionic liquids. High ionic conductivity and increased surface area was obtained by the effective combination of gold nanoclusters and ILC nanocomposites. L-dopa is considered one of the main prescription drugs used to treat Parkinson’s disease. In addition, two-drug therapy with L-dopa and carbidopa has been shown to be effective and promising to avoid the disadvantages of L-dopa monotherapy for patients with Parkinson’s disease. The developed Au/CILCE can be used to detect L-dopa in human serum in the concentration range of 0.1- to 90 μM, with detection and quantification limits of 4.5 and 15.0 nM, respectively. Sensitive detection of L-dopa can be done simultaneously in the presence of carbidopa with a low detection limit (Nada et al., 2020). Perovskite materials such as LaNiO3, LaFeO3, and LaCoO3 were used for modifying the CPEs for the analysis of neurotransmitters by Jasmine et al. These materials significantly increased the electrochemical active area of the electrode and reduced the charge transfer resistance of the modified electrode. Further the perovskite modified electrode could enhance the thermal stability and surface features such as phase formation and morphology. The modified electrodes showed a high current response, enabling nanomolar sensing of neurotransmitters such as DA, 5-HT, AP, and tyrosine (TYR). All the electrochemical parameters of the modified electrode showed remarkable advancement associated with the electrochemical reaction of the abovementioned analytes. The perovskite-modified CPE (LNO MCPE) showed a wide linear range, high sensitivity, excellent selectivity, and wide linear concentration range are the superiority of the electrode and this enables simultaneous detection and quantification of four neurotransmitters (Jasmine et al., 2021). Salimi et al. synthesized sol-gel incorporated carbon composite electrode (CCE) by mixing carbon powder with a sol-gel precursor such as methyltrimethoxysilane, without the addition of electron transfer mediators or specific reagents. This sensor has been shown to be usable for simultaneous measurement neurotransmitters such as DA and adrenaline, in the presence of other biomolecules like UA and AA. Compared to other common carbon-based electrodes, especially boron-doped diamond, glassy carbon, graphite, and CPE, the developed CCE was found to be significantly more reversible to dopamine. Direct electrochemical oxidation of AA, UA and catecholamines on carbon composite electrodes was investigated and the oxidation peaks of UA, AA, and catecholamines are well separated with good sensitivity. The analytical utility of this sensor has been evaluated and this method seems to be highly sensitive to detect biomolecules in urine and serum samples with inherent stability and integrity (Abdollah et al., 2006).
Miniaturization of devices make fast and sensitive detection of analytes from nano or micro volume of real sample is very much applicable in the fields of biomedicine and forensic medicine. Shui et al. manufactured an electrochemical sensor (MWCNTCPE/PDDARGO) based on a poly (diallyl-dimethyl-ammonium) chloride-reduced graphene oxide (PDDARGO)-modified multi-walled carbon nanotube carbon paste electrode (MWCNTCPE) and integrated it into a microfluidic device. Detailed studies show that the PDDARGO and MWCNT hybrid nanomaterials have a dependent effect on the electrode-catalyzed reaction of DA and 5-hydroxytryptamine (5-HTA), enabling sensitive detection. The fabricated electrode has ability to quantify DA sensitively and selectively and 5-HTA in microliter doses of rat plasma suggests that it could be used to monitor bioactive substances both in vivo and in vitro. The device showed wide range of concentration, with very high sensitivity, stability, and excelled reproducibility (Zhenping et al., 2018). Mazloum Ardakani et al. reported a chemically modified CPE containing bis-hydroquinone (DOH) for the electrode-catalyzed oxidation of DA. Kinetic parameters of the modified CPE such as the oxidation diffusion coefficient and electron transfer coefficient (α = 0.33) on the surface of DOH were also incorporated. Under optimum conditions (pH-7.0), DA oxidation on the surface of the fabricated electrode has been found to occur at a positive potential about 290 mV lower than the bare CPE. The oxidation peak current exhibited a linear variation depends on DA concentration, and the linear analysis curve was obtained in the SWV range of 3.0 × 105–2 × 103 M DA. The detection limit was determined to be 3.2 × 106 M. This method was also used to determine the DA of a drug injection using a standard addition method (Hadi et al., 2019). Tetradecyltrimethylammonium bromide (TTAB) immobilized CPE for EP and 5-HT in the presence of AA in phosphoric acid was fabricated by Kumara Swamy et al. Proposed method applied for simultaneous study and measurement of the neurochemicals in buffer solution (PBS) with pH 7.4. Anode peaks for EP, 5-HT, and AA were observed at scanning rates of 50 mVs−1 at 198 mV, 363 mV, and 17 mV, respectively. In TTABMCPE, the peak currents of all electroactive molecules increased. It was found that EP and 5HT can be measured simultaneously with good sensitivity even in the presence of high concentrations of AA. Interference studies have shown that modified electrodes have excellent selectivity for measuring EP in the presence of large excesses of AA and 5HT. The difference in oxidation peak potential between EP-AA and EP-5HT was about 215 and 165 mV, respectively. Voltammetric resolution is large enough to determine AA, EP, and 5HT individually. The detection limit of the modified electrode was 0.12 μM by differential pulse voltammetry technology. The developed method was applied to the measurement of EP in synthetic samples with satisfactory results [63]. Table 2 is representing an outline of the review which highlights CPE modified sensors for important neurotransmitters reported in the literature so far.
A simplistic and comprehensive assessment of carbon paste-based electrochemical sensors for neurotransmitters has been outlined. Considerable number of electrochemical techniques have been reported in the literature to quantify the neurochemicals from real samples due to extensive need of these sensors in the clinical field. The development of a very simple point-of-care device is essential to detect and quantify these molecules in biological samples. Carbon paste-based electrochemical sensors have very much attention in the current scenario of electrochemical research because of the cheapness of the electrode material and superior electron transfer kinetics than other carbon-based electrodes. Modification of the carbon-paste electrode seems to have led to the elimination of confounding effects in real samples. The practical application and selectivity of the neurotransmitter electrochemical sensor can be further improved by placing greater emphasis on nanomaterial fabrication by appropriately combining various advanced nanomaterials. A very low detection limit, high sensitivity and selectivity can be obtained by combining metal organic framework and nanoparticle modified electrodes. Apart from the innovative methodologies developed, most other sensors cannot reach this level. Even so, most nanocomposite methods used somewhat complicated strategies such as combining two or three nanostructures, making this method difficult to commercialize. This review describes the advantages of carbon paste-based materials in the design and construction of various electrochemical sensors with electrochemical properties and in the electrochemical detection of neurochemicals from different perspectives. This review aims to accelerate current development in this area by taking the future development of neurotransmitter electrochemical sensors to a better level and exploring the benefits of carbon paste-based electrode materials.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge Amrita Vishwa Vidyapeetham, Amritapuri Campus for the support to carry out our research work.
Abdollah, S., Hussein, M., and Rahman, H. (2006). Simultaneous Determination of Ascorbic Acid, Uric Acid and Neurotransmitters with a Carbon Ceramic Electrode Prepared by Sol-Gel Technique. Talanta 70 (4), 823–832.
Atta, N. F., Galal, A., El-Ads, E. H., and Galal, A. E. (2020). Efficient Electrochemical Sensor Based on Gold Nanoclusters/Carbon Ionic Liquid Crystal for Sensitive Determination of Neurotransmitters and Anti-parkinson Drugs. Adv. Pharm. Bull. 10, 46–55. doi:10.15171/apb.2020.006
Bakker, E., and Telting-Diaz, M. (2002). Electrochemical Sensors. Anal. Chem. 74 (12), 2781–2800. doi:10.1021/ac0202278
Bodur, O. C., Dinç, S., Özmen, M., and Arslan, F. (2021). A Sensitive Amperometric Detection of Neurotransmitter Acetylcholine Using Carbon Dot‐modified Carbon Paste Electrode. Biotechnol. Appl. Biochem. 68, 20–29. doi:10.1002/bab.1886
Chandra, S., Siraj, S., and Wong, D. K. Y. (2017). Recent Advances in Biosensing for Neurotransmitters and Disease Biomarkers Using Microelectrodes. ChemElectroChem 4, 822–833. doi:10.1002/celc.201600810
Cheraghi, S., Taher, M. A., and Karimi-Maleh, H. (2017). Highly Sensitive Square Wave Voltammetric Sensor Employing CdO/SWCNTs and Room Temperature Ionic Liquid for Analysis of Vanillin and Folic Acid in Food Samples. J. Food Compos. Analysis 62, 254–259. doi:10.1016/j.jfca.2017.06.006
David, I. G., Popa, D.-E., and Buleandra, M. (2017). Pencil Graphite Electrodes: A Versatile Tool in Electroanalysis. J. Anal. Methods Chem. 2017, 1–22. doi:10.1155/2017/1905968
Deepa, G. P., Naveen, M., Gokavi, A. M. B., and Nandibewoor, S. T. (2016). Anal. Bioanal. Electrochem. 8, 78–91.
El-Ries, M. A., Abou Attia, F. M., and Ibrahim, S. A. (2000). AAS and Spectrophotometric Determination of Propranolol HCl and Metoprolol Tartrate. J. Pharm. Biomed. Analysis 24 (2), 179–187. doi:10.1016/S0731-7085(00)00408-8
El-Ries, M. A., Abou-Sekkina, M. M., and Wassel, A. A. (2002). Polarographic Determination of Propranolol in Pharmaceutical Formulation. J. Pharm. Biomed. Analysis 30 (3), 837–842. doi:10.1016/S0731-7085(02)00087-0
El-Saharty, Y. S. (2003). Simultaneous High-Performance Liquid Chromatographic Assay of Furosemide and Propranolol HCL and its Application in a Pharmacokinetic Study. J. Pharm. Biomed. Analysis 33 (4), 699–709. doi:10.1016/S0731-7085(03)00229-2
Giuliani, J. G., Benavidez, T. E., Duran, G. M., Vinogradova, E., Rios, A., and Garcia, C. D. (2016). Development and Characterization of Carbon Based Electrodes from Pyrolyzed Paper for Biosensing Applications. J. Electroanal. Chem. (Lausanne) 765, 8–15. doi:10.1016/j.jelechem.2015.07.055
Gölcü, A. (2008). New, Simple, and Validated UV-Spectrophotometric Method for the Estimation of Some Beta Blockers in Bulk and Formulations. J. Anal. Chem. 63 (6), 538–543. doi:10.1134/S106193480806004X
Gowda, B. G., Melwanki, M. B., Seetharamappa, J., and Srinivasa Murthy, K. C. (2002). Spectrophotometric Determination of Isoniazid in Pure and Pharmaceutical Formulations. Anal. Sci. 18 (7), 839–841. doi:10.2116/analsci.18.839
Gupte, V., and Luthra, U. (2017). Analytical Techniques for Serratiopeptidase: A Review. J. Pharm. Analysis 7, 203–207. doi:10.1016/j.jpha.2017.03.005
Hadi, B., Mohadeseh, S., and Somayeh, T. (2019). Different Electrochemical Sensors for Determination of Dopamine as Neurotransmitter in Mixed and Clinical Samples: A Review. Anal. Bioanal. Chem. Res. 6 (1), 81–96.
Hassan, S. S. M., Abou-Sekkina, M. M., El-Ries, M. A., and Wassel, A. A. (2003). Polymeric Matrix Membrane Sensors for Sensitive Potentiometric Determination of Some β-blockers in Pharmaceutical Preparations. J. Pharm. Biomed. Analysis 32 (1), 175–180. doi:10.1016/S0731-7085(03)00015-3
Heinze, J. (1984). Cyclic Voltammetry-"Electrochemical Spectroscopy". New Analytical Methods(25). Angew. Chem. Int. Ed. Engl. 23 (11), 831–847. doi:10.1002/anie.198408313
Idowu, O. S., Adegoke, O. A., and Olaniyi, A. A. (2004). Colorimetric Assay of Propranolol Tablets by Derivatization: Novel Application of Diazotized 4-Amino-3,5-Dinitrobenzoic Acid (ADBA). J. AOAC Int. 87 (3), 573–578. doi:10.1093/jaoac/87.3.573
Jasmine, T., Anitha, P. K., Tony, T., and Nygil, T. (2021). The Influence of B-Site Cation in LaBO3 (B= Fe, Co, Ni) Perovskites on the Nanomolar Sensing of Neurotransmitters. Sensors Actuators B Chem. 332, 129362.
Joseph, W., and Alain, W. (1996). Zeolite-modified Carbon Paste Electrode for Selective Monitoring of Dopamine. J. Electroanal. Chem. 407, 183–187.
Li, H.-X., Xu, X.-L., Chen, H., Zhang, S., and Kong, J.-L. (2012). Fabrication of Molecularly Imprinted Electrochemical Sensor for Selective Detection of Propranolol Hydrochloride. Chin. J. Anal. Chem. 40 (6), 817–822. doi:10.1016/S1872-2040(11)60550-1
Lourencao, B. C., Silva, T. A., Fatibello-Filho, O., and Swain, G. M. (2014). Voltammetric Studies of Propranolol and Hydrochlorothiazide Oxidation in Standard and Synthetic Biological Fluids Using a Nitrogen-Containing Tetrahedral Amorphous Carbon (ta-C:N) Electrode. Electrochimica Acta 143, 398–406. doi:10.1016/j.electacta.2014.08.008
Madhusudhana, , Manasa, G., Bhakta, A. K., Mekhalif, Z., and Mascarenhas, R. J. (2020). Bismuth-nanoparticles Decorated Multi-Wall-Carbon-Nanotubes Cast-Coated on Carbon Paste Electrode; an Electrochemical Sensor for Sensitive Determination of Gallic Acid at Neutral pH. Mater. Sci. Energy Technol. 3, 174–182. doi:10.1016/j.mset.2019.10.001
Mandler, D., and Kraus-Ophir, S. (2011). Self-assembled Monolayers (SAMs) for Electrochemical Sensing. J. Solid State Electrochem 15, 1535–1558. doi:10.1007/s10008-011-1493-6
Martín, A., López, M. Á., González, M. C., and Escarpa, A. (2015). Multidimensional Carbon Allotropes as Electrochemical Detectors in Capillary and Microchip Electrophoresis. Electrophoresis 36, 179–194. doi:10.1002/elps.201400328
Mathew, G., Dey, P., Das, R., Chowdhury, S. D., Paul Das, M., Neppolian, B., et al. (2018). Direct Electrochemical Reduction of Hematite Decorated Graphene Oxide (α-Fe2O3@erGO) Nanocomposite for Selective Detection of Parkinson's Disease Biomarker. Biosens. Bioelectron. 115, 53–60. doi:10.1016/j.bios.2018.05.024
Mohammad, M. A., Hadi, B., Mohammad, K. A., Fakhradin, M., and Mohammad, A. A. (2010). New Strategy for Simultaneous and Selective Voltammetric Determination of Norepinephrine, Acetaminophen and Folic Acid Using ZrO2 Nanoparticles-Modified Carbon Paste Electrode. Sensors Actuators B Chem. 151 (26), 243–249.
Muñoz de la Peña, A., Salinas, F., and Durán, M. S. (1991). Simultaneous Determination of Propranolol and Hydralazine by Derivative Synchronous Spectrofluorimetry. Anal. Chim. Acta 255 (2), 317–323. doi:10.1016/0003-2670(91)80062-X
Nada, F. A. (2012). Electrochemical Determination of Neurotransmitters Using Gold Nanoparticles on Nafion/Carbon Paste Modified Electrode. J. Electrochem. Soc. 159, 765. doi:10.1149/2.004210jes
Nada, F. A., Ahmed, G., Abu-Attia, F. M., and Azab, S. M. (2011). Simultaneous Determination of Paracetamol and Neurotransmitters in Biological Fluids Using a Carbon Paste Sensor Modified with Gold Nanoparticles. Mat. Chem. 21, 13015–13024.
Nada, F. A., Ahmed, G., Ekram, H. E. A., and Aya, E. G. (2020). Efficient Electrochemical Sensor Based on Gold Nanoclusters/Carbon Ionic Liquid Crystal for Sensitive Determination of Neurotransmitters and Anti-parkinson Drugs. Adv. Pharm. Bull. 10 (1), 46–55.
Pérez Ruiz, T., Martínez-Lozano, C., Tomás, V., and Carpena, J. (1998). Simultaneous Determination of Propranolol and Pindolol by Synchronous Spectrofluorimetry. Talanta 45 (5), 969–976. doi:10.1016/S0039-9140(97)00203-8
Purushothama, H. T., Nayaka, Y. A., Vinay, M. M., Manjunatha, P., Yathisha, R. O., and Basavarajappa, K. V. (2018). Pencil Graphite Electrode as an Electrochemical Sensor for the Voltammetric Determination of Chlorpromazine. J. Sci. Adv. Mat. Devices. 3, 161–166. doi:10.1016/j.jsamd.2018.03.007
Ramakrishnan, S., Pradeep, K. R., Raghul, A., Senthilkumar, R., Rangarajan, M., and Kothurkar, N. K. (2015). One-step Synthesis of Pt-Decorated Graphene-Carbon Nanotubes for the Electrochemical Sensing of Dopamine, Uric Acid and Ascorbic Acid. Anal. Methods 7, 779–786. doi:10.1039/c4ay02487g
Ramesh, K. C., Gowda, B. G., Seetharamappa, J., and Keshavayya, J. (2003). Indirect Spectrofluorimetric Determination of Piroxicam and Propranolol Hydrochloride in Bulk and Pharmaceutical Preparations. J. Anal. Chem. 58 (10), 933–936. doi:10.1023/A:1026171515492
Rao, H., Chen, M., Ge, H., Lu, Z., Liu, X., Zou, P., et al. (2017). A Novel Electrochemical Sensor Based on Au@PANI Composites Film Modified Glassy Carbon Electrode Binding Molecular Imprinting Technique for the Determination of Melamine. Biosens. Bioelectron. 87, 1029–1035. doi:10.1016/j.bios.2016.09.074
Rapado-Martínez, I., García-Alvarez-Coque, M. C., and Villanueva-Camañas, R. M. (1997). Liquid Chromatographic Procedure for the Evaluation of β-blockers in Pharmaceuticals Using Hybrid Micellar Mobile Phases. J. Chromatogr. A 765 (2), 221–231. doi:10.1016/S0021-9673(96)00919-3
Raril, C., and Manjunatha, J. G. (2018). Carbon Nanotube Paste Electrode for the Determination of Some Neurotransmitters: A Cyclic Voltammetric Study. Mod. Chem. Appl. 06, 3. doi:10.4172/2329-6798.1000263
Rejithamol, R., and Beena, S. (2021). Phenyl Hydrazine and 2,4-dinitrophenyl Hydrazine-Based Polymeric Materials for the Electrochemical Quantification of Thrombotonin. MRS Adv. 6, 750–757. doi:10.1557/s43580-021-00116-y
Rejithamol, R., Rajasree, G. K., and Beena, S. (2020). Disposable Pencil Graphite Electrode Decorated with a Thin Film of Electro-Polymerized 2, 3, 4, 6, 7, 8, 9, 10-octahydropyrimido [1, 2-a] Azepine for Simultaneous Voltammetric Analysis of Dopamine, Serotonin, and Tryptophan. Mater. Phys. Chem. 258, 123857.
Saifeldin, M. S. (2020). Electrochemical Detection of Neurotransmitter Dopamine: A Review. Int. J. Electrochem. Sci. 15, 599–612. doi:10.20964/2020.01.61
Salem, H. (2002). Spectrophotometric Determination of β-adrenergic Blocking Agents in Pharmaceutical Formulations. J. Pharm. Biomed. Analysis 29 (3), 527–538. doi:10.1016/S0731-7085(02)00100-0
Santhy, A., Beena, S., and Rajasree, G. K. (2021). Carbon-based Electrodes as Avscaffold for the Electrochemical Sensing of Pharmaceuticals. Amsterdam, Netherlands: Elsevier.
Sartori, E. R., Barbosa, N. V., Faria, R. C., and Fatibello-Filho, O. (2011). Conductometric Determination of Propranolol Hydrochloride in Pharmaceuticals. Eclet. Quím. 36 (1), 110–122. doi:10.1590/S0100-46702011000100008
Sartori, E. R., Medeiros, R. A., Rocha-Filho, R. C., and Fatibello-Filho, O. (2010). Square-wave Voltammetric Determination of Propranolol and Atenolol in Pharmaceuticals Using a Boron-Doped Diamond Electrode. Talanta 81 (4–5), 1418–1424. doi:10.1016/j.talanta.2010.02.046
Sasya, M., Jayanth, B. K., Dermot, B., Madoka, T., Inam, U. A., John, B. B. R., et al. (2020). “Nano”: An Emerging Avenue in Electrochemical Detection of Neurotransmitters. ACS Chem. Neurosci. 11, 4024. doi:10.1021/acschemneuro.0c00355
Sehit, E., and Altintas, Z. (2020). Significance of Nanomaterials in Electrochemical Glucose Sensors: An Updated Review (2016-2020). Biosens. Bioelectron. 159, 112165. doi:10.1016/j.bios.2020.112165
Sharath Shankar, S., and Kumara Swamy, B. E. (2014). Detection of Epinephrine in Presence of Serotonin and Ascorbic Acid by TTAB Modified Carbon Paste Electrode: A Voltammetric Study. Int. J. Electrochem. Sci. 9, 1321–1339.
Sharath Shankar, S., Kumara Swamy, B. E., Pandurangachar, M., Chandra, U., Chandrashekar, B. N., Manjunatha, J. G., et al. (2010). Electrocatalytic Oxidation of Dopamine on Acrylamide Modified Carbon Paste Electrode- A Voltammetric Study. J. Electrochem. Sci. 5, 944–954.
Shi, T., Wu, G., Jin, Q., and Wang, X. (2020). Camellia Oil Authentication: A Comparative Analysis and Recent Analytical Techniques Developed for its Assessment. A Review. Trends Food Sci. Technol. 97, 88–99. doi:10.1016/j.tifs.2020.01.005
Szuplewska, A., Kulpińska, D., Dybko, A., Chudy, M., Jastrzębska, A. M., Olszyna, A., et al. (2020). Future Applications of MXenes in Biotechnology, Nanomedicine, and Sensors. Trends Biotechnol. 38, 264–279. doi:10.1016/j.tibtech.2019.09.001
Tabrizi, A. B. (2007). A Simple Spectrofluorimetric Method for Determination of Piroxicam and Propranolol in Pharmaceutical Preparations. J. Food Drug Analysis 15 (3), 242–248.
Umesh, C., Kumara Swamy, B. E., Mahanthesha, K. R., Vishwanath, C. C., and Sherigara, B. S. (2013). Poly(malachite Green) Film-Based Carbon Paste Electrode Sensor for the Voltammetric Investigation of Dopamine. Chem. Sensors 3, 1–6.
Umesh, C., Kumara Swamy, B. E., Ongera, G., and Sherigara, B. S. (2010). Voltammetric Resolution of Dopamine in the Presence of Ascorbic Acid and Uric Acid at Poly (Calmagite) Film Coated Carbon Paste Electrode. Electrochimica Acta 55 (24), 7166–7174.
Vadivaambigai, A., Senthilvasan, P. A., Kothurkar, N., and Rangarajan, M. (2015). Graphene-oxide-based Electrochemical Sensor for Salicylic Acid. Nanosci. Nanotechnol. Lett. 7, 140–146. doi:10.1166/nnl.2015.1909
Vedhi, C., Selvanathan, G., Arumugam, P., and Manisankar, P. (2009). Electrochemical Sensors of Heavy Metals Using Novel Polymer-Modified Glassy Carbon Electrodes. Ionics 15, 377–383. doi:10.1007/s11581-008-0277-1
Willander, M., Klason, P., Yang, L. L., Al‐Hilli, S. M., Zhao, Q. X., and Nur, O. (2008). ZnO Nanowires: Chemical Growth, Electrodeposition, and Application to Intracellular Nano‐sensors. Phys. Status Solidi C. 5, 3076–3083. doi:10.1002/pssc.200779232
Wring, S. A., and Hart, J. P. (1992). Chemically Modified, Carbon-Based Electrodes and Their Application as Electrochemical Sensors for the Analysis of Biologically Important Compounds. A Review. Analyst 117, 1215–1229. doi:10.1039/AN9921701215
Zhenping, L., Mingliang, J., Jieping, C., Ruiwen, N., Pengfei, L., Guofu, Z., et al. (2018). Electrochemical Sensor Integrated Microfluidic Device for Sensitive and Simultaneous Quantification of Dopamine and 5-hydroxytryptamine. Sensors Actuators B Chem. 273 (10), 873–883. doi:10.1016/j.snb.2018.06.123
Keywords: carbon paste electrode (CPE), neurotransmitters, voltammetry, nanoparticles, sensors
Citation: Rejithamol R and Beena S (2022) Carbon Paste Electrochemical Sensors for the Detection of Neurotransmitters. Front. Sens. 3:901628. doi: 10.3389/fsens.2022.901628
Received: 22 March 2022; Accepted: 25 April 2022;
Published: 10 May 2022.
Edited by:
Sharath Shankar Sarojini, Thomas Jefferson University, United StatesReviewed by:
Tiago Almeida Silva, Universidade Federal de Viçosa, BrazilCopyright © 2022 Rejithamol and Beena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Rejithamol, cmVqaXRoYW1vbHJAYW0uYW1yaXRhLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.