
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Reprod. Health, 17 January 2025
Sec. Andrology
Volume 6 - 2024 | https://doi.org/10.3389/frph.2024.1515166
Introduction: Male infertility, often attributed to insufficient production of healthy and active sperm, can be exacerbated by electromagnetic radiation emitted from mobile phones, which disrupts normal spermatogenesis and leads to a notable decline in sperm quality. The main targets of mobile phone-induced damage in the testes are Leydig cells, seminiferous tubules, and sperm cells. The aim of this systematic literature review is to identify histopathological changes in the testes due to mobile phone radiation exposure and to examine its effects on sperm parameters in experimental animals.
Methods: In this systematic review, an extensive literature search was conducted across databases such as PubMed, ScienceDirect, Hinari, and Google scholar.
Results: A total of 752 studies were identified for screening, and 18 studies were deemed eligible for data extraction. Studies have identified histopathological alterations in testicular tissue caused by mobile phone radiation, such as reduced seminiferous tubule diameter, tunica albuginea and germinal epithelial thickness, Leydig cell hypoplasia, and increased intertubular space. Consistent exposure to mobile phone radiation has been shown to significantly reduce sperm count, motility, and viability, while also increasing abnormal sperm morphology in male rats, mice, and rabbits.
Conclusion: Animal studies indicate that electromagnetic radiation from mobile phones can negatively impact testicular tissue and sperm parameters, including sperm count, motility, viability, and morphology. As a precaution, preventive measures are recommended to minimize potential risks from mobile phone exposure, and further research is needed to fully understand its effects on human reproductive health.
Infertility is the inability to conceive after one year of consistent, unprotected intercourse (1). Around 35% of infertility cases are linked to male factors (2). Commonly, male infertility stems from insufficient production of healthy and active sperm (2, 3). Several risk factors linked to male infertility include genetic abnormalities, blockage of genital ducts, varicocele, erectile dysfunction, and impotence (4). Environmental factors like heat, chemicals, radiation, alcohol, and smoking also play a role in reducing fertility (5, 6).
Over the last two decades, mobile phones have become essential to daily life, with their use increasing dramatically (7). Mobile phones operate within a spectrum of frequencies ranging from 450 to 2,700 MHz, emitting electromagnetic radiation (EMR) as they function (8). The specific absorption rate (SAR) measures the amount of radiofrequency energy absorbed by tissues from mobile phones. Depending on the model, SAR values for cell phones range from 0.12 to 1.6 W/kg body weight (9). When mobile phones are kept in pockets near the scrotum, the testes can absorb the emitted electromagnetic radiation. This has raised concerns about potential health effects on male reproductive organs, particularly the testes (10, 11). Several studies have indicated that the testes, specifically the Leydig cells, are particularly sensitive to exposure to electromagnetic radiation (12).
The impact of mobile phone on male reproduction remains uncertain due to conflicting results from various studies. Yet, it is believed that the EMR emitted by mobile phones might disrupt normal sperm production, potentially decreasing sperm quality. Studies suggest that the use of mobile phone could affect semen quality, impacting sperm count, motility, viability, and serum testosterone levels, possibly playing a role in male infertility (7, 13–15). Animal studies indicate that mobile phone exposure can lead to harmful changes in the testes and negatively affect male germ cells (16–19). Some researchers have found no harmful effects from exposure to EMR emitted by mobile phones, noting no histological changes in rat testes or alterations in serum testosterone levels (20, 21).
The testes, located in the scrotum outside the abdominal cavity, require a lower temperature for proper function and sperm production. Keeping a mobile phone in your trouser pockets and using it for extended period may elevate testicular temperature, potentially causing hyperthermia and oxidative stress (22). Oxidative stress in sperm cells, resulting in impaired fertilization ability and DNA damage, is associated with decreased fertility (23).
The testes have two primary components: the seminiferous tubules, which are responsible for sperm production, and the Leydig cells, have role for male sex hormone production. Sperm production, regulated by Y chromosome genes, generally takes about 54 days in rats from the spermatogonia stage (24). Additionally, it takes approximately 12–21 days for sperm to travel from the testis to the epididymis and then to the ejaculatory duct. During this time, sperm mature in the epididymis, acquiring motility. Leydig cells produce testosterone, which plays a crucial role in regulating spermatogenesis (10, 25).
The testes, essential for sperm development and maturation, are highly susceptible to radiation, potentially leading to genetic damage (21). Sperm cellular membranes are rich in polyunsaturated fatty acids (PUFAs), making them vulnerable to oxidative damage from reactive oxygen species (ROS), which can result in lipid peroxidation. This process undermines membrane integrity and reduces sperm motility (23). Mobile phone radiation mainly impacts the Leydig cells, seminiferous tubules, and spermatozoa. This exposure lowers testosterone production, interferes with sperm production, and damages sperm DNA (26).
Histological examination of the testis reveals seminiferous tubules, which are hexagonal or rounded in shape, separated by interstitial connective tissue. Inside these tubules, different stages of spermatogenic cells are found, but only Sertoli cells and spermatogonia from the seminiferous epithelium are situated adjacent to the basement membrane. Leydig cells, characterized by large, acidophilic cytoplasm, reside in the interstitial tissue between seminiferous tubules. The germinal epithelium contains layers of spermatogenic cells, including spermatogonia, primary and secondary spermatocytes, as well as early (round) and late (elongated). Spermatozoa are free in the lumen (27, 28).
Spermatogenesis is a synchronized, intricate, and lengthy process that takes place in the germinal epithelium of the testes (29). Testes are sensitive to stressors, both internal and external, and exposure to EMR can disrupt germinal cells at various differentiation stages, potentially causing infertility (30). This systematic literature review aims to identify histopathological changes in the testes due to mobile phone radiation exposure and to examine its effects on sperm parameters in experimental animals.
The study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analysis (PRISMA) 2020 guidelines. It reviewed research reports on the histopathological effects of mobile phone radiation on the testes and sperm parameters. A thorough literature search was performed using databases like PubMed, ScienceDirect, Hinari, and Google Scholar, along with manual searches of reference lists from relevant articles and university websites to identify additional studies.
The following key words and terms were utilized to identify relevant studies: “histopathology”, “testes”, “sperm parameters”, “mobile phone exposure”, “sperm count”, “sperm motility”, “sperm viability”, “sperm morphology”, “electromagnetic radiation”, “experimental animals”.
✓ Type of studies: animal studies with English language
✓ Only studies that included a control or comparator group were selected
✓ Year of studies: 2000 to 2021 were included in the study
✓ Participants: rats (Sprague-Dawley and Wistar rat strains), Albino mice and rabbits
✓ Complete articles on the topic of interest were included
✓ Incomplete and abstract only articles, and reviews are omitted from the review.
✓ Some articles with redundant ideas were also omitted by taking the most recent one.
✓ Radiation exposure characteristics (e.g., frequency, duration)
✓ Testicular histopathologic changes (e.g., diameter of seminiferous tubules, thickness of tunica albuginea, number of Sertoli and Leydig cells, degeneration of germinal epithelium, etc.)
✓ Sperm parameters (e.g., sperm count, motility, morphology, and viability)
All the included studies were examined in detail and a reported result from each animal studies such as histopathological changes and sperm parameters of animals of exposed and control group were abstracted. Then the data was analyzed using qualitative analysis method and finally presented in text and tables.
A total of 752 articles were identified through keyword searches. Three hundred seven duplicate articles and an additional 376 after screening of titles and abstract, and 51 articles after screening the full of content of the studies were excluded based on the selection criteria. Finally, a total of 18 articles which fulfilled the inclusion criteria have been reviewed (Figure 1).
Research has explored the tissue changes in the testes resulting from mobile phone EMR exposure. These alterations are influenced by factors such as exposure length, the specific absorption rate (SAR), and the energy levels of the EMR (Table 1).
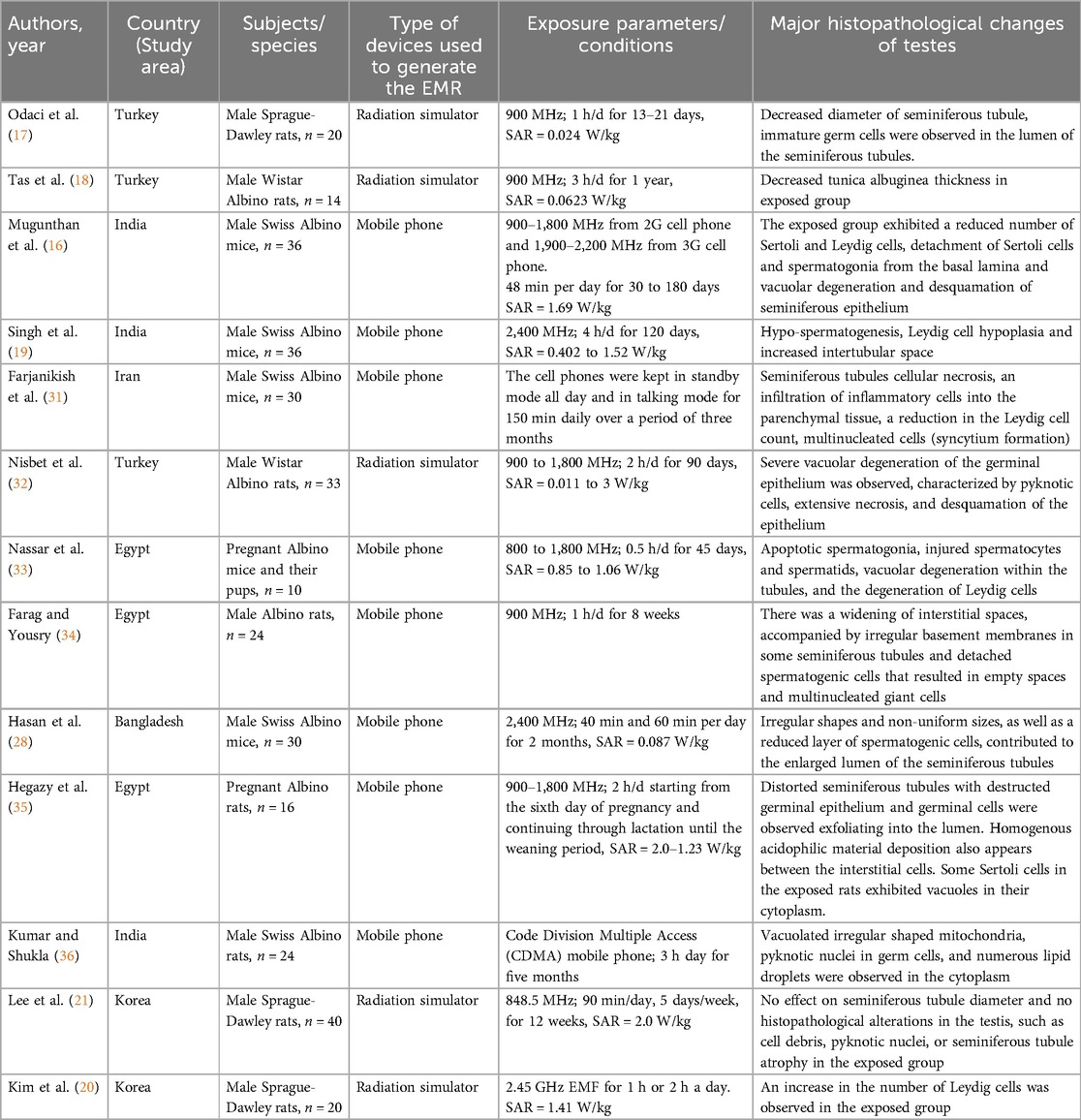
Table 1. Characteristics of studies included in the review that shows histopathological changes of mobile phone radiation exposure on the testes.
Exposure to extremely low-frequency irradiation may result in decreased sperm count and compromised reproductive function in the testes (37). Electromagnetic radiation can enhance oxidative stress by disrupting the balance between the production of reactive oxygen species (ROS) and the body's antioxidant defense mechanisms can lead to cellular damage (38). Due to high metabolic rates and cell replication in the testes, oxidative stress presents a considerable threat, especially in the seminiferous tubules. Exposure to EMR and heat can weaken the blood-testis barrier, resulting in degeneration of spermatogonia (39).
Extended exposure to 2.4 GHz EMR induces changes in the overall morphology of rat testes, resulting in a decrease in the diameter of seminiferous tubules was observed in the exposed rats (40). EMR exposure decreased both the diameter of the seminiferous tubules and the thickness of the germinal epithelium (17, 41). Saygin et al. indicated that short-term exposure to 2.45 GHz EMR did not result in any changes to the diameter of the seminiferous tubules (42). The difference in exposure duration between the studies could account for this variation. In addition, Lee et al. also found that exposure to 848.5 MHz RF for 12 weeks had no effect on seminiferous tubule diameter (21).
Tas et al. found that a reduction in tunica albuginea thickness in rats subjected to prolonged exposure to 900 MHz radiofrequency radiation from mobile phones (18). Dasdag et al. similarly found that a reduction in tunica albuginea thickness was observed in the exposed rats (40). The tunica albuginea's contractile properties help propel spermatozoa from the testes into the epididymis (43). Decreased production of Type I, Type III, and Type V collagens, as well as decreased fibroblast proliferation capacity, could lead to the thinning of the tunica albuginea (44).
Prolonged exposure to 2G cell phone radiation in mice caused a marked decrease in testis weight and seminiferous tubule diameters. Additionally, exposure to 2G radiation in mice led to a significant reduction in the number of Sertoli and Leydig cells, resulting in a significant drop in serum testosterone levels (45). Additional studies have indicated that cell phone EMR can cause Leydig cell hypoplasia and an increase in intertubular space (19, 31, 36, 46–48). Leydig cells are particularly vulnerable to EMR, which may adversely affect their structure and function, leading to reduced serum testosterone levels (49). In contrast, research by Kim et al. indicated that prolonged exposure of rats to 2.45 GHz radiation resulted in a rise in the number of Leydig cells and higher serum testosterone levels (20). Another study also indicated that the mean number of Sertoli cells in the seminiferous tubule showed no significant difference between the exposed and control groups (21).
Exposure to EMR negatively impacts the structure of the germinal epithelium within seminiferous tubules. Spermatogenic cells in the epithelium were observed to be widely separated by empty spaces, with nuclei condensation evident in dividing cells. The exposed group displayed numerous atypical tubules at various stages of sperm development, characterized by disorganized or absent germinal epithelium. Exposing the testes to 1800 MHz EMR resulted in abnormal cellular morphology in rats, characterized by severe vacuolar degeneration of the germinal epithelium was observed, along with the presence of pyknotic cells and significant necrosis and desquamation of the epithelium (32). A related study similarly detected vacuolation and giant cells in the germinal epithelium, along with the presence of abnormal cells in the lumen of the seminiferous tubules within the exposed group (50).
Nassar et al. examined the impacts of exposure to non-ionizing radiation from mobile phones on mice pups during both the intrauterine period and after birth. They observed various histopathological abnormalities in the testes, including disorganization and damage to germ cells located in the seminiferous tubules. The study further showed the occurrence of apoptotic spermatogonia, compromised spermatocytes and spermatids, intra-tubular vacuolar degeneration, and the degeneration of Leydig cells (33). Naggar et al. found that exposed male rats to 950 MHz EMR for three hours each day over a period of two months led to degeneration, disorganization, and atrophy in certain seminiferous tubules, along with expanded interstitial spaces. They also observed ruptured basement membranes with detached cells, reduced cellularity, and significantly diminished sperm count within the seminiferous tubule lumens (48). In contrast, another study conducted by Lee et al. exposed male rats to 848.5 MHz CDMA cellular phone phone-based RF of for 12weeks, and found no histopathological changes, including cell debris, pyknotic nuclei, or seminiferous tubule atrophy, in the exposed group (21).
Exposing mice to mobile phones in standby mode all day and in talking mode for 150 min each day over 90 days led to histopathological alterations in their testes. The study revealed cellular necrosis in seminiferous tubules and infiltration of inflammatory cells into the parenchymal tissue. Additionally, some necrotized tubules displayed the presence of multinucleated cells, indicating syncytium formation (31). Another study conducted in male rats exhibited irregular seminiferous tubules, a reduced number of spermatogonia, and the presence of giant multinucleated cells, and sparse primary spermatocytes with densely condensed nuclei. Additionally, degenerated spermatocytes were observed within the lumens of seminiferous tubules in rats exposed to EMR (10).
Farag and Yusry's study illustrated distorted histological architecture in certain seminiferous tubules, characterized by widened interstitial spaces. Additionally, irregular basement membranes were observed, along with separated spermatogenic cells, leaving behind empty spaces. Furthermore, giant cells with multiple nuclei were noted (34). The widening of interstitial spaces may result from impaired spermatogenesis, leading to a reduction in the height of the germinal epithelium alongside the diameter of the seminiferous tubules. Multinucleated giant cells could arise from the opening of cytoplasmic bridges develop between progeny cells, resulting in the merging of their cellular contents (51).
Histopathological analysis of mouse testes exposed to 60 min of EMR from a 4G cell phone revealed irregularly shaped and non-uniformly sized seminiferous tubules. These tubules exhibited fewer layers of spermatogenic cells, resulting in larger lumens devoid of spermatozoa (28). Esa et al. also found that EMR induced notable alterations within the seminiferous tubules of the mice that were exposed, leading to irregular shapes and dimensions (52). Chauhan et al. found alterations in the epithelial lining of seminiferous tubules, along with a reduction in the size of the tubule lumens, along with reduced cell population, in mice exposed to radiation (53).
Hegazy et al. explored the impacts of cell phone radiation exposure occurring both before and after birth on rat testicular development, uncovering degenerative alterations. These changes included distorted seminiferous tubules with disrupted germinal epithelium and the shedding of germinal cells into the lumen. Additionally, deposition of homogeneous acidophilic material was noted among the interstitial cells (35). Exfoliated germinal cells may result from disrupted connections between Sertoli cells and developing germ cells (51). The appearance of homogeneous acidophilic material in some tubules may result from reduced phagocytic activity of Sertoli cells (34).
Exposure to a mobile phone for five months induces ultrastructural alterations in rat testicular tissue. Findings include irregularly shaped, vacuolated mitochondria, pyknotic nuclei in germ cells, and an abundance of lipid droplets in the cytoplasm (36). Pyknotic nuclei observed in germ cells could be attributed to the degeneration of germ cells at various developmental stages following radiation exposure. Additionally, another study noted the presence of vacuoles in the cytoplasm of certain Sertoli cells in exposed rats (35). Vacuoles seen in Sertoli cells may be due to the buildup of lipid droplets that nourish germ cells (54).
Research on experimental animals such as rats and mice has demonstrated that exposure to mobile phone radiation negatively impacts sperm parameters, including a significant reduction in epididymal sperm count, motility, viability, and an increase in abnormal sperm morphology (Table 2).
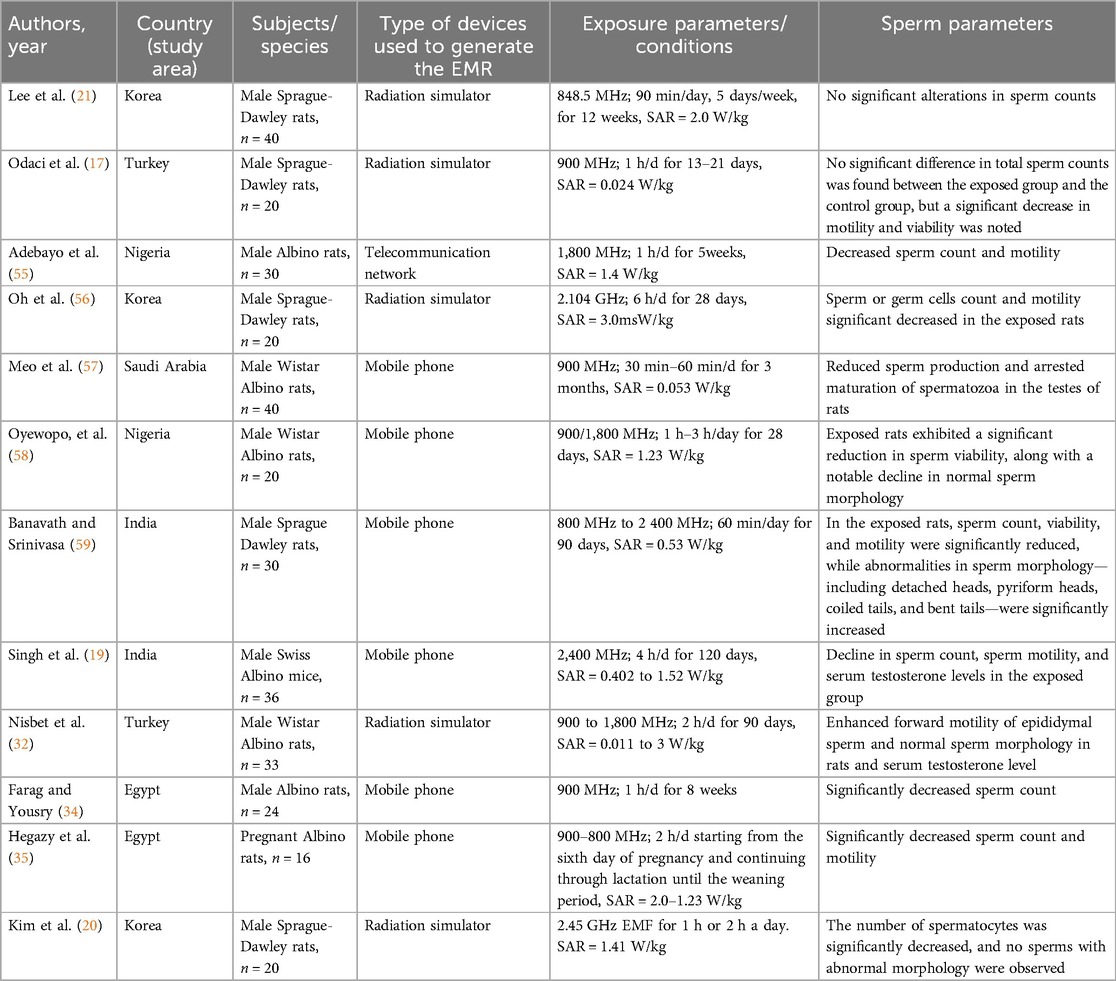
Table 2. Overview of the studies included in the review that demonstrate the effects of mobile phone radiation exposure on sperm parameters.
In rats, sperm is primarily collected through epididymal retrieval. Due to their small body size, the testes can readily and regularly move between the abdomen and scrotum through the inguinal canal (60). Mobile radiation exposure in male rats caused a significant reduction in both epididymal sperm count and motility (61). Another study revealed that exposure to mobile phones for 6 h daily over 5 days leading to a reduction in the rapid progressive motility of sperm cells (14). Odaci et al. examined the effects of prenatal exposure to 900 MHz EMR on rat testicular health and epididymal sperm quality, finding reduced sperm motility and immature germ cells in the seminiferous tubule lumen of exposed rats (17).
Several studies also reported decreased sperm count and motility in rats and mice exposed to non-ionizing radiation (27, 55, 62–65). Exposure to EMR adversely impacts sperm parameters, causing significant alterations in the sperm cell cycle due to impaired Leydig and Sertoli cells that are crucial for cell proliferation (66). EMR exposure alters cellular enzyme activity, leading to reduced production of adenosine triphosphate (ATP), essential for sperm motility. Experimental research indicates that mobile phone radiation elevates oxygen free radical levels, leading to oxidative stress (67, 68). Oxidative stress in testicular tissue impairs Leydig cell function (69) and disrupts the ability of the germinal epithelium to produce normal sperm cells or undergo spermatogenesis (70). The localized thermal effects of EMR may contribute to a decrease in sperm count (71).
Long-term exposure to mobile phone radiation may harm spermatogenesis. The study found a marked decrease in sperm cell count, along with reduced motility in rats exposed to radiation (56). This could be due to a reduction in Leydig cell count, responsible for secreting testosterone, which in turn promotes spermatogenesis. Long-term exposure to mobile phone radiation caused hypospermatogenesis and arrested the maturation of spermatozoa in the testes of Wistar albino rats (57). This could be attributed to hormonal disruption induced by EMR. Meo et al. examined the effects of mobile phone radiation on serum testosterone levels in Wistar albino rats, revealing a significant reduction in testosterone levels among those exposed to radiation (72). Mobile phone radiation impacts reproductive morphology and alters hormone levels, such as serum testosterone and FSH, which are vital for spermatogenesis and the maturation of spermatozoa (73, 74). Conversely, another study reported no significant alterations in sperm counts including the number of spermatocytes and round spermatids, during spermatogenesis in rats exposed to mobile phone EMR (21).
Several studies have also observed a marked reduction in sperm viability among exposed rats (58, 59, 75–78). EMR could impact cell function by altering ion channel structure and plasma membrane integrity. The decrease in sperm viability may be attributed to EMF-induced disruptions in sperm cell membrane mechanisms regulating ion flow, particularly sodium and potassium, ultimately affecting water content and sperm viability (79).
Hamdi et al. explored the impact of EMR exposure during the developmental stages of mice on testicular tissues in adulthood. The research identified several sperm abnormalities, including double-tailed sperms, sperms without tails, sperm exhibiting abnormal head shapes, and those with cytoplasmic droplets (27). Another study indicated a notable rise in aberrations in sperm morphology, including detached heads, pyriform heads, coiled tails, and bent tails, among rats exposed to EMR (59). Studies have reported DNA damage in sperm cells following EMR exposure to the testes. This damage may correlate with the abnormalities seen in the sperm head and the mitochondrial sheath of the sperm tail (46, 80). However, a study by Kim et al. on the long-term exposure of rats to a 2.45 GHz electromagnetic field found no significant effects on sperm morphology (20). Defective sperm function is a major cause of male infertility. Excessive exposure of sperm to mobile phone radiation disrupts sperm function by affecting the mitochondria located in the mid-piece of the sperm tail, which are crucial for ATP production. This disruption leads to the generation of high levels of reactive oxygen species (ROS), contributing to decreased sperm motility. Studies have shown alterations in microtubule arrangement following exposure to mobile phone radiation. In experiments with EMR-exposed rats, significant changes in microtubules were observed, resulting in abnormalities in the sperm tail and adversely affecting motility. Additionally, radiation can disrupt the acrosome, potentially impairing spermatozoa's ability to penetrate oocytes, thus contributing to infertility (23, 81).
The included studies varied significantly in terms of experimental design, radiation exposure protocols, animal models, and histopathologic assessment methods. This heterogeneity limited the ability to draw consistent conclusions and precluded a meta-analysis. The studies used parametric tests without verifying the assumptions like normality and homogeneity of variances, which might introduce biases and affect the reliability of the findings. The studies also had small sample sizes, which may affect the statistical power and generalizability of the results.
Animal studies indicate that electromagnetic radiation from mobile phones can negatively impact testicular tissue histology and various sperm parameters, potentially affecting sperm count, motility, viability, and morphology. Therefore, it is advisable to exercise caution and implement preventive measures to reduce the potential risks associated with mobile phone use. Additionally, further research is essential to gain a comprehensive understanding of the effects of mobile phone radiation on human reproductive health.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
EA: Conceptualization, Data curation, Methodology, Supervision, Visualization, Writing – original draft, Writing – review & editing. SA: Data curation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors would like to express their heartfelt gratitude to all the colleagues in the Biomedical Sciences department at the College of Medicine and Health Sciences of Wollo University for their invaluable suggestions and support during the manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. 2016. Multiple definitions of infertility. Available online at: https://www.who.int/reproductivehealth/topics/infertility/multiple-definitions/en/ (accessed 21 November).
2. Khaki A, Fathi AF, Nouri M, Khaki AA, Ozanci CC, Ghafari NM, et al. The effects of ginger on spermatogenesis and sperm parameters of rat. Iran J Reprod Med. (2009) 7(1):7–12.
3. Olea N, Fernandez MF. Chemicals in the environment and human male fertility. Occup Environ Med. (2007) 64:430–1. doi: 10.1136/oem.2007.033621
4. Mohammadi F, Nikzad H, Taherian A, Mahabadi JA, Saleh M. Effects of herbal medicine on male infertility. Anat Sci. (2013) 10(4):3–16.
5. Pacey A. Environmental and lifestyle factors associated with sperm DNA damage. Hum Fertil. (2010) 13(4):189–93. doi: 10.3109/14647273.2010.531883
6. Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. (2008) 14(3):243–58. doi: 10.1093/humupd/dmn004
7. Gorpinchenko I, Nikitin O, Banyra O, Shulyak A. The influence of direct mobile phone radiation on sperm quality. Cent European J Urol. (2014) 67(1):65–71. doi: 10.5173/ceju.2014.01.art14
8. Agarwal A, Desai NR, Makker K, Varghese A, Mouradi R, Sabanegh E, et al. Effects of radiofrequency electromagnetic waves (RF-EMW) from cellular phones on human ejaculated semen: an in vitro pilot study. Fertil Steril. (2009) 92(4):1318–25. doi: 10.1016/j.fertnstert.2008.08.022
9. Kesari KK, Kumar S, Behari J. Mobile phone usage and male infertility in Wistar rats. Indian J Exp Biol. (2010) 48:987–92.21299041
10. Bin-Meferij MM, El-kott AF. The radioprotective effects of Moringa oleifera against mobile phone electromagnetic radiation-induced infertility in rats. J Clin Exp Med. (2015) 8(8):12487–97.
11. Karaman MI, Gokce AM, Koca O, Karaman B, Ozturk MI, Yurdakul N, et al. The effects of electromagnetic waves emitted by the cell phones on the testicular tissue. Arch Ital Urolrol. (2014) 30:274–7. doi: 10.4081/aiua.2014.4.274
12. Xu G, Intano GW, McCarrey JR, Walter RB, McMahan CA, Walter CA. Recovery of a low mutant frequency after ionizing radiation-induced mutagenesis during spermatogenesis. Mutat Res. (2008) 654(2):150–7. doi: 10.1016/j.mrgentox.2008.05.012
13. Kilgallon SJ, Simmons LW. Image content influences men’s semen quality. Biol Lett. (2005) 1:253–5. doi: 10.1098/rsbl.2005.0324
14. Davoudi M, Brossner C, Kuber W. The influence of electromagnetic waves on sperm motility. Urol Urogynaecol. (2002) 19:18–22.
15. Fejes I, Závaczki Z, Szöllősi J, Koloszár S, Daru J, Kovacs L, et al. Is there a relationship between cell phone use and semen quality? Arch Androl. (2005) 51(5):385–93. doi: 10.1080/014850190924520
16. Mugunthan N, Anbalagan J, Samy AS, Rajanarayanan S, Meenachi S. Effects of chronic exposure to 2G and 3G cell phone radiation on mice testis-A randomized controlled trial. Int J Curr Res Rev. (2015) 7:36–47.
17. Odacı E, Hancı H, Yuluğ E, Türedi S, Aliyazıcıoğlu Y, Kaya H, et al. Effects of prenatal exposure to a 900 MHz electromagnetic field on 60-day-old rat testis and epididymal sperm quality. Biotech Histochem. (2016) 91(1):9–19. doi: 10.3109/10520295.2015.1060356
18. Tas M, Dasdag S, Akdag MZ, Cirit U, Yegin K, Seker U, et al. Long-term effects of 900 MHz radiofrequency radiation emitted from mobile phone on testicular tissue and epididymal semen quality. Electromagn Biol Med. (2014) 8378(3):216–22. doi: 10.3109/15368378.2013.801850
19. Singh H, Sharma M, Yadav KC, Dhatwalia SK. Effect of 3G/4G mobile phone radiations on mice testis. Poll Res. (2021) 40:6–8.
20. Kim JY, Kim HT, Moon KH, Shin HJ. Long – term exposure of rats to a 2.45 GHz electromagnetic field: effects on reproductive function. J Urol. (2007) 48(12):1308–14.
21. Lee HJ, Pack JK, Kim TH, Kim N, Choi SY, Lee JS, et al. The lack of histological changes of CDMA cellular phone-based radio frequency on rat testis. Bioelectromagnetics. (2010) 31(7):528–34. doi: 10.1002/bem.20589
22. Eroschenko VP. diFior’s Atlas of Histology with Functional Correlations. 11 ed. Baltimore, MD: Lippincott Williams & Wilkins (2007).
23. De Iuliis GN, Newey RJ, King BV, Aitken RJ. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One. (2009) 4:e6446. doi: 10.1371/journal.pone.0006446
24. Perrard MH, Sereni N, Schluth-Bolard C, Blondet A, Giscard d’Estaing S, Plotton I, et al. Complete human and rat ex vivo spermatogenesis from fresh or frozen testicular tissue. Biol Reprod. (2016) 95(4):1–10. doi: 10.1095/biolreprod.116.142802
25. Agarwal A, Singh AH, Kesari K. Cell phones and male infertility: a review of recent innovations in technology and consequences. Int Braz J Urol. (2011) 37(4):432–54. doi: 10.1590/S1677-55382011000400002
26. Depinder F, Makkler K, Agarwal A. Cell phones and male infertility: dissecting the relationship. Reprod Biomed Online. (2007) 3:266–70. doi: 10.1016/S1472-6483(10)60338-0
27. Hamdi BA, Roshangar L, Khaki AA, Soleimani-rad J. Histological study of testes and sperm parameters in adult mice exposed to 50 Hz electromagnetic field during developmental period. Ann Biol Res. (2011) 2(5):455–62.
28. Hasan I, Amin T, Alam R, Rafiqul M. Hematobiochemical and histopathological alterations of kidney and testis due to exposure of 4G cell phone radiation in mice. Saudi J Biol Sci. (2021) 28(5):2933–42. doi: 10.1016/j.sjbs.2021.02.028
29. Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol. (2003) 107. doi: 10.1186/1477-7827-1-107
30. Rostamzadeh A, Mohammadi M, Ahmadi R, Nazari A, Ghaderi O, Anjomshoa M. Evaluation of mouse embryos produced in vitro after electromagnetic waves exposure; morphometric study. Electron Physician. (2016) 8:1701. doi: 10.19082/1701
31. Farjanikish G, Esmaeeli-Sani S, Mohammadi P. Effects of the long term exposure to mobile phone on testicular histology and serum level of testosterone in mice. Sci J Kurdistan Univ Med Sci. (2018) 23(4):110–8.
32. Nisbet HO, Nisbet C, Akar A, Cevik M, Karayigit MO. Effects of exposure to electromagnetic field (1.8/0.9 GHz) on testicular function and structure in growing rats. Res Vet Sci. (2012) 93(2):1001–5. doi: 10.1016/j.rvsc.2011.10.023
33. Nassar SA, Algazeery A, Ahmed GAS, El-maaty WAA. Histological, immunohistochemical and molecular alterations in immature mice testes due to chronic exposure to Mobile phone radiofrequency radiation. Egypt J Hosp Med. (2020) 78:128–35. doi: 10.21608/ejhm.2020.68482
34. Farag EA, Yousry MM. Effect of mobile phone electromagnetic waves on rat testis and the possible ameliorating role of naringenin : a histological study. Egypt J Histol. (2018) 41(1):108–21. doi: 10.21608/EJH.2018.7526
35. Hegazy AA, Ahmad MM, Almotaleb NAA, Aziz JA. Prenatal and postnatal exposure to cell phone radiation and its possible impact on the development of albino rat testicular tissue light and electron microscopic study. Egypt J Histol. (2022) 45(3):908–26. doi: 10.21608/EJH.2021.72870.1464
36. Kumar P, Shukla V. Ultrastructural changes in rat testicular tissue after whole body exposure to electromagnetic radiation emitted from mobile phones. J Int Acad Res Multidiscip. (2014) 2(1):518–26.
37. Luo Q, Li J, Cui X, Yan J, Zhao Q, Xiang C. The effect of Lycium barbarum polysaccharides on the male rats' reproductive system and spermatogenic cell apoptosis exposed to low-dose ionizing irradiation. J Ethnopharmacol. (2014) 154(1):249–58. doi: 10.1016/j.jep.2014.04.013
38. Baharara J, Zafar-Balanejad S, Kamareh E, Asadi-Samani M. The effects of green tea extract on teratogenicity induced by low frequency electromagnetic field on bone marrow Balb/C mice embryo. J Herbmed Pharmacol. (2014) 3(1):47–51.
39. Sowa P, Sieron-Stoltny K, Cieslar G, Sieron A. Impact of electromagnetic field generated by mobile phone on prooxidant-antioxidant balance in selected internal organs of rats. PIERS Proc. (2013):1903–37.
40. Dasdag S, Taş M, Akdag MZ, Yegin K. Effect of long-term exposure of 2.4 GHz radiofrequency radiation emitted from Wi-Fi equipment on testes functions. Electromagn Biol Med. (2015) 34(1):37–42. doi: 10.3109/15368378.2013.869752
41. Ozguner M, Koyu A, Cesur G, Ural M, Ozguner F, Gokcimen A, et al. Biological and morphological effects on the reproductive organ of rats after exposure to electromagnetic field. Saudi Med J. (2005) 26:405–10.15806208
42. Saygin M, Caliskan S, Karahan N, Koyu A, Gumral N, Uguz AC, et al. Testicular apoptosis and histopathological changes induced by a 2.45 GHz electromagnetic field. Toxicol Ind Health. (2011) 27(5):455–63. doi: 10.1177/0748233710389851
43. Middendorff R, Müller D, Mewe M, Mukhopadhyay AK, Holstein AF, Davidoff MS, et al. The tunica albuginea of the human testis is characterized by complex contraction and relaxation activities regulated by cyclic GMP. J Clin Endocrinol Metab. (2002) 87(7):3486–99. doi: 10.1210/jcem.87.7.8696
44. Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. (2006) 168(6):1861–8. doi: 10.2353/ajpath.2006.051302
45. Mugunthan N, Anbalagan J, Meenachi S. Effects of long term exposure to a 2G cell phone radiation (900–1900 MHz) on mouse testis. Int J Sci Res. (2014) 3:523–9.
46. Kumar S, Nirala JP, Behari J, Paulraj R. Effect of electromagnetic irradiation produced by 3G mobile phone on male rat reproductive system in a simulated scenario. Indian J Exp Biol. (2014) 52(9):890–7.25241589
47. Khayyat LI. The histopathological effects of an electromagnetic field on the kidney and testis of mice. Eur Asian J Bio Sci. (2011) 5:103–9. doi: 10.5053/ejobios.2011.5.0.12
48. El-Naggar MI, El-Sagheer AS, Ebaid AE. The possible protective effect of vitamin E on adult albino rat’s testes exposed to electromagnetic field emitted from a conventional cellular phone. Egypt J Hosp Med. (2019) 74(4):873–84. doi: 10.21608/ejhm.2019.25267
49. Wang SM, Wang DW, Peng RY, Gao YB, Yang Y, Hu WH, et al. Effect of electromagnetic pulse irradiation on structure and function of Leydig cell in mice. Natl J Androl. (2003) 9(5):327–30.
50. Zareen N. Testicular morphology: effects of mobile phone induced electromagnetic fields on mice testes. Professional Med J. (2009) 16(2):289–92. doi: 10.29309/TPMJ/2009.16.02.2945
51. Weyden VD, Arends MJ, Chausiaux OE. Loss of TSLC1 causes male infertility due to a defect at the spermatid stage of spermatogenesis. Mol Cell Biol. (2006) 26:3595–609. doi: 10.1128/MCB.26.9.3595-3609.2006
52. Esa PD, Suryandari DA, Sari P. Effect of extremely low frequency electromagnetic fields on the diameter of seminiferous tubules in mice, in. J Phys Conf Ser. (2018) 062043. doi: 10.1088/1742-6596/1073/6/062043
53. Chauhan P, Verma HN, Sisodia R, Kesari KK. Microwave radiation (2.45 GHz)-induced oxidative stress: whole-body exposure effect on histopathology of Wistar rats. Electromagn Biol Med. (2017) 36:20–30. doi: 10.3109/15368378.2016.1144063
54. Wong CH, Cheng CY. The blood testis barrier: its biology, regulation, and physiological role in spermatogene-sis. Curr Top Dev Biol. (2005) 71:263–96. doi: 10.1016/S0070-2153(05)71008-5
55. Adebayo EA, Adeeyo AO, Ogundiran MA, Olabisi O. Bio-physical effects of radiofrequency electromagnetic radiation (RF-EMR) on blood parameters, spermatozoa, liver, kidney and heart of albino rats. J King Saud Univ Sci. (2019) 31(4):813–21. doi: 10.1016/j.jksus.2018.11.007
56. Oh JJ, Byun SS, Lee SE, Choe G, Hong SK. Effect of electromagnetic waves from mobile phones on spermatogenesis in the era of 4G-LTE. Biomed Res Int. (2018) 2018(1):1801798. doi: 10.1155/2018/1801798
57. Meo SA, Arif M, Rashied S, Khan MM, Vohra MS, Usmani AM, et al. Hypospermatogenesis and spermatozoa maturation arrest in rats induced by mobile phone radiation. J Coll Physicians Surg Pakistan. (2011) 21(5):262–5.
58. Oyewopo AO, Olaniyi SK, Oyewopo CI, Jimoh AT. Radiofrequency electromagnetic radiation from cell phone causes defective testicular function in male Wistar rats. Andrologia. (2017) 49(10):e12772. doi: 10.1111/and.12772
59. Banavath AN, Srinivasa SN. Ameliorative effect of Punica granatum on sperm parameters in rats exposed to mobile radioelectromagnetic radiation. Asian Pacific J Reprod. (2021) 10(5):225–31. doi: 10.4103/2305-0500.326720
60. Dasdag S, Zulkuf Akdag M, Aksen F, Yılmaz F, Bashan M, Mutlu Dasdag M, et al. Whole body exposure of rats to microwaves emitted from a cell phone does not affect the testes. Bioelectromagnetics. (2003) 24:182–8. doi: 10.1002/bem.10083
61. Guan M, Tang W, Hang J, Wang H, Jiang X, Zhu H. Effects of mobile phone radiation on semen quality of rat. Chin J Androl. (2012) 4:23–5.
62. Bahaodini A, Owjfard M, Tamadon A, Jafari SM. Low frequency electromagnetic fields long-term exposure effects on testicular histology, sperm quality and testosterone levels of male rats. Asian Pac J Reprod. (2015) 4(3):195–200. doi: 10.1016/j.apjr.2015.06.001
63. Mailankot M, Kunnath AP, Jayalekshmi H, Koduru B, Valsalan R. Radio frequency electromagnetic radiation (RF-EMR) from GSM (0.9/1.8 GHz) mobile phones induces oxidative stress and reduces sperm motility in rats. Clinics. (2019) 64(6):561–5. doi: 10.1590/S1807-59322009000600011
64. Ghanbari M, Mortazavi SB, Khavanin A, Khazaei M. The effects of cell phone waves (900 MHz-GSM band) on sperm parameters and total antioxidant capacity in rats. J Fertility Steril. (2013) 7:21–8.
65. Mortazavi SMJ, Tavassoli AR, Ranjbari F, Moammaiee P. Effects of laptop computers’ electromagnetic field on sperm quality. J Reprod Infertil. (2010) 11(4):251–8.
66. Kesari KK, Kumar S, Behari J. Effects of radiofrequency electromagnetic wave exposure from cellular phones on the reproductive pattern in male Wistar rats. Appl Biochem Biotechnol. (2011) 164:546–59. doi: 10.1007/s12010-010-9156-0
67. Meral I, Mert H, Mert N, Deger Y, Yoruk I, Yetkin A, et al. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. (2007) 1169:120–4. doi: 10.1016/j.brainres.2007.07.015
68. Oktem F, Ozguner F, Mollaoglu H, Koyu A, Efkan U. Oxidative damage in the kidney induced by 900-MHz-emitted mobile phone: protection by melatonin. J Arch Med Res. (2005) 36:350–5. doi: 10.1016/j.arcmed.2005.03.021
69. Hales DB, Allen JA, Shankara T, Janus P, Buck S, Diemer T, et al. Mitochondrial function in leydig cell steroidogenesis. Ann N Y Acad Sci. (2005) 1061(1):120–34. doi: 10.1196/annals.1336.014
70. Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. J Human Reprod Update. (2001) 7:473–81. doi: 10.1093/humupd/7.5.473
71. Makker K, Varghese A, Desai NR, Mouradi R, Agarwal A. Cell phones: modern man’s nemesis? Reprod BioMed Online. (2009) 18:148–57. doi: 10.1016/S1472-6483(10)60437-3
72. Meo SA, Al-Drees AM, Husain S, Khan MM, Imran MB. Effects of mobile phone radiation on serum testosterone in Wistar albino rats. Saudi Med J. (2010) 30(8):869–73.
73. Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. (2010) 365:1557–69. doi: 10.1098/rstb.2009.0258
74. Kliesch S. Testosterone and infertility. Urologe Artz. (2010) 49:32–6. doi: 10.1007/s00120-009-2195-x
75. La Vignera S, Condorelli RA, Vicari E, D’Agata R, Calogero AE. Effects of the exposure to mobile phones on male reproduction: a review of the literature. J Androl. (2012) 33(3):350–6. doi: 10.2164/jandrol.111.014373
76. Yu G, Bai Z, Song C, Cheng Q, Wang G, Tang Z, et al. Current progress on the effect of mobile phone radiation on sperm quality: an updated systematic review and meta-analysis of human and animal studies. Environ Pollut. (2021) 282:116952. doi: 10.1016/j.envpol.2021.116952
77. Adams JA, Galloway TS, Mondal D, Esteves SC, Mathews F. Effect of mobile telephones on sperm quality: a systematic review and meta-analysis. Environ Int. (2014) 70:106–12. doi: 10.1016/j.envint.2014.04.015
78. Liu K, Li Y, Zhang G, Liu J, Cao J, Ao L, et al. Association between mobile phone use and semen quality: a systemic review and meta-analysis. Andrology. (2014) 2:491–501. doi: 10.1111/j.2047-2927.2014.00205.x
79. Ayrapetyan SN. Cell aqua medium as a primary target for the effect of electromagnetic fields. In: Ayrapetyan SN, Markov MS, editors. BIOELECTROMAGNETICS Current Concepts: The Mechanisms of the Biological Effect of Extremely High Power Pulses. Dordrecht: Springer Netherlands (2006). p. 31–63.
80. Kumar S, Behari J, Sisodia R. Influence of electromagnetic fields on reproductive system of male rats. Int J Rad Biol. (2013) 89(3):147. doi: 10.3109/09553002.2013.741282
Keywords: histopathology, mobile phone radiation, testes, sperm parameters, lab animals
Citation: Assefa EM and Abdu SM (2025) Histopathologic effects of mobile phone radiation exposure on the testes and sperm parameters: a systematic literature review of animal studies. Front. Reprod. Health 6:1515166. doi: 10.3389/frph.2024.1515166
Received: 25 October 2024; Accepted: 30 December 2024;
Published: 17 January 2025.
Edited by:
Ruben Dario Motrich, National Scientific and Technical Research Council (CONICET), ArgentinaReviewed by:
Nicolas Ramirez, CCT CONICET Córdoba, ArgentinaCopyright: © 2025 Assefa and Abdu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ebrahim Msaye Assefa, ZWJyYWhpbW1zYXllQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.