- 1Kinderwunschzentrum Ulm, Ulm, Germany
- 2Department of Urology, University Hospital of Ulm, Ulm, Germany
- 3Sartorius Stedim Cellca GmbH, Ulm, Germany
- 4Department of Gynaecology and Obstetrics, University Hospital of Ulm, Ulm, Germany
Objectives: Endometrial scratching (ES) and/or intravenous intralipid therapy (in cases of increased uterine natural killer cells, uNKs) are still conducted in several fertility centers as “add-on” treatments in patients undergoing ART, although convincing evidence for beneficial effects is lacking.
Study design: In this retrospective study, associations between ES treatment or additional intralipid therapy and pregnancy and live birth rates of 1,546 patients undergoing 2,821 IVF-/ICSI-treatment cycles with fresh or frozen embryo transfers in a German fertility-center between 1st January 2014 and 31th May 2017 were analyzed.
Results: Overall pregnancy and live birth rates for all 2,821 treatment cycles (468 cycles with ES) were 32.8% and 23.5%. There were no statistically significant differences in pregnancy or live birth rates between first treatment cycles with and without ES (p = 0.915 and p = 0.577) or between second cycles following an unsuccessful first cycle with and without ES (p = 0.752 and p = 0.623). These results were confirmed using multivariable generalized estimating equations (GEE) models accounting for non-independency of multiple treatment cycles per patients that included all cycles and showed no significant effect of ES on pregnancy (p = 0.449) or live birth rates (p = 0.976). Likewise, a GEE model revealed no significant effect of intralipid treatment on pregnancy (p = 0.926) and live birth rates (p = 0.727).
Conclusions: Our results reveal no evidence that ES increases the pregnancy or live birth rates in women undergoing their first or further IVF cycle with fresh or frozen embryo transfer. Intralipid treatment was also not beneficial. Even if patients explicitly ask for it, these procedures are not recommended outside of clinical studies.
Introduction
In Germany, between 1997 and 2020 over 364 thousand children were born with assisted reproductive technologies (ART), such as IVF and ICSI, but success rates reached a plateau of approximately 32% per fresh and 30% per frozen embryo transfer (1). Implantation success depends on various factors, like cytokines, interleukins, growth-factors, macrophages and decidualization of stromal cells regulating trophoblast invasion (2, 3). Already in 1907, L. Loeb showed in guinea pigs that endometrial injury - termed endometrial scratching (ES) - increases proliferation of decidual cells (3). ES is a local endometrial trauma caused by a catheter which aspirates mucosal tissue, thereby disrupting the endometrial integrity (4–6). It is assumed that ES leads to an improved synchronization between endometrium and transferred embryo, thereby increasing implantation success (4). However, biological and molecular mechanisms induced by ES are still mostly unknown.
Although Nastri et al. demonstrated an encouraging increase in pregnancy and live birth rates after ES in patients with ART therapy (4), heterogeneous results of other studies challenged the benefit of ES and its applicability in routinely clinical practice (3). Several randomized controlled trials (RCTs) and meta-analyses have now demonstrated the lacking benefit of ES (7–11), but there seems to be a positive effect in certain subgroups (12, 13). ES is still offered and conducted in several fertility centers to patients with recurrent implantation failure (RIF) or miscarriages. Patients often actively ask their physicians for an ES procedure, hoping to increase their pregnancy chances, even if there is no clear evidence for beneficial effects.
Besides ES, other “add-on” treatments such as immune therapies [e.g., intralipids, corticosteroids, Granulocyte-Colony Stimulating Factor (G-CSF) or intravenous immunoglobulin], endometrial receptivity array (ERA), uterine artery vasodilation, and human chorionic gonadotropin instillation) try to improve IVF success rates. However, benefits of these add-ons are often not evidenced yet, and therefore use of these treatments is controversially discussed (14, 15).
For further clarity, we retrospectively evaluated data from a fertility center in Ulm between 1/2014 and 5/2017, when ES treatment was extensively performed due to unclear data at that time. This analysis investigates the impact of ES in patients undergoing ART and compares pregnancy and livebirth rates of patients in the first, second or further fresh or frozen-embryo transfer cycles. Moreover, we analyzed certain subgroups of patients that might benefit from ES. Additionally, we examined potential beneficial effects of intralipid treatment with special attention to patients with enhanced uNK cells detected immunohistochemically in ES samples.
Materials and methods
Institutional review board (IRB) approval: This study was authorized by the ethics commission of the “Landesärztekammer Baden-Württemberg” (B-F-2017-129).
This retrospective study is based on all ART treatments conducted in “Kinderwunschzentrum Ulm, Einsteinstraße” between 1/2014 and 5/2017. Inclusion criteria were IVF-/, ICSI- fresh or frozen-embryo transfers. Exclusion criteria were multiple pregnancy and missing data of potential previous ES. Stimulation prior fresh embryo transfer was either performed using an antagonist protocol (FSH/LH: Gonal-F®, Puregon®, Bemfola®; Ovaleap®, Pergoveris®, Menogon®; antagonists: Orgalutran®, Cetrotide®) with individually patient adapted FSH-levels or an agonist protocol (FSH/LH as mentioned; down regulation: Synarela® nasal-spray). Frozen embryo transfers were performed in an artificial cycle (oral Estrifam® stepup-protocol until ≥8 mm endometrial size, luteal phase induction with progesterone (Utrogest®, Lutinus®, Progestan®, Famenita®, Prolutex®). Fresh or frozen embryo transfers were performed at day 2/3 in the 4-/8-cell-stage or at day 5 at blastocyst-stage. Following a detailed patient informed consent, ES was performed either in the luteal phase of the pre-cycle of IVF/ICSI or in the mid-luteal phase of the cycle prior frozen-transfer. Endometrial tissue was extracted with a “Pipelle de Cornier” catheter in an outpatient setting in a fertility center in Ulm (city in the South of Germany) and tissue samples were examined histologically in Ulm and Jena (laboratories in two cities in Germany). The immunohistochemical analyzes of uNK cells (CD56-positive) were performed in Jena.
Patients previously identified with increased uNK cells (>300 uNK cells/mm2) were offered an off-label intralipid infusion therapy. After written informed consent, patients received intralipid intravenously (50 mL intralipid 20% in 100 mL physiological sodium chloride solution, over 1.5–2 h) once in the previous menstrual cycle, another application at the day of egg retrieval and, if applicable, with a positive pregnancy test.
Descriptive statistics included absolute and relative frequency, mean, standard deviation (SD), median, interquartile range, minimum (min) and maximum (max). Associations between categorical variables and differences between patients with and without ES regarding pregnancy and live birth rates were analyzed by chi-square test. If expected frequencies in cells of 2 × 2 crosstabs were less than five, Fisher's exact test was used instead of chi-square test. Differences between patients with and without ES regarding quantitative data were analyzed with Mann Whitney U-test. The effect of ES on pregnancy and live birth rates was analyzed separately for the first treatment cycle of each patient received within the study period, for second treatment cycles following an unsuccessful first treatment cycle, and for third treatment cycles following two unsuccessful treatment cycles. Furthermore, we used Generalized estimating equations (GEE) models (an extension of generalized linear models) to be able to incorporate all treatment cycles into the analyses despite the fact that repeated treatment cycles for the same patient are not independent events. We ran binary logistic models with the dependent variables pregnancy (yes/no) or live birth (yes/no), specifying a binomial probability distribution with a logit link function and incorporating patient ID as subject effect for a repeated measures design. Statistical models for assessment of the effect of ES on pregnancy and live birth rates included ES (yes/no), embryo transfer type (fresh/frozen), patient age (years), patient BMI (kg/m2) and partner age (years) as independent predictor variables. Statistical models for assessment of the effect of intralipid therapy on pregnancy and live birth rates (only cases with ES) included only intralipid therapy (yes/no) as independent predictor variable.
A significance level of α = 0.05 was used throughout and - given that the analyses presented here are part of a retrospective explorative study - no adjustments for multiple testing were made.
Results
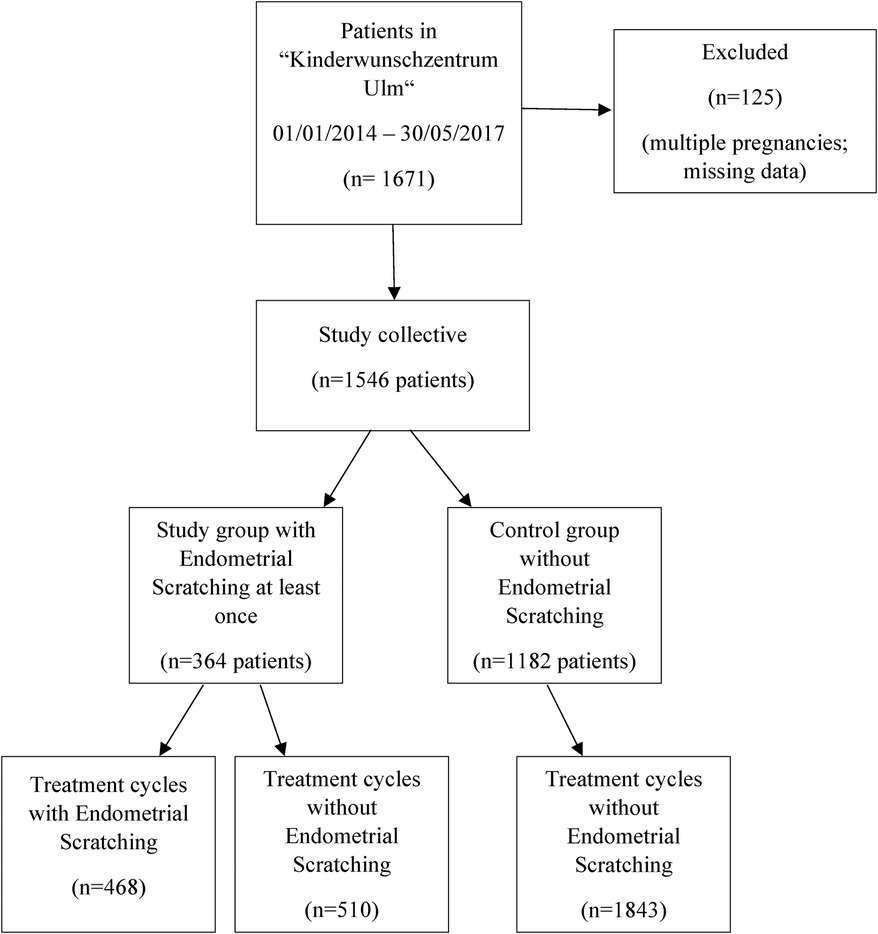
Overall, data of 1,671 patients were collected and 1,546 patients were finally included. Of those, more than 50% were older than 35 years, 58% had no previous pregnancy and 70% had not given birth before. 1,546 patients underwent 2,821 treatment cycles (median 1 treatment cycle, range 1–10 treatment cycles) with more than 90% receiving less than four treatment cycles. In total, 364 (23.5%) patients were subject to at least one ES procedure during the study period (range 1–6 treatment cycles with ES), while in 1,182 (76.5%) patients ES was never applied (Figure 1). Table 1 compares the baseline patient characteristics at the first ART treatment. Patients with at least one ES procedure were comparable to patients without ES regarding age, partner age, BMI, gravida, parity and anti-Müllerian hormone (AMH), but had significantly more treatment cycles, with 26.1% vs. 4.0% of patients with more than three treatment cycles overall (Table 1).
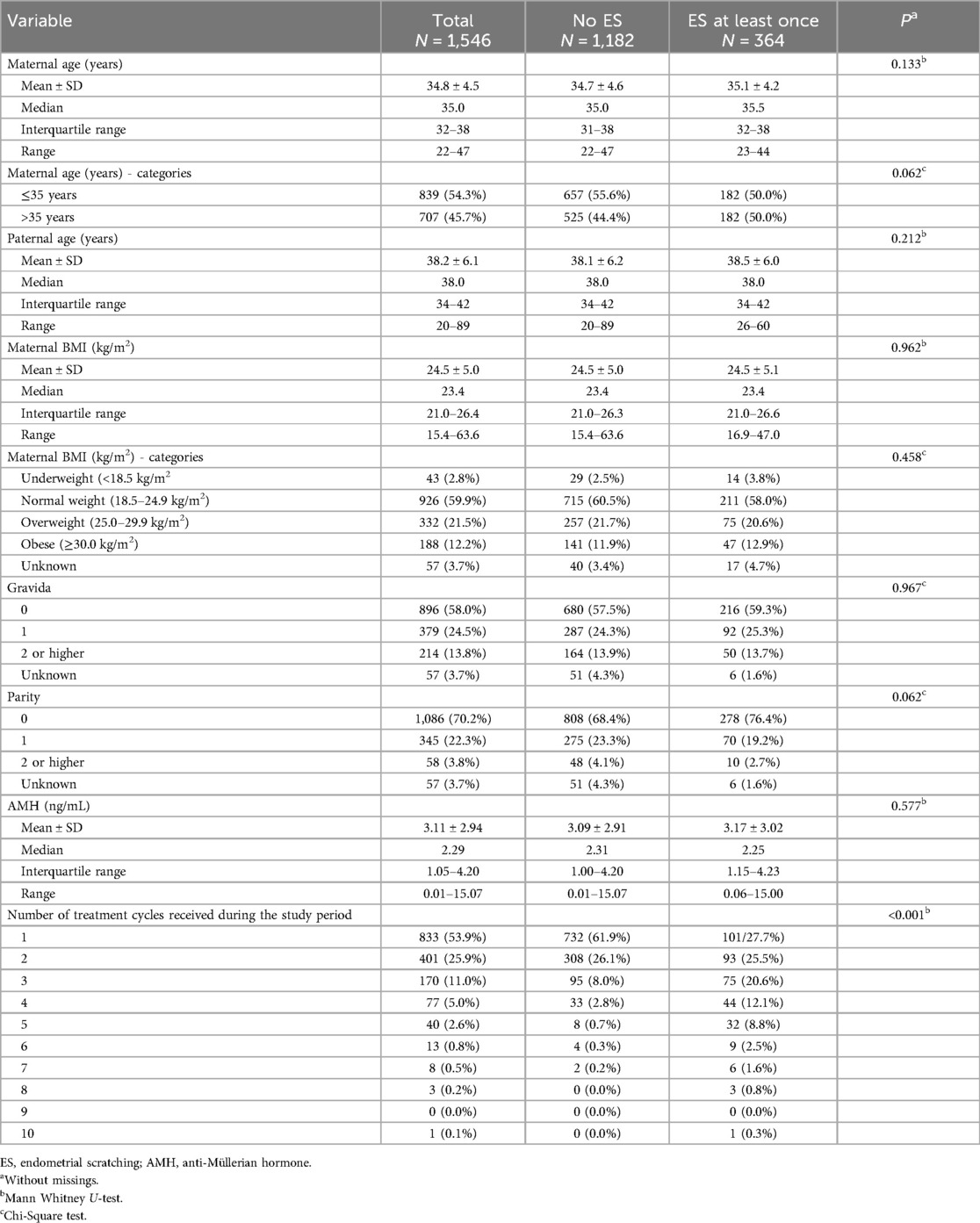
Table 1. Baseline characteristics for 1,546 patients that received at least one ART treatment during the study period.
Out of the 2,821 treatment cycles conducted during the study period, 468 (16.6%) included an ES procedure. Table 2 compares the basic features of the single treatment cycles between treatment cycles with and without ES, showing similar parameters regarding the proportion of fresh or frozen embryo transfers, number of fertilized oocytes, frozen fertilized oocytes and embryos transferred per evaluated cycle.
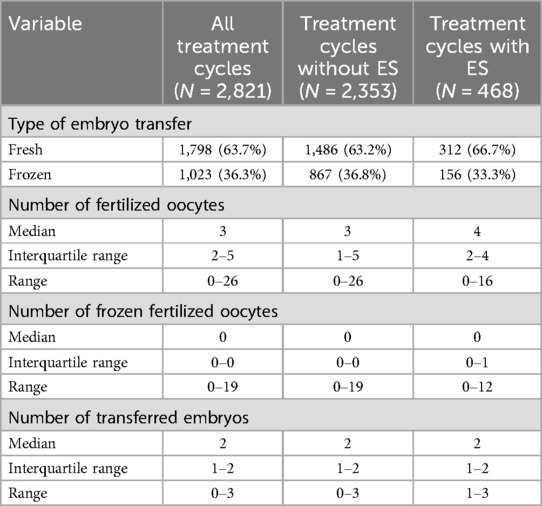
Table 2. Basic features for 2,821 ART treatment cycles according to the use of endometrial scratching.
Fresh or frozen embryo transfers were mostly performed at day 2/3 in the 4-/8-cell-stage or as blastocysts at day 5. In fresh embryo transfer cycles, transfer rate at day 5 was 61.5% (192 of 312) with ES and 59.5% (884 of 1,486) without ES. In frozen embryo transfers, day 0 or 5 transfer rate was 80.8% (126 of 156) with ES and 78.4% (680 of 867) without ES.
Overall, 1,175 of the 2,821 treatment cycles represent the first ART cycle of a patient, of which 111 (9.4%) and 1,064 (90.6%) were conducted with and without ES, respectively. (Some patients had received their first ART cycle outside the study and the exact number of the treatment cycle was unknown for 351 out of the 2,821 included treatment cycles). Pregnancy rate in the first treatment cycle was 33.3% (95% CI 24.6%–42.1%) with ES and 33.8% (95% CI 31.0%–36.7%) without ES, showing no significant difference (p = 0.915; see Figure 2A). Subgroup analysis for fresh or frozen embryo transfers could also not reveal any benefit for patients undergoing ES (Table 3). Likewise, there was no significant difference in the overall live birth rates between first treatment cycles with and without ES (with ES: 27.0%, 95% CI 18.8%–35.3%; without ES: 24.6%, 95% CI 22.0%–27.2%; p = 0.577; see Figure 2A); the same result was obtained when live birth rates were compared separately for fresh or frozen embryo transfers (Table 3).
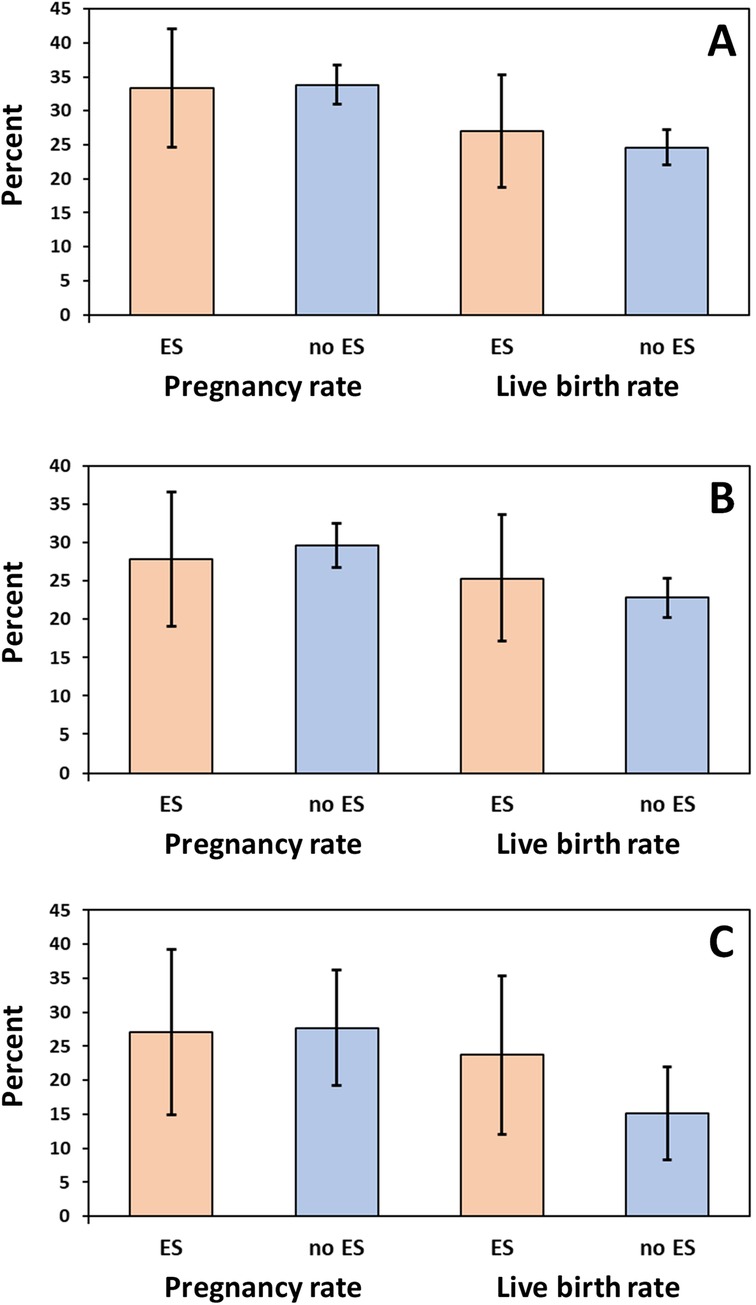
Figure 2. Pregnancy and live birth rates (%) in (A) first treatment cycles (n = 1,175), (B) second treatment cycles following a first unsuccessful treatment cycle (n = 457), and (C) third treatment cycles following a first and second unsuccessful treatment cycle (n = 178). ES, endometrial scratching. Error bars show 95% confidence intervals.
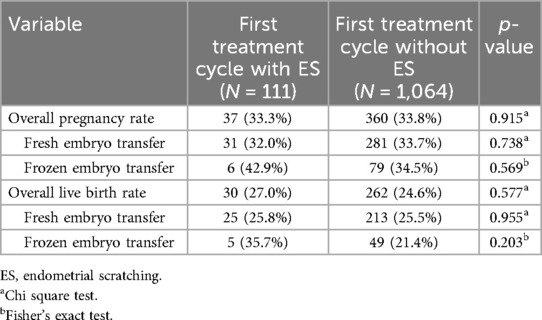
Table 3. Pregnancy and live birth rates of women undergoing assisted reproduction in their first treatment cycle.
Overall, 457 (58.7%) of the 778 patients with one unsuccessful first treatment cycle (no pregnancy) underwent a second cycle during the study course. The pregnancy rate was 27.8% (95% CI 18.0–37.7%) in the 79 patients with ES vs. 29.6% (95% CI 25.0%–34.2%) in the 378 patients without ES (p = 0.752). Likewise, live birth rates did not differ significantly between second treatment cycles with or without ES following unsuccessful first treatment cycles (25.3%, 95% CI 15.7%–34.9% vs. 22.8%, 95% CI 18.5%–27.0%; p = 0.623; see Figure 2B). As for the first treatment cycle, there were also no significant differences with regard to pregnancy and live birth rates when the comparisons were performed separately for fresh or frozen embryo transfers (Table 4).
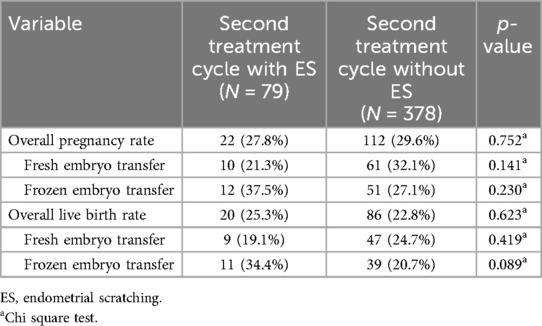
Table 4. Pregnancy and live birth rates of women undergoing a second assisted reproduction treatment after an unsuccessful first treatment cycle.
Overall, 178 (55.1%) of the 323 patients with two unsuccessful treatment cycles (no pregnancy) underwent a third cycle during the study course. The pregnancy rate was 27.1% (95% CI 14.9–39.3%) in the 59 patients with ES vs. 27.7% (95% CI 19.3%–36.2%) in the 119 patients without ES (p = 0.931). Likewise, live birth rates did not differ significantly between second treatment cycles with or without ES following two unsuccessful treatment cycles (23.7%, 95% CI 12.0%–35.4% vs. 15.1%, 95% CI 8.3%–22.0%; p = 0.159; see Figure 2C). As for the first or second treatment cycle, there were also no significant differences regarding pregnancy and live birth rates when comparisons were performed separately for fresh or frozen embryo transfers (Table 5).
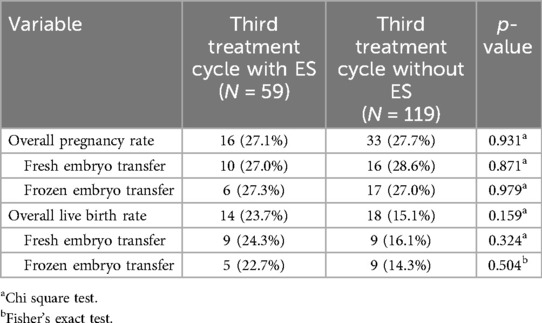
Table 5. Pregnancy and live birth rates of women undergoing a third assisted reproduction treatment after two unsuccessful treatment cycles.
Pregnancy and live birth rates were 34.5% and 25.4% for all 1,486 treatment cycles with fresh embryo transfer but without ES, 29.8% and 22.8% for all 312 treatment cycles with fresh embryo transfer and ES, 31.4% and 21.1% for all 867 treatment cycles with frozen embryo transfer but without ES, and 30.1% and 21.2% for all 156 treatment cycles with frozen embryo transfer and ES.
To account for non-independency of the data (as many patients received more than one treatment cycle), we analyzed the data set including all treatment cycles using an adjusted multivariable GEE model approach (explained in detail in the method section) with the variables ES, type of embryo transfer, patient age, patient BMI and partner age as independent predictor variables for the outcome variables pregnancy rate and live birth rate. There was no significant effect of ES (yes vs. no) on pregnancy rate (adjusted odds ratio 0.92, 95% CI 0.74–1.15, p = 0.449) or live birth rates (adjusted odds ratio 1.00, 95% CI 0.78–1.29, p = 0.976). Both pregnancy and live birth rate were significantly affected by maternal age at the time of the first treatment cycle received within the study, with decreasing odds for success with increasing age (pregnancy rate: adjusted odds ratio 0.93, 95% CI 0.90–0.95, p < 0.001; live birth rate: adjusted odds ratio 0.92, 95% CI 0.90–0.94, p < 0.001), indicating that with each year a woman gets older the odds for pregnancy or live birth decrease by 7% to 8%. While there was no significant effect of the type of embryo transfer (frozen vs. fresh) on pregnancy rate (adjusted odds ratio 0.87, 95% CI 0.73–1.03, p = 0.101), treatment cycles with frozen embryo transfer were associated with significantly decreased odds for live birth compared to fresh embryo transfers (adjusted odds ratio 0.79, 95% CI 0.65–0.96, p = 0.018).
Information on intralipid application was available for 450 of 468 treatment cycles with an ES procedure, and intralipid was applied in 113 (24.1%) of these cases. Pregnancy and live birth rates were 30.1% and 23.9% in the 113 treatment cycles with an ES procedure and intralipid therapy, and 30.6% and 22.3% in the 337 treatment cycles with an ES procedure but without intralipid therapy. A GEE analysis accounting for non-independency of multiple data obtained from the same patient (see methods) showed no significant effect of intralipid (yes vs. no) on pregnancy rates (odds ratio 0.98, 95% CI 0.61–1.57, p = 0.926) or live birth rates (odds ratio 1.10, 95% CI 0.65–1.84, p = 0.727).
ES tissue samples were examined immunohistochemically for uNK cells in 262 patients and in 79 (30.2%) patients an increase of uNK cells was found, with a mean value of absolute uNK cell number of 413.5 ± 131.8 (median 376 cells, range 304–1,111 cells). Both pregnancy and live birth rates were higher in the 67 patients with increased uNKs cells in ES biopsies and application of intralipid compared to the 12 patients with increased uNKs cells but no subsequent infusion therapy (34.3% vs. 8.3% and 28.4% vs. 0.0%, respectively). However, at least partly due to the small sample size, these differences were not significant (p = 0.094 and p = 0.060, respectively).
Discussion
Here, we analyzed retrospectively outcome data of patients treated by ES. Our data revealed no significant differences in pregnancy and live birth rates between patients undergoing ART with ES compared to the control group without ES, even not by considering different subgroups like patient collectives with one or more previous unsuccessful embryo transfers and fresh or frozen embryo transfers. Thus, our large retrospective single-center study adds to the data casting severe doubts on the efficacy of ES, and we are convinced that it is important to make all existing data available to protect patients from potentially unnecessary therapies. Moreover, these therapies can lead to negative side effects, cause extra self-paid costs, are time consuming, promise doubtful false hope while lacking any beneficial effects (11).
In recent years, several RCTs, systematic reviews, and meta-analyses have been published, still reporting controversial results of potentially beneficial effects of ES for improving the reproductive outcomes of infertile women (16, 17). Even if several large RCTs reported no clear evidence for improved live birth rates following ES and the ESHRE add-ons working group reported that ES is currently not recommended for routine clinical use (15), a lot of fertility specialists still offer this procedure to their patients, mostly in cases of recurrent implantation failure (RIF), as demonstrated in a survey study in Australia, New Zealand, and the UK (9, 18).
Our results are in line with the majority of the already published data, which also did not find any significant increase of pregnancy and live birth rates following ES in different contexts (10, 14, 19–24). A recent Cochrane analysis emphasized that even if ES does not affect the chance of miscarriages, it is a slightly painful, unpleasant procedure with small amount of bleeding and in the overall view of available data there is no current evidence to support the routine use of ES for women undergoing IVF (18). Another current Cochrane meta-analysis demonstrated that evidence is insufficient to support ES in women undergoing IUI or attempting to conceive via sexual intercourse (7). Additionally, subgroup analysis of patients with one or two preceding unsuccessful embryo transfers did not show a significant benefit of endometrial scratching in general, which corresponds to data of the current literature (14, 22, 25).
Some previous trials showing a statistically significant benefit of ES were either small or had a higher risk of confounding bias (e.g., missing randomization or overestimation of the treatment effect by early termination of the study) (4, 26, 27). In a meta-analysis, Nahshon and colleges showed improved pregnancy and livebirth rates especially among younger subgroups and in studies that conducted ES twice (28). However, potential confounding factors such as embryo quality were not considered, and they could not prove a significant effect of ES when analyzing studies that solely included patients with two or more previously failed cycles (28), which is in line with our results. Another meta-analysis has shown that ES performed once during the follicular phase of the same cycle of IUI may improve clinical pregnancy rates and further RCTs are suggested on specific populations to ultimately identify the appropriate time of invasion and whether certain subgroups might benefit from ES (12, 29). However, as long as the situation is still unclear, a routinely daily practice of ES should be avoided (15, 22).
Furthermore, in our present study, we could not see any beneficial effects regarding pregnancy or life birth rates of patients treated intravenously with intralipid in cases of increased uNK cells detected in ES biopsies.
Previous studies have shown that abnormal increased expression of NK cells surface markers in peripheral blood, endometrial and uNK cells and higher concentrations of distinct T-lymphocytes are involved in RIF and recurrent miscarriage (RM), assuming that NK cells activity is important for a physiological pregnancy (30–38). In particular, uNK cells seem to play an important role in trophoblast invasion and angiogenesis and represent about 70% of immune cells at the feto-maternal interface (39). However, so far presented data on uNK cells are controversial, not only due to missing standardized diagnostic but also proper reference amounts (40).
Besides ES, immune therapies like intralipid, corticosteroids, Granulocyte-Colony Stimulating Factor (G-CSF) or intravenous immunoglobulin in addition to IVF therapies are widespread and try to improve the success of life birth rates (14). Intralipid is an emulsion of soybean-oil, glycerin egg and phospholipids, usually used as infusion therapy for patients not tolerating oral nutrition. Additionally, intralipid is thought to modulate immune functions and in a mouse model it has been shown to decrease spontaneous abortions (41). In general, intralipid is well tolerated with rare adverse side effects (42), nevertheless complications like allergic reactions, hyperthermia or jaundice have been described (43–45). Depending on the institution, intralipid treatment as “add-on” in ART therapies costs approximately 100–300 US$/€ (14). It was postulated that pre-pregnancy immunomodulatory treatments using intralipid in patients with RM might be helpful for women with abnormal uterine and/or peripheral blood NK cells and establish an immune environment which is supportive for fetal development by recruitment and expression of pro-inflammatory cytokines (34, 36, 46). Singh and colleges have shown in women with previous implantation failure after IVF/ICSI that intralipid increased implantation and live birth rate (47), but the effects are still discussed controversial, and further studies are needed to establish evidence-based guidelines (30, 48). A recently published review and meta-analysis demonstrated that although intralipid is not recommended as a routine treatment for RM or implantation failure, and the presence of abnormal uNK cells as target marker needs further evaluation, a selected patient collective could benefit of intralipid (49). It is assumed, that only patients whose reproductive failure has an identifiable immunologic factor would respond to immunotherapies (50), however the appropriate selection of these subgroups remains challenging.
As valid data are still missing, defining a causal relationship between uNK cells and RIF and RM, as well as a beneficial effect of immunomodulators like intralipid (51–53), immunology testing and treatment outside of well-structured clinical studies is currently not recommended (14). Also the ESHRE add-ons working group remarked that immunomodulating treatments, such as Intralipid, IVIG, rh-LIF, PBMCs, and anti-TNF, are not recommended (15). Although not evidenced, many infertile patients are willing to try anything that might help to increase their chances to get pregnant, despite proven inefficacy, possible side-effects and extra costs for these procedures (54). It also seems to be a psychological problem for infertile couples and for their counselors to discard “potentially useful” naturalized therapies (55).
Of course, we are aware that our retrospective study has its limitations, as it is not a RCT, which is the present gold standard. However, the large size, inclusion of both uNK cell determinations and intralipid therapy, and the incorporation of subgroup analyses with regard to fresh or frozen embryo transfers and treatment cycles following one or two previous unsuccessful ART attempts result in important data that add to the available information necessary to assess the role of ES in ART.
Prospectively, there could be other beneficial effects by further histological, microbiological, or transcriptome analyses of ES material. E.g. detection of an increased amount of uterine plasma cells in chronical endometritis, analyses of a disturbed uterine microbiome, or significant differential gene expression patterns. These might lead to treatments with antibiotics, reconstitution of a physiological endometrial flora, determination of the best time window of endometrial receptivity, or detection of an appropriate secretome profile, respectively (56, 57). Recently, also other approaches were discussed, like intrauterine instillation of growth hormones or autologous platelet rich plasma (PRP) (58). However, all these new methods lack evidence so far.
Conclusion
Based on our results, there is no clear evidence that ES increases the probability of pregnancy or live birth rates in patients undergoing ART. Even within various subgroup analyses, regarding first or further treatment cycles and fresh or frozen-embryo transfer, we could not detect any clear statistically significant beneficial effects of ES. Furthermore, no positive effect was detectable following an intravenous intralipid therapy. Considering our results together with the majority of the current available literature, we cannot recommend ES and intralipid therapies as valid “add-on” treatment in daily clinical reproductive medicine practice outside of well-designed clinical trials, even if patients might ask their attending physicians for it.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics commission of the “Landesärztekammer Baden-Württemberg” (B-F-2017-129). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PM: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. NS-M: Data curation, Formal Analysis, Methodology, Validation, Writing – review & editing. FG: Data curation, Methodology, Supervision, Writing – review & editing. TF: Formal Analysis, Investigation, Methodology, Software, Validation, Writing – review & editing. KH: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. KB: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication is funded by the BMBF (Bundesministerium für Bildung und Forschung - FKZ 01GR2301).
Acknowledgments
We are grateful to all staff members of the “Kinderwunschzentrum Ulm”, Einsteinstr. 59, Ulm/Germany for their support during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ES, endometrial scratching; uNK cells, uterine natural killer cells; IVF, in vitro fertilization; ICSI, intracytoplasmic sperm injection; ART, assisted reproductive technologies; CI, confidence interval; RCT, randomised controlled trial; RIF, recurrent implantation failure; G-CSF, granulocyte-colony stimulating factor; ERA, endometrial receptivity array; AMH, anti-Müllerian hormone; RM, recurrent miscarriages; IUI, intrauterine insemination.
References
1. dir-jahrbuch-2021-deutsch-1.pdf. Available online at: https://www.deutsches-ivf-register.de/perch/resources/dir-jahrbuch-2021-deutsch-1.pdf (accessed March 5, 2023)
2. Coughlan C, Ledger W, Wang Q, Liu F, Demirol A, Gurgan T, et al. Recurrent implantation failure: definition and management. Reprod Biomed Online. (2014) 28:14–38. doi: 10.1016/j.rbmo.2013.08.011
3. Ko JKY, Ng EHY. Scratching and IVF: any role? Curr Opin Obstet Gynecol. (2016) 28:178–83. doi: 10.1097/GCO.0000000000000264
4. Nastri CO, Ferriani RA, Raine-Fenning N, Martins WP. Endometrial scratching performed in the non-transfer cycle and outcome of assisted reproduction: a randomized controlled trial. Ultrasound Obstet Gynecol. (2013) 42:375–82. doi: 10.1002/uog.12539
5. Senocak GC, Yapca OE, Borekci B. Comparison of pregnancy rates between patients with and without local endometrial scratching before intrauterine insemination. J Gynecol Obstet Hum Reprod. (2017) 46:687–90. doi: 10.1016/j.jogoh.2017.09.003
6. van Hoogenhuijze NE, Torrance HL, Mol F, Laven JSE, Scheenjes E, Traas M, et al. Endometrial scratching in women with implantation failure after a first IVF/ICSI cycle; does it lead to a higher live birth rate? The SCRaTCH study: a randomized controlled trial (NTR 5342). BMC Womens Health. (2017) 17:47. doi: 10.1186/s12905-017-0378-y
7. Bui BN, Lensen SF, Gibreel A, Martins WP, Torrance H, Broekmans FJ. Endometrial injury for pregnancy following sexual intercourse or intrauterine insemination. Cochrane Database Syst Rev. (2022) 10:CD011424. doi: 10.1002/14651858.CD011424.pub4
8. Lensen S, Osavlyuk D, Armstrong S, Stadelmann C, Hennes A, Napier E, et al. A randomized trial of endometrial scratching before in vitro fertilization. N Engl J Med. (2019) 380:325–34. doi: 10.1056/NEJMoa1808737
9. Lensen SF, Armstrong S, Gibreel A, Nastri CO, Raine-Fenning N, Martins WP. Endometrial injury in women undergoing in vitro fertilisation (IVF). Cochrane Database Syst Rev. (2021) 6:CD009517. doi: 10.1002/14651858.CD009517.pub4
10. Metwally M, Chatters R, Pye C, Dimairo M, White D, Walters S, et al. Endometrial scratch to increase live birth rates in women undergoing first-time in vitro fertilisation: RCT and systematic review. Health Technol Assess Winch Engl. (2022) 26:1–212. doi: 10.3310/JNZT9406
11. van Hoogenhuijze NE, van Eekelen R, Mol F, Schipper I, Groenewoud ER, Traas M, et al. Economic evaluation of endometrial scratching before the second IVF/ICSI treatment: a cost-effectiveness analysis of a randomized controlled trial (SCRaTCH trial). Hum Reprod Oxf Engl. (2022) 37:254–63. doi: 10.1093/humrep/deab261
12. Kang Y, Wang Z, Yang Y, Liang H, Duan X, Gao Q, et al. Impact of endometrial scratching on reproductive outcome in patients: a systematic review and meta-analysis. Medicine (Baltimore). (2022) 101:e30150. doi: 10.1097/MD.0000000000030150
13. Vitagliano A, Noventa M, Saccone G, Gizzo S, Vitale SG, Laganà AS, et al. Endometrial scratch injury before intrauterine insemination: is it time to re-evaluate its value? Evidence from a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. (2018) 109:84–96.e4. doi: 10.1016/j.fertnstert.2017.09.021
14. Lensen S, Shreeve N, Barnhart KT, Gibreel A, Ng EHY, Moffett A. In vitro fertilization add-ons for the endometrium: it doesn’t add-up. Fertil Steril. (2019) 112:987–93. doi: 10.1016/j.fertnstert.2019.10.011
15. ESHRE Add-ons Working Group, Lundin K, Bentzen JG, Bozdag G, Ebner T, Harper J, Le Clef N, et al. Good practice recommendations on add-ons in reproductive medicine. Hum Reprod. (2023) 38:2062–104. doi: 10.1093/humrep/dead184
16. Palomba S, Macklon N. Endometrial scratching: is it all over? Reprod Biomed Online. (2022) 44:583–5. doi: 10.1016/j.rbmo.2022.03.018
17. van Hoogenhuijze NE, Lahoz Casarramona G, Lensen S, Farquhar C, Kamath MS, Kunjummen AT, et al. Endometrial scratching in women undergoing IVF/ICSI: an individual participant data meta-analysis. Hum Reprod Update. (2023) 29(6):721–40. doi: 10.1093/humupd/dmad014
18. Sarwari M, Beilby K, Hammarberg K, Hickey M, Lensen S. Endometrial scratching in Australia, New Zealand and the United Kingdom (UK): a follow-up survey. Hum Fertil (Camb). (2023) 26(3):599–604. doi: 10.1080/14647273.2021.1995902
19. Berntsen S, Hare KJ, Løssl K, Bogstad J, Palmø J, Prætorius L, et al. Endometrial scratch injury with office hysteroscopy before IVF/ICSI: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. (2020) 252:112–7. doi: 10.1016/j.ejogrb.2020.06.034
20. Crosby DA, Glover LE, Downey P, Mooney EE, McAuliffe FM, O’Farrelly C, et al. The impact of accurately timed mid-luteal endometrial injury in nulligravid women undergoing their first or second embryo transfer. Ir J Med Sci. (2021) 190:1071–7. doi: 10.1007/s11845-020-02414-0
21. Santamaria X, Katzorke N, Simón C. Endometrial “scratching”: what the data show. Curr Opin Obstet Gynecol. (2016) 28:242–9. doi: 10.1097/GCO.0000000000000279
22. van Hoogenhuijze NE, Kasius JC, Broekmans FJM, Bosteels J, Torrance HL. Endometrial scratching prior to IVF; does it help and for whom? A systematic review and meta-analysis. Hum Reprod Open. (2019) 2019(1):hoy025. doi: 10.1093/hropen/hoy025
23. Wong TY, Lensen S, Wilkinson J, Glanville EJ, Acharya S, Clarke F, et al. Effect of endometrial scratching on unassisted conception for unexplained infertility: a randomized controlled trial. Fertil Steril. (2022) 117:612–9. doi: 10.1016/j.fertnstert.2021.12.009
24. Yeung TWY, Chai J, Li RHW, Lee VCY, Ho PC, Ng EHY. The effect of endometrial injury on ongoing pregnancy rate in unselected subfertile women undergoing in vitro fertilization: a randomized controlled trial. Hum Reprod. (2014) 29:2474–81. doi: 10.1093/humrep/deu213
25. Shahrokh-Tehraninejad E, Dashti M, Hossein-Rashidi B, Azimi-Nekoo E, Haghollahi F, Kalantari V. A randomized trial to evaluate the effect of local endometrial injury on the clinical pregnancy rate of frozen embryo transfer cycles in patients with repeated implantation failure. J Fam Reprod Health. (2016) 10:108–14.
26. Maged AM, Rashwan H, AbdelAziz S, Ramadan W, Mostafa WAI, Metwally AA, et al. Randomized controlled trial of the effect of endometrial injury on implantation and clinical pregnancy rates during the first ICSI cycle. Int J Gynecol Obstet. (2018) 140:211–6. doi: 10.1002/ijgo.12355
27. Matsumoto Y, Kokeguchi S, Shiotani M. Effects of endometrial injury on frozen-thawed blastocyst transfer in hormone replacement cycles. Reprod Med Biol. (2017) 16:196–9. doi: 10.1002/rmb2.12031
28. Nahshon C, Sagi-Dain L, Dirnfeld M. The impact of endometrial injury on reproductive outcomes: results of an updated meta-analysis. Reprod Med Biol. (2020) 19:334–49. doi: 10.1002/rmb2.12348
29. Vitagliano A, Vitale SG, Cianci A, Ferrero S, Barra F, Andrisani A, et al. Endometrial scratching for infertility: the never-ending story. J Gynecol Obstet Hum Reprod. (2020) 49:101743. doi: 10.1016/j.jogoh.2020.101743
30. Canella PRBC, Barini R, de Carvalho PO, Razolli DS. Lipid emulsion therapy in women with recurrent pregnancy loss and repeated implantation failure: the role of abnormal natural killer cell activity. J Cell Mol Med. (2021) 25:2290–6. doi: 10.1111/jcmm.16257
31. Dakhly DMR, Bayoumi YA, Sharkawy M, Gad Allah SH, Hassan MA, Gouda HM, et al. Intralipid supplementation in women with recurrent spontaneous abortion and elevated levels of natural killer cells. Int J Gynaecol Obstet. (2016) 135:324–7. doi: 10.1016/j.ijgo.2016.06.026
32. Faas MM, de Vos P. Uterine NK cells and macrophages in pregnancy. Placenta. (2017) 56:44–52. doi: 10.1016/j.placenta.2017.03.001
33. Farghali MM, El-Kholy A-LG, Swidan KH, Abdelazim IA, Rashed AR, El-Sobky E, et al. Relationship between uterine natural killer cells and unexplained repeated miscarriage. J Turk Ger Gynecol Assoc. (2015) 16:214–8. doi: 10.5152/jtgga.2015.0082
34. Fukui A, Kamoi M, Funamizu A, Fuchinoue K, Chiba H, Yokota M, et al. NK cell abnormality and its treatment in women with reproductive failures such as recurrent pregnancy loss, implantation failures, preeclampsia, and pelvic endometriosis. Reprod Med Biol. (2015) 14:151–7. doi: 10.1007/s12522-015-0207-7
35. Karami N, Boroujerdnia MG, Nikbakht R, Khodadadi A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J Reprod Immunol. (2012) 95:87–92. doi: 10.1016/j.jri.2012.06.005
36. Lédée N, Vasseur C, Petitbarat M, Chevrier L, Vezmar K, Dray G, et al. Intralipid® may represent a new hope for patients with reproductive failures and simultaneously an over-immune endometrial activation. J Reprod Immunol. (2018) 130:18–22. doi: 10.1016/j.jri.2018.09.050
37. Sacks G, Yang Y, Gowen E, Smith S, Fay L, Chapman M. Detailed analysis of peripheral blood natural killer cells in women with repeated IVF failure. Am J Reprod Immunol. (2012) 67:434–42. doi: 10.1111/j.1600-0897.2012.01105.x
38. Tang A-W, Alfirevic Z, Turner MA, Drury JA, Small R, Quenby S. A feasibility trial of screening women with idiopathic recurrent miscarriage for high uterine natural killer cell density and randomizing to prednisolone or placebo when pregnant. Hum Reprod Oxf Engl. (2013) 28:1743–52. doi: 10.1093/humrep/det117
39. Lash GE, Bulmer JN. Do uterine natural killer (uNK) cells contribute to female reproductive disorders? J Reprod Immunol. (2011) 88:156–64. doi: 10.1016/j.jri.2011.01.003
40. Vomstein K, Feil K, Strobel L, Aulitzky A, Hofer-Tollinger S, Kuon R-J, et al. Immunological risk factors in recurrent pregnancy loss: guidelines versus current state of the art. J Clin Med. (2021) 10:869. doi: 10.3390/jcm10040869
41. Clark DA. Intralipid as treatment for recurrent unexplained abortion? Am J Reprod Immunol. (1994) 32:290–3. doi: 10.1111/j.1600-0897.1994.tb01128.x
42. Martini AE, Jasulaitis S, Fogg LF, Uhler ML, Hirshfeld-Cytron JE. Evaluating the utility of intralipid infusion to improve live birth rates in patients with recurrent pregnancy loss or recurrent implantation failure. J Hum Reprod Sci. (2018) 11:261–8. doi: 10.4103/jhrs.JHRS_28_18
43. Hansen LM, Hardie BS, Hidalgo J. Fat emulsion for intravenous administration: clinical experience with intralipid 10%. Ann Surg. (1976) 184:80–8. doi: 10.1097/00000658-197607000-00014
44. Kamath KR, Berry A, Cummins G. Acute hypersensitivity reaction to intralipid. N Engl J Med. (1981) 304:360.7442781
45. Ogawa A, Samoto M, Takahashi K. Soybean allergens and hypoallergenic soybean products. J Nutr Sci Vitaminol (Tokyo). (2000) 46:271–9. doi: 10.3177/jnsv.46.271
46. Kuon RJ, Müller F, Vomstein K, Weber M, Hudalla H, Rösner S, et al. Pre-pregnancy levels of peripheral natural killer cells as markers for immunomodulatory treatment in patients with recurrent miscarriage. Arch Immunol Ther Exp (Warsz). (2017) 65:339–46. doi: 10.1007/s00005-017-0457-7
47. Singh N, Davis AA, Kumar S, Kriplani A. The effect of administration of intravenous intralipid on pregnancy outcomes in women with implantation failure after IVF/ICSI with non-donor oocytes: a randomised controlled trial. Eur J Obstet Gynecol Reprod Biol. (2019) 240:45–51. doi: 10.1016/j.ejogrb.2019.06.007
48. Kieu V, Lantsberg D, Mizrachi Y, Stern C, Polyakov A, Teh WT. A survey study of endometrial receptivity tests and immunological treatments in in vitro fertilisation (IVF). Aust N Z J Obstet Gynaecol. (2022) 62:306–11. doi: 10.1111/ajo.13466
49. Kumar P, Marron K, Harrity C. Intralipid therapy and adverse reproductive outcome: is there any evidence? Reprod Fertil. (2021) 2:173–86. doi: 10.1530/RAF-20-0052
50. Coulam CB. Intralipid treatment for women with reproductive failures. Am J Reprod Immunol. (2021) 85:e13290. doi: 10.1111/aji.13290
51. Achilli C, Duran-Retamal M, Saab W, Serhal P, Seshadri S. The role of immunotherapy in in vitro fertilization and recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. (2018) 110:1089–100. doi: 10.1016/j.fertnstert.2018.07.004
52. Seshadri S, Sunkara SK. Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis. Hum Reprod Update. (2014) 20:429–38. doi: 10.1093/humupd/dmt056
53. Sfakianoudis K, Rapani A, Grigoriadis S, Pantou A, Maziotis E, Kokkini G, et al. The role of uterine natural killer cells on recurrent miscarriage and recurrent implantation failure: from pathophysiology to treatment. Biomedicines. (2021) 9:1425. doi: 10.3390/biomedicines9101425
54. Braga DPAF, Setti AS, Borges E. Ethics and IVF add-ons: we need to talk about it. JBRA Assist Reprod. (2022) 26:371–3. doi: 10.5935/1518-0557.20220030
55. Paulson RJ. Cognitive dissonance in infertility treatment: why is it so difficult to discard disproven therapies, like the endometrial scratch? FS Rep. (2022) 3:85. doi: 10.1016/j.xfre.2022.05.005
56. Mackens S, Santos-Ribeiro S, Racca A, Daneels D, Koch A, Essahib W, et al. The proliferative phase endometrium in IVF/ICSI: an in-cycle molecular analysis predictive of the outcome following fresh embryo transfer. Hum Reprod Oxf Engl. (2020) 35:130–44. doi: 10.1093/humrep/dez218
57. Wang D-Y, Tian L, Shen D, Yang Z-J. Histological component quantification for the evaluation of endometrial receptivity in women with natural cycles undergoing in vitro fertilization/intracytoplasmic sperm injection. Taiwan J Obstet Gynecol. (2017) 56:368–70. doi: 10.1016/j.tjog.2017.04.019
Keywords: endometrial scratching, endometrial injury, intralipid, uterine natural killer cells, repeated implantation failure
Citation: Mrosk P, Sandi-Monroy N, Gagsteiger F, Friedl TWP, Hancke K and Bundschu K (2024) Endometrial scratching and intralipid treatment—no general recommendations. Front. Reprod. Health 6:1505842. doi: 10.3389/frph.2024.1505842
Received: 3 October 2024; Accepted: 13 November 2024;
Published: 27 November 2024.
Edited by:
Johannes Ott, Medical University of Vienna, AustriaReviewed by:
Panagiotis Tsiartas, Karolinska Institutet (KI), SwedenGedis Grudzinskas, Self-employed, London, United Kingdom
Copyright: © 2024 Mrosk, Sandi-Monroy, Gagsteiger, Friedl, Hancke and Bundschu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karin Bundschu, a2FyaW4uYnVuZHNjaHVAdW5pa2xpbmlrLXVsbS5kZQ==
†ORCID:
Karin Bundschu
orcid.org/0000-0001-9329-8958
 Paolina Mrosk1,2
Paolina Mrosk1,2 Karin Bundschu
Karin Bundschu