- 1Obstetrics and Gynecology Department, Shahid Beheshti University of Medical Science, Tehran, Iran
- 2Obstetrics and Gynecology Department, Tehran Medical Science University, Tehran, Iran
- 3Department of Gynecology and Obstetrics, Arash Women's Hospital, Tehran University of Medical Sciences, Tehran, Iran
- 4Department of Endocrinology and Female Infertility, Reproductive Biomedicine Research Center, Royan Institute for Reproductive Biomedicine, ACECR, Tehran, Iran
- 5Breast Disease Research Center (BDRC), Tehran University of Medical Sciences, Tehran, Iran
- 6Armaghan Infertility Center, Mashhad Medical Science University, Mashhad, Iran
- 7Animal Biotechnology Department, Reproductive Biomedicine Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran
- 8Obstetrics and Gynecology Department, Alzahra Hospital, Tabriz, Iran
- 9IVF Department, Hamedan Medical Science University, Hamedan, Iran
- 10Fertility Department, Merck Serono Middle East, Dubai, United Arab Emirates
- 11IVF Unit, Fertility Center Hamburg, Hamburg, Germany
Introduction: Numerous consensus documents worldwide address luteinizing hormone (LH) supplementation in controlled ovarian stimulation, yet to the best of our knowledge, only one consensus paper has been published in the Arab region. This study presents a Delphi consensus by seven Iranian infertility experts, offering real-world clinical perspectives. The aim was to develop evidence-based opinions on LH's role alongside FSH in various aspects of assisted reproductive technology (ART), including LH levels, monitoring, r-hLH use, and suggested activity.
Methods: Employing the Delphi consensus approach, the Iran consensus unfolded in three steps. In Step 1, eight out of 10 statements gained approval, while two unclear statements were removed. In Step 2, the 20-member extended panel voted on the remaining eight statements.
Results: Only one (statement 3) lacked consensus (55% agreement), prompting a modification. The revised statement (noted as statement 3′) obtained an 83% agreement.
Discussion: The clinical perspectives included in this consensus complement clinical guidelines and policies that help further improve treatment outcomes, especially for patients with FSH and LH deficiencies.
Introduction
Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are gonadotropins secreted by the pituitary gland under the pulsatile stimulus of gonadotropin-releasing hormone (GnRH) (1). Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) play a complementary role in follicle development and ovulation. FSH initiates follicular growth, while LH acts at the follicle growth level, contributing to follicle maturation, fertilization and embryo quality (2). It affects the endometrium by promoting the decidualization of endometrial stromal cells and embryo implantation (3). Thus, a decrease or deficiency in the production or action of these gonadotrophins might compromise gametogenesis and gonadal steroid production, thereby reducing both female fertility and outcomes of medically assisted reproduction (MAR) (4, 5).
While the decrease in LH and FSH levels has been somehow extensively studied in the literature, their lack of action has been less documented (4, 6). LH and FSH deficiency may be congenital or acquired, functional/reversible, or permanent and may exhibit different degrees of severity. Several contributing factors have been identified and may explain the deficiency, including variability and impairment in gonadotropin-releasing hormone (GnRH) frequency, amplitude peaks and pulses, genetic variants in genes coding the gonadotropins and their receptors, and altered signaling pathways (7–9). Other identified demographic and clinical factors may also contribute to gonadotropin deficiency, such as advanced age, comorbidities (e.g., diabetes, thyroid disorders, eating disorders, excessive exercise, and tumors and related treatments), the use of contraceptive pills (10–15).
Several consensus documents have been developed around the globe regarding LH supplementation in controlled ovarian stimulation (16–18). Nevertheless, and to the best of the authors' knowledge, only one consensus paper has been published in the Arab region (19). In particular, a Delphi consensus provides a real-world clinical perspective from several experts, contributing to improved patient management and follow-up within a patient-tailored strategy (20, 21). In that perspective, seven Iranian experts in infertility management gathered to discuss and develop evidence-based opinions and statements regarding the LH role when co-administered with FSH in several aspects of assisted reproductive technology (ART).
Consensus methodology and inclusion criteria for the panel of experts
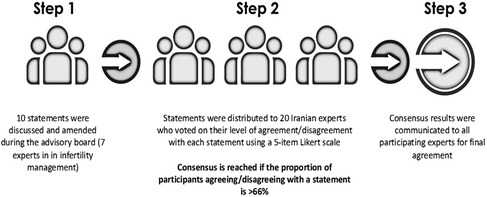
The Iran consensus was developed according to the Delphi consensus methodology and was achieved over three steps (Figure 1).
Step 1
A panel of seven infertility experts, each affiliated with distinct medical universities based in Tehran, Hamadan, Isfahan, and East Azerbaijan province in Iran, were gathered with a scientific coordinator from Germany, an active member of the American Society of Reproductive Medicine, and a founding member of the European Society of Human Reproduction, for an interactive group discussion regarding ten statements proposed by the scientific coordinator and supported by updated references (Table 1). Statements were drafted, discussed, and amended by the experts' committee, when necessary, according to the available scientific evidence and current clinical practice.
Step 2
The statements were then distributed to 20 infertility experts before the voting session, who voted on their level of agreement or disagreement with each statement using a 5-point Likert scale: 1 (absolutely disagree), 2 (disagree), 3 (agree), 4 (more than agree), and 5 (absolutely agree) (20, 35). Consensus was reached if the proportion of participants agreeing or disagreeing with a statement was >66%. Statements that did not reach consensus were updated and sent again for voting.
Step 3
Based on the outcomes of Step 2, the revised statements were communicated to all participating experts for final agreement. The present manuscript was written based on the group discussion; it was reviewed by all experts, who incorporated their experience regarding the role of LH in ART.
Results of the consensus and recommendations
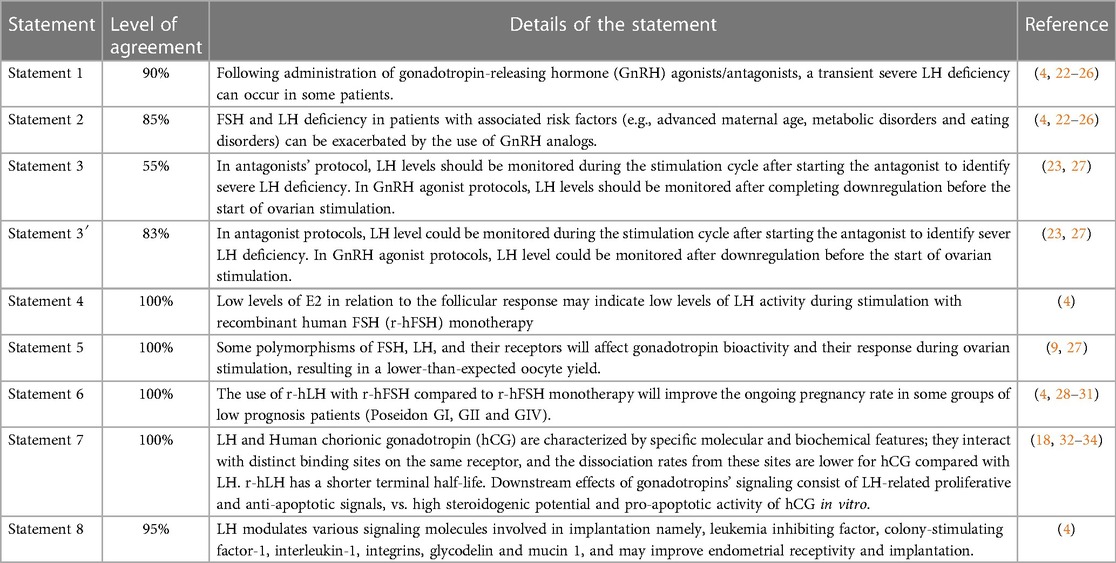
In Step 1, eight out of ten statements were approved after discussion and modification. Two redundant or deemed unclear statements were removed. The remaining eight statements were then voted in Step 2 by the 20-member extended panel. Only statement 3 did not reach a consensus (55% agreement); thus, the committee suggested a modification. This new statement (noted statement 3′) obtained an 83% agreement afterward. Details are presented in Table 1 and Figure 2.
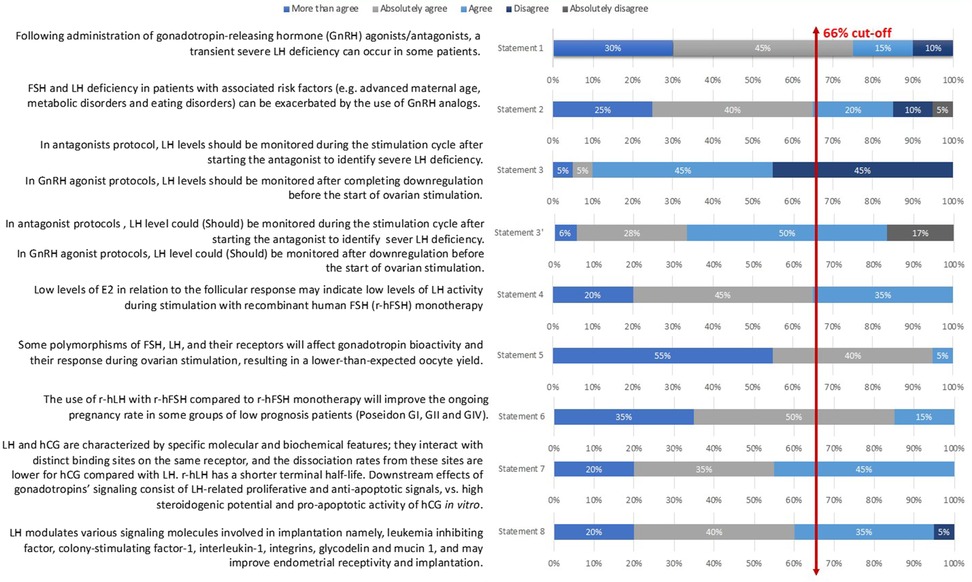
Statement 1: Following the administration of gonadotropin-releasing hormone (GnRH) agonists/antagonists, a transient severe LH deficiency can occur in some patients. This statement reached a 90% level of total agreement among the extended expert panel (Figure 2).
Discussion statement 1
GnRH agonists and antagonists are used during ovarian stimulation (OS) to enable the clinical retrieval of the maximum number of oocytes. They can induce a transient deficiency in LH and FSH, effectively preventing premature ovulation. The analogs exert different mechanisms of action on endogenous gonadotropins, causing either a gradual (GnRH agonists) or an abrupt (GnRH antagonist) suppression (4, 36, 37). Whether with agonists or antagonists, residual LH levels are usually enough to support steroidogenesis and allow OS following the administration of recombinant FSH (r-FSH); however, a severe deficiency can occur in some patients (4, 22). In GnRH agonist cycles, due to the reduced gonadotropin production, a severe deficiency can be observed when the LH levels drop below a threshold value (ranging from < 1.5–0.5 IU/L LH, according to the literature) (28, 38, 39). Several studies demonstrated that standard long GnRH agonist protocols followed by an OS with r-FSH led to significant severe LH deficiency, seen in up to almost 50% of normogonadotropic women (39, 40). Such observations were associated with higher early pregnancy loss (39) and lower live birth rates (40). One hypothesis that can be put forward to explain such results is that the abrupt drop in LH levels during OS might be related to lower E2 production by the follicles and, consequently, lower circulating E2 levels (4, 22).
Although less frequently observed, LH suppression can also be documented in GnRH antagonist protocols, with detrimental effects on the quality and quantity of eggs (41, 42), thus altering treatment outcomes. In 2014, Kol reported that LH was over-suppressed in 26% of women, and these patients had a significantly lower increase in E2 during the first 24 h after antagonist administration compared to women who were not over-suppressed. Nevertheless, some authors suggested that severe suppression is not observed in all patients but only in some subgroups, such as, but not limited to, patients with advanced maternal age. Moreover, negative reproductive outcomes were postulated to result from the magnitude of suppression over time vs. the baseline rather than a drop in the absolute LH levels, as previously mentioned (43). The addition of r-LH seems to reverse the effect of the reported deficiency (4, 44).
Statement 2: FSH and LH deficiency in patients with associated risk factors (e.g., advanced maternal age, metabolic disorders, and eating disorders) can be exacerbated by the use of GnRH analogs.
This statement had an 85% level of total agreement among the extended expert panel (Figure 2).
Discussion statement 2
Aging, metabolic diseases (including diabetes or thyroid disorders), and eating disorders (including obesity and anorexia) are widely recognized to significantly influence gonadotropin secretion and action, mainly by affecting the hypothalamic-pituitary axis (4, 45). Hence, FSH and LH deficiencies already observed in these patients, especially women with advanced maternal age, seem to be exacerbated by the administration of GnRH analogs, probably due to the transient gonadotropin deficiency (Bosch et al. 2021). In that context, identifying and treating the underlying disorders is paramount to restoring reproductive function and improving stimulation outcomes (4, 11, 46). Thus, several reports, including women aged 35 to 40 years old, demonstrated that the r-hFSH:r-hLH co-supplementation led to higher implantation (and oocytes maturation) and birth rates as compared to r-hFSH monotherapy (4, 47–49)
Statement 3: In antagonist protocols, LH levels could be monitored during the stimulation cycle after starting the antagonist to identify severe LH deficiency. In GnRH agonist protocols, LH levels could be monitored after downregulation before the start of ovarian stimulation.
The statement that was voted during the Step 1 expert meeting consisted of the following: “In antagonists’ protocol, LH levels should be monitored during the stimulation cycle after starting the antagonist to identify severe LH deficiency; In GnRH agonist protocols, LH levels should be monitored after completing downregulation before the start of ovarian stimulation” (Statement 3 in Table 1 and Figure 2). Only 1 of 7 (14%) experts disagreed on it during the voting session. Nevertheless, only 55% of the Step 2 expert board agreed with the statement as stated earlier; therefore, it did not reach a consensus. The committee of seven Iranian experts then suggested the following changes: “In antagonist protocols, LH levels could be monitored during the stimulation cycle after starting the antagonist to identify severe LH deficiency; In GnRH agonist protocols, LH levels could be monitored after downregulation before the start of ovarian stimulation” arguing that LH monitoring is not mandatory but could be used to improve the quality of the cycle and clinical outcomes (Statement 3′ in Table 1 and Figure 2). The final statement was then resent to the 20 experts for voting and reached an 83% level of total agreement.
Discussion statement 3
FSH and LH are both essential components for folliculogenesis; this is the concept of “two cell–two gonadotropin” described in the literature (50). By stimulating the theca cells in the ovary, LH plays a critical role in androgen production, thus facilitating estradiol production and FSH activity (23).
Therefore, determining LH levels is recommended if a severe LH deficiency is anticipated. Hence, in antagonist protocols, some subgroups of patients with LH drop (as described in Statement 1) would require r-hLH supplementation from the beginning of the cycle; these include women at an advanced reproductive age (36–39 years old) and women with adequate pre-stimulation ovarian reserve parameters and an unexpected hypo-responders to r-hFSH monotherapy (27). Moreover, if LH levels remain low, they reflect a state of severe deficiency, and patients might not have an adequate response to GnRH agonists.
In GnRH agonist protocols, LH level measurements could rather be performed before ovarian stimulation. A systematic review (27) noted a severe mid-follicular LH deficiency in 7%–48% of normogonadotropic women undergoing OS, which might affect the ovarian response to r-hFSH monotherapy. It also highlighted controversial results related to pregnancy outcomes, with some studies reporting early pregnancy loss and reduced fertilization rates when LH levels were 0.5–0.7 IU/L (39, 51), while others did not observe any difference (27, 38, 52). Further research is necessary to clarify these findings. In Iran, LH levels are not measured routinely; however, in the case of FSH and LH deficiencies due to GnRH analogs, the 5 IU/L is considered a deficiency.
Of note, evidence suggested that the absolute LH serum level might not correctly reflect LH deficiency but rather the magnitude of suppression over time compared to the baseline (43). Therefore, when investigating LH deficiency, clinicians should focus on exploring the difference in LH levels before and after the administration of GnRH analogs (delta) rather than relying solely on a simple cut-off value.
Statement 4: Low levels of E2 in relation to the follicular response may indicate low levels of LH activity during stimulation with recombinant human FSH (r-hFSH) monotherapy. This statement had a total agreement from the extended panel (100%; Figure 2).
Discussion statement 4
During the first meeting, experts stated that, in the Iranian practice, the measure of E2 levels might not be feasible due to cost constraints. Nevertheless, regardless of this economic issue, estradiol levels should be monitored 2–3 times during the cycle in ovarian stimulation protocols, and for poor responders, testing more than three times may be necessary. Evidence from the literature suggested that low E2 levels might be considered relevant endocrine endpoints for LH and FSH deficiencies (4). E2 could reflect the effect of LH on steroidogenesis in both theca and granulosa cells; thus, low levels of E2 that do not match the size and number of follicles (E2/oocyte ratio) might suggest low levels of LH activity during OS.
Statement 5: Some polymorphisms of FSH, LH, and their receptors will affect gonadotropin bioactivity and their response during ovarian stimulation, resulting in a lower-than-expected oocyte yield. This statement had a 100% level of agreement among the extended expert panel (Figure 2).
Discussion statement 5
Cumulative evidence highlighted interindividual differences in ovarian response to gonadotropin stimulation related to polymorphisms in genes encoding for the gonadotropins or their receptors (4, 7, 53). A recent systemic review with meta-analysis (53) highlighted that several single nucleotide polymorphisms (SNP), especially in the gene encoding the FSH receptor, FSHR, have been shown to modulate ovarian response and are among the best candidates to be selected as markers to predict individual response to OS.
The SNP FSHR rs6166 (c.2039G > A; p.Asn680Ser) was extensively studied in the literature. Studies have shown that this polymorphism could also affect OS. Indeed, a recent Delphi consensus related to this polymorphism reported that the Ser/Ser genotype was associated with a reduced sensitivity of the FSHR to exogenous FSH (7). Moreover, patients carrying two copies of the variant Ser allele required higher amounts of gonadotropin during OS, had higher basal levels of FSH, and produced fewer oocytes and fewer metaphase II oocytes in response to OS than Asn/Asn or Asn/Ser patients (7). Interestingly, a randomized controlled trial showed that increasing the FSH dose might revert this reduced sensitivity (54).
The rs6165 (c.919G > A; p.Thr307Ala) polymorphism is another SNP in the FSHR in strong linkage disequilibrium with rs6166: patients with the AA genotype had a significantly higher number of retrieved oocytes, a higher number of metaphase II oocytes, and necessitated a shorter duration of gonadotropin stimulation as compared to the other groups of patients (53).
The FSHR rs1394205 (c.-29G > A) polymorphism, located in the promoter region, has also been extensively studied and suggested as a critical marker to predict ovarian response in assisted reproductive technology (9). Studies in specific ethnic populations have demonstrated that homozygote AA patients had lower ovarian sensitivity and produced significantly fewer oocytes, thus necessitating significantly higher FSH consumption to achieve an adequate OS as compared with GA and GG patients (53).
A recent multicenter, multinational prospective study (2016–2019), which enrolled 366 predicted normal responders from Vietnam, Belgium, and Spain, yielded controversial results regarding genetic susceptibility in response to OS. Thus, the authors failed to reproduce the previously published genetic correlations since none of the studied SNPs (rs6165, rs6166, and rs1394205) was significantly associated with the late follicular phase serum progesterone or estradiol levels (55).
Other genetic variants in the gene encoding the FSH beta subunit (FSHB rs10835638; c.-211G > T), the luteinizing hormone b-chain (LHB), and the LH/choriogonadotropin receptor (LHCGR) might also affect ovarian stimulation, but more evidence is required to confirm their implication (4, 7, 53).
Statement 6: The use of r-hLH with r-hFSH compared to r-hFSH monotherapy will improve the ongoing pregnancy rate in some groups of low prognosis patients (Poseidon GI, GII and GIV). This statement had a total agreement (100%) from the extended panel (Figure 2).
Discussion statement 6
Several studies have explored the role of r-hLH in ovarian stimulation for ART. A systematic review from 2018 concluded that rhLH supplementation might be beneficial, particularly in two groups of patients, i.e., (1) women with adequate prestimulation ovarian reserve parameters (Antral follicle count- AFC ≥5, Anti-Mullerian Hormone- AMH ≥ 1.2 ng/mL) and an unexpected hyporesponse to r-hFSH monotherapy (unexpected poor or suboptimal ovarian response; Poseidon Groups 1 (age < 35 years) and 2 (age ≥ 35 years) and (2) women with advanced maternal age (35–39 years old), including those from the Poseidon Group 4 (Age ≥ 35 years, with poor ovarian reserve prestimulation parameters: AFC <5 & AMH < 1.2 ng/mL) (27, 56). In all other cases, the results remain controversial and require further research to confirm the need for rhFSH supplementation (27). This supplementation with rhLH seems to have an added value for pregnancy outcomes. Indeed, a literature review with metanalysis, including 12 randomized control trials, showed that using r-hLH with r-hFSH as compared to the hFSH alone yielded higher pregnancy and implantation rates, especially in GnRH agonist protocols, while evidence is still debatable with GnRH antagonist protocols (6, 44). A recent in vitro study tested whether the addition of LH to FSH affects the response of granulosa lutein cells collected from poor-, sub-, and normoresponder women undergoing MAR. These cell lines are an excellent model to evaluate the co-administration of both LH and FSH since they express receptors for the two gonadotropins. Primary endpoints consisted of cAMP and progesterone production. The results showed that LH addition in the poor-responder and sub-responder groups enabled some recovery of the FSH-induced cAMP and progesterone production since these were similar to those observed in normoresponder women (57).
Statement 7: LH and hCG are characterized by specific molecular and biochemical features; they interact with distinct binding sites on the same receptor, and the dissociation rates from these sites are lower for hCG compared with LH. r-hLH has a shorter terminal half-life. Downstream effects of gonadotropins' signaling consist of LH-related proliferative and anti-apoptotic signals, vs. high steroidogenic potential and pro-apoptotic activity of hCG in vitro.
Statement 7 had a 100% level of agreement among the extended expert panel (Figure 2).
Discussion statement 7
Several studies, systemic reviews, and meta-analyses discussed comparatively the molecular and biochemical features of LH and hCG. LH and hCG consist of heterodimeric glycoproteins that share a common alpha-subunit but a distinct beta-subunit. Due to these similarities, both hormones can bind to the same receptor, i.e., the LH chorionic gonadotropin receptor (LHCGR), but their pharmacokinetic characteristics and molecular responses are somewhat different (5, 18, 32). Hence, r-hLH has a shorter terminal elimination half-life, estimated to be around 10 h, as compared to the 28–31 h for the hCG after intravenous administration (18, 58, 59). Moreover, signaling pathways following the receptor stimulation are distinct as well. LH works as a partial agonist for progesterone, with a proliferative and antiapoptotic action. It exerts its action mainly through the activation of kinases (extracellular signal-regulated kinase ½ [pERK1/2] and protein kinase B (AKT)-dependent pathways). hCG, oppositely, displays a notable steroidogenic and proapoptotic potential, along with a decreased proliferation of granulosa cells, mainly via the upregulation of the cAMP/protein kinase A (PKA) and caspase-3 pathways. However, this apoptotic effect of hCG seems to be masked by the action of estrogen in vivo (5, 18).
Statement 8: LH modulates various signaling molecules involved in implantation, namely leukemia inhibiting factor, colony-stimulating factor-1, interleukin-1, integrins, glycodelin, and mucin 1, and may improve endometrial receptivity and implantation. This last statement had a 95% total level agreement by the extended expert panel (Figure 2).
Discussion statement 8
Evidence highlighted that LH receptors are expressed in the human endometrium (epithelial and stromal cells) and that LH can affect uterine receptivity independently of ovarian function (4, 60). Moreover, a study has shown that patients with low endogenous LH levels would have a disturbed endometrial maturation that can be rescued by a mid-cycle administration of exogenous hCG or LH, which would stimulate LH receptors (60). Several factors seem to be involved in the endometrial function that enables the implantation process, including leukemia inhibiting factor, colony-stimulating factor-1, interleukin-1, integrins, glycodelin, and mucin 1 (MUC1) (4, 60, 61)
Limitations and strength
The statements only represent the collective opinion of the experts included. Moreover, the consensus was reached based on references selected by the scientific adviser who might have omitted relevant information. Furthermore, not all statements reached 100% agreement, with some statements reaching consensus even though some participants disagreed with them. Some of these statements might also evolve with new evidence emerging from randomized controlled studies. Nevertheless, and despite these limitations, this Delphi consensus provides a real-world clinical perspective on the LH supplement in COS from group of Iranian Expert.
Conclusion
The clinical perspectives included in this consensus supplement clinical guidelines and policies that help to further improve treatment outcomes especially patients with FSH & LH deficiency.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for this study in accordance with the local legislation and institutional requirements. Written informed consent from the program evaluation focus group participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SS: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. AA: Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. NeM: Writing – original draft, Writing – review & editing. NaM: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. SM: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. RF: Formal Analysis, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This project was sponsored and funded by Merck Serono Middle East, Dubai, United Arab Emirates, an affiliate of Merck KGaA. The sponsor was involved early in the process, defining the overarching topic to be discussed, but did not participate in the development of the statements or in any of the meetings or discussions involved in developing the Delphi consensus. The statements were, therefore, developed independently of the industry sponsor. The authors from Merck Serono Middle East, Dubai, United Arab Emirates, were only involved in the development of the manuscript, critically revising it for important intellectual content, especially in the Introduction, Results and Discussion sections, but could not alter the consensus statements in any way.
Acknowledgments
Authors would like to thank Science PRO sarl, Lebanon for their support in the medical writing and editing of the article.
Conflict of interest
All authors had received honoraria from Merck for participating in this consensus.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AFC, antral follicle count; AG, agonists; AMH, anti-mullerian hormone; ART, assisted reproductive technology; COS, controlled ovarian stimulation; E2, estradiol; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; hCG, human chorionic gonadotropin; IU, international unit; LH, luteinizing hormone; LHB, luteinizing hormone b-chain; LHCGR, LH/choriogonadotropin receptor; MAR, medically assisted reproduction; MUC1, mucin 1; OS, OVARIAN stimulation; r-hLH, recombinant human luteinizing hormone; r-hFSH, recombinant human follicle-stimulating hormone; Rc, receptor; SNP, single nucleotide polymorphism.
References
1. Duggavathi R, Murphy BD. Development. Ovulation signals. Science. (2009) 324(5929):890–1. doi: 10.1126/science.1174130
2. Hall JE. Chapter 7—neuroendocrine control of the menstrual cycle. In: Strauss JF, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology, Eighth ed. Philadelphia: Elsevier; 2019. p. 149–66.e5.
3. Freis A, Germeyer A, Jauckus J, Capp E, Strowitzki T, Zorn M, et al. Endometrial expression of receptivity markers subject to ovulation induction agents. Arch Gynecol Obstet. (2019) 300(6):1741–50. doi: 10.1007/s00404-019-05346-y
4. Bosch E, Alviggi C, Lispi M, Conforti A, Hanyaloglu AC, Chuderland D, et al. Reduced FSH and LH action: implications for medically assisted reproduction. Hum Reprod. (2021) 36(6):1469–80. doi: 10.1093/humrep/deab065
5. Casarini L, Santi D, Brigante G, Simoni M. Two hormones for one receptor: evolution, biochemistry, actions, and pathophysiology of LH and hCG. Endocr Rev. (2018) 39(5):549–92. doi: 10.1210/er.2018-00065
6. Younis JS, Laufer N. Recombinant luteinizing hormone supplementation to recombinant follicle stimulating hormone therapy in gonadotropin releasing hormone analogue cycles: what is the evidence? Curr Med Res Opin. (2018) 34(5):881–6. doi: 10.1080/03007995.2017.1417827
7. Conforti A, Tuttelmann F, Alviggi C, Behre HM, Fischer R, Hu L, et al. Effect of genetic variants of gonadotropins and their receptors on ovarian stimulation outcomes: a delphi consensus. Front Endocrinol (Lausanne). (2021) 12:797365. doi: 10.3389/fendo.2021.797365
8. Huang Y, Zhao Y, Yu Y, Li R, Lin S, Zhang C, et al. Altered amphiregulin expression induced by diverse luteinizing hormone receptor reactivity in granulosa cells affects IVF outcomes. Reprod Biomed Online. (2015) 30(6):593–601. doi: 10.1016/j.rbmo.2015.03.001
9. Riccetti L, De Pascali F, Gilioli L, Santi D, Brigante G, Simoni M, et al. Genetics of gonadotropins and their receptors as markers of ovarian reserve and response in controlled ovarian stimulation. Best Pract Res Clin Obstet Gynaecol. (2017) 44:15–25. doi: 10.1016/j.bpobgyn.2017.04.002
10. Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, et al. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. N Engl J Med. (1974) 291(17):861–5. doi: 10.1056/NEJM197410242911701
11. Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. (2010) 363(4):365–71. doi: 10.1056/NEJMcp0912024
12. Li RH, Ng EH. Management of anovulatory infertility. Best Pract Res Clin Obstet Gynaecol. (2012) 26(6):757–68. doi: 10.1016/j.bpobgyn.2012.05.004
13. Shangold MM. Causes, evaluation, and management of athletic oligo-/amenorrhea. Med Clin North Am. (1985) 69(1):83–95. doi: 10.1016/S0025-7125(16)31058-6
14. Strotmeyer ES, Steenkiste AR, Foley TP Jr, Berga SL, Dorman JS. Menstrual cycle differences between women with type 1 diabetes and women without diabetes. Diabetes Care. (2003) 26(4):1016–21. doi: 10.2337/diacare.26.4.1016
15. Tanriverdi F, Kelestimur F. Hypothalamic-Pituitary Diseases. Switzerland: Springer (2018). Available online at: https://link.springer.com/content/pdf/10.1007/978-3-319-44444-4.pdf
16. Wong PC, Qiao J, Ho C, Ramaraju GA, Wiweko B, Takehara Y, et al. Current opinion on use of luteinizing hormone supplementation in assisted reproduction therapy: an Asian perspective. Reprod Biomed Online. (2011) 23(1):81–90. doi: 10.1016/j.rbmo.2011.03.023
17. Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Hum Reprod Update. (2006) 12(4):333–40. doi: 10.1093/humupd/dml001
18. Orvieto R, Venetis CA, Fatemi HM, D'Hooghe T, Fischer R, Koloda Y, et al. Optimising follicular development, pituitary suppression, triggering and luteal phase support during assisted reproductive technology: a delphi consensus. Front Endocrinol (Lausanne). (2021) 12:675670. doi: 10.3389/fendo.2021.675670
19. Al-Izzi F, Al-Obaidi M, Sabah Ahmed E, Sami Khunda S, Abdulkareem Wahd S, Yousif Shamdeen M, et al. Strategies to improve outcomes of ovulation induction and assisted reproductive technology (ART): the Iraqi consensus. Glob J Reprod Med. (2023) 9(4):555770. doi: 10.19080/GJORM.2023.09.555770
20. Barrett D, Heale R. What are delphi studies? Evid Based Nurs. (2020) 23(3):68–9. doi: 10.1136/ebnurs-2020-103303
21. Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. (2021) 11(4):116–29. doi: 10.5662/wjm.v11.i4.116
22. Conforti A, Esteves SC, Di Rella F, Strina I, De Rosa P, Fiorenza A, et al. The role of recombinant LH in women with hypo-response to controlled ovarian stimulation: a systematic review and meta-analysis. Reprod Biol Endocrinol. (2019) 17(1):18. doi: 10.1186/s12958-019-0460-4
23. Zhang W, Liu Z, Liu M, Li J, Guan Y. Is it necessary to monitor the serum luteinizing hormone (LH) concentration on the human chorionic gonadotropin (HCG) day among young women during the follicular-phase long protocol? A retrospective cohort study. Reprod Biol Endocrinol. (2022) 20(1):24. doi: 10.1186/s12958-022-00888-4
24. Hugues JN, Masse-Laroche E, Reboul-Marty J, Boiko O, Meynant C, Cedrin-Durnerin I. Impact of endogenous luteinizing hormone serum levels on progesterone elevation on the day of human chorionic gonadotropin administration. Fertil Steril. (2011) 96(3):600–4. doi: 10.1016/j.fertnstert.2011.06.061
25. Sonntag B, Kiesel L, Nieschlag E, Behre HM. Differences in serum LH and FSH levels using depot or daily GnRH agonists in controlled ovarian stimulation: influence on ovarian response and outcome of ART. J Assist Reprod Genet. (2005) 22(7-8):277–83. doi: 10.1007/s10815-005-5998-8
26. Westergaard LG, Wiberg N, Andersen CY, Laursen SB, Kliem A, Westergaard JG, et al. Circulating concentrations of placenta protein 14 during the natural menstrual cycle in women significantly reflect endometrial receptivity to implantation and pregnancy during successive assisted reproduction cycles. Hum Reprod. (1998) 13(9):2612–9. doi: 10.1093/humrep/13.9.2612
27. Alviggi C, Conforti A, Esteves SC, Andersen CY, Bosch E, Buhler K, et al. Recombinant luteinizing hormone supplementation in assisted reproductive technology: a systematic review. Fertil Steril. (2018) 109(4):644–64. doi: 10.1016/j.fertnstert.2018.01.003
28. Humaidan P, Bungum M, Bungum L, Yding Andersen C. Effects of recombinant LH supplementation in women undergoing assisted reproduction with GnRH agonist down-regulation and stimulation with recombinant FSH: an opening study. Reprod Biomed Online. (2004) 8(6):635–43. doi: 10.1016/S1472-6483(10)61643-4
29. Vihko KK, Kujansuu E, Morsky P, Huhtaniemi I, Punnonen R. Gonadotropins and gonadotropin receptors during the perimenopause. Eur J Endocrinol. (1996) 134(3):357–61. doi: 10.1530/eje.0.1340357
30. Wide L, Naessen T, Sundstrom-Poromaa I, Eriksson K. Sulfonation and sialylation of gonadotropins in women during the menstrual cycle, after menopause, and with polycystic ovarian syndrome and in men. J Clin Endocrinol Metab. (2007) 92(11):4410–7. doi: 10.1210/jc.2007-1342
31. Zumoff B, Strain GW, Miller LK, Rosner W. Twenty-four-hour mean plasma testosterone concentration declines with age in normal premenopausal women. J Clin Endocrinol Metab. (1995) 80(4):1429–30. doi: 10.1210/jcem.80.4.7714119
32. Casarini L, Lispi M, Longobardi S, Milosa F, La Marca A, Tagliasacchi D, et al. LH And hCG action on the same receptor results in quantitatively and qualitatively different intracellular signalling. PLoS One. (2012) 7(10):e46682. doi: 10.1371/journal.pone.0046682
33. Casarini L, Riccetti L, De Pascali F, Nicoli A, Tagliavini S, Trenti T, et al. Follicle-stimulating hormone potentiates the steroidogenic activity of chorionic gonadotropin and the anti-apoptotic activity of luteinizing hormone in human granulosa-lutein cells in vitro. Mol Cell Endocrinol. (2016) 422:103–14. doi: 10.1016/j.mce.2015.12.008
34. Riccetti L, De Pascali F, Gilioli L, Poti F, Giva LB, Marino M, et al. Human LH and hCG stimulate differently the early signalling pathways but result in equal testosterone synthesis in mouse leydig cells in vitro. Reprod Biol Endocrinol. (2017) 15(1):2. doi: 10.1186/s12958-016-0224-3
35. Ng J. Delphi method: a qualitative approach for quantitative results. Value Health. (2018) 21:S54. doi: 10.1016/j.jval.2018.04.447
36. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH antagonist versus long agonist protocols in IVF: a systematic review and meta-analysis accounting for patient type. Hum Reprod Update. (2017) 23(5):560–79. doi: 10.1093/humupd/dmx017
37. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. (2017) 108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005
38. Balasch J, Vidal E, Penarrubia J, Casamitjana R, Carmona F, Creus M, et al. Suppression of LH during ovarian stimulation: analysing threshold values and effects on ovarian response and the outcome of assisted reproduction in down-regulated women stimulated with recombinant FSH. Hum Reprod. (2001) 16(8):1636–43. doi: 10.1093/humrep/16.8.1636
39. Westergaard LG, Laursen SB, Andersen CY. Increased risk of early pregnancy loss by profound suppression of luteinizing hormone during ovarian stimulation in normogonadotrophic women undergoing assisted reproduction. Hum Reprod. (2000) 15(5):1003–8. doi: 10.1093/humrep/15.5.1003
40. Lahoud R, Al-Jefout M, Tyler J, Ryan J, Driscoll G. A relative reduction in mid-follicular LH concentrations during GnRH agonist IVF/ICSI cycles leads to lower live birth rates. Hum Reprod. (2006) 21(10):2645–9. doi: 10.1093/humrep/del219
41. Huirne JA, van Loenen AC, Schats R, McDonnell J, Hompes PG, Schoemaker J, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod. (2005) 20(2):359–67. doi: 10.1093/humrep/deh601
42. Kol S. Individualized treatment from theory to practice: the private case of adding LH during GnRH antagonist-based stimulation protocol. Clin Med Insights Reprod Health. (2014) 8:59–64. doi: 10.4137/CMRH.S17788
43. Kol S, Homburg R. Change, change, change: hormonal actions depend on changes in blood levels. Hum Reprod. (2008) 23(5):1004–6. doi: 10.1093/humrep/den061
44. Conforti A, Esteves SC, Humaidan P, Longobardi S, D'Hooghe T, Orvieto R, et al. Recombinant human luteinizing hormone co-treatment in ovarian stimulation for assisted reproductive technology in women of advanced reproductive age: a systematic review and meta-analysis of randomized controlled trials. Reprod Biol Endocrinol. (2021) 19(1):91. doi: 10.1186/s12958-021-00759-4
45. Hayes F, Dwyer A, Pitteloud N. Hypogonadotropic hypogonadism (HH) and gonadotropin therapy. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, editors. Endotext. South Dartmouth (MA): MDText.com, Inc.Copyright © 2000-2022, MDText.com, Inc. (2000).
46. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional hypothalamic amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. (2017) 102(5):1413–39. doi: 10.1210/jc.2017-00131
47. Bosch E, Labarta E, Crespo J, Simon C, Remohi J, Pellicer A. Impact of luteinizing hormone administration on gonadotropin-releasing hormone antagonist cycles: an age-adjusted analysis. Fertil Steril. (2011) 95(3):1031–6. doi: 10.1016/j.fertnstert.2010.10.021
48. Hill MJ, Levens ED, Levy G, Ryan ME, Csokmay JM, DeCherney AH, et al. The use of recombinant luteinizing hormone in patients undergoing assisted reproductive techniques with advanced reproductive age: a systematic review and meta-analysis. Fertil Steril. (2012) 97(5):1108–14 e1. doi: 10.1016/j.fertnstert.2012.01.130
49. Matorras R, Prieto B, Exposito A, Mendoza R, Crisol L, Herranz P, et al. Mid-follicular LH supplementation in women aged 35–39 years undergoing ICSI cycles: a randomized controlled study. Reprod Biomed Online. (2009) 19(6):879–87. doi: 10.1016/j.rbmo.2009.09.016
50. Falck B. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol Scand Suppl. (1959) 47(163):1–101. doi: 10.1111/j.1748-1716.1960.tb01823.x
51. Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod. (2002) 17(8):2016–21. doi: 10.1093/humrep/17.8.2016
52. Fleming R, Rehka P, Deshpande N, Jamieson ME, Yates RW, Lyall H. Suppression of LH during ovarian stimulation: effects differ in cycles stimulated with purified urinary FSH and recombinant FSH. Hum Reprod. (2000) 15(7):1440–5. doi: 10.1093/humrep/15.7.1440
53. Alviggi C, Conforti A, Santi D, Esteves SC, Andersen CY, Humaidan P, et al. Clinical relevance of genetic variants of gonadotrophins and their receptors in controlled ovarian stimulation: a systematic review and meta-analysis. Hum Reprod Update. (2018) 24(5):599–614. doi: 10.1093/humupd/dmy019
54. Behre HM, Greb RR, Mempel A, Sonntag B, Kiesel L, Kaltwasser P, et al. Significance of a common single nucleotide polymorphism in exon 10 of the follicle-stimulating hormone (FSH) receptor gene for the ovarian response to FSH: a pharmacogenetic approach to controlled ovarian hyperstimulation. Pharmacogenet Genomics. (2005) 15(7):451–6. doi: 10.1097/01.fpc.0000167330.92786.5e
55. Neves AR, Vuong NL, Blockeel C, Garcia S, Alviggi C, Spits C, et al. The effect of polymorphisms in FSHR gene on late follicular phase progesterone and estradiol serum levels in predicted normoresponders. Hum Reprod. (2022) 37(11):2646–54. doi: 10.1093/humrep/deac193
56. Grisendi V, Mastellari E, La Marca A. Ovarian reserve markers to identify poor responders in the context of poseidon classification. Front Endocrinol (Lausanne). (2019) 10:281. doi: 10.3389/fendo.2019.00281
57. Sperduti S, Paradiso E, Anzivino C, Lazzaretti C, Limoncella S, D'Alessandro S, et al. LH increases the response to FSH in granulosa-lutein cells from sub/poor-responder patients in vitro. Hum Reprod. (2023) 38(1):103–12. doi: 10.1093/humrep/deac246
58. le Cotonnec JY, Porchet HC, Beltrami V, Munafo A. Clinical pharmacology of recombinant human luteinizing hormone: part I. Pharmacokinetics after intravenous administration to healthy female volunteers and comparison with urinary human luteinizing hormone. Fertil Steril. (1998) 69(2):189–94. doi: 10.1016/S0015-0282(97)00501-3
59. Trinchard-Lugan I, Khan A, Porchet HC, Munafo A. Pharmacokinetics and pharmacodynamics of recombinant human chorionic gonadotrophin in healthy male and female volunteers. Reprod Biomed Online. (2002) 4(2):106–15. doi: 10.1016/S1472-6483(10)61927-X
60. Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. (2003) 7(1):59–64. doi: 10.1016/S1472-6483(10)61729-4
Keywords: Delphi consensus, ovarian stimulation, ART, LH supplementation, Iran, expert
Citation: Salehpour S, Aleyasin A, Moini A, Mousavifar N, Mohammadhossein N, Abdollahi Fard S, Marzie S, Mohammadzadeh M and Fischer R (2024) Luteinizing hormone supplementation in controlled ovarian stimulation: the Iran Delphi consensus. Front. Reprod. Health 6:1397446. doi: 10.3389/frph.2024.1397446
Received: 7 March 2024; Accepted: 1 April 2024;
Published: 9 May 2024.
Edited by:
Shevach Friedler, Barzilai Medical Center, IsraelReviewed by:
Kulvinder Kochar Kaur, Kulvinder Kaur Centre For Human Reproduction, IndiaAkmal El-Mazny, Cairo University, Egypt
© 2024 Salehpour, Aleyasin, Moini, Mousavifar, Mohammadhossein, Abdollahi Fard, Marzie, Mohammadzadeh and Fischer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saghar Salehpour, c2FnaGFyLnNhbGVocG91cjIwMTRAZ21haWwuY29t
 Saghar Salehpour
Saghar Salehpour Ashraf Aleyasin2
Ashraf Aleyasin2 Nasresfahani Mohammadhossein
Nasresfahani Mohammadhossein