
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Reprod. Health , 24 August 2023
Sec. HIV and STIs
Volume 5 - 2023 | https://doi.org/10.3389/frph.2023.1238813
This article is part of the Research Topic Multipurpose Prevention Technologies: Call for Innovative Strategies to Address Critical Priorities and Gaps View all 13 articles
With less than half of the 15-year Sustainable Development Goal (SDG) agenda remaining, progress toward the goal of ensuring healthy lives and promoting wellbeing for all has slowed, stalled, and in some cases reversed. UNAIDS has targets of 95% of people at risk of human immunodeficiency virus (HIV) infection using appropriate, prioritized, person-centered and effective combination prevention options and 95% of women of reproductive age having their HIV and sexual and reproductive health service needs met by 2025 (1). However, in 2021, there were 1.5 million new HIV infections, far from the 2025 target of 370,000 (2); 121 million unintended pregnancies, nearly half of all pregnancies globally (3); and 374 million new infections with one of four curable sexually transmitted infections (STIs) (4), compounding the risk of HIV acquisition. Most of this burden is borne by low- and middle-income countries (L/MICs). The COVID-19 pandemic pushed progress further off-track. For example, an estimated 12 million women were unable to access family planning services due to the pandemic, resulting in 1.4 million unintended pregnancies (5). With additional factors such as climate change driving migration and conflict, and the cost-of-living crisis constraining budgets, there is even greater need for streamlined services and improved, affordable technologies to meet diverse health needs across different contexts (6).
Novel forms of multipurpose prevention technologies (MPTs) are a game-changing innovation for the simultaneous prevention of unintended pregnancy, HIV, and/or STIs. By addressing multiple health needs at once, MPTs have the potential to better meet the needs of the populations they seek to serve and accelerate progress toward global goals. However, technological innovation alone is insufficient to achieve global impact. Addressing timely and equitable access is key for novel technologies to reach populations in need and realize their full potential. There is an opportunity to learn from other technologies that have emerged in recent decades. On average, it has taken eight to ten years for HIV medicines approved by the United States Food and Drug Administration (US FDA) to become accessible in L/MICs (7). With the urgent need to accelerate progress toward SDG31 (8), we cannot afford preventable mortality and morbidity caused by this lag in access.
An end-to-end (E2E) approach in the context of addressing health innovations aligns relevant stakeholders across sectors, including product developers, generic manufacturers, funders, regulators, health agencies, governments and communities, on a common strategy to bring a product from research and development to uptake and adoption by communities (Figure 1). An E2E approach has a holistic view of the actions and commitments necessary to facilitate equitable access and must be initiated at the earliest stages of product development. Waiting until products reach the market to plan for equitable access often leaves it too late to address the root causes of high prices and slow commercialization in L/MICs (7). The full potential of any given MPT can best be realized if an E2E approach is taken to ensure the conditions to achieve equitable access are established rapidly—including in advance of products being available—and in a sustainable manner.
An E2E approach goes beyond advancing products in pipeline and looks ahead at the roadmap to suitable, affordable and quality supply for accelerated introduction and adoption. This approach prevents and removes potential roadblocks associated with intellectual property, facilitates timely regulatory approvals; anticipates supply pathways; coordinates with country stakeholders for integration into guidelines; and maximizes the engagement of affected communities (6). The following sections outline key considerations for an E2E approach for MPTs, divided into the phases before, during and after regulatory approval.
Figure 1 is a linear depiction of the steps required, however in practice many of these steps overlap to expedite timelines.
The research and development phase for MPTs is a critical time for developers to consider end-user preferences and to chart a product's access pathways to ensure the final product will be both acceptable and accessible to all who could benefit. Investors will similarly need to prioritize which products to support based on end-user preferences and access profiles. User preferences vary widely, however some studies have shown a general preference for discreet and familiar provider-administered long-acting products, such as injectables and implants (9). That said, as one MPT will not meet the needs or preferences of all potential users, this prioritization needs to be balanced with the objective of having a range of MPTs available to enable choice. Condoms are the only MPT currently available, and while they have been and continue to be pivotal in the global agenda to prevent HIV, STI, and unintended pregnancy, they do not meet the preferences of all potential users. This speaks to the need to have a range of different MPTs available, and to establish feedback mechanisms from users to inform the design of future products.
Identifying and mitigating potential barriers to access for end-users, such as affordability and availability, also needs to be initiated by product developers and those financing these innovations in parallel with research and development efforts. The complexity of combining active pharmaceutical ingredients and the technologies involved in MPTs may imply cost differences with the standard-of-care products they aim to replace, especially for formulations with extended release, and may also limit the number and location of manufacturers able to produce them (9). Cost-effectiveness estimates and potential impact need to be considered from early stages to inform which MPTs in the pipeline are prioritized, and developers may need to be incentivized by global health funders to investigate cost-reduction strategies. Global health funders can play an important role by embedding access terms, such as sharing of intellectual property, into funding agreements for research and development or considering market shaping support, such as guaranteeing minimum volumes of procurement in early introduction phases (see Box 1).
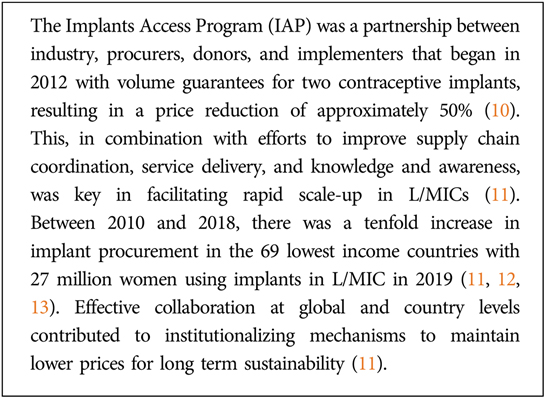
Box 1. Long-acting contraceptives have demonstrated the potential for partnerships to catalyze a healthy market for L/MIC access.
Intellectual property may also limit who can manufacture MPTs, especially considering the additional intellectual property involved compared to single-indication products. Early planning by developers to establish licensing mechanisms that enable a generic manufacturing base will not only help overcome intellectual property barriers and improve supply security; it will also enable greater affordability. For example, the Medicines Patent Pool (MPP), an organization founded by Unitaid, employs a model that facilitates non-exclusive, voluntary licensing of life-saving medicines for L/MICs (14). While MPTs would require special considerations for voluntary licensing, this model could help address such access barriers. Engagement between industry partners and MPP prior to regulatory approval accelerates access once approval is obtained. Voluntary licenses may not enable access for all L/MICs, however, and other mechanisms may need to be explored to overcome intellectual property barriers (15)2.
Early planning is necessary to ensure regulatory pathways do not cause delays in access to MPTs. Regulatory requirements for MPTs are yet to be clearly defined, which may cause challenges for MPT developers as they are planning what data to generate for approvals. There have been reviews of key regulatory guidance documents and their applicability to MPTs, however there will not be one single regulatory pathway for MPTs (9). Regulatory approval requirements often differ across Stringent Regulatory Authorities and national regulators in L/MICs, and requirements will also vary across MPTs depending on the components. For example, the Dual Prevention Pill under development only requires a bioequivalence study, rather than Phase 3 safety and efficacy trials because both oral dose products are separately approved and their drug-drug interactions well-studied (16).
Developers and regulators are therefore encouraged to engage early on in these discussions, so that approval processes can be executed without delay once products in the pipeline reach this stage. Developers are also encouraged to engage with the World Health Organization (WHO) technical areas regarding guidance recommendations; and the WHO Prequalification program (17) including the WHO Collaborative Procedure for Accelerated Registration (18), to accelerate access to and registration of critical quality-assured products in L/MICs. It is generally expected that developers widely register and supply quality-assured products soon after a recommendation or approval is obtained by a normative body and/or regulatory entity.
In order to have rapid introduction and scale up once approved, both supply and demand side strategies need to be considered including policy and implementation guidance (see Box 2). MPTs will break the mold of the silos that healthcare systems typically operate in. Supply chains for family planning and HIV commodities are currently disconnected, and financing and procurement streams are typically separated by governments and donors as well. Establishing the budget, procurement mechanisms, and supply and delivery pathways for MPTs will require collaboration among diverse stakeholders, including industry, governments, global health funders, and procurement agencies. This will have major implications for the scope of responsibilities of health departments as MPTs become available, ranging from monitoring and evaluation to health provider training. In addition to the adaptations country health systems will need to make to integrate MPTs, country-specific efforts will also be required to obtain national market authorization and guidelines for sustainable implementation in national programs. Implementation research in early adopter countries will play a critical role in generating real-world evidence to inform recommendations at the national and international level and enable broader scale up. In parallel with research, MPT awareness-raising by and with policymakers, medical associations and advocates through convenings, publications and interest group formations can accelerate uptake once products achieve government commitment to scale (9).
Meaningful innovation requires more than new and improved products; it also necessitates finding novel strategies to collaborate and overcome barriers to equitable access for products to have global impact (6). This article highlights the need for a range of stakeholders to collaboratively address product and market characteristics, such as affordability, supply capacity, intellectual property, regulatory pathways, and ease of use, to enable prompt community adoption. While lessons can be learned from existing products, some considerations will be unique to MPTs and it is imperative that the global community align on how to address these and plan far in advance so that MPTs can reach L/MICs without delay once available. Platforms that bring together donors, agencies, governments, affected communities and advocates to ensure an accelerated, sustainable, and collaborative approach is taken to making MPTs equitably accessible will be essential. If planning to remove access barriers does not start early on, there will be a myriad of challenges impeding timely uptake and scale in L/MICs. As the pipeline of MPTs continues to grow and advance, now is the time to plan for an E2E approach to equitable access.
All authors contributed to the article and approved the submitted version.
Sections of this article built upon the MPTs Landscape and Potential for Low- and Middle-Income Countries published in 2021, funded by Unitaid and the Children's Investment Fund Foundation.
We would like to acknowledge Bethany Young Holt and Susanna Moore (Initiative for MPTs), Joseph Romano (NWJ Group), and Ariane van der Sraten (ASTRA Consulting) for preparing the landscape assessment noted above, and all experts and key informants consulted for their valuable contributions.
At the time of publication, AC, CP-C and CS were employed by Unitaid and TB was employed by Children’s Investment Fund Foundation.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1Sustainable Development Goal 3 is to ensure health lives and promote well-being for all at all ages. This includes targets relevant to MPTs such as target 3.3 (fight communicable diseases) and 3.7 (universal access to sexual and reproductive care, family planning and education).
2Note that while this resource refers to COVID-19 therapeutics, the mechanisms outlined to overcome intellectual property barriers can be applied to other products.
1. UNAIDS. UNAIDS Targets 2025. (2023). Available at: https://aidstargets2025.unaids.org/ (Accessed May 23, 2023).
2. UNAIDS. Global AIDS Update 2022: (2022). Available at: https://www.unaids.org/sites/default/files/media_asset/2022-global-aids-update-summary_en.pdf (Accessed May 23, 2023).
3. UNFPA. State of the World Population 2022: Seeing the Unseen: The case for action in the neglected crisis of unintended pregnancy. (2022). Available at: https://www.unfpa.org/sites/default/files/pub-pdf/EN_SWP22%20report_0.pdf
4. Chlamydia, gonorrhoea, syphilis and trichomoniasis. World Health Organization. Fact sheets: Sexually transmitted infections. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)
5. Luchsinger, Gretchen. No Exceptions, No Exclusions: Realizing Sexual and Reproductive Health, Rights and Justice for All. High-Level Commission on the Nairobi Summit on ICPD25 Follow-up. (2021). Available at: www.nairobisummiticpd.org/publication/no-exceptions-no-exclusions
6. Unitaid. Strategy 2023–2027. (2023). Available at: https://unitaid.org/assets/Unitaid_Strategy_2023-2027.pdf
7. Pérez Casas C. (2015). Accelerating access for optimized regimens. WHO ICASA Non-Abstract Driven Session HIV Treatment: What’s New in the Pipeline. Harare, Zimbabwe.
8. The Global Goals. Good Health and Wellbeing. Available at: https://www.globalgoals.org/goals/3-good-health-and-well-being/ (Accessed May 23, 2023).
9. The IMPT; Unitaid; CIFF. Multipurpose Prevention Technologies (MPTs): Technology Landscape and Potential for Low- and Middle-Income Countries. (2021). Available at: https://unitaid.org/assets/Multipurpose-Prevention-Technologies-Technology-Landscape-and-Potential-for-Low-and-Middle-Income-Countries.pdf
10. Clinton Health Access Initiative. Case Study: Expanding global access to contraceptive implants. (2015). Available at: https://clintonhealthaccess.org/wp-content/uploads/2015/08/Case-Study_LARC.pdf
11. Braun R, Grever A. Scaling up access to implants: a summative evaluation of the implants access program. Glob Health Sci Pract. (2020) 8(2):205–19. doi: 10.9745/GHSP-D-19-00383
12. Rademacher K, Sripipatana T, Danna K, Sitrin D, Brunie A, Williams KM, et al. What have we learned? Implementation of a shared learning agenda and access strategy for the hormonal intrauterine device. Glob Health Sci Pract. (2022) 10(5):e2100789. doi: 10.9745/GHSP-D-21-00789
13. Weinberger M, Eva G, Gold J, Bellows N, Reidy M, Sanders R, et al. LEAP: landscape and projection of reproductive health supply needs. Reprod Health Suppl Coalition. (2021).
14. Medicines Patent Pool. Available at: https://medicinespatentpool.org/ (Accessed June 5, 2023).
15. Unitaid; WHO; Medicines Law & Policy. Improving access to novel COVID-19 treatments: A briefing to Member States on how to navigate interfaces between public health and intellectual property. (2023). Available at: https://www.who.int/publications/m/item/improving-accessto-novel-covid-19treatments
16. Friedland B, Mathur S, Haddad L. The Promise of the Dual Prevention Pill: A Framework for Development and Introduction. (2021). Available at: https://www.frontiersin.org/articles/10.3389/frph.2021.682689/full
17. World Health Organization. WHO Prequalification. (2023). Available at: https://extranet.who.int/pqweb/ (Accessed May 23, 2023).
18. World Health Organization. Collaborative Procedure for Accelerated Registration. (2023). Available at: https://extranet.who.int/pqweb/medicines/collaborative-procedure-accelerated-registration (Access May 23, 2023).
19. Philippe Duneton, Karin Timmermans, Carmen Pérez Casas. Unitaid Submission to The United Nations Secretary-General’s High-Level Panel On Access To Medicines: Accelerating Access To Innovation: Lessons Learned By Unitaid. (2016). Available at: http://www.unsgaccessmeds.org/inbox/2016/2/26/unitaidb (Accessed May 23, 2023).
20. AVAC. PrEPWatch. (2023). Available at: https://www.prepwatch.org/ (Accessed May 23, 2023).
21. AVAC; Clinton Health Access Initiative. Lessons From Oral PrEP Programs and Their Implications for Next Generation Prevention. (2021). Available at: https://www.prepwatch.org/wp-content/uploads/2021/11/PrEP_LearningReportNov21.pdf (Access May 12, 2023).
Keywords: multipurpose prevention technologies (MPTs), equitable access, low- and middle-income countries (LMICs), HIV, contraception, sexually transmitted infections (STIs)
Citation: Cameron A-I, Scott C, Pérez-Casas C and Barker T (2023) End-to-end approach to ensuring equitable access to multipurpose prevention technologies in low- and middle-income countries. Front. Reprod. Health 5:1238813. doi: 10.3389/frph.2023.1238813
Received: 12 June 2023; Accepted: 10 August 2023;
Published: 24 August 2023.
Edited by:
Bethany Young Holt, CAMI Health | Initiative for MPTs| Public Health Institute, United StatesReviewed by:
Margaret Kasaro, UNC Global Projects Zambia, Zambia© 2023 Cameron, Scott, Pérez-Casas and Barker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anne-Isabelle Cameron Y2FtZXJvbmFuQHVuaXRhaWQud2hvLmludA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.