
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Reprod. Health, 24 April 2023
Sec. HIV and STIs
Volume 5 - 2023 | https://doi.org/10.3389/frph.2023.1125159
This article is part of the Research TopicMultipurpose Prevention Technologies for HIV, STIs & PregnanciesView all 13 articles
 Sammy Gakero Gachigua1
Sammy Gakero Gachigua1 Robinson Karuga1
Robinson Karuga1 Anne Ngunjiri1
Anne Ngunjiri1 Courtney Jarrahian2
Courtney Jarrahian2 Patricia S. Coffey2
Patricia S. Coffey2 Maggie Kilbourne-Brook2*
Maggie Kilbourne-Brook2* Lilian Otiso1
Lilian Otiso1
Background: Microarray patches (MAPs), a novel drug delivery system, are being developed for HIV pre-exposure prophylaxis (PrEP) delivery and as a multipurpose prevention technology (MPT) to protect from both HIV and unintended pregnancy. Prevention technologies must meet the needs of target audiences, be acceptable, easy to use, and fit health system requirements.
Methodology: We explored perceptions about MAP technology and assessed usability, hypothetical acceptability, and potential programmatic fit of MAP prototypes using focus group discussions (FGD), usability exercises, and key informant interviews (KII) among key populations in Kiambu County, Kenya. Adolescent girls and young women (AGYW), female sex workers (FSW), and men who have sex with men (MSM) assessed the usability and acceptability of a MAP prototype. Male partners of AGYW/FSW assessed MAP acceptability as partners of likely users. We analyzed data using NVivo, applying an inductive approach. Health service providers and policymakers assessed programmatic fit. Usability exercise participants applied a no-drug, no-microneedle MAP prototype and assessed MAP features.
Results: We implemented 10 FGD (4 AGYW; 2 FSW; 2 MSM; 2 male partners); 47 mock use exercises (19 AGYW; 9 FSW; 8 MSM; 11 HSP); and 6 policymaker KII. Participants reported high interest in MAPs due to discreet and easy use, long-term protection, and potential for self-administration. MAP size and duration of protection were key characteristics influencing acceptability. Most AGYW preferred the MPT MAP over an HIV PrEP-only MAP. FSW saw value in both MAP indications and voiced need for MPTs that protect from other infections. Preferred duration of protection was 1–3 months. Some participants would accept a larger MAP if it provided longer protection. Participants suggested revisions to the feedback indicator to improve confidence. Policymakers described the MPT MAP as “killing two birds with one stone,” in addressing AGYW needs for both HIV protection and contraception. An MPT MAP is aligned with Kenya's policy of integrating health care programs.
Conclusions: MAPs for HIV PrEP and as an MPT both were acceptable across participant groups. Some groups valued an MPT MAP over an HIV PrEP MAP. Prototype refinements will improve usability and confidence.
In Kenya, the most recent estimated prevalence of HIV among adults was 4.9% in 2019 (1, 2), marking it as the country with the twelfth highest rate of HIV globally. In the same year, the estimated HIV prevalence for women aged 15–49 was more than twice as high as that for men aged 15–49 (6.6% vs. 3.1%). HIV infection rates among young people (15–24) accounted for 35% of new infections, with two-thirds of cases among adolescent girls and young women (AGYW) (3, 4). Also, the most recent national statistics (from 2016) showed an HIV prevalence of 18.2% among men who have sex with men (MSM) and 29.3% among female sex workers (FSW) (5). Reducing HIV infection rates among these populations is crucial for HIV epidemic control.
Likewise, unintended pregnancies in Kenya continue to be a public health burden. Although Kenya had a high contraceptive prevalence rate of about 58% for married women in 2020 (6), national survey data demonstrated that unmet need for family planning was highest among young women 20–29 years old (33%), followed by adolescent girls 15–19 years old (23%) (7). The high proportion of sexually active AGYW with unmet need for family planning in Kenya has led to a high number of unintended or mistimed pregnancies, unsafe abortions, and maternal deaths (8–10). Women, particularly AGYW, who face a persistent unmet need for contraception tend to have a higher risk of HIV infection (11–13). The high incidence of HIV among AGYW is exacerbated by low uptake of HIV prevention methods, such as pre-exposure prophylaxis (PrEP) (14, 15).
New drug delivery systems are being developed to address the challenges users experience (16–18) with PrEP delivery through daily oral pills. For example, a microarray patch (MAP) is being developed to deliver an antiretroviral (ARV) for HIV PrEP, as well as alongside a hormonal contraceptive as a multipurpose technology (MPT) for women. These ARV MAPs in development have the potential to offer protection for 1 month to 3 months, depending on the active pharmaceutical ingredient (API). The MAPs have the potential for easy, discreet, and self-administered protection that could improve uptake of and adherence to HIV PrEP. Qualitative research in 2016 with South African women and health care providers indicated that an ideal HIV PrEP solution should be discreet, long acting (3–6 months), highly effective, possible to self-administer, and protect users against not only HIV but also other sexually transmitted infections and pregnancy (19). A 2019/2020 assessment in South Africa and Uganda that explored user/stakeholder preferences regarding the MAP for HIV PrEP and as an MPT (20) generated recommendations for refinements to the MAP prototype, including the feedback indicator, to improve ease of use. Using the refined MAP prototype, we conducted this early-stage product development assessment in Kenya to continue exploring user and stakeholder needs and preferences for product features that could influence acceptability, usability, and programmatic fit for a MAP delivering HIV PrEP and as an MPT.
The ARV MAPs in development are designed to have multiple arrays, each containing hundreds of tiny (<1 mm) microneedles. Each array on the MAP has a corresponding dome above it that the user would depress to apply the patch to the skin; collectively, the domes serve as a “feedback indicator” to confirm successful application. The MAP projections would gently pierce the skin and begin to dissolve. First, the base of the projections dissolve, separating from the patch backing (Figure 1). After a specified wear time, the MAP backing and feedback domes would be removed and discarded. Next, the projections would fully dissolve in the skin, releasing the API into systemic circulation (21, 22). The targeted wear time for the HIV PrEP and MPT MAP is less than 20 min, at which point the MAP's adhesive layer would be discarded.
The MAP prototype used in this assessment (Figure 2) was representative of the aforementioned MAP currently being developed to deliver an ARV for HIV PrEP and as an MPT for delivery of both an ARV and a hormonal contraceptive. The MAP prototype had 8 feedback indicator domes indicating where microneedle arrays would be located (if it were a marketed product containing drugs). After the MAP prototype was applied to the skin, the user pressed on each dome until the domes inverted. The MAP prototypes used in this assessment were “looks like/feels like” prototypes—they did contain any microneedles and did not contain any drugs.
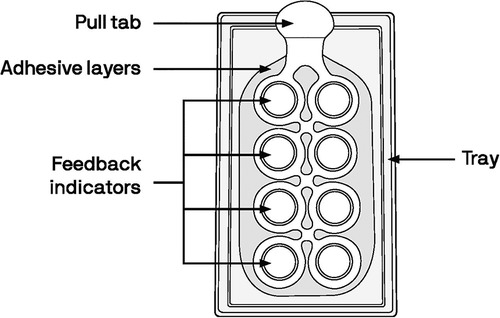
Figure 2. Diagram of the MAP prototype used in this assessment; dimensions were 14.5 cm × 6.25 cm (5.75 inches by 2.5 inches).
This descriptive exploratory study was conducted in Kenya, a country known as a leader in PrEP rollout, but where barriers continue to exist for currently available HIV prevention methods (23, 24). Within Kenya, Kiambu County was selected as the study site because it has a large proportion of AGYW, FSW, and MSM. Kiambu County hosts a high concentration of institutions of higher learning and corollary large populations of AGYW. The proximity of Kiambu County to Nairobi has resulted in a high rate of urbanization and related increase in the study populations of interest (25). In addition, the county provides access to peri-urban and rural settings. Importantly, service delivery points that serve AGYW, FSW, and MSM with HIV testing services were accessible and interested in study collaboration, as they are all owned and managed by LVCT Health.
The objectives of the study were to assess (a) usability of a MAP prototype (no drug or microneedles) for ease of use and design features, (b) hypothetical acceptability of MAP technology for HIV PrEP and/or as an MPT, and (c) potential programmatic fit of MAP technology within the Kenya health care system. Study participants included adolescent girls (AG, 15–17 years) and young women (YW, 18–24 years); FSW; MSM; male sexual partners of AGYW and FSW; health service providers; and national and county policymakers and managers. MSM assessed the MAP prototype for HIV prevention only.
Sampling for qualitative data collection considered the homogeneity of the populations and followed general guidance around the sample sizes required to reach saturation (26). The research team worked with staff at the five participating health facilities to select AGYW, FSW, male sexual partners of AGYW and FSW, and MSM participants for focus group discussions (FGD) via purposive typical case sampling whereby every third client from each of the target populations was asked to participate. Male sexual partners of YW were recruited via referral from their partners. We also purposively sampled national stakeholders and county-level health managers with experience in PrEP and contraceptive service provision and commodity management to assess the potential programmatic fit of MAPs for HIV PrEP and as an MPT.
We based our sample size (n = 14 per user group) for the mock use exercise on general guidance about appropriate sample sizes for usability testing (27). Because self-care products in Kenya, such as HIV self-testing (28), are usually delivered initially in health facilities where providers are able to assist, we also engaged providers to evaluate usability of the prototype via a simulated use exercise in which they were asked to provide additional support to mock clients.
Primary data collection included a mock use exercise with a MAP prototype followed by in-depth interviews and a self-administered questionnaire with AG, YW, FSW, MSM, and health service providers. In addition, FGD were held with AGYW, FSW, male partners of AGYW and FSW, and MSM. Participants in these two data collection efforts were distinct from one another. Finally, key informant interviews were conducted with county and national stakeholders. In both data collection efforts, user perceptions and preferences about product features that could affect acceptability were explored, including MAP size, duration of protection, site of application, wear time, feedback indicator, and packaging. The mock use exercise consisted of orienting the participants to the MAP prototype through an informed consent process. Participants who consented were given instructions for use (IFU) (Supplementary material S1) and a MAP prototype and were asked to follow the instructions to apply the MAP on their body. During this activity, the participants were encouraged to “think out loud” and describe what they were doing. Simultaneously, a research assistant observed the mock use to document use errors, difficulties, close calls, and surprises across all steps of MAP application (handling, opening package, using instructions, practicing applying the patch, activating the feedback indicator, removing the patch). An in-depth interview was conducted to capture user perspectives after mock use, including a survey in which participants rated perceived importance and relative satisfaction with MAP features. Data collection methods are summarized in Table 1.
The data collection instruments were translated into Kiswahili and pretested to identify ambiguity and clarify language. The FGD guide and semi-structured interview questionnaire were pretested with AGYW, FSW, MSM, and male partners at drop-in centers and DREAMS program sites in Nairobi County. The key informant interview questionnaire was pretested with providers and stakeholders in facilities in Nairobi County that did not participate in this study.
Data were collected in “safe spaces” identified by the facilities (where they usually meet confidentially with clients to discuss health issues). When a safe space was not available, clients were asked to suggest a meeting place within their community and interviewers assessed the location prior to the meetings to check whether it was conducive for data collection according to interview and research ethics requirements.
Qualitative data were transcribed verbatim in Microsoft Word (Microsoft Corporation, USA), translated as necessary, and analyzed applying an inductive approach using NVivo™ R (QSR International Pty Ltd; Doncaster, Australia). A coding framework was developed, initially based on the study objectives and then expanded in a data analysis workshop. Coding and qualitative data analysis were done collaboratively by the research team, noting comparisons (where appropriate) for the different target populations.
Quantitative data (ranking of MAP features and satisfaction from the self-administered questionnaire and mock use exercise observation checklist) were analyzed using Microsoft Excel version 2016 or a rainbow spreadsheet (29) with filters for user populations and usability steps. Data were cleaned and reviewed after entry into the spreadsheets; incomplete, inaccurate, or irrelevant data were identified and rectified after consultation with at least two members of the research team.
The AMREF Ethics and Scientific Review Committee granted ethics approval for this study (approval number: P770/2020). Letters confirming approval to conduct the study were shared with county partners and clinic and drop-in center sites after meetings to sensitize the county representatives on the proposed study. The Kiambu County Health Research Unit also granted approval to conduct the research (reference number: KIAMBU/HRDU/22/03/08/RA_OTISO). Written informed consent was obtained for all study procedures, including audio, photo, and video recording, as warranted by each data collection method.
Between February and April 2022, we collected data from 47 participants in a mock use exercise and conducted ten FGD and six stakeholder interviews (Table 2). The stakeholder interviews were conducted with three county managers and three national managers.
The research team observed 47 participants during simulated use of the MAP prototype (Table 3). Observations were recorded as either correct use or one of the four standardized categories employed in usability testing (30). The majority of the 47 participants reviewed the instructions before engaging in the mock exercise. The four participants who did not review the instructions experienced user errors. Successful completion of all steps in the mock use exercise was low for all user groups, ranging from 13% for MSM to 46% for providers. About a quarter of AG (29%), YW (25%), and FSW (22%) successfully accomplished the mock use exercise.
The most problematic task observed for all population groups except providers was cleaning of the application site (both user error and difficulty). For AGYW, the second highest number of user errors and difficulties was observed for understanding wear time. For FSW and MSM, the second highest number of user errors and difficulties was observed for pressing down firmly on all feedback indicator domes, one at a time, until each dome crushed. Health service providers were observed to have the most challenges with pressing down firmly on all indicator domes one at a time until each dome crushed (use errors and difficulties) and removing the MAP layer from the skin (difficulty only). Two FSW showed extreme difficulty when using the MAP; one mistook the MAP for an HIV test kit and the other was not literate.
After the mock use exercise, participants reported their perceived importance (“not important”, “undecided”, or “important”) and satisfaction (“dissatisfied”, “neutral”, or “satisfied”) about a set of MAP features (Figure 3 and Supplementary material S2). The feedback indicator, patch size, and wear time were the features with the largest gaps between importance and satisfaction, indicating product design alignments that will need to be made in future product iterations.
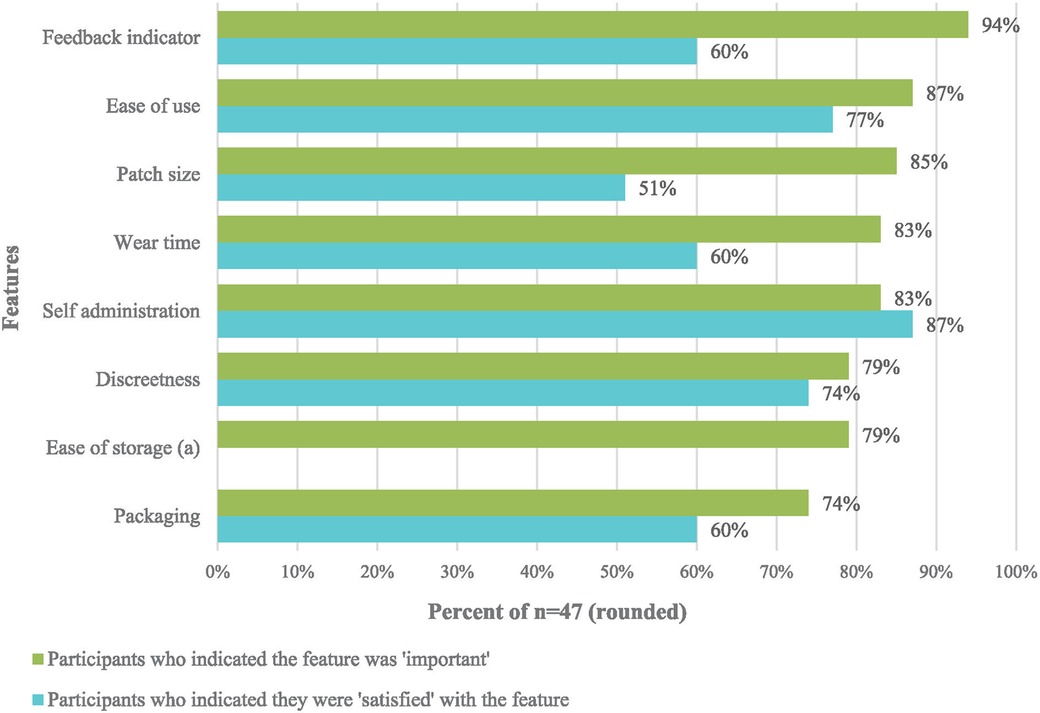
Figure 3. Differences between “important” and “satisfied” for MAP features after mock use by AGYW, FSW, MSM, and health service providers (n = 47).
All FGD participants (AGYW, FSW, MSM, male partners) and mock use participants AGYW, FSW, MSM, providers) expressed their willingness to use a MAP for either HIV protection or as an MPT when it became available. Men shared opinions from the perspective of their own hypothetical use of an HIV PrEP MAP and perspectives about women using HIV PrEP MAP or MPT MAP. The convenience of the method and longer projected wear time were noted as advantages particularly when compared to existing methods of protection (Table 4). Conversely, a few male partners noted that despite their positive perception of the MAP, they could be suspicious that somebody using it might have an HIV infection.
Preferences around the MAP design feature set varied among user groups (Table 5). More detailed results about each feature are also discussed below.
In general, AGYW reported that the current size of the MAP was acceptable. For example:
“I haven’t seen anything that is wrong with the size because it can easily be covered.” YW, mock use exercise [IDIYW002].
Service providers and FGD participants felt the size of the MAP should be smaller. They suggested the MAP should be small enough to be carried easily, possibly in a pocket or purse.
“It can be smaller, so that when you put it in your handbag, someone should not see that it is something funny. It should look like something smart, small.” FSW, FGD [FGDFSW001]
“It's too long…some clients would not like to be seen by their partners…it would raise some alarm or queries.” Provider, mock use exercise [IDIHSP001].
“I wish it was smaller. It is big in that when you carry it you can’t put it in a smaller purse.” Male partner of AGYW, FGD [FGDMSP001].
In particular, FGD participants recommended the MAP be about 7 cm × 7 cm (about 2.75 inches square), similar in size to a nicotine patch, deworming medicine patch, or Elastoplast (bandage).
Most participants preferred a short period of wear time (≤10 min) because it would be reasonable, convenient for busy lifestyles, and allow discreet application. Adolescent girls preferred the shortest wear time. A few participants mentioned that they preferred longer wear times (30–60 min or even 24 h), primarily to ensure that the drug had been fully delivered.
“One minute. It should be something that works fast.” AG, FGD [IDIAG001].
“10 min is good for me. It is a reasonable time, anyone can get those ten minutes. Let's say you go to work in the morning and wake up at 7am, you can still be able to get the ten minutes to apply the patch. Ten minutes is okay.” YW, mock use [IDIYW003].
“I think those 10 min are okay, because even if you are at home, and you have bathed and put it on, by the time you finish getting ready, those 10 min will have passed. I think that time is okay those 10 min will not prevent you from doing your normal business.” FSW, FGD [FGDFSW001].
“If am applying it to a client it should take ten minutes. It should not take long since there might be other clients waiting.” Provider, mock use [IDIHSP002].
“Because you can set apart five to ten minutes knowing that you are applying some medication and then after ten minutes you go and do your chores.” MSM, mock use [IDIMSM001].
The majority of AGYW preferred at least a 1-month duration of protection because it offered greater convenience and flexibility within their sexual and reproductive life. All providers proposed that the duration of protection should last between 1 and 3 months. One provider explained that shorter periods would not cure the problem of non-compliance with PrEP, and concomitantly, longer periods would reduce the burden of regular client visits to health facilities.
“I would say so to reduce clients from coming back to the facility. My cry is actually for the clients since as a provider I will always be here. As you can see calling clients is sometimes hectic. They say they are not available on certain days.” Provider, mock use [IDIHSP002].
FSW stated their preference for longer periods of protection than AGYW. It appeared that longer periods were favored because the FSW had had experience with injectable contraceptives (3-month protection) and/or contraceptive implants that had much longer periods of protection.
“In fact, not even for 1 month; if I find for a whole year, I can be very happy.” FSW, FGD [FGDFSW001].
Providers and policymakers expressed preference for a longer-term duration of protection (3–6 months) to improve protection and simplify resupply.
However, MSM and FSW also said having even 1 week of protection would be better than oral daily pills for HIV protection.
After mock use, most participants preferred the MAP application site to be on the thigh (large surface, discreet) or the forearm or upper arm (ease of access, convenient). In FGD, the three most preferred sites were the upper arm, lower arm, or thigh. AG also mentioned the stomach as a third preference and YW mentioned under the breast and the rib cage as being appropriate application sites. Most providers preferred the inside upper arm, similar to placement for contraceptive implants (ease of access, does not invade client's privacy). Some mock use participants (AGYW) were confused about where to apply the MAP and wanted more guidance.
There was a general consensus among stakeholders that the MAP technology, whether HIV only or as an MPT, was a revolutionary innovation whose introduction would be very timely within the Kenya health care system. Overall, stakeholders preferred the MPT MAP over the HIV-only MAP. Stakeholders noted that the MPT MAP aligned well with the integrated health services policy currently in place in Kenya (31).
“I think the combined would work better because it will make the integration of service easier. That this is a PrEP and a family planning and then it is dealing with two birds with one stone. I like the integration part, because we are looking at integrating services and integrating HIV to other services. So, you are killing two birds with one stone. At least that it does so we don’t have to deal with issues of unwanted pregnancies and abortions and complications of unwanted pregnancies and everything so that's a plus for the women. As we know, we are dealing with two pandemics here, the pandemic of unintended pregnancies and the pandemic of HIV especially women between 15 and 24 years. So having a product that helps you to address those two things together, it is very beneficial. From our data we can see we are getting many new HIV infections in that age. Then we are currently focusing on service integration, integrating PrEP into SRH and SRH into PrEP. We are currently trying to put systems in place to actualize integration.” Female, national-level key informant interview [IDIKII002].
Additionally, stakeholders noted that an MPT MAP would save client time, reduce stigma associated with HIV prevention by combining with less stigmatized family planning services, reduce the pill burden associated with taking oral PrEP and of taking multiple drugs at different times and the service provider/facility burden of dispensing them, and be attractive to AGYW who are keen to prevent pregnancy at the expense of HIV protection. The stakeholders felt that an MPT MAP would be received with high enthusiasm/interest among AGYW, FSW, and providers. On the other hand, it was noted that the HIV PrEP MAP would be preferred by male users and female clients who had intolerance for hormonal contraceptives.
In general, stakeholders perceived the MAP technology favorably and identified product benefits as being its potential for discreetness, ease of application, long-term protection, and self-administration. For example, the simplified administration offered by the MAP would provide flexibility in terms of who can deliver the MAP and where it can be distributed/delivered, thus helping improve the overall efficiency of health services. Stakeholders mentioned that the MAP technology could likely improve PrEP uptake by redressing the major problems associated with oral PrEP.
“Uptake of PrEP in the country is still low. Let us be honest, oral PrEP uptake is still low and one key challenge is the small things the end users raised were not addressed. The issue of the rattling sound of the tablet, the tablet is so big, the color looks like an ARV, you know, those small things. So, I’m very excited about this new product.” Female, national-level key informant interview [IDIKII002].
Stakeholders agreed that the minimum duration of protection should be 3 months, to coincide with the recommended HIV retesting period and the schedule for injectable contraception. They also felt that MAPs should come in different durations of protection (3 months, 6 months, and 1 year) to address the needs of different users and that the package color should indicate duration.
Stakeholders identified multiple access points for a MAP product, including both facility-based and home-based self-care options.
“For me, I see this product having a broad spectrum of delivery points. I see it beyond the current delivery point like for the oral PrEP. I see it as a self-empowering product which should be delivered at the comfort of somebody's home or even privacy. Just like a HIV self-test kit, buy in the chemist, go with it at home, when I am free, test.” Female, national-level key informant interview [IDIKII002].
Several potential risks and/or unanswered questions were raised by stakeholders. These included the need for clarifying information about which drug(s) would be used in the MAP and the impact/safety; how to reverse the long-term MAP HIV prevention and contraceptive drugs in the body if they started having adverse effects on users; how the MAP feedback indicator would ensure that potential users have the optimal dosage of the prescribed drug(s); the sensation of using microneedles, including pain and side effects on skin; how to build user confidence that medication is actually delivered if no sensation; and the potential impact of repeated MAP applications (possible scarring). Stakeholders also raised the possibility of improper MAP use, both from the perspective of the technology and the intended user group (AG).
“So, there is a risk of misuse because maybe of a client not understanding that if the duration of the product is 30 days. You see there is nothing being left there (nothing left inside the skin like the depo); how do you convince me that just that one application has left enough drugs to protect me for 30 days, you might find some people repeating the administration and that is a risk.” Female, national-level key informant interview [IDIKII001].
“I foresee this spilling over even in schools because it is very easy to administer. Those in school may opt for such a product and, as I had said, the policy currently for the in-school is age-appropriate information and abstinence, but because it is easy to administer this product, it will be easily administered even in school. We will not have a way of monitoring…how will we monitor that? So, I do not know how we will restrict this product to ensure that it is only maybe those children who can consent…in our country it is 18 years; it is only accessible to those above 18 years.” Female, national-level key informant interview [IDIKII003].
A final concern, raised by policymakers, was the possible environmental impact of improper waste disposal.
“Yes, plastic is harmful for the environment and that is why am suggesting there should be instructions on how to dispose it. We can also explain to the client how to dispose; is it burning it, or?” Provider, mock use [IDIHSP002].
In our study, all user groups expressed willingness to use MAPs as a stand-alone HIV prevention technology. Users showed a strong preference for MAP technology over other currently available HIV prevention methods (oral PrEP, condoms). Women, providers, and stakeholders expressed strong interest in an MPT MAP—sometimes in preference to a stand-alone HIV PrEP MAP. Some potential users also expressed preference for the contraceptive MAP concept over existing contraceptives because it would be easy to use, discreet, and self-administered. The strong interest in the MAP technology was related primarily to its potential for discreet use and self-administration, ease of application, and long-term protection. MAP size and duration of protection were seen as key characteristics influencing acceptability. Some participants—especially MSM/FSW, who preferred long-term protection—would accept a larger MAP if it provided longer protection. Most AGYW preferred the MPT MAP over an HIV PrEP-only MAP and preferred 1-month protection. This finding in support of an MPT option is consistent with the results of the Tablets, Ring, Injections as Options (TRIO) study in which young women aged 18–30 in Kenya and South Africa perceived high value for an MPT (32, 33), and preferred a longer duration as well as discreet protection (34, 35). Similarly, results from a discrete choice experiment among women and adolescents in South Africa reported likely limited uptake and health impact among adolescent women unless the new PrEP products also provide pregnancy protection (33, 36). An assessment of the potential for MPTs in Nigeria, South Africa, and Uganda also found that 93% of women surveyed preferred an MPT product to either an HIV-only or contraceptive-only product (37). Because Kenyan AGYW seeking contraception frequently have high HIV risk (38), an MPT option could be particularly beneficial for this user group. Policymakers in this study also noted that the MPT MAP could address needs of AGYW who are keen on avoiding pregnancy but also are at risk of HIV.
FSW often have overlapping burdens: high risk of HIV, unmet need for contraception, and increased likelihood of contracting a sexually transmitted infection (39). FSW saw value in both MAP indications and voiced a need for MPTs that protect from other infections besides HIV. FSW wanted duration of protection consistent with injectable contraception. Other user groups, such as women living with HIV, have also identified a longer-acting injectable as preferred over daily oral tablets when a multipurpose technology concept offered an antiretroviral for HIV treatment co-administered with a hormonal contraceptive (40). However, this may reflect respondent bias in that injectable contraception is well accepted in Kenya, thereby making it a familiar benchmark technology.
Male partner support can be an important influence on AGYW and FSW who are interested in using HIV PrEP (41–44). In this study, male partners were supportive of MAP use, although they mentioned that partner use of an MPT MAP might give rise to suspicions around partner fidelity and serostatus. Male partners of young Kenyan and South African women in the TRIO study, while being supportive of MPT use generally, also expressed similar concerns about product use disclosure (45).
MSM viewed the HIV PrEP MAP as being a viable option, which is noteworthy given that MSM in Kenya show low adherence to a daily PrEP regimen (46, 47). A recent programmatic surveillance of PrEP program rollout in Kenya also showed substantial missed opportunities for PrEP initiation for MSM, as well as high levels of PrEP discontinuation at 1 month (48). In other studies (49, 50), MSM have noted their preferences for longer-acting PrEP options. Use of novel delivery platforms such as the MAP could be important for MSM, because PrEP discontinuation is not uncommon.
Providers and stakeholders preferred an MPT MAP and expressed that an MPT MAP could ease workload in health facilities and is aligned with Kenya's policy of integrating health care programs. The MPT MAP was identified by stakeholders as a revolutionary technology that has the potential to “kill two birds with one stone.” Enthusiasm for MPTs has been documented by health care providers in Kenya and South Africa where one South African nurse explained that provision of an MPT to YW could “kill two birds with one stone” (51). This finding strongly parallels our study findings, with one stakeholder using the exact same language to illustrate their point. Regardless of type of MAP (i.e., HIV PrEP, MPT as well as a contraceptive MAP), stakeholders felt that the technology could ease the burden of existing methods for users, providers, and the health care system.
In this study, Kenyan participants evaluated a second-generation prototype design that had been refined based on participant experiences with earlier prototypes in South Africa and Uganda (20). The MAP prototype used in the Kenya assessment was designed to be scaled for different numbers of arrays, depending on API potency. The second-generation prototype had an improved feedback indicator that was optimized for ease of use through (a) reduced number of handling tabs; (b) larger arrays, enabling fewer domes to press; (c) material chosen for optimized inversion force; (d) refined dome design to ensure no rebound; and (e) cutouts to increase flexibility (to accommodate different body locations).
The mock use exercise identified several areas in need of further product iteration. Most participants (70%) across all user groups had some difficulty understanding the IFU, in part due to low literacy and poor comprehension of images. Participants recommended simplifying the language and making the graphics more distinct to improve clarity. Importantly, users need a MAP orientation and demonstration before use. This is similar to the MAP assessment in Uganda, where participants who were oriented to the MAP solely by the IFU experienced more difficulty using the MAP during mock use. In contrast, mock use participants in South Africa were recruited from FGD where they saw a demonstration of the MAP and had an opportunity to familiarize themselves with the device, resulting in a more successful user experience. Even with the refinements made to the current MAP prototype and IFU to improve clarity and ease of use, the Kenya results indicate that the potential user will benefit from an orientation and product demonstration before their first use.
Although most participants (74%) were able to place the MAP on the skin correctly, potential users across all groups wanted more guidance on exact placement of the MAP on the skin. YW displayed the most difficulty in this regard. Mitigation for this would be to integrate more direct counsel on freedom of choice for the application site when orienting the potential user to the device. Alternatively, a specific site could be identified as the best possible location and potential users could be instructed to apply in that location.
Across all groups, some participants (34%) struggled with activating the MAP feedback indicator by, for example, not crushing all domes or not pressing firmly enough to completely crush the domes. Providers were able to complete this step successfully, yet they felt that it took too much effort to crush the domes. This design issue could be remedied by providing more explanation on how the MAP works during orientation and/or in the IFU. Investigating the use of more pliable dome material and how it might result in reliable insertion of the MAP projections would be another potential option to address this concern.
Most users (73%) had difficulty understanding wear time of the device because they found interpreting the clock on the IFU challenging. Providers were able to interpret the clock correctly; however, they noted that it may be difficult for their clients. Using digital time in the IFU may help overcome this issue.
Some participants (30%) had difficulty removing the MAP layer, which could be resolved by providing a pull tab. Most participants (79%) understood instructions for device disposal, and some felt that disposal could be a health hazard. Information on safe disposal will be added to the IFU.
This study indicates that additional refinements are needed to optimize the MAP prototype, including the feedback indicator. These refinements could improve confidence in appropriate delivery, especially for low-literate and AGYW user groups, particularly if they have not had specific counseling about the MAP before use. Similar to results from the South Africa and Uganda assessments (20), these results from Kenya show a strong interest in a MAP as a drug delivery platform and desire for an MPT MAP that is long acting.
The use of mixed methods in this study strengthens the robust nature of the findings. The study is limited by the relatively small geographic distribution of participants (only from Kiambu County); however, participants from varied settings (urban, peri-urban, and rural) were included. Preferences about a contraceptive MAP were inferred from responses about an MPT MAP. Specific questions about a contraceptive MAP were deleted from data collection tools because results from the instrument pretesting indicated that no additional insights would be collected with contraceptive-specific questions. Data were analyzed with a focus on user group rather than these varied settings, so some additional nuance and learning may still be uncovered. Because the usability testing was not with a microarray patch containing microneedles, some skin reactions (from wearing the patch), side effects (e.g., pain) at the wear site could not be assessed thus biasing potential user acceptability more favorably. Additionally, many of the MSM and some of the FSW appeared to have been intoxicated when they participated in the mock use exercise, potentially affecting their ability to perform adequately. On the other hand, their level of sobriety may be representative of their real-life situations and data collected would reflect that reality. Nonetheless, engaging users and stakeholders in early-stage product development gives an opportunity to refine MAP design to better meet user needs. Users/stakeholders want to be part of the process of developing new products and moving them forward, and this type of mixed methods assessment offers an ideal opportunity for this involvement.
Participants reported high potential acceptability of MAP as a drug delivery system for both for HIV PrEP and as an MPT. Health service providers and policymakers felt MAP could be integrated into the HIV and family planning health care systems. Some potential target audiences seemed to value a MPT MAP over an HIV PrEP-only MAP—specifically, AGYW, health service providers, and policymakers. Reducing the overall MAP size and number of arrays would likely improve acceptability, the feasibility of which is dependent on successfully formulating a higher-potency ARV to be delivered by MAP. Further prototype modifications, such as refining the feedback indicator to provide greater confidence of successful application and instructing users when to remove the MAP, are recommended to improve confidence and acceptability.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by AMREF Ethics and Scientific Review Committee (approval number: P770/2020) and Kiambu County Health Research Unit (reference number: KIAMBU/HRDU/22/03/08/RA_OTISO). The patients/participants provided their written informed consent to participate in this study.
SGG, RK, AN, MK-B, LO: contributed to the design and implementation of the study and analysis of the results. CJ: provided financial and technical oversight of the study. PSC: prepared the initial draft. All authors contributed to the article and approved the submitted version.
The MAPs for PrEP project was made possible by the generous support of the American people through the United States Agency for International Development (USAID) through the United States President's Emergency Plan for AIDS Relief (PEPFAR), under the terms of Cooperative Agreement #AID-OAA-A-17-00015. This study in Kenya was funded through a sub-award research grant from the Bill & Melinda Gates Foundation (ID: GAT.572767-01680386-SUB) and a USAID subaward through PATH (ID: AID.2211-01625549-SUB). The contents of this article are the responsibility of the authors and do not necessarily reflect the views of the Bill & Melinda Gates Foundation, USAID, PEPFAR, or the United States government.
The authors thank the girls, women, and men who shared their time and perspectives to explore MAP acceptability in Kenya. In addition, we would like to acknowledge the excellent effort undertaken by the LVCT Health research assistants, specifically Patricia Okoth, Cynthia Owino, Audrey Okoth, Millicent Kagure, and Rene Mulupi. A summary of this research was presented in an oral poster at the International Workshop on HIV & Adolescence 2022, held in Cape Town, South Africa, October 5–6, 2022.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2023.1125159/full#supplementary-material.
1. UNAIDS. Country factsheets: Kenya HIV and AIDS estimates (2021). Available at: https://www.unaids.org/en/regionscountries/countries/kenya (Accessed December 1, 2022).
2. Statista. Ranking of countries with the highest prevalence of HIV in 2000 and 2021 (2022). Available at: https://www.statista.com/statistics/270209/countries-with-the-highest-global-hiv-prevalence/ (Accessed December 1, 2022).
3. UNICEF. Kenya HIV and AIDS programme (2021). Available at: https://www.unicef.org/kenya/hiv-and-aids (Accessed December 1, 2022).
4. National AIDS and STI Control Programme (NASCOP). Preliminary KENPHIA 2018 report (2020). Available at: https://phia.icap.columbia.edu/wp-content/uploads/2020/04/KENPHIA-2018_Preliminary-Report_final-web.pdf (Accessed December 1, 2022).
5. PEPFAR. Kenya country operational plan (COP) 2017 strategic direction summary (2017). Available at: https://copsdata.amfar.org/SDS/2017/Kenya.pdf (Accessed December 1, 2022).
6. FP2030. Data: Kenya modern contraceptive prevalence rate for married women (2020). Available at: https://fp2030.org/kenya-old (Accessed December 1, 2022).
7. Kenya National Bureau of Statistics, ICF Macro. Kenya demographic and health survey 2014 (2015). Available at: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf (Accessed December 1, 2022).
8. African Population and Health Research Center. What it takes: Meeting unmet need for family planning in East Africa (2018). Available at: https://aphrc.org/publication/what-it-takes-meeting-unmet-need-for-family-planning-in-east-africa/ (Accessed December 1, 2022).
9. Republic of Kenya Ministry of Health. National family planning costed implementation plan 2017–2020 (2017). Available at: https://fp2030.org/sites/default/files/2017-2020_Kenya_Family_Planning_CIP_0%5B1%5D.pdf (Accessed December 1, 2022).
10. Mumah JN, Kabiru CW, Mukiira C, Brinton J, Mutua M, Izugbara C, et al. Unintended pregnancies in Kenya: A country profile (2014). Available at: https://aphrc.org/wp-content/uploads/2019/07/APHRC-country-step-up-report-Final.pdf (Accessed December 1, 2022).
11. Ziraba A, Orindi B, Muuo S, Floyd S, Birdthistle IJ, Mumah J, et al. Understanding HIV risks among adolescent girls and young women in informal settlements of Nairobi, Kenya: lessons for DREAMS. PLoS One. (2018) 13(5):e0197479. doi: 10.1371/journal.pone.0197479
12. Madise NJ, Ziraba AK, Inungu J, Khamadi SA, Ezeh A, Zulu EM, et al. Are slum dwellers at heightened risk of HIV infection than other urban residents? Evidence from population-based HIV prevalence surveys in Kenya. Health Place. (2012) 18:1144–52. doi: 10.1016/j.healthplace.2012.04.003
13. Republic of Kenya Ministry of Health. National AIDS control council. Kenya HIV estimates: report 2018 (2018). Available at: https://nacc.or.ke/wp-content/uploads/2018/11/HIV-estimates-report-Kenya-20182.pdf (Accessed December 1, 2022).
14. Heck CJ, Mathur S, Alwang’a H, Daniel OM, Obanda R, Owiti M, et al. Oral PrEP consultations among adolescent girls and young women in Kisumu county, Kenya: insights from the DREAMS program. AIDS Behav. (2022) 26(8):2516–30. doi: 10.1007/s10461-022-03590-z
15. Tapsoba JD, Cover J, Obong’o C, Brady M, Cressey TR, Mori K, et al. Continued attendance in a PrEP program despite low adherence and non-protective drug levels among adolescent girls and young women in Kenya: results from a prospective cohort study. PLoS Med. (2022) 19(9):e1004097. doi: 10.1371/journal.pmed.1004097
16. Sidebottom D, Ekström AM, Strömdahl S. A systematic review of adherence to oral pre-exposure prophylaxis for HIV–how can we improve uptake and adherence? BMC Infect Dis. (2018) 18(1):581. doi: 10.1186/s12879-018-3463-4
17. Eakle R, Weatherburn P, Bourne A. Understanding user perspectives of and preferences for oral PrEP for HIV prevention in the context of intervention scale-up: a synthesis of evidence from sub-Saharan Africa. J Int AIDS Soc. (2019) 22(Suppl 4):e25306. doi: 10.1002/jia2.25306
18. Sila J, Larsen AM, Kinuthia J, Owiti G, Abuna F, Kohler PK, et al. High awareness, yet low uptake, of pre-exposure prophylaxis among adolescent girls and young women within family planning clinics in Kenya. AIDS Patient Care STDS. (2020) 34(8):336–43. doi: 10.1089/apc.2020.0037
19. Rein-Weston A, Jarrahian C, Blair B, Moyo Y, Creelman B, Saxon E, et al. Acceptability of microarray patches for delivery of HIV Pre-Exposure Prophylaxis (PrEP) among women and health care providers in South Africa (2016). Available at: https://media.path.org/documents/VAC_hiv_prep_poster.pdf (Accessed December 1, 2022).
20. Kilbourne-Brook M, Ismail A, Magni S, Fellows T, Katahoire AR, Ayebare F, et al. User assessment of a microarray patch for HIV PrEP and as a multipurpose prevention technology for HIV and pregnancy prevention: perspectives from Uganda and South Africa (oral abstract of HIVR4P virtual, January 27–28 and February 3–4, 2021). Abstract OA04.01. Available at: https://onlinelibrary.wiley.com/doi/10.1002/jia2.25659 (Accessed December 1, 2022).
21. PATH. Microarray patches for drug delivery (2016). Available at: https://www.path.org/resources/microarray-patches-for-drug-delivery/ (Accessed December 1, 2022).
22. Vora LK, Moffatt K, Tekko IA, Paredes AJ, Volpe-Zanutto F, Mishra D, et al. Microneedle array systems for long-acting drug delivery. Eur J Pharm Biopharm. (2021) 159:44–76. doi: 10.1016/j.ejpb.2020.12.006
23. Jackson-Gibson M, Ezema AU, Orero W, Were I, Ohiomoba RO, Mbullo PO, et al. Facilitators and barriers to HIV Pre-Exposure Prophylaxis (PrEP) uptake through a community-based intervention strategy among adolescent girls and young women in seme sub-county, Kisumu, Kenya. BMC Public Health. (2021) 21(1):1284. doi: 10.1186/s12889-021-11335-1
24. Thomson K, Baeten J, Mugo N, Bekker L, Celum C, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. (2016) 11(1):18–26. doi: 10.1097/COH.0000000000000207
25. Republic of Kenya Ministry of Health, National AIDS Control Council. Kenya HIV county profiles (2016). Available at: https://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf (Accessed December 1, 2022).
26. McKenna GG, Emily N, Kevin M. How many focus groups are enough? Building an evidence base for nonprobability sample sizes. Field Methods. (2017) 29(1):3–22. doi: 10.1177/1525822X16639015
27. Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. (2003) 35(3):379–83. doi: 10.3758/bf03195514
28. Republic of Kenya Ministry of Health, National AIDS & STI Control Programme. HIV self-testing: an operational manual for the delivery of HIV self-testing services in Kenya (2017). Available at: https://livinggoods.org/wp-content/uploads/2019/06/HIV-Self-Testing-Manual.pdf (Accessed December 1, 2022).
29. Cabrera-Mieles Z, for Medium UX Collective. The rainbow sheet: a visual method for research analysis (2019). Available at: https://uxdesign.cc/the-rainbow-sheet-a-visual-method-for-research-analysis-a7e7d2011058 (Accessed December 1, 2022).
30. US Food and Drug Administration. Applying human factors and usability engineering to medical devices guidance for industry and food and drug administration staff (2016). Available at: https://www.fda.gov/media/80481/download (Accessed December 1, 2022).
31. Republic of Kenya Ministry of Health. Kenya health policy 2014–2030 (2014). Available at: https://www.ncikenya.or.ke/documents/kenya-health-policy.pdf (Accessed December 1, 2022).
32. Minnis AM, Browne EN, Boeri M, Agot K, van der Straten A, Ahmed K, et al. Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. (2019) 80(4):394–403. doi: 10.1097/QAI.0000000000001945
33. Vickerman P, Quaife M, Kilbourne-Brook M, Mvundura M, Eakle R, Terris-Prestholt F. HIV prevention is not all about HIV–using a discrete choice experiment among women to model how the uptake and effectiveness of HIV prevention products may also rely on pregnancy and STI protection. BMC Infect Dis. (2020) 20(1):704. doi: 10.1186/s12879-020-05399-4
34. Minnis AM, Krogstad E, Shapley-Quinn MK, Agot K, Ahmed K, Danielle Wagner L, et al. Giving voice to the end-user: input on multipurpose prevention technologies from the perspectives of young women in Kenya and South Africa. Sex Reprod Health Matters. (2021) 29(1):1927477. doi: 10.1080/26410397.2021.1927477
35. van der Straten A, Agot K, Ahmed K, Weinrib R, Browne EN, Manenzhe K, et al. TRIO study team. The Tablets, Ring, Injections as Options (TRIO) study: what young African women chose and used for future HIV and pregnancy prevention. J Int AIDS Soc. (2018) 21(3):e25094. doi: 10.1002/jia2.25094
36. Quaife M, Eakle R, Cabrera Escobar MA, Vickerman P, Kilbourne-Brook M, Mvundura M, et al. Divergent preferences for HIV prevention: a discrete choice experiment for multipurpose HIV prevention products in South Africa. Med Decis Making. (2018) 38(1):120–33. doi: 10.1177/0272989X17729376
37. Ipsos. Assessing the potential of MPTs in South Africa, Uganda and Nigeria: summary booklet (2014). Available at: http://www.theimpt.org/documents/BMGF-AssessingMPTSolutions-Booklet.pdf (Accessed December 1, 2022).
38. Nyaboe E, Larsen A, Sila J, Kinuthia J, Owiti G, Abuna F, et al. Contraceptive method mix and HIV risk behaviors among Kenyan adolescent girls and young women seeking family planning services: implications for integrating HIV prevention. Front Reprod Health. (2021) 3:667413. doi: 10.3389/frph.2021.667413
39. Bowring AL, Ampt FH, Schwartz S, Stoové MA, Luchters S, Baral S, et al. HIV pre-exposure prophylaxis for female sex workers: ensuring women's family planning needs are not left behind. J Int AIDS Soc. (2020) 23(2):e25442. doi: 10.1002/jia2.25442
40. Bernard C, Jakait B, Fadel WF, Mocello AR, Onono MA, Bukusi EA, et al. Preferences for multipurpose technology and non-oral methods of antiretroviral therapy among women living with HIV in western Kenya: a survey study. Front Glob Womens Health. (2022) 3:869623. doi: 10.3389/fgwh.2022.869623
41. Agot K, Hartmann M, Otticha S, Minnis A, Onyango J, Ochillo M, et al. “I didn’t support PrEP because I didn’t know what it was”: inadequate information undermines male partner support for young women’s pre-exposure prophylaxis use in western Kenya. Afr J AIDS Res. (2022) 21(3):221–30. doi: 10.2989/16085906.2022.2049831
42. Montgomery ET, van der Straten A, Stadler J, Hartmann M, Magazi B, Mathebula F, et al. Male partner influence on women’s HIV prevention trial participation and use of pre-exposure prophylaxis: the importance of “understanding”. AIDS Behav. (2015) 19(5):784–93. doi: 10.1007/s10461-014-0950-5
43. Roberts ST, Nair G, Baeten JM, Palanee-Philips T, Schwartz K, Reddy K, et al. Impact of male partner involvement on women’s adherence to the dapivirine vaginal ring during a phase III HIV prevention trial. AIDS Behav. (2020) 24(5):1432–42. doi: 10.1007/s10461-019-02707-1
44. Jani N, Mathur S, Kahabuka C, Makyao N, Pilgrim N. Relationship dynamics and anticipated stigma: key considerations for PrEP use among Tanzanian adolescent girls and young women and male partners. PLoS One. (2021) 16(2):e0246717. doi: 10.1371/journal.pone.0246717
45. Wagner LD, Minnis AM, Shea J, Agot K, Ahmed K, van der Straten A. Female and male partner perspectives on placebo multipurpose prevention technologies (MPTs) used by women in the TRIO study in South Africa and Kenya. PLoS One. (2022) 17(5):e0265303. doi: 10.1371/journal.pone.0265303
46. Wahome EW, Graham SM, Thiong’o AN, Mohamed K, Oduor T, Gichuru E, et al. PrEP uptake and adherence in relation to HIV-1 incidence among Kenyan men who have sex with men. EClinicalMedicine. (2020) 26:100541. doi: 10.1016/j.eclinm.2020.100541
47. Kimani M, van der Elst EM, Chirro O, Wahome E, Ibrahim F, Mukuria N, et al. “I wish to remain HIV negative”: pre-exposure prophylaxis adherence and persistence in transgender women and men who have sex with men in coastal Kenya. PLoS One. (2021) 16(1):e0244226. doi: 10.1371/journal.pone.0244226
48. Were D, Musau A, Mutegi J, Ongwen P, Manguro G, Kamau M, et al. Using a HIV prevention cascade for identifying missed opportunities in PrEP delivery in Kenya: results from a programmatic surveillance study. J Int AIDS Soc. (2020) 23(Suppl 3):e25537. doi: 10.1002/jia2.25537
49. Bailey RC, Kimani M, Kabuti R, Gumbe E, Otieno G, Kimani J, et al. URCHOICE: preferences for Pre-Exposure Prophylaxis (PrEP) options for HIV prevention among Kenyan men who have sex with men and transgender women in Nairobi, Kisumu and the coast. AIDS Behav. (2022) 27(1):25–36. doi: 10.1007/s10461-022-03741-2
50. Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV pre-exposure prophylaxis among potential user groups: a multinational study. PLoS One. (2012) 7(1):e28238. doi: 10.1371/journal.pone.0028238
Keywords: microarray patch, HIV PrEP, multipurpose prevention, contraception, Kenya, health product development, acceptability, microneedle patch
Citation: Gachigua SG, Karuga R, Ngunjiri A, Jarrahian C, Coffey PS, Kilbourne-Brook M and Otiso L (2023) Microarray patch for HIV prevention and as a multipurpose prevention technology to prevent HIV and unplanned pregnancy: an assessment of potential acceptability, usability, and programmatic fit in Kenya. Front. Reprod. Health 5:1125159. doi: 10.3389/frph.2023.1125159
Received: 16 December 2022; Accepted: 4 April 2023;
Published: 24 April 2023.
Edited by:
Andrea Ries Thurman, Eastern Virginia Medical School, United StatesReviewed by:
Ariane van der Straten, University of California, United States© 2023 Gachigua, Karuga, Ngunjiri, Jarrahian, Coffey, Kilbourne-Brook and Otiso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maggie Kilbourne-Brook bWtpbGJvdUBwYXRoLm9yZw==
Abbreviations AGYW, adolescent girls and young women; API, active pharmaceutical ingredient; ARV, antiretroviral; DREAMS, determined, resilient, empowered, AIDS-free, mentored and safe; FGD, focus group discussions; FSW, female sex workers; HIV, human immunodeficiency virus; IFU, instructions for use; MAP, microarray patch; MPT, multipurpose prevention technology; MSM, men who have sex with men; PrEP, pre-exposure prophylaxis.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.