
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Reprod. Health , 28 July 2022
Sec. Adolescent Reproductive Health and Well-being
Volume 4 - 2022 | https://doi.org/10.3389/frph.2022.887736
This article is part of the Research Topic Empowering Pregnant Adolescents: Addressing Health Behaviors and Socio-Cultural Determinants View all articles
 Harris Onywera1,2,3,4,5*
Harris Onywera1,2,3,4,5* Sikhumbuzo A. Mabunda6,7,8
Sikhumbuzo A. Mabunda6,7,8 Anna-Lise Williamson1,2,6
Anna-Lise Williamson1,2,6 Zizipho Z. A. Mbulawa2,6,9,10*
Zizipho Z. A. Mbulawa2,6,9,10*Background: Genital human papillomavirus (HPV) is the most common sexually transmitted virus in most populations globally. Adolescent girls and young women (AGYW) remain a key population group at risk for HPV infection. However, the risk factors of HPV infection among AGYW, especially in sub-Saharan Africa, are a subject of little investigation in published literature. Here, we investigated the factors associated with HPV infection among unvaccinated South African AGYW with a high HPV burden (prevalence: 76.1%).
Methods: We retrospectively recruited 213 AGYW learners (aged 15–25 years) from a previous cross-sectional study, the HPV Education Intervention Study, conducted in the Eastern Cape, South Africa. Sexually transmitted infections (STIs), bacterial pathobionts, genital ulcers (due to infectious causes), candidiasis, and bacterial vaginosis (BV) in the self-collected vaginal specimens were determined using the Allplex™ Panel Assays. Statistical analyses were performed using STATA v16.1. Continuous and categorical variables were computed by t-test /Wilcoxon rank-sum test and Chi-square/Fisher's exact tests, respectively. Logistic regression was used to determine the univariable predictors of HPV infection.
Results: The overall detection rate of any viral STI, bacterial STI, pathobiont, genital ulcer, candidiasis, and BV among the AGYW was 75.0, 34.4, 90.7, 14.4, 26.9, and 43.6%, respectively. The main factors associated with HPV infection were alcohol consumption (p = 0.005), infection with any and multiple Candida species (p = 0.011 and 0.006, respectively), Candida albicans infection (p = 0.010), Ureaplasma urealyticum pathobiont infection (p = 0.044), BV-associated bacteria (specifically Atopobium vaginae: p = 0.039, BV-associated bacteria 2: p = 0.021, Gardnerella vaginalis: p = 0.021, Megasphaera type 1: p = 0.037), and BV (p = 0.011).
Conclusions: Our study, albeit not necessarily generalizable, found social behavior as well as specific vaginal microbes as correlates of HPV infection among AGYW in South Africa. There is a need to investigate HPV epidemiology in other AGYW populations. The factors associated with genital HPV infection among AGYW burdened with HPV infection necessitate the need to formulate and implement population-specific public health strategies for creating HPV awareness and reducing its risk.
Cervical cancer (CC) is the second most common female cancer in women aged 15–44 years in the world, with its magnitude of difference in incidence by geography often striking (1). Sub-Saharan Africa (SSA) bears the highest burden of CC. Worldwide data has consistently reported that genital HPV infection is the most common sexually transmitted infection (STI) in most populations (1, 2), and that persistence of high-risk HPV (HR-HPV) infection causes anogenital cancers (2)—including CC (3). There is extensive overlap between geographical regions significantly burdened with CC and genital HPV infection. Epidemiological studies have found that HPV infection exhibits non-uniform geographical distribution, with SSA having the highest global prevalence (country prevalence ranges from 8.5 to 74.6% among women with normal cervical cytology) (1, 2, 4). Studies have noted a link between HPV infection and the age of women (1, 2, 4). These studies have observed that, whatever the geographical region and period of investigation, the early peak of HPV infection occurs at adolescent and young adult ages (<25 years). Thereafter, HPV prevalence declines with increasing age as observed globally (1, 2). However, in Africa, a second peak, though less pronounced, occurs in older women (≥45 years) (1, 2).
Globally, adolescent girls and young women (AGYW) remain a key sub-population most vulnerable to STIs, including HIV and HPV (4–13). Of note, a study that collated data from various sources in the U.S. found that 48.1% of the reported STIs occurred among youth (aged 15–24 years), of which at least half (50.5%) had incident HPV infections (14). A cross-sectional, multicentric, nationwide Brazilian survey found that 54.6 and 38.6% of sexually active unvaccinated AGYW had HPV and HR-HPV infections, respectively (8). Among indigenous Panamanian AGYW learners, HPV prevalence was found to be 33.2% (10). It has been noted that, among AGYW, the cumulative prevalence of HPV infection can exceed 80%, and that persistent HPV infection, to a lesser extent, is associated with cervical cytologic abnormalities (11). HPV infections among AGYW in SSA are significantly higher (4–6, 12), including in rural settings (12), than in the general populace of SSA and the rest of the globe (1, 2). South Africa still has scarce data on HPV infection among AGYW. The few published studies have reported that HPV and HR-HPV prevalence among AGYW can be as high as over 70 and 50% (4, 6, 12), respectively. These observations are confirmed and reinforced by the current high prevalence of HPV (76.1%) and HR-HPV (54.5%) AGYW from the Eastern Cape Province of South Africa (15). Published data on South African AGYW have also reported that multiple HPV infection varies by geographical area (5). In these HPV studies on South African cohort of AGYW, the determinants of HPV infection remain inconsistent and largely unexplored.
The determinants of HPV infection among women, including AGYW, appear to be very similar. The early peak in HPV prevalence often coincides with age at first sexual intercourse, which occurs during adolescent and young adult ages for most women. Both adolescent age and sexual debut in adolescence have been linked to prevalent HPV (16) and incident HR-HPV infection (17). Epidemiological studies have convincingly shown that HPV infection is rare before the first coitus (7, 13, 18, 19), and that HPV transmission in virgins is partly because of non-penetrative sexual contact (13) or douching habits (7). The rapid acquisition of HPV among AGYW after first coitus emphasizes the importance of vaccinating young girls and adolescents against HPV prior to early sexual debut. Other common risk factors (such as condomless vaginal sex, douching habits, and STIs) for HPV infection have been acknowledged (5, 12, 13, 16, 17, 19–21). Aside from these factors, vaginal microecology is gaining prominence on its role on HPV infection. In a meta-analysis, Candida albicans and bacterial vaginosis (BV, a vaginal syndrome-induced by an overgrowth of anaerobic bacteria) were identified as protective and risk factors for HPV infection, respectively (21). BV-associated bacteria (e.g., Gardnerella vaginalis) and vaginal microflora with paucity of keystone Lactobacillus spp. (e.g., Lactobacillus cripatus) are considered less protective against HPV infection (22–24). However, studies of the association of HPV (including HR-HPV) infection with vaginal microecology, specifically Candida spp. (20, 25) and Lactobacillus spp. (22, 23, 26), have yielded inconsistent and conflicting results. Hitherto, there are conflicting reports over the relationship of the relative abundance or dominance of L. iners with HPV infection; non-significant (24), positive (22, 27), and inverse associations (26) have all been reported. This could be ascribed to inter-study heterogeneity and clonal lineages of L. iners. Vaginal microecology and HPV have been associated with cervical intraepithelial neoplasia (21). Thus, more studies are needed to evaluate and understand the relationship between vaginal microecology and HPV infection, if we are to identify modifiable risk factors for HPV and CC prevention.
In Southern Africa, CC remains the most common cancer affecting women aged 15 to 44 years (1). Prophylactic HPV vaccines have been licensed in several countries as a primary preventive strategy for cervical HPV disease. Currently, two HPV vaccines are licensed in South Africa: (i) Cervarix®–a bivalent vaccine targeting two HR-HPV types (HPV16 and HPV18, responsible for majority of cancer, including CC), and (ii) Gardasil®4—a quadrivalent vaccine targeting HPV16, HPV18, and two low-risk HPV types (HPV6 and HPV11, the most common causative agents of genital warts) (28). Gardasil®4, administered in three doses (0, 1–2, 6 month schedule), was successfully used in an HPV vaccination demonstration programme in South Africa targeting girls aged 9–12 years (29). Nevertheless, in April 2014, South Africa's national Department of Health introduced a school-based HPV vaccination programme; with the administration of a two-dose regimen (6 months apart schedule) of the Cervarix®, primarily targeting girls aged ≥9 years in their fourth year of elementary (primary) school (Grade 4, equivalent to 4th Grade in the U.S. and Year 5 in the UK) (30). To realize the greatest impact of the HPV vaccination programme, the World Health Organization (WHO) recommends that the vaccines be administered to young girls (9–13 years) prior to sexual debut, hence before exposure to HPV infection (28). Most AGYW in South Africa do not have access to the HPV vaccine programme since only girls in Grade 4 in some public schools are prioritized (31). For AGYW aged 15–25 years who may not benefit from catch-up vaccination programmes, an understanding of the modifiable risk factors may help to reduce the duration of HPV infection. To address this, information on HPV epidemiology among AGYW need to be collected.
We are currently aware of the high HPV prevalence, including the genotypes targeted by Gardasil®9 (a non-avalent HPV vaccine) among unvaccinated AGYW in rural Eastern Cape (15), yet HPV risk factors among AGYW remain unknown. Thus, the aim of the present study was to determine the factors associated with genital HPV infection among AGYW in the Eastern Cape. This study may help formulate and implement evidence-based policies designed to create and intensify awareness of risk factors for HPV infection among AGYW in SSA.
This was a retrospective cross-sectional descriptive study among female learners who had participated in a parent study known as the HPV Education Intervention Study (32) that was conducted between April and May 2019. All the study participants were from two high schools in Chris Hani District Municipality, Eastern Cape Province (South Africa). The participating high schools were randomly selected, and they belong to quintile one (no-fee paying schools) South African Department of Education quintile ranking. Participants were invited to and recruited from the nearest primary care facilities. Upon being conversant with the study, participants filled out a detailed questionnaire, mostly with closed-format questions about their demographics, sexual behavior, alcohol consumption, and smoking habits. All information collected from the study participants was anonymised and de-identified prior to analysis. Participants did not include their identities on the questionnaires. To be eligible for participation in the present study, the female had to be aged ≥15 and ≤25 years, and sexually experienced. Any AGYW who was menstruating or pregnant at the time of vaginal specimen collection on the day of the visit was excluded from the study. In the assessment of the factors associated with HPV infection, only AGYW with valid HPV results as performed by the HPV genotyping assay (described in a later subsection) were included.
Details regarding specimen collection used for evaluation of STIs, vaginal bacterial pathobionts, genital ulcers, and BV are published elsewhere (15). A pathobiont is a potentially pathological organism which, under normal microenvironment lives as a non-harming symbiont but causes pathogenesis under an altered ecosystem condition. In brief, with regard to sample collection, a health professional trained the AGYW on the self-sampling technique using the Evalyn® Brush (Rovers® Medical Devices B.V., Oss, Netherlands) as shown in the information leaflet. Specimen was collected by inserting the white brush as far as possible into the vagina after assuming a comfortable stance (either while standing or squatting), and then rotating the plunger 360° five times. The brush was carefully removed from the vagina and capped back after being pulled by the pink plunger into the transparent casing. Brushes with self-collected vaginal specimens were stored at room temperature and shipped to the HPV Laboratory at the University of Cape Town, South Africa for laboratory processing.
HIV counseling and testing was performed at the primary care facilities by either qualified clinic staff or HIV lay counselor. HIV test was done using a rapid antibody screening test using blood from a finger prick. All newly diagnosed HIV-infected AGYW were followed-up according to the Department of Health protocol for management of HIV/AIDS in adults.
Nucleic acid from each specimen was isolated as detailed elsewhere (15) using MagNA Pure Compact (Roche Molecular Systems, Inc., Branchburg, NJ, USA) and MagNA Pure Compact Nucleic Acid Isolation Kit (Roche Molecular Systems, Inc., Branchburg, NJ, USA), according to the manufacturer's instructions.
HPV DNA testing in the specimens was performed using the Roche Linear Array HPV Genotyping Assay (Roche Molecular Systems, Inc., Branchburg, NJ, USA) according to the manufacturers' instructions. The primers for this assay are designed to amplify a 450-bp fragment of the L1 region of 13 high-risk and 24 low-risk HPV genotypes [as stated elsewhere (15)] as well as a 268-bp fragment of the β-globin gene. The β-globin gene was amplified concurrently to monitor and assess cellular adequacy, extraction, and amplification for each processed specimen.
To detect STIs, bacterial pathobionts, genital ulcers, candidiasis, and BV in the vaginal swabs, we used a multiplex real-time PCR assay, the Allplex™ Panel Assays (Seegene Inc., Seoul, Korea), according to the manufacturers' instructions. The Allplex™ STI Essential Assay (Seegene Inc., Seoul, Korea) detects and identifies the following two bacterial STIs (Chlamydia trachomatis and Neisseria gonorrhoeae), four pathobionts (Mycoplasma genitalium, Mycoplasma hominis, Ureaplasma parvum, and Ureaplasma urealyticum) and one parasitic pathogen (Trichomonas vaginalis). The pathobionts that we assessed are believed to be emerging sexually transmitted pathogens (33, 34).
Genital ulcers due to infectious causes were diagnosed using the Allplex™ Genital Ulcer Assay (Seegene Inc., Seoul, Korea) whose panel consists of the following pathogens: herpes simplex virus type 1 (HSV-1), HSV-2, cytomegalovirus (CMV), varicella-zoster virus (VZV), C. trachomatis serovar L. (causes lymphogranuloma venereum, LGV), Treponema pallidum (causes syphilis), and Haemophilus ducreyi (causes chancroid).
The Allplex™ Candidiasis Assay (Seegene Inc., Seoul, Korea) panel consisted of seven Candida spp. These included Candida albicans, Candida dubliniensis, Candida glabrata, Candida krusei, Candida lusitaniae, Candida parapsilosis, and Candida tropicalis.
The panel for BV diagnosis in the Allplex™ Bacterial Vaginosis Assay (Seegene Inc., Seoul, Korea) is designed to (i) quantitatively detect Lactobacillus spp. (L. crispatus, Lactobacillus gasseri, and Lactobacillus jensenii), G. vaginalis, and Atopobium vaginae, and (ii) qualitatively detect Megasphaera type 1, Bacteroides fragilis, BV-associated bacteria 2 (BVAB2), and Mobiluncus spp. (Mobiluncus mulieris and Mobiluncus curtisii).
In each of the above-mentioned four-panel assays, an internal control (an endogenous human gene) was coamplified simultaneously with the target DNA sequence to monitor sample adequacy, nucleic acid extraction, and check for any possible PCR inhibition. RNase-free water and a mixture of pathogen clones were used as negative and positive controls, respectively. Each 20.0 μl reaction consisted of 15.0 μl PCR mastermix and 5.0 μl of template or controls (either negative or positive control). The Allplex™ Panel Assays were all run on a real-time PCR instrument and results were examined using CFX96™ Real-time PCR Detection System (Bio-Rad)—CFX Manager™ Software-IVD v1.6 and CFX96™ Dx System (Bio-Rad)—CFX Manager™ Dx Software v3.1. Seegene Viewer Software (Seegene Inc., Seoul, Korea) was used to interpret data according to the manufacturer's instructions.
Data were captured in Microsoft Excel 2016 (Microsoft Corporation, Seattle, USA) and exported to STATA v16.1 (Stata Corp LP, College Station, Texas, USA) for analysis. Numerical data were explored using the Shapiro Wilk test for normality. Numerical variables that were normally distributed are summarized using the minimum, maximum, mean, and standard deviation. Conversely, numerical variables that were skewed were summarized using the median and interquatile range (IQR). The two-sample t-test with equal variances was used to compare the mean age of participants by HPV status, whereas the Wilcoxon rank-sum test was used to compare all other medians of numerical variables by HPV status.
Percentages and frequencies were used to summarize categorical variables. The Chi-squared and Fisher's exact tests were used to compare two categorical variables. For the chi-squared test to be used, the expected frequencies needed to be ≥5, unless if the number of rows being compared exceeded 2 rows, wherein no more than 20% of the rows could have an expected frequency of <5 and no cell in the table could have an expected frequency of <1. Where these conditions were not met, the Fisher's exact test was used.
Logistic regression was used to determine the univariable predictors of HPV infection as well as history of vaginal discharge or itching, and genital ulcers, blisters, or warts. The odds ratio (OR) is the measure of association used and the 95% confidence interval is used to show the precision of estimates. A p-value of < 0.05 is used as the level of significance.
The baseline characteristics of the 220 AGYW that were finally enrolled in this study are summarized in Table 1 using median (IQR) and proportion (%). A majority of the AGYW were aged 17–20 years (78.7%). Only one young woman (0.5%) was aged 25 years. and were in at least Grade 10 (equivalent to 10th Grade or Sophomore Year in the U.S. and Year 11 in the UK: 85.8%). The median age of sexual debut and frequency of vaginal sex in the past 1 month were 16 years and 2, respectively. Approximately 67.8% of the AGYW were currently on contraception, with condom being the most frequently used contraception (37.3%) by the participant and/or sexual partner. About three-quarters (73.1%) of the AGYW had circumcised male sexual partners. Approximately two-thirds (64.5%) and one-fifth (20.6%) of the AGYW had ever experienced vaginal discharge (or itching) and had genital ulceration (including blisters and warts), respectively.
The prevalences of STIs, pathobionts, genital ulcers, candidiasis, and BV among the AGYW are summarized in Table 2. The proportion of any viral STI was high (75.0%). The proportions of these STIs were as follows: HIV (5.3%), HPV (76.1), HSV-1 (0.5%), and HSV-2 (6.0%). The overall prevalence of genital ulcers due to infectious causes (including HSV-1 and HSV-2) was 14.4%, with only one woman (0.5%) having genital ulcers due to multiple infectious causes (CMV and HSV-2 coinfection). CMV was the most common cause of genital ulcers (8.4%). None of the women had genital ulcers associated with VZV, LGV, syphilis, and/or chancroid. Only 26.9% of the AGYW had any detectable fungi (C. albicans: 23.6%, C. glabrata: 4.8%, and C. lusitaniae: 1.4%). Multiple fungal infections were at least 8 times more prevalent than single fungal infections (89.3 vs. 10.7%).
The overall detection rate of any bacterial STI among the AGYW was 34.4% (C. trachomatis: 29.8% and N. gonorrhoeae: 12.1%). Single bacterial STI (infection with either C. trachomatis or N. gonorrhoeae) was more common compared to coinfections (78.4 vs. 21.6%). Most of the AGYW had any pathobiont (overall prevalence: 90.7%—U. parvum: 66.8%, M. hominis: 55.8%, U. urealyticum: 43.7%, and M. genitalium: 6.5%), with 66.2% of them having multiple infections. Figure 1 shows the distributions of any detectable pathobiont according to multiplicity of infection (whether single or multiple infections) among AGYW.
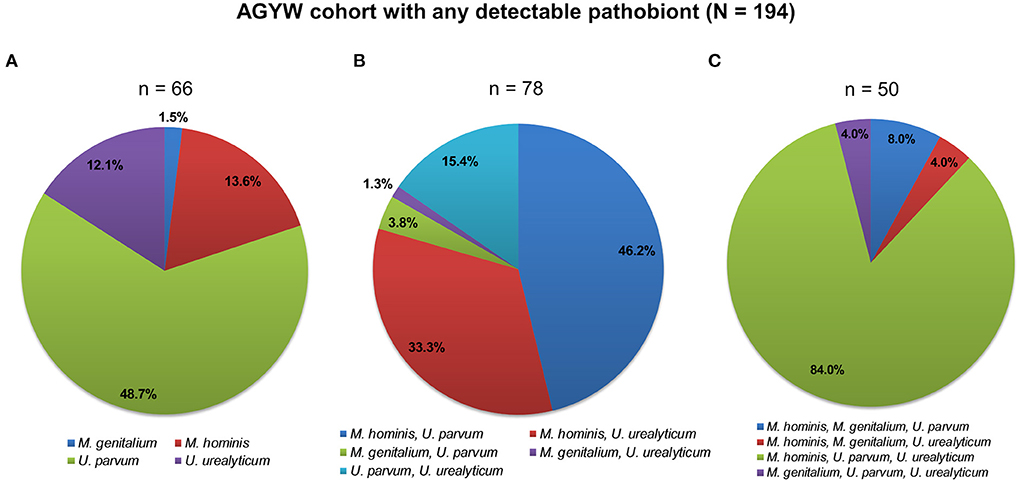
Figure 1. Patterns of bacterial pathobiont infections among 194 AGYW. AGYW with (A) single infections, (B) dual infections, and (C) triple infections. The number of AGYW in each group is in parentheses. In total, 195 AGYW had any detectable pathobiont (M. genitalium, M. hominis, U. parvum, or U. urealyticum). One participant, not included in any of the two-dimensional pie charts showing the patterns of infections, had all the four examined pathobionts detected.
Among the single pathobiont infections, U. parvum was the most detected (48.7%). Coinfection with M. hominis and U. parvum was most frequent among dual infections. Simultaneous infection with M. hominis, U. parvum, and U. urealyticum was the commonest infection with three pathobionts. Only one participant had infections with all the pathobionts. Analogous patterns of infections were observed according to the HPV status of the AGYW (Supplementary Figure 1).
Only 9.2% of the AGYW had protozoal STI (T. vaginalis). Lastly, 44.0% of the AGYW had normal vaginal microflora. Of these, 67.7% had vaginal microflora with appreciable numbers of Lactobacillus spp. Among AGYW with abnormal microflora, 43.6 and 12.4% had BV and intermediate microflora, respectively.
Of the 220 AGYW, only 213 (96.7%) had valid HPV results as performed by the HPV genotyping assay. A statistical comparison of the baseline characteristics of the 213 AGYW with and without HPV infection is summarized in Table 3. There were statistically significant differences with regard to alcohol consumption (p = 0.004), number of lifetime sexual partners (p = 0.049), frequency of vaginal sex in the past 1 month (p = 0.005), and detectability of the following: any Candida spp. (p = 0.007), C. albicans (p = 0.007), multiplicity of Candida infection (p = 0.013), any bacterial STI/pathobiont (p = 0.007), any pathobiont (p = 0.004; specifically U. urealyticum: p = 0.042), BV-associated bacteria (specifically A. vaginae: p = 0.037, BVAB2: p = 0.019, G. vaginalis: p = 0.020, Megasphaera type 1: p = 0.034), and BV (p = 0.032).
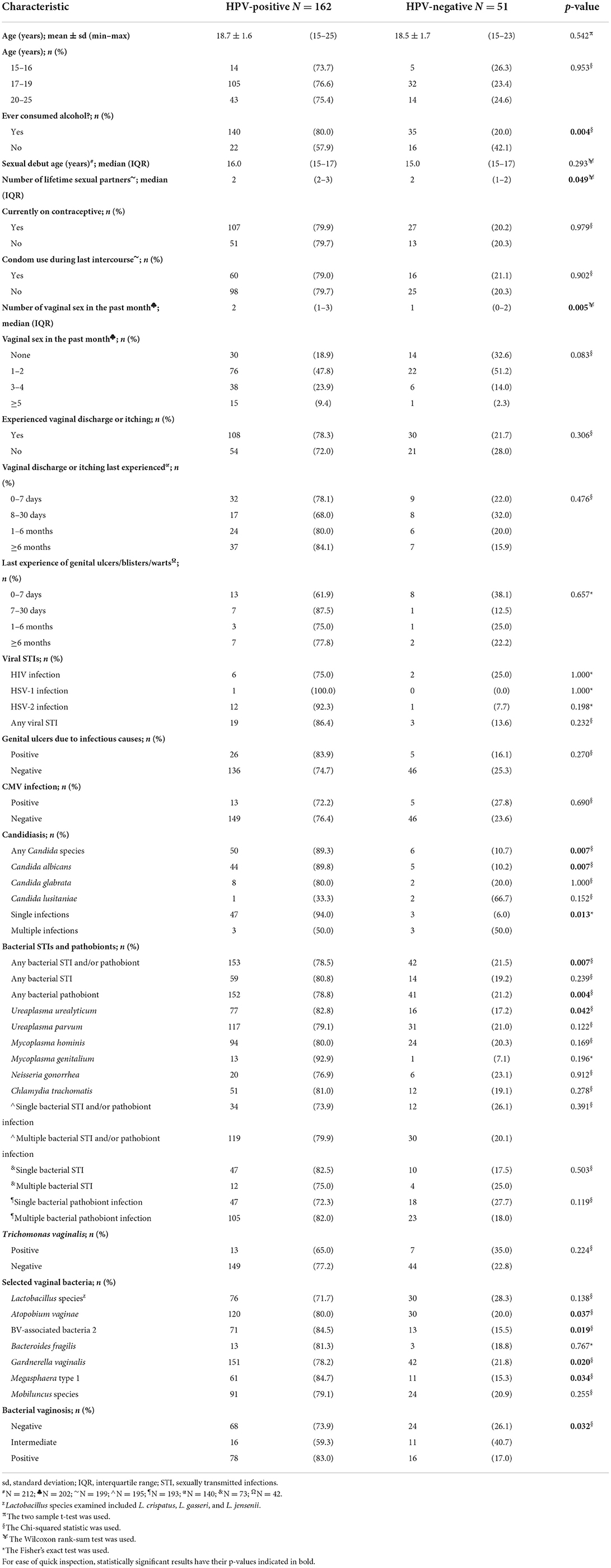
Table 3. Comparison of baseline characteristics between HPV-negative and HPV-positive AGYW in South Africa.
Overall, HPV-positive AGYW had significantly higher prevalence or frequency of the aforesaid variables compared to HPV-negative AGYW. None of the 213 AGYW with and without HPV infection were coinfected with HIV and HSV-1/2.
Next, we used logistic regression to determine the factors associated with HPV infection, vaginal discharge or itching, and genital ulcers, blisters, or warts. Table 4 shows that the main factors that were associated with HPV infection comprised alcohol consumption (p = 0.005), infection with any Candida spp. (p = 0.011), C. albicans infection (p = 0.010), multiple Candida infection (p = 0.006), infection with any bacterial STI/pathobiont (p = 0.010), detectability of any pathobiont (p = 0.004—mostly U. urealyticum: p = 0.044), BV-associated bacteria (specifically A. vaginae: p = 0.039, BVAB2: p = 0.021, G. vaginalis: p = 0.021, Megasphaera type 1: p = 0.037), and BV (p = 0.011). It is worthwhile to mention that, although not statistically significant, alcohol consumption (p = 0.057), consumption of different alcoholic drinks (p = 0.0712), and vaginal sex in the past month (p = 0.057) were more common in HPV-positive AGYW than HPV-negative AGYW.
Finally, we investigated whether bacterial STI, candidiasis, BV and genital ulcer panel positivity were associated with vaginal discharge or itching, and genital ulcers, blisters, or warts. These results are tabulated in Table 5. Self-reported history of vaginal discharge or itching was significantly associated with alcohol consumption (p = 0.005), infection with any Candida spp. (p = 0.031), C. albicans infection (p = 0.014), and A. vaginae (p = 0.006). None of the analyzed variables was a predictor of self-reported genital ulcers, blisters, or warts.
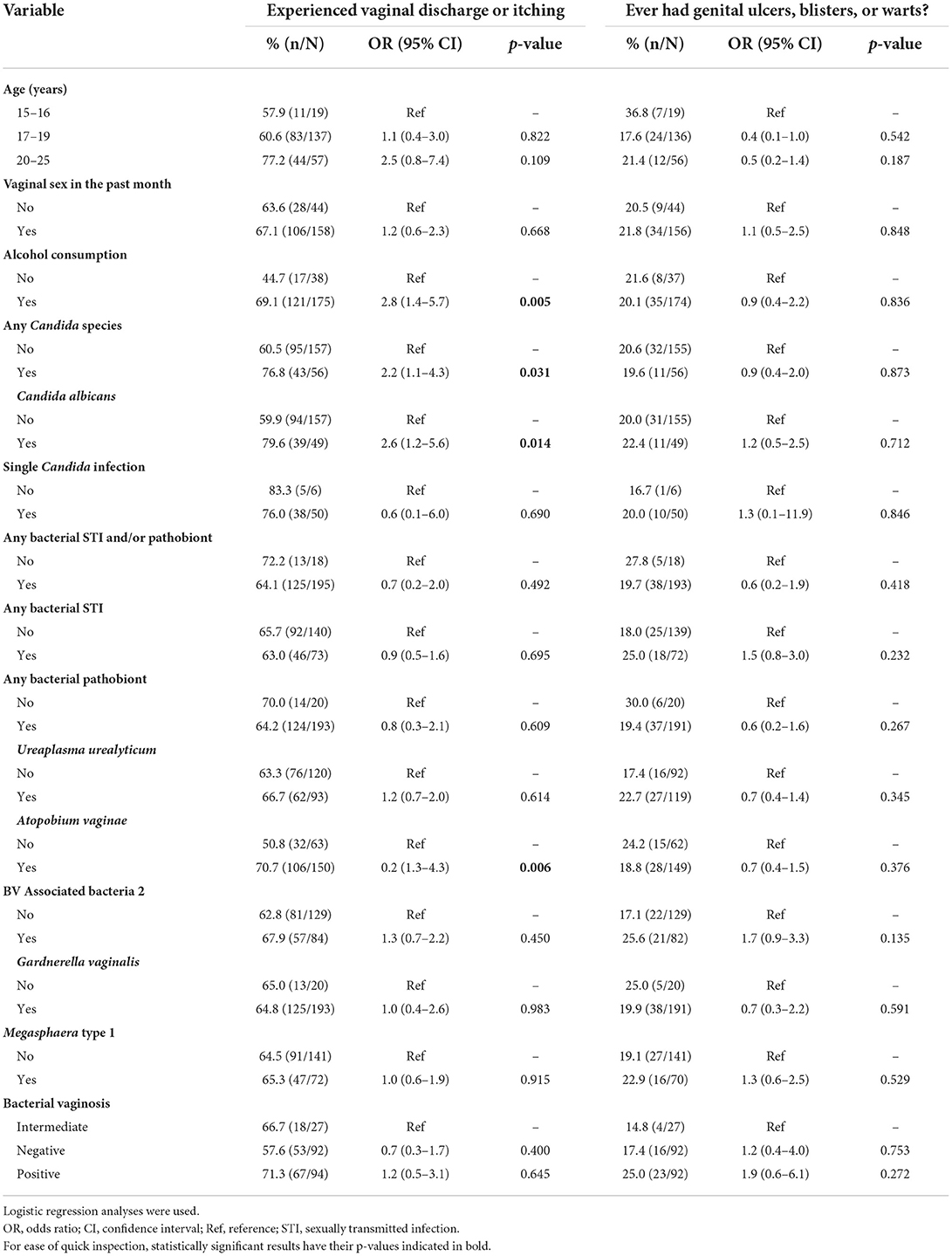
Table 5. Factors associated with self-reported vaginal discharge or itching, and genital ulcers, blisters, or warts.
STIs, including HPV infection, in AGYW is a pressing global health problem (6, 9, 10); resulting in significantly reduced quality of life of infected individuals. In South Africa, there is little to no available regional and nationwide data on the factors associated with HPV infection among AGYW. Therefore, in the present cross-sectional study, we assessed sociodemographic, behavioral, clinical, and microbiological factors associated with the highly prevalent (76%) HPV infection among AGYW in the rural Eastern Cape.
We identified a wide variety of STIs and pathobionts. The prevalence of any bacterial STI (34%), N. gonorrhea (7%), and C. trachomatis (30%) were intermediate to those reported among South African AGYW from Cape Town (60, 14, and 43%, respectively) and Johannesburg (19, 5, and 17%, respectively) (35). Our data add to the consensus that, after HPV infection, C. trachomatis is the second most prevalent STI among AGYW in South Africa (5, 6, 9, 35) and other regions (10, 14). Syphilis, chancroid, and LGV, were not detected whereas T. vaginalis, M. genitalium, HIV, HSV-1, HSV-2, and candidiasis were detected at low rates (1–10%), comparable to AGYW cohorts from different regions in South Africa (5, 9, 35). Overall, the distribution pattern of viral and bacterial STIs and pathobionts among the AGYW resembles that previously reported among Eastern Cape women aged ≥30 years attending community-based clinics or CC screenings (36). However, the prevalences of these microbes, including infections, are higher among AGYW compared to the aforesaid Eastern Cape women (37); thus, reflecting high-risk sexual behavior among AGYW. We also affirmed that BV prevalence (44%) is high among South African AGYW, and regionally ranges from 36 to 51% (35). We find the presence of a wide range of STIs among the HPV-burdened unvaccinated AGYW learners worrying, as it may compromise HPV prevention and control. Studies have already found a positive relationship of HPV with other STIs (20, 21, 36). A small cohort study on U.S. adolescents noted that HPV infections were acquired earlier than other STIs (18). This probably corresponds to the early peak of HPV infection. If indeed HPV infections among AGYW precede most of the other STIs, then it makes sense to ensure that, as many young girls as possible are vaccinated against HPV and educated about human reproductive health prior to their sexual debuts. Perhaps these may substantially reduce the burden of HPV and other STIs.
Owing to the high prevalence of HPV infection in our study cohort (15), current and projected (2030) high caseload of CC in Southern Africa (1, 2), variations of HPV risk factors by community (geography) (5, 8, 12, 16), and recent reporting of HPV-related cervical cytologic abnormalities among women aged ≥18 years in rural Eastern Cape (38), we sought to evaluate the determinants of HPV infection among these AGYW. In univariable analysis, alcohol consumption, candidiasis, U. urealyticum, BV and specific BV-associated bacteria were strong predictors of HPV infection. We also found that besides alcohol consumption, vaginal discharge or itching was mainly associated with specific vaginal microbes, all of which were associated with HPV infection. A previous study on Swedish cohort aged 17–50 years noted that vaginal discharge and itching were more common in women with vulvovaginal HPV infection (39). However, our study found no direct association between vaginal HPV infection and vaginal discharge or itching. Abnormal vaginal discharge and itching are probably because of vaginal infections or syndromes, including BV, an independent predictor of HPV.
Alcohol consumption is a common behavior associated with HPV infection (16). It may lead to irrational choices, including high-risk sexual activities (e.g., engaging in condomless vaginal sex) that increase the risk for STIs (40). A survey that estimated alcohol-related health problems among U.S. college students (aged 18–24 years) had 8.4% of the respondents attributing the cause of engaging condomless vaginal sex to alcohol consumption (41). However, in our study, condom use was not a determinant of HPV infection, yet there was a trend toward association of both the frequency of alcohol consumption and consumption of different alcoholic drinks in the past month with HPV infection. This could have been influenced by biased self-reporting of condom use, particularly on events that occurred under the influence of alcohol. We know that alcohol consumption may cause memory impairment and blackouts (42). In congruent with other public health experts (43, 44), our findings suggest that didactic public health campaigns advocating for the reduction of high-risk sexual behavior among AGYW are warranted to curtail HPV infection. School-based interventions with risk-modification counseling can control HPV infection, for example, by improving HPV knowledge and awareness (32), intention to use condoms, and uptake of HPV vaccine among adolescents (45). Currently, there is noticeable poor knowledge of HPV, its associated risk factors, and prevention among AGYW (43, 44), including in our study population (32) and other parts of SSA (31).
There is still a gap between the relationship between vaginal microecology (fungi and bacteria in our context) and HPV infection. There have been mixed findings with regards to the association of candidiasis with HPV and HR-HPV infections (20, 21, 25). We found that, in contrast to a previous report (21), C. albicans significantly predicted HPV infection. The observed differences probably reflect methodological and true population differences, which include variations in host epithelial cells (46) and failure to resolve dimorphic switches of C. albicans (47). Under normal scenario, Candida spp. are human commensal microflora that live in harmony with normal microflora. We think that their ability to form biofilm matrix partially account for their inverse association with HPV infection. This is because the dispersed form of C. albicans is more virulent than mature C. albicans biofilms (47). C. albicans positive association with HPV is, perhaps contributed by adhesion and cellular damage to mucosal epithelia (46, 47). A breeched epithelial barrier may then act as a gateway for HPV and replication in the basal layer (48).
The associations of specific BV-associated bacteria, BV, and pathobionts with HPV infection are in common with other reports (21, 23). Serving as an example, Mbulawa et al. (5) reported an association between BV and HPV among AGYW from Cape Town and Johannesburg in South Africa. These findings are not surprising as a majority of women from SSA, including AGYW from South Africa, have cervicovaginal microflora devoid of appreciable quantities of the protective lactobacilli (35, 49). Furthermore, vaginal microflora with higher abundances of keystone lactobacilli are thought to be protective against HPV (HR-HPV) infection whereas those with BV-associated bacteria (including A. vaginae) or BV are not (10, 22). In the only study to date of cervicovaginal microflora and HPV infection in South Africa, higher relative abundances of A. vaginae and G. vaginalis [believed to initiate BV development (50)] were correlated with HPV infection (including HR-HPV) (49). A. vaginae and sialidase gene from G. vaginalis have been associated with persistent HPV infection (27). The mechanisms that BV-associated bacteria use to contribute to HPV infection partly parallels that of C. albicans. An in vitro model found that besides BV-associated G. vaginalis manifesting propensity to displace protective lactobacilli from HeLa epithelial cells, caused cytopathogenic changes on these epithelial cells (50). Cervicovaginal microflora with G. vaginalis dominance has been associated with host epithelial barrier disruption and impaired wound healing (51). As earlier explained, HPV infection of the basal layer requires disruption of epithelial barriers.
With regards to pathobionts, U. urealyticum, has been associated with both increased HPV infection (21) and young women age (≤25 years) (52). It is possible that U. urealyticum is also co-acquired during the early peak of HPV infection. Also, this assumption is reinforced by the finding that U. urealyticum is more frequent in women with BV than healthy women (53) and the possibility of heterosexual exchange of BV-associated bacteria (54). Ureaplasma/Mycoplasma infections may be risk factors for persistent HR-HPV infection and could be associated with the development of precancerous cervical lesions (55). U. urealyticum could be causing pathogenicity by synergistic effects with other pathobionts and BV associated bacteria. This theory stems from the association of U. urealyticum/U. parvum coinfection with incident C. trachomatis (52) and the perspective that U. urealyticum might have better survival in symbiotic relationships with BV-associated bacteria (53). In-depth studies are required to delineate the role of U. urealyticum and other pathobionts in HPV infection. Since these pathogens are ubiquitously present in the urogenital tract in both healthy and diseased individuals (52, 53), their quantitative and not only qualitative molecular detection should be considered in order to improve their predictive values (56). Limited knowledge of the relationship between vaginal microecology and HPV infection remains a key “Achilles heel” of HPV control programmes. Significant associations of specific vaginal microecology with HPV infection underline the value to screen for and manage aberrant vaginal microflora (i.e., those with bacteria indicative of BV) within an integrated model for HPV control among AGYW.
The major strengths of our study are the rich participant information and the utility of a culture-independent approach for the detection and/or quantification of vital organisms in the vaginal microflora. Molecular-based methods perform better than the culture method, which, for instance, cannot discriminate between U. urealyticum and U. parvum. Consequently, such culture results are inaccurately reported as U. urealyticum instead of ureaplasmas (56). Our findings should be considered in light of the following study limitations. In addition to its cross-sectional nature, our study included only AGYW from two schools (recruited from convenient primary care facilities) in the rural Eastern Cape, therefore may have limited its casual inferences and generalizability. Since the vaginal specimens were self-collected by inserting the brush as far as possible into the vagina after assuming a comfortable position, inter-individual differences (inconsistent sampling) in anatomical site sampled cannot be ruled out. This may have affected our results since the vaginal milieu comprises different anatomical sites, which are composed of varying microbial community composition (57). Another study limitation is the inability of the multiplex real-time PCR assay to distinguish between bacterial colonization and pathogenic infection when examining the pathobionts. We do not rule out the possibility of bias since we relied on self-reported questionnaire. Finally, we did not adjust for possible confounders for HPV infection, including sexual networks. Although not investigated, the observed HPV prevalence and its factors associated with HPV infection could somewhat be mediated by (i) poor HPV vaccine acceptance and coverage (30, 31), and (ii) poor community knowledge of HPV infection, prevention, and its consequences (31, 32, 43, 44). Notwithstanding the study limitations, our study leads us to believe that HPV education should be urgently integrated in the high school educational curriculum to confront the high HPV burden among AGYW in SSA.
Our study is the first to evaluate the factors associated with HPV infection among AGYW learners in the Eastern Cape Province of South Africa. Our study, albeit not necessarily generalizable, yet consistent with published literature from other female sub-populations, found specific factors (including vaginal microecology) associated with prevalent HPV infection among unvaccinated AGYW in South Africa. The association of HPV infection and vaginal discharge or itching with specific microbes in the vaginal microflora underlines the necessity of incorporating such microbes in the surveillance and management of vaginal syndromes and infections, including HPV infection among AGYW. But first, further surveillances are needed to: (i) accurately investigate HPV epidemiology and its determinants in other AGYW populations, and (ii) adequately resolve their roles in HPV infection. To conclude, the findings from our study have the potential to inform comprehensive evidence-based interventions and programmes which, increase the public health awareness of HPV infection, its risk factors, and ramifications among AGYW in SSA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The parent study – The HPV Education Intervention Study – was reviewed and approved by the Human Research Ethics Committee at the University of Cape Town, South Africa (HREC: 369/2015) and the Eastern Cape Province of South Africa was granted by the Eastern Cape Provincial Health Research Committee (EC_2016RP29_562). Moreover, Eastern Cape's Department of Education and Department of Health also granted us permission to conduct the study. Written informed consent to participate in parent study was provided by the participants' legal guardian/next of kin. For the present study, which sought to include a molecular test of a panel of vaginal microbes, ethical clearance was secured from the Human Research Ethics Committee at Walter Sisulu University, South Africa (protocol number: 044/2020).
A-LW and ZM: conceptualization and resources. ZM: methodology, validation, supervision, project administration, and funding acquisition. HO and SM: formal analysis. HO, SM, A-LW, and ZM: investigation and writing—review and editing. HO, SM, and ZM: data curation. HO: writing—original draft preparation and visualization. All authors contributed to the article and approved the submitted version.
The present work was funded by research grants of the South African Medical Research Council (SIR Grant Number: 384709 and SAMRC/UCT Gynaecological Cancer Research Centre) and the National Health Laboratory Service Research Trust. HO was funded by the University of Cape Town's Faculty of Health Sciences Postdoctoral Research Fellowship and the South African National Research Foundation Postdoctoral Grantholder Bursary (Grant Number: 64815).
We are indebted to all the AGYW participants of the HPV Education Intervention Study and Dr. Nontuthuzelo I. Somdyala (South African Medical Research Council) for her contributions toward of the study conception, design of the study, protocol compilation of the HPV Education Intervention Study. We are also grateful to the Eastern Cape's Department of Education, Ngcobo sub-District office, school principals, school teachers, and the school management for their involvement in the study. We acknowledge the support from the office of the Department of Education Social Support Service (Mrs. Kape and Mrs. Mpomane) and the Department of Health Ngcobo sub-District (Mrs. Mbulawa, Mrs. Manqinana, and former and current managers). Finally, we thank the members of the HPV and Microbiome Group with unfeignedly delight for their invaluable advices and constructive criticisms regarding the manuscript.
Author HO is employed by Tunacare Services Health Providers Limited, Nairobi, Kenya.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The findings, views, conclusions, and recommendations expressed in this article are those of the authors and do not necessarily represent the views of the funders and institutes affiliated with the authors and/or those mentioned in the “Acknowledgments” section above.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2022.887736/full#supplementary-material
Supplementary Figure 1. Patterns of bacterial pathobiont infections according to vaginal HPV status among 192 AGYW. HPV-positive AGYW with (A) single infections, (B) dual infections, (C) triple infections; and HPV-negative AGYW with (D) single infections, (E) dual infections, (F) triple infections. The number of AGYW in each group is in parentheses. Of all the AGYW with information on HPV status, 193 had any detectable pathobiont (M. genitalium, M. hominis, U. parvum, or U. urealyticum). One HPV-positive participant, not included in any of the two-dimensional pie charts showing the patterns of infections, had all the four examined pathobionts detected.
β, beta; μ, micro; AGYW, adolescent girls and young women; AIDS, acquired immunodeficiency syndrome; bp, base pair; BV, bacterial vaginosis; BVAB, bacterial vaginosis-associated bacteria 2; CC, cervical cancer; CI, confidence interval; CMV, cytomegalovirus; DNA, deoxyribonucleic acid; HIV, human immunodeficiency virus; HPV, human papillomavirus; HR-HPV, high-risk human papillomavirus; HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; IQR, interquartile range; LGV, lymphogranuloma venereum; OR, odds ratio; PCR, polymerase chain reaction; spp., species (plural); SSA, sub-Saharan Africa; STI, sexually transmitted infection; VZV, varicella-zoster virus; WHO, World Health Organization.
1. Bruni L, Albero G, Serrano B, Mena M, Collado J, Gómez, et al. Human Papillomavirus and Related Diseases in the World. Summary Report 22 October 2021. Barcelona: ICO Information Centre on HPV Cancer (HPV Information Centre) (2021). Available online at: https://hpvcentre.net/statistics/reports/XWX.pdf
2. Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. (2012) 30(Suppl. 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055
3. Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. (2002) 55:244–65. doi: 10.1136/jcp.55.4.244
4. Richter K, Dreyer G. Paradigm shift needed for cervical cancer: HPV infection is the real epidemic. S Afr Med J. (2013) 103:290–2. doi: 10.7196/SAMJ.6936
5. Mbulawa ZZA, van Schalkwyk C, Hu NC, Meiring TL, Barnabas S, Dabee S, et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS ONE. (2018) 13:e0190166. doi: 10.1371/journal.pone.0190166
6. Giuliano AR, Botha MH, Zeier M, Abrahamsen ME, Glashoff RH, van der Laan LE, et al. High HIV, HPV, and STI prevalence among young Western Cape, South African women: EVRI HIV prevention preparedness trial. J Acquir Immune Defic Syndr. (2015) 68:227–35. doi: 10.1097/QAI.0000000000000425
7. Houlihan CF, de Sanjose S, Baisley K, Changalucha J, Ross DA, Kapiga S, et al. Prevalence of human papillomavirus in adolescent girls before reported sexual debut. J Infect Dis. (2014) 210:837–45. doi: 10.1093/infdis/jiu202
8. Wendland EM, Villa LL, Unger ER, Domingues CM, Benzaken AS, Group PO-BS. Prevalence of HPV infection among sexually active adolescents and young adults in Brazil: the POP-Brazil study. Sci Rep. (2020) 10:4920. doi: 10.1038/s41598-020-61582-2
9. Kaida A, Dietrich JJ, Laher F, Beksinska M, Jaggernath M, Bardsley M, et al. A high burden of asymptomatic genital tract infections undermines the syndromic management approach among adolescents and young adults in South Africa: implications for HIV prevention efforts. BMC Infect Dis. (2018) 18:499. doi: 10.1186/s12879-018-3380-6
10. Gabster A, Pascale JM, Cislaghi B, Francis SC, Weiss HA, Martinez A, et al. High prevalence of sexually transmitted infections, and high-risk sexual behaviors among indigenous adolescents of the Comarca Ngabe-Bugle, Panama. Sex Transm Dis. (2019) 46:780–787. doi: 10.1097/OLQ.0000000000001070
11. Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. (2005) 191:182–92. doi: 10.1086/426867
12. Ebrahim S, Mndende XK, Kharsany AB, Mbulawa ZZ, Naranbhai V, Frohlich J, et al. High burden of human papillomavirus (HPV) infection among young women in KwaZulu-Natal, South Africa. PLoS ONE. (2016) 11:e0146603. doi: 10.1371/journal.pone.0146603
13. Winer RL. Genital human papillomavirus infection: incidence and risk factors in a cohort of female university students. Am J Epidemiol. (2003) 157:218–26. doi: 10.1093/aje/kwf180
14. Weinstock H, Berman S, Cates W Jr. Sexually transmitted diseases among American: youth incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. (2004) 36:6–10. doi: 10.1363/3600604
15. Mbulawa ZZA, Somdyala NI, Mabunda SA, Williamson A-L. High human papillomavirus prevalence among females attending high school in the Eastern Cape Province of South Africa. PLoS ONE. (2021) 16:e0253074. doi: 10.1371/journal.pone.0253074
16. Bell MC, Schmidt-Grimminger D, Jacobsen C, Chauhan SC, Maher DM, Buchwald DS. Risk factors for HPV infection among American Indian and white women in the Northern Plains. Gynecol Oncol. (2011) 121:532–6. doi: 10.1016/j.ygyno.2011.02.032
17. Tounkara FK, Teguete I, Guedou FA, Talbot D, Traore CB, Behanzin L, et al. Type-specific incidence, persistence and factors associated with human papillomavirus infection among female sex workers in Benin and Mali, West Africa. Int J Infect Dis. (2021) 106:348–57. doi: 10.1016/j.ijid.2021.04.008
18. Weaver B, Tu W, Shew M, Qadadri B, Tong Y, Denski C, et al. Acquisition of first human papillomavirus infection related to first vaginal intercourse and other sexually transmitted infections in adolescent women. J Adolesc Health. (2011) 48:S11–2. doi: 10.1016/j.jadohealth.2010.11.031
19. Igidbashian S, Boveri S, Bottari F, Vidal Urbinati A, Preti E, Casadio C, et al. Prevalence and risk factors of human papillomavirus infection in 18-year-old women: baseline report of a prospective study on human papillomavirus vaccine. J Low Genit Tract Dis. (2017) 21:4–8. doi: 10.1097/LGT.0000000000000268
20. Menon S, Broeck DV, Rossi R, Ogbe E, Harmon S, Mabeya H. Associations between vaginal infections and potential high-risk and high-risk human papillomavirus genotypes in female sex workers in western Kenya. Clin Ther. (2016) 38:2567–77. doi: 10.1016/j.clinthera.2016.10.005
21. Liang Y, Chen M, Qin L, Wan B, Wang H. A meta-analysis of the relationship between vaginal microecology, human papillomavirus infection and cervical intraepithelial neoplasia. Infect Agent Cancer. (2019) 14:29. doi: 10.1186/s13027-019-0243-8
22. Brotman RM, Shardell MD, Gajer P, Tracy JK, Zenilman JM, Ravel J, et al. Interplay between the temporal dynamics of the vaginal microbiota and human papillomavirus detection. J Infect Dis. (2014) 210:1723–1733. doi: 10.1093/infdis/jiu330
23. Gao W, Weng J, Gao Y, Chen X. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect Dis. (2013) 13:271. doi: 10.1186/1471-2334-13-271
24. Reimers LL, Mehta SD, Massad LS, Burk RD, Xie X, Ravel J, et al. The cervicovaginal microbiota and its associations with human papillomavirus detection in HIV-infected and HIV-uninfected women. J Infect Dis. (2016) 214:1361–9. doi: 10.1093/infdis/jiw374
25. Rodriguez-Cerdeira C, Sanchez-Blanco E, Alba A. Evaluation of association between vaginal infections and high-risk human papillomavirus types in female sex workers in Spain. ISRN Obstet Gynecol. (2012) 2012:240190. doi: 10.5402/2012/240190
26. Dareng EO, Ma B, Famooto AO, Akarolo-Anthony SN, Offiong RA, Olaniyan O, et al. Prevalent high-risk HPV infection and vaginal microbiota in Nigerian women. Epidemiol Infect. (2016) 144:123–37. doi: 10.1017/S0950268815000965
27. Di Paola M, Sani C, Clemente AM, Iossa A, Perissi E, Castronovo G, et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk human papillomavirus infection. Sci Rep. (2017) 7:10200. doi: 10.1038/s41598-017-09842-6
28. World Health Organization (WHO). Human papillomavirus vaccines WHO position paper. Wkly Epidemiol Rec. (2009) 84:117–32. Available online at: https://apps.who.int/iris/handle/10665/241310
29. Moodley I, Tathiah N, Mubaiwa V, Denny L. High uptake of Gardasil vaccine among 9 - 12-year-old schoolgirls participating in an HPV vaccination demonstration project in KwaZulu-Natal, South Africa. S Afr Med J. (2013) 103:318–21. doi: 10.7196/SAMJ.6414
30. Delany-Moretlwe S, Kelley KF, James S, Scorgie F, Subedar H, Dlamini NR, et al. Human papillomavirus vaccine introduction in South Africa: implementation lessons from an evaluation of the national school-based vaccination campaign. Glob Health Sci Pract. (2018) 6:425–38. doi: 10.9745/GHSP-D-18-00090
31. Russell VL, Ogilvie G, Beksinska M, Nyrenda M, Mitchell-Foster S, Lavoie J, et al. Human papillomavirus and cervical cancer risk perception and vaccine acceptability among adolescent girls and young women in Durban, South Africa. S Afr Med J. (2020) 110:887–93. doi: 10.7196/SAMJ.2020.v110i9.14367
32. Mbulawa ZZA, Somdyala NI, Mabunda SA, Williamson AL. Effect of human papillomavirus (HPV) education intervention on HPV knowledge and awareness among high school learners in Eastern Cape Province of South Africa. J Cancer Educ. (2021). doi: 10.1007/s13187-021-02090-3. [Epub ahead of print].
33. Sethi S, Singh G, Samanta P, Sharma M. Mycoplasma genitalium: an emerging sexually transmitted pathogen. Indian J Med Res. (2012) 136:942–55.
34. Taylor-Robinson D, Furr PM. Update on sexually transmitted mycoplasmas. Lancet. (1998) 351:S12–5. doi: 10.1016/S0140-6736(98)90004-6
35. Lennard K, Dabee S, Barnabas SL, Havyarimana E, Blakney A, Jaumdally SZ, et al. Microbial composition predicts genital tract inflammation and persistent bacterial vaginosis in adolescent South African women. Infect Immun. (2018) 86:e00410–7. doi: 10.1128/IAI.00410-17
36. Taku O, Brink A, Meiring TL, Phohlo K, Businge CB, Mbulawa ZZA, et al. Detection of sexually transmitted pathogens and co-infection with human papillomavirus in women residing in rural Eastern Cape, South Africa. PeerJ. (2021) 9:e10793. doi: 10.7717/peerj.10793
37. Taku O, Onywera H, Mbulawa ZZA, Businge CB, Meiring TL, Williamson A-L. Molecular identification of cervical microbes in HIV-negative and HIV-positive women in an African setting using a customized bacterial vaginosis microbial DNA quantitative PCR (qPCR) array. Microbiol Spectr. (2022) 10:e0222921. doi: 10.1128/spectrum.02229-21
38. Taku O, Mbulawa ZZA, Phohlo K, Garcia-Jardon M, Businge CB, Williamson AL. Distribution of human papillomavirus (HPV) genotypes in HIV-negative and HIV-positive women with cervical intraepithelial lesions in the Eastern Cape Province, South Africa. Viruses. (2021) 13. doi: 10.3390/v13020280
39. Bodén E, Eriksson A, Rylander E, von Schoultz B. Clinical characteristics of papillomavirus-vulvovaginitis. A new entity with oncogenic potential. Acta Obstet Gynecol Scand. (1988) 67:147–51. doi: 10.3109/00016348809004188
40. Cerwonka ER, Isbell TR, Hansen CE. Psychosocial factors as predictors of unsafe sexual practices among young adults. AIDS Educ Prev. (2000) 12:141–53.
41. Hingson RW, Heeren T, Zakocs RC, Kopstein A, Wechsler H. Magnitude of alcohol-related mortality morbidity among U.S. college students ages 18-24. J Stud Alcohol. (2002) 63:136–44. doi: 10.15288/jsa.2002.63.136
42. White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. (2003) 27:186–96.
43. Harries J, Moodley J, Barone MA, Mall S, Sinanovic E. Preparing for HPV vaccination in South Africa: key challenges and opinions. Vaccine. (2009) 27:38–44. doi: 10.1016/j.vaccine.2008.10.033
44. Thanasas I, Lavranos G, Gkogkou P, Paraskevis D. Understanding of young adolescents about HPV infection: how health education can improve vaccination rate. J Cancer Educ. (2020) 35:850–9. doi: 10.1007/s13187-019-01681-5
45. Grandahl M, Rosenblad A, Stenhammar C, Tyden T, Westerling R, Larsson M, et al. School-based intervention for the prevention of HPV among adolescents: a cluster randomised controlled study. BMJ Open. (2016) 6:e009875. doi: 10.1136/bmjopen-2015-009875
46. Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. (2010) 12:248–71. doi: 10.1111/j.1462-5822.2009.01394.x
47. Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. (2013) 4:119–28. doi: 10.4161/viru.22913
48. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. (2003) 16:1–17. doi: 10.1128/CMR.16.1.1-17.2003
49. Onywera H, Williamson AL, Mbulawa ZZA, Coetzee D, Meiring TL. The cervical microbiota in reproductive-age South African women with and without human papillomavirus infection. Papillomavirus Res. (2019) 7:154–63. doi: 10.1016/j.pvr.2019.04.006
50. Castro J, Alves P, Sousa C, Cereija T, Franca A, Jefferson KK, et al. Using an in-vitro biofilm model to assess the virulence potential of bacterial vaginosis or non-bacterial vaginosis Gardnerella vaginalis isolates. Sci Rep. (2015) 5:11640. doi: 10.1038/srep11640
51. Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, et al. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog. (2016) 12:e1005889. doi: 10.1371/journal.ppat.1005889
52. Marovt M, Kese D, Kotar T, Kmet N, Miljkovic J, Soba B, et al. Ureaplasma parvum and Ureaplasma urealyticum detected with the same frequency among women with and without symptoms of urogenital tract infection. Eur J Clin Microbiol Infect Dis. (2015) 34:1237–45. doi: 10.1007/s10096-015-2351-8
53. Rumyantseva T, Khayrullina G, Guschin A, Donders G. Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagn Microbiol Infect Dis. (2019) 93:227–31. doi: 10.1016/j.diagmicrobio.2018.10.001
54. Zozaya M, Ferris MJ, Siren JD, Lillis R, Myers L, Nsuami MJ, et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome. (2016) 4:16. doi: 10.1186/s40168-016-0161-6
55. Ji Y. Co-infections with human papillomavirus and mycoplasma/Ureaplasma spp. in women with abnormal cervical cytology. Res Rep Gynaecol Obstet. (2017) 1:1–3. Available online at: https://www.alliedacademies.org/articles/coinfections-with-human-papillomavirus-and-mycoplasmaureaplasma-spp-inwomen-with-abnormal-cervical-cytology.html
56. Horner P, Donders G, Cusini M, Gomberg M, Jensen JS, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women - a position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. (2018) 32:1845–51. doi: 10.1111/jdv.15146
Keywords: HPV, determinants, risk factors, adolescent girls and young women (AGYW), South Africa
Citation: Onywera H, Mabunda SA, Williamson A-L and Mbulawa ZZA (2022) Microbiological and behavioral determinants of genital HPV infections among adolescent girls and young women warrant the need for targeted policy interventions to reduce HPV risk. Front. Reprod. Health 4:887736. doi: 10.3389/frph.2022.887736
Received: 01 March 2022; Accepted: 05 July 2022;
Published: 28 July 2022.
Edited by:
Ronel Sewpaul, Human Sciences Research Council, South AfricaReviewed by:
Admire Takuranenhamo Chikandiwa, Wits Health Consortium (WHC), South AfricaCopyright © 2022 Onywera, Mabunda, Williamson and Mbulawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harris Onywera, aGFycmlzLm9ueXdlcmFAdWN0LmFjLnph; Zizipho Z. A. Mbulawa, eml6aXBoby5tYnVsYXdhQG5obHMuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.