
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
DATA REPORT article
Front. Reprod. Health , 11 July 2022
Sec. Assisted Reproduction
Volume 4 - 2022 | https://doi.org/10.3389/frph.2022.886277
 Haiyan Wang1,2†
Haiyan Wang1,2† Aiqing Han3†
Aiqing Han3† Shiyuan Jiang1†
Shiyuan Jiang1† Dan Cao1
Dan Cao1 Yangyu Jiang1
Yangyu Jiang1 Lin Sun2
Lin Sun2 Na Zou4
Na Zou4 Shiying Tao1
Shiying Tao1 Xiaoou Xue5
Xiaoou Xue5 Xiaoguang Shao2*
Xiaoguang Shao2* Jian Li1*
Jian Li1*Infertility has become a worldwide public health problem, affecting 12.5 to 15 in 100 couples of reproductive ages annually in China (1). Diagnosis of diminished ovarian reserve (DOR), a predominant contributor to infertility, has increased from 19% in 2004 to 26% in 2011 in the United States, while similar trends have emerged in China (2). Aging is directly associated with physiological DOR, whereas endometriosis and some diseases contribute to pathological DOR (3). However, the pathophysiological mechanisms of DOR remain unclear.
The assisted reproductive technique (ART) has been broadly used to improve reproductive outcomes for women with infertility. Nevertheless, DOR is also a big challenge in ART, which addresses reductions in oocyte quantity and quality, poor ovarian response (POR), high cancellation rate, and recurrent pregnancy loss (RPL) (4, 5).
The number of oocytes and high-quality embryos acquired was crucial across all in vitro fertilization (IVF) laboratories in ART. Progress is being made in exploring the pathophysiologic mechanisms of poor-quality embryos and discovering therapeutic strategies for improving the embryo quality of women with DOR (6, 7).
Recently, investigators have demonstrated some potential correlations between serum homocysteine (Hcy) level and embryo quality (8). In brief, Hcy concentration correlates with oocyte and embryo qualities in patients with infertility and polycystic ovary syndrome (PCOS). Hyper-homocysteinemia (hHcy) has been identified as a risk factor for unexplained infertility (9, 10). Furthermore, aging (≥39 years) and high levels of plasma Hcy are strongly associated with thrombotic events during IVF. Mild-to-moderate hHcy is an independent risk factor for thromboembolic disorders (11), implantation failure in patients undergoing IVF (9), and early pregnancy loss (12). However, to our knowledge, no study has yet assessed the correlation between serum Hcy level and embryo quality in patients with DOR undergoing IVF.
In the presented study, we analyzed the medical records of infertile women with DOR who underwent ART at our center to reveal the possible correlation between clinical characteristics and embryo quality. Thus, our objective was to provide evidence for predicting embryo quality and suggesting potential therapeutic approaches by evaluating Hcy levels in patients undergoing IVF treatment.
A retrospective cohort study was conducted using 3,390 medical record data on IVF cycles from Dalian Municipal Women and Children's Medical Center (Group) from January 2015 to December 2020. The flowchart is shown in Figure 1. The inclusion criteria were (1) women aged 20~49 years old, (2) patients who underwent IVF/ intracytoplasmic sperm injection (ICSI) for the first time, and (3) patients with serum basal follicle-stimulating hormone (FSH) ≥ 10 mIU/ml, anti- Müllerian hormone (AMH) < 1.1 ng/ml, or antral follicle count (AFC) < 7 (1–3 months of the IVF cycle).
Patients were excluded if any of the following conditions were present: (1) premature ovarian failure (POF) or primary ovarian insufficiency (POI), (2) acute cardiovascular disease, renal function damage, or malignant tumor, and (3) chromosome abnormality by preimplantation genetic screening.
Stimulation protocols were selected based on the individual patient's conditions. Standard ovarian stimulation with gonadotrophins was performed using gonadotrophin-releasing hormone (GnRH) antagonist protocol, mini-stimulation protocol, or natural protocol. Oocyte retrieval was performed 34~36 h after triggering with hCG, a GnRH agonist, or a combined hCG and GnRH agonist under transvaginal ultrasound guidance.
Cases with no oocyte obtained or ovulation occurring at the time of egg collection were excluded. Oocytes were fertilized using either IVF or intracytoplasmic sperm injection (ICSI) depending on the semen parameters of the husbands per the standard protocols in the centers. The fertilization was confirmed by the presence of two pronuclei at 16~18 h after conventional insemination or ICSI. Three days after oocyte retrieval, an embryo with at least seven cells and Grades 1 and 2 was defined as good quality. Embryos with at least six cells and fragments of <50% were frozen. All good embryos were frozen or vitrified using the Cryotop method as cleavage-stage embryos on Day 3, or as blastocysts on Day 5 or 6 according to the standard operating protocol.
The DX1800 automatic chemiluminescence instrument (Beckman Coulter Company, USA) was used for hormone determination, and an automatic biochemical analyzer (Hitachi Company, Japan) was used to detect blood lipid levels. The selection of fertilization method and embryo score, according to pronuclear stage score, development speed, number of blastomeres, size, morphology, cytoplasm, fragment ratio, and embryo quality score of the cleavage stage, was divided into four grades: Grade 1 embryo: blastomere equality, regular morphology, bright, and no fragment; Grade 2 embryo: blastomere unequal in size and/or fragmentation of <10%. Grade 3 embryo: 10–50% fragments; and Grade 4 embryo: fragmentation >50%. Embryos with more than six cells in Grades 1-2 on Day 3 after egg collection were defined as high-quality embryos. Embryos with <30% fragments are available and can be transplanted or frozen (Figure 1).
A total of 1,023 data met the criteria. The data were divided into poor-quality embryos group and high-quality embryos group according to the embryo status. We summarized the demographic and clinical characteristics (age, infertility years, infertility type, AFC, and BMI), medical history, reproductive hormone parameters (AMH, FSH, LH, PRL, and E2), and carbohydrate and lipid metabolic indexes (CHO, TG, LP, LDL, HDL, fasting insulin (FINS), Glucose, and Hcy).
Descriptive statistics were presented as mean ± standard deviation (SD) for continuous variables. Categorical variables were presented as frequency and percentage (%). Normally distributed data were analyzed using Student's t-test. Non-parametric variables were compared using the Mann-Whitney U test. Categorical variables were compared using the chi-square or Fisher's exact tests. Binary logistic regression analysis with backward stepwise elimination of candidate variables was used to measure the association between independent and outcome variables. Results were presented as Odds ratios (ORs) with 95% confidence intervals (CIs) and area under the curves (AUCs).
Data were analyzed using SPSS version 20 (IBM SPSS, Inc., Chicago, IL, USA). Comparisons were interpreted as significant when associated with P < 0.05. The ROC curves and AUC values were calculated using the pROC package of R statistical software (Version 4.1.0). The scatter box diagram was drawn by python software (Version 3.8.3).
A total of 1,023 patients with DOR were included in this study. Of these, 540 cases were with high-quality embryos (the number of high-quality embryos, number ≥1) and 483 cases were with poor-quality embryos (no high-quality embryos, number = 0). The demographic characteristics of the two groups were similar (Table 1). However, the level of serum Hcy was significantly higher in the poor-quality embryo group compared with the high-quality embryo group (P < 0.05). In addition, the levels of serum AMH and PRL were statistically different between the two groups.
Impressed with the higher Hcy level in the poor-quality embryo group, we analyzed the relationship between the number of high-quality embryos and the level of serum Hcy. The level of serum Hcy negatively correlated with the number of high-quality embryos (Figure 2). A high level of serum Hcy might be one of the key factors that triggers the decline in embryo quality.
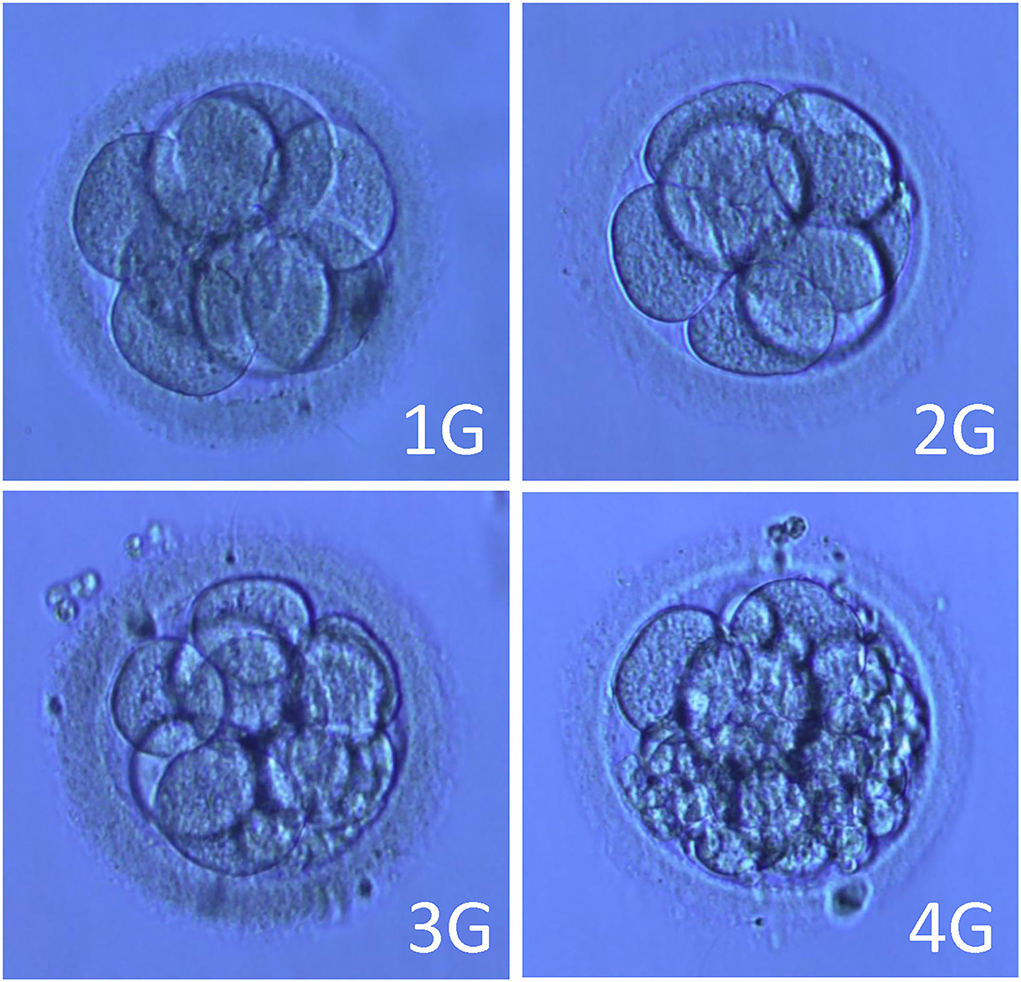
Figure 2. Pictures of embryos of different grades. Note: 1G (Grade 1 embryo): blastomere equality, regular morphology, bright, no fragment. 2G (Grade 2 embryo): blastomere unequal in size and/or fragmentation <10%. 3G (Grade 3 embryo): 10–50% fragments. 4G (Grade 4 embryo): fragmentation > 50%.
To further evaluate the Hcy levels in patients with different embryo quality, we perform subgroup analysis. Patients with serum Hcy>15 μmol/L were at higher risk of having poor-quality embryos (82.1%) compared to patients with serum Hcy <8 μmol/L (43.9%) and at 8~15 μmol/L (48.1%) (Table 2; χ2 = 15.859, P < 0.001).
Since the above results showed a negative correlation between the serum Hcy and high-quality embryos, a logistic regression analysis was performed to measure the association between independent variables and high-quality embryos. Patients who were older (OR = 0.960, 95% CI = 0.926–0.996, P < 0.05) or with higher Hcy level (OR = 0.901, 95% CI = 0.847–0.959, P < 0.05) had lower risk of having high-quality embryos (Table 3). The logistic regression model was established and the formula was followed (in the formula, p indicates the probability of having high-quality embryo; x1 and x2 are explanatory variables, representing the values of age and Hcy, respectively).
The AUC for using this formula to differentiate age and Hcy was 0.53 and 0.543, respectively (Figure 3).
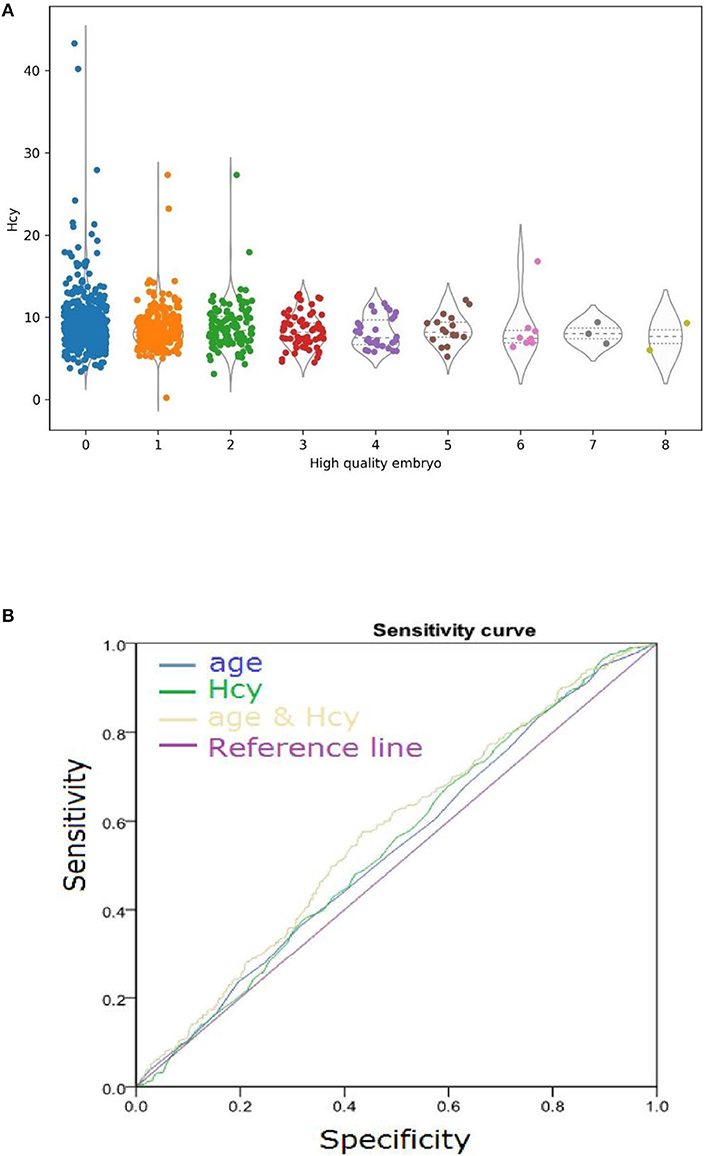
Figure 3. (A) Serum Hcy levels negatively correlated with high-quality embryo number. Horizontal lines represent the median and interquartile range (IQR) of the data. (B) Diagnostic Performance of embryo quality. Comparison of model performance for predicting embryo quality using variables age and Hcy independently and using variables age and Hcy simultaneously.
According to the latest national census of China, the fertility rate significantly decreased in 2020 (1.3 children per woman). One reason is that more Chinese young people tend to postpone marriage and childbearing to later reproductive years; the other reason is that the number of infertile couples has increased (13, 14). Furthermore, the Chinese government released a two-child policy encouraging families to have a second child in January 2016. To sum up the above reasons, the reproductive need in women of advanced age (≥ 40) has been increasing dramatically, thus leading to higher demands for receiving ART in recent years. Unfortunately, DOR has become a huge challenge for reproductive medicine. The published results showed that about 20% of infertile women suffered from DOR in China (15–17). DOR induces not only infertility but also poor fertility outcomes even when in vitro fertilization-embryo transfer (IVF-ET) is used (18).
The goal of ART is to deliver a healthy baby. Therefore, it is essential to evaluate ovarian reserve, oocyte quality, and embryo quality. In clinical practice, there is a standard scheme for definite DOR, in which age, baseline AFC (<5–7), baseline AMH (<0.5~1.1 ng/ml), and baseline FSH (>10 mIU/ml) are considered (19, 20). However, the existing research showed that these parameters are not good predictors of embryo quality or pregnancy in controlled ovarian stimulation cycles in ART processing (6, 21, 22). Therefore, we designed a project to explore the potential correlation between embryo quality and potential parameters from medical records.
Our study showed that the level of serum AMH was significantly higher while PRL and Hcy were statistically lower in patients with high-quality embryos compared to those with poor-quality embryos. The role of AMH level as a biomarker of embryo quality remains controversial (23–26). Most researchers argued that serum AMH concentrations fluctuate for three days after stimulating with standard hormone-based drugs. PRL, a 198 amino acid polypeptide hormone, is secreted from the anterior pituitary. Its secretion could be affected by stress, pain, trauma, ingesting food, and pregnancy (27, 28). Hence, some researchers do not believe it might affect the outcome of IVF (29). Hcy, a sulfur-containing non-essential amino acid generated from the metabolism of the methionine and cysteine, plays a vital role in remethylation and trans-sulphuration. Previous studies have shown that a high serum Hcy level might lead to a reduction in the number of oocytes, suppress oocyte maturity, and reduce embryo quality (30–32). In order to reveal the correlation between AMH, PRL, Hcy, and embryo quality, a logistic regression analysis model was built for the analysis. The results demonstrated that only serum Hcy level correlated with embryo quality. In particular, a high Hcy level coupled with aging might partly reduce embryo quality. Our findings suggested that Hcy might be a sensitive marker for predicting embryo quality in patients with DOR of advanced age who underwent IVF. In other words, a high serum level of Hcy might reduce the embryo quality.
The Hcy is an intermediate product of methionine metabolism via methylation catalyzed by various enzymes, including betaine-homocysteine S-methyltransferase (BHMT), glycine N-methyltransferase (GNMT), guanidinoacetate N-methyltransferase (GAMT), cystathionine β-synthase (CBS), and γ-cystathionase (33, 34). These reactions depend on folate and B-vitamins, including vitamin B-2, B-6, and B-12 as cofactors at different steps of the metabolic pathways (35, 36). The main catabolic product of Hcy is cysteine, which is helped by CBS and γ-cystathionase. Thus, we can assume that a moderate supply of B-vitamin, folate, and cysteine in our diet might be beneficial to obtaining high-quality embryos in IVF (37). A high-methionine diet could induce hyperhomocysteinemia, which is related to some pathological statuses, including infertility (28).
In conclusion, the presented study demonstrated that high serum Hcy level is linked to poor-quality embryos. Moreover, Hcy coupled with age might be a sensitive marker for embryo quality after IVF. In connection with this result, we assumed that improving the metabolism of Hcy will benefit the success of assisted reproduction.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Dalian Municipal Women and Children's Medical Center (#No. 2020010). The Ethics Committee waived the requirement of written informed consent for participation.
Conceived and designed the experiments: HW, AH, XS, and JL. Performed the experiments: DC, YJ, LS, NZ, and ST. Analyzed the data: AH and SJ. Contributed reagents, materials, and analysis tools: XS, JL, XX, and ST. Wrote the manuscript: HW, AH, and JL. The final manuscript was read and approved by all authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely acknowledge the assistance given by the Beijing University of Chinese Medicine and Dalian Women and Children's Medical Group, Dalian, China, in this study.
Hcy, homocysteine; DOR, Diminished ovarian reserve; IVF, in vitro fertilization; ICSI, Intracytoplasmic sperm injection; AMH, Anti-Müllerian hormone; FSH, Follicle stimulating hormone; AFC, Antral follicle count; POR, Poor ovarian response; POF, Premature ovarian failure; POI, Primary ovarian insufficiency; LH, Luteinizing hormone; PRL, Prolactin; E2, Estradiol; CHO, Cholesterol; TG, Triglyceride; LP, Lipoprotein; LDL, Low density lipoprotein; ART, Assisted reproductive technique; BMI, Body mass index; Gn, Gonadotrophin; 2PN, Two pronuclear; IQR, interquartile range; hHcy, Hyper-homocysteinemia.
1. Liu J, Larsen U, Wyshak G. Prevalence of primary infertility in China: in-depth analysis of infertility differentials in three minority province/autonomous regions. J Biosoc Sci. (2005) 37:55–74. doi: 10.1017/S0021932003006461
2. Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the Society for Assisted Reproductive Technology Clinic Outcomes Reporting System. Fertility and sterility (2015) 104:612–19.e3. doi: 10.1016/j.fertnstert.2015.05.017
3. D.K. Shah. Diminished ovarian reserve and endometriosis: insult upon injury. Semin Reprod Med. (2013) 31:144–9. doi: 10.1055/s-0032-1333479
4. Ata B, Seyhan A, Seli E. Diminished ovarian reserve versus ovarian aging: overlaps and differences. Curr Opin Obstet Gynecol. (2019) 31:139–47. doi: 10.1097/GCO.0000000000000536
5. Gurtcheff SE, Klein NA. Diminished ovarian reserve and infertility. Clin Obstet Gynecol. (2011) 54:666–74. doi: 10.1097/GRF.0b013e3182353c65
6. Scheffer JB, Carvalho RF, Aguiar APS, Machado IJM, Franca JB, Lozano DM, et al. Which ovarian reserve marker relates to embryo quality on day 3 and blastocyst; age, AFC, AMH? JBRA Assisted Reprod. (2021) 25:109–14. doi: 10.5935/1518-0557.20200060
7. Musters AM, van Wely M, Mastenbroek S, Kaaijk EM, Repping S, van der Veen F, et al. The effect of recombinant LH on embryo quality: a randomized controlled trial in women with poor ovarian reserve. Hum Reprod. (2012) 27:244–50. doi: 10.1093/humrep/der371
8. Wang F, Sui X, Xu N, Yang J, Zhao H, Fei X, et al. The relationship between plasma homocysteine levels and MTHFR gene variation, age, and sex in Northeast China. Niger J Clin Pract. (2019) 22:380–5.
9. Razi Y, Eftekhar M, Fesahat F, Dehghani Firouzabadi R, Razi N, et al. Concentrations of homocysteine in follicular fluid and embryo quality and oocyte maturity in infertile women: a prospective cohort. J Obstet Gynaecol. (2021) 41:588–93. doi: 10.1080/01443615.2020.1785409
10. Berker B, Kaya C, Aytac R, Satiroglu H. Homocysteine concentrations in follicular fluid are associated with poor oocyte and embryo qualities in polycystic ovary syndrome patients undergoing assisted reproduction. Hum Reproduct (Oxford, England). (2009) 24:2293–302. doi: 10.1093/humrep/dep069
11. Grandone E, Colaizzo D, Vergura P, Cappucci F, Vecchione G, Lo Bue A, et al. Age and homocysteine plasma levels are risk factors for thrombotic complications after ovarian stimulation. Hum Reprod. (2004) 19:1796–9. doi: 10.1093/humrep/deh346
12. Agrawal A, Ilango K, Singh PK, Karmakar D, Singh GP, Kumari R, et al. Age dependent levels of plasma homocysteine and cognitive performance. Behav Brain Res. (2015) 283:139–44. doi: 10.1016/j.bbr.2015.01.016
13. Zheng D, Zhou Z, Li R, Wu H, Xu S, Kang Y, et al. Consultation and treatment behaviour of infertile couples in China: a population-based study. Reprod Biomed Online. (2019) 38:917–25. doi: 10.1016/j.rbmo.2018.11.034
14. Liu Y, Kong XD, Wu QH, Li G, Song L, Sun YP. Karyotype analysis in large-sample infertile couples living in Central China: a study of 14965 couples. J Assist Reprod Genet. (2013) 30:547–53. doi: 10.1007/s10815-013-9964-6
15. Zhou SJ, Sun TC, Song LL, Yang M, Sun XP, Tian L. The status and comparison of ovarian reserve between fertile and infertile healthy Chinese women of reproductive age. Medicine. (2021) 100:e25361. doi: 10.1097/MD.0000000000025361
16. Qin X, Gong Y, Yu F, Song J, Dong S, Zhang R, et al. A comparison of the efficacy and safety of traditional Chinese medicine in preconditioning patients with diminished ovarian reserve that would undergo In vitro fertilization: A network meta-analysis protocol. Medicine. (2021) 100:e24408. doi: 10.1097/MD.0000000000024408
17. Teng B, Peng J, Ong M, Qu X. Successful pregnancy after treatment with Chinese herbal medicine in a 43-year-old woman with diminished ovarian reserve and multiple uterus fibrosis: a case report. Medicines (Basel). (2017) 4:7. doi: 10.3390/medicines4010007
18. Gozuyesil E, Karacay Yikar S, Nazik E. An analysis of the anxiety and hopelessness levels of women during IVF-ET treatment. Perspect Psychiatr Care. (2020) 56:338–46. doi: 10.1111/ppc.12436
19. de Carvalho BR, Rosa e Silva AC, Rosa e Silva JC, dos Reis RM, Ferriani RA, Silva de Sa MF. Ovarian reserve evaluation: state of the art. J Assist Reprod Genet. (2008) 25:311-22. doi: 10.1007/s10815-008-9241-2
20. Xu H, Shi L, Feng G, Xiao Z, Chen L, Li R, et al. An Ovarian Reserve Assessment Model Based on Anti-Mullerian Hormone Levels, Follicle-Stimulating Hormone Levels, and Age: Retrospective Cohort Study. J Med Internet Res. (2020) 22:e19096. doi: 10.2196/19096
21. Bonilla-Musoles F, Castillo JC, Caballero O, Perez-Panades JF, Bonilla JF, Dolz M, et al., Predicting ovarian reserve and reproductive outcome using antimullerian hormone (AMH) and antral follicle count (AFC) in patients with previous assisted reproduction technique (ART) failure. Clin Exp Obstet Gynecol. (2012) 39:13–8.
22. Broer SL, Dolleman M, Opmeer BC, Fauser BC, Mol BW, Broekmans FJ. AMH and AFC as predictors of excessive response in controlled ovarian hyperstimulation: a meta-analysis. Hum Reprod Update. (2011) 17:46–54. doi: 10.1093/humupd/dmq034
23. Morales HSG, Lopez GGP, Cortes DV, Torres GCR, Hernandez HS, Guiot ML, et al. Evaluation of the anti-mullerian hormone and its association with embryo quality in advanced reproductive treatments in a Latin American population. JBRA Assisted Reproduct. (2021) 26:50–2. doi: 10.5935/1518-0557.20210050
24. Korkidakis A, Cho KK, Albert A, Au J, Mellon J, Dunne CM. Anti-Mullerian hormone and embryo quality as determined by time-lapse imaging. Minerva Ginecol. (2020) 72:132–7. doi: 10.23736/S0026-4784.20.04546-3
25. Bhide P, Escriba M, Srikantharajah A, Joshi H, Gudi A, Shah A, et al. Anti-Mullerian hormone (AMH) and embryo quality assessed by time-lapse imaging (TLI): a cross-sectional observational study. Arch Gynecol Obstet. (2017) 296:583–7. doi: 10.1007/s00404-017-4453-2
26. Lin WQ, Yao LN, Zhang DX, Zhang W, Yang XJ, et al. The predictive value of anti-Mullerian hormone on embryo quality, blastocyst development, and pregnancy rate following in vitro fertilization-embryo transfer (IVF-ET). J Assist Reprod Genet. (2013) 30:649–55. doi: 10.1007/s10815-013-9973-5
27. Majumdar A, Mangal NS. Hyperprolactinemia. J Hum Reprod Sci. (2013) 6:168–75. doi: 10.4103/0974-1208.121400
28. Sonigo C, Young J. Binart Hyperprolactinemia N, and infertility: a new physiopathological approach. Med Sci. (2013) 29:242–4. doi: 10.1051/medsci/2013293004
29. Kamel A, Halim AA, Shehata M, AlFarra S, El-Faissal Y, Ramadan W, et al. Changes in serum prolactin level during intracytoplasmic sperm injection, and effect on clinical pregnancy rate: a prospective observational study. BMC Pregnancy Childbirth. (2018) 18:141. doi: 10.1186/s12884-018-1783-4
30. Altmae S, Stavreus-Evers A, Ruiz JR, Laanpere M, Syvanen T, Yngve A, et al. Variations in folate pathway genes are associated with unexplained female infertility. Fertil Steril. (2010) 94:130–7. doi: 10.1016/j.fertnstert.2009.02.025
31. Liu L, Lin Z, Lin P, Jiang Z. Association between serum homocysteine level and unexplained infertility in in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI): A retrospective, hospital-based, case-control study. J Clin Lab Anal. (2020) 34:e23167. doi: 10.1002/jcla.23167
32. Jerzak M, Putowski L, Baranowski W. Homocysteine level in ovarian follicular fluid or serum as a predictor of successful fertilization. Ginekol Pol. (2003) 74:949–52.
33. Skovierova H, Vidomanova E, Mahmood S, Sopkova J, Drgova A, Cervenova T, et al. The molecular and cellular effect of homocysteine metabolism imbalance on human health. Int J Mol Sci. (2016) 17:1733. doi: 10.3390/ijms17101733
34. Schalinske KL, Smazal AL. Homocysteine imbalance: a pathological metabolic marker. Adv Nutr. (2012) 3:755–62. doi: 10.3945/an.112.002758
35. Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. (2017) 14:78. doi: 10.1186/s12986-017-0233-z
36. M. Drozdzik. [Clinical significance of modulating homocysteine metabolism]. Pol Tyg Lek. (1992) 47:972–4.
Keywords: homocysteine, in vitro fertilization (IVF), diminished ovarian reserve (DOR), embryo quality, aged
Citation: Wang H, Han A, Jiang S, Cao D, Jiang Y, Sun L, Zou N, Tao S, Xue X, Shao X and Li J (2022) Homocysteine Level Related to Age Is Associated With Embryo Quality in Women Who Had IVF With Diminished Ovarian Reserve. Front. Reprod. Health 4:886277. doi: 10.3389/frph.2022.886277
Received: 28 February 2022; Accepted: 09 June 2022;
Published: 11 July 2022.
Edited by:
Jan Tesarik, MARGen Clinic, SpainReviewed by:
Chuanling Tang Tang, Shanghai First Maternity and Infant Hospital, ChinaCopyright © 2022 Wang, Han, Jiang, Cao, Jiang, Sun, Zou, Tao, Xue, Shao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Li, bGlqaWFuQGJ1Y20uZWR1LmNu; Xiaoguang Shao, c2hhb3hpYW9ndWFuZzAzQHNpbmEuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.