- 1Institute of Medicine, Tribhuvan University, Kathmandu, Nepal
- 2Nepal Development Society, Bharatpur, Nepal
Background: Preterm birth is a significant cause of neonatal death globally. Nepal is in the 20th position in the world, with the highest rate of preterm deliveries. The risk factors of preterm birth have not been fully identified and established in Nepal. The study aims to identify risk factors of preterm birth among women who underwent delivery in a tertiary maternal hospital in Nepal.
Methods: This study employed a hospital-based matched case-control study design. The case included women who delivered before 37 weeks of gestation, and women who delivered between 37 and 42 weeks of gestation served as controls. The ratio of the case to control was 1:2, and matching was done for the type of delivery. The first author collected the data in the Paropakar Maternity and Women's Hospital between December 2015 and January 2016. Face-to-face interviews were conducted using a structured questionnaire. Backward conditional logistic regression was performed to identify the independent risk factors of preterm birth.
Results: Antihelminthic treatment during pregnancy was found to be protective for preterm birth. Women performing intensive physical work during their pregnancy and women exposed to indoor air pollution were more likely to have a preterm birth than women not performing intensive physical work and women not exposed to indoor pollution, respectively.
Conclusions: Women who had not consumed antihelminthic drugs per protocol, those exposed to indoor air pollution, and those who performed intensive work during pregnancy were at higher risk for preterm birth. Maternal health programs can encourage women to consume antihelminthic drugs, take proper rest during pregnancy, and prevent indoor pollution exposure.
Introduction
An estimated 15 million babies (more than 1 in 10 babies) are born preterm globally (1). Death of infants is common if they are preterm (2), and almost 1 million children die each year globally due to complications related to preterm birth (1). Preterm birth is any childbirth that occurs before 37 weeks of pregnancy or before 259 days of gestation since the first day of the woman's last menstrual period (3). Based on gestational age, preterm birth is categorized as extremely preterm occurring before 28 weeks; very preterm occurring at 28–31 weeks; moderate preterm occurring between 32 and 33 weeks; and late preterm occurring at 34 to <37 weeks of gestation (4). Preterm infants have higher rates of respiratory distress, apnea, temperature instability, seizures, hypoglycemia, and feeding difficulties. Many survivors suffer from neuro-developmental impairments, including cerebral palsy, intellectual disability, sensory impairments, and behavioral problems, including attention, visual processing, academic progress, and executive function (5).
The majority of preterm births (~85%) occur in Africa and the South-East Asia region (6). Nepal is a low-income South Asian country whose neonatal mortality rate (NMR) is 21 and infant mortality rate (IMR) is 32 per thousand live births according to the Nepal Demographic Health Survey (NDHS) 2016. Through the decades of investment in public health programs, maternal and child survival has improved over the years in the country. For instance, IMR has decreased from 78 deaths per thousand live births to 32 deaths in 2016 (7). However, Nepal has the 20th highest rate of preterm deliveries in the world (8), and there are no adequate data on prevalence and mortality associated with preterm birth (9); even if available, they are not collected and reported using standard definitions (10). In 2010, the worldwide estimates of preterm birth stated that 14 out of 100 babies born in Nepal were preterm resulting in around 1 million preterm births and 10,000 neonatal deaths due to complications of preterm birth (1). In 2015, the UNICEF report revealed that the leading cause of neonatal deaths in Nepal was prematurity (30.8 percent of the total neonatal deaths) (11).
Previous literature has identified a research gap in identifying risk factors of preterm birth in low-income countries, including Nepal (6, 12, 13). Therefore, it is crucial to systematically identify the risk factors of preterm birth among Nepalese women. In this context, this study aimed to determine the risk factors of preterm births among newly delivered women attending a tertiary-level maternity hospital in Nepal.
Methods
Study Design and Study Population
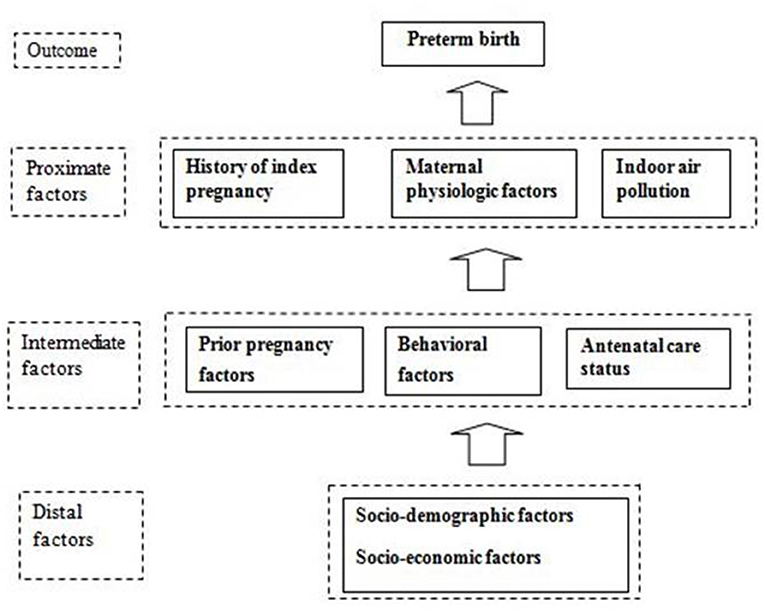
This study employed an institution-based retrospective case-control study design. The study participants were women of reproductive age of 15–49 years who had recently delivered a baby in the hospital. Cases were the women who delivered a single live newborn before 37 weeks of gestation. Controls were the women who delivered a single live newborn at or after 37 weeks and before 42 weeks of gestation, matched to the cases by type of delivery. This study was conducted in Paropakar Maternity and Women's Hospital (PMWH), located in Kathmandu, Nepal. The PMWH is the only tertiary-level public maternity hospital in the country. The conceptual framework of the study is shown in Figure 1. Data collection was done in December 2015 and January 2016. The study obtained ethical approval from the Institutional Review Committee of the Institute of Medicine, Tribhuvan University. Study participants provided written informed consent before the data collection.
Sample Size Calculation and Sampling Technique
We calculated the sample size taking power at 80%, confidence level as 95%, a ratio of control to case as 2:1, an odds ratio of 3.23, and the percentage of controls exposed as 7.8 (14). Considering a 10% non-response rate, 94 cases and 188 controls were calculated as the final sample size for the study. We used the StatCalc application of Epi Info version 7 (Centers for Disease Control and Prevention, USA) to calculate the sample size.
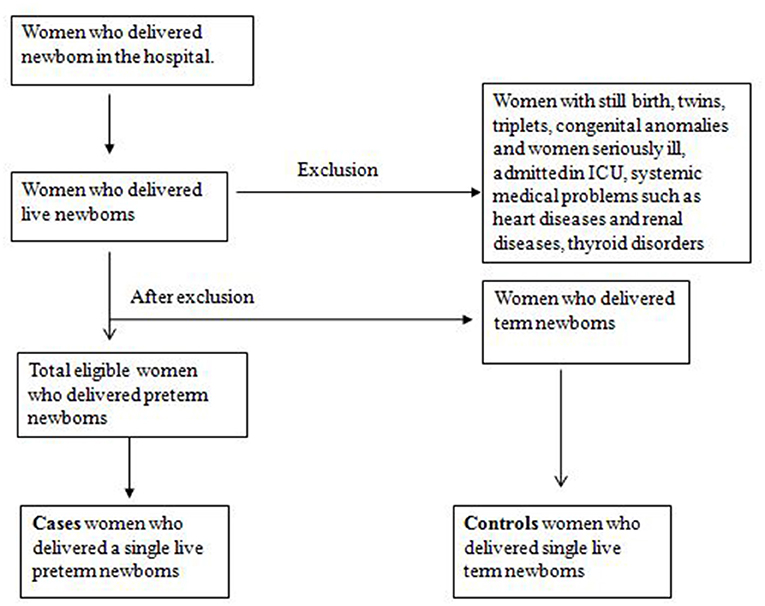
The confinement books of the labor room and birthing center and in/out record book of the post-operative ward were checked daily to identify the birth of preterm newborns by vaginal delivery and the preterm newborns by lower section cesarean section (LSCS). The eligible controls matched to a case by type of delivery, delivering immediately after a case, were searched, and approached in postnatal ward A and ward B of the hospital and recruited for the study. The sampling strategy is presented in Figure 2. We selected two matched controls by type of delivery for every case. If the case delivered a preterm newborn by vaginal delivery, two controls who underwent vaginal delivery immediately after the case were selected. Similarly, if the case delivered preterm newborns by LSCS, we immediately selected two controls delivered by LSCS.
Study Variables
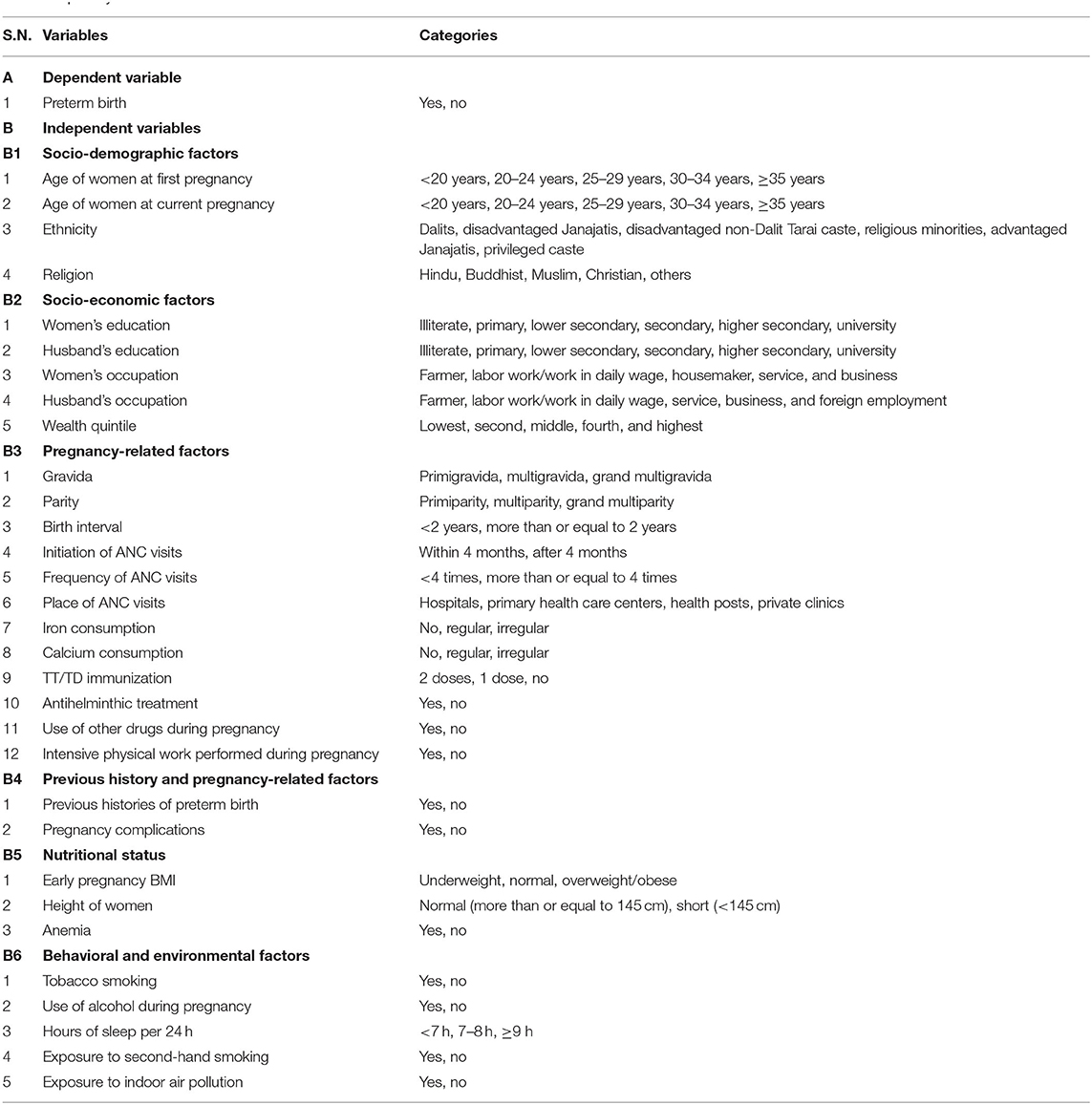
The outcome variable of the study was preterm birth. Independent variables included socio-demographic and socio-economic factors, pregnancy-related factors, previous history and antenatal complications-related factors, nutritional status, and behavioral and environmental factors. The study variables are presented in Table 1. Similarly, operational definitions of some variables are described in Supplementary Material File 1.
Operational Definition of the Variables
Iron Consumption
It was classified as no for women who did not consume iron tablets in their current pregnancy.
Iron consumption was marked as regular for women who started consuming iron at the start of the 4th month and were taking it daily, and irregular for women who did not take iron at the start of 4th month or women who were not consuming iron daily.
Calcium Consumption
Calcium consumption was classified as no for women who did not consume calcium tablets in their current pregnancy, regular for women who took calcium at the start of 4th month and were taking it daily, and irregular for women who did not take calcium at the start of 4th month or women who were not consuming it daily.
Drug Use
This was classified as the use of any kind of drug during pregnancy except folic acid, iron, calcium, and albendazole. It was dichotomized as yes or no.
Intensive Work Performed During Pregnancy
This was defined as any heavy work the women performed during pregnancy. This included fetching water with large buckets; lifting heavy loads; and washing clothes/utensils for long and labor-intensive work (construction work). For bivariate analysis, this variable was classified as performed intensive work and not performed intensive work.
Exposure to Indoor Air Pollution
It was adopted from the Nepal Demographic Health Survey 2011. Exposure to indoor air pollution was defined as women who reported that they cook inside the home using solid fuel (coal, lignite, charcoal, wood, and other traditional materials such as agricultural crop waste and animal dung).
Data Collection Methods
Participants were invited to participate in a face-to-face interview using a structured questionnaire which took around 30 min. Information was also abstracted referring to the ultrasonography (USG) report for the period of gestation, antenatal care (ANC) card for antenatal, medical, and reproductive histories, prenatal records for complications during pregnancy, and postnatal medical records for conditions of newborns. We adapted the study tool from NDHS, 2011 (15).
Data Management and Analysis
Collected data were entered in EpiData version 3.1 and analyzed using IBM Statistical Package for Social Science (SPSS) version 20 (IBM SPSS Statistics, USA). After importing data from EpiData, we conducted data checking, cleaning, editing, and recoding before analysis. We then conducted descriptive analysis in terms of frequency tables by splitting cases and controls. Frequency and percentages were calculated for the categorical variables and mean, and standard deviation (SD) was calculated for continuous variables. Chi-square test was performed to determine the difference in the exposure between cases and controls. Those variables with p-value < 0.1 were then fitted into the multivariable logistic regression model to identify the predictors of pre-term birth.
Backward conditional logistic regression analysis was performed and the probability of removal was set at p > 0.1. We carried out Hosmer and Lemeshow goodness of fit to ensure that the model was fit. The analysis was fitted in the backward logistic regression model, logit P (X) = β0+ β1X1 + β2X2 + β3X3 + β4X4 + …………. + βiXi, where P (X) is the log odds of the outcome variable, β0 is constant, βi is the regression coefficient, and Xi is the exposure variable.
Before fitting into the multivariable regression model, we checked the multi-collinearity of the variables, and the maximum variance inflation factor (VIF) obtained were 29.76 and 13.97 for birth spacing and parity, respectively. After removing the variable “birth spacing” from the model, the maximum VIF was 8.79 for parity, which was fitted in the model.
A total of 14 variables showed statistical significance in the bivariate analysis: Age of women at current pregnancy, educational status of women, gravida, parity, calcium consumption, antihelminthic treatment, use of drugs in pregnancy, intensive work during pregnancy, early pregnancy BMI, history of preterm birth, pregnancy-related problems, sleep hours per 24 h, exposure to second-hand smoking, and exposure to indoor air pollution. We fitted 13 variables into the multivariable regression model. Variable “history of preterm birth” was removed due to insufficient data in the control group.
Results
The proportions of extremely preterm (<28 weeks), very preterm (28– <32 weeks), moderate preterm (32– <34weeks), and late preterm (34– <37) in this study were 5.3, 12.8, 10.6, and 71.3%, respectively.
Socio-Demographic and Socio-Economic Characteristics
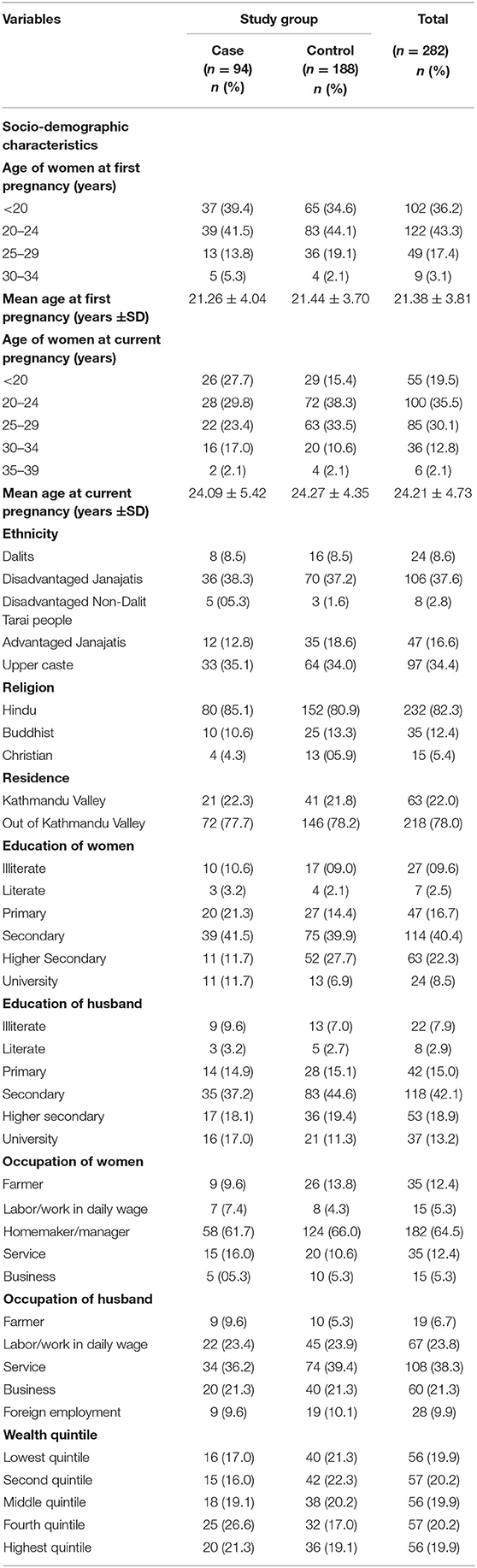
The mean age of study participants at their first pregnancy and current pregnancy were 21 and 24 years, respectively for both case and control groups. The majority (82.3%) of the study participants were Hindu. More than one-third of the study participants (37.6%) were from the disadvantaged Janajatis ethnic group with almost equal proportions in both case and control groups. Two out of five participants had completed secondary level education with similar proportions in cases and controls. Nearly two-thirds of the participants (64.5%) were homemakers with a slightly higher proportion in the control (66%) group than in the case (61.7%) group. One out of five study participants were in the lowest wealth quintile with more controls (21.3%) in the lowest wealth quintile than cases (17.0%) (Table 2).
Pregnancy-Related Characteristics
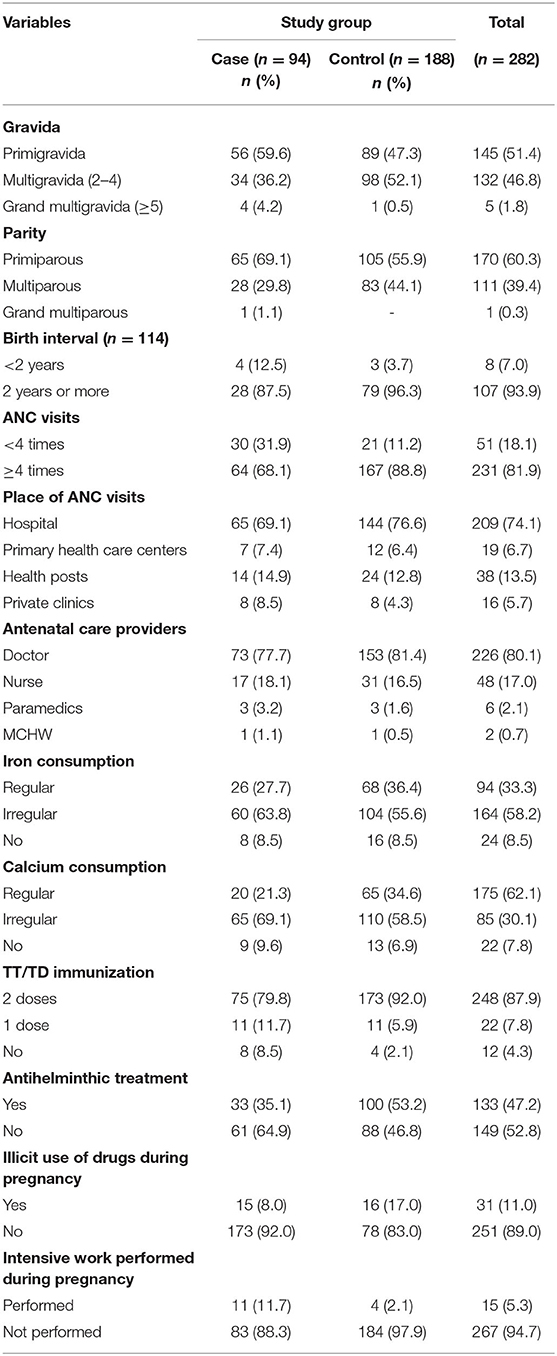
Among the case group women, 59.6% were primigravida, 36.2% were multigravida, and 4.3% were grand multigravida, while in the control group women, 47.3% were primigravida, 52.1% were multigravida, and 0.5% were grand multigravida. In the case group, 69.1% of women were primiparous, 29.8% were multiparous, and 1.1% were grand multiparous, while in the control group, 55.9% of women were primiparous and 44.1% were multiparous. Among multiparous and grand multiparous women, 12.5% of the case group and 3.7% of the control group reported <2 years of birth interval. Regarding pregnancy care, 31.9% of case group women and 11.2% of control group women had ANC check-ups <4 times. Likewise, 77.7% of case group women and 81.4% of control group women received ANC check-ups from doctors. The proportion of women who consumed iron regularly was 27.7% in the case group and 36.4% in the control group. Similarly, the proportion of women who consumed calcium regularly was 21.3% in the case group and 34.6% in the control group. Also, 8.5% of case group women were not immunized with tetanus toxoid (TT)/tetanus-diphtheria (TD) vaccine compared with 2.1% of control group women; and 64.9% of the case group women and 46.8% of the control group women did not have antihelminthic treatment. More women in the case group (11.7%) than those in the control group (2.1%) performed intensive work during pregnancy (Table 3).
Previous Histories and Antenatal Complication-Related Characteristics
Among study participants, 2.1% of women in the case group and 1.1% in the control group had a previous history of stillbirth; 10.6% of the case group and 8.5% of the control group women had a history of spontaneous abortion, and 9.6% of the women in the case group and 9% of the women in the control group had a history of induced abortion. Similarly, 6.4% of the women in the case group had a history of preterm birth compared to 0.5% in the control group. Also, 1.1% of the case group women and 1.6% of the control group had a history of delivering a baby with low birth weight.
The proportion of women who had antenatal complications was 45.7% in the case group and 30.9% among control group women. Likewise, 7.4% of the case group women had vaginal bleeding, while no women in the control group had vaginal bleeding. A similar finding was reported in the case of eclampsia, where 4.3% of the case group women had eclampsia, with none had it in the control group. The proportion of women who had severe gestational hypertension was 2.1% in the case group women and 0.5% in the control group. An almost equal proportion of the case group women (1.1%) and control group women (1.6%) had oligohydramnios while 6.4% of the case group women and 3.2% of the control had a urine infection during their pregnancy (Table 4).
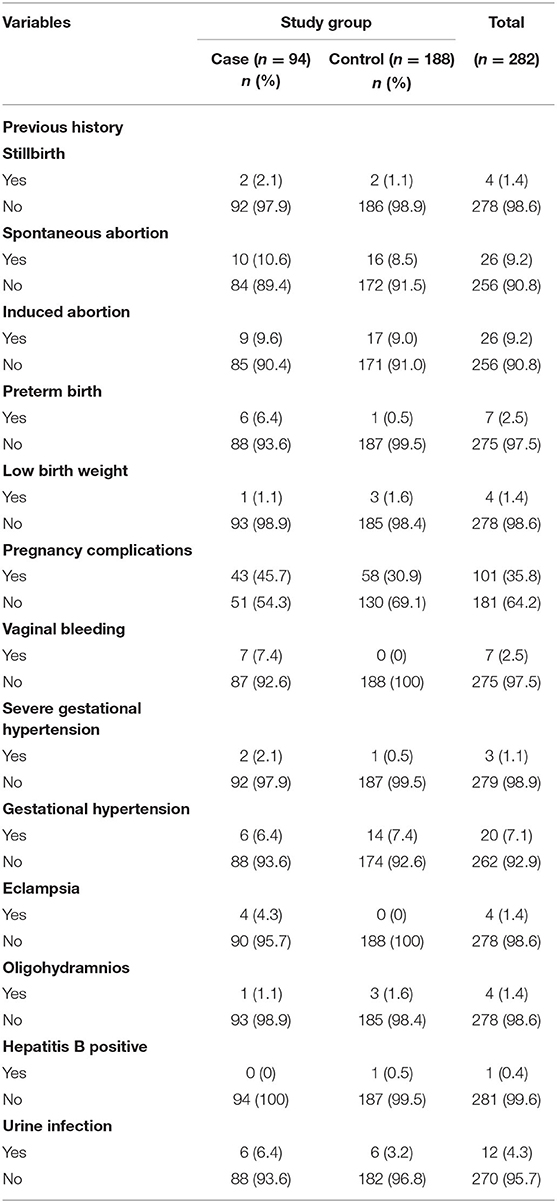
Table 4. Previous histories and antenatal complication-related characteristics of the study participants.
Nutritional Status
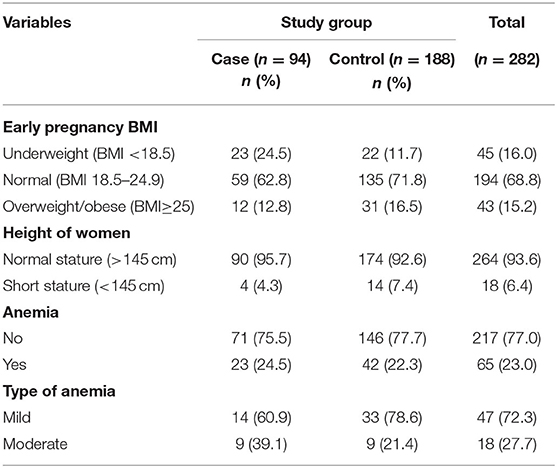
Among women in the case group, 24.5% were underweight and 12.8% were obese compared to 11.7% underweight and 16.5% obese in the control group women. More women in the control group (7.4%) had a short stature than those in the case group (4.3%). Women in the case group women (24.5%) had a higher proportion of anemia than those in the control group (22.3%) (Table 5).
Behavioral and Environmental Characteristics
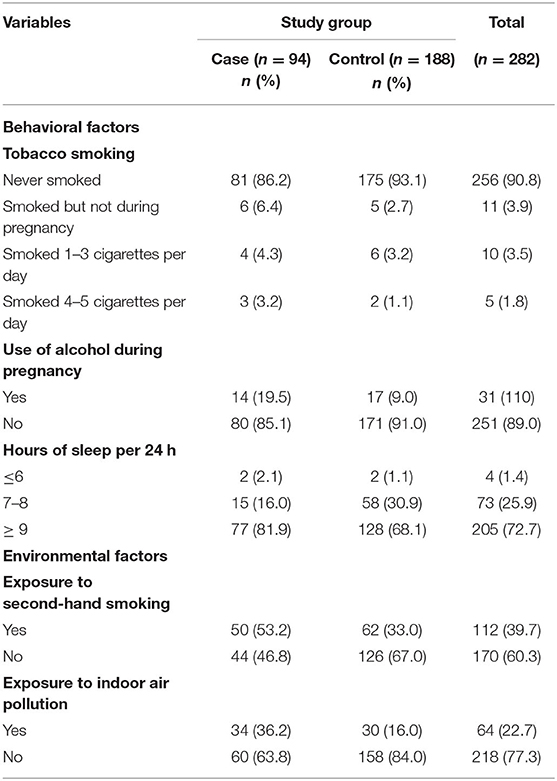
Among study participants, 86.2% in the case group and 93.1% in the control group had no smoking history. Among those who smoked, 3.2% of the case and 1.1% of the control group women smoked 4–5 cigarettes per day. One out of five women in the case group and one in 10 in the control group had a history of drinking alcohol during pregnancy. A nearly equal proportion of the case (2.1%) and control group women (1.1%) had <6 h of sleep.
A higher proportion of the case group (53.2%) was exposed to second-hand smoking than the control group (33%). Similarly, 36.2% of the case group and 16% of the control group were exposed to indoor air pollution (Table 6).
Risk Factors for Preterm Birth
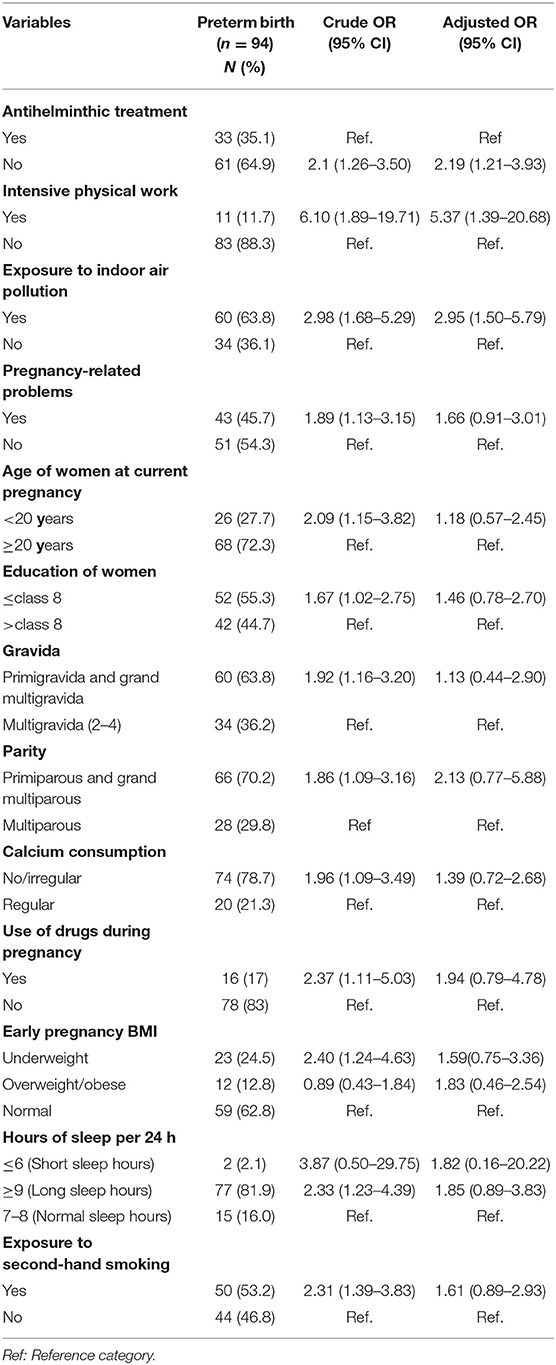
In adjusted analysis, antihelminthic treatment, intensive work performed during pregnancy, and exposure to indoor air pollution were identified as independent risk factors of preterm birth.
Those women who did not receive antihelminthic treatment had higher odds for preterm birth (AOR: 2.19, 95% CI: 1.21–3.93) than women who had antihelminthic treatment. Similarly, women who performed intensive work during their pregnancy were five times more likely to have a preterm birth than those who did not do intensive work (AOR: 5.37, 95% CI:1.39–20.68). In addition, exposure to indoor air pollution increased the risk of preterm birth (AOR: 2.95, 95% CI: 1.50–5.79).
Based on the findings, the final equation can be derived as
Preterm birth = −3.122 (constant) + 0.737 × X1 + 1.643 × X2 ++ 1.120 × X3
Where, X1 = antihelminthic treatment; X2 = intensive work performed during pregnancy; and X3 = exposure to indoor air pollution.
The final equation had a Nagelkerke R2 value of 0.325, showing that a 33% change in the outcome variable (preterm birth) was explained by exposure variables, i.e., no antihelminthic treatment during pregnancy, intensive work performed during pregnancy, and exposure to indoor air pollution. Hosmer and Lemeshow's goodness of fit was not significant (p = 0.221), indicating that the model fits the data (Table 7).
Discussion
This study identified risk factors of preterm birth among newborns in Nepal by using a case-control study design. Women who did not receive antihelminthic treatment during pregnancy, those exposed to indoor air pollution, and those who performed intensive work during pregnancy were identified as risk factors of preterm birth on our study of Nepalese women delivering in the tertiary hospital of Nepal.
The finding that women who did not have antihelminthic treatment were at higher risk of preterm birth was contrary to the Cochrane review (16) and Canadian study (17), which showed no association between antihelminthic treatment and preterm birth. However, very little information is available on the effect of antihelminthic treatment on preterm birth (18). Further research might be helpful to understand the relationship between antihelminthic treatment and preterm birth.
The risk of preterm birth was higher in women exposed to solid fuel while cooking. Our findings are consistent with the multicentric observational study done in Nepal (19) and other studies that provide evidence of solid fuels for cooking and increased risk of preterm birth (19, 20, 20–22). The combustion of biomass fuel emits high concentrations of airborne particulate matter and toxic chemicals, including carbon monoxide (CO), nitrogen dioxide (NO2), and sulfur dioxide (SO2). The oxygen content of maternal blood gets reduced when these pollutants are absorbed into the maternal bloodstream. Subsequently, oxygen delivery to the placenta is reduced, resulting in preterm delivery. It is biologically plausible that exposure to solid fuel smoke increases the risk of preterm birth (21). However, our findings and those of Liu et al. differ. The study done by Liu et al. in China showed no significant associations between solid fuels for cooking and preterm birth (23).
Our study showed that those women who performed intensive physical activity during their pregnancy had a significant association with the risk of preterm birth. A previous systematic review and meta-analysis showed that physically demanding work is associated with an increased risk of preterm birth (24). Studies done in Italy (25) and Iran (26) also found an increased risk of preterm birth in women employed in heavy work (25).
In our study, age of the women, ethnicity, religion, education, occupation, wealth quintile, gravid, parity, ANC visits, iron consumption, calcium consumption, TT/TD immunization, pregnancy-related problems, use of drugs during pregnancy, early pregnancy BMI, the height of women, anemia during pregnancy, past pregnancy-related characteristics, smoking, alcohol intake during pregnancy, sleep hours, and exposure to second-hand smoking did not show any significant association with preterm birth. As evidence on the predictors of preterm birth is limited in Nepal, we suggest additional studies to validate the findings.
There are some strengths and limitations of our study. The major strength of this study is that it employs a case-control study design. In the study, the recruitment of control immediately after the case helped to minimize the risk of selection bias. Also, we retrieved some of the clinical information from the maternity service register which helped reduce the recall bias. One of the study's limitations was that this study was conducted in a hospital in Kathmandu Valley and thus cannot be generalized to other parts of the country. Similarly, the classification of term birth and preterm birth was based on the women's recall of their LMP date, which might have affected the findings. The study does not distinguish between spontaneous and medically indicated preterm birth. The study did not assess whether the study participants had a helminthic infection. Only the administration of antihelminthic treatment was evaluated. Also, this study did not analyze other risk factors such as the mother's mental health status and other environmental factors such as heavy metal and pesticides.
Conclusion
This study found that preterm birth was protective in women who had consumed antihelminthic drugs as per ANC protocol. Exposure to indoor air pollution and intense physical activity during pregnancy were also significantly associated with preterm birth. Based on the study findings, we recommend that maternal health programs consider factors such as counseling pregnant women for antihelminthic consumption, taking rest during pregnancy, and reducing exposure to indoor pollutants. Further prospective cohort studies are needed in the future to identify the causes of preterm birth.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board, Tribhuwan University Teaching Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RA contributed to the design and implementation of the research, to the analysis of the results, and to the writing of the manuscript. PK contributed to the analysis of data and writing the manuscript. HB contributed to the analysis of data, writing the manuscript, and review of the article. AA contributed to the overall supervision of the research and review of the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge the cooperation of Paropakar Maternity and Women's Hospital staff while conducting this study. We would like to thank all the study participants for their participation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frph.2021.697419/full#supplementary-material
Abbreviations
ANC, antenatal care; AOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; C/S, cesarean section; ICU, intensive care unit; NDHS, Nepal Demographic Health Survey; OR, odds ratio; PCA, principal component analysis; PTB, preterm birth; RR, relative risk; SPSS, Statistical Package for Social Sciences; TD, tetanus diphtheria; TT, tetanus toxoid; VIF, variance inflation factor; WHO, World Health Organization.
References
1. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
2. Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. (2006) 118:1566–73. doi: 10.1542/peds.2006-0860
3. WHO. WHO recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Acta Obstetr Gynaecol. (1977). 56:7.
4. Moutquin JM. Classification and heterogeneity of preterm birth. Int J Obstetr Gynaecol. (2003) 110:30–3. doi: 10.1016/s1470-0328(03)00021-1
5. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. (2008) 371:261–9. doi: 10.1016/S0140-6736(08)60136-1
6. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. (2010) 88:31–8. doi: 10.2471/BLT.08.062554
8. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. (2012) 379:2151–61. doi: 10.1016/S0140-6736(12)60560-1
9. Howson C, Merialdi M, Lawn J, Requejo J, Say L. March of Dimes White Paper on Preterm Birth: The Global and Regional Toll. Wilmington, DE: March of dimes foundation (2009). p.13.
10. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A-B, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10:S2. doi: 10.1186/1742-4755-10-S1-S2
12. Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, Group GR. Global report on preterm birth and stillbirth (1 of 7): definitions, description of the burden and opportunities to improve data. BMC Pregn Childbirth. (2010) 10:S1. doi: 10.1186/1471-2393-10-S1-S1
13. Sather M, Fajon A-V, Zaentz R, Rubens CE. Global report on preterm birth and stillbirth (5 of 7): advocacy barriers and opportunities. BMC Pregn Childbirth. (2010) 10:1–10. doi: 10.1186/1471-2393-10-S1-S5
14. Rao CR, de Ruiter LE, Bhat P, Kamath V, Kamath A, Bhat V. A case-control study on risk factors for preterm deliveries in a secondary care hospital, southern India. Int Scholar Res Notices. (2014) 2014:935982. doi: 10.1155/2014/935982
16. Haider BA, Humayun Q, Bhutta ZA. Effect of administration of antihelminthics for soil transmitted helminths during pregnancy. Cochr Datab Syst Rev. (2009) 18:CD005547. doi: 10.1002/14651858.CD005547.pub2
17. Gyorkos TW, Larocque R, Casapia M, Gotuzzo E. Lack of risk of adverse birth outcomes after deworming in pregnant women. Pediatr Infect Dis J. (2006) 25:791–4. doi: 10.1097/01.inf.0000234068.25760.97
18. Barros FC, Bhutta ZA, Batra M, Hansen TN, Victora CG, Rubens CE, et al. Global report on preterm birth and stillbirth (3 of 7): evidence for effectiveness of interventions. BMC Pregn Childbirth. (2010) 10:S3. doi: 10.1186/1471-2393-10-S1-S3
19. Gurung A, Wrammert J, Sunny AK, Gurung R, Rana N, Basaula YN, et al. Incidence, risk factors and consequences of preterm birth–findings from a multicentric observational study in Nepal. Arch Public Health. (2020) 78:64. doi: 10.21203/rs.2.22050/v2
20. Wylie BJ, Coull BA, Hamer DH, Singh MP, Jack D, Yeboah-Antwi K, et al. Impact of biomass fuels on pregnancy outcomes in central East India. Environ Health. (2014) 13:1. doi: 10.1186/1476-069X-13-1
21. Jiang M, Qiu J, Zhou M, He X, Cui H, Lerro C, et al. Exposure to cooking fuels and birth weight in Lanzhou, China: a birth cohort study. BMC Public Health. (2015) 15:712. doi: 10.1186/s12889-015-2038-1
22. Hussein H, Shamsipour M, Yunesian M, Hasanvand MS, Fotouhi A. Association of adverse birth outcomes with exposure to fuel type use: a prospective cohort study in the northern region of Ghana. Heliyon. (2020) 6:e04169. doi: 10.1016/j.heliyon.2020.e04169
23. Liu W, Huang C, Cai J, Wang X, Zou Z, Sun C. Household environmental exposures during gestation and birth outcomes: A cross-sectional study in Shanghai, China. Sci Tot Environ. (2018) 615:1110–8. doi: 10.1016/j.scitotenv.2017.10.015
24. Van Beukering M, Van Melick M, Mol B, Frings-Dresen M, Hulshof C. Physically demanding work and preterm delivery: a systematic review and meta-analysis. Int Arch Occup Environ Health. (2014) 87:809–34. doi: 10.1007/s00420-013-0924-3
25. Di Renzo GC, Giardina I, Rosati A, Clerici G, Torricelli M, Petraglia F, et al. Maternal risk factors for preterm birth: a country-based population analysis. Eur J Obstetr Gynecol Reprod Biol. (2011) 159:342–6. doi: 10.1016/j.ejogrb.2011.09.024
Keywords: preterm birth, risk factors, Nepal, delivery, newborn
Citation: Acharya R, Khanal P, Bhattarai HK and Amatya A (2021) Risk Factors of Preterm Birth in Nepal: A Hospital-Based Matched Case-Control Study. Front. Reprod. Health 3:697419. doi: 10.3389/frph.2021.697419
Received: 19 April 2021; Accepted: 25 June 2021;
Published: 30 August 2021.
Edited by:
Stefania Papatheodorou, School of Public Health and Harvard University, United StatesReviewed by:
Felipe Vadillo-Ortega, Universidad Nacional Autónoma de México, MexicoMatthew J. Blitz, Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, United States
Chau-Ren Jung, China Medical University, Taiwan
Copyright © 2021 Acharya, Khanal, Bhattarai and Amatya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richa Acharya, cmVlY2hhLmFjaGFyeWFAZ21haWwuY29t
 Richa Acharya
Richa Acharya Pratik Khanal
Pratik Khanal Hari Krishna Bhattarai
Hari Krishna Bhattarai Archana Amatya
Archana Amatya