
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Remote Sens. , 23 May 2022
Sec. Acoustic Remote Sensing
Volume 3 - 2022 | https://doi.org/10.3389/frsen.2022.862435
This article is part of the Research Topic Acoustic Remote Sensing of Cetacean and Pinniped Populations View all 13 articles
Passive acoustic methods enable remote monitoring of marine species and habitats. These methods can be applied to investigate distribution and abundance of populations, to evaluate behavioral and physiological states of individuals, and to inform management efforts for animals that live in hard-to-reach places. Spotted seals (Phoca largha) inhabit high-latitude, light-limited sub-Arctic and Arctic waters and move seasonally with unstable sea ice. They are high trophic level predators vulnerable to changing conditions associated with environmental warming. At present, an incomplete characterization of the spotted seal vocal repertoire limits our ability to monitor this species acoustically. Captive studies can inform passive acoustic efforts by describing fundamental features of species-typical vocalizations emitted by known individuals. These features include acoustic parameters as well as developmental, seasonal, and sex-specific patterns in vocal behavior. Here, we studied several male spotted seals in captivity from age 6 months through adulthood (10 years). Vocal behavior was scored daily and opportunistically recorded. The production of underwater calls emerged during sexual maturation, at age 4. To evaluate vocal repertoire and fine-scale temporal patterns of sound production in adult seals, an underwater acoustic recorder was continuously deployed with two seals at age 7 years. The spotted seals produced at least eight distinctive underwater call types with dominant energy below 1 kHz. The amplitude of the most common vocalization was ∼140 dB re 1 μPa (sound pressure level at 1 m). There was a marked peak in vocal activity in springtime, prior to onset of the annual molt. This period coincided with increased aggressive behavior, presence of a notable musky odor, and urogenital swelling indicative of heightened reproductive status. These results from developing male spotted seals reared in human care confirm the production of recognizable, stereotypic underwater calls associated with the breeding season. Description of vocal behavior improves knowledge of this species’ biology, and informs the potential use of autonomous acoustic recorders to track the presence and movements of free-ranging spotted seals in remote habitats.
Passive acoustic methods provide powerful tools for studying the distribution and relative abundance of marine mammals. In some cases, such methods are the only feasible way to consistently monitor otherwise inaccessible underwater environments (e.g., Stirling et al., 1983; Mellinger et al., 2007; Frouin-Mouy et al., 2019). However, in order to utilize passive acoustics, details of vocal behavior must be available for the target species.
Spotted seals (Phoca largha, Pallas 1811)—like other phocid (true) seals—likely rely on underwater vocalizations for territorial defense, mate attraction, and competitor deterrence. However, the acoustic ecology of spotted seals remains largely unknown. This knowledge gap can be attributed to their association with unstable pack ice in polar regions, their presumed aquatic breeding habits, and their overlapping distribution in sub-Arctic regions with the closely related harbor seal (Phoca vitulina). A few prior studies have evaluated vocal activity in this species. In the 1970s and early 1980s, a male-female pair was observed in captivity1; the resulting long-term data set included an initial description of vocalizations (Beier and Wartzok, 1979; Gailey-Phipps, 1984). While this account focused primarily on reproductive behavior, it did identify up to eight potential underwater call types. These sounds ranged in duration from tens of milliseconds up to several seconds, with dominant frequencies between 500 and 3,500 Hz. Vocalizations were produced by the adult male and female throughout the year and predominantly during the breeding season, with highest calling rates between February and mid-April.
More recently, there has been one study of airborne sound production in captive spotted seals (Zhang et al., 2016) and two field studies of underwater sound production in wild individuals (Yang et al., 2017; Yang et al., 2022). The latter described four call types detected from groups of spotted seals in the wild. These underwater calls had simple, pulsed structures, were generally short in duration (<300 ms on average), and had dominant energy between approximately 300 and 500 Hz. Two of the call types observed—the growl and the drum—were consistent with underwater calls described previously for captive individuals (Beier and Wartzok, 1979; Gailey-Phipps, 1984). These recordings of free-ranging seals in Liaodong Bay, China, obtained during the breeding season, provide important ecological context to descriptions of spotted seal vocal activity. However, field recordings are typically made in variable ambient conditions and at various distances from vocalizing individuals, which can complicate efforts to describe the source characteristics of specific calls. Furthermore, these valuable but opportunistic data were collected from groups of seals recorded over just a few days, and thus cannot provide information about the age or sex of calling individuals or temporal patterns in calling behavior.
The limited available data describing underwater sound production in spotted seals suggests that acoustic monitoring could be useful for this species. However, there is variability between these reports; limited information concerning age, sex, and calling rate of individuals; and a lack of high-quality, species-typical call exemplars to inform species identification and automated call detection. As a result, spotted seals cannot yet reasonably be included in passive acoustic monitoring in Arctic and sub-Arctic regions. Such efforts have proven valuable for investigating seasonal occurrence and movement patterns for related species including bearded (Erignathus barbatus), ringed (Pusa hispida), and ribbon seals (Histriophoca fasciata) in similar environments (e.g., Stirling et al., 1983; Miksis-Olds and Parks, 2011; MacIntyre et al., 2013; Frouin-Mouy et al., 2019), as well as for seals in the Antarctic (e.g., Klinck et al., 2010; Van Opzeeland et al., 2010). With expected industrialization and militarization of Arctic waters as sea ice retreats, the ability to monitor seals acoustically will likely be of increasing importance for conservation efforts.
In this study, our aims were to build upon prior research with this species to describe temporal and developmental patterns in vocal activity over scales of days, seasons, and years, and to characterize the fundamental features of species-typical underwater vocalizations in spotted seals.
The primary subjects of this study were two male spotted seals identified as Amak (NOA0006675) and Tunu (NOA0006674), who were observed in captivity from age 6 months through 10 years. Both seals were stranded as newborn pups (<48 h old) in Alaska in April 2010, and were rehabilitated at the Alaska SeaLife Center in Seward. They were subsequently transferred to Long Marine Laboratory at the University of California Santa Cruz in September 2010 to participate in behavioral research. In December 2015 these 5-year-old seals were transferred back to the Alaska SeaLife Center, where they continued to contribute to cooperative studies of health and physiology. At both facilities, Amak and Tunu were trained using operant conditioning methods and positive reinforcement to voluntarily participate in husbandry and research sessions. They were provided a daily diet of freshly thawed fish and vitamin supplements that was established to maintain optimal health.
Supplemental data were obtained from one additional male spotted seal, Kunik (NOA0010310), who stranded as a pup in spring 2015 and was rehabilitated and then housed separately at the Alaska SeaLife Center.
Bioacoustics research was conducted with the approval and oversight of the Institutional Animal Care and Use Committees at the University of California Santa Cruz and the Alaska SeaLife Center; with authorization from the National Marine Fisheries Service of the United States under marine mammal research permits 14535, 18902, and 23554; and with expressed support from the Ice Seal Committee, a tribally authorized Alaska Native co-management organization. Research was conducted opportunistically and without harm to seals.
The emergence and development of vocal behavior were evaluated as Amak and Tunu transitioned from young of the year (<1 year old) seals to sexually mature adults. For the first 5 years of this study, these two spotted seals were housed outdoors at Long Marine Laboratory in flow-through seawater pools (10–18°C) with adjacent haul-out space. While they had occasional exposure to other pinnipeds—including California sea lions (Zalophus californianus), harbor seals, and ringed seals—the spotted seals were typically housed together and all data were obtained in the absence of other conspecifics.
We scored vocal activity daily between age 6 months and 5 years. The two seals were monitored 7 days per week, 365 days per year, intermittently during daylight hours. Trained observers noted the presence or absence of airborne and underwater vocalizations produced by each seal so that the proportion of days per month with observed vocal behavior could be evaluated over time. Vocalizations were clearly audible to human listeners within 30 m of the seals’ living enclosure.
During April and May of 2014 and 2015, when Amak and Tunu were 4–5 years old, we obtained close-range (≤4 m) recordings of their spontaneous underwater calls. These opportunistic recordings were made in a circular, partially in-ground, epoxy-lined concrete pool of 1.8 m depth and 7.6 m diameter. A self-powered 2270 sound analyzer (sampling rate 48 kHz; Brüel & Kjær A/S) was paired with a calibrated TC4032 low-noise hydrophone (0.01–80 kHz, ±2.5 dB; Teledyne Reson) fixed at a depth of 1 m. Only one seal was in the pool during each recording session, and he was observed from a viewing blind so that his distance and orientation to the hydrophone could be noted for each spontaneous vocalization. Efforts were made to minimize background noise (e.g., by reducing water flow and nearby activity) during recordings. Median ambient noise spectral density levels in the environment—measured with the TC4032 hydrophone and 2270 sound analyzer—generally decreased with increasing frequency from 81 dB re (1 μPa)2/Hz at 40 Hz to 39 dB re (1 μPa)2/Hz at 2 kHz.
Recordings were visually and aurally reviewed in Adobe Audition and subjectively scored. Excellent-quality calls with signal-to-noise ratio (SNR) > 10 dB were analyzed in Raven Pro 1.5 (Center for Conservation Bioacoustics, Cornell Lab of Ornithology, Ithaca, NY). Received sound pressure level (SPL, dB re 1 μPa) was measured over the 90% duration of each call and referenced to a calibration tone generated with a 42AA pistonphone (GRAS Sound and Vibration) and recorded on the same system. Amplitudes for recordings obtained 1 m from the hydrophone receiver were considered source levels.2 For recordings made between 1 and 4 m, a simple propagation loss equation was used to estimate the SPL at 1 m; transmission loss was calculated as 20logR based on the short distances over which signals propagated.
We collected detailed husbandry and acoustic data for the two sexually mature spotted seals (age 5–10 years); these were later reviewed to describe vocal behavior and associated reproductive status. At the Alaska SeaLife Center these seals were housed in an outdoor exhibit with a large, irregularly shaped concrete pool (7.3 m length, 5.3 m diameter, 5.3 m depth) with adjacent haul-out space, which was filled with flow-through natural seawater (ambient temperature 4–11°C). No other marine mammals were typically housed in this pool.
In addition to continued documentation of the presence and absence of vocalizations, we monitored other behavioral indicators of reproductive status daily in the adult seals. The occurrence of a strong odor/musk was routinely tracked beginning at age 5 (in January 2016), while apparent urogenital swelling was scored from age 6 (January 2017). Prior to this, qualitative husbandry records were maintained that included notations of these and other indicators of breeding season.
The presence and absence of vocal activity, odor/musk, and urogenital swelling were also monitored daily for the younger seal Kunik between December 2015 and April 2020 (age 8 months to 5 years).
To document year-round temporal patterns in vocal activity and enable characterization of the vocal repertoire of the two sexually mature male seals, we deployed an underwater acoustic recorder for approximately 14 months in the seals’ living enclosure at the Alaska SeaLife Center. This research was conducted from March 2017 to April 2018, when the seals were ∼7–8 years old.
A SoundTrap 202 or 300 STD self-powered acoustic recorder (0.02–60 kHz, +/- 3 dB; Ocean Instruments) was used to record the spontaneous underwater vocalizations of the two spotted seals. The instrument was programmed to record 30 min every hour with a sampling rate of 48 or 96 kHz and high preamplifier gain setting. The SoundTrap was mounted in the enclosure at approximately 4.5 m depth within a water-filled PVC pipe in a concrete base, which left only the hydrophone exposed.
A visual-aural inspection of waveforms and spectrograms was conducted in Adobe Audition for the entire acoustic data set. Vocalizations were defined as continuous units of biological sound separated by silent periods, and environmental noises were excluded from analysis. If other marine mammals were placed temporarily in the spotted seals’ primary living enclosure, this was noted in the husbandry records and the corresponding acoustic data were excluded from analysis.
Manual call counts from 24 30-min acoustic files (12 h of recordings) per day were used to evaluate temporal patterns in vocal activity. The number of calls in every 30-min file was noted. To investigate seasonal changes in vocal activity, the mean number of calls per 30 min file was evaluated for each month of the data set. The mean number of calls per 30 min file for each hourly interval was summarized monthly to reveal diel patterns in calling behavior.
Acoustic files were reviewed by trained observers (n = 16), with secondary quality control checks performed on 20% of data files. Every detected call was logged and perceptually classified as one of eight potential sound types; categories were based on consistent spectral and temporal features and followed prior studies when applicable. These call types were identified as burp, drum, growl, grunt, knock, moan, pulse, and rumble. A few other sounds were observed in the data set; these were noted but not included in further acoustic analyses due to low encounter rates or poor SNRs that prevented spectrographic analysis. Each vocalization was scored for subjective quality. Complete vocalizations labeled as excellent-quality signals (SNR >10 dB, all parameters of the spectral contour identifiable) were then extracted for further analysis to formally describe the eight perceptual call types (n = 20 for each type). For these calls, 17 spectral and temporal features were measured by a single analyst using Raven Pro 1.5: total duration, 90% duration, center frequency, inter-quartile range bandwidth (and 1st and 3rd quartile frequencies), 90% bandwidth (and lower and upper frequency bounds), peak frequency, 3-dB bandwidth (and lower and upper frequency bounds), 10-dB bandwidth (and lower and upper frequency bounds), and aggregate entropy. Minimum and maximum frequency were not estimated since they were not always discernable within ambient noise; 90%, 3-dB, and 10-dB bandwidths and quartiles thus provided more robust frequency measures for these calls. Temporal parameters were measured from the waveform, while spectral parameters were measured from either the spectrum or spectrogram [Hann window; DFT size 4096 or 8192 samples depending on sampling rate (85.5 ms); 90% overlap; 3-dB filter bandwidth 16.9 Hz]. Spectral features were always measured over the 90% call duration to avoid subjective measurement of call duration.3
Additional acoustic parameters were measured for a subset of calls. Specifically, the presence or absence of harmonics was noted, and the number of harmonics and fundamental frequency were determined when applicable (for moans, pulses, growls, and rumbles). For call types that occurred in bouts (knocks and burps), the inter-unit interval, number of units per bout, and number of units per second were measured.
Discriminant function analysis (DFA) was used to validate the eight perceptual vocal types based on the 17 acoustic parameters measured for all call categories.4 A cross-validated DFA using the leave-one-out method was computed, which provided a classification matrix indicating how well the measured variables separated into the pre-assigned call categories. A DFA (without cross-validation) was run to plot these vocalizations in acoustic space, with the eight perceptual call types as group identifiers and the acoustic measurements as discriminant variables. A one-way ANOVA (not assuming equal variances) was used to test for significant differences between linear discriminants LD1 and LD2.
Patterns of in-air and underwater call production are depicted in Figure 1 for spotted seals Amak and Tunu between ages 6 months and 5 years. The developing seals produced airborne vocalizations year-round, beginning in their first year of life. These calls were variable, generally short-duration sounds resembling grunts and moans (see Section 3.2.2. for description of underwater call types). Their observed vocal behavior increased over successive years, with annual peaks in calling evident in spring and summer, reaching a maximum near the onset of the annual molt (see Figure 1). In particular, the production of underwater calls showed clear developmental and seasonal patterns. Stereotyped underwater vocal behavior emerged for both seals at age four, with calls produced most often in April.
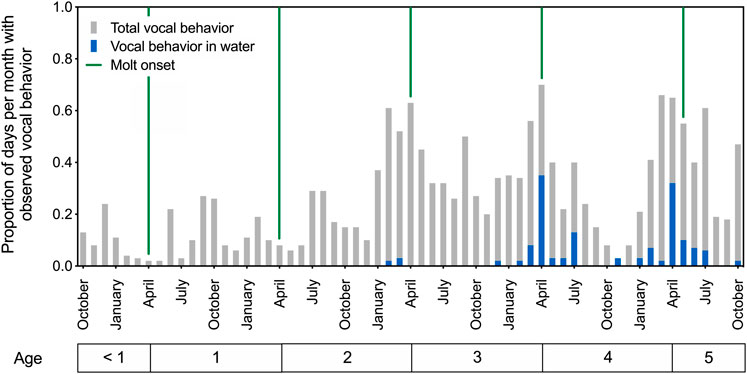
FIGURE 1. Patterns of call production for two developing male spotted seals from ages 6 months to 5 years. The proportion of days per month with observed vocal behavior is plotted over time, with each bar representing a 1-month period and providing averaged data for the two seals. Green vertical lines indicate the onset of annual molt and presumed end of the breeding season. Total vocal behavior (in air or water) increased as the seals aged, and underwater vocal production emerged with presumed sexual maturity around age 4. Peak underwater calling activity was observed in April at ages 4 and 5.
In 2014 and 2015, when the seals were ages 4–5 years, 199 spontaneous underwater calls were opportunistically recorded with visual confirmation. Of these, 91% were perceptually categorized as growls and the remainder as grunts or moans. Source level analysis was conducted for the growl vocalization based on 140 high-quality growls recorded at known distance (≤4 m) during April and May 2015, when the spotted seals were 5 years old. Estimated sound pressure level at 1 m over the 90% call duration was 142 ± 6 dB re 1 μPa (95% confidence interval 142–143 dB re 1 μPa; range 129–161 dB re 1 μPa).
Similar to the early observational data, results for the two adult seals showed a peak in vocal activity in the spring, prior to the yearly molt and overlapping with the reported breeding season for this species (reviewed in Boveng et al., 2009). The springtime period prior to the onset of molt also coincided with increased aggressive behavior, urogenital swelling, and a notable musky odor for these two seals (Figure 2, upper and middle panels). Husbandry staff described this odor as pungent and heavy in the air; reminiscent of a skunk’s spray and at times very metallic; and easily transferred to clothing after contact with urine, bowel movements, saliva, or mucus (personal communication, J. Kim). Figure 2 (lower panel) tracks the proportion of days per month with observed breeding status indicators for Kunik as he developed from the first year of life to a sexually mature 5-year-old male.
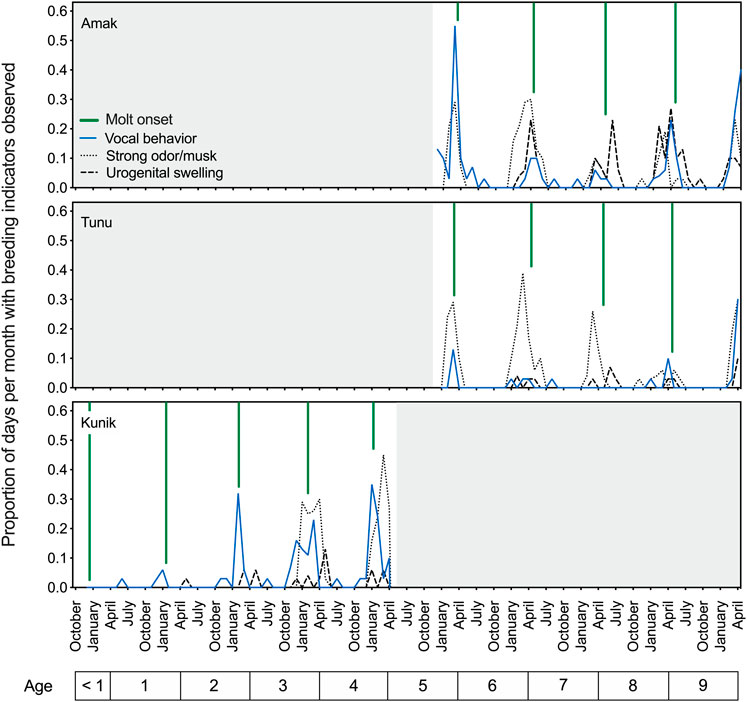
FIGURE 2. Temporal patterns in breeding indicators observed in three male spotted seals over an interval of 5 years (December 2015 to April 2020). The proportion of days per month when vocal behavior (in air or water), strong musky odor, or urogenital swelling were noted by husbandry staff are plotted as separate lines over time, and are referenced to the age of each seal (noted below). Across individuals, there is clear temporal overlap between these three cues, with their annual peaks also corresponding well with the presumed breeding season for this species and declining around molt onset (denoted by the green vertical lines) each year. These descriptive husbandry data (scored daily) demonstrate the observed presence of three breeding indicators over time and provide a relative indication of their magnitudes.
Husbandry data opportunistically confirmed the production of sperm—as evidenced by presence in urine samples—for all three seals (at ages 5–10) between mid-February and early June, providing further indication of heightened reproductive status during this time of year.
A total of 86,072 vocalizations were identified in 4,486 h of year-round acoustic recordings from the two adult seals. The maximum number of vocalizations in any 30-min recording interval was 411 (13.7 calls/min), which occurred in March (14 March 2018 at 0330). This file also contained the most knock bouts of any 30-min file: 239 bouts comprising 709 individual knocks. During times of peak vocal activity, calling rates for the two male seals typically reached 7.6 calls per minute. However, 74% of files contained no calls. Although these results are essentially pooled across subjects, the acoustic data did show evidence of two individuals calling: at times, overlapping vocalizations of different types or amplitudes were observed, which presumably were produced by the two seals simultaneously.
There was a strong seasonal pattern in underwater calling behavior for the adult seals (Figure 3). Peak call production was observed in April, during the suspected breeding season. Subsequently, calling rate remained high before dropping off precipitously with the onset of the annual molt around 15 May. Few (or no) vocals were produced each hour during summer or fall, with rates of call production beginning to increase again in February and March. This overall pattern is shown in Figure 3 for all vocalizations, but the same general trend was observed for each call type. This annual timing of vocal activity is consistent with that reported by Gailey-Phipps (1984) for two captive spotted seals. Note that while vocal activity data reported in Figure 3 are related to those presented in Figure 2, vocal counts cannot be compared quantitatively. The observational data (Figure 2) were obtained during daylight hours by husbandry staff, while the more continuous acoustic data (Figure 3) were recorded throughout 24-h periods regardless of nearby activity.
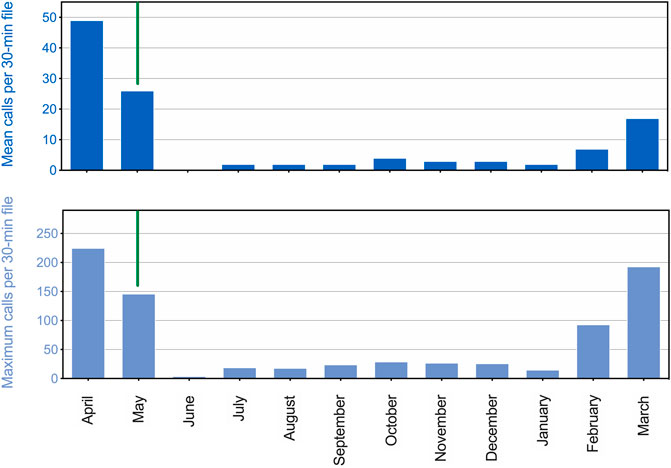
FIGURE 3. Seasonal patterns in call production for two adult male spotted seals. The mean number of calls per 30-min file is shown for a 12-month period (April 2017 through March 2018) in the upper panel. The maximum number of calls per 30-min file per day was also averaged over each month, and is shown in the lower panel. A vertical green line separates the end of the suspected breeding season (left) from the onset of the molting period (right), which began on 15 May 2017.
The acoustic recordings revealed clear diel patterns in calling behavior (Figure 4). Underwater calls were detected throughout day and night, but calling rate was highest during nighttime hours; this was especially evident between February and May, when overall rates of call production increased substantially. During this period, extended series of vocalizations (particularly growls) were often observed in the data set, with the majority of calls produced between 2100 and 0600 overnight. Diel patterns are shown in Figure 4 for all vocalizations combined, but the same general trend was observed for each call type. Notably, although staff hours varied throughout the year based on available daylight, observed peaks in diel calling behavior consistently occurred in darkness, when husbandry staff were offsite.
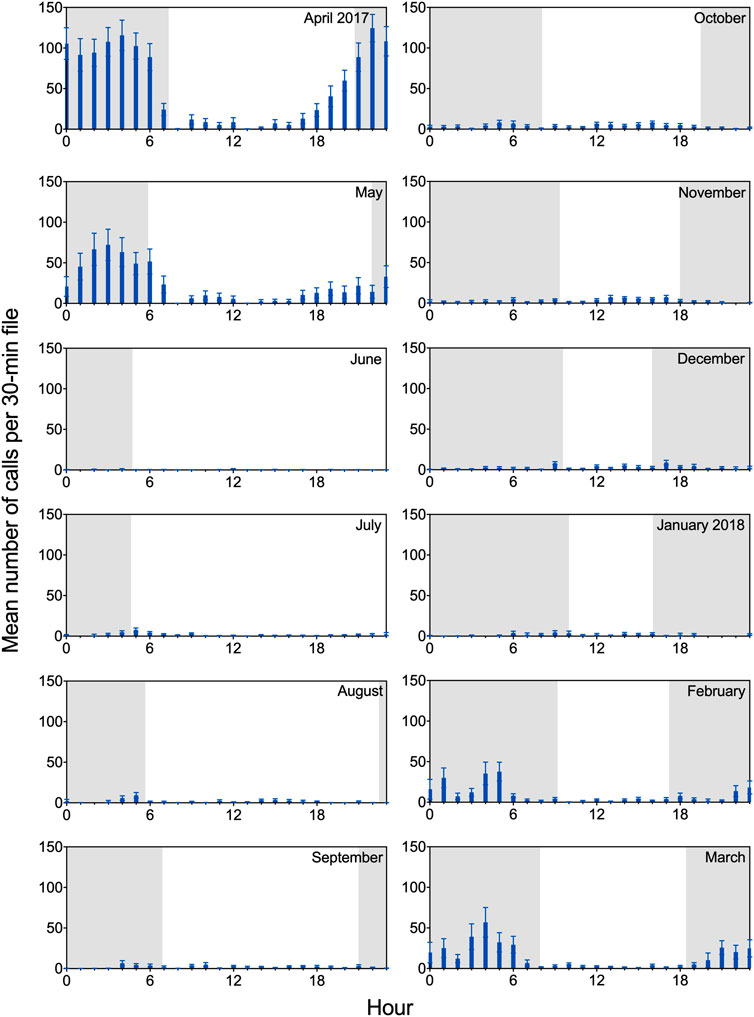
FIGURE 4. Diel patterns in calling for two adult male spotted seals. Mean (and SEM) number of calls per 30-min file is shown for a 12-month period (April 2017 through March 2018), binned in hourly intervals. Each month includes 427–744 30-min files. Shading represents photoperiod, with nighttime intervals shaded. Observed peaks in diel calling behavior occurred during darkness when husbandry staff were offsite. For example, for the month of April—when maximum calling rate was observed—Amak and Tunu typically received their daily training sessions between 0800 and 1700, while maximum vocal activity occurred between 2100 and 0600. Molt occurred between 15 May and 20 June.
The two spotted seals exhibited a vocal repertoire of at least eight distinctive call types, as described individually below and shown in Figure 5; Table 1, and Supplementary Audio S1. These underwater vocalizations were predominantly low in frequency—with most energy emitted below 1,000 Hz—and had total durations ranging from 0.07 to 6.5 s.
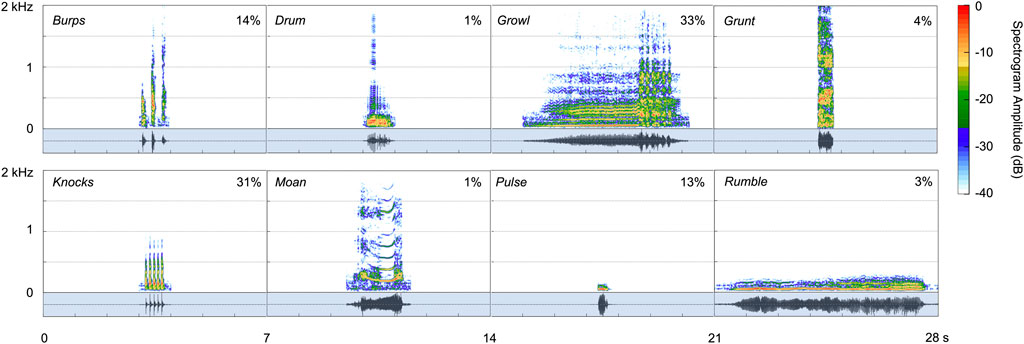
FIGURE 5. Spectrograms for the eight call types produced under water by two male spotted seals (Hanning window, sampling rate 48 kHz, window length 4,096 points, 90% overlap). Corresponding waveforms, shown in the shaded regions below the spectrograms, represent the relative amplitude of each vocalization. The proportion of each call type in the data set (out of 86,072 vocalizations identified) is shown as a percentage in each panel.
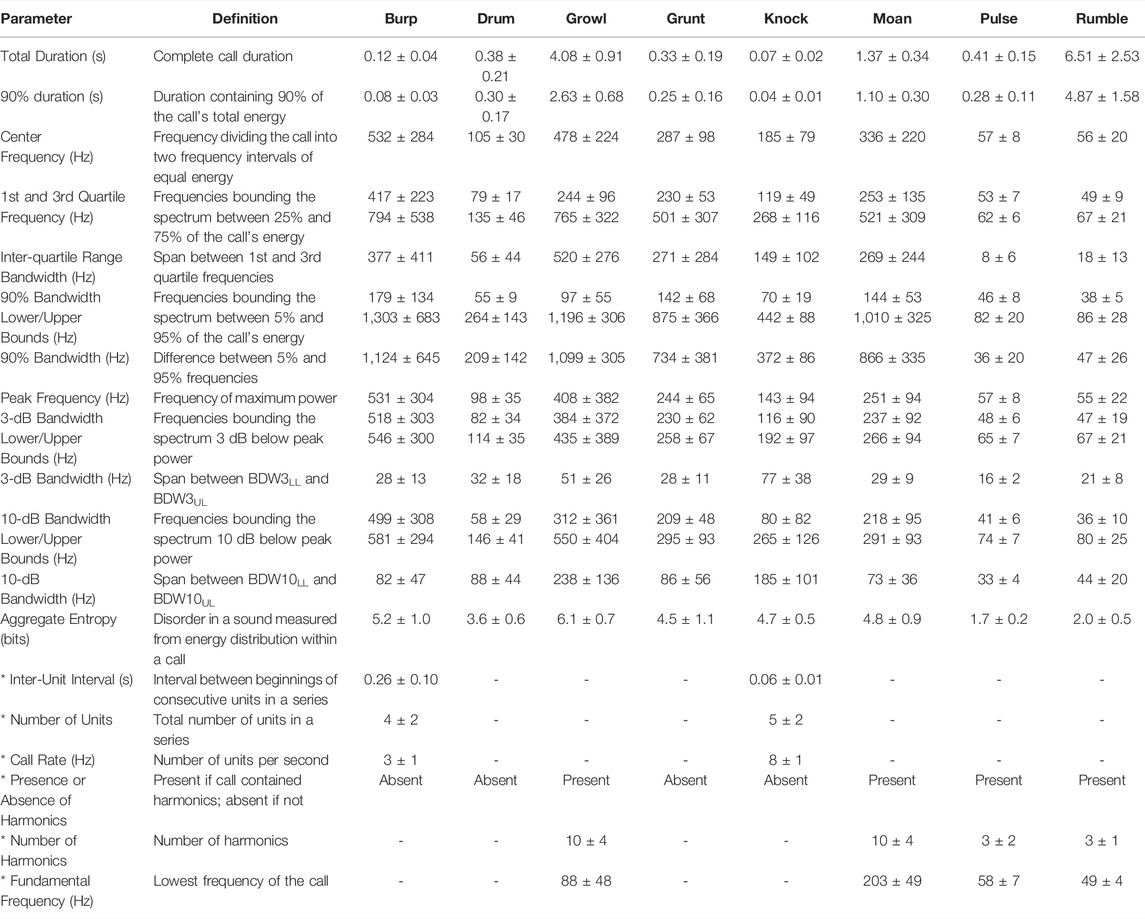
TABLE 1. Mean (±SD) values of acoustic parameters measured for eight call types produced under water by two male spotted seals. Only high-quality calls with SNR >10 dB were analyzed (n = 20 per call type). All parameters were measured with Raven Pro 1.5 software (Hann window; DFT size 4,096 or 8,192 samples depending on sampling rate (85.5 ms); 90% overlap; 3-dB filter bandwidth 16.9 Hz). Asterisks (*) denote call variables that were either discrete or those that were measured only for certain call types; these parameters were used to describe vocalizations but were not used in the DFA to validate call types.
Growls (see Supplementary Video S1) were guttural calls with an initial harmonic component and a terminal series of pulses—qualitatively similar to the growls and roars produced by wild and captive spotted seals (Beier and Wartzok, 1979; Gailey-Phipps, 1984; Yang et al., 2017) and harbor seals (Hanggi and Schusterman, 1994; Casey et al., 2021), respectively. These relatively long-duration (90% duration: 2.6 s), broadband calls contained 90% of their energy between 97 and 1,196 Hz and had a peak frequency of 408 Hz. Growls had the highest aggregate entropy—a measure of the disorder of a sound—of all call types (6.1 bits). This was the most common vocalization for the adult spotted seals, as it was when they began producing underwater calls, with growls comprising 33% of detected vocalizations. Similarly, this was the most common call produced by captive spotted seal males observed in prior studies (Beier and Wartzok, 1979; Gailey-Phipps, 1984).
Knocks were the second most common call type, making up 31% of identified vocalizations. These very brief, impulsive calls (90% duration: 0.04 s)—which sound like someone knocking rapidly on a wooden door—could appear as a single element but were most often produced in bouts of two or more units with an inter-unit interval of 0.06 s; the average number of knocks per bout was 4, and the maximum number in series was 30 during the year-long deployment. Knocks have been recorded from wild spotted seals, with bouts including 3 to 50 pulses (Yang et al., 2017; Yang et al., 2022).
Burps were short-duration calls that appeared singularly or in bouts. Individual burps were longer than knocks (90% duration: 0.08 s) and the units within a bout were more spaced out in time (inter-unit interval: 0.26 s). Two burps were produced per bout on average, with a maximum of 32 in series during the year-long deployment. These calls had the widest 90% bandwidth of any call type (1,124 Hz, extending from 179–1,303 Hz) and high aggregate entropy (5.2 bits)—second only to growls. Burps comprised 14% of all identified vocalizations. They have not been described previously for captive or wild spotted seals.
Pulses—another new vocal type—were fairly brief (90% duration: 0.28 s), very low-frequency (peak frequency: 57 Hz) sounds that made up 13% of all calls. These narrowband (tonal) signals had the lowest aggregate entropy (1.7 bits) of all call types.
Grunts were harsh, broadband calls with peak frequency of 244 Hz. They were similar to burps but longer in duration (90% duration: 0.25 s) and did not occur in bouts. Grunts, which have been reported for other captive spotted seals (Beier and Wartzok, 1979; Gailey-Phipps, 1984), comprised 4% of detected calls.
Rumbles (3% of calls produced) were very low in frequency (peak frequency: 55 Hz), but of much longer duration (90% duration: 4.9 s) than pulses. They had some harmonic structure and most of their energy fell below 100 Hz (90% bandwidth lower/upper bounds: 38–86 Hz). They were often associated with pulses. This is the first time this distinctive call type has been described.
The drum was a pulsed call with peak frequency of 98 Hz. This vocalization made up just 1% of all calls in the data set, and was typically associated with bouts of knocks. This vocal type has been recorded from wild spotted seals (Yang et al., 2017) and other captive individuals (Beier and Wartzok, 1979; Gailey-Phipps, 1984).
Moans were tonal calls with clear harmonic structure (10 harmonics on average) and a fundamental frequency of 203 Hz. They were of moderate duration (90% duration: 1.1 s). Moans were the least common call type, with 819 identified (1% of 86,072 calls), and have not been detected from other spotted seals.
The DFA (no cross-validation) indicated that these eight perceptual call types separated well into multivariate acoustic space, and revealed significant differences among vocal types (LD1: F (7,64) = 139, p < 0.001; LD2: F (7,63) = 385, p < 0.001). Seven functions were extracted, with LD1 and LD2 accounting for 51 and 32% of total variance, respectively (Figure 6). LD1 was most strongly correlated with the 90% duration of the call. In Figure 6, this was reflected by relatively long-duration vocals like rumbles and growls sorting toward the right side of the plot, while shorter calls like knocks and burps fell farther to the left. LD2 was most strongly correlated with aggregate entropy. More broadband calls like growls sorted close to the top of Figure 6, while more tonal calls (e.g., pulses) appeared nearer to the bottom.
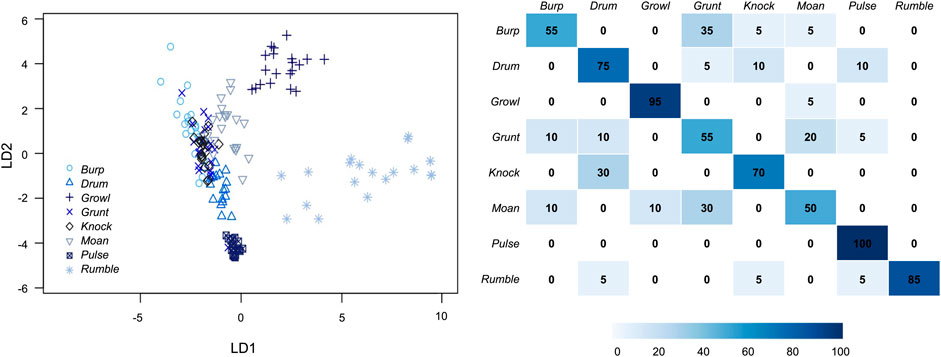
FIGURE 6. Discriminant function analysis scatterplot (left) and cross-validated DFA matrix (right) for the eight call types produced under water by male spotted seals (n = 20 for each call type). Seventeen acoustic parameters were included in the DFA (see Table 1). LD1 was most strongly correlated with 90% call duration and explained 51% of the total variance. LD2 was most strongly correlated with aggregate entropy and explained 32% of the total variance. The cross-validated DFA classification matrix (right) shows the percent of correctly classified calls by type, with darker colors indicating higher correct classification rates. The eight call types were accurately classified by the cross-validated DFA at a significantly higher rate than would be expected by chance (12.5%).
The cross-validated DFA assigned 73% of vocalizations to their designated perceptual classes—with all call types correctly categorized at a rate higher than predicted by chance (12.5%; Figure 6). Correct classification rates varied from 50% (moans) to 100% (pulses); it is possible that increased sample size would further improve discrimination between call types. These results confirmed that the spotted seal males had a vocal repertoire of at least eight call types with recognizable, distinguishable acoustic features.
Our findings provide insight into the emergence and development of vocal behavior in young spotted seals, and seasonal and annual patterns of sound production. The male spotted seals started vocalizing in air in their first year of life, but the number of vocalizations they produced per month increased over time and notable underwater vocal behavior began at age 4 with the onset of presumed sexual maturation (Naito and Nishiwaki, 1972; Burns, 1973; Boveng et al., 2009). As adults, these seals had an underwater vocal repertoire of at least eight discrete call types, all of which were predominantly low in frequency. A few of these have been described previously, although the vocalizations identified herein tended to be longer in duration and lower in frequency than those reported in earlier studies—this difference may be a function of sampling equipment, distance from the vocalizing individual, or background noise in the recording environment. It is also possible that repertoire differences are related to geographic variation, which has been observed for phocid species including harbor and bearded seals (e.g., Cleator et al., 1989; Van Parijs et al., 2003; Risch et al., 2007).
The ability to observe known individuals in controlled settings provides the unique opportunity to examine vocal repertoire acquisition. The two older spotted seals in this study produced a variety of stereotypical vocalizations throughout the year—and particularly during the breeding season—in the absence of adult males or females of their species. The similarity of several of their call types to those reported previously for both captive and wild individuals suggests that species-typical vocal development occurs despite rearing in acoustic isolation from conspecifics. While additional research would be useful, our results imply that vocal behavior in spotted seals arises from innate developmental processes that do not require specific auditory inputs from the environment. Whether social exposure influences vocal behavior within wild populations remains unknown.
This study focused on male spotted seals observed and recorded throughout development. Among seals, males of aquatically breeding species produce underwater calls primarily during the breeding season (e.g., Thomas and DeMaster, 1982; Riedman, 1990; Van Parijs et al., 1999, Van Parijs et al., 2001; Van Opzeeland et al., 2010; Casey et al., 2021; Sills et al., 2021). The production of underwater calls by adult females has been confirmed for individuals of some species, including captive spotted, bearded, leopard (Hydrurga leptonyx), harp (Pagophilus groenlandicus), and ringed seals (Beier and Wartzok, 1979; Gailey-Phipps, 1984; Rogers et al., 1996; Serrano 2001; Mizuguchi et al., 2016a; Mizuguchi et al., 2016b), and wild Weddell seals (Leptonychotes weddellii; Thomas and Kuechle, 1982; Oetelaar et al., 2003). Here, the findings are limited to male vocal development and behavior. Additional studies are needed to improve understanding of the acoustic behavior of female seals.
The patterns of sound production documented in this study can be considered relative to physiological cycles in spotted seals. Although precise timing varies latitudinally, spotted seals complete predictable cycles of reproduction and molting each year. Adult seals form male-female pair bonds prior to whelping, and males apparently guard small territories throughout the reproductive period (Burns et al., 1972; Burns, 1973; Boveng et al., 2009; Burns, 2009). In the Bering Sea and northern Sea of Okhotsk, most females give birth to their pups in April and nurse for 2–4 weeks prior to weaning (Burns et al., 1972; Naito and Nishiwaki, 1972; Burns, 1973; Burns, 2009). Breeding takes place shortly thereafter, primarily in late April and early May (reviewed in Boveng et al., 2009). The two males in this study produced calls most often in April, coinciding with peak pupping season and the start of the breeding season for their wild counterparts. Subsequently, with the onset of the annual molt, free-ranging seals dramatically change their haul-out behavior and begin spending considerably more time out of water (reviewed in Boveng et al., 2009; C. Reichmuth unpublished data). This explains the precipitous drop in vocal production observed for spotted seals Amak and Tunu between May and June, which likely also corresponds to a change in hormonal status (Sills et al., 2021).
The clear diel pattern in acoustic behavior for these two seals is similar to trends reported for several polar phocids (e.g., Thomas and DeMaster, 1982; Cleator et al., 1989; Van Parijs et al., 2001; Van Opzeeland et al., 2010; Frouin-Mouy et al., 2016, 2019), in addition to the harbor seal (e.g., Van Parijs et al., 1999; Nikolich et al., 2018). Unlike in studies of free-ranging seals, however, this increase in calls during nighttime hours can definitively be attributed to higher individual calling rates as opposed to a change in seal abundance. Furthermore, the observed pattern was not a response to variation in female movement or behavior, as it occurred in the absence of conspecific females. Beyond this, it is unclear what endogenous or environmental factors may have resulted in higher vocal activity for these two male seals at night.
When considering the biological significance of vocal behavior, sound production is often evaluated with respect to phylogeny and ecology. Recent investigations have suggested that evolutionary relatedness among seals is not a predictor of similar acoustic behavior (Terhune, 2019). Instead, repertoire size and vocal complexity in male seals have been linked to life history factors including mating system and predation risk (Stirling and Thomas, 2003; Terhune, 2019). While this study revealed several additional vocal types—and thus a larger repertoire—than previously identified for this species, the known spotted seal repertoire is still relatively small compared to the extensive variety of calls produced by harp and Weddell seals, for example (see Terhune, 2019). Given that spotted seals are annually monogamous and widely dispersed on the pack ice during the short breeding season (Burns et al., 1972; Burns, 1973; Boveng et al., 2009; Burns, 2009), they may not rely on elaborate acoustic displays to attract mates or defend areas occupied by receptive females. However, the spotted seal repertoire is, surprisingly, larger than that reported for the closely related but promiscuous harbor seal. Harbor seals produce five different call types and seem to rely primarily on one repetitive vocalization during acoustic displays—a roar that is similar to the spotted seal growl (e.g., Hanggi and Schusterman, 1994; Van Parijs et al., 1997; Casey et al., 2021).
The function of specific call types cannot be determined in the present study, as acoustic recordings were not paired with systematic evaluations of behavioral context. However, as expected, the production of underwater calls by two male seals was associated primarily with the breeding season, peaking in spring prior to onset of the annual molt. The emergence of regular vocalizations during sexual maturation, seasonal timing linked to pupping and breeding in wild individuals, and correlation with other behavioral indicators of reproductive status suggest that vocal activity is related to reproduction in this species. Consistent with this view, previous studies have reported that growls and drums are associated with underwater copulatory behavior and that growls and grunts serve in territorial boundary affirmation in this species (Beier and Wartzok, 1979; Gailey-Phipps, 1984). The second most common vocal type, the knock, is comparable to the knocks, taps, and claps of walruses (Odobenus rosmarus) and grey (Halichoerus grypus), ringed, hooded (Cystophora cristata), and harp seals (e.g., Møhl et al., 1975; Ray and Watkins, 1975; Stirling et al., 1987; Asselin et al., 1993; Ballard and Kovacs, 1995; Kunnasranta et al., 1996; McCulloch, 2000; Rautio et al., 2009; Mizuguchi et al., 2016a; Hocking et al., 2020; Larsen and Reichmuth, 2021). Although the function of impulsive signals for these species remains incompletely understood, their use by multiple ice-associated species is noteworthy. Additional, targeted behavioral research could resolve the functional significance of different vocalizations and their role in life history events such as breeding.
Of particular interest in the present study is the novel observation of a notable musky odor from the adult males each spring, coincident with peak vocal activity. While ringed seal males are known to produce a strong odor via facial glands during the breeding season (Hardy et al., 1991; Ryg et al., 1992), this phenomenon has not been described for other phocid seals. Reports from subsistence hunters suggest that male spotted seals occasionally develop an odor in late spring and early summer, although one that is less noticeable to humans than the intense seasonal odor of ringed seals (B. Ahmasuk, personal communication). The production of seasonal scent cues by mature males could be related to territorial or female resource marking in spotted seals, as it is for ringed seals. Thus, both olfactory and vocal cues may be relevant to the breeding behavior of this species.
To consider sound production holistically, it is necessary to evaluate vocal behavior in the context of auditory capabilities. Hearing sensitivity has been measured for spotted seals—underwater audiograms are available for the same two individuals that were the subjects of the present study (Sills et al., 2014). Spotted seals can detect acoustic signals in water from below 100 Hz to above 70 kHz, with a gradual roll-off in sensitivity on the low-frequency end. They have a broad range of best hearing (thresholds within 20 dB of best sensitivity) between ∼300 Hz and 56 kHz. In terms of their vocalizations, most energy falls below 1,000 Hz with peak frequencies between 55 and 531 Hz. Thus, their vocal energy is largely encompassed within the low-frequency region of most sensitive hearing. While precise tuning between the frequency range of vocalizations and that of sensitive hearing is often assumed, seals are likely listening under water for cues to aid in predator avoidance, prey detection, and passive orientation in the environment (Schusterman et al., 2000) in addition to communication signals. Although spotted seals are able to hear frequencies much higher than those contained in their sound emissions, species-typical hearing certainly supports their ability to detect the low-frequency vocalizations of conspecific seals.
From an applied perspective, this study provides necessary information to implement passive acoustic methods for spotted seals. The eight call types described herein can be attributed to this species when recorded in the wild, and details of the spectral and temporal features of these calls can aid in automatic detector development to facilitate the processing of large data sets. This is true moving forward, but can also be applied to acoustic data collected previously in Arctic and sub-Arctic regions; it is likely that spotted seal vocalizations are present in existing acoustic recordings, which could be reanalyzed to learn more about the presence and distribution of this species.
In addition to call characteristics, temporal patterns in vocal behavior can inform the application of passive acoustic methods. The lower rates of call production observed for much of the year suggest that acoustic monitoring may be most effectively applied during springtime for this species. On a diel scale, sampling focused during nighttime hours is likely to be most productive. Importantly, these observed trends emphasize that a lack of detected calls does not indicate an absence of spotted seals; rather, seals may be present but silent based on time of day or year, sex, or age class. Mean calling rate per hour—and variation in this value seasonally and throughout the day—can also provide insight into cue rates for density estimation of spotted seals, as cue rates are not yet available for wild individuals.
In terms of the suitability of spotted seal vocalizations for passive acoustic monitoring, there are several considerations. With regard to detection, the low-frequency nature of these calls means that they will propagate well in their underwater environment. However, this may make them more difficult to detect within background noise in certain ambient conditions. Their source levels (∼140 dB re 1 μPa, this study; see also Yang et al., 2022)5 are higher than those reported for ringed seals (Cummings et al., 1984) and generally similar to source level estimates for harbor seals (Casey et al., 2016; Matthews et al., 2017). Bearded seals reportedly emit calls as loud as 178 dB re 1 μPa (Cummings et al., 1983 as cited in Richardson et al., 1995; see also Fournet et al., 2021) and large whales in similar environments (e.g., Bowhead whales Balaena mysticetus) produce low-frequency sounds with SPLs exceeding 156 dB re 1 μPa (Clark and Johnson, 1984; Cummings and Holliday, 1987). In representative sub-Arctic environments, spotted seal vocalizations might be expected to travel hundreds to thousands of meters before falling below ambient conditions (see, e.g., Sills J. et al., 2017; Sills J. M. et al., 2017).
Regarding species identification, while spotted seals produce several distinctive calls, there is some potential for confusion with sympatric species. As mentioned previously, knocks may be confused with those of walruses or ringed seals in certain areas; conversely, this should not be an issue for grey, hooded, or harp seals, as their ranges do not overlap with the spotted seal’s distribution in the seasonally ice-covered seas of the North Pacific. The similarity of the growl to the harbor seal roar could be a complicating factor in areas where these seals occur together, though differences in their vocal repertoires may allow resolution. The growl and the roar are quite similar. While there is variability across individuals and populations (Hanggi and Schusterman, 1994; Van Parijs et al., 1999), harbor seal roars are predominantly low-frequency, broadband calls with peak frequencies between 400 and 800 Hz and 90% bandwidths spanning from ∼120 to 1,660 Hz (Hanggi and Schusterman, 1994; Casey et al., 2021). They last about 4 s in duration and have a terminal series of 3-8 pulses (Hanggi and Schusterman, 1994; Casey et al., 2021). Although it is possible that in-depth acoustic analyses could enable parsing of roars from growls based on one or more acoustic features, habitat and time of year should provide more clarity. These two species only overlap geographically in the southern portion of the spotted seal’s range—in the southern Bering Sea and between the Kamchatka Peninsula and northern Japan—and harbor seals breed approximately 2 months later than spotted seals (Burns, 2009). While harbor seals may utilize freshwater icebergs in some areas, they haul out mainly on land and are typically associated with rocks and beaches along the coastline, in contrast to the preferred sea ice habitat of spotted seals (Burns, 2009). Discrimination between these two species should thus be possible based on differences in distribution, timing of physiological cycles, and habitat preference. These clues (among others) may improve the accuracy of species identification when evaluating passive acoustic data sets.
Ultimately, these findings from captive seals will inform targeted field work for this minimally studied marine mammal species, and can enable the use of passive acoustic methods to track free-ranging spotted seals in remote habitats.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: DRYAD, doi: 10.7291/D1QM3G.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committees at the University of California Santa Cruz and the Alaska SeaLife Center.
JS and CR developed this study together. JS was responsible for data collection and analyses and led manuscript preparation. CR facilitated data collection, was responsible for funding and animals, and contributed to manuscript preparation.
Research was supported by the National Oceanic and Atmospheric Administration’s Alaska Pinnipeds Program (NA15NMF4390166, NA16NMF4390027, NA19NMF4390083) and by the International Association of Oil and Gas Producers, through their E&P Joint Industry Programme on Sound and Marine Life (22-07-23).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This long-term study was made possible by the dedication of many individuals at Long Marine Laboratory (UC Santa Cruz) and the Alaska SeaLife Center. In particular, we thank K. Parnell and J. Linossier for statistical advice and assistance with spectrograms; R. Ventura and S. Knaub for their efforts to record calls from the developing seals; B. Russell and the ASLC diving teams for acoustic data collection with the adult seals; J. Sullivan, S. Knaub, and J. Kim and the LML and ASLC husbandry teams for their careful characterization of the physiological indicators associated with breeding; J. Einsweiler for leading quality assurance checks of the manually annotated data; M. Meranda for obtaining opportunistic underwater video footage of the adult seals; and A. Rouse for managing the husbandry databases at both facilities. J. Einsweiler, C. Lew, M. Hartwick, R. Hobbs, A. Taylor, B. Giorgione, K. Rodriguez, C. Birch, K. Appler, M. Garcia, E. Levy, E. Ashton, S. Santich, V. Zobell, D. Scheik, and N. Wilson contributed countless hours for data review and processing. We are also grateful to P. Madsen and B. Southall for sharing acoustic equipment in the early stages of this study, and to R. Sills for assistance with data handling. The transfer of the two spotted seals from California to Alaska was supported by a donation from Shell Alaska Venture, and we especially thank L. Brzuzy for his support. A. Whiting, P. Boveng, J. Crawford, and H. Frouin-Mouy offered helpful input into species behavior and potential passive acoustic applications. Portions of this work were presented by JS at the Alaska Marine Science Symposium (January 2022), the 178th Meeting of the Acoustical Society of America (San Diego, CA, December 2019), the 173rd Meeting of the Acoustical Society of America (Boston, MA, June 2017), and the 21st Biennial Conference on Marine Mammals (San Francisco, CA, December 2015).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsen.2022.862435/full#supplementary-material
Supplementary Video S1 | Opportunistic footage of male spotted seal Tunu producing the growl vocalization in water. This was the most common call type recorded from the adult male seals (33% of 86,072 detected calls). Videos were obtained with a GoPro HERO8.
Supplementary Audio S1 | Exemplars of the eight underwater call types produced by adult male spotted seals, as shown in Figure 5.
1These two individuals were obtained as young of the year (<1 year old) seals from the northern Bering Sea.
2The orientation of the vocalizing animal relative to the hydrophone was typically between 0 and 90°, and varied up to 180°. Thus, the reported SPLs should be considered apparent source levels that approximate source levels measured on axis with the caller. As the spectral content of vocalizations was predominantly <1 kHz, these sounds would not be highly directional. Additionally, as these recordings were made in a pool, SPLs are an estimate of the source levels that would be measured in a free field.
3For all knocks (and some burps), the 90% duration was shorter than the minimum window dictated by the sampling rate. The minimum window length (0.085 s) was used for analysis in these cases, and was always within 0.06 s of the actual 90% duration.
4The “mass” and “seewave” packages in RStudio v.1.2.5019 and R v.3.6.1 (R Core Team 2019) were used for DFA analysis and to create spectrogram figures, respectively.
5Received levels for growls measured from wild spotted seals recorded within 30 m were 142 dB re 1 μPa on average (median SPL between 50 and 4,000 Hz, range 131–152 dB re 1 μPa; Yang et al., 2022). Based on these received levels, the source levels of these calls were likely higher.
Asselin, S., Hammill, M. O., and Barrette, C. (1993). Underwater Vocalizations of Ice Breeding Grey Seals. Can. J. Zool. 71, 2211–2219. doi:10.1139/z93-310
Ballard, K. A., and Kovacs, K. M. (1995). The Acoustic Repertoire of Hooded Seals (Cystophora Cristata). Can. J. Zool. 73, 1362–1374. doi:10.1139/z95-159
Beier, J. C., and Wartzok, D. (1979). Mating Behaviour of Captive Spotted Seals (Phoca largha). Anim. Behav. 27, 772–781. doi:10.1016/0003-3472(79)90013-7
Boveng, P. L., Bengtson, J. L., Buckley, T. W., Cameron, M. F., Dahle, S. P., Kelly, B. P., et al. (2009). Status Review of the Spotted Seal (Phoca largha),” NOAA Technical Memorandum NMFS-AFSC- 200. Washington D.C: US Department of Commerce.
Burns, J. J., and Alaska, p. (1973). Marine Mammal Report. Project Progress Report, XIII. Juneau, AK: Department of Fish and Game, 29.
Burns, J. J. (2009). “Harbor Seal and Spotted Seal,” in Encyclopedia of Marine Mammals. Editors W. F. Perrin, B. Würsig, and J. G. M. Thewissen. 2nd Ed. (San Diego, CA: Academic Press), 533–542. doi:10.1016/b978-0-12-373553-9.00126-7
Burns, J. J., Ray, G. C., Fay, F. H., and Shaughnessy, P. D. (1972). Adoption of a Strange Pup by the Ice‐inhabiting Harbor Seal, Phoca vitulina Largha. J. Mammal. 53, 594–598. doi:10.2307/1379048
Casey, C., Sills, J. M., Knaub, S., Sotolotto, K., and Reichmuth, C. (2021). Lifelong Patterns of Sound Production in Two Seals. Aquat. Mamm. 47, 499–514. doi:10.1578/am.47.5.2021.499
Casey, C., Sills, J., and Reichmuth, C. (2016). Source Level Measurements for Harbor Seals and Implications for Estimating Communication Space. Proc. Mtgs. Acoust. 27, 010034. doi:10.1121/2.0000353
Clark, C. W., and Johnson, J. H. (1984). The Sounds of the Bowhead Whale, Balaena Mysticetus, during the Spring Migrations of 1979 and 1980. Can. J. Zool. 62, 1436–1441. doi:10.1139/z84-206
Cleator, H. J., Stirling, I., and Smith, T. G. (1989). Underwater Vocalizations of the Bearded Seal (Erignathus Barbatus). Can. J. Zool. 67, 1900–1910. doi:10.1139/z89-272
Cummings, W. C., Holliday, D. V., Ellison, W. T., and Graham, B. J. (1983). Technical Feasibility of Passive Acoustic Location of Bowhead Whales in Population Studies off Point Barrow, Alaska. San Diego, CA, for North Slope Borough, Barrow, AK: Rep. from Tracor Appl. Sci.
Cummings, W. C., Holliday, D. V., and Lee, B. J. (1984). Potential Impacts of Man-Made Noise on Ringed Seals: Vocalizations and Reactions,” Outer Continental Shelf Environmental Assessment Program, Final Report. OCS Study MMS 86-0021; NTIS PB87-107546. Anchorage, AK: NOAA.
Cummings, W. C., and Holliday, D. V. (1987). Sounds and Source Levels from Bowhead Whales off Pt. Barrow, Alaska. J. Acoust. Soc. Am. 82, 814–821. doi:10.1121/1.395279
Fournet, M. E. H., Silvestri, M., Clark, C. W., Klinck, H., and Rice, A. N. (2021). Limited Vocal Compensation for Elevated Ambient Noise in Bearded Seals: Implications for an Industrializing Arctic Ocean. Proc. R. Soc. B 288, 20202712. doi:10.1098/rspb.2020.2712
Frouin-Mouy, H., Mouy, X., Berchok, C. L., Blackwell, S. B., and Stafford, K. M. (2019). Acoustic Occurrence and Behavior of Ribbon Seals (Histriophoca Fasciata) in the Bering, Chukchi, and Beaufort Seas. Polar Biol. 42 (4), 657–674. doi:10.1007/s00300-019-02462-y
Frouin-Mouy, H., Mouy, X., Martin, B., and Hannay, D. (2016). Underwater Acoustic Behavior of Bearded Seals (Erignathus Barbatus) in the Northeastern Chukchi Sea, 2007–2010. Mar. Mamm. Sci. 32, 141–160.
Gailey-Phipps, J. J. (1984). Acoustic Communication and Behavior of the Spotted Seal (Phoca largha. PhD Dissertation. Baltimore, MD, USA: The Johns Hopkins University.
Hanggi, E. B., and Schusterman, R. J. (1994). Underwater Acoustic Displays and Individual Variation in Male Harbour Seals, Phoca vitulina. Anim. Behav. 48, 1275–1283. doi:10.1006/anbe.1994.1363
Hardy, M. H., Roff, E., Smith, T. G., and Ryg, M. (1991). Facial Skin Glands of Ringed and Grey Seals, and Their Possible Function as Odoriferous Organs. Can. J. Zool. 69, 189–200. doi:10.1139/z91-029
Hocking, D. P., Burville, B., Parker, W. M. G., Evans, A. R., Park, T., and Marx, F. G. (2020). Percussive Underwater Signaling in Wild Gray Seals. Mar. Mam. Sci. 36, 728–732. doi:10.1111/mms.12666
Klinck, H., Mellinger, D. K., Klinck, K., Hager, J., Kindermann, L., and Boebel, O. (2010). Long-range Underwater Vocalizations of the Crabeater Seal (Lobodon Carcinophaga). J. Acoust. Soc. Am. 128, 474–479. doi:10.1121/1.3442362
Kunnasranta, M., Hyvärinen, H., and Sorjonen, J. (1996). Underwater Vocalizations of Ladoga Ringed Seals (Phoca Hispida Ladogensis Nordq.) in Summertime. Mar. Mamm. Sci. 12, 611–618.
Larsen, O. N., and Reichmuth, C. (2021). Walruses Produce Intense Impulse Sounds by Clap-Induced Cavitation during Breeding Displays. R. Soc. open Sci. 8, 210197. doi:10.1098/rsos.210197
MacIntyre, K. Q., Stafford, K. M., Berchok, C. L., and Boveng, P. L. (2013). Year-round Acoustic Detection of Bearded Seals (Erignathus Barbatus) in the Beaufort Sea Relative to Changing Environmental Conditions, 2008-2010. Polar Biol. 36, 1161–1173. doi:10.1007/s00300-013-1337-1
Matthews, L. P., Parks, S. E., Fournet, M. E. H., Gabriele, C. M., Womble, J. N., and Klinck, H. (2017). Source Levels and Call Parameters of Harbor Seal Breeding Vocalizations Near a Terrestrial Haulout Site in Glacier Bay National Park and Preserve. J. Acoust. Soc. Am. 141, EL274–EL280. doi:10.1121/1.4978299
McCulloch, S. (2000). The Vocal Behaviour of the Grey Seal (Halichoerus Grypus). St. Andrews, UK: Ph.D. dissertation, University of St. Andrews.
Mellinger, D., Stafford, K., Moore, S., Dziak, R., and Matsumoto, H. (2007). An Overview of Fixed Passive Acoustic Observation Methods for Cetaceans. Oceanog. 20, 36–45. doi:10.5670/oceanog.2007.03
Miksis-Olds, J., and Parks, S., E. (2011). Seasonal Trends in Acoustic Detection of Ribbon Seal (Histriophoca Fasciata) Vocalizations in the Bering Sea. Aquat. Mamm. 37, 464–471. doi:10.1578/am.37.4.2011.464
Mizuguchi, D., Tsunokawa, M., Kawamoto, M., and Kohshima, S. (2016b). Sequential Calls and Associated Behavior in Captive Bearded Seals (Erignathus Barbatus). J. Acoust. Soc. Am. 140, 3238. doi:10.1121/1.4970242
Mizuguchi, D., Tsunokawa, M., Kawamoto, M., and Kohshima, S. (2016a). Underwater Vocalizations and Associated Behavior in Captive Ringed Seals (Pusa Hispida). Polar Biol. 39, 659–669. doi:10.1007/s00300-015-1821-x
Møhl, B., Terhune, J. M., and Ronald, K. (1975). Underwater Calls of the Harp Seal, Pagophilus Groenlandicus. Rapp. P.-v. Réun. Cons. Int. Explor. Mer. 169, 533–543.
Naito, Y., and Nishiwaki, M. (1972). The Growth of Two Species of the Harbour Seal in the Adjacent Waters of Hokkaido. Sci. Rep. Whales Res. Inst. 24, 127–144.
Nikolich, K., Frouin-Mouy, H., and Acevedo-Gutiérrez, A. (2018). Clear Diel Patterns in Breeding Calls of Harbor Seals (Phoca vitulina) at Hornby Island, British Columbia, Canada. Can. J. Zool. 96 (11), 1236–1243. doi:10.1139/cjz-2018-0018
Oetelaar, M. L., Terhune, J. M., and Burton, H. R. (2003). Can the Sex of a Weddell Seal (Leptonychotes Weddellii) Be Identified by its Surface Call? Aquat. Mamm. 29, 261–267. doi:10.1578/016754203101024194
R Development Core Team, (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rautio, A., Niemi, M., Kunnasranta, M., Holopainen, I. J., and Hyvärinen, H. (2009). Vocal Repertoire of the Saimaa Ringed Seal (Phoca Hispida Saimensis) during the Breeding Season. Mar. Mamm. Sci. 25, 920–930. doi:10.1111/j.1748-7692.2009.00299.x
Ray, G. C., and Watkins, W. A. (1975). Social Function of Underwater Sounds in the Walrus Odobensus Rosmarus. Rapp. P.-v. R&n. Cons. Int. Explor. Mer. 169, 524–526.
Richardson, W. J., Greene, C. R., Malme, C. I., and Thomson, D. H. (1995). Marine Mammals and Noise. San Diego, CA: Academic.
Risch, D., Clark, C. W., Corkeron, P. J., Elepfandt, A., Kovacs, K. M., Lydersen, C., et al. (2007). Vocalizations of Male Bearded Seals, Erignathus barbatus: Classification and Geographical Variation. Anim. Behav. 73, 747–762.
Rogers, T. L., Cato, D. H., and Bryden, M. M. (1996). Behavioral Significance of Underwater Vocalizations of Captive Leopard Seals, Hydurga Leptonyx. Mar. Mammal. Sci. 12, 414–427. doi:10.1111/j.1748-7692.1996.tb00593.x
Ryg, M., Solberg, Y., Lydersen, C., and Smith, T. G. (1992). The Scent of Rutting Male Ringed Seals (Phoca Hispida). J. Zoology 226, 681–689. doi:10.1111/j.1469-7998.1992.tb07509.x
Schusterman, R. J., Kastak, D., Levenson, D. H., Reichmuth, C. J., and Southall, B. L. (2000). Why Pinnipeds Don't Echolocate. J. Acoust. Soc. Am. 107, 2256–2264. doi:10.1121/1.428506
Serrano, A. (2001). New Underwater and Aerial Vocalizations of Captive Harp Seals (Pagophilus Groenlandicus). Can. J. Zool. 79, 75–81. doi:10.1139/z00-182
Sills, J. M., Southall, B. L., and Reichmuth, C. (2014). Amphibious Hearing in Spotted Seals (Phoca largha): Underwater Audiograms, Aerial Audiograms and Critical Ratio Measurements. J. Exp. Biol. 217, 726–734. doi:10.1242/jeb.097469
Sills, J. M., Southall, B. L., and Reichmuth, C. (2017). The Influence of Temporally Varying Noise from Seismic Air Guns on the Detection of Underwater Sounds by Sealsa). J. Acoust. Soc. Am. 141, 996–1008. doi:10.1121/1.4976079
Sills, J., Parnell, K., Ruscher, B., Lew, C., Kendall, T., and Reichmuth, C. (2021). Underwater Hearing and Communication in the Endangered Hawaiian Monk Seal Neomonachus Schauinslandi. Endang. Species. Res. 44, 61–78. doi:10.3354/esr01092
Sills, J., Reichmuth, C., and Whiting, A. (2017). Acoustic Habitat Utilized by Ice-Living Seals: Hearing and Masking in Natural Noise Environments. J. Acoust. Soc. Am. 141, 4002. doi:10.1121/1.4989175
Stirling, I., Calvert, W., and Cleator, H. (1983). Underwater Vocalizations as a Tool for Studying the Distribution and Relative Abundance of Wintering Pinnipeds in the High Arctic. Arctic 36, 262–274. doi:10.14430/arctic2275
Stirling, I., Calvert, W., and Spencer, C. (1987). Evidence of Stereotyped Underwater Vocalizations of Male Atlantic Walruses (Odobenus Rosmarus Rosmarus). Can. J. Zool. 65, 2311–2321. doi:10.1139/z87-348
Stirling, I., and Thomas, J. A. (2003). Relationships between Underwater Vocalizations and Mating Systems in Phocid Seals. Aquat. Mamm. 29, 227–246. doi:10.1578/016754203101024176
Terhune, J. M. (2019). The Underwater Vocal Complexity of Seals (Phocidae) Is Not Related to Their Phylogeny. Can. J. Zool. 97, 232–240. doi:10.1139/cjz-2018-0190
Thomas, J. A., and DeMaster, D. P. (1982). An Acoustic Technique for Determining Diurnal Activities in Leopard (Hydrurga Leptonyx) and Crabeater (Lobodon Carcinophagus) Seal. Can. J. Zool. 60, 2028–2031. doi:10.1139/z82-260
Thomas, J. A., and Kuechle, V. B. (1982). Quantitative Analysis of Weddell Seal (Leptonychotes Weddelli) Underwater Vocalizations at McMurdo Sound, Antarctica. J. Acoust. Soc. Am. 72, 1730–1738. doi:10.1121/1.388667
Van Opzeeland, I., Van Parijs, S., Bornemann, H., Frickenhaus, S., Kindermann, L., Klinck, H., et al. (2010). Acoustic Ecology of Antarctic Pinnipeds. Mar. Ecol. Prog. Ser. 414, 267–291. doi:10.3354/meps08683
Van Parijs, S. M., Corkeron, P. J., Harvey, J., Hayes, S. A., Mellinger, D. K., Rouget, P. A., et al. (2003). Patterns in the Vocalizations of Male Harbor Seals. J. Acoust. Soc. Am. 113 (6), 3403–3410. doi:10.1121/1.1568943
Van Parijs, S. M., Hastie, G. D., and Thompson, P. M. (1999). Geographical Variation in Temporal and Spatial Vocalization Patterns of Male Harbour Seals in the Mating Season. Anim. Behav. 58, 1231–1239. doi:10.1006/anbe.1999.1258
Van Parijs, S. M., Kovacs, K. M., and Lydersen, C. (2001). Spatial and Temporal Distribution of Vocalising Male Bearded Seals: Implications for Male Mating Strategies. Behaviour 138, 905–922.
Van Parijs, S. M., Thompson, P. M., Tollit, D. J., and Mackay, A. (1997). Distribution and Activity of Male Harbour Seals during the Mating Season. Anim. Behav. 54 (1), 35–43. doi:10.1006/anbe.1996.0426
Yang, L., Xu, X., and Berggren, P. (2022). Spotted Seal Phoca largha Underwater Vocalisations in Relation to Ambient Noise. Mar. Ecol. Prog. Ser. 683, 209–220. doi:10.3354/meps13951
Yang, L., Xu, X., Zhang, P., Han, J., Li, B., and Berggren, P. (2017). Classification of Underwater Vocalizations of Wild Spotted Seals (Phoca largha) in Liaodong Bay, China. J. Acoust. Soc. Am. 141 (3), 2256–2262. doi:10.1121/1.4979056
Keywords: vocalization, passive acoustic monitoring, call repertoire, acoustic ontogeny, phocid, arctic
Citation: Sills JM and Reichmuth C (2022) Vocal Behavior in Spotted Seals (Phoca largha) and Implications for Passive Acoustic Monitoring. Front. Remote Sens. 3:862435. doi: 10.3389/frsen.2022.862435
Received: 25 January 2022; Accepted: 21 April 2022;
Published: 23 May 2022.
Edited by:
Ilse Catharina Van Opzeeland, Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research (AWI), GermanyReviewed by:
William Halliday, Wildlife Conservation Society, CanadaCopyright © 2022 Sills and Reichmuth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jillian M. Sills, am1zaWxsc0B1Y3NjLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.