- 1Center for Stroke Rehabilitation Research, Kessler Foundation, West Orange, NJ, United States
- 2Department of Physical Medicine and Rehabilitation, New Jersey Medical School, Rutgers University, Newark, NJ, United States
- 3Department of Orthopaedic Surgery, Occupational Therapy Division, School of Medicine, Duke University, Durham, NC, United States
- 4Department of Psychology & Neuroscience, Dalhousie University, Halifax, NS, Canada
- 5Department of Neurology, University of Texas Southwestern Medical Center, Dallas, TX, United States
- 6Departments of Psychiatry and Psychology & Neuroscience, Dalhousie University, Halifax, NS, Canada
The potential of using prism adaptation for treating spatial neglect (SN) was questioned when recent meta-analyses found inconsistent evidence. However, analyses of clinical datasets support the use of prism adaptation treatment (PAT) in reducing SN and improving function. The main objective of this review is to evaluate the current state of the evidence of PAT therapeutic effects, identify knowledge gaps, and make suggestions to guide further research and support clinical decision-making. We used the framework of the National Institutes of Health (NIH) Stage Model for Behavioral Intervention Development which provides guidance on best practices for developing effective behavioral interventions that can be implemented in real-world settings. This model emphasizes the interplay between mechanisms underlying therapeutic effects (“who” should receive the treatment and “how” best does it work?) and considerations of adaptability and feasibility in real-world settings. The present critical review led to the following conclusion: the use of the NIH Stage Model reveals the heterogeneity of PAT studies and challenges in advancing PAT as an effective intervention. The key mechanisms such as prism strength, treatment intensity, arm visibility and activities during treatment, and evaluation methods lack consensus. Therefore, clinical research teams must continue to collect evidence to determine critical mechanisms and the optimal protocol. Further research identifying the optimal PAT protocol is needed before another meta-analysis on PAT's clinical efficacy should be conducted again.
Introduction
Spatial neglect (SN) is a disorder characterized by difficulties with reporting, responding, or orienting to information presented or mentally represented on the contralesional side of space (1–4). SN is caused by damage to neural networks critical to spatial processing and attention control (5–8), affecting multiple perceptual modalities (9–12) and thus multiple cognitive and motor functions (4, 13–16). Symptom presentations of SN are independent of primary sensory or motor defects (2, 17, 18). For example, conjugate eye deviation toward the ipsilesional side of space at rest (19, 20) and gaze preference toward the ipsilesional side during visual exploration, independent of vision or the passive range of motion of the eyes, are hallmark signs of SN (14, 21). While symptoms in the visual modality are most observable and reported, symptoms in the auditory, tactile, and proprioceptive modalities have been documented and can be disabling as well (9–12, 22, 23).
In addition to the presence of SN in different modalities, there is much heterogeneity in specific symptom presentation. Individuals with SN show substantial bias toward the ipsilesional side of space while appearing to “neglect” the contralesional side of space, although some implicit processing can be confirmed with certain tasks (24). The ipsilesional bias can be based on either egocentric (in relation to the person) or allocentric (in relation to an object in any spatial location) frames of reference (25, 26); for example, persons with left-sided egocentric SN may fail to locate objects in the space to or on the left side of their body, and those with left-sided allocentric SN may be unable to detect information appearing in the space to or on the left side of individual objects regardless where the objects are located in the left or right side of the person’s body. Symptoms also can be manifested in spatial regions on or of the body (i.e., personal space), within arms' reach (peri-personal space), and/or beyond arms' reach (extra-personal space) (27–29). Furthermore, not only the external space but also the mental space is affected; individuals with SN have difficulties retrieving information represented in the contralesional side of mental space (16, 30, 31). Finally, adding to the variability in SN symptom presentations, these symptoms can fluctuate depending on the internal mental capacity (e.g., fatigue level; focused vs. dual-tasking) (32, 33) and task requirement (e.g., erasing vs. marking targets; searching for vs. arranging objects; reporting vs. marking the center of a horizontal line) (34–36). Anosognosia for SN is also part of the syndrome, and a majority of patients with SN have poor self-awareness of their symptoms, overestimate their performance in tasks that require spatial ability (37), and thus may be unable to fully engage in therapy activities of strategy training (38).
There is currently no gold standard for how to screen, assess, or diagnose SN (39–41). According to a scoping review performed in 2018 (42), 292 SN tests (spanning across impairment and functional levels) have been published with the literature growing since then. This growing collection of measures reflects, at least in part, the challenges of measuring SN due to the heterogeneity of the disorder as well as the unpredictable variability in symptom presentations as described above. In other words, patients may show SN symptoms on certain tests but not all tests (43, 44), and the estimated incidence of SN varies depending on diagnostic methods (45), creating large variability in our understanding of its prevalence and long-term recovery (45).
Two common outcome measures have tried to account for this heterogeneity by including multiple tests—the conventional subtests of the behavioral inattention test (BIT-c) with six paper-based tests (viz., line crossing, star cancellation, letter cancellation, figure copying, line bisection, and free drawing) (46, 47) and the Catherine Bergego scale (CBS) (48) on its original questionnaire format or using a standardized procedure through Kessler Foundation neglect assessment process (KF-NAP) (49). BIT-c measures SN at the impairment level in the peri-personal space. CBS measures the impact of SN severity at the functional level in a range of daily activities including gaze orientation, limb awareness, auditory attention, personal belongings, dressing, grooming, navigation, collisions, meals, and cleaning after meals. With all things considered (e.g., diagnostic methods, injured cerebral hemisphere, and neglected side of space), overall SN occurs in approximately 30% of individuals who have had a unilateral stroke within the first 3 months (45). One-third of these individuals continue experiencing SN symptoms at the chronic stage when spontaneous improvement is highly unlikely (6, 26, 50, 51).
There are a variety of non-pharmaceutical treatments for SN (52–54), such as prism adaptation treatment (PAT), optokinetic stimulation combined with smooth pursuit (55, 56), visual scanning training (57), limb activation (58), visual imagery training (59), hemifield eye patching (60), neck vibration (61), vestibular stimulation (41), and transcranial magnetic stimulation (62). Among them, PAT is considered a promising option (63, 64). It is important to note that prism adaptation had been documented for decades before it was applied to treating SN. In his study published in 1963 (65), Harris asked participants, with unspecified neurological backgrounds, to point to a central visual target 90 times while wearing wedged prism lenses that shifted the visual field to the left or right by approximately 11°. Harris then observed aftereffects after prism removal in different pointing tasks, including pointing to visual targets without feedback (i.e., without seeing their upper limb) and with eyes closed, pointing to auditory targets, or simply pointing straight ahead (65). The aftereffects consisted of participants making pointing errors that were now biased toward the side of space opposite to the visual shift that had been induced by prism lenses. For example, after adapting to rightward-shifting prisms, when participants removed the prisms then leftward aftereffects occurred. Illustrations of prism adaptation and its aftereffects can be found in several published articles (66–70). Many replicated Harris's observations and expanded on the basic mechanisms of prism adaptation in healthy individuals (71–75). Then in 1998, a study led by Rossetti was published (67), which demonstrated prism adaptation as a potential treatment for SN. In the study, participants with left-sided SN made 50 pointing movements to visual targets while wearing wedged prism lenses that shifted the visual field to the right by 10° (experimental condition) or flat lenses that induced no visual displacement (sham-control condition). Similar to those reported by Harris (65), leftward aftereffects (i.e., increased pointing toward the left) were now observed after prism removal. More importantly, Rossetti et al. also observed changes in neuropsychological tests sensitive to SN symptoms, showing a reduction of left-sided SN symptoms immediately after prism removal and 2 h later (67). Rossetti et al. were the first to demonstrate the therapeutic effects of prism adaptation on SN. This led to the development of the treatment, i.e., PAT.
In general, PAT requires individuals with SN to complete a brief, repetitive arm reaching visual-guided exercise while temporarily wearing prism lenses that shift the visual field toward the egocentric ipsilesional side of space. Once the goggles are removed, the aftereffects from prism exposure induce an opposite bias in the contralesional direction. The therapeutic effect of PAT is hypothesized to be generated through implicit sensorimotor adaptation training, requiring no development of explicit strategies or new skills, and the benefits can be multimodal and across domains beyond sensorimotor activities (76–78). Interestingly, the potential of using PAT for treating SN was questioned by recent meta-analyses (53, 79–81) but supported by recent analyses of clinical datasets (82–86). Data generated from prospective clinical trials and data retrospectively extracted from clinical quality improvement practices are fundamentally different, and analysis methods are different too. Nonetheless, conflicting conclusions—both for and against the clinical implementation of PAT—are concerning or at least confusing for clinicians who rely on published evidence to guide their practice. In this critical synthesis and narrative review, we aim to achieve three objectives: to evaluate the current state of the evidence of PAT therapeutic effects based on recently published meta-analyses and clinical data analyses; identify knowledge gaps through critical comments; make suggestions to guide further research and to support clinical decision making based on the current status of PAT research findings. In particular, much of our efforts in this critical review are guided through the US National Institutes of Health (NIH) Stage Model for Behavioral Intervention Development (87).
Does prism adaptation treatment work?
The groundbreaking study by Rossetti et al. (67) continues to inspire research and development of PAT for SN over the last 26 years, and a number of systematic reviews using meta-analysis methods have been published in recent years to seek evidence of PAT therapeutic effects. The latest 2021 Cochrane review on SN treatments, led by Longley (53), analyzed findings of PAT in eight RCTs of 257 participants and found no evidence for short- or longer-term therapeutic effects at either the SN-related impairment or functional level. Also published in 2021 were two other meta-analyses: Qiu et al. (80) found null effects in seven RCTs of 211 participants on the BIT-c or CBS when tested immediately or in the long term. In contrast, Li et al. (79) reviewed eight studies (244 participants) and reported on a series of meta-analyses of six studies that PAT resulted in benefits on the BIT-c or star cancellation test in the short term, while no effect was found for CBS. More recently, Szekely et al. (81) conducted a systematic review of published work up to June 2021 and a series of meta-analyses and found no evidence for PAT reducing SN measured using BIT-c (16 studies, 430 participants) or CBS (8 studies, 250 participants) immediately after treatment. While their broad criteria led to notable variability in the included patient characteristics or treatment protocols, including number of sessions (ranging from 1 to 20) and prism strength (5–17° of visual angle shifted by prisms), Szekely et al. found no relationship between these variables and treatment effect sizes. Thus, while acknowledging their conclusions were limited by the lack of coherence of the studies and the need for a more standardized approach in future work with well-controlled trials and sufficient sample sizes, Szekely et al. (81) suggested that since no short-term benefits were found, it was unlikely that long-term benefits would exist. Indeed, while the lack of well-controlled designs and sufficient sample sizes are important issues underlying negative results, the lack of consensus on PAT mechanisms and therapeutic protocol among the studies are also critical issues that make conclusions from meta-analyses problematic, i.e., the lack of evidence should not be interpreted as lack of effect. This logic is ignored in the conclusion by Szekely's emphasis on lack of benefit, rather than highlighting the need for better research methodology and standard therapeutic protocols to obtain stronger evidence.
Inconsistent conclusions are also highlighted through positive results from other evidence-gathering approaches. While RCTs and meta-analyses are regarded as a high level of evidence, real-world evidence of a treatment's use is also crucial for assessing the beneficial impacts of the treatment. An RCT is designed and conducted with a list of inclusion and exclusion criteria, controlled delivery of the intervention, and strict manualized protocols. However, in translating these findings to clinical care, therapy is individualized according to patient symptoms and goals of rehabilitation, and treatment is conducted within a multidisciplinary environment. The ultimate goal of developing PAT is to integrate it into regular clinical care, and analyzing large-scale clinical data is a necessary step toward this goal.
The latest real-world evidence of PAT therapeutic effects, based on the largest sample size reported to date, was part of a quality improvement initiative that implemented PAT for the treatment of SN conducted from June 2017 to March 2021 in 16 rehabilitation hospitals across 11 states in the United States (88). Occupational therapists were trained to use two standardized tools for assessing SN and using PAT in their regular clinical practice and, importantly, document the scores and usage. The assessment tool was KF-NAP (89, 90), and the treatment tool was Kessler Foundation prism adaptation treatment (KF-PAT) (49). KF-NAP provides standardized methods for functional evaluation of SN based on the CBS (as briefly mentioned above), and KF-PAT provides standardized procedures and equipment to administer and facilitate PAT. Once therapists finished training, they used the tools in their regular practice—thus, it was designed as a quality improvement project to improve clinical care by implementing standard assessment and treatment. At the end of the project (88), information available in medical records and therapist notes (i.e., clinical data) was analyzed retrospectively (84–86, 91, 92). A total of 4,454 individuals were assessed using KF-NAP, 82% of them were stroke survivors, and 55% of the total sample had SN (CBS > 0) within the first week admitted to rehabilitation hospitals (91).
Based on these clinical data, one analysis explored the beneficial impact of PAT on rehabilitation outcomes measured using the Functional Independence Measure (FIM), which was the most commonly used functional outcome measure in inpatient rehabilitation programs within the United States until 2019 (86). The analysis identified two groups of patients with SN (n = 156 per group) where one group received 8–12 sessions of PAT and the other did not receive PAT at certain sites. The two groups were matched in age, baseline severity in SN, and disability (measured using CBS and FIM at admission, respectively). The result showed a three-point difference in total FIM between groups (η2 = 0.035, p = 0.02), suggesting that PAT was associated with better rehabilitation outcomes (86). Another analysis of the clinical data (84) looked into treatment intensity and found that receiving more daily sessions of PAT was correlated with greater reduction of SN, as measured in CBS (n = 520; b = 0.16, SE = 0.06, p = 0.006) and greater improvement in FIM (n = 1720; b = 0.47, SE = 0.09, p < 0.001) after controlling for age, sex, type of brain injury, and relevant clinical characteristics. Moreover, patients who received eight or more PAT sessions showed greater CBS reduction as the frequency of PAT sessions increased (i.e., fewer days between two consecutive sessions). More PAT sessions also correlated with better FIM improvement in self-care, sphincter control, and transfers (84). Thus, this series of analyses provided practice-based evidence (93) supporting the use of PAT in inpatient rehabilitation settings.
Answering “does PAT work?” through the NIH Stage Model for Behavioral Intervention Development
The terminology describing the effects of PAT is crucial in answering the question: “does PAT work?”. In the present article, “a therapeutic effect” is defined as a reduction of SN severity and/or symptoms after PAT, regardless of the presence of a control condition. A therapeutic effect can be at the impairment level (measured using neuropsychological tests) or functional level (measured using ecological assessments). When using “efficacy” and “effectiveness” to describe a therapeutic effect, a control condition must be included in the analysis. According to the US National Institutes of Health (NIH) definition, while both efficacy and effectiveness refer to the strength of a therapeutic effect, efficacy is used when the therapy is provided “under ideal and controlled circumstances” (94) while effectiveness is used “in clinical practice in the real world” (95).
The goal of the present article is to address the question “does PAT work?” through a new perspective on the current state of PAT clinical research. This new perspective is derived from an NIH five-stage framework that provides guidance on best practices for developing effective behavioral interventions that can be implemented in real-world settings (Figure 1a) (87). Table 1 outlines the details of the stages. As emphasized in the NIH Stage Model, behavioral interventions are defined by the mechanisms governing their effects. Of note, the NIH Stage Model differs from the US Food and Drug Administration (FDA) Clinical Research Phases (96). The use of “phases” is ubiquitous in non-pharmaceutical clinical trials and has been criticized, and alternative approaches have been proposed (97, 98). Drug development is a linear process (Figure 1b), and developing a non-pharmaceutical behavioral intervention such as PAT, in contrast, is iterative and multidirectional (Figure 1a). Development of a behavioral intervention proceeds through an interplay between mechanisms underlying therapeutic effects (“who” should receive the treatment and “how” best does it work?) and considerations of adaptability and feasibility in real-world settings. Thus, defining and refining mechanisms are considered of both scientific and practical value in the NIH Stage Model. In addition, intervention development must also include validating materials (e.g., devices and therapist manuals) and methods (e.g., step-by-step procedures) used for administering the intervention; in other words, the fidelity of the planned intervention is crucial. The NIH Stage Model provides guidance on the goals of the research and who should be involved at the different stages, but the principles of the intervention should be a focus at each step. The usefulness of the NIH Stage Model has been reviewed (99, 100).
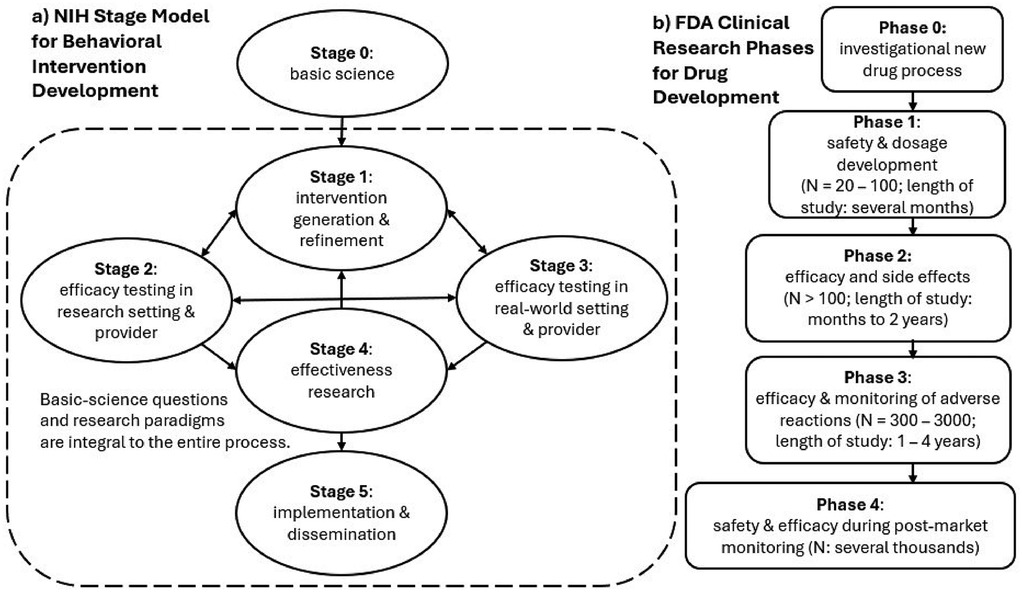
Figure 1. (a) NIH Stage Model for Behavioral Intervention Development vs. (b) FDA Clinical Research Phases for Drug Development. The NIH Stage Model emphasizes that questions of mechanisms of behavior change are relevant to every Stage. In addition, while stages are labeled from 1 to 5, the directions of progress are not linear from 1 to 5. The arrowheads can be bidirectional and point from different stages.
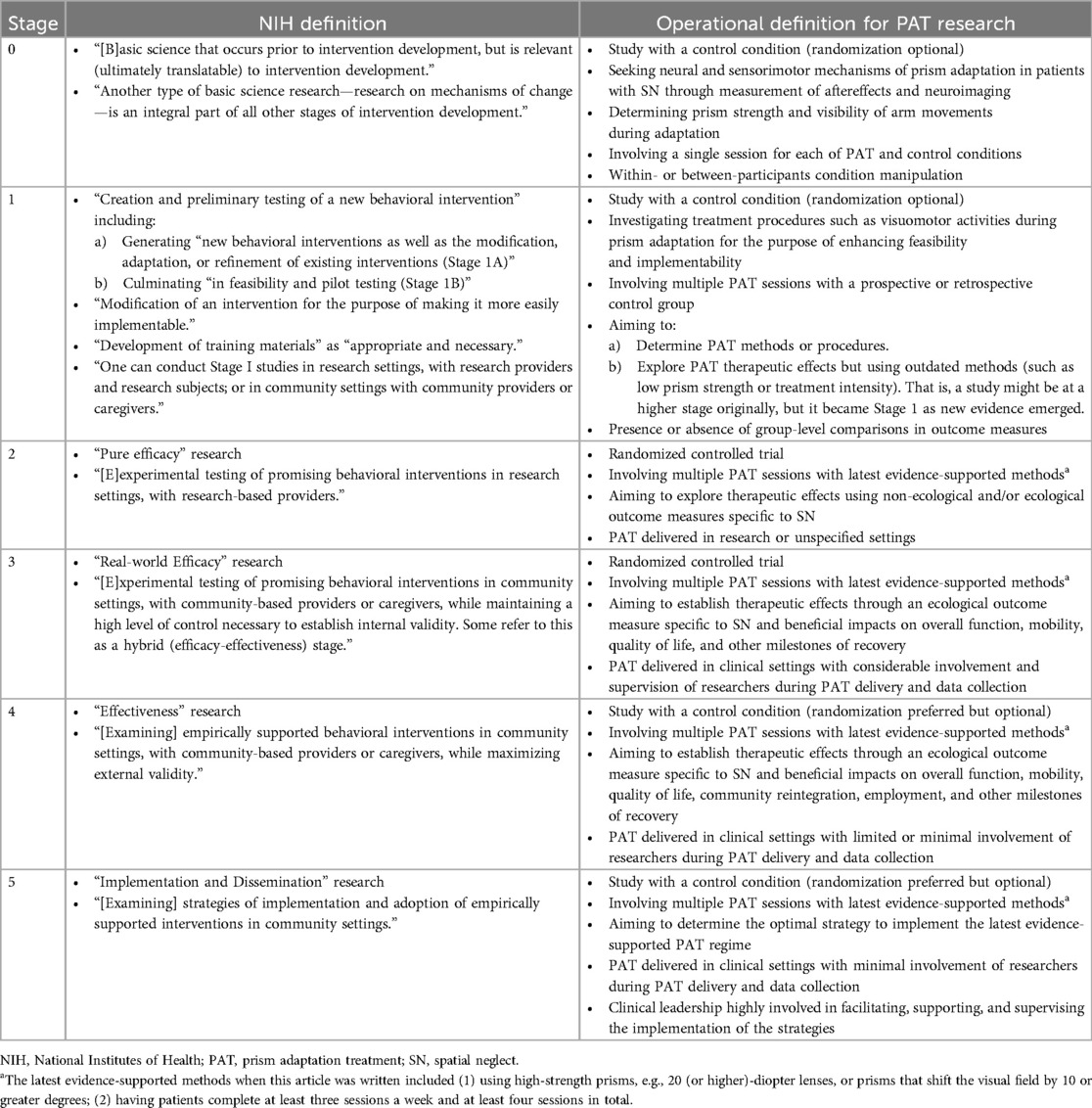
Table 1. NIH stage model for behavioral intervention development. Quotes are extracted directly from the NIH webpage.
To reiterate, the NIH Stage Model highlights the consideration of the underlying mechanisms or principles of treatment to enhance the potency and implementation of the treatment. These principles focus on identifying “who” should benefit, “how” does treatment works, and the fidelity with which the treatment is applied. Keeping these principles at the forefront of studies is ideally needed for developing a successful therapy. Certain key elements are relevant to these principles, including measurement of SN for diagnosis and evaluating outcomes (“who”), prism strength/presence of aftereffects, treatment intensity, arm visibility, and activities during prism adaptation (“how”). The impacts of these elements on intervention effects in SN are reviewed below.
Key elements of prism adaptation treatment
Defining SN and measuring outcomes
The fact that there is no gold standard for SN measurement (39, 40) is reflected in the PAT literature where various SN tests and criteria have been used as the operational definition of SN diagnosis and outcome measures in different studies. While BIT-c and CBS are commonly used for outcome measures, studies differ in screening criteria, which may or may not involve BIT-c or CBS. Some studies excluded patients with mild SN (101–103) to potentially ensure the detectability of therapeutic effects after PAT. However, it is unknown what level of SN severity is likely to benefit from PAT, which is a critical question for enhancing treatment potency. In addition, using BIT-c or CBS to determine SN severity is not sufficient to capture the heterogeneity of SN. A variety of subtypes exist, as briefly reviewed at the beginning of this article, in terms of reference frames (egocentric vs. allocentric), regions of space based on the distance from the examinees' body (personal, peri-personal, and extra-personal space), or whether a symptom presentation primarily depends on perceptual input or motor output. Few PAT studies have focused on whether certain symptoms or subtypes would derive differential benefits from PAT and thus enhance treatment effects [cf. (104)].
Measurement of PAT outcomes requires that the instruments used are reliable and sensitive to any treatment effects. Thus, the heterogeneity of SN also has an impact in terms of matching tests to symptoms as they may not have the same responsiveness to changes in certain patients. In addition, ceiling effects (BIT-c) or floor effects (CBS) of common scales need to be considered. Some studies also add measures less directly related to SN per se to explore to what extent PAT may have beneficial impacts on rehabilitation outcomes and other aspects of patients' lives. Studies have assessed abilities to perform daily tasks (105, 106), for example, using the Functional Independence Measure (FIM) (107), and estimated the likelihood of returning home after PAT (86). However, very few studies have investigated other outcome measures indicative of fall risk, quality of life, community reintegration, employment, and other milestones of brain injury rehabilitation. While the choice of outcome measures may be related to the purpose of the study, a lack of consensus can make developing general principles difficult.
Prism strength
Prism strength is measured in diopter, and the amount of visual shift, measured in visual angle degree, is also commonly reported. Prism lenses used in PAT are worn by individuals with SN during a session guided by a therapist and removed after the session, and it is important to note that prism strength is not tailored to individual patients. Thus, regardless of SN severity, all patients wear lenses with the same prism strength in a given PAT study. This one-size-fits-all approach is rather different from other clinical approaches using prescribed prisms to correct visual field cuts, double vision, or other visual impairments. In these cases, the prescriptions are used to personalize the visual assistance needed during all waking hours. In the context of PAT, prisms are only used to shift the visual field during a short period of time (e.g., 10–20 min) to induce sensorimotor adaptation and aftereffects, and aftereffects can fade away in minutes to hours after prism removal. To our knowledge, the question of personalization of prisms in PAT related to SN severity has not yet been addressed.
The importance of prism strength is related to the detectability of a therapeutic effect. While there is yet no systematic study examining the relationship between prism strength and the size of PAT therapeutic effects, a relationship can be inferred across studies with different prism strengths. Two randomized sham-controlled studies using 10-diopter prism lenses (inducing 6° visual shift) (108) or 5° shifting prism lenses (109) did not find PAT effects on the BIT-c or CBS. Studies using higher prism strength with at least a 10° shift showed positive, albeit mixed, results (see later sections of the article). Prism strength determines the detectability of prism aftereffects. The size of an aftereffect is correlated with, but usually less than, the prism strength, although there can be much individual variation. Tasks to measure prism aftereffects are usually done without visual feedback to prevent de-adaptation, including pointing straight ahead with eyes closed or pointing at visual targets with eyes open, but with the vision of the limb completely occluded. The latter is often referred to as open-loop pointing. Facchin et al. (110) showed that the open-loop pointing aftereffect was approximately 40% of the prism-induced visual shift. That is, while a set of 20-diopter prism goggles shifts the visual field by 11.4° horizontally to one side (non-neglected side) during prism adaptation, the aftereffect is only on average 4.5° to the other side (neglected side) of space. This size of aftereffect is in contrast to a 1° aftereffect reported after only 10-diopter (5.7°) prisms were used (108).
The inclusion of aftereffect screening may be important as patients who do not demonstrate measurable aftereffects are unlikely to benefit from PAT, as undetectable aftereffects may suggest the basic neural mechanism underlying sensorimotor adaptation is impaired. Prism adaptation requires the involvement of the cerebellum and the visual, motor, and parietal cortices (111, 112). If this fundamental neural mechanism is impaired and aftereffects are not generated, then the therapeutic effects based on changes to related brain areas are unlikely to be detected (measured using neuropsychological tests and ecological assessments) (76). Some studies and treatment protocols, but not all, thus exclude patients whose prism aftereffects fail to meet a certain criterion (49, 113–115). For example, in the KF-PAT protocol (49) used in several studies and clinical practices (82, 83, 88, 101, 102, 116), aftereffects are measured using open-loop pointing and straight-ahead pointing with eyes closed. In this protocol, an aftereffect is defined when an after-adaptation performance is more toward the neglected side of space in comparison to the before-adaptation performance. If patients do not demonstrate aftereffects in either pointing measure, in the first three consecutive sessions, continuing PAT is not recommended. This variability in aftereffect generation and the need for measurement can be seen in a study by Ten Brink et al. (115), who measured the aftereffect after PAT with open-loop pointing by asking patients to point to a central visual target presented briefly in front of them before closing their eyes and pointing. Patients who did not show an aftereffect greater than 3 cm toward the neglected side of space after the adaptation procedure (100 pointing movements while wearing prisms) were required to repeat the adaptation procedure with prisms with 50 more pointing movements. Twelve (35%) of the participants in the treatment group failed to reach the 3 cm criterion in more than 50% of the sessions. Thus, there is a need to measure aftereffects and their relations to PAT therapeutic effects, and yet the presence or the extent of an aftereffect has been variably measured and used in different studies, making comparison across studies more difficult.
Arm visibility and adaptation activities when wearing prisms
The extent of visibility of arm reaching movements while wearing prisms may be important to the size of aftereffects (117) or therapeutic effects (118), although the research is inconsistent (110), due, at least in part, to what aftereffect or outcome measure is used. There are two major approaches related to arm visibility—terminal and concurrent exposure. Terminal exposure restricts vision of the reaching arm and only allows patients to see a few centimeters of the final movement trajectory as the fingertip gets close to the target. Concurrent exposure allows patients to see the latter one-third to half of the arm movement (part of the forearm, hand, and finger) or the entire limb reaching toward the target or during functional activities. In both approaches, immediate visual feedback of the final performance is available to participants.
A small number of studies compared terminal exposure and concurrent exposure directly in individuals with SN while the concurrent-exposure condition differed in the amount of limb visible among the studies. Facchin et al. (119) used a within-participant study design where participants were given either terminal or concurrent exposure to an 11.3° visual shift in a counterbalanced order in one session. Concurrent exposure allowed almost the entire limb visible while pointing to targets. No difference was seen in the size of open-loop pointing aftereffects or in the results of neuropsychological tests for SN (119). Two other studies compared the two procedures with multi-session PAT administration using prism lenses inducing 10° of visual shift: Ladavas et al. (118) compared terminal exposure, concurrent exposure (pointing toward a visual target with the latter half of arm movement being visible), and no prism adaptation in three groups of participants, who completed 10 PAT sessions over 2 weeks, in a randomized controlled trial (RCT). Ladavas et al. found no difference in open-loop pointing aftereffects between the two exposure conditions, but a greater reduction of SN symptoms of impairment and behavioral performance on standard SN tests in the terminal exposure group compared to the concurrent exposure group. Thus, while Ladavas et al. could not draw a direct link between differences in aftereffects and therapeutic effects, terminal exposure resulted in greater therapeutic effects than concurrent exposure. Fortis et al. (120) used a randomized, crossover study design where one procedure was administered for 10 sessions (2 sessions a day) within a week, followed by the other procedure. Fortis et al.'s procedure for concurrent exposure was integrated with functional activities that required arm-reaching movements toward visual targets (e.g., coin collection and card sorting). Fortis et al. found no difference in the results of SN tests at the impairment or functional level and general functional improvement (120). Thus, the small but diverse literature has not determined whether and how the beneficial effects of PAT are associated with the extent to which arm movements are visible to patients during adaptation.
In terms of activities performed when wearing prisms, a finger pointing task to simple targets, e.g., dots or lines, either with terminal or concurrent exposure, is the most common task reported in the literature following Rossetti et al. (67) Variations to this approach do exist, however. As described above, Fortis et al. (120) used a variety of functional reaching tasks during concurrent exposure. Other activities include using a pen to mark targets on paper (102, 121), a digital stylus to reach targets on a touch screen (115, 122), and a controller, represented as a virtual fingertip, to reach targets in immersive virtual reality (123). When in an MRI scanner, various setups have been used with patients for finger pointing (124, 125) or imagined pointing (126). All different activities, except for the imagined pointing, follow the same principle—the arm reaching toward a visible target followed by immediate visual feedback, repeatedly for 50–100 times per PAT session. Methods can differ, however, in terms of task, visual angle of workspace, and number of targets or responses. Whether any of these methods are more effective than the others in terms of inducing aftereffects or PAT therapeutic effects remains mostly untested.
Treatment intensity
Few prospective studies have been conducted to directly investigate what number and frequency of PAT sessions are sufficient to result in short-term SN reduction or long-term functional improvement. Nonetheless, the literature provides some insights. While most RCTs have followed the once-daily 2-week treatment regimen (e.g., 10 sessions in total), Goedert et al. (127) conducted a secondary analysis of an RCT and found 4–6 sessions over 2 weeks might be as sufficient as 10 sessions over 2 weeks for lasting effects shown on BIT-c. In contrast, Rode et al. (70) using a low treatment intensity of one session a week over 4 weeks (four sessions in total) showed no PAT effects on BIT-c or FIM, suggesting treatment intensity can be an important factor.
The importance of treatment intensity may be related to the underlying neural mechanisms of prism adaptation. During prism adaptation and measurement of aftereffects, circuits between the cerebellum and motor cortex are activated, and bilateral parietal cortices and several parts of the intact hemisphere are involved as well (66, 112, 126). A working hypothesis proposes that after repeated and frequent sessions, functional connectivity between the parietal cortex and other parts of the brain may be formed, leading to symptom changes with lasting effects beyond eye–hand coordination and visuomotor behaviors (66, 76). Therefore, in line with the evidence cited above, theoretically, the number and frequency of PAT sessions matter and deserve more investigation.
Studies included in the recent meta-analyses
None of the recent meta-analyses of prospective RCTs (53, 79–81), when selecting studies through systematic reviews, considered key elements of PAT—prism strength, arm visibility and activity when wearing prisms, and treatment intensity—except for outcome measures. Longley et al. (53) included RCTs that measured functional abilities as outcomes. Qiu et al. (80) included RCTs that used BIT-c or CBS as an outcome measure. Li et al. (79) included RCTs that used BIT-c, CBS, FIM, star cancellation test, line bisection, or reading as an outcome measure. Szekely et al. (81) included studies with any control condition and an outcome measure that was CBS, BIT-c, or a cancellation test. However, circumstances of when, why, and for whom are critical for effective behavioral intervention development according to the NIH Stage Model.
To develop a broader perspective of PAT studies and how the variability of research might affect the four recent meta-analyses (53, 79–81), we categorized studies that were included in those meta-analyses based on the NIH Stage Model. We used the stage descriptions in the NIH Stage Model (87) as guidelines and operationalized each stage for the context of PAT clinical research focused on SN rehabilitation (Table 1). A total of 22 studies were included in the four recent meta-analyses (53, 79–81). For the purpose of the exercise, we extracted information from 20 of those studies that were written in English and that examined PAT therapeutic effects by comparing PAT to a control condition. Therefore, one study (128) reviewed by Szekely et al. (81) was excluded because it was not written in English. Another study (129) included in Longley et al. (53) was also excluded because there was no control condition but two comparative conditions using different experimental treatments.
Among the 20 studies, 4 studies (105, 108, 113, 115) were included in all recent systematic reviews. Three studies (70, 102, 109) were included in any three reviews. Three studies (118, 130, 131) were included in any two reviews. Ten studies (67, 101, 114, 116, 132–137) were only included in Szekely et al. (81). Each author of the present article independently reviewed the full-text publications of these 20 studies. Stage labels were determined after multiple group meetings following the operational definitions of the NIH Stage Model for PAT research (Table 1). As summarized in Table 2, three studies (67, 134, 136) were categorized as Stage 0. There were eight Stage 1 studies (70, 108, 109, 116, 118, 130, 135, 137) and five Stage 2 studies (102, 113, 114, 131, 133). One study (132) was categorized as Stage 1 or 2 because it was designed like a Stage 2 study but the analysis did not compare outcomes between the PAT and control groups, which made the study fall into Stage 1 for method feasibility. Three RCTs (101, 105, 115) met the Stage 3 criteria. Within each stage category, studies differed in SN diagnosis, time post incident at baseline, age, prism strength, limb visibility when wearing prisms, treatment intensity in terms of the total number of sessions and frequency of sessions, and outcome measures (Table 2). Thus, the studies were varied within each development stage and even more different across stages.
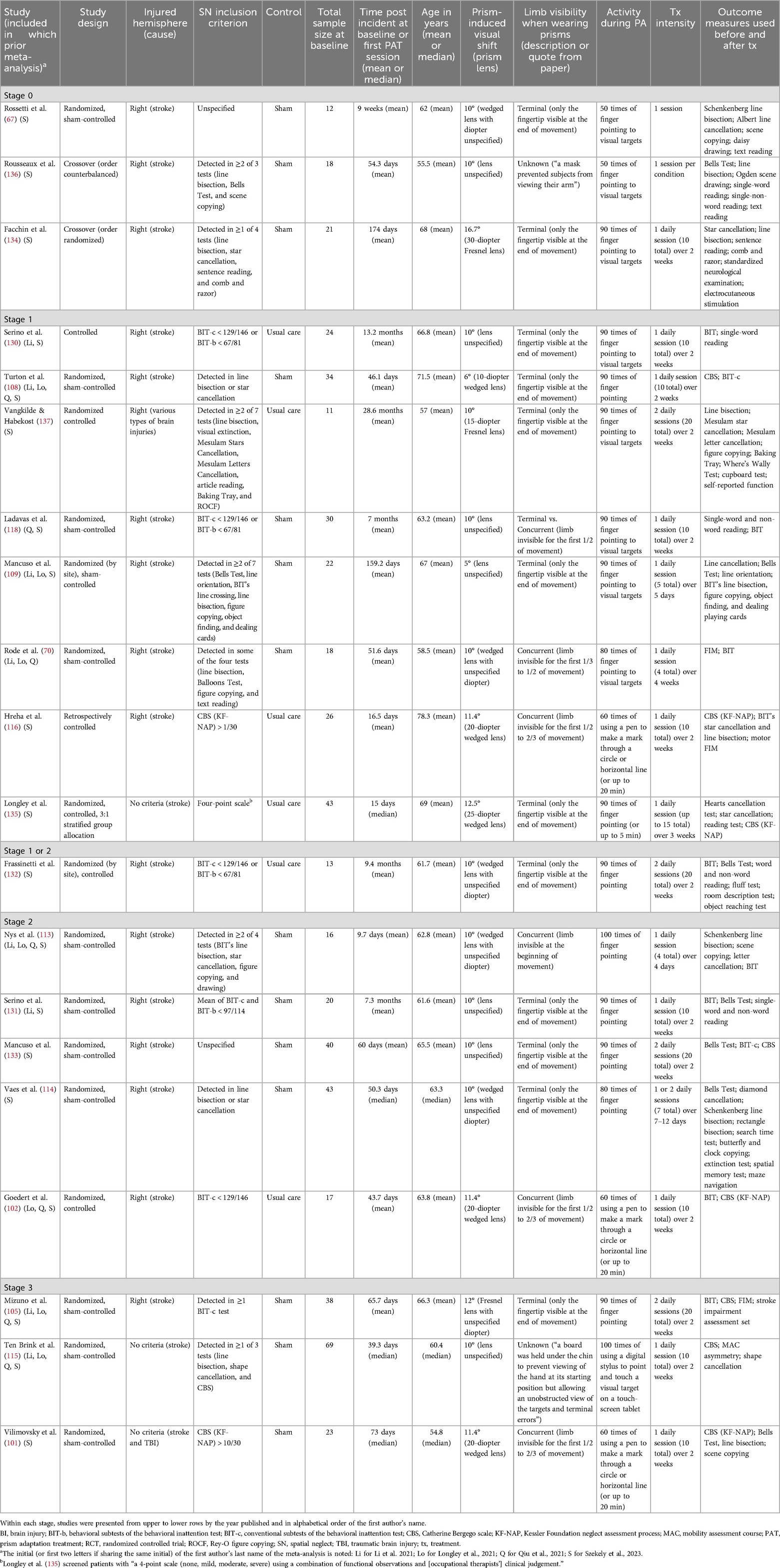
Table 2. Studies categorized as Stage 0, 1, 2, and 3 based on the operational definition of the NIH Stage Model for Behavioral Intervention Development.
For example, the three studies categorized as Stage 3 (i.e., “real-world efficacy” trials) were all sham-controlled RCTs; however, experimental groups received a variety of PAT with different prism strength but potentially comparable (10–12° visual field shift), different arm reach activities (finger pointing to visual targets vs. hand-holding devices to touch or mark visual stimuli) with different extents of limb visibility during prism adaptation, and different treatment intensity (20 sessions over 2 weeks, 10 sessions over 2 weeks). While Mizuno et al. (105) integrated PAT as part of the regular practice, Ten Brink et al. (115) and Vilimovsky et al. (101) had patients complete PAT as an additional treatment on top of regular therapy. What was considered regular could vary considerably given that these RCTs were conducted in three different countries where rehabilitation care services are provided differently. Regarding participant selection, all the RCTs included stroke survivors, and Vilimovsky et al. (101) included two individuals with traumatic brain injury (one in each group). Mizuno et al. (105) included patients with right brain damage and excluded patients with left brain damage, while Ten Brink et al. (115) and Vilimovsky et al. (101) included individuals based on SN diagnosis regardless of which hemisphere was damaged (Table 2). In terms of participants' age and time post-brain injury, Mizuno et al.'s (105) cohort appeared older than the others, and Vilimovsky et al.'s (101) sample may have received PAT later than the others' time post-incident. In short, although these three RCTs met the Stage 3 criteria, they were qualitatively diverse, reflecting the lack of consensus regarding SN diagnosis and treatment methods with PAT.
Thus, unsurprisingly, there was considerable heterogeneity of studies (Table 2) included in recently published meta-analyses (53, 79–81), resulting in null or some effects. In other words, sometimes PAT works and sometimes it does not, without providing the circumstances of when and why PAT works and for whom. However, circumstances of when, why, and for whom are critical for effective behavioral intervention development according to the NIH Stage Model.
Discussion
Circumstances (development stages) matter
High heterogeneity is a major limitation of meta-analyses that have been conducted in recent years (53, 79–81). Szekely et al. (81), for example, recognized the difficulty in combining the results of various studies and the small numbers of included studies in each of their meta-analyses, which impeded the efforts to include variables as candidate moderators. Szekely et al. (81) suggested that one solution may be the standardization of treatment protocols and outcome measures. However, other factors contribute to the high heterogeneity as revealed in our categorization exercise guided through the NIH Stage Model (Table 2). One of Szekely et al.'s meta-analyses was focused on three RCTs, led by Turton (108), Mizuno (105), and Ten Brink (115) (order by year published), because they met the highest quality standard of research conduct determined by the authors (81) and used the same outcome measure, i.e., CBS. The standard, however, did not take treatment apparatus, procedure, or intensity into account. One of the studies (108), which used low-strength prism lenses (shifting visual field by 6°), was categorized as Stage 1 because it contributed to the understanding of PAT and enabled the suggestion for using high-strength prism lenses that shift visual field by at least 10°. The other two studies, by Mizuno et al. (105) and Ten Brink et al. (115), were categorized as Stage 3. Pulling studies at different development stages contributes to the heterogeneity of data sources.
Lunven et al. (138) promptly responded after the publication of Szekely et al. and suggested that the null effect resulting from Szekely et al.'s analyses may have been washed out because the RCTs included in the meta-analyses did not consider individual differences in lesioned brain areas or impaired brain connectivity, which may have played significant roles in mediating PAT effects (66, 121, 126). This critique can be expanded to another factor that may affect PAT therapeutic effects and thus a meta-analysis result, which is the timing of PAT provision relative to time post-brain damage because brain connectivity changes over time (7, 139). No RCT has yet prospectively triaged patients based on their profiles regarding SN symptoms or brain lesions while it is well known that SN is heterogenous. Thus, more studies are required.
More research is needed before standardizing treatment protocols
While much has been learned about PAT since Rossetti et al.'s groundbreaking study (67), it is not nearly enough to result in any standardized best practice recommendations for the use of PAT as an SN therapy. To put it in the framework of the NIH Stage Model (Figure 1a), treatment refinement and modification studies (Stage 1) combined with efficacy testing (Stage 2) and neural mechanism exploration based on individual patients' brain connectivity profiles (all stages) are needed to determine a treatment protocol appropriate for a specific cohort of patients with SN. In addition, it is essential to involve the intended users (i.e., clinicians and management leaders in rehabilitation care services) in determining a treatment protocol that is not only scientifically sound but also practically feasible, which is one of the lessons learned from the implementation project by Hreha et al. (88).
More research is needed before standardizing outcome measures
As reviewed above and summarized in Table 2, CBS and BIT-c are relatively popular among PAT studies, but it does not necessarily mean that CBS or BIT-c has been used following the same methods in different studies or either is ideal for measuring outcomes.
Standardization of the administration and scoring of outcome measures is critical when combining studies in a meta-analysis, and it is unclear whether this is always accomplished. Regarding the administration of CBS, for example, all three studies categorized as Stage 3 used CBS as an outcome measure. Vilimovsky et al. (101) followed KF-NAP, and per protocol, patients were assessed by an occupational therapist with each session completed during one single visit. The two other studies followed the CBS questionnaire format. Mizuno et al. (105) did not specify how, or by what discipline, the assessment was administered, while Ten Brink et al. (115) stated that nurses, occupational therapists, and physical therapists shared the responsibility. In terms of scoring, the final CBS score is a prorated score (to account for unrated items), rather than the total score (simple sum of rated items), which is the method used to quantify SN severity regardless of how the CBS is administered. Unrated items are usually due to patients’ cognitive or motor impairment (92). However, many studies, such as Mizuno et al. (105), did not specify whether the prorated score was used as the outcome measure.
The large variability in SN can affect how well an outcome measure aligns with symptoms and their changes. The BIT-c is limited in capturing a range of changes in SN symptoms partly because it is confined in the peri-personal space defined by a regular letter-sized or A4 paper. In addition, the BIT-c total score is heavily weighted on cancellation tests, accounting for 89% of the total score of 146 (46) or 72% of the total score of 181 (with more scoring details for figure copying and representational drawings) (47). While CBS is more sensitive than many neuropsychological or non-ecological assessments such as BIT-c (44, 140), it cannot capture SN symptoms beyond the 10 items during other daily activities such as reading, wayfinding in unfamiliar environments, medication management activities, and crossing streets. Thus, CBS may be insensitive to long-term changes in SN as patients regain function over months or years. Studies are required to develop ecological outcome measures sensitive to chronic SN before conducting future PAT trials focused on long-term effects. More efforts are needed to identify and standardize an outcome measure or test battery for future RCTs evaluating SN treatment efficacy and effectiveness.
More research is needed before conducting another meta-analysis
PAT is not ready for meta-analysis focused on clinical efficacy, based on the NIH Stage Model for Behavioral Intervention Development. This conclusion is derived from the fact that the optimal combination of key elements that define “who” should be included and “how” it works in PAT are not yet determined. In other words, knowledge gaps exist in every key element of PAT that should be included in efficacy and effectiveness research. There is no consensus on “who” (e.g., defining SN and outcome measures and taking into account individual patient heterogeneity such as brain lesion profile, age, and time post-stroke) or “how” (e.g., developing consensus on PAT protocols, including prism strength, arm visibility, and treatment intensity). Only when a PAT protocol is ready with all the key elements validated to be contributing to therapeutic effects, should the protocol be examined for clinical efficacy. While there are standardized PAT protocols published by different research groups, there is no consensus. This is a major contributor to a lack of consistent findings from recent meta-analyses, and clinicians should interpret meta-analysis evidence very cautiously.
The practice-based evidence of PAT is encouraging (82–86), based on retrospective analyses of clinical data, and can potentially be categorized as Stage 4 (Table 1). While those studies do not answer all the questions regarding PAT effectiveness for the same reasons stated above for prospective studies, findings can inform the designs and planning of new prospective studies to further refine certain key elements.
Conclusion
Research provides guidance and helps clinicians evaluate whether a treatment, such as PAT, may be beneficial to patients. The current knowledge established in the literature provides a general direction for prism strength (at least 20 diopters or shifting visual field by at least 10°) insights into treatment intensity (at least three sessions per week and at least four sessions in total) and suggested the use of prism aftereffects for screening for PAT eligibility. Repeated (50–100) visuomotor activities that require arm movement toward a visible target are necessary when wearing prisms, but arm visibility (terminal vs. concurrent exposure) and types of visuomotor activities need further research to determine how they contribute to PAT therapeutic effects.
No project, to our knowledge, has been planned to examine different strategies to implement PAT clinically. Few cognitive rehabilitative therapies have ever reached Stage 5, and most established interventions and treatments are adopted by certain clinical practices and implemented organically. We advocate for more research and implementation projects involving rehabilitation practitioners, using the NIH principles of developing behavioral interventions as a path toward generating real-world evidence for clinical effectiveness, guiding the clinical use of PAT.
Author contributions
PC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. KH: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. CM: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. AS: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. GE: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rich TJ, Williams LJ, Bowen A, Eskes GA, Hreha K, Checketts M, et al. An international and multidisciplinary consensus on the labeling of spatial neglect using a modified Delphi method. Arch Rehabil Res Clin Transl. (2024) 6(2):100343. doi: 10.1016/j.arrct.2024.100343
2. Heilman KM, Valenstein E. Mechanisms underlying hemispatial neglect. Ann Neurol. (1979) 5(2):166–70. doi: 10.1002/ana.410050210
3. Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. 5th ed. New York: Oxford University (2012). p. 296–348.
4. Rode G, Pagliari C, Huchon L, Rossetti Y, Pisella L. Semiology of neglect: an update. Ann Phys Rehabil Med. (2017) 60(3):177–85. doi: 10.1016/j.rehab.2016.03.003
5. Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. (2011) 34:569–99. doi: 10.1146/annurev-neuro-061010-113731
6. Karnath HO, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: a longitudinal study. Brain. (2011) 134(Pt 3):903–12. doi: 10.1093/brain/awq355
7. Umarova RM, Beume L, Reisert M, Kaller CP, Kloppel S, Mader I, et al. Distinct white matter alterations following severe stroke: longitudinal DTI study in neglect. Neurology. (2017) 88(16):1546–55. doi: 10.1212/WNL.0000000000003843
8. Moore MJ, Hearne L, Demeyere N, Mattingley JB. Comprehensive voxel-wise, tract-based, and network lesion mapping reveals unique architectures of right and left visuospatial neglect. Brain Struct Funct. (2023) 228(9):2067–87. doi: 10.1007/s00429-023-02702-2
9. Marsh EB, Hillis AE. Dissociation between egocentric and allocentric visuospatial and tactile neglect in acute stroke. Cortex. (2008) 44(9):1215–20. doi: 10.1016/j.cortex.2006.02.002
10. Vallar G, Guariglia C, Nico D, Bisiach E. Spatial hemineglect in back space. Brain. (1995) 118(Pt 2):467–72. doi: 10.1093/brain/118.2.467
11. Gutschalk A, Dykstra A. Auditory neglect and related disorders. Handb Clin Neurol. (2015) 129:557–71. doi: 10.1016/B978-0-444-62630-1.00031-7
12. Hillis AE, Chang S, Heidler-Gary J, Newhart M, Kleinman JT, Davis C, et al. Neural correlates of modality-specific spatial extinction. J Cogn Neurosci. (2006) 18(11):1889–98. doi: 10.1162/jocn.2006.18.11.1889
13. Punt TD, Riddoch MJ. Motor neglect: implications for movement and rehabilitation following stroke. Disabil Rehabil. (2006) 28(13-14):857–64. doi: 10.1080/09638280500535025
14. Kaufmann BC, Cazzoli D, Pflugshaupt T, Bohlhalter S, Vanbellingen T, Muri RM, et al. Eyetracking during free visual exploration detects neglect more reliably than paper-pencil tests. Cortex. (2020) 129:223–35. doi: 10.1016/j.cortex.2020.04.021
15. Tegner R, Levander M. Through a looking glass: a new technique to demonstrate directional hypokinesia in unilateral neglect. Brain. (1991) 114:1943–51. doi: 10.1093/brain/114.4.1943
16. Salvato G, Sedda A, Bottini G. In search of the disappeared half of it: 35 years of studies on representational neglect. Neuropsychology. (2014) 28(5):706–16. doi: 10.1037/neu0000062
17. Heilman KM, Watson RT. Mechanisms underlying the unilateral neglect syndrome. Adv Neurol. (1977) 18:93–106.411356
18. Heilman KM, Bowers D, Coslett HB, Whelan H, Watson RT. Directional hypokinesia: prolonged reaction times for leftward movements in patients with right hemisphere lesions and neglect. Neurology. (1985) 35(6):855–9. doi: 10.1212/WNL.35.6.855
19. Fruhmann-Berger M, Pross RD, Ilg U, Karnath HO. Deviation of eyes and head in acute cerebral stroke. BMC Neurol. (2006) 6:23. doi: 10.1186/1471-2377-6-23
20. Fruhmann-Berger M, Karnath HO. Spontaneous eye and head position in patients with spatial neglect. J Neurol. (2005) 252(10):1194–200. doi: 10.1007/s00415-005-0831-y
21. Kortman B, Nicholls K. Assessing for unilateral spatial neglect using eye-tracking glasses: a feasibility study. Occup Ther Health Care. (2016) 30(4):344–55. doi: 10.1080/07380577.2016.1208858
22. Schindler I, Clavagnier S, Karnath HO, Derex L, Perenin MT. A common basis for visual and tactile exploration deficits in spatial neglect? Neuropsychologia. (2006) 44(8):1444–51. doi: 10.1016/j.neuropsychologia.2005.12.003
23. Nijboer TC, Ten Brink AF, van der Stoep N, Visser-Meily JM. Neglecting posture: differences in balance impairments between peripersonal and extrapersonal neglect. Neuroreport. (2014) 25(17):1381–5. doi: 10.1097/WNR.0000000000000277
24. Driver J, Vuilleumier P. Perceptual awareness and its loss in unilateral neglect and extinction. Cognition. (2001) 79(1-2):39–88. doi: 10.1016/S0010-0277(00)00124-4
25. Medina J, Kannan V, Pawlak MA, Kleinman JT, Newhart M, Davis C, et al. Neural substrates of visuospatial processing in distinct reference frames: evidence from unilateral spatial neglect. J Cogn Neurosci. (2009) 21(11):2073–84. doi: 10.1162/jocn.2008.21160
26. Moore MJ, Vancleef K, Riddoch MJ, Gillebert CR, Demeyere N. Recovery of visuospatial neglect subtypes and relationship to functional outcome six months after stroke. Neurorehabil Neural Repair. (2021) 35(9):823–35. doi: 10.1177/15459683211032977
27. Whitehouse CE, Green J, Giles SM, Rahman R, Coolican J, Eskes GA. Development of the Halifax visual scanning test: a new measure of visual-spatial neglect for personal, peripersonal, and extrapersonal space. J Int Neuropsychol Soc. (2019) 25(5):490–500. doi: 10.1017/S135561771900002X
28. McIntosh RD, Brodie EE, Beschin N, Robertson IH. Improving the clinical diagnosis of personal neglect: a reformulated comb and razor test. Cortex. (2000) 36(2):289–92. doi: 10.1016/S0010-9452(08)70530-6
29. Aimola L, Schindler I, Simone AM, Venneri A. Near and far space neglect: task sensitivity and anatomical substrates. Neuropsychologia. (2012) 50(6):1115–23. doi: 10.1016/j.neuropsychologia.2012.01.022
30. Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. (1978) 14(1):129–33. doi: 10.1016/S0010-9452(78)80016-1
31. Boccia M, Di Vita A, Palermo L, Committeri G, Piccardi L, Guariglia C. The way to “left” Piazza del Popolo: damage to white matter tracts in representational neglect for places. Brain Imaging Behav. (2018) 12(6):1720–9. doi: 10.1007/s11682-018-9839-7
32. Blini E, Romeo Z, Spironelli C, Pitteri M, Meneghello F, Bonato M, et al. Multi-tasking uncovers right spatial neglect and extinction in chronic left-hemisphere stroke patients. Neuropsychologia. (2016) 92:147–57. doi: 10.1016/j.neuropsychologia.2016.02.028
33. Halligan PW, Marshall JC, Wade DT. Diminution and enhancement of visuo-spatial neglect with sequential trials. J Neurol. (1993) 240(2):117–20. doi: 10.1007/BF00858728
34. Mark VW, Kooistra CA, Heilman KM. Hemispatial neglect affected by non-neglected stimuli. Neurology. (1988) 38(8):1207–11. doi: 10.1212/WNL.38.8.1207
35. Guariglia C, Palermo L, Piccardi L, Iaria G, Incoccia C. Neglecting the left side of a city square but not the left side of its clock: prevalence and characteristics of representational neglect. PLoS One. (2013) 8(7):e67390. doi: 10.1371/journal.pone.0067390
36. Harvey M, Kramer-McCaffery T, Dow L, Murphy PJS, Gilchrist ID. Categorisation of ‘perceptual’ and ‘premotor’ neglect patients across different tasks: is there strong evidence for a dichotomy? Neuropsychologia. (2002) 40(8):1387–95. doi: 10.1016/S0028-3932(01)00202-0
37. Chen P, Toglia J. Online and offline awareness deficits: anosognosia for spatial neglect. Rehabil Psychol. (2019) 64(1):50–64. doi: 10.1037/rep0000207
38. Chen P, Zanca J, Esposito E, Barrett AM. Barriers and facilitators to current rehabilitation care of individuals with spatial neglect: a qualitative study of professional views. Arch Rehabil Res Clin Transl. (2021) 3(2):100122. doi: 10.1016/j.arrct.2021.100122
39. Moore M, Milosevich E, Beisteiner R, Bowen A, Checketts M, Demeyere N, et al. Rapid screening for neglect following stroke: a systematic search and European Academy of Neurology recommendations. Eur J Neurol. (2022) 29(9):2596–606. doi: 10.1111/ene.15381
40. Checketts M, Mancuso M, Fordell H, Chen P, Hreha K, Eskes GA, et al. Current clinical practice in the screening and diagnosis of spatial neglect post-stroke: findings from a multidisciplinary international survey. Neuropsychol Rehabil. (2021) 31(9):1495–526. doi: 10.1080/09602011.2020.1782946
41. Wheeler C, Smith LJ, Sakel M, Wilkinson D. A systematic review of vestibular stimulation in post-stroke visual neglect. Neuropsychol Rehabil. (2024):1–33. doi: 10.1080/09602011.2024.2338603
42. Williams LJ, Kernot J, Hillier SL, Loetscher T. Spatial neglect subtypes, definitions and assessment tools: a scoping review. Front Neurol. (2021) 12:742365. doi: 10.3389/fneur.2021.742365
43. Lindell AB, Jalas MJ, Tenovuo O, Brunila T, Voeten MJM, Hamalainen H. Clinical assessment of hemispatial neglect: evaluation of different measures and dimensions. Clinical Neuropsychologist. (2007) 21(3):479–97. doi: 10.1080/13854040600630061
44. Azouvi P, Samuel C, Louis-Dreyfus A, Bernati T, Bartolomeo P, Beis JM, et al. Sensitivity of clinical and behavioural tests of spatial neglect after right hemisphere stroke. J Neurol Neurosurg Psychiatry. (2002) 73(2):160–6. doi: 10.1136/jnnp.73.2.160
45. Esposito E, Shekhtman G, Chen P. Prevalence of spatial neglect post stroke: a systematic review. Ann Phys Rehabil Med. (2021) 64(5):101459. doi: 10.1016/j.rehab.2020.10.010
46. Halligan PW, Cockburn J, Wilson BA. The behavioural assessment of visual neglect. Neuropsychol Rehabil. (1991) 1(1):5–32. doi: 10.1080/09602019108401377
47. Halligan PW, Robertson I, Pizzamiglio L, Homberg V, Weber E, Bergego C. The laterality of visual neglect after right hemisphere damage. Neuropsychol Rehabil. (1991) 1(4):281–301. doi: 10.1080/09602019108402259
48. Azouvi P, Marchal F, Samuel C, Morin L, Renard C, Louis-Dreyfus A, et al. Functional consequences and awareness of unilateral neglect: study of an evaluation scale. Neuropsychol Rehabil. (1996) 6(2):133–50. doi: 10.1080/713755501
49. Chen P, Hreha K. Kessler Foundation Prism Adaptation Treatment 2020 Manual. Wood Dale, IL: Stoelting (2020).
50. Nijboer TC, Kollen BJ, Kwakkel G. Time course of visuospatial neglect early after stroke: a longitudinal cohort study. Cortex. (2013) 49(8):2021–7. doi: 10.1016/j.cortex.2012.11.006
51. Berger MF, Johannsen L, Karnath HO. Subcortical neglect is not always a transient phenomenon: evidence from a 1-year follow-up study. J Clin Exp Neuropsychol. (2009) 31(5):617–23. doi: 10.1080/13803390802403672
52. Chen P, Pitteri M, Gillen G, Ayyala H. Ask the experts how to treat individuals with spatial neglect: a survey study. Disabil Rehabil. (2018) 40(22):2677–91. doi: 10.1080/09638288.2017.1347720
53. Longley V, Hazelton C, Heal C, Pollock A, Woodward-Nutt K, Mitchell C, et al. Non-pharmacological interventions for spatial neglect or inattention following stroke and other non-progressive brain injury. Cochrane Database Syst Rev. (2021) 7(7):CD003586. doi: 10.1002/14651858.CD003586.pub4
54. Pizzamiglio L, Guariglia C, Antonucci G, Zoccolotti P. Development of a rehabilitative program for unilateral neglect. Restor Neurol Neurosci. (2006) 24(4-6):337–45.17119308
55. Kerkhoff G. Successful return to professional work after neglect, extinction, and spatial misperception—three long-term case studies. Neuropsychol Rehabil. (2021) 31(6):837–62. doi: 10.1080/09602011.2020.1738248
56. Kerkhoff G, Bucher L, Brasse M, Leonhart E, Holzgraefe M, Volzke V, et al. Smooth pursuit “bedside” training reduces disability and unawareness during the activities of daily living in neglect: a randomized controlled trial. Neurorehabil Neural Repair. (2014) 28(6):554–63. doi: 10.1177/1545968313517757
57. Weinberg J, Diller L, Gordon WA, Gerstman LJ, Lieberman A, Lakin P, et al. Visual scanning training effect on reading-related tasks in acquired right brain damage. Arch Phys Med Rehabil. (1977) 58(11):479–86.931586
58. Eskes GA, Butler B, McDonald A, Harrison ER, Phillips SJ. Limb activation effects in hemispatial neglect. Arch Phys Med Rehabil. (2003) 84(3):323–8. doi: 10.1053/apmr.2003.50012
59. Welfringer A, Leifert-Fiebach G, Babinsky R, Brandt T. Visuomotor imagery as a new tool in the rehabilitation of neglect: a randomised controlled study of feasibility and efficacy. Disabil Rehabil. (2011) 33(21-22):2033–43. doi: 10.3109/09638288.2011.556208
60. Aparicio-Lopez C, Garcia-Molina A, Garcia-Fernandez J, Lopez-Blazquez R, Ensenat-Cantallops A, Sanchez-Carrion R, et al. Cognitive rehabilitation with right hemifield eye-patching for patients with sub-acute stroke and visuo-spatial neglect: a randomized controlled trial. Brain Inj. (2015) 29(4):1–7. doi: 10.3109/02699052.2014.995230
61. Kamada K, Shimodozono M, Hamada H, Kawahira K. Effects of 5 min of neck-muscle vibration immediately before occupational therapy on unilateral spatial neglect. Disabil Rehabil. (2011) 33(23-24):2322–8. doi: 10.3109/09638288.2011.570411
62. Yang NYH, Fong KNK, Li-Tsang CWP, Zhou D. Effects of repetitive transcranial magnetic stimulation combined with sensory cueing on unilateral neglect in subacute patients with right hemispheric stroke: a randomized controlled study. Clin Rehabil. (2017) 31(9):1154–63. doi: 10.1177/0269215516679712
63. Yang NY, Zhou D, Chung RC, Li-Tsang CW, Fong KN. Rehabilitation interventions for unilateral neglect after stroke: a systematic review from 1997 through 2012. Front Hum Neurosci. (2013) 7:187. doi: 10.3389/fnhum.2013.00187
64. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47(6):e98–e169. doi: 10.1161/STR.0000000000000098
65. Harris CS. Adaptation to displaced vision: visual, motor, or proprioceptive change? Science. (1963) 140(3568):812–3. doi: 10.1126/science.140.3568.812
66. Boukrina O, Chen P. Neural mechanisms of prism adaptation in healthy adults and individuals with spatial neglect after unilateral stroke: a review of fMRI studies. Brain Sci. (2021) 11(11):1468. doi: 10.3390/brainsci11111468
67. Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, et al. Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature. (1998) 395(6698):166–9. doi: 10.1038/25988
68. Prablanc C, Panico F, Fleury L, Pisella L, Nijboer T, Kitazawa S, et al. Adapting terminology: clarifying prism adaptation vocabulary, concepts, and methods. Neurosci Res. (2020) 153:8–21. doi: 10.1016/j.neures.2019.03.003
69. Chen P, Landar V, Noce N, Hreha K. Prism adaptation treatment for spatial neglect post brain tumor removal: a case report. Hong Kong J Occup Ther. (2020) 33(1):25–9. doi: 10.1177/1569186120921472
70. Rode G, Lacour S, Jacquin-Courtois S, Pisella L, Michel C, Revol P, et al. Long-term sensorimotor and therapeutical effects of a mild regime of prism adaptation in spatial neglect. A double-blind RCT essay. Ann Phys Rehabil Med. (2015) 58(2):40–53. doi: 10.1016/j.rehab.2014.10.004
71. Taub E, Goldberg LA. Prism adaptation: control of intermanual transfer by distribution of practice. Science. (1973) 180(4087):755–7. doi: 10.1126/science.180.4087.755
72. Redding GM, Wallace B. Sources of “overadditivity” in prism adaptation. Percept Psychophys. (1978) 24(1):58–62. doi: 10.3758/BF03202974
73. Redding GM, Wallace B. Intermanual transfer of prism adaptation. J Mot Behav. (2008) 40(3):246–62. doi: 10.3200/JMBR.40.3.246-264
74. Kornheiser AS. Adaptation to laterally displaced vision: a review. Psychol Bull. (1976) 83(5):783–816. doi: 10.1037/0033-2909.83.5.783
75. Redding GM, Wallace B. Adaptive eye-hand coordination: implications of prism adaptation for perceptual-motor organization. In: Proteau L, Elliott D, editors. Vision and Motor Control. North Holland: Elsevier Science Publishers B.V. (1992). p. 105–27.
76. Panico F, Rossetti Y, Trojano L. On the mechanisms underlying prism adaptation: a review of neuro-imaging and neuro-stimulation studies. Cortex. (2020) 123:57–71. doi: 10.1016/j.cortex.2019.10.003
77. Champod AS, Frank RC, Taylor K, Eskes GA. The effects of prism adaptation on daily life activities in patients with visuospatial neglect: a systematic review. Neuropsychol Rehabil. (2018) 28(4):491–514. doi: 10.1080/09602011.2016.1182032
78. Jacquin-Courtois S, O’Shea J, Luaute J, Pisella L, Revol P, Mizuno K, et al. Rehabilitation of spatial neglect by prism adaptation: a peculiar expansion of sensorimotor after-effects to spatial cognition. Neurosci Biobehav Rev. (2013) 37(4):594–609. doi: 10.1016/j.neubiorev.2013.02.007
79. Li J, Li L, Yang Y, Chen S. Effects of prism adaptation for unilateral spatial neglect after stroke: a systematic review and meta-analysis. Am J Phys Med Rehabil. (2021) 100(6):584–91. doi: 10.1097/PHM.0000000000001598
80. Qiu HD, Wang JY, Yi WC, Yin ZF, Wang HX, Li JA. Effects of prism adaptation on unilateral neglect after stroke: an updated meta-analysis of randomized controlled trials. Am J Phys Med Rehabil. (2021) 100(3):259–65. doi: 10.1097/PHM.0000000000001557
81. Szekely O, Ten Brink AF, Mitchell AG, Bultitude JH, McIntosh RD. No short-term treatment effect of prism adaptation for spatial neglect: an inclusive meta-analysis. Neuropsychologia. (2023) 189:108566. doi: 10.1016/j.neuropsychologia.2023.108566
82. Gillen RW, Harmon EY, Weil B, Fusco-Gessick B, Novak PP, Barrett AM. Prism adaptation treatment of spatial neglect: feasibility during inpatient rehabilitation and identification of patients most likely to benefit. Front Neurol. (2022) 13:803312. doi: 10.3389/fneur.2022.803312
83. Vilimovsky T, Chen P, Hoidekrova K, Slavicek O, Harsa P. Prism adaptation treatment predicts improved rehabilitation responses in stroke patients with spatial neglect. Healthcare. (2022) 10(10):2009. doi: 10.3390/healthcare10102009
84. Chen P, Hreha K, Gonzalez-Snyder C, Rich TJ, Gillen RW, Parrott D, et al. Impacts of prism adaptation treatment on spatial neglect and rehabilitation outcome: dosage matters. Neurorehabil Neural Repair. (2022) 36(8):500–13. doi: 10.1177/15459683221107891
85. Rich TJ, Pylarinos M, Parrott D, Chen P. Prism adaptation treatment for right-sided and left-sided spatial neglect: a retrospective case-matched study. Arch Rehabil Res Clin Transl. (2023) 5(2):100263. doi: 10.1016/j.arrct.2023.100263
86. Chen P, Diaz-Segarra N, Hreha K, Kaplan E, Barrett AM. Prism adaptation treatment improves inpatient rehabilitation outcome in individuals with spatial neglect: a retrospective matched control study. Arch Rehabil Res Clin Transl. (2021) 3(3):100130. doi: 10.1016/j.arrct.2021.100130
87. National Institutes of Health. NIH Stage Model for Behavioral Intervention Development (2022). Available online at: https://www.nia.nih.gov/research/dbsr/nih-stage-model-behavioral-intervention-development (Accessed December 08, 2023).
88. Hreha K, Barrett AM, Gillen RW, Gonzales-Snyder C, Masmela J, Chen P. The implementation process of two evidence-based protocols: a spatial neglect network initiative. Front Health Servic. (2022) 2:839517. doi: 10.3389/frhs.2022.839517
89. Chen P, Hreha K. KF-NAP 2015 Manual. West Orange, N.J., USA: Kessler Foundation (2015). Available online at: https://www.kflearn.org/courses/kf-nap-2015-manuals
90. Chen P, Chen CC, Hreha K, Goedert KM, Barrett AM. Kessler Foundation neglect assessment process uniquely measures spatial neglect during activities of daily living. Arch Phys Med Rehabil. (2015) 96(5):869–76. doi: 10.1016/j.apmr.2014.10.023
91. Chen P, Hreha K. Spatial neglect not only occurs after stroke but also after traumatic brain injury. Ann Phys Rehabil Med. (2023) 66(8):101778. doi: 10.1016/j.rehab.2023.101778
92. Rich TJ, Hreha KP, Barrett AM, Parrott D, Chen P. The effect of missed items on the reliability of the Kessler Foundation neglect assessment process. Arch Phys Med Rehabil. (2022) 103(11):2145–52. doi: 10.1016/j.apmr.2022.01.165
93. Horn SD, DeJong G, Deutscher D. Practice-based evidence research in rehabilitation: an alternative to randomized controlled trials and traditional observational studies. Arch Phys Med Rehabil. (2012) 93(8 Suppl):S127–37. doi: 10.1016/j.apmr.2011.10.031
94. NIH National Center for Advancing Translational Sciences. Efficacy. Available online at: https://toolkit.ncats.nih.gov/glossary/efficacy/ (Accessed July 16, 2024).
95. NIH National Center for Advancing Translational Sciences. Effectiveness. Available online at: https://toolkit.ncats.nih.gov/glossary/effectiveness/ (Accessed July 16, 2024).
96. US Food and Drug Administration. Drug Development Process. (2018). Available online at: https://www.fda.gov/patients/drug-development-process/step-3-clinical-research (Accessed April 22, 2024).
97. Dobkin BH. Progressive staging of pilot studies to improve phase III trials for motor interventions. Neurorehabil Neural Repair. (2009) 23(3):197–206. doi: 10.1177/1545968309331863
98. Robey RR. A five-phase model for clinical-outcome research. J Commun Disord. (2004) 37(5):401–11. doi: 10.1016/j.jcomdis.2004.04.003
99. Onken LS, Kaskie B. Implementation science at the National Institute on Aging: the principles of it. Public Policy Aging Rep. (2022) 32(1):39–41. doi: 10.1093/ppar/prab034
100. Onken LS, Carroll KM, Shoham V, Cuthbert BN, Riddle M. Reenvisioning clinical science: unifying the discipline to improve the public health. Clin Psychol Sci. (2014) 2(1):22–34. doi: 10.1177/2167702613497932
101. Vilimovsky T, Chen P, Hoidekrova K, Petioky J, Harsa P. Prism adaptation treatment to address spatial neglect in an intensive rehabilitation program: a randomized pilot and feasibility trial. PLoS One. (2021) 16(1):e0245425. doi: 10.1371/journal.pone.0245425
102. Goedert KM, Chen P, Foundas AL, Barrett AM. Frontal lesions predict response to prism adaptation treatment in spatial neglect: a randomised controlled study. Neuropsychol Rehabil. (2020) 30(1):32–53. doi: 10.1080/09602011.2018.1448287
103. Umeonwuka CI, Roos R, Ntsiea V. Clinical and demographic predictors of unilateral spatial neglect recovery after prism therapy among stroke survivors in the sub-acute phase of recovery. Neuropsychol Rehabil. (2023) 33(10):1624–49. doi: 10.1080/09602011.2022.2131582
104. Goedert KM, Chen P, Boston RC, Foundas AL, Barrett AM. Presence of motor-intentional aiming deficit predicts functional improvement of spatial neglect with prism adaptation. Neurorehabil Neural Repair. (2014) 28(5):483–92. doi: 10.1177/1545968313516872
105. Mizuno K, Tsuji T, Takebayashi T, Fujiwara T, Hase K, Liu M. Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: a randomized, controlled trial. Neurorehabil Neural Repair. (2011) 25(8):711–20. doi: 10.1177/1545968311407516
106. Shiraishi H, Muraki T, Itou YSA, Hirayama K. Prism intervention helped sustainability of effects and ADL performances in chronic hemispatial neglect: a follow-up study. Neurorehabilitation. (2010) 27(2):165–72. doi: 10.3233/nre-2010-0593
107. Centers for Medicare & Medicaid Services. The Inpatient Rehabilitation Facility—Patient Assessment Instrument (IRF-PAI) Training Manual. (2012).
108. Turton AJ, O'Leary K, Gabb J, Woodward R, Gilchrist ID. A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychol Rehabil. (2010) 20(2):180–96. doi: 10.1080/09602010903040683
109. Mancuso M, Pacini M, Gemignani P, Bartalini B, Agostini B, Ferroni L, et al. Clinical application of prismatic lenses in the rehabilitation of neglect patients: a randomized controlled trial. Eur J Phys Rehabil Med. (2012) 48(2):197–208.22318363
110. Facchin A, Folegatti A, Rossetti Y, Farne A. The half of the story we did not know about prism adaptation. Cortex. (2019) 119:141–57. doi: 10.1016/j.cortex.2019.04.012
111. Calzolari E, Bolognini N, Casati C, Marzoli SB, Vallar G. Restoring abnormal aftereffects of prismatic adaptation through neuromodulation. Neuropsychologia. (2015) 74:162–9. doi: 10.1016/j.neuropsychologia.2015.04.022
112. Chapman HL, Eramudugolla R, Gavrilescu M, Strudwick MW, Loftus A, Cunnington R, et al. Neural mechanisms underlying spatial realignment during adaptation to optical wedge prisms. Neuropsychologia. (2010) 48(9):2595–601. doi: 10.1016/j.neuropsychologia.2010.05.006
113. Nys GMS, de Haan EHF, Kunneman A, de Kort PLM, Dijkerman HC. Acute neglect rehabilitation using repetitive prism adaptation: a randomized placebo-controlled trial. Restor Neurol Neurosci. (2008) 26(1):1–12.18431002
114. Vaes N, Nys G, Lafosse C, Dereymaeker L, Oostra K, Hemelsoet D, et al. Rehabilitation of visuospatial neglect by prism adaptation: effects of a mild treatment regime. A randomised controlled trial. Neuropsychol Rehabil. (2018) 28(6):899–918. doi: 10.1080/09602011.2016.1208617
115. Ten Brink AF, Visser-Meily JMA, Schut MJ, Kouwenhoven M, Eijsackers ALH, Nijboer TCW. Prism adaptation in rehabilitation? No additional effects of prism adaptation on neglect recovery in the subacute phase poststroke: a randomized controlled trial. Neurorehabil Neural Repair. (2017) 31(12):1017–28. doi: 10.1177/1545968317744277
116. Hreha K, Gillen G, Noce N, Nilsen DM. The feasibility and effectiveness of using prism adaptation to treat motor and spatial dysfunction in stroke survivors with multiple incidents of stroke. Top Stroke Rehabil. (2018) 25(4):305–11. doi: 10.1080/10749357.2018.1437937
117. Redding GM, Rossetti Y, Wallace B. Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev. (2005) 29(3):431–44. doi: 10.1016/j.neubiorev.2004.12.004
118. Ladavas E, Bonifazi S, Catena L, Serino A. Neglect rehabilitation by prism adaptation: different procedures have different impacts. Neuropsychologia. (2011) 49(5):1136–45. doi: 10.1016/j.neuropsychologia.2011.01.044
119. Facchin A, Bultitude JH, Mornati G, Peverelli M, Daini R. A comparison of prism adaptation with terminal versus concurrent exposure on sensorimotor changes and spatial neglect. Neuropsychol Rehabil. (2020) 30(4):613–40. doi: 10.1080/09602011.2018.1484374
120. Fortis P, Maravita A, Gallucci M, Ronchi R, Grassi E, Senna I, et al. Rehabilitating patients with left spatial neglect by prism exposure during a visuomotor activity. Neuropsychology. (2010) 24(6):681–97. doi: 10.1037/a0019476
121. Chen P, Goedert KM, Shah P, Foundas AL, Barrett AM. Integrity of medial temporal structures may predict better improvement of spatial neglect with prism adaptation treatment. Brain Imaging Behav. (2014) 8(3):346–58. doi: 10.1007/s11682-012-9200-5
122. Smit M, Van der Stigchel S, Visser-Meily JM, Kouwenhoven M, Eijsackers AL, Nijboer TC. The feasibility of computer-based prism adaptation to ameliorate neglect in sub-acute stroke patients admitted to a rehabilitation center. Front Hum Neurosci. (2013) 7:353. doi: 10.3389/fnhum.2013.00353
123. Ramos AA, Horning EC, Wilms IL. Simulated prism exposure in immersed virtual reality produces larger prismatic after-effects than standard prism exposure in healthy subjects. PLoS One. (2019) 14(5):e0217074. doi: 10.1371/journal.pone.0217074
124. Crottaz-Herbette S, Fornari E, Notter MP, Bindschaedler C, Manzoni L, Clarke S. Reshaping the brain after stroke: the effect of prismatic adaptation in patients with right brain damage. Neuropsychologia. (2017) 104:54–63. doi: 10.1016/j.neuropsychologia.2017.08.005
125. Saj A, Cojan Y, Vocat R, Luaute J, Vuilleumier P. Prism adaptation enhances activity of intact fronto-parietal areas in both hemispheres in neglect patients. Cortex. (2013) 49(1):107–19. doi: 10.1016/j.cortex.2011.10.009
126. Saj A, Cojan Y, Assal F, Vuilleumier P. Prism adaptation effect on neural activity and spatial neglect depend on brain lesion site. Cortex. (2019) 119:301–11. doi: 10.1016/j.cortex.2019.04.022
127. Goedert KM, Zhang JY, Barrett AM. Prism adaptation and spatial neglect: the need for dose-finding studies. Front Hum Neurosci. (2015) 9:243. doi: 10.3389/fnhum.2015.00243
128. Hauer B, Quirbach A. On the economy and effectiveness of prism adaptation as therapy for unilateral neglect. Zeitschrift für Neuropsychologie. (2007) 18(3):171–81. doi: 10.1024/1016-264X.18.3.171
129. Choi HS, Kim DJ, Yang YA. The effect of a complex intervention program for unilateral neglect in patients with acute-phase stroke: a randomized controlled trial. Osong Public Health Res Perspect. (2019) 10(5):265–73. doi: 10.24171/j.phrp.2019.10.5.02
130. Serino A, Angeli V, Frassinetti F, Ladavas E. Mechanisms underlying neglect recovery after prism adaptation. Neuropsychologia. (2006) 44(7):1068–78. doi: 10.1016/j.neuropsychologia.2005.10.024
131. Serino A, Barbiani M, Rinaldesi ML, Ladavas E. Effectiveness of prism adaptation in neglect rehabilitation: a controlled trial study. Stroke. (2009) 40(4):1392–8. doi: 10.1161/STROKEAHA.108.530485
132. Frassinetti F, Angeli V, Meneghello F, Avanzi S, Ladavas E. Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain. (2002) 125(Pt 3):608–23. doi: 10.1093/brain/awf056
133. Mancuso M, Capitani D, Ferroni L, Caputo M, Bartalini B, Abbruzzese L, et al. Efficacy of prisms in neglect treatment: a randomized single blind study. Int J Phys Med Rehabil. (2016) 4:355. doi: 10.4172/2329-9096.1000355
134. Facchin A, Sartori E, Luisetti C, De Galeazzi A, Beschin N. Effect of prism adaptation on neglect hemianesthesia. Cortex. (2019) 113:298–311. doi: 10.1016/j.cortex.2018.12.021
135. Longley V, Woodward-Nutt K, Turton AJ, Stocking K, Checketts M, Bamford A, et al. A study of prisms and therapy in attention loss after stroke (SPATIAL): a feasibility randomised controlled trial. Clin Rehabil. (2023) 37(3):381–93. doi: 10.1177/02692155221134060
136. Rousseaux M, Bernati T, Saj A, Kozlowski O. Ineffectiveness of prism adaptation on spatial neglect signs. Stroke. (2006) 37(2):542–3. doi: 10.1161/01.STR.0000198877.09270.e8
137. Vangkilde S, Habekost T. Finding Wally: prism adaptation improves visual search in chronic neglect. Neuropsychologia. (2010) 48(7):1994–2004. doi: 10.1016/j.neuropsychologia.2010.03.020
138. Lunven M, Toba MN, Bartolomeo P. Prism adaptation therapy in spatial neglect: the importance of connectional anatomy. Neuropsychologia. (2023) 188:108640. doi: 10.1016/j.neuropsychologia.2023.108640
139. Ramsey LE, Siegel JS, Baldassarre A, Metcalf NV, Zinn K, Shulman GL, et al. Normalization of network connectivity in hemispatial neglect recovery. Ann Neurol. (2016) 80(1):127–41. doi: 10.1002/ana.24690
Keywords: efficacy, effectiveness, behavioral intervention, therapeutic treatment, rehabilitative therapy, spatial exploration, sensorimotor adaptation, unilateral spatial neglect
Citation: Chen P, Hreha K, MacPhee C, Salter A and Eskes GA (2025) Does prism adaptation treatment reduce spatial neglect and improve function?. Front. Rehabil. Sci. 6:1539887. doi: 10.3389/fresc.2025.1539887
Received: 5 December 2024; Accepted: 21 January 2025;
Published: 5 February 2025.
Edited by:
Francesco Lena, Mediterranean Neurological Institute Neuromed (IRCCS), ItalyReviewed by:
Laura Jennie Smith, Queen Mary University of London, United KingdomCarolina Colomer, Servicio Neurorrehabilitación Hospitales Vithas, Spain
Copyright: © 2025 Chen, Hreha, MacPhee, Salter and Eskes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peii Chen, cGNoZW5Aa2Vzc2xlcmZvdW5kYXRpb24ub3Jn
 Peii Chen
Peii Chen Kimberly Hreha3
Kimberly Hreha3 Catrina MacPhee
Catrina MacPhee Gail A. Eskes
Gail A. Eskes