
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Rehabil. Sci. , 28 March 2025
Sec. Rehabilitation for Musculoskeletal Conditions
Volume 6 - 2025 | https://doi.org/10.3389/fresc.2025.1538336
This article is part of the Research Topic Advancing Muscle Health: From Technical and Clinical Research to Practice View all 3 articles
 Antimo Moretti1
Antimo Moretti1 Federica Tomaino1
Federica Tomaino1 Marco Paoletta2
Marco Paoletta2 Sara Liguori1,2*
Sara Liguori1,2* Silvia Migliaccio3
Silvia Migliaccio3 Mariangela Rondanelli4
Mariangela Rondanelli4 Angelo Di Iorio5
Angelo Di Iorio5 Raffaello Pellegrino6
Raffaello Pellegrino6 Davide Donnarumma7
Davide Donnarumma7 Daniele Di Nunzio7
Daniele Di Nunzio7 Giuseppe Toro1
Giuseppe Toro1 Francesca Gimigliano2
Francesca Gimigliano2 Maria Luisa Brandi8
Maria Luisa Brandi8 Giovanni Iolascon1
Giovanni Iolascon1
Sarcopenia is the age-related loss of skeletal muscle mass and function. Recently, research has focused on defining diagnostic criteria for this condition, now recognized as a muscle disease with a specific identifying code (ICD-10: M62.84). The diagnostic process for sarcopenia involves several stages, including the use of dedicated questionnaires and objective measurements of muscle strength and mass. According to international guidelines, therapeutic exercise is recommended to improve muscle mass, muscle strength, and physical performance. However, much of the supporting evidence comes from studies on non-sarcopenic elderly patients. Among types of therapeutic exercise, guidelines mainly emphasize muscle strengthening. The prescription of therapeutic exercise must consider the clinical and functional conditions of the patient (e.g., the presence of severe sarcopenia) and patient preferences. Muscle strengthening should target large muscle groups and include low-intensity resistance exercise for strength improvement, or high-intensity resistance exercise for additional benefits in muscle mass and function. Evidence suggests that an ideal therapeutic exercise program for sarcopenic patients should be multimodal, incorporating muscle strengthening, aerobic exercise, and balance control programs. This approach could enhance patient adherence by offering variety. Although multimodal therapeutic exercise improves muscle mass and function, these benefits can be lost during prolonged physical inactivity. Therefore, the exercise prescription must define intensity, volume (repetitions and sets), frequency, rest intervals, and duration, tailored to the type of exercise. Aerobic training programs improve endurance and optimize mitochondrial function. Balance training, important for reducing the risk of falls, should be done at least three times a week. Muscle strengthening should be done at least two days a week, starting at 50%–60% of 1 repetition maximum (RM) and progressing to 60%–80% of 1 RM, with approximately 10 exercises per session. Adopting comprehensive prescription protocols, such as those proposed in this paper, can significantly aid in the functional recovery and well-being of patients with sarcopenia.
Sarcopenia is a degenerative and progressive disorder of skeletal muscle characterized by a reduction in muscle mass and function (1). This age-related disease has significant consequences, including an increased risk of falls, mobility limitations, decreased independence in daily activities, and higher mortality rates (2). At socio-economic level, the estimated cost of hospitalization for people with sarcopenia was very high, reaching over USD $40 billion (3). This increased economic burden is due to greater odds of hospitalization and on average more hospital stays in sarcopenic individuals compared to people without sarcopenia.
Despite these impacts, identifying sarcopenia is challenging due to the lack of a universal operational definition. However, a case-finding strategy might be effective, as suggested by both the European Working Group on Sarcopenia in Older People 2 (EWGSOP2) (1) and the Asian Working Group for Sarcopenia (AWGS) (4). Both groups proposed using the SARC-F questionnaire as a screening tool (1, 5). If a patient scores higher than 4 on the SARC-F, further assessment of muscle strength using a handheld dynamometer is required (6). An alternative test for muscle strength assessment is the sit-to-stand test, which measures the time taken to stand up and sit down five times consecutively without using the upper limbs (7). To confirm a diagnosis of sarcopenia, it is essential to measure muscle mass using dual-energy x-ray absorptiometry (DXA) or bioelectrical impedance analysis (BIA) (8, 9). Additionally, assessing the severity of sarcopenia involves testing physical performance with specific tools, such as the 4-meter walking speed, the Short Physical Performance Battery (10), the Time Up and Go test, or the 400-meter walk test (11). Table 1 summarizes the diagnostic cut-offs for each parameter according to the EWGSOP2 and the AWGS recommendations.
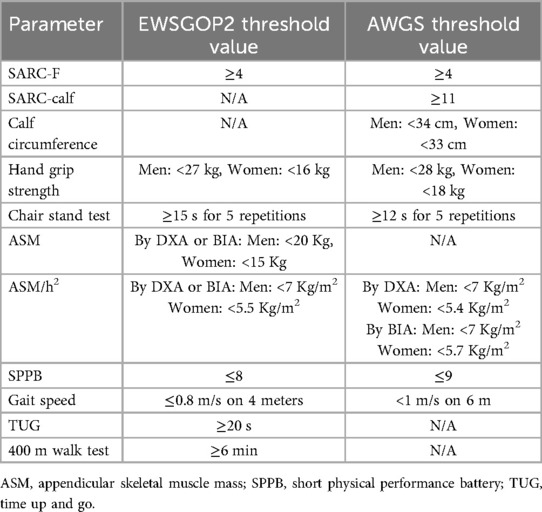
Table 1. Key indicators used for the diagnosis of sarcopenia according to European and Asian working groups on sarcopenia.
The appropriate management of sarcopenic patients encompasses nutritional and physical exercise interventions (12–14). To date, current evidence tends to recommend a higher intake doses of key nutrients, including protein (25–30 g per meal), leucine (77.8 mg/kg/day for men and 78.2 mg/kg/day for woman), and vitamins (D, C, and E), among other essential elements (15, 16). In the future, complex hybrid nutritional supplements will be developed to personalize the nutritional experience based on the metabolic status of each individual patient (17). Currently, no specific pharmacological treatments for sarcopenia have been approved by international regulatory agencies (18), making exercise the frontline treatment against age-related muscle wasting (19). Physical exercise stimulates the production of various myokines that have anti-inflammatory and anabolic effects (20). It activates specific pathways, including the activation of adenosine monophosphate-activated protein kinase (AMPK), which enhances myofiber hypertrophy, mitochondrial biogenesis, and angiogenesis (21). Additionally, regular progressive resistance and aerobic exercise promotes reduction of adipose tissue improving insulin sensitivity and glucose metabolism. Furthermore, physical activity has cognitive benefits, such as increasing cerebral flow and elevating levels of neurotrophic factors like BDNF and insulin-like growth factor-1 (IGF-1), while decreasing neurotoxic factors such as C-reactive protein, cortisol, and interleukin-6 (IL-6), as well as modulating other inflammatory cytokines (22).
The need for this expert opinion also arises from the increased interest for this condition following its inclusion in the International Classification of Diseases as a muscle pathology (ICD-10: M62.84) (23, 24).
Current guidelines strongly recommend exercise for managing sarcopenia (14). However, the studies included in this guideline did not involve patients diagnosed with sarcopenia, according to the subsequently published EWSGOP2 and AWGS criteria, limiting their applicability. This manuscript aims to address this knowledge gap by emphasizing the need for tailored physical exercise guidelines for people affected by primary sarcopenia and providing recommendations for their implementation in clinical practice.
We collected and assessed scientific evidence on physical exercise in sarcopenia. We considered for the search strategy studies including patients with diagnosis of sarcopenia according to the criteria of the EWGSOP2 or the AWGS. The included studies were randomized controlled trials (RCTs), systematic review and meta-analyses of RCTs, addressing the efficacy of physical exercise for sarcopenia compared to other interventions, such as nutritional approaches, or no intervention. The primary outcomes were muscle mass, strength, physical performance, and risk of falls. The literature search was conducted via the PubMed database from inception until December 31st, 2023. The following set of MeSH terms was used: “Sarcopenia”, “Exercise” and ‘Resistance training’. Two independent reviewers (M.P. and S.L.) evaluated abstracts and full texts based on established inclusion criteria.
Most studies suggest prescribing resistance exercise (RE) alone or in combination with other exercise modalities. Undoubtedly, RE is a cornerstone intervention in managing sarcopenia, although proposed protocols vary widely among the studies.
In a systematic umbrella review, (14 studies, 7 of which conducted a meta-analysis), Beckwée and al (25). suggest that low-load resistance training (LIRT) with intensity up to 50% of one-repetition maximum (1RM) improves muscle strength. More in detail, LIRT is primarily focused on building endurance and improving muscle efficiency, making it suitable for activities requiring sustained effort over long periods. Conversely, high-intensity resistance training (HIRT) at 80% of 1RM maximizes strength gains. The HIRT is geared toward maximizing muscle strength and size, leveraging the benefits of heavier loads and lower repetitions. Depending on an individual's fitness goals, choosing between LIRT and HIRT can significantly influence the physiological outcomes and overall training effectiveness. Regarding volume, frequency, and duration, authors recommend performing 1–4 sets of 8–15 repetitions, 2–3 times per week, over 6–12 weeks. Evidence also supports multimodal exercise, combining RE, aerobic training, and balance exercises, as well as blood flow restriction (BFR) training. Balance training involves exercises designed to improve stability and coordination, yielding significant biological effects across various body systems. This training enhances neuromuscular control by improving communication between the nervous system and muscles, which boosts proprioception, coordination, and reaction times, thereby reducing fall risk. Moreover, balance training engages multiple muscle groups, strengthening stabilizer muscles and promoting overall muscular development and endurance (22).
BFR is a technique that creates a controlled environment in which venous blood flow is restricted while maintaining arterial blood flow to the targeted muscles. This method allows individuals to achieve significant muscle adaptations with lighter weights, reducing injury risk and minimizing stress on joints. By restricting venous outflow, BFR training promotes metabolic buildup in muscles, through a localized metabolic accumulation in muscle tissue, creating a hypoxic environment that stimulates hypertrophic signaling pathways. The hypoxic environment in BFR training promotes metabolite buildup, such as lactate, which enhances growth hormone production and fast-twitch fiber recruitment. These effects, driven by mTORC1 and MAPK pathways, facilitate hypertrophy and strength gains even at low intensities (≥20% 1RM), making low-load BFR a safer alternative to high-load resistance training (26, 27). Compared to LIRT, BFR appears more effective for enhancing muscle strength at low intensity but remains training less effective than HIRT.
Among RE modalities, kettlebell training (KT) stands out for its dynamic nature and full body engagement (28), making it suitable for older patients (29). Chen HT et al. (29) studied 33 elderly women with sarcopenia (65–75 years) divided into a KT group and a control group (CON). The KT group followed an 8-week training program, while the CON group maintained their usual lifestyle. At 8 and 12 weeks, the KT group demonstrated significant improvements in muscle mass, sarcopenia index, grip strength, back strength, and peak expiratory flow (PEF), with effects persisting at 4 weeks. Serum high-sensitivity C-reactive protein (hs-CRP) levels were also significantly lower in the KT group. The KT protocol involved kettlebells at 60%–70% of 1RM, with 11 movements targeting major muscle groups, incorporating chairs for safety. Progression ranged from basic to advanced exercises, with resistance adjusted based on individual capacity. Resistance was adjusted based on individual physical capacity, providing gradual increases when repetitions exceeded 10 and decreased when they fell below 8. Load and planning changed every two weeks, involving both upper and lower limbs. The frequency of workouts included a 48-hour interval between sessions. This approach enhances safety, inclusivity, and compliance in sarcopenic individuals. Despite all these benefits, exercise selection should consider individual limitations, and while KT offers dynamic and full-body engagement, its suitability depends on the individual's ability to safely perform the activity.
In osteosarcopenia, a condition characterized by the concurrent presence of both sarcopenia and osteopenia/osteoporosis, RE appears both feasible and safe. The FrOST study, a one-year RCT involving 43 elderly men (73–91 years), examined the effects of twice-weekly high-intensity RE combined with whey protein, vitamin D, and calcium supplementation (30). Resistance training is structured into four progressive phases, each increasing in difficulty. It includes a high-intensity dynamic resistance training (DRT) regimen that emphasizes single-set training at high intensity and effort, targeting both major and minor muscle groups. The exercise intensity is organized around specific repetition ranges (5–7 or 8–10) and effort levels, defined as work to failure (non-repetition maximum, nRM). The exercise group showed significant preservation of lumbar spine bone mineral density (BMD), increased skeletal muscle mass index (SMI), and improved hip extensor strength. In contrast, the control group experienced significant reduction in spine BMD and SMI, underscoring the detrimental effects of neglecting high intensity RE in osteosarcopenia management. Given the impact of exercise mode and selection on bone adaptations, variations in movement patterns, loading strategies, and multi-directional exercises should be integrated to optimize skeletal benefits. For optimal results in improving bone mineral density (BMD) at the neck of the femur, as reported by Benedetti et al. (31), it is recommended to engage in progressive resistance exercise for the lower limbs at least three times a week over the course of a year. This type of strength training effectively increases bone density at specific sites, particularly the neck of the femur and lumbar spine, with benefits sustained in the short to medium term.
The meta-analysis conducted by Shen et al. (32) highlights the efficacy of RE alone or combined with aerobic exercise and balance training (BT), in improving quality of life in elderly patients with sarcopenia. Combined nutritional and exercise interventions significantly improve grip strength compared to exercise alone. Additionally, combining RE with BT is the most effective approach for enhancing physical performance.
Liang et (33). further explored this in a single-blind RCT involving very elderly individuals (80–99 years). A 12-week program combining BT and RE significantly improved functional independence, as assessed by the Barthel Index, though it did not significantly reduce fall incidence.
Aerobic exercise complements RE by enhancing mitochondrial function, muscle endurance and cardiovascular health. For sarcopenic patients, a recommended regimen includes 30 min per day, three or more times per week, for at least five months. Progression is essential, starting with low intensity exercise (40% of maximum heart rate) and advancing to moderate (50%–60%) and high-intensity (>60% of maximum heart rate) phases over time. Activities such as walking, jogging, cycling, swimming, dancing, and tai chi are commonly recommended (34–37).
In the meta-analysis of 42 RCTs (3,728 elderly participants) conducted by Shen et al. (32), aerobic exercise alone or with nutritional interventions improved quality of life compared to non-exercise interventions. Adding balance or aerobic training to RE is particularly effective for physical performance improvement.
When comparing nutritional interventions plus RE to RE alone, Tokuda et al. demonstrated that supplementation with essential amino acids (EAA) and tea catechins (TCC) following RE may enhance skeletal muscle mass (SMM) in older adults with sarcopenia. However, supplementation with EAA alone after RE did not provide additional benefits beyond RE alone (38).
The molecular mechanisms underlying exercise benefits remain under investigation. Robinson et al. (37) demonstrated that high-intensity interval training (HIIT) improves insulin sensitivity, skeletal muscle mitochondrial respiration, lean mass, and aerobic capacity in both young and elderly individuals. HIIT induces greater increases in gene transcripts, particularly in mitochondrial proteins, than other training modes, suggesting its potential to reverse age-related mitochondrial decline. These adaptations include enhanced mitochondrial biogenesis, improved muscle mass and function.
Physical activity offers extramuscular benefits through ‘exerkins’, signaling molecules released in response to muscle contractions (39). These molecules, such as angiopoietin-1, FGF21, and IL-6 play autocrine, paracrine, and endocrine roles, influencing cardiovascular, metabolic, immune, and neurological health. These findings highlight exercise’ holistic benefits, making it indispensable in sarcopenia management.
Practical recommendations for managing primary sarcopenia and severe sarcopenia are summarized in Figures 1, 2. A proposed session, tailored for patients with primary sarcopenia and severe sarcopenia, is presented in Figures 3, 4.
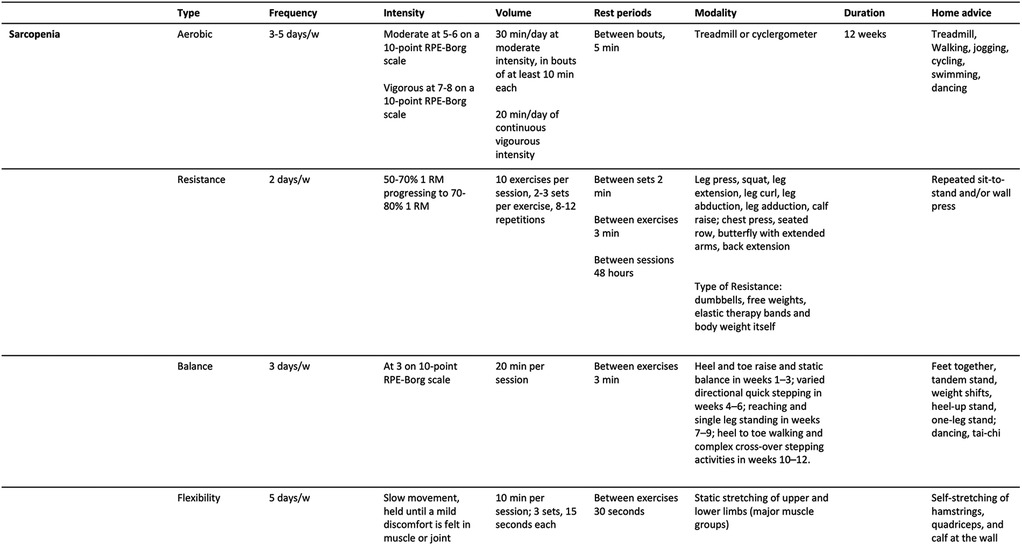
Figure 1. Practical recommendations for therapeutic exercise prescription for people with primary sarcopenia.
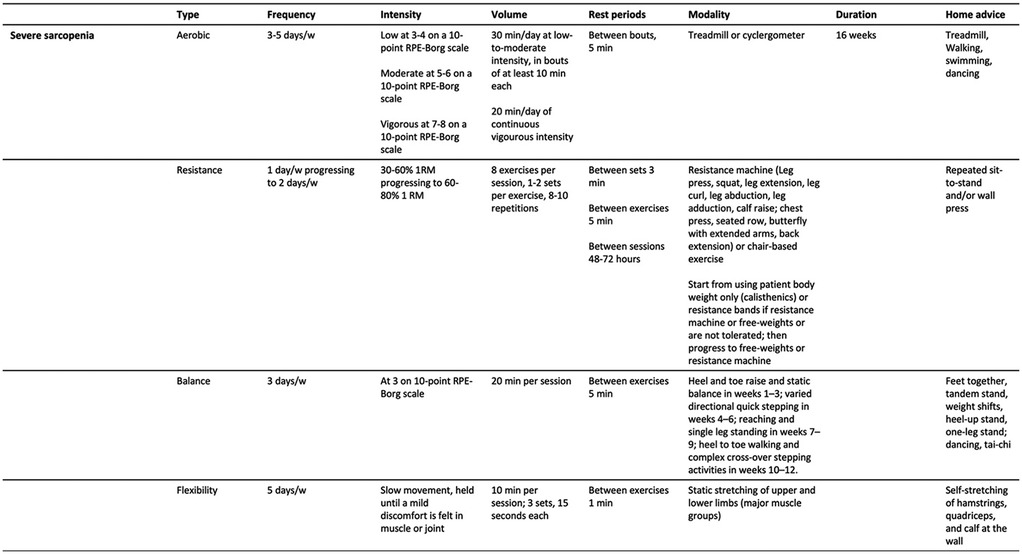
Figure 2. Practical recommendations for therapeutic exercise prescription for people with primary sarcopenia (severe sarcopenia).
These recommendations provide a comprehensive framework for translating scientific advancements into clinical practice. When prescribing therapeutic exercise, it is crucial to personalize the regimen based on individual needs and specific patient conditions. Baseline functional assessments, along the EWGSOP2/AWGS criteria form the foundations for targeted prescriptions. To identify and assess the severity of sarcopenia, various tools can be employed, such as the SARC-F questionnaire, muscle strength measurements like the handgrip strength test, and other evaluations, including walking speed and the SPPB. The Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD) emphasizes tailoring exercise programs to align with patient's goals and preferences to improve adherence, since particularly give the commonly low levels of physical activity among the elderly (40). This approach is particularly crucial when considering the commonly low levels of physical activity among the elderly population. By customizing exercise plans to reflect the unique interests, motivations, and physical capabilities of older adults, healthcare providers can foster a greater sense of ownership and commitment to their physical activities program. Strategies to increase physical activity participation among older adults should focus on raising awareness of the benefits while also addressing and minimizing the perceived risks associated with physical activity (41). Finally, providing a video and brochure about the exercise, as well as using phone or email reminders, could improve the integration of certain activities at home. On the other side, when it is possible, patient adherence can be significantly improved by fostering social interaction and support through organized group exercise sessions, that actively involve caregivers, family members, and friends. These collaborative activities not only create a sense of community and belonging but also encourage individuals to remain committed to their health and fitness goals.
Factors such as intensity, volume, and progression should be carefully considered. Resistance exercise should play a key role in the prescription, serving as both as a preventive and therapeutic intervention. The most important training principles include progressive overload, specificity, individualization, and periodization. Considering the different sub-types of RE available (i.e., traditional, cluster-set RE, suspension, high-speed RE, etc), low-load power-based training, which emphasizes faster movement execution and shorter rest periods between sets, can be particularly beneficial, especially in cases of advanced sarcopenia (42).
However, it is important to note that while physical exercise provides significant benefits in managing sarcopenia, excessive exercise raises concerns regarding the relationship among volume, intensity, and cardiovascular risks. Careful evaluation of the balance between exercise quantity and intensity is essential, especially when considering potential cardiovascular complications. These complications may include accelerated coronary artery calcification, myocardial fibrosis, atrial fibrillation, and an increased risk of sudden cardiac death, particularly in the elderly (43).
A major challenge in implementing exercise interventions for older adults and sarcopenic patients is limited compliance, often due to personal, family, or occupational commitments. Consequently, evidence of maintaining muscle health after exercise training periods is essential. A recent narrative review suggests that muscle strength and size can be preserved for up to 32 weeks with as few as 2 sessions per week and 2–3 sets per exercise, provided exercise intensity is maintained (44). While most evidence supports muscle strengthening for managing sarcopenia, the optimal type of RE remains inadequately defined, particularly concerning isometric vs. dynamic contractions. This represents an unmet need, as different types of muscle actions may yield distinct outcomes in terms of both effectiveness and safety. For example, older adults participating in an eccentric training program not only demonstrated greater preservation of exercise-induced muscular adaptations compared with other training modalities, but also maintained power and strength for up to 3 months of detraining. These findings have significant implications for managing sarcopenia (45).
The diversity of physical abilities in the elderly population with sarcopenia requires individualized intervention strategies. For instance, for frail patients who struggle with conventional physical exercises, vibrational therapy has emerged as a promising alternative, as highlighted by a systematic review and meta-analysis (46). Vibration utilizes mechanical oscillations to improving muscle function by enhancing excitatory signaling from muscle spindles while reducing the inhibitory response from the Golgi tendon organ to the motoneuron pool (47). The mechanical stimulus generated is believed to engage proprioceptive spinal reflexes, thereby VT can be directed at specific muscles through two primary approaches: whole-body vibration, where participants either squat or stand on vibrating platforms, and local vibration, which is applied superficially to the targeted muscle (48).
Sarcopenia, a significant cause of disability in the elderly, requires early diagnosis and comprehensive intervention. Accurate assessment, beginning as early as age 50, based on mobility, muscle strength, and body composition, is crucial. Alongside nutritional strategies, therapeutic exercise emerges as a cornerstone of effective management. Multimodal exercise, which incorporates various exercise modalities, is preferable to single-modality programs. This preference is driven by the fact that single-modality exercises can more easily lead to muscle fatigue, be less enjoyable, and present challenges in maintaining long-term adherence. In contrast, a multimodal approach offers variety, making the training experience more enjoyable and sustainable over time (40). While RE remains an indispensable intervention to counteract the gradual loss of muscle mass, strength, and performance characteristic of severe sarcopenia, the multimodal exercise approach appears to be the most effective and suitable strategy for managing this condition. These recommendations, in addition to outlining practical prescription principles, are firmly rooted in evidence-based medicine (EBM).
In conclusion, early diagnosis and the adoption of an integrated approach from the earliest signs are imperative for managing sarcopenia. This approach underscores the essential synergy among clinical assessments, advanced diagnostic tools, and personalized interventions. Only through such a comprehensive strategy can we hope to counteract the development and the progression of primary sarcopenia, thereby reducing associated disability and mortality.
AM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. FT: Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. SM: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. MR: Supervision, Writing – original draft, Writing – review & editing. AD: Methodology, Supervision, Writing – original draft, Writing – review & editing. RP: Supervision, Writing – original draft, Writing – review & editing. DDo: Writing – original draft, Writing – review & editing. DDi: Writing – original draft, Writing – review & editing. GT: Writing – original draft, Writing – review & editing. FG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. MB: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. GI: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Funded by the European Union - Next Generation EU, Mission 4 Component 1-Project code 2022MSE59K_01-CUP B53D23020510006.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393(10191):2636–46. Erratum in: Lancet. 2019;393(10191):2590. doi: 10.1016/S0140-6736(19)31138-9
2. Greco EA, Pietschmann P, Migliaccio S. Osteoporosis and sarcopenia increase frailty syndrome in the elderly. Front Endocrinol (Lausanne). (2019) 10:255. doi: 10.3389/fendo.2019.00255
3. Goates S, Du K, Arensberg MB, Gaillard T, Guralnik J, Pereira SL. Economic impact of hospitalizations in US adults with sarcopenia. J Frailty Aging. (2019) 8:93–9. doi: 10.14283/JFA.2019.10
4. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012
5. Malmstrom TK, Morley JE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia. J Am Med Dir Assoc. (2013) 14(8):531–2. doi: 10.1016/j.jamda.2013.05.018
6. Teraž K, Marusic U, Kalc M, Šimunič B, Pori P, Grassi B, et al. Sarcopenia parameters in active older adults—an eight-year longitudinal study. BMC Public Health. (2023) 23(1):917. doi: 10.1186/s12889-023-15734-4
7. Pinheiro PA, Carneiro JA, Coqueiro RS, Pereira R, Fernandes MH. “Chair stand test” as simple tool for sarcopenia screening in elderly women. J Nutr Health Aging. (2016) 20(1):56–9. doi: 10.1007/s12603-016-0676-3
8. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. (2008) 11(5):566–72. doi: 10.1097/MCO.0b013e32830b5f23
9. Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the geelong osteoporosis study. Calcif Tissue Int. (2014) 94(4):363–72. doi: 10.1007/s00223-013-9830-7
10. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lowerextremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. (1995) 332(9):556–61. doi: 10.1056/NEJM199503023320902
11. Bischoff HA, Stahelin HB, Monsch AU, Iversen MD, Weyh A, von Dechend M, et al. Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing. (2003) 32(3):315–20. doi: 10.1093/ageing/32.3.315
12. Bauer J, Morley JE, Schols AMWJ, Ferrucci L, Cruz-Jentoft AJ, Dent E, et al. Sarcopenia: a time for action. An SCWD position paper. J Cachexia Sarcopenia Muscle. (2019) 10(5):956–61. doi: 10.1002/jcsm.12483
13. Uchitomi R, Oyabu M, Kamei Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. (2020) 12(10):3189. doi: 10.3390/nu12103189
14. Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, et al. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. (2018) 22(10):1148–61. doi: 10.1007/s12603-018-1139-9
15. Liu S, Zhang L, Li S. Advances in nutritional supplementation for sarcopenia management. Front Nutr. (2023) 10:1189522. doi: 10.3389/fnut.2023.1189522
16. Szwiega S, Pencharz PB, Rafii M, Lebarron M, Chang J, Ball RO, et al. Dietary leucine requirement of older men and women is higher than current recommendations. Am J Clin Nutr. (2021) 113:410–9. doi: 10.1093/ajcn/nqaa323
17. Kortesniemi M, Noerman S, Kårlund A, Raita J, Meuronen T, Koistinen V, et al. Nutritional metabolomics: recent developments and future needs. Curr Opin Chem Biol. (2023) 77:102400. doi: 10.1016/j.cbpa.2023.102400
18. Iolascon G, Moretti A, De Sire A, Liguori S, Toro G, Gimigliano F. Pharmacological therapy of sarcopenia: past, present and future. Clin Cases Miner Bone Metab. (2018) 15(3):407–15.
19. Iolascon G, Di Pietro G, Gimigliano F, Mauro GL, Moretti A, Giamattei MT, et al. Physical exercise and sarcopenia in older people: position paper of the Italian society of orthopaedics and medicine (OrtoMed). Clin Cases Miner Bone Metab. (2014) 11(3):215–21.25568656
20. Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. (2018) 15(12):731–43. doi: 10.1038/s41569-018-0065-1
21. Shah SZA, Zhao D, Hussain T, Yang L. Role of the AMPK pathway in promoting autophagic flux via modulating mitochondrial dynamics in neurodegenerative diseases: insight into prion diseases. Ageing Res Rev. (2017) 40:51–63. doi: 10.1016/j.arr.2017.09.004
22. Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. (2019) 15(7):383–92. doi: 10.1038/s41574-019-0174-x
23. Anker SD, Morley JE, von Haehling S. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. (2016) 7(5):512–4. doi: 10.1002/jcsm.12147
24. Wiegmann S, Felsenberg D, Armbrecht G, Dietzel R. Longitudinal changes in muscle power compared to muscle strength and mass. J Musculoskelet Neuronal Interact. (2021) 21(1):13–25.33657752
25. Beckwée D, Delaere A, Aelbrecht S, Baert V, Beaudart C, Bruyere O, et al. Exercise interventions for the prevention and treatment of sarcopenia. A systematic umbrella review. J Nutr Health Aging. (2019) 23(6):494–502. doi: 10.1007/s12603-019-1196-8
26. Hughes L, Paton B, Rosenblatt B, Gissane C, Patterson SD. Blood flow restriction training in clinical musculoskeletal rehabilitation: a systematic review and meta-analysis. Br J Sports Med. (2017) 51(13):1003–11. doi: 10.1136/bjsports-2016-097071
27. Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. (2015) 45(2):187–200. doi: 10.1007/s40279-014-0264-9
28. Meigh NJ, Keogh JWL, Schram B, Hing WA. Kettlebell training in clinical practice: a scoping review. BMC Sports Sci Med Rehabil. (2019) 11:19. doi: 10.1186/s13102-019-0130-z
29. Chen HT, Wu HJ, Chen YJ, Ho SY, Chung YC. Effects of 8-week kettlebell training on body composition, muscle strength, pulmonary function, and chronic low-grade inflammation in elderly women with sarcopenia. Exp Gerontol. (2018) 112:112–8. doi: 10.1016/j.exger.2018.09.015
30. Kemmler W, Kohl M, Fröhlich M, Jakob F, Engelke K, von Stengel S, et al. Effects of high-intensity resistance training on osteopenia and sarcopenia parameters in older men with osteosarcopenia-one-year results of the randomized controlled franconian osteopenia and sarcopenia trial (FrOST). J Bone Miner Res. (2020) 35(9):1634–44. doi: 10.1002/jbmr.4027
31. Benedetti MG, Furlini G, Zati A, Letizia Mauro G. The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed Res Int. (2018) 2018:4840531. doi: 10.1155/2018/4840531
32. Shen Y, Shi Q, Nong K, Li S, Yue J, Huang J, et al. Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14(3):1199–211. doi: 10.1002/jcsm.13225
33. Liang Y, Wang R, Jiang J, Tan L, Yang M. A randomized controlled trial of resistance and balance exercise for sarcopenic patients aged 80–99 years. Sci Rep. (2020) 10(1):18756. doi: 10.1038/s41598-020-75872-2
34. Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. (2019) 132:42–9. doi: 10.1016/j.freeradbiomed.2018.08.035
35. Ziaaldini MM, Marzetti E, Picca A, Murlasits Z. Biochemical pathways of sarcopenia and their modulation by physical exercise: a narrative review. Front Med (Lausanne). (2017) 4:167. doi: 10.3389/fmed.2017.00167
36. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American College of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. (2009) 41(7):1510–30. doi: 10.1249/MSS.0b013e3181a0c95c
37. Robinson MM, Dasari S, Konopka AR, Johnson ML, Manjunatha S, Esponda RR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. (2017) 25(3):581–92. doi: 10.1016/j.cmet.2017.02.009
38. Tokuda Y, Mori H. Essential amino acid and tea catechin supplementation after resistance exercise improves skeletal muscle mass in older adults with sarcopenia: an open-label, pilot, randomized controlled trial. J Am Nutr Assoc. (2023) 42(3):255–62. doi: 10.1080/07315724.2022.2025546
39. Chow LS, Gerszten RE, Taylor JM, Pedersen BK, van Praag H, Trappe S, et al. Exercises in health, resilience, and disease. Nat Rev Endocrinol. (2022) 18(5):273–89. doi: 10.1038/s41574-022-00641-2
40. Negm AM, Lee J, Hamidian R, Jones CA, Khadaroo RG. Management of sarcopenia: a network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. (2022) 23(5):707–14. doi: 10.1016/j.jamda.2022.01.057
41. Franco MR, Tong A, Howard K, Sherrington C, Ferreira PH, Pinto RZ, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. (2015) 49(19):1268–76. doi: 10.1136/bjsports-2014-094015
42. Cannataro R, Cione E, Bonilla DA, Cerullo G, Angelini F, D'Antona G. Strength training in elderly: an useful tool against sarcopenia. Front Sports Act Living. (2022) 4:950949. doi: 10.3389/fspor.2022.950949
43. Eijsvogels TMH, Thompson PD, Franklin BA. The “extreme exercise hypothesis”: recent findings and cardiovascular health implications. Curr Treat Options Cardiovasc Med. (2018) 20(10):84. doi: 10.1007/s11936-018-0674-3
44. Spiering BA, Mujika I, Sharp MA, Foulis SA. Maintaining physical performance: the minimal dose of exercise needed to preserve endurance and strength over time. J Strength Cond Res. (2021) 35(5):1449–58. doi: 10.1519/JSC.0000000000003964
45. Baxter BA, Baross AW, Ryan DJ, Kay AD. Effects of detraining on neuromuscular function and structural adaptations following once- or twice-weekly eccentric resistance training in older adults. Aging Clin Exp Res. (2024) 36(1):177. doi: 10.1007/s40520-024-02828-1
46. Wu S, Ning HT, Xiao SM, Hu MY, Wu XY, Deng HW, et al. Effects of vibration therapy on muscle mass, muscle strength and physical function in older adults with sarcopenia: a systematic review and meta-analysis. Eur Rev Aging Phys Act. (2020) 17:14. doi: 10.1186/s11556-020-00247-5
47. Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. (2011) 32(2):75–99. doi: 10.1055/s-0030-1268010
Keywords: sarcopenia, exercise, resistance training, aerobic training, balance training, multimodal exercise
Citation: Moretti A, Tomaino F, Paoletta M, Liguori S, Migliaccio S, Rondanelli M, Di Iorio A, Pellegrino R, Donnarumma D, Di Nunzio D, Toro G, Gimigliano F, Brandi ML and Iolascon G (2025) Physical exercise for primary sarcopenia: an expert opinion. Front. Rehabil. Sci. 6:1538336. doi: 10.3389/fresc.2025.1538336
Received: 2 December 2024; Accepted: 17 March 2025;
Published: 28 March 2025.
Edited by:
Dustin J. Oranchuk, University of Colorado, United StatesReviewed by:
Michael Harris-Love, University of Colorado Anschutz Medical Campus, United StatesCopyright: © 2025 Moretti, Tomaino, Paoletta, Liguori, Migliaccio, Rondanelli, Di Iorio, Pellegrino, Donnarumma, Di Nunzio, Toro, Gimigliano, Brandi and Iolascon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Liguori, cy5saWd1b3JpQGhvdG1haWwuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.