
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Rehabil. Sci. , 28 March 2025
Sec. Medical and Surgical Rehabilitation
Volume 6 - 2025 | https://doi.org/10.3389/fresc.2025.1535138
This article is part of the Research Topic Advances on Participation Perspective in Rehabilitation Sciences View all 5 articles
 Dmitry Rozenberg1,2*
Dmitry Rozenberg1,2* Sherrie Logan3,†
Sherrie Logan3,† Sahar Sohrabipour4
Sahar Sohrabipour4 Nicholas Bourgeois5,‡
Nicholas Bourgeois5,‡ Anita Cote6,7
Anita Cote6,7 Robin Deliva8
Robin Deliva8 Astrid De Souza7
Astrid De Souza7 Rienk de Vries3
Rienk de Vries3 Maoliosa Donald9
Maoliosa Donald9 Manoela Ferreira3
Manoela Ferreira3 Donna Hart3
Donna Hart3 Megha Ibrahim Masthan10
Megha Ibrahim Masthan10 Tania Jaundis-Ferreira11
Tania Jaundis-Ferreira11 Sandrine Juillard12,13
Sandrine Juillard12,13 Michael Khoury14
Michael Khoury14 Afsana Lallani3
Afsana Lallani3 Diana Mager15
Diana Mager15 Istvan Mucsi16,17
Istvan Mucsi16,17 Ani Orchanian-Cheff18
Ani Orchanian-Cheff18 Jennifer L. Reed19,20,21
Jennifer L. Reed19,20,21 Puneeta Tandon22
Puneeta Tandon22 Karthik Tennankore23
Karthik Tennankore23 Elaine Yong3
Elaine Yong3 Lisa Wickerson1,24
Lisa Wickerson1,24 Sunita Mathur25,26,†
Sunita Mathur25,26,†
Solid organ transplantation (SOT) is a life-saving procedure for those with end-stage organ dysfunction. The main goals of SOT are to improve quality of life and daily function, which are supported by pre- and post-transplant rehabilitation. In-person rehabilitation programs have traditionally been the standard-of-care for delivering rehabilitation for SOT patients. Many programs have adopted a virtual delivery model [telerehabilitation (TR)], an approach that has become increasingly used given restrictions to in-person delivery during the COVID-19 pandemic. Presently, TR programs are being used both clinically and in research with variable practices. A 2-day virtual meeting held in February 2023 brought together over 30 Canadian adult and pediatric researchers, clinicians, and patient and family partners across SOT. The meeting objectives were: (1) To facilitate knowledge exchange and dialogue in TR between patient partners, healthcare professionals, researchers, and key stakeholders, and (2) Identify gaps in clinical practice and research in TR. The discussion focused on delivery methods of TR, digital tools, facilitators and barriers of TR, and the effects of TR on physical and mental health in both adult and pediatric populations. This meeting report incorporates a narrative literature review of SOT and rehabilitation articles in the last 20 years. Future directions in TR are highlighted leading to the development of key research priorities targeted towards improved delivery of TR in SOT patients.
Transplantation is a well-established procedure for end-stage organ disease known to improve health-related quality of life (HRQL) and survival (1, 2). In 2022, a total of 2,936 solid organ transplants (SOT), including adult and pediatric kidney, liver, heart, lung, and pancreas, were performed in Canada (3) and a total of 42,887 SOT in the United States (4). However, SOT candidates have impairments in functional capacity that does not return to predicted levels post-transplantation resulting in reduced physical activity levels and impairments in HRQL (5–7).
Traditional facility-based rehabilitation programs not only help with recovery in exercise capacity, muscle strength, and HRQL post-transplant, but also mitigate physical deconditioning pre-transplant (8–10). The level of physical function pre- and post-transplant is associated with physiological benefits (improved skeletal muscle function, bone density, and metabolic factors) (11–13), shorter hospitalizations (14), lower surgical complications (15) and hospital readmissions (16), and improved post-transplant survival (16, 17). However, facility-based programs have faced challenges with respect to accessibility and uptake as we emerge from the COVID-19 pandemic with increased in-person activities, which have accelerated telerehabilitation (TR) initiatives (18–20).
Telerehabilitation programs have emerged as a promising alternative to in-person rehabilitation programs in SOT populations (21–25), which is the delivery of rehabilitation through various telecommunication strategies, such as videoconferencing, phone calls, or internet applications (26). TR can enhance access to medically underserviced populations (27–29), reduce financial and time constraints for patients geographically distant from a transplant centre (30, 31) and may help improve accessibility (28, 32).
There is emerging evidence on the feasibility and effectiveness of TR programs on adult and pediatric patient outcomes with several ongoing trials (33–36). Despite limited evidence, TR was rapidly adopted during the COVID-19 pandemic with a strong positive response from the patient partner community. The optimal structure of TR programs such as individual versus group training, synchronous versus asynchronous, and the balance of virtual and in-person assessments remains unclear. Further, practical aspects of exercise progression, technological support and equipment needed for safe physiological monitoring and effective delivery of TR pre- and post-transplant in adult and pediatric populations requires additional investigation. Other challenges in the field of TR include patient and provider digital literacy, digital access in rural areas, data privacy and governance (37, 38). Thus, there are several questions related to training, technological support and implementation that require ongoing study in SOT (30).
To more thoroughly understand the delivery of TR in SOT, a virtual meeting was held to identify important facilitators, barriers, and care priorities for rehabilitation for adult and pediatric transplant candidates, recipients, caregivers, and healthcare providers. The objectives of the meeting were: (1) To facilitate knowledge exchange and dialogue between patient and family partners, clinicians, researchers, and other key partners as it relates to TR, and (2) Identify gaps in clinical practice and research in TR to improve delivery of TR in SOT patients.
A two-day virtual meeting titled “Telerehabilitation in Solid Organ Transplantation in Canada: Celebrating Achievements and Designing the Future” was held through Zoom in February 2023. The meeting was held in English for 5-hours each day with over 30 attendees, including researchers, healthcare providers, adult and pediatric patient partners, and caregivers. A pre-meeting with the principal applicants and co-applicants of the Canadian Institute of Health Research funded grant was held in October 2022 to finalize the list of participants, speakers, and the meeting agenda (Supplementary Appendix A1). Details on invitation of meeting participants can be found in Supplementary Appendix A2.
The meeting included 3 presentations by patient partners regarding their transplant and rehabilitation experiences, and 18 presentations by researchers, clinical investigators, and patient partners on key adult (n = 13) and pediatric (n = 5) topics in TR, including the evolving landscape of TR, delivery methods, user experiences with TR, technological considerations, and clinical applications of TR tools, Supplementary Appendix A1. Facilitated, small group discussions were held in breakout rooms, then larger group discussions with all attendees, and interaction among attendees using Google Jamboard (a tool that allowed attendees to write their reflections in a shared document). The meeting was recorded for reference and a summary of the discussion is outlined in Supplementary Appendix A2.
A literature search was conducted by a librarian to identify key papers in the field using Ovid Medline on June 24, 2024. A combination of key words and subject headings for SOT and rehabilitation, including various study designs such as review articles, cross-sectional or cohort studies were searched to ensure key papers were included. To maximize the literature included in our narrative review, studies had to report on at least one aspect of TR or some consideration of rehabilitation delivery in SOT or chronic disease populations. Only studies with full-text available in English and published in the last 20-years were included, Supplementary Appendix A3. Case reports were excluded.
The results from the meeting are synthesized and grouped into four main topics: (1) Delivery methods and safety of TR; (2) Digital tools and available applications; (3) Barriers and facilitators to TR delivery; and (4) Evaluation of physical and mental health with exercise, as in Figure 1. The topics pertained to both adult and pediatric SOT candidates and recipients with a dedicated pediatric section are summarized below.
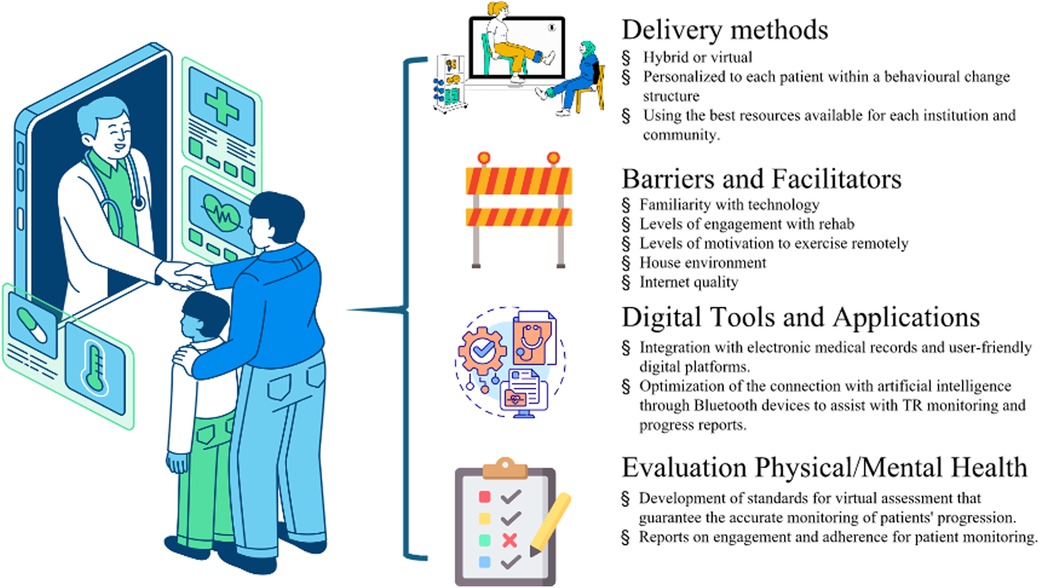
Figure 1. Aspects that should be considered for successful telerehabilitation in solid organ transplantation. Credits from the left and top to bottom: Reproduced with permission from “Lined Isometric Online Pediatric Consultation” by M. Wallflower; “Expressive Lined Virtual Healthcare” by Dianne Rosario; “Isolated Construction Barrier Flat Design” by Iconsy, licensed under Free Content License. Reproduced with permission from “2D Customizable Thin Line Icon Health Records Access Concept” by bsd studio; “Evaluation” by Uniconlabs, licensed under Pro Content License. Figure was generated using stock images from Canva.
Several models of TR delivery were discussed at the meeting, specifically synchronous (i.e., direct observation) and asynchronous programs, optimal timing of TR implementation and duration, balance between in-person and virtual visits, equipment, and digital requirements. A variety of videoconference platforms (i.e., MS Teams, Zoom, Vivify Health) (21, 35, 39) have been utilized across TR with good uptake. Furthermore, technological considerations of TR include access to a computer or portable electronic device with potential video capability, access to the internet and wearable or ancillary devices.
Safety considerations were discussed at the meeting. Specifically, several programs undertook a hybrid approach where the initial assessment was conducted on site followed by training using TR. The ability to perform synchronous monitoring during exercise and having an emergency action plan (e.g., phone nearby, caregiver present at home, doors unlocked in case emergency personnel need entry) were highlighted as important safety considerations, especially with higher risk transplant populations such as those with cardiovascular disease (40), or those with higher falls risk (41). The participant's ability to access technical support or an alternative contact number for the healthcare provider was highlighted as a key safety aspect. An environmental safety survey of the home environment, either done virtually or as a home visit, to evaluate falls risk (e.g., loose rugs, appropriate chair height/stability) was suggested (42). It was also suggested that patients who experience hospitalizations or significant changes in health status be re-evaluated by a healthcare professional prior to re-starting their program.
Digital applications can be utilized to facilitate TR. These applications can capture changes in exercise routines and adherence given possible setbacks such as infections, hospital admissions and musculoskeletal injuries (43). Some adult SOT recipients expressed a desire to be able to share their exercise data with healthcare providers, their peers or family members.
Healthcare providers expressed that they wanted tools that were evidence-based, had the availability of an exercise library, and the ability to integrate these exercises with digital applications. Furthermore, proficiency in using digital tools, adequate training, and ability to use the tools at multiple transplant sites were highlighted as important priorities by healthcare providers (44). Healthcare providers and researchers expressed the importance of integrating the patient perspective in designing, testing and implementing these digital applications.
A web-based platform (Heal-Me) was presented as an example of a multidisciplinary program developed with input from patients to support nutrition and exercise programming through videoconferencing for individuals with chronic conditions, including SOT patients (45, 46). An updated version has also been adapted to provide mental health support, including mind-body movement and chronic disease skills management (47, 48). Another program developed for chronic kidney disease patients (Kidney BEAM) (49, 50) provides an opportunity for participants to join live classes or virtual groups to chat with patients and providers, and more importantly, create a sense of community with benefits in both physical and mental health (51).
Generally, most patient partners reported a positive experience after participating in TR. Specifically, they enjoyed using physical activity trackers, such as Fitbits, which increased their motivation to exercise and served as a potential incentive to participate in TR programs offering Fitbits. Further, patients stated that they enjoyed being able to complete exercises at home instead of on-site, as it allowed them to save time and money on travel.
A number of perspectives on TR were discussed during the meeting. The emphasis on TR throughout the transplant journey was an important consideration in the SOT population. It was highlighted that TR should be personalized, flexible in terms of delivery options, and be able to combine both physical and mental health support. Several facilitators and barriers are described below.
Facilitators to TR were highlighted during the meeting, Table 1. Specifically, the ability to tailor the program to the unique needs and desires of patients, accounting for the variability that may exist in preferences for individual versus group exercises. Secondly, involving patients when developing digital applications with consideration of visual, hearing and language challenges was discussed. The ability to provide mental health support through TR was echoed as an important strategy for overall well-being. Furthermore, having a “transplant mentor,” described as an SOT recipient who has gone through the process, may prove to be beneficial in providing motivational support through their lived experiences (52). Other facilitators to TR included having strong multidisciplinary support, well developed educational programs with effective and safe exercises, involvement of caregivers with TR support, and providing incentives (i.e., the ability keep a physical activity tracker after use). Also, focusing on the transition period from hospital to home allows in-person rehab to be performed initially followed by TR at home.
Some drawbacks of TR included challenges in learning the physical exercises virtually, the possibility that some programs may not be able to provide a personalized approach, and decreased motivation with perceived loss of community with an asynchronous program, and potential restrictions with internet accessibility in some rural communities (53). The safety, validity and adaptation of several in-person assessments to the remote environment (i.e., six-minute walk test) should be an area for further investigation (54). Additional barriers to TR include lack of technological access, potential language barriers, and program level factors, as shown in Table 1.
Some research programs provided participants with tablets if needed to facilitate access to TR (55). Potential risk factors that may warrant closer supervision, including the potential need for facility-based programs, include the presence of unstable heart disease, fluctuations in anticoagulation levels, limited digital literacy, and presence of frailty (56, 57). Motivation was also cited as an important consideration with some recipients keen to minimize healthcare visits post-transplant. Furthermore, it remains unclear whether certain physiological outcomes can be achieved in a similar manner (i.e., target heart rate zones) with TR as with in-person exercises. Table 2 provides several research gaps identified with TR.

Table 2. Future research priorities and questions identified to address gaps in telerehabilitation (TR).
The evaluation of physical and mental well-being in the virtual environment were discussed. The need for an in-person assessment was addressed as several physical assessments (i.e., six-minute walk test) and clinical impressions (i.e., frailty) have more in person standardized procedures. However, frailty assessments have been carried out in transplant candidates virtually and may be an important baseline assessment for assessing reversibility (58). Patient reported outcome measures can be completed on various electronic platforms and may provide useful information related to physical functioning, psychological symptoms, and overall physical and mental health (59, 60). In fact, TR creates an opportunity to provide rehabilitation to vulnerable or frail transplant patients who may not have been able to participate in a center-based program due to travel or physical limitations. Furthermore, wearable devices (i.e., physical activity trackers, oximeters, daily activity logs) may provide an opportunity to guide exercise training and monitor progress throughout TR, but need to consider that some of these devices are not medical grade and may lack accuracy (61). The integration of mental health and nutritional supports in TR may have a synergistic benefit with exercise training. The group felt that evaluating the contribution of TR on physiological benefits (i.e., exercise capacity, strength, balance) and clinical outcomes (i.e., hospitalization data, readmissions, infections, graft function) may be helpful in understanding its effects on health-care utilization, as there is a paucity of literature in this area (Table 2).
The specific benefits, challenges and research questions for the pediatric SOT population were also discussed. Telerehabilitation programs for pediatric transplants have demonstrated improvements in strength and self-confidence at the end of a 12–16 week resistance program (24). However, researchers identified recruitment and program enjoyment as challenges among youth (25). Breakout session discussions and presentations by youth and clinicians reinforced the importance of individualizing the program based on the developmental age of the child to promote enjoyment, improve adherence and enhance motivation. Depending on the age of the individual, other discussions to increase enjoyment included family involvement, peer support, games or activities with rewards and virtual reality. For example, an ongoing randomized crossover feasibility trial of a video-game linked TR exercise platform (known as MedBIKE), which is currently underway for 10–18 year old heart transplant recipients was discussed (36). Adolescence was highlighted as a period of rapid change in personal growth that may hinder motivation to participate (62, 63).
Physical literacy was highlighted by researchers and clinicians during the workshop as an important component to develop positive physical activity habits, exercise self-efficacy and promote pediatric neurodevelopment (64). However, workshop participants also cautioned that too much focus on the educational benefits of physical activity within a TR program could reduce the pleasure for youth. Thus, a greater emphasis on physical literacy may help promote enjoyment in physical activity (65). One clinician indicated that the pre-transplant phase is ideal for TR recruitment, as families are eager to minimize frailty and health consequences before surgery (8, 66). Furthermore, pre-transplant TR can help improve confidence with physical activity and prepare patients and families for the post-transplant period.
Parental concerns regarding safety of exercise and physical activity post-transplant are potential barriers that may require mitigation strategies for TR programs (67, 68). However, caregivers participating in the workshop mentioned that their confidence in safety improved as they observed their children participating in sporting activities as previously described in liver transplant recipients (67). Parental and sibling participation in TR was observed to be important in improving adherence in liver recipients (69). However, caregiver mental health barriers, such as post-traumatic stress disorder, anxiety or depression related to their child's transplant journey, may require additional preventative support for families pre-transplant in integrating physical activity (67, 68). Moreover, pediatric SOT recipients typically have a much more heterogeneous array of pre-transplant diagnoses. Thus, each child must be considered individually with respect to safety and ability to participate in various TR programs. Age- and ability-specific individualizations are essential to ensure the appropriate delivery of TR for pediatric SOT recipients.
Assessment tools, including wearables (oximeters, fitness activity trackers) (70), quality of life and fatigue scales (PedsQL 4.0, PedsQL Multidimensional Fatigue Scale) (71, 72), physical activity questionnaire (PAQ-C or A) (73), musculoskeletal strength [Bruininks-Oseretsky Test of Motor Proficiency (BOT-2) (74), functional testing, FitnessGram] and communication platforms (WelTel, Zoom) (75, 76) were identified by researchers, clinicians and patients/caregivers as useful to monitor health, performance, changes in fitness and activity levels and engagement in TR programs. However, if patient families are required to absorb costs associated with assessment tools (wearables, online programs), they may be a barrier for lower-income families and equitable access is an important consideration (43).
A number of important knowledge gaps remain regarding TR in pediatric SOT recipients. First, there is a paucity of randomized control trials evaluating the safety and effectiveness of exercise delivery. In addition, various modalities of exercise programs have not been adequately compared in pediatric participants such as high-intensity interval training which has been shown to be superior to moderate intensity continuous exercise in adult heart transplant recipients (77). While pediatric transplant recipients are known to be quite sedentary with suboptimal physical activity levels (78), barriers to increasing activity and the potential for TR programs to have sustained improvements in activity and self-efficacy require further study.
By creating an environment for communication and knowledge exchange during our two-day virtual meeting with SOT patient partners, caregivers, clinicians, and SOT rehabilitation researchers, we were able to identify key research questions and priorities in the field of TR with the goals of improving transplant outcomes and patient-centered research within a public healthcare system. The meeting discussion focused on delivery methods of TR, digital tools, facilitators and barriers of TR, and effects of TR on physical and mental health in both adult and pediatric populations.
Several models of TR were discussed at the meeting that have been adopted in the last several years. Most clinical and research TR programs combine a hybrid approach with in person and virtual assessments and training (79, 80). The optimal balance between in-person and virtual assessment and training remains unclear, but also depends on the needs of patients and transplant center resources. Furthermore, the timing of TR in the post-transplant period and how to utilize the transition period in hospital to engage patients on some of the technological and equipment requirements for TR remain to be determined. In addition, it is important to account for the non-linear trajectories in transplant populations, given frequent infections, hospital admissions, and management of underlying comorbidities (81–83). TR holds a great deal of promise as telehealth has been shown in many chronic disease populations to improve treatment adherence, increase the ability to capture measurements, and promote self-management (57, 84). TR also offers an opportunity for greater accessibility to exercise professionals who are familiar with SOT patients and their needs, which was a common barrier expressed by SOT recipients related to physical activity resources (43).
There are a number of digital health tools that are publically available and those that have been developed by individual clinical and research programs. These technological advancements hold promise with integration into TR programs as they can provide a broad range of physiological monitoring (i.e., heart rate, oxygen), nutritional support, information on organ-related function, and optimal strategies for informing the healthcare team (43). Furthermore, digital resources should be complementary and provide flexibility in selecting required features for TR accounting for the variable health changes in SOT recipients. Digital health applications can help promote greater self-monitoring and independence among patients pre and post-SOT, which can aid TR programs in monitoring their patients virtually (44).
The clinical sustainability of TR programs is an important consideration. Specifically, consideration for TR utilization beyond the immediate training period or the optimal integration of digital applications and tools still needs to be defined. The two-day meeting highlighted variability in both clinical and research practices in the delivery of TR (virtual vs. in-person training), degree of supervision (synchronous vs. asynchronous), and number of participants (individual vs. group classes). Given the evolving field of TR in the last few years, there are no existing guidelines that provide guidance on the integration of TR or telehealth for SOT recipients (85). Furthermore, there remain questions regarding start-up costs for programs and patients as it relates to exercise equipment, digital resources, and health-care personnel availability. The funding availability for TR programs remains to be defined and is certainly variable across SOT programs and geographic locations.
The need for developing standardized safety protocols for TR was highlighted to ensure that appropriate guidelines are in place for both patients and providers. The common elements among ongoing studies evaluating the feasibility and safety of TR in SOT are as follows: (1) having an emergency action plan in place (2) education on technical support as needed (3) environmental safety survey, and (4) support of caregivers (34, 35, 79). Reassuringly, no serious adverse events have been reported with TR in several SOT populations (21, 24, 33, 86). Future work in the area of TR is needed to evaluate and create safety guidelines in the delivery and evaluation of TR outcomes in SOT.
Another important priority that was identified throughout the two-day meeting was the need for mental health support for SOT candidates and recipients and for these resources to be integrated into TR during both the pre- and post-transplant period to help with adherence and adaptation of new health conditions. Previous research found that structured exercise itself had positive effects on mental health for patients both pre- and post-transplant (80, 87); however, the need for formal mental health support beyond physical exercise was emphasized, such as through counselling and peer support. In addition, the timing and progression of TR in the post-transplant period needs to be considered in the context of the transplant experience given the variability in both physical and emotional experiences leading up to the transplant, peri-operative period, and functional recovery for patients, which will influence readiness to participate in physical activity and exercise training (43). In addition, the availability of some of these supports for caregivers of transplant recipients was articulated by several stakeholders during the meeting, given some of the emotional challenges that caregivers may experience. Furthermore, peer support for caregivers and transplant patients was expressed as one potential strategy to assist with mental health challenges.
The association between participation in TR with pre- and post-transplant clinical outcomes such as hospitalizations and mortality have not been evaluated. However, there is evidence in transplant recipients that low physical HRQL in kidney recipients is associated with reduced survival (88, 89). In addition to physical health, mental and general well-being have been shown to be associated with graft and overall survival in renal transplant recipients (13, 88, 90). In liver transplant recipients, exercise is associated with improved exercise capacity, physical function, and HRQL, but long-term sustainability and association with clinical outcomes remain unclear (20). Thus, TR may help identify patients at higher risk of functional decline and potentially provide earlier opportunities for intervention through rehabilitation. However, the optimal timing and duration of TR pre and post-transplantation remains to be defined.
There are a number of research questions in TR that were identified at the meeting (Table 2). There is an increased need to evaluate the optimal delivery strategy for TR. Specifically, a focus is needed on the actual delivery of TR, including optimal monitoring strategies, integration of TR into electronic medical records, and development of flexible digital platforms (e.g., applications or web-based tools) that are sustainable and up-to-date with the ability to evaluate the quality of TR delivery. Potential areas for future research include creating flexible TR programs that can be personalized, performing economic evaluation of preventative lifestyle factors that could be facilitated with TR, or using virtual reality (91) and/or artificial intelligence (AI) to enhance TR, and assist with TR recommendations. To date, there have been no studies on the use of virtual reality or AI in TR for SOT patients, therefore highlighting an exciting area of future research in this patient population, especially among adolescent and young adult SOT recipients. Digital health applications that incorporate multifaceted health domains such as exercise guidance, mental health supports, nutrition, organ specific features, and the capability of sharing information with family, peers and healthcare providers require further evaluation. Importantly, collaboration among SOT TR programs, researchers, clinicians, and patient partners was identified as a future priority to enhance knowledge mobilization and engagement in collaborative research opportunities. These collaborations would allow sharing of resources and experiences to improve TR and patient outcomes.
Even though not discussed at the 2-day meeting, it is important to highlight several future considerations in TR related to cost-effectiveness, healthcare provider training and policy considerations. TR has been shown to be feasible and helpful in providing increased access to rehabilitation across a number of chronic conditions in adult and pediatric populations relative to traditional in-person rehabilitation practices (92). There have been only several studies that have evaluated cost of TR in cardiac, respiratory and musculoskeletal settings, all of which have favoured TR over standard rehabilitation (93–97). However, to our knowledge the cost-effectiveness or long-term sustainability of TR in SOT candidates or recipients has not been evaluated to-date despite the sudden implementation during the COVID-19 pandemic (21, 98). Further, the training of healthcare providers, as it relates to online communication and technological skills, has been shown to be an important factor in healthcare provider uptake, satisfaction, and implementation of TR (92, 99). It is important that organizations utilizing TR have the appropriate technological infrastructure (i.e., equipment, standardized platforms) and informatics support for both providers and patients. This is a key consideration from a health policy sustainability standpoint that funding is available for organizations to provide appropriate technological training and support for effective TR delivery for providers, patients and caregivers (100), highlighted in Supplementary Table S2. Based on the literature (92, 100), many of the facilitators and barriers are common across chronic conditions including the SOT population. However, the optimal delivery structure of TR in SOT populations (balance of in-person vs. virtual program), funding for infrastructure support, and cost-effectiveness requires further evaluation. There are several considerations in the SOT population such as greater travel distance from healthcare center, increased infectious concerns with immunosuppression, and frequent clinical appointments that may favor TR compared to other chronic or surgical conditions.
There are similar limitations worth highlighting. Firstly, the views in this report were from participants affiliated with the CDTRP with diverse representation across SOT centers, organ types, adult and pediatric populations, and varied experience with TR; however, the views expressed may differ across jurisdictions and healthcare settings. Second, we utilized a narrative literature review without a critical analysis of the included articles; thus, limiting our ability to comment on study validity. Lastly, the 2-day virtual meeting did not discuss cost-effectiveness or implementation in low or middle income countries, which are important considerations in future studies.
Telerehabilitation has emerged as an important intervention post COVID-19 pandemic in the SOT population. A few TR models of care have been applied both in the clinical and research settings across SOT recipients, but further research with regards to their cost, effectiveness, optimal delivery and association with clinical outcomes is needed. Several important considerations for TR in transplantation were identified in the meeting. The use of digital applications, wearables, and health tools is promising in facilitating TR, but further refinement on organ specific and lifestyle needs will need to be considered. The sustainability of TR beyond the immediate training period, along with multidisciplinary resources such as mental health and nutritional support, were highlighted as key resources for TR effectiveness. Future work will need to explore opportunities for increased clinical and research collaboration across centers in TR delivery, digital applications, and collaborative funding resources to develop the required evidence for TR sustainability.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
DR: Conceptualization, Data curation, Validation, Writing – original draft, Writing – review & editing. SL: Conceptualization, Data curation, Validation, Writing – review & editing. SS: Conceptualization, Data curation, Validation, Writing – review & editing. NB: Conceptualization, Validation, Writing – review & editing. AC: Conceptualization, Validation, Writing – review & editing. RD: Conceptualization, Validation, Writing – review & editing. AD: Conceptualization, Validation, Writing – review & editing. Rd: Conceptualization, Validation, Writing – review & editing. MD: Conceptualization, Validation, Writing – review & editing. MF: Conceptualization, Validation, Writing – review & editing. DH: Conceptualization, Validation, Writing – review & editing. MI: Conceptualization, Validation, Writing – review & editing. TJ-F: Conceptualization, Validation, Writing – review & editing. SJ: Conceptualization, Validation, Writing – review & editing. MK: Conceptualization, Validation, Writing – review & editing. AL: Conceptualization, Validation, Writing – review & editing. DM: Conceptualization, Validation, Writing – review & editing. IM: Conceptualization, Validation, Writing – review & editing. AO-C: Conceptualization, Validation, Writing – review & editing. JR: Conceptualization, Validation, Writing – review & editing. PT: Conceptualization, Validation, Writing – review & editing. KT: Conceptualization, Validation, Writing – review & editing. EY: Conceptualization, Validation, Writing – review & editing. LW: Conceptualization, Validation, Writing – review & editing. SM: Conceptualization, Data curation, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Canadian Institute of Health Research Planning and Dissemination Grant [PCS 183364]. DR receives research support from the Sandra Faire and Ivan Fecan Professorship in Rehabilitation Medicine and Temerty Faculty of Medicine.
We thank the Canadian Donation Transplant Research Program (CDTRP) for providing preparation and meeting support, specifically Manuel Escoto, Patricia Gongal, Erika Kathe Croft, Stéphanie Larivière, and Demitra Yotis. We also acknowledge the generous contribution of CDTRP for its in-kind funding support and logistical operations of the meeting. We also acknowledge our collaborators Kathryn Armstrong, Yaron Avitzur, Mamatha Bhat, Tom Blydt-Hansen, Darlene Reid, Daniel Santa Mina, Simon Urschel, Rhea Varughese, and our patient partner collaborators Sandra Holdsworth, and Karina Prevost. We are very grateful to Ashley Logan and Addison McArthur for sharing their lived experiences during the two-day meeting.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2025.1535138/full#supplementary-material
1. Black CK, Termanini KM, Aguirre O, Hawksworth JS, Sosin M. Solid organ transplantation in the 21st century. Ann Transl Med. (2018) 6(20):409. doi: 10.21037/atm.2018.09.68
2. Feurer ID, Speroff T, Harrison C, Wright Pinson C. Health-related quality of life before and after solid organ transplantation. Measurement consideration, reported outcomes, and future directions. Minerva Chir. (2002) 57(3):257–71.12029219
3. Canadian Institute for Health Information. e-statistics on organ transplants in Canada (2022). Available at: https://www.cihi.ca/en/summary-statistics-on-organ-transplants-wait-lists-and-donors (Accessed January 13, 2024).
4. UNOS Transplant. Available at: https://unos.org/news/2022-organ-transplants-again-set-annual-records/ (Accessed January 13, 2024).
5. Leung TC, Ballman KV, Allison TG, Wagner JA, Olson LJ, Frantz RP, et al. Clinical predictors of exercise capacity 1 year after cardiac transplantation. J Heart Lung Transplant. (2003) 22(1):16–27. doi: 10.1016/s1053-2498(02)00475-8
6. Painter P, Krasnoff J, Paul SM, Ascher NL. Physical activity and health-related quality of life in liver transplant recipients. Liver Transpl. (2001) 7(3):213–9. doi: 10.1053/jlts.2001.22184
7. Saeed I, Rogers C. Murday A, Steering Group of the UK Cardiothoracic Transplant Audit. Health-related quality of life after cardiac transplantation: results of a UK national survey with norm-based comparisons. J Heart Lung Transplant. (2008) 27(6):675–81. doi: 10.1016/j.healun.2008.03.013
8. Janaudis-Ferreira T, Mathur S, Deliva R, Howes N, Patterson C, Räkel A, et al. Exercise for solid organ transplant candidates and recipients: a joint position statement of the Canadian society of transplantation and CAN-RESTORE. Transplantation. (2019) 103(9):e220–38. doi: 10.1097/TP.0000000000002806
9. Janaudis-Ferreira T, Tansey CM, Mathur S, Blydt-Hansen T, Lamoureaux J, Räkel A, et al. The effects of exercise training in adult solid organ transplant recipients: a systematic review and meta-analysis. Transpl Int. (2021) 34(5):801–24. doi: 10.1111/tri.13848
10. Hume E, Ward L, Wilkinson M, Manifield J, Clark S, Vogiatzis I. Exercise training for lung transplant candidates and recipients: a systematic review. Eur Respir Rev. (2020) 29(158):200053. doi: 10.1183/16000617.0053-2020
11. Wickerson L, Mathur S, Brooks D. Exercise training after lung transplantation: a systematic review. J Heart Lung Transplant. (2010) 29(5):497–503. doi: 10.1016/j.healun.2009.12.008
12. Cohen E, Korah M, Callender G, Belfort de Aguiar R, Haakinson D. Metabolic disorders with kidney transplant. Clin J Am Soc Nephrol. (2020) 15(5):732–42. doi: 10.2215/CJN.09310819
13. Ahmed A, Cote A, Lui S, Blydt-Hansen TD. Height-adjusted lean body mass and its associations with physical activity and kidney function in pediatric kidney transplantation. Pediatr Transplant. (2022) 26(1):e14128. doi: 10.1111/petr.14128
14. Li M, Mathur S, Chowdhury NA, Helm D, Singer LG. Pulmonary rehabilitation in lung transplant candidates. J Heart Lung Transplant. (2013) 32(6):626–32. doi: 10.1016/j.healun.2013.04.002
15. Dos Santos Mantovani M, Coelho de Carvalho N, Archangelo TE, Modelli de Andrade LG, Pires Ferreira Filho S, de Souza Cavalcante R, et al. Frailty predicts surgical complications after kidney transplantation. A propensity score matched study. PLoS One. (2020) 15(2):e0229531. doi: 10.1371/journal.pone.0229531
16. Courtwright AM, Salomon S, Fuhlbrigge A, Divo M, Rosas IO, Camp PC, et al. Predictors and outcomes of unplanned early rehospitalization in the first year following lung transplantation. Clin Transplant. (2016) 30(9):1053–8. doi: 10.1111/ctr.12787
17. Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, et al. Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol. (2018) 69(5):1164–77. doi: 10.1016/j.jhep.2018.06.017
18. Schoo E, Gustaw T, Barbalinardo C, Rodrigues N, Zameni Y, Mathur S, et al. Solid organ transplant recipients’ opinions of pre- and post-transplant supervised exercise programmes: a brief report. Physiother Can. (2017) 69(2):178–83. doi: 10.3138/ptc.2016-18EP
19. Gustaw T, Schoo E, Barbalinardo C, Rodrigues N, Zameni Y, Motta VN, et al. Physical activity in solid organ transplant recipients: participation, predictors, barriers, and facilitators. Clin Transplant. (2017) 31(4). doi: 10.1111/ctr.12929
20. Dunn MA, Rogal SS, Duarte-Rojo A, Lai JC. Physical function, physical activity, and quality of life after liver transplantation. Liver Transpl. (2020) 26(5):702–8. doi: 10.1002/lt.25742
21. Wickerson L, Helm D, Gottesman C, Rozenberg D, Singer LG, Keshavjee S, et al. Telerehabilitation for lung transplant candidates and recipients during the COVID-19 pandemic: program evaluation. JMIR Mhealth Uhealth. (2021) 9(6):e28708. doi: 10.2196/28708
22. Lambooy S, Krishnasamy R, Pollock A, Hilder G, Gray NA. Telemedicine for outpatient care of kidney transplant and CKD patients. Kidney Int Rep. (2021) 6(5):1265–72. doi: 10.1016/j.ekir.2021.02.016
23. Tian M, Wang B, Xue Z, Dong D, Liu X, Wu R, et al. Telemedicine for follow-up management of patients after liver transplantation: cohort study. JMIR Med Inform. (2021) 9(5):e27175. doi: 10.2196/27175
24. Chen AC, Ramirez FD, Rosenthal DN, Couch SC, Berry S, Stauffer KJ, et al. Healthy hearts via live videoconferencing: an exercise and diet intervention in pediatric heart transplant recipients. J Am Heart Assoc. (2020) 9(3):e013816. doi: 10.1161/JAHA.119.013816
25. Grishin NK, De Souza AM, Fairbairn J, Sheel AW, Puterman E, Blydt-Hansen T, et al. An 8-week virtual exercise training program for pediatric solid organ transplant recipients. Pediatr Exerc Sci. (2023) 36(3):135–45. doi: 10.1123/pes.2023-0066
26. Peretti A, Amenta F, Tayebati SK, Nittari G, Mahdi SS. Telerehabilitation: review of the state-of-the-art and areas of application. JMIR Rehabil Assist Technol. (2017) 4(2):e7. doi: 10.2196/rehab.7511
27. Davis AM, Sampilo M, Gallagher KS, Dean K, Saroja MB, Yu Q, et al. Treating rural paediatric obesity through telemedicine vs. telephone: outcomes from a cluster randomized controlled trial. J Telemed Telecare. (2016) 22(2):86–95. doi: 10.1177/1357633X15586642
28. Marcin JP, Ellis J, Mawis R, Nagrampa E, Nesbitt TS, Dimand RJ. Using telemedicine to provide pediatric subspecialty care to children with special health care needs in an underserved rural community. Pediatrics. (2004) 113(1 Pt 1):1–6. doi: 10.1542/peds.113.1.1
29. Nesbitt TS, Marcin JP, Daschbach MM, Cole SL. Perceptions of local health care quality in 7 rural communities with telemedicine. J Rural Health. (2005) 21(1):79–85. doi: 10.1111/j.1748-0361.2005.tb00066.x
30. Hezer B, Massey EK, Reinders MEJ, Tielen M, van de Wetering J, Hesselink DA, et al. Telemedicine for kidney transplant recipients: current state, advantages, and barriers. Transplantation. (2024) 108(2):409–20. doi: 10.1097/TP.0000000000004660
31. Udayaraj UP, Watson O, Ben-Shlomo Y, Langdon M, Anderson K, Power A, et al. Establishing a tele-clinic service for kidney transplant recipients through a patient-codesigned quality improvement project. BMJ Open Qual. (2019) 8(2):e000427. doi: 10.1136/bmjoq-2018-000427
32. World Confederation for Physical Therapy. Rehabilitation and the vital role of physiotherapy response to COVID-19 (2020). Available at: world.physio/sites/default/files/2020-07/COVID19-Briefing-Paper-2-Rehabilitation.pdf (Accessed March 01, 2025).
33. Wickerson L, Rozenberg D, Singer LG, Mathur S. Early change in lower limb strength and function in lung transplant patients after center-based and telerehabilitation. J Cardiopulm Rehabil Prev. (2023) 43(1):55–60. doi: 10.1097/HCR.0000000000000728
34. Vendetti ML, Esther Moon SJ, Imes CC, Hergenroeder A, Sciurba F, Lendermon E, et al. Design of lung transplant go (LTGO): a randomized controlled trial evaluating the efficacy of a telerehabilitation behavioral exercise intervention to improve physical activity, physical function, and blood pressure control after lung transplantation. Contemp Clin Trials Commun. (2023) 33:101097. doi: 10.1016/j.conctc.2023.101097
35. Rozenberg D, Santa Mina D, Nourouzpour S, Camacho Perez E, Stewart BL, Wickerson L, et al. Feasibility of a home-based exercise program for managing posttransplant metabolic syndrome in lung and liver transplant recipients: protocol for a pilot randomized controlled trial. JMIR Res Protoc. (2022) 11(3):e35700. doi: 10.2196/35700
36. Spence CM, Foshaug R, Rowland S, Krysler A, Conway J, Urschel S, et al. Evaluating a telemedicine video game-linked high-intensity interval training exercise programme in paediatric heart transplant recipients. CJC Pediatr Congenit Heart Dis. (2023) 2(4):198–205. doi: 10.1016/j.cjcpc.2023.04.001
37. Blandford A, Wesson J, Amalberti R, AlHazme R, Allwihan R. Opportunities and challenges for telehealth within, and beyond, a pandemic. Lancet Glob Health. (2020) 8(11):e1364–5. doi: 10.1016/S2214-109X(20)30362-4
38. Tsutsui M, Gerayeli F, Sin DD. Pulmonary rehabilitation in a post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2021) 16:379–91. doi: 10.2147/COPD.S263031
39. Sarmento A, Adodo R, Hodges G, Webber SC, Sanchez-Ramirez DC. Virtual pulmonary rehabilitation approaches in patients with post COVID syndrome: a pilot study. BMC Pulm Med. (2024) 24(1):139. doi: 10.1186/s12890-024-02965-3
40. Salisbury C, O'Cathain A, Thomas C, Edwards L, Gaunt D, Dixon P, et al. Telehealth for patients at high risk of cardiovascular disease: pragmatic randomised controlled trial. Br Med J. (2016) 353:i2647. doi: 10.1136/bmj.i2647
41. Bernocchi P, Giordano A, Pintavalle G, Galli T, Ballini Spoglia E, Baratti D, et al. Feasibility and clinical efficacy of a multidisciplinary home-telehealth program to prevent falls in older adults: a randomized controlled trial. J Am Med Dir Assoc. (2019) 20(3):340–6. doi: 10.1016/j.jamda.2018.09.003
42. Hergenroeder AL, Willey B, Vendetti M, Dabbs AD. Exercise progression protocol for lung transplant GO: a multicomponent telerehab exercise intervention for patients after lung transplantation. Cardiopulm Phys Ther J. (2023) 34(1):2–12. doi: 10.1097/CPT.0000000000000203
43. Mathur S, Janaudis-Ferreira T, Hemphill J, Cafazzo JA, Hart D, Holdsworth S, et al. User-centered design features for digital health applications to support physical activity behaviors in solid organ transplant recipients: a qualitative study. Clin Transplant. (2021) 35(12):e14472. doi: 10.1111/ctr.14472
44. Handler L, Jaloul P, Clancy J, Cuypers B, Muir J, Hemphill J, et al. A qualitative study of the perspectives of healthcare professionals on features of digital health interventions to support physical activity in solid organ transplant recipients. Prog Transplant. (2023) 33(1):43–9. doi: 10.1177/15269248221145039
45. Tandon P, Purdy G, Ismond KP, Cruz C, Etruw E, Suderman K, et al. Heal-me PiONEer (personalized online nutrition and exercise): an RCT assessing 2 levels of app-based programming in individuals with chronic disease. Contemp Clin Trials. (2022) 118:106791. doi: 10.1016/j.cct.2022.106791
46. Ismond KP, Cruz C, Limon-Miro AT, Low G, Prado CM, Spence JC, et al. An open label feasibility study of a nutrition and exercise app-based solution in cirrhosis. Can Liver J. (2024) 7(1):5–15. doi: 10.3138/canlivj-2023-0011
47. Watt M, Hyde A, Spence JC, Wright GM, Vander Well S, Johnson E, et al. The feasibility and acceptability of an online mind-body wellness program for patients with primary biliary cholangitis. Can Liver J. (2023) 6(3):314–31. doi: 10.3138/canlivj-2022-0045
48. Peerani F, Watt M, Ismond KP, Whitlock R, Ambrosio L, Hotte N, et al. A randomized controlled trial of a multicomponent online stress reduction intervention in inflammatory bowel disease. Therap Adv Gastroenterol. (2022) 15:17562848221127238. doi: 10.1177/17562848221127238
49. Mayes J, Billany RE, Vadaszy N, Young HML, Castle EM, Bishop NC, et al. The rapid development of a novel kidney-specific digital intervention for self-management of physical activity and emotional well-being during the COVID-19 pandemic and beyond: kidney beam. Clin Kidney J. (2021) 15(3):571–3. doi: 10.1093/ckj/sfab239
50. Young HML, Castle EM, Briggs J, Walklin C, Billany RE, Asgari E, et al. The development and internal pilot trial of a digital physical activity and emotional well-being intervention (kidney BEAM) for people with chronic kidney disease. Sci Rep. (2024) 14(1):700. doi: 10.1038/s41598-023-50507-4
51. Greenwood SA, Young HML, Briggs J, Castle EM, Walklin C, Haggis L, et al. Evaluating the effect of a digital health intervention to enhance physical activity in people with chronic kidney disease (kidney BEAM): a multicentre, randomised controlled trial in the UK. Lancet Digit Health. (2024) 6(1):e23–32. doi: 10.1016/S2589-7500(23)00204-2
52. Pomey MP, Gallego FB, Affdal A, Fortin MC. Peer mentoring as an avenue to explore in kidney transplantation: kidney transplant Recipients’ perspectives on peer mentoring. Transplant Direct. (2021) 7(3):e672. doi: 10.1097/TXD.0000000000001130
53. Ko D, Dierker J, Stouff R, Senier L. Telehealth experience among liver and kidney transplant recipients: a mixed methods study. Transpl Int. (2023) 36:11819. doi: 10.3389/ti.2023.11819
54. Wickerson LM, de Paula Ferreira M, Rozenberg D, Mathur S, Singer LG. In-Person versus remote 6-minute walk and incremental shuttle walk distances in advanced lung disease. Respir Care. (2024) 69(5):557–65. doi: 10.4187/respcare.11417
55. Schenkel FA, Barr ML, McCloskey CC, Possemato T, O'Conner J, Sadeghi R, et al. Use of a bluetooth tablet-based technology to improve outcomes in lung transplantation: a pilot study. Am J Transplant. (2020) 20(12):3649–57. doi: 10.1111/ajt.16154
56. Md Fadzil NH, Shahar S, Rajikan R, Singh DKA, Mat Ludin AF, Subramaniam P, et al. A scoping review for usage of telerehabilitation among older adults with mild cognitive impairment or cognitive frailty. Int J Environ Res Public Health. (2022) 19(7):4000. doi: 10.3390/ijerph19074000
57. Huuskes BM, Scholes-Robertson N, Guha C, Baumgart A, Wong G, Kanellis J, et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic. Transpl Int. (2021) 34(8):1517–29. doi: 10.1111/tri.13934
58. de Paula Ferreira M, Chowdhury N, Wickerson L, Ross H, Selzner N, Kim SJ, et al. Feasibility of virtual assessment of physical frailty in solid organ transplant recipients: a single center, observational study. Int J Telerehabil. (2022) 14(1):e6447. doi: 10.5195/ijt.2022.6447
59. Short H, Al Sayah F, Churchill K, Keogh E, Warner L, Ohinmaa A, et al. The use of EQ-5D-5l as a patient-reported outcome measure in evaluating community rehabilitation services in Alberta, Canada. Health Qual Life Outcomes. (2023) 21(1):125. doi: 10.1186/s12955-023-02207-w
60. Tang E, Yantsis A, Ho M, Hussain J, Dano S, Aiyegbusi OL, et al. Patient-Reported outcome measures for patients with CKD: the case for patient-reported outcomes measurement information system (PROMIS) tools. Am J Kidney Dis. (2024) 83(4):508–18. doi: 10.1053/j.ajkd.2023.09.007
61. Lu L, Zhang J, Xie Y, Gao F, Xu S, Wu X, et al. Wearable health devices in health care: narrative systematic review. JMIR Mhealth Uhealth. (2020) 8(11):e18907. doi: 10.2196/18907
62. Stilley CS, Lawrence K, Bender A, Olshansky E, Webber SA, Dew MA. Maturity and adherence in adolescent and young adult heart recipients. Pediatr Transplant. (2006) 10(3):323–30. doi: 10.1111/j.1399-3046.2005.00473.x
63. Patterson C, So S, Schneiderman JE, Stephens D, Stephens S. Physical activity and its correlates in children and adolescents post-liver transplant. Pediatr Transplant. (2016) 20(2):227–34. doi: 10.1111/petr.12662
64. Mohammad S, Alonso EM. Approach to optimizing growth, rehabilitation, and neurodevelopmental outcomes in children after solid-organ transplantation. Pediatr Clin N Am. (2010) 57(2):539–57. doi: 10.1016/j.pcl.2010.01.014
65. Rees R, Kavanagh J, Harden A, Shepherd J, Brunton G, Oliver S, et al. Young people and physical activity: a systematic review matching their views to effective interventions. Health Educ Res. (2006) 21(6):806–25. doi: 10.1093/her/cyl120
66. Anthony SJ, Annunziato RA, Fairey E, Kelly VL, So S, Wray J. Waiting for transplant: physical, psychosocial, and nutritional status considerations for pediatric candidates and implications for care. Pediatr Transplant. (2014) 18(5):423–34. doi: 10.1111/petr.12305
67. Patterson C, So S, DeAngelis M, Ghent E, Southmayd D, Carpenter C. Physical activity experiences in children post-liver transplant: developing a foundation for rehabilitation interventions. Pediatr Transplant. (2018) 22(4):e13179. doi: 10.1111/petr.13179
68. Feldman AG, Neighbors K, Mukherjee S, Rak M, Varni JW, Alonso EM. Impaired physical function following pediatric LT. Liver Transpl. (2016) 22(4):495–504. doi: 10.1002/lt.24406
69. Hager A, Boule N, Pritchard L, Hodgetts S, Noga M, Guo Y, et al. Sarcopenia in children post liver transplant: development of a home-based video program to support muscle strength and function-A pre-post controlled pilot study. Clin Transplant. (2024) 38(9):e15455. doi: 10.1111/ctr.15455
70. Voss C, Harris KC. Physical activity evaluation in children with congenital heart disease. Heart. (2017) 103(18):1408–12. doi: 10.1136/heartjnl-2017-311340
71. Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the pediatric quality of life inventory version 4.0 generic core scales in healthy and patient populations. Med Care. (2001) 39(8):800–12. doi: 10.1097/00005650-200108000-00006
72. Varni JW, Limbers CA, Bryant WP, Wilson DP. The PedsQL multidimensional fatigue scale in type 1 diabetes: feasibility, reliability, and validity. Pediatr Diabetes. (2009) 10(5):321–8. doi: 10.1111/j.1399-5448.2008.00482.x
73. Janz KF, Lutuchy EM, Wenthe P, Levy SM. Measuring activity in children and adolescents using self-report: PAQ-C and PAQ-A. Med Sci Sports Exerc. (2008) 40(4):767–72. doi: 10.1249/MSS.0b013e3181620ed1
74. Bruininks RH, Bruininks BD. Bruininks-Oseretsky Test of Motor Proficiency Second Edition Manual. Minneapolis, MN: Pearson Assessments (2005).
75. Rathgeber SL, Hutchison SM, De Souza AM, Lester R, Blydt-Hansen T, Human DG, et al. A text messaging intervention and quality of life in adolescents with solid organ transplants. Pediatr Transplant. (2022) 26(3):e14219. doi: 10.1111/petr.14219
76. Meredith M, Welk G. Fitnessgram Test Administration Manual. 2nd ed Chapmaign, IL: Human Kinetics (1999).
77. Nytrøen K, Rolid K, Andreassen AK, Yardley M, Gude E, Dahle DO, et al. Effect of high-intensity interval training in de novo heart transplant recipients in Scandinavia. Circulation. (2019) 139(19):2198–211. doi: 10.1161/CIRCULATIONAHA.118.036747 Erratum in: Circulation. 2019;140(17):e737. doi: 10.1161/CIR.0000000000000739.
78. Banks L, Dipchand AI, Manlhiot C, Millar K, McCrindle BW. Factors associated with low physical activity levels following pediatric cardiac transplantation. Pediatr Transplant. (2012) 16(7):716–21. doi: 10.1111/j.1399-3046.2012.01706.x
79. Pedersini P, Picciolini S, Di Salvo F, Toccafondi A, Novembre G, Gualerzi A, et al. The Exercise aNd hEArt transplant (ENEA) trial - a registry-based randomized controlled trial evaluating the safety and efficacy of cardiac telerehabilitation after heart transplant. Contemp Clin Trials. (2024) 136:107415. doi: 10.1016/j.cct.2023.107415
80. Wickerson L, Grewal R, Singer LG, Chaparro C. Experiences and perceptions of receiving and prescribing rehabilitation in adults with cystic fibrosis undergoing lung transplantation. Chron Respir Dis. (2023) 20:14799731221139293. doi: 10.1177/14799731221139293
81. Levine MA, Schuler T, Gourishankar S. Complications in the 90-day postoperative period following kidney transplant and the relationship of the Charlson comorbidity index. Can Urol Assoc J. (2017) 11(12):388–93. doi: 10.5489/cuaj.4378
82. Khandoga A, Thomas M, Kleespies A, Kühnke L, Andrassy J, Habicht A, et al. Surgical complications and cardiovascular comorbidity - substantial non-immunological confounders of survival after living donor kidney transplantation. Surgeon. (2019) 17(2):63–72. doi: 10.1016/j.surge.2018.04.005
83. Alrawashdeh M, Zomak R, Dew MA, Sereika S, Song MK, Pilewski JM, et al. Pattern and predictors of hospital readmission during the first year after lung transplantation. Am J Transplant. (2017) 17(5):1325–33. doi: 10.1111/ajt.14064
84. Omboni S, McManus RJ, Bosworth HB, Chappell LC, Green BB, Kario K, et al. Evidence and recommendations on the use of telemedicine for the management of arterial hypertension: an international expert position paper. Hypertension. (2020) 76(5):1368–83. doi: 10.1161/HYPERTENSIONAHA.120.15873
85. Raina R, Shah R, Marks SD, Johnson JN, Nied M, Bhatt GC, et al. The effects of COVID-19 on pediatric and adult solid organ transplant recipients and the emergence of telehealth. Pediatr Transplant. (2023) 27(4):e14490. doi: 10.1111/petr.14490
86. Ziebell D, Stark M, Xiang Y, Mckane M, Mao C. Virtual cardiac fitness training in pediatric heart transplant patients: a pilot study. Pediatr Transplant. (2023) 27(1):e14419. doi: 10.1111/petr.14419
87. Baranyi A, Krauseneck T, Rothenhausler HB. Overall mental distress and health-related quality of life after solid-organ transplantation: results from a retrospective follow-up study. Health Qual Life Outcomes. (2013) 11:15. doi: 10.1186/1477-7525-11-15
88. Molnar-Varga M, Molnar MZ, Szeifert L, Kovacs AZ, Kelemen A, Becze A, et al. Health-related quality of life and clinical outcomes in kidney transplant recipients. Am J Kidney Dis. (2011) 58(3):444–52. doi: 10.1053/j.ajkd.2011.03.028
89. Tsarpali V, Midtvedt K, Lønning K, Bernklev T, Åsberg A, von der Lippe N, Reisæter AV, Heldal K. Poor physical function trajectory predicts impaired patient survival in older recipients of deceased donor kidneys: a prospective cohort study. Transplant Direct. 2022;8(11):e1374. doi: 10.1097/TXD.0000000000001374
90. Prihodova L, Nagyova I, Rosenberger J, Roland R, Groothoff JW, Majernikova M, et al. Health-related quality of life 3 months after kidney transplantation as a predictor of survival over 10 years: a longitudinal study. Transplantation. (2014) 97(11):1139–45. doi: 10.1097/01.TP.0000441092.24593.1e
91. Fregna G, Schincaglia N, Baroni A, Straudi S, Casile A. A novel immersive virtual reality environment for the motor rehabilitation of stroke patients: a feasibility study. Front Robot AI. (2022) 9:906424. doi: 10.3389/frobt.2022.906424
92. Nizeyimana E, Joseph C, Plastow N, Dawood G, Louw QA. A scoping review of feasibility, cost, access to rehabilitation services and implementation of telerehabilitation: implications for low- and middle-income countries. Digit Health. (2022) 8:20552076221131670. doi: 10.1177/20552076221131670
93. Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Effect of comprehensive cardiac telerehabilitation on one-year cardiovascular rehospitalization rate, medical costs and quality of life: a cost-effectiveness analysis. Eur J Prev Cardiol. (2016) 23(7):674–82. doi: 10.1177/2047487315602257
94. Tousignant M, Moffet H, Nadeau S, Mérette C, Boissy P, Corriveau H, et al. Cost analysis of in-home telerehabilitation for post-knee arthroplasty. J Med Internet Res. (2015) 17(3):e83. doi: 10.2196/jmir.3844
95. Fatoye F, Gebrye T, Fatoye C, Mbada CE, Olaoye MI, Odole AC, et al. The clinical and cost-effectiveness of telerehabilitation for people with nonspecific chronic low back pain: randomized controlled trial. JMIR Mhealth Uhealth. (2020) 8(6):e15375. doi: 10.2196/15375
96. Pastora-Bernal JM, Martin-Valero R, Baron-Lopez FJ. Cost analysis of telerehabilitation after arthroscopic subacromial decompression. J Telemed Telecare. (2018) 24(8):553–9. doi: 10.1177/1357633X17723367
97. Burge AT, Cox NS, Holland AE, McDonald CF, Alison JA, Wootton R, et al. Telerehabilitation compared to center-based pulmonary rehabilitation for people with chronic respiratory disease: economic analysis of a randomized, controlled clinical trial. Ann Am Thorac Soc. (2024) 22(1):47–53. doi: 10.1513/AnnalsATS.202405-549OC
98. Carrigan I, Mathur S, Bourgeois N, Dieudé M, Fantus D, Gongal P, et al. Updates in kidney transplantation from the 2022 Banff-Canadian society of transplantation joint meeting: conference report. Can J Kidney Health Dis. (2023) 10:20543581231209185. doi: 10.1177/20543581231209185
99. Peel NM, Russell TG, Gray LC. Feasibility of using an in-home video conferencing system in geriatric rehabilitation. J Rehabil Med. (2011) 43(4):364–6. doi: 10.2340/16501977-0675
Keywords: transplantation, rehabilitation, exercise, physical activity, telerehabilitation
Citation: Rozenberg D, Logan S, Sohrabipour S, Bourgeois N, Cote A, Deliva R, De Souza A, de Vries R, Donald M, Ferreira M, Hart D, Ibrahim Masthan M, Jaundis-Ferreira T, Juillard S, Khoury M, Lallani A, Mager D, Mucsi I, Orchanian-Cheff A, Reed JL, Tandon P, Tennankore K, Yong E, Wickerson L and Mathur S (2025) Establishment of emerging practices and research priorities for telerehabilitation in solid organ transplantation: meeting report and narrative literature review. Front. Rehabil. Sci. 6:1535138. doi: 10.3389/fresc.2025.1535138
Received: 26 November 2024; Accepted: 17 February 2025;
Published: 28 March 2025.
Edited by:
Mert Doğan, Akdeniz University, TürkiyeCopyright: © 2025 Rozenberg, Logan, Sohrabipour, Bourgeois, Cote, Deliva, De Souza, de Vries, Donald, Ferreira, Hart, Ibrahim Masthan, Jaundis-Ferreira, Juillard, Khoury, Lallani, Mager, Mucsi, Orchanian-Cheff, Reed, Tandon, Tennankore, Yong, Wickerson and Mathur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dmitry Rozenberg, ZG1pdHJ5LnJvemVuYmVyZ0B1aG4uY2E=
†These authors share senior authorship
‡All subsequent authors listed in alphabetical order based on surname
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.