- 1Doctor of Physical Therapy Division, Department of Orthopaedics, Duke University, Durham, NC, United States
- 2Department of Population Health Sciences, Duke University, Durham, NC, United States
- 3Duke Clinical Research Institute, Duke University, Durham, NC, United States
- 4Department of Veterans Affairs Medical Center, Cleveland, OH, United States
- 5Departments of Bioengineering & Neurosurgery, University of Pennsylvania, Philadelphia, PA, United States
- 6Department of Physical Therapy, University of Alabama at Birmingham, Birmingham, AL, United States
Background: Matching disease and treatment mechanisms is a goal of the Precision Medicine Initiative. Pro- and anti-inflammatory cytokines (e.g., Tumor Necrosis Factor-alpha, Transforming Growth Factor-beta, and Interleukin-2, 10, and 12) have gained a significant amount of interest in their potential role in persistent pain for musculoskeletal (MSK) conditions. Manual therapy (MT) and exercise are two guideline-recommended approaches for treating MSK conditions. The objective of this narrative overview was to investigate of the effects of MT and exercise on pro- and anti-inflammatory cytokines and determine the factors that lead to variability in results.
Methods: Two reviewers evaluated the direction and variabilities of MT and exercise literature. A red, yellow, and green light scoring system was used to define consistencies.
Results: Consistencies in responses were seen with acute and chronic exercise and both pro- and anti-inflammatory cytokines. Chronic exercise is associated with a consistent shift towards a more anti-inflammatory cytokine profile (Transforming Growth Factor-beta, and Interleukin-2 and 13, whereas acute bouts of intense exercise can transiently increase pro-inflammatory cytokine levels. The influence of MT on cytokines was less commonly studied and yielded more variable results.
Conclusion: Variability in findings is likely related to the subject and their baseline condition or disease, when measurement occurs, and the exercise intensity, duration, and an individual's overall health and fitness.
Introduction
The Precision Medicine Initiative is an emerging approach for disease prevention and treatment that considers individual differences in people's genetic background, environments, and lifestyles (1). To date, most precision medicine-based approaches for musculoskeletal-related (MSK-related) pain disorders remain in the early stages of development and implementation. Nonetheless, recent successes (2–4) have elevated interests and have highlighted the importance of matching predictive biomarkers such as pro- and anti-inflammatory cytokines, especially for matching MSK-related conditions with dedicated treatment mechanisms (5–7). Conceptually, by exploring pro- and anti-inflammatory cytokines in patients, we may be able to tailor treatments and dosages, thus improving post-treatment responses (8–10).
Interventions such as manual therapy (MT) (e.g., manipulation, mobilization and massage) and exercise (e.g., resistance and aerobic) are typically recommended by clinical guidelines, as first-line approaches for management of MSK-related pain (11, 12). Both approaches have theoretically different mechanisms by which they provide clinical efficacy. Manual therapy activates both peripheral and central physiological mechanisms, which have shown to reduce pain, muscle hypertonicity, and improve mobility (13). Exercise occurs in many forms but is commonly labeled as resistance exercise or aerobic. Resistance exercise leads to increases in muscle fiber size and neural adaptations, thus improving strength and endurance (13). Aerobic exercise releases endorphins, improves blood flow, and reduces hypoxia and macrophage infiltration (14).
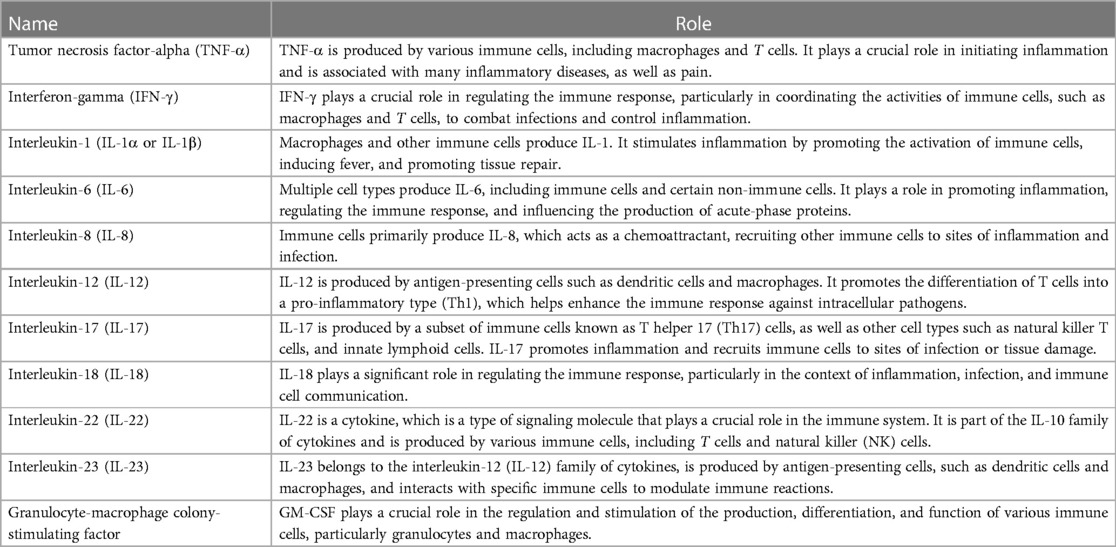
Cytokines are a large family of small-secreted proteins produced by numerous cells that function independently and interact with immune-related cells. Other terms within the cytokine's family include lymphokines, monokines, chemokines, and interleukins, which reflect the cells from which they are generated (8, 15). Cytokines are essential in regulating the human immune response (15) and are broadly categorized as pro-inflammatory (Table 1) or anti-inflammatory (Table 2) (15–17).
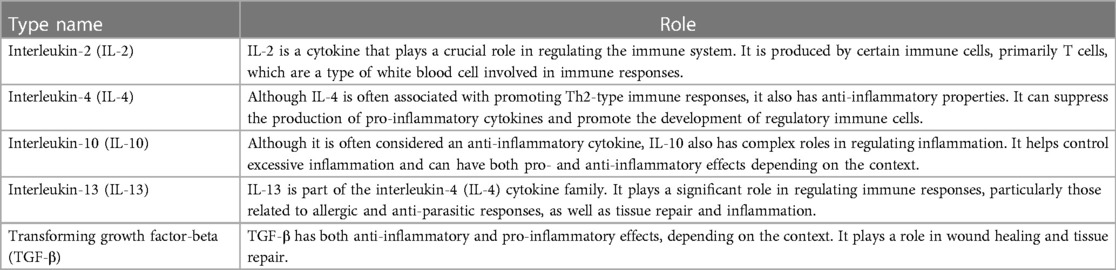
Pro- and anti-inflammatory cytokines have contrasting effects on inflammation and immune reactions, and over different temporal phases. Pro-inflammatory cytokines promote inflammation, aimed at eliminating harmful agents and damaged cells, as well as initiating tissue repair. Pro-inflammatory cytokines also directly or indirectly sensitize nociceptors both peripherally and centrally (18) and may play a role in depression, anxiety, sleep disturbances (19) and cognitive deficits (18). Whereas inflammation serves a protective function initially, excessive or prolonged inflammation can lead to tissue damage and contribute to various chronic diseases such as autoimmune disorders, allergies, neuronal dysfunction, psychiatric disorders and certain types of cancers (8, 15). Anti-inflammatory cytokines (e.g., Transforming Growth Factor-beta, Interleukin-4, 10 and 13) act as counterbalances to pro-inflammatory cytokines (e.g., Tumor Necrosis Factor-alpha, Interleukin-1, 6, 8 and 12), helping to prevent excessive inflammation and promoting immune system homeostasis (8). Some cytokines can act as either pro- or anti-inflammatory, depending on the physiological circumstance or both in selected situations (8).
Cytokines are known to play a role in MSK-related pain (5–7). Low-grade inflammation is associated with the severity of low back and neck pain (18, 20–23) and specific conditions such as intervertebral disc degeneration has a complex inflammatory response within its pathological processes, with elevated levels of pro-inflammatory cytokines (24). Several pro-inflammatory mediators have been identified as playing a role in rotator cuff tendinopathy (25) and general shoulder pain (26). Dominate nociplastic pain conditions such as fibromyalgia exhibit different circulating cytokine profiles versus healthy controls (27). Conflicting evidence exists regarding the role of inflammatory cytokines in knee osteoarthritis (28).
Prescription drugs are used to target specific pro- and anti-inflammatory cytokines. Adalimumab (brand names Humira, Amgevita, Hyrimoz, Idacio, Imraldi, and Yuflyma), which is used to treat conditions such as Crohn's disease and rheumatoid arthritis, targets and inhibits tumor necrosis factor-alpha (TNF-α). Tocilizumab (brand name Actemra), which is used to treat rheumatoid and systemic juvenile arthritis, targets interleukin-6 (IL-6) receptors. Prednisolone is used in treatment of a number of inflammatory conditions and can suppress the production of pro-inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α). Anti-TNF-α therapy agents are used in alleviating intervertebral disc degeneration, especially in inhibiting extracellular matrix degradation and reducing inflammatory responses (24).
Laboratory and preclinical studies have shown that MT and exercise can modulate pro- and anti-inflammatory cytokines as well (29–38); however, the extent of the modulation and the direction of influence (i.e., increase or decrease in cytokine levels) is largely unknown. The purpose of this narrative overview is to investigate of the effects of MT and exercise on pro- and anti-inflammatory cytokines. We reviewed and summarized the current pre-clinical and clinical evidence evaluating MT and exercise influences on cytokines and included studies that outlined the direction of the influence as well as factors that might explain why differences were present in the literature.
Materials and methods
Design
The study used a narrative overview design. Narrative overviews are useful as they compile large amounts of information together into a structured, transferable format that includes identification of gaps in knowledge and present trends (39–41). Narrative overviews are especially useful for (1) developing theories of how an intervention works, why and for whom; (2) developing a preliminary synthesis of findings of included studies; or (3) exploring relationships in the data (42). Unlike systematic reviews, narrative overviews rarely include a risk of bias score and routinely do not involve an automated systematic search (39).
Search strategy
We included articles published in PubMed, and those referenced in systematic reviews. Systematic reviews were also included, if they reported the direction of the influence of MT or exercise on cytokines. A biomedical librarian was consulted in August of 2023 to assist in the structure of the search. The search string included keywords (“inflammatory cytokines” OR “pro-inflammatory cytokines” OR “anti-inflammatory cytokines”) AND (exercise OR “physical activity” OR “exercise therapy” OR “exercise training”) AND (manual therapy OR “massage therapy” OR “manipulative therapy” OR “chiropractic therapy”).
Study inclusion criteria
We included studies that incorporated short-term or longer-term applications of MT and/or exercise (acute and chronic) and measured cytokine changes after application. All experimental designs were considered, including both pre-clinical and clinical research, with any follow-up period. We included all approaches used in collection and measurement of inflammatory cytokines [e.g., enzyme-linked immunosorbent assay (ELISA), urine, etc.] (43).
Subject inclusion criteria
We included both animal and human studies, and did not restrict papers to those involving MSK-related disorders, since there are very few in the literature. Since one of the goals of this narrative was to determine the influence of MT and exercise on cytokines, we included studies with animal models and humans with metabolic disorders, of all ages, healthy controls, and individuals who had known inflammatory conditions such as fibromyalgia.
Selection of specific pro- and anti-inflammatory cytokines
We limited pro- and anti-inflammatory cytokines to those routinely captured on an Enzyme-linked immunosorbent assay (ELISA) that are thought to have high relevance in various biological processes and diseases (Tables 1, 2).
Selection of specific MT and exercise interventions
We included all forms of acute and chronic (regular) MT and exercise and did not restrict studies based on parameters of intervention application. We did not evaluate the fidelity of the MT and/or exercise interventions.
Specific measures-direction of influence
Direction of influence (e.g., how MT and exercise influenced changes in cytokines) was recorded using a novel green, yellow and red-light assessment, independently by two authors (CC, DK). A score of “green light” represented one or more studies that (consistently) showed a defined pattern of either decreasing or increasing plasma cytokine levels after application of MT or exercise. A score of “red light” suggested there was one or more studies that did not find any change (either from baseline or against a comparator) in cytokines after application of MT or exercise (in other words, MT and exercise did not influence the cytokine). A score of “yellow light” either consisted of “mixed” or conflicting information (e.g., involving an increase, decrease, or no-response) across two or more studies. Studies could have a green, yellow or red light and a designation of “limited” evidence, if there are few studies that have explored the role of MT and exercise on the cytokine of interest.
Specific measures-factors that identified variations in findings
Two authors (CC, DK) used a modified form of thematic coding to explain factors that might explain why differences were present in the literature. In particular, we evaluated potential differences in: (1) follow up timing, (2) subject health, (3) types of MT and exercise applications used, including force, intensity, etc., (4) how long the interventions were provided, and (5) how the cytokines were measured.
Results
Study inclusion
Our search identified 4,434 studies involving exercise and cytokines (128 systematic reviews) and 2,437 studies involving MT and cytokines (28 systematic reviews). Variability across studies was very high; studies involved preclinical and clinical research, animal and human models, and a wide range of MT and exercise applications. To summarize the evidence in this overview, we included comparative trials, pre-post studies, and systematic reviews.
Evidence for pro-inflammatory cytokine modulation with manual therapy and exercise
Table 3 outlines the current evidence for MT and exercise for modulation of pro-inflammatory cytokines (29–54, 56–82, 91–110). A majority of studies found that acute (short-term) exercise may lead to transient changes in both pro- and anti-inflammatory cytokine levels, whereas chronic (regular) exercise of various forms, especially at moderate intensities, is generally associated with a reduction in pro-inflammatory cytokine profiles. There is mixed evidence that TNF-a, IFN-y, IL-6, and IL-8 are influenced by MT approaches. The direction of the influence is conflicting. Outside of IL-18 and IL-22, which have presently not been studied, the remaining pro-inflammatory markers do not appear to be influenced by MT—although there are a limited number of studies that have investigated such relationships.
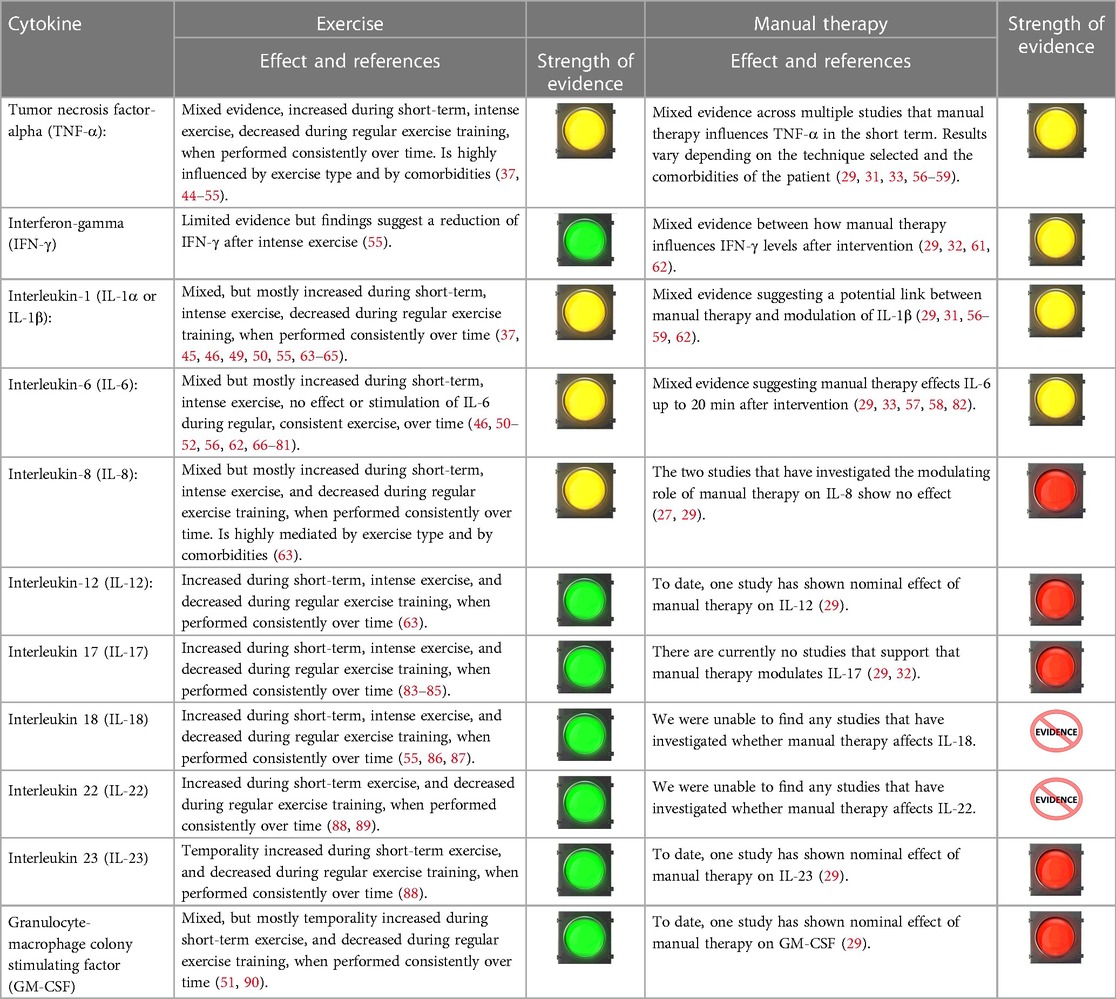
Table 3. Evidence suggesting an association between exercise, manual therapy and Pro-inflammatory cytokines.
Evidence for anti-inflammatory cytokine modulation with manual therapy and exercise
Table 4 outlines the current evidence for MT and exercise for modulation of anti-inflammatory cytokines (29–31, 33, 44, 47, 49–51, 53, 55, 58, 61–63, 66, 67, 77, 79, 80, 91, 101, 111–142). Current evidence suggests acute exercise tends to increase anti-inflammatory cytokines (i.e., IL-10, IL-13, and TGF-β). Studies suggest that IL-2 and IL-4 increase with MT, whereas values are mixed or unstudied in other cytokines.
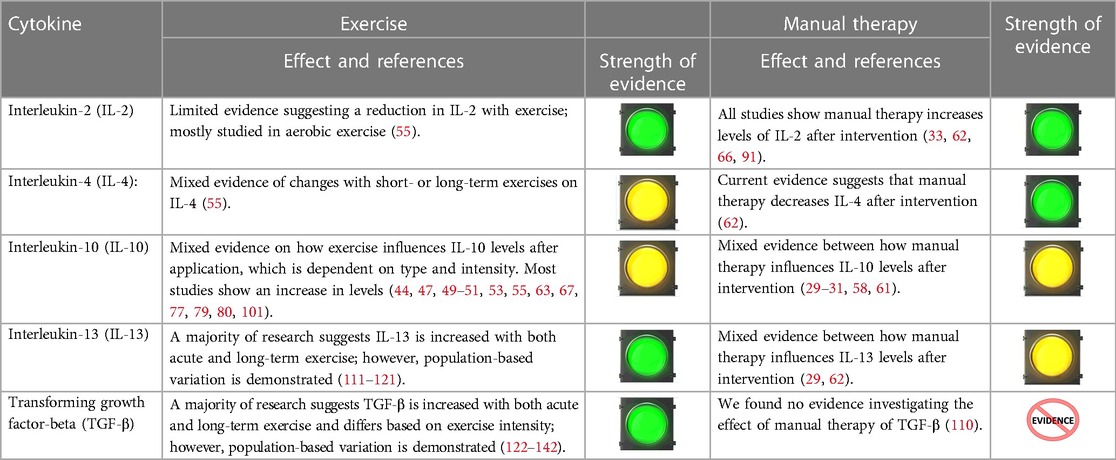
Table 4. Evidence suggesting an association between exercise, manual therapy and anti-inflammatory cytokines.
Factors that identified variations in findings
For exercise and pro-inflammatory cytokines, the amount of change depends on various factors, including the subject and their baseline condition or disease, when measurement occurs, and the exercise intensity, duration, and an individual’s overall health and fitness. The variations in findings for pro-inflammatory cytokines and MT may be explained by differences in techniques used, differences in intensities and variations in subject populations, including baseline condition, context of the MT provided, when measurement occurs, and follow-up times. For anti-inflammatory cytokines, similar types of variations present in pro-inflammatory literature were likely the reason for mixed results in selected cases.
Discussion
The purpose of the study was to investigate the effects of MT and exercise on pro- and anti-inflammatory cytokines. We were particularly interested in measuring direction of the influence as well as factors that might explain why differences were present in the literature. As anticipated, we found notably more studies investigating the role of exercise on cytokine levels/profile, as compared to MT approaches indicating the need for additional investigation. The experimental design types were highly variant, involving both pre-clinical and clinical studies, with study participants with and without health-related conditions.
Exercise and MT and their influence on cytokines
There was a notable trend toward an increase in pro-inflammatory cytokine levels with acute exercise, and a reduction in cytokine levels with longer-term, chronic exercise (51, 55, 60, 63, 83–90). A consistent pattern involving increases emerged for anti-inflammatory cytokines with acute and regular aerobic and resistance exercise (111–142). Generally, moderate-intensity aerobic exercise and resistance training is associated with anti-inflammatory effects; however, excessive or intense exercise without adequate recovery appeared to increase inflammation over the short term. This suggests that exercise may be useful as a moderator for inflammation that occurs as a result of infections, injuries, or stress, but dosing (intensity and timing) is critical.
The most accurate way to summarize the role of MT on cytokine profiles based on this review is to indicate that there is insufficient evidence to draw conclusions. In many studies investigating MT, results were mixed (29–31, 33, 56, 57, 59, 62, 82). The inconsistency across MT types, study designs, and dosages likely played a role in these mixed results. In addition, many of the subjects included were poorly described and it was unclear of the role of pathology on the biomarker response. Further study is warranted, but only if dosage rates are somewhat comparable to clinical applications assuring that they are at a level that might be therapeutically beneficial.
Factors that identified variations in findings
We found that multiple factors seemed to influence response from exercise and MT (e.g., exercise intensity, duration, and an individual's overall health and fitness). The literature suggests that characteristics such as genetics, sex, age, and body composition interact with the immune system thereby influencing the inflammatory response (143–145). Intrinsic factors such as sleep quality (146–148), diet (149, 150), anxiety (151–153), depression (154, 155), fear (156), and stress (154, 157, 158) have all shown to associate with inflammatory mediator expression. Further, placebo/expectation-based effects have also shown to moderate inflammation in conditions including asthma, irritable bowel syndrome, chronic fatigue syndrome, and multiple sclerosis (159). Extrinsic factors have also been shown to influence neuroimmune responses including: auditory input (160), lighting (161), environmental pollution (162), time of day which measurement occurs (146, 163), and temperature (164). These confounding factors can demonstrate variability from day to day and therefore should be accounted for within mechanistic studies with measurement at baseline and re-assessment to establish association with mechanistic outcome.
Challenges in measuring pro- and anti-inflammatory cytokines
Variability in mechanism-specific research on inflammatory cytokines stems from the complex interplay of the neurological, endocrine, and immune systems that regulate them. Population-based differences in immunologic profiles have also been established and should be accounted for when assessing variability in mechanistic trials. Differences have been demonstrated between healthy controls and individuals with pain therefore limiting the applicability of several of the included trials (155, 165). Further complicating interpretation is evidence that even in individuals experiencing pain, different inflammatory pain disorders demonstrate different cytokine profiles that mediate inflammation (likely associated with crosstalk) (166). In-vitro studies have been proposed to identify specific local mechanistic responses by removing crosstalk and limiting systemic influences; however, that approach limits real world applicability in-vivo studies (167–169). Animal studies allow for more control over extrinsic stimuli potentially impacting outcomes; however, differences between animal models and humans are likely (169, 170).
The immune system does not act in isolation but rather in conjunction with the nervous and endocrine systems (171, 172). These systems all demonstrate connectivity and responsiveness to physical, psychological, physiological and viral stress (173). This multisystem interface attempts to establish balance between inflammatory and anti-inflammatory mechanisms (171, 172). An example of this response occurs when inflammatory mediators are released in response to peripheral tissue injury sensitizing peripheral nerve endings, nociceptors release neuropeptides attempting to modulate various inflammatory mediators to protect and heal the injured tissue (163, 174).
Further complicating the interaction between these systems is the fact that cytokines can influence one another and replicate action at other sites, which has been termed “crosstalk” (167, 175). This phenomenon is not specific to one class of cytokine with influence reaching beyond inflammation and peripheral sensitization. Interleukin-6 type cytokines have shown to have crosstalk with IL-12, IL-35, IL-1, TNFα, glucocorticoids, glucagon, and insulin (159). Cytokines also have effects on Apolipoprotein E (glycosylated protein) influencing neurodegenerative and autoimmune disorders (176), dendritic cells (175), and leptin (177). The cytokines outlined within this review do not act specifically, but act systemically (178–180). When studying and accounting for crosstalk, it is crucial that researchers optimize assays, use multiple assays to avoid background signal and nonspecific interactions, and consider the use of bioinformatics tools and larger sample sizes to improve precision.
Study limitations
By its nature, the narrative overview design allows for qualitative interpretations from authors who summarize the research which increases the risk for subjectivity. Nonetheless, our objectives were two-fold and included an assessment accounting for the variability observed. Although there are consistent trends in the role of acute and chronic exercise on pro- and anti-inflammatory cytokines, studies included animal models and human studies without health compromise. This is a limitation that is worth noting. Further, a lack of a comprehensive systematic selection process increases risk of missing key studies. Despite this, we include nearly 80 studies that investigated cytokines, including systematic reviews, which we feel represents an accurate overview of the literature.
Recommendations for future research
Future studies investigating the role of MT or exercise on pro- and anti-inflammatory cytokines need to control for confounding variables and contextual influences to the whatever extent is possible. Pre-clinical efficacy studies may better control for these variables; however, being less reflective of clinical practice limits the applicability of such work. Clinical studies can be performed with careful controls for factors such as timing and contextual influences. Future studies should investigate subjects with specific MSK-disorders, since cytokine changes depend on the health/disease of the individual. Further, study of single cytokines is likely of less benefit, given the complicated constellation of their interplay and crosstalk occurs between cytokines. The use of cytokine response phenotypes is likely to provide greater value when determining the transferability of findings to clinical populations. Studies should attempt to correlate clinical value of mechanistic response to determine whether the changes that occur are of benefit.
Author contributions
CC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. DK: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. WC: Writing – original draft, Writing – review & editing. BW: Writing – original draft, Writing – review & editing. WR: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This was a NIH U24 supported research study [1U24AT011969-01, Force-Based Manipulation network (ForceNet)]; Administering institutes or centers National Center for Complementary and Integrative Health, https://reporter.nih.gov/search/Hq4YC-ZDk0yDq2PgxIHgVA/project-details/10450926; National Institute of Neurological Disorders and Stroke (NINDs). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hartl D, de Luca V, Kostikova A, Laramie J, Kennedy S, Ferrero E, et al. Translational precision medicine: an industry perspective. J Transl Med. (2021) 19(1):245. doi: 10.1186/s12967-021-02910-6
2. Stevens TW, Matheeuwsen M, Lönnkvist MH, Parker CE, Wildenberg ME, Gecse KB, et al. Systematic review: predictive biomarkers of therapeutic response in inflammatory bowel disease-personalised medicine in its infancy. Aliment Pharmacol Ther. (2018) 48(11-12):1213–31. doi: 10.1111/apt.15033
3. Guo H, Meng Y, Peng J, Yang X. Post-operative adjuvant immunotherapy with cytokine-induced killer and dendritic cells for hepatocellular carcinoma: a systematic review and meta-analysis. Asian J Surg. (2023) 46(6):2433–6. doi: 10.1016/j.asjsur.2022.12.049
4. Negaresh R, Motl RW, Mokhtarzade M, Dalgas U, Patel D, Shamsi MM, et al. Effects of exercise training on cytokines and adipokines in multiple sclerosis: a systematic review. Mult Scler Relat Disord. (2018) 24:91–100. doi: 10.1016/j.msard.2018.06.008
5. McKinley TO, Gaski GE, Billiar TR, Vodovotz Y, Brown KM, Elster EA, et al. Patient-specific precision injury signatures to optimize orthopaedic interventions in multiply injured patients (PRECISE STUDY). J Orthop Trauma. (2022) 36(Suppl 1):S14–20. doi: 10.1097/BOT.0000000000002289
6. Fields AJ, Dudli S, Schrepf A, Kim A, Pham B, Gallego E, et al. Protocol for biospecimen collection and analysis within the BACPAC research program. Pain Med. (2023) 24(Suppl 1):S71–80. doi: 10.1093/pm/pnac197
7. Dagostino C, De Gregori M, Gieger C, Manz J, Gudelj I, Lauc G, et al. Validation of standard operating procedures in a multicenter retrospective study to identify -omics biomarkers for chronic low back pain. PLoS One. (2017) 12(5):e0176372. doi: 10.1371/journal.pone.0176372
8. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. (2007) 45(2):27–37. Spring. doi: 10.1097/AIA.0b013e318034194e
9. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. (2020) 214:108393. doi: 10.1016/j.clim.2020.108393
10. Colombi A, Testa M. The effects induced by spinal manipulative therapy on the immune and endocrine systems. Medicina (Kaunas). (2019) 55(8):448. doi: 10.3390/medicina55080448
11. George SZ, Fritz JM, Silfies SP, Schneider MJ, Beneciuk JM, Lentz TA, et al. Interventions for the management of acute and chronic low back pain: revision 2021. J Orthop Sports Phys Ther. (2021) 51(11):CPG1–CPG60. doi: 10.2519/jospt.2021.0304
12. Blanpied PR, Gross AR, Elliott JM, Devaney LL, Clewley D, Walton DM, et al. Neck pain: revision 2017. J Orthop Sports Phys Ther. (2017) 47(7):A1–A83. doi: 10.2519/jospt.2017.0302
13. McDevitt AW, O'Halloran B, Cook CE. Cracking the code: unveiling the specific and shared mechanisms behind musculoskeletal interventions. Arch Physiother. (2023) 13(1):14. doi: 10.1186/s40945-023-00168-3
14. Song Y, Jia H, Hua Y, Wu C, Li S, Li K, et al. The molecular mechanism of aerobic exercise improving vascular remodeling in hypertension. Front Physiol. (2022) 13:792292. doi: 10.3389/fphys.2022.792292
15. Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. (2000) 117(4):1162–72. doi: 10.1378/chest.117.4.1162
16. Justiz Vaillant AA, Qurie A. Interleukin. [Updated 2022 Aug 22]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK499840/
17. Armstrong SA, Herr MJ. Physiology, Nociception. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK551562/
18. Klyne DM, Barbe MF, Hodges PW. Systemic inflammatory profiles and their relationships with demographic, behavioural and clinical features in acute low back pain. Brain Behav Immun. (2017) 60:84–92. doi: 10.1016/j.bbi.2016.10.003
19. Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. (2013) 246:199–229. doi: 10.1016/j.neuroscience.2013.04.060
20. Generaal E, Vogelzangs N, Macfarlane GJ, Geenen R, Smit JH, Dekker J, et al. Basal inflammation and innate immune response in chronic multisite musculoskeletal pain. Pain. (2014) 155:1605–12. doi: 10.1016/j.pain.2014.05.007
21. Kadow T, Sowa G, Vo N, Kang JD. Molecular basis of intervertebral disc degeneration and herniations: what are the important translational questions? Clin Orthop Relat Res. (2015) 473(6):1903–12. doi: 10.1007/s11999-014-3774-8
22. Hener KL, France CR, Tros Z, Mei Ng H, Pigeon WR. Chronic low back pain, sleep disturbance, and interleukin-6. Clin J Pain. (2011) 27:35–41. doi: 10.1097/AJP.0b013e3181eef761
23. Teodorczyk-Injeyan JA, Triano JJ, McGregor M, Woodhouse L, Injeyan HS. Elevated production of inflammatory mediators including nociceptive chemokines in patients with neck pain: a cross-sectional evaluation. J Manipulative Physiol Ther. (2011) 34(8):498–505. doi: 10.1016/j.jmpt.2011.08.010
24. Pan H, Li H, Guo S, Wang C, Long L, Wang X, et al. The mechanisms and functions of TNF-α in intervertebral disc degeneration. Exp Gerontol. (2023) 174:112119. doi: 10.1016/j.exger.2023.112119
25. Struzik S, Czarkowska-Paczek B, Wyczalkowska-Tomasik A, Maldyk P, Paczek L. Selected clinical features fail to predict inflammatory gene expressions for TNF-α, TNFR1, NSMAF, Casp3 and IL-8 in tendons of patients with rotator cuff tendinopathy. Arch Immunol Ther Exp (Warsz). (2021) 69(1):6. doi: 10.1007/s00005-021-00610-z
26. George SZ, Parr JJ, Wallace MR, Wu SS, Borsa PA, Dai Y, et al. Inflammatory genes and psychological factors predict induced shoulder pain phenotype. Med Sci Sports Exerc. (2014) 46(10):1871–81. doi: 10.1249/MSS.0000000000000328
27. O'Mahony LF, Srivastava A, Mehta P, Ciurtin C. Is fibromyalgia associated with a unique cytokine profile? A Systematic Review and Meta-Analysis. Rheumatology (Oxford). (2021) 60(6):2602–14. doi: 10.1093/rheumatology/keab146
28. Dainese P, Mahieu H, De Mits S, Wittoek R, Stautemas J, Calders P. Associations between markers of inflammation and altered pain perception mechanisms in people with knee osteoarthritis: a systematic review. RMD Open. (2023) 9(2):e002945. doi: 10.1136/rmdopen-2022-002945
29. Duarte FCK, Funabashi M, Starmer D, Partata WA, West DWD, Kumbhare DA, et al. Effects of distinct force magnitude of spinal manipulative therapy on blood biomarkers of inflammation: a proof of principle study in healthy young adults. J Manipulative Physiol Ther. (2022) 45(1):20–32. doi: 10.1016/j.jmpt.2022.03.012
30. Barbe MF, Panibatla ST, Harris MY, Amin M, Dorotan JT, Cruz GE, et al. Manual therapy with rest as a treatment for established inflammation and fibrosis in a rat model of repetitive strain injury. Front Physiol. (2021) 12:755923. doi: 10.3389/fphys.2021.755923
31. Lutke Schipholt IJ, Coppieters MW, Meijer OG, Tompra N, de Vries RBM, Scholten-Peeters GGM. Effects of joint and nerve mobilisation on neuroimmune responses in animals and humans with neuromusculoskeletal conditions: a systematic review and meta-analysis. Pain Rep. (2021) 6(2):e927. doi: 10.1097/PR9.0000000000000927
32. Sornkayasit K, Jumnainsong A, Phoksawat W, Eungpinichpong W, Leelayuwat C. Traditional Thai massage promoted immunity in the elderly via attenuation of senescent CD4+ T cell subsets: a randomized crossover study. Int J Environ Res Public Health. (2021) 18(6):3210. doi: 10.3390/ijerph18063210
33. Teodorczyk-Injeyan JA, Triano JJ, Gringmuth R, DeGraauw C, Chow A, Injeyan HS. Effects of spinal manipulative therapy on inflammatory mediators in patients with non-specific low back pain: a non-randomized controlled clinical trial. Chiropr Man Therap. (2021) 29(1):3. doi: 10.1186/s12998-020-00357-y
34. Ma Y, He M, Qiang L. Exercise therapy downregulates the overexpression of TLR4, TLR2, MyD88 and NF-κB after cerebral ischemia in rats. Int J Mol Sci. (2013) 14(2):3718–33. doi: 10.3390/ijms14023718
35. Zwagerman N, Plumlee C, Guthikonda M, Ding Y. Toll-like receptor-4 and cytokine cascade in stroke after exercise. Neurol Res. (2010) 32(2):123–6. doi: 10.1179/016164109X12464612122812
36. Choi DH, Kwon IS, Koo JH, Jang YC, Kang EB, Byun JE, et al. The effect of treadmill exercise on inflammatory responses in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. J Exerc Nutrition Biochem. (2014) 18(2):225–33. doi: 10.5717/jenb.2014.18.2.225
37. McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 Is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. (2004) 36(11):1876–83. doi: 10.1249/01.MSS.0000145465.71269.10
38. Booth S, Florida-James GD, McFarlin BK, Spielmann G, O'Connor DP, Simpson RJ. The impact of acute strenuous exercise on TLR2, TLR4 and HLA.DR expression on human blood monocytes induced by autologous serum. Eur J Appl Physiol. (2010) 110(6):1259–68. doi: 10.1007/s00421-010-1616-2
39. Green BN, Johnson CD, Adams A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. (2006) 5(3):101–17. Autumn. doi: 10.1016/S0899-3467(07)60142-6
40. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. (2009) 26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x
41. Rozas LW, Klein WC. The value and purpose of the traditional qualitative literature review. J Evid Based Soc Work. (2010) 7(5):387–99. doi: 10.1080/15433710903344116
42. Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme. Swindon: Economic and Social Research Council (ESRC) (2006). doi: 10.13140/2.1.1018.4643
43. Amsen D, de Visser KE, Town T. Approaches to determine expression of inflammatory cytokines. Methods Mol Biol. (2009) 511:107–42. doi: 10.1007/978-1-59745-447-6_5
44. Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. (2012) 44(11):2099–110. doi: 10.1249/MSS.0b013e3182644984
45. Lira FS, Rosa JC, Pimentel GD, Tarini VA, Arida RM, Faloppa F, et al. Inflammation and adipose tissue: effects of progressive load training in rats. Lipids Health Dis. (2010) 9:109. doi: 10.1186/1476-511X-9-109
46. Robinson E, Durrer C, Simtchouk S, Jung ME, Bourne JE, Voth E, et al. Short-term high-intensity interval and moderate-intensity continuous training reduce leukocyte TLR4 in inactive adults at elevated risk of type 2 diabetes. J Appl Physiol. (2015) 119(5):508–16. doi: 10.1152/japplphysiol.00334.2015
47. Cheng YY, Kao CL, Ma HI, Hung CH, Wang CT, Liu DH, et al. SIRT1-related Inhibition of pro-inflammatory responses and oxidative stress are involved in the mechanism of nonspecific low back pain relief after exercise through modulation of toll-like receptor 4. J Biochem. (2015) 158(4):299–308. doi: 10.1093/jb/mvv041
48. Fashi M, Agha Alinejad H, Asilian Mahabadi H. The effect of aerobic exercise in ambient particulate matter on lung tissue inflammation and lung cancer. Iran J Cancer Prev. (2015) 8(3):e2333. doi: 10.17795/ijcp2333
49. Zanchi NE, Lira FS, de Siqueira Filho MA, Rosa JC, de Oliveira Carvalho CR, Seelaender M, et al. Chronic low frequency/low volume resistance training reduces pro-inflammatory cytokine protein levels and TLR4 mRNA in rat skeletal muscle. Eur J Appl Physiol. (2010) 109(6):1095–102. doi: 10.1007/s00421-010-1456-0
50. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol. (1999) 515(Pt 1):287–91. doi: 10.1111/j.1469-7793.1999.287ad.x
51. Nieman DC, Konrad M, Henson DA, Kennerly K, Shanely RA, Wallner-Liebmann SJ. Variance in the acute inflammatory response to prolonged cycling is linked to exercise intensity. J Interferon Cytokine Res. (2012) 32(1):12–7. doi: 10.1089/jir.2011.0038
52. Marklund P, Mattsson CM, Wåhlin-Larsson B, Ponsot E, Lindvall B, Lindvall L, et al. Extensive inflammatory cell infiltration in human skeletal muscle in response to an ultraendurance exercise bout in experienced athletes. J Appl Physiol (1985). (2013) 114(1):66–72. doi: 10.1152/japplphysiol.01538.2011
53. Brenner IK, Natale VM, Vasiliou P, Moldoveanu AI, Shek PN, Shephard RJ. Impact of three different types of exercise on components of the inflammatory response. Eur J Appl Physiol Occup Physiol. (1999) 80(5):452–60. doi: 10.1007/s004210050617
54. Bernecker C, Scherr J, Schinner S, Braun S, Scherbaum WA, Halle M. Evidence for an exercise induced increase of TNF-α and IL-6 in marathon runners. Scand J Med Sci Sports. (2013) 23(2):207–14. doi: 10.1111/j.1600-0838.2011.01372.x
55. Ayari S, Abellard A, Carayol M, Guedj É, Gavarry O. A systematic review of exercise modalities that reduce pro-inflammatory cytokines in humans and animals’ models with mild cognitive impairment or dementia. Exp Gerontol. (2023) 175:112141. doi: 10.1016/j.exger.2023.112141
56. Zhu GC, Tsai KL, Chen YW, Hung CH. Neural mobilization attenuates mechanical allodynia and decreases proinflammatory cytokine concentrations in rats with painful diabetic neuropathy. Phys Ther. (2018) 98(4):214–22. doi: 10.1093/ptj/pzx124
57. Degenhardt BF, Johnson JC, Fossum C, Andicochea CT, Stuart MK. Changes in cytokines, sensory tests, and self-reported pain levels after manual treatment of low back pain. Clin Spine Surg. (2017) 30(6):E690–701. doi: 10.1097/BSD.0000000000000231
58. Licciardone JC, Kearns CM, Hodge LM, Bergamini MV. Associations of cytokine concentrations with key osteopathic lesions and clinical outcomes in patients with nonspecific chronic low back pain: results from the OSTEOPATHIC trial. J Am Osteopath Assoc. (2012) 112(9):596–605. doi: 10.7556/jaoa.2012.112.9.596
59. Teodorczyk-Injeyan JA, Injeyan HS, Ruegg R. Spinal manipulative therapy reduces inflammatory cytokines but not substance P production in normal subjects. J Manipulative Physiol Ther. (2006) 29(1):14–21. doi: 10.1016/j.jmpt.2005.10.002
60. Millard A-L, Valli PV, Stussi G, Mueller NJ, Yung GP, Seebach JD. Brief exercise increases peripheral blood NK cell counts without immediate functional changes, but impairs their responses to ex vivo stimulation. Front Immunol. (2013) 4:125. doi: 10.3389/fimmu.2013.00125
61. Song XJ, Huang ZJ, Song WB, Song XS, Fuhr AF, Rosner AL, et al. Attenuation effect of spinal manipulation on neuropathic and postoperative pain through activating endogenous anti-inflammatory cytokine interleukin 10 in rat spinal cord. J Manipulative Physiol Ther. (2016) 39(1):42–53. doi: 10.1016/j.jmpt.2015.12.004
62. Rapaport MH, Schettler P, Breese C. A preliminary study of the effects of a single session of Swedish massage on hypothalamic-pituitary-adrenal and immune function in normal individuals. J Altern Complement Med. (2010) 16(10):1079–88. doi: 10.1089/acm.2009.0634
63. Light AR, White AT, Hughen RW, Light KC. Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J Pain. (2009) 10(10):1099–112. doi: 10.1016/j.jpain.2009.06.003
64. Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, et al. The physiological regulation of toll-like receptor expression and function in humans. J Physiol. (2005) 563(Pt 3):945–55. doi: 10.1113/jphysiol.2004.081224
65. Azizbeigi K, Azarbayjani MA, Peeri M, Agha-alinejad H, Stannard S. The effect of progressive resistance training on oxidative stress and antioxidant enzyme activity in erythrocytes in untrained men. Int J Sport Nutr Exerc Metab. (2013) 23(3):230–8. doi: 10.1123/ijsnem.23.3.230
66. Teodorczyk-Injeyan JA, McGregor M, Ruegg R, Injeyan HS. Interleukin 2-regulated in vitro antibody production following a single spinal manipulative treatment in normal subjects. Chiropr Osteopat. (2010) 18:26. doi: 10.1186/1746-1340-18-26
67. Neubauer O, Sabapathy S, Lazarus R, Jowett JBM, Desbrow B, Peake JM, et al. Transcriptome analysis of neutrophils after endurance exercise reveals novel signaling mechanisms in the immune response to physiological stress. J Appl Physiol. (2013) 114(12):1677–88. doi: 10.1152/japplphysiol.00143.2013
68. Ghosh S, Lertwattanarak R, Garduno Jde J, Galeana JJ, Li J, Zamarripa F, et al. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci. (2015) 70(2):232–46. doi: 10.1093/gerona/glu067
69. Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, Cuevas MJ, González-Gallego J. TLR4-mediated Blunting of inflammatory responses to eccentric exercise in young women. Mediators Inflamm. (2014) 2014:479395. doi: 10.1155/2014/479395
70. Liao P, Zhou J, Ji LL, Zhang Y. Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. (2010) 298(3):R599–607. doi: 10.1152/ajpregu.00480.2009
71. Radom-Aizik S, Zaldivar FP Jr, Haddad F, Cooper DM. Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun. (2014) 39:121–9. doi: 10.1016/j.bbi.2014.01.003
72. Tanaka Y, Kawanishi N, Shiva D, Tsutsumi N, Uchida M, Kitamura H, et al. Exhaustive exercise reduces tumor necrosis factor-alpha production in response to lipopolysaccharide in mice. Neuroimmunomodulation. (2010) 17(4):279–86. doi: 10.1159/000290044
73. Li H, Geib RW. Exploring the use of five color flow cytometry to examine the effect of acute tai chi practice on pro inflammatory monocyte subtypes—biomed 2013. Biomed Sci Instrum. (2013) 49:209–15.23686202
74. Ortega E, Hinchado MD, Martin-Cordero L, Asea A. The effect of stress-inducible extracellular Hsp72 on human neutrophil chemotaxis: a role during acute intense exercise. Stress. (2009) 12(3):240–9. doi: 10.1080/10253890802309853
75. Connolly PH, Caiozzo VJ, Zaldivar F, Nemet D, Larson J, Hung SP, et al. Effects of exercise on gene expression in human peripheral blood mononuclear cells. J Appl Physiol (1985). (2004) 97(4):1461–9. doi: 10.1152/japplphysiol.00316.2004
76. Spiropoulos A, Goussetis E, Margeli A, Premetis E, Skenderi K, Graphakos S, et al. Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med. (2010) 48(2):199–203. doi: 10.1515/CCLM.2010.034
77. de Gonzalo-Calvo D, Dávalos A, Montero A, García-González Á, Tyshkovska I, González-Medina A, et al. Circulating inflammatory miRNA signature in response to different doses of aerobic exercise. J Appl Physiol (1985). (2015) 119(2):124–34. doi: 10.1152/japplphysiol.00077.2015
78. Stelzer I, Kröpfl JM, Fuchs R, Pekovits K, Mangge H, Raggam RB, et al. Ultra-endurance exercise induces stress and inflammation and affects circulating hematopoietic progenitor cell function. Scand J Med Sci Sports. (2015) 25(5):e442–50. doi: 10.1111/sms.12347
79. Ulven SM, Foss SS, Skjølsvik AM, Stadheim HK, Myhrstad MC, Raael E, et al. An acute bout of exercise modulate the inflammatory response in peripheral blood mononuclear cells in healthy young men. Arch Physiol Biochem. (2015) 121(2):41–9. doi: 10.3109/13813455.2014.1003566
80. Wadley AJ, Chen YW, Lip GY, Fisher JP, Aldred S. Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J Sports Sci. (2016) 34(1):1–9. doi: 10.1080/02640414.2015.1035666
81. Degerstrøm J, Østerud B. Increased inflammatory response of blood cells to repeated bout of endurance exercise. Med Sci Sports Exerc. (2006) 38(7):1297–303. doi: 10.1249/01.mss.0000227315.93351.8d
82. Roy RA, Boucher JP, Comtois AS. Inflammatory response following a short-term course of chiropractic treatment in subjects with and without chronic low back pain. J Chiropr Med. (2010) 9(3):107–14. doi: 10.1016/j.jcm.2010.06.002
83. Machado OAS, Diniz VLS, Passos MEP, de Oliveira HH, Santos-Oliveira LC, Alecrim AL, et al. Physical exercise increases global and gene-specific (interleukin-17 and interferon-γ) DNA methylation in lymphocytes from aged women. Exp Physiol. (2021) 106(9):1878–85. doi: 10.1113/EP089673
84. Sugama K, Suzuki K, Yoshitani K, Shiraishi K, Kometani T. IL-17, neutrophil activation and muscle damage following endurance exercise. Exerc Immunol Rev. (2012) 18:116–27.22876724
85. Ernberg M, Christidis N, Ghafouri B, Bileviciute-Ljungar I, Löfgren M, Bjersing J, et al. Plasma cytokine levels in fibromyalgia and their response to 15 weeks of progressive resistance exercise or relaxation therapy. Mediators Inflamm. (2018) 2018:3985154. doi: 10.1155/2018/3985154
86. Ding Y, Xu X. Anti-inflammatory effect of exercise training through reducing inflammasome activation-related inflammatory cytokine levels in overweight/obese populations: a systematic review and meta-analysis. Complement Ther Clin Pract. (2022) 49:101656. doi: 10.1016/j.ctcp.2022.101656
87. Wołyniec W, Ratkowski W, Renke J, Renke M. Changes in novel AKI biomarkers after exercise. A systematic review. Int J Mol Sci. (2020) 21(16):5673. doi: 10.3390/ijms21165673
88. Dimauro I, Grazioli E, Lisi V, Guidotti F, Fantini C, Antinozzi C, et al. Systemic response of antioxidants, heat shock proteins, and inflammatory biomarkers to short-lasting exercise training in healthy male subjects. Oxid Med Cell Longev. (2021) 2021:1938492. doi: 10.1155/2021/1938492
89. Ramos JS, Dalleck LC, Stennett RC, Mielke GI, Keating SE, Murray L, et al. Effect of different volumes of interval training and continuous exercise on interleukin-22 in adults with metabolic syndrome: a randomized trial. Diabetes Metab Syndr Obes. (2020) 13:2443–53. doi: 10.2147/DMSO.S251567
90. Kanda K, Sugama K, Hayashida H, Sakuma J, Kawakami Y, Miura S, et al. Eccentric exercise-induced delayed-onset muscle soreness and changes in markers of muscle damage and inflammation. Exerc Immunol Rev. (2013) 19:72–85.23977721
91. Teodorczyk-Injeyan JA, Injeyan HS, McGregor M, Harris GM, Ruegg R. Enhancement of in vitro interleukin-2 production in normal subjects following a single spinal manipulative treatment. Chiropr Osteopat. (2008) 16:5. doi: 10.1186/1746-1340-16-5
92. Kovanur-Sampath K, Mani R, Cotter J, Gisselman AS, Tumilty S. Changes in biochemical markers following spinal manipulation-a systematic review and meta-analysis. Musculoskelet Sci Pract. (2017) 29:120–31. doi: 10.1016/j.msksp.2017.04.004
93. Rodriguez-Miguelez P, Lima-Cabello E, Martinez-Florez S, Almar M, Cuevas MJ, Gonzalez-Gallego J. Hypoxia-inducible factor-1 modulates the expression of vascular endothelial growth factor and endothelial nitric oxide synthase induced by eccentric exercise. J Appl Physiol. (2015) 118(8):1075–83. doi: 10.1152/japplphysiol.00780.2014
94. Rosa JC, Lira FS, Eguchi R, Pimentel GD, Venancio DP, Cunha CA, et al. Exhaustive exercise increases inflammatory response via toll like receptor-4 and NF-kappaBp65 pathway in rat adipose tissue. J Cell Physiol. (2011) 226(6):1604–7. doi: 10.1002/jcp.22490
95. Zbinden-Foncea H, Raymackers JM, Deldicque L, Renard P, Francaux M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med Sci Sports Exerc. (2012) 44(8):1463–72. doi: 10.1249/MSS.0b013e31824e0d5d
96. Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, Cuevas MJ, Gonzalez-Gallego J. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J Appl Physiol. (2012) 112(12):2011–8. doi: 10.1152/japplphysiol.01499.2011
97. Nickel T, Emslander I, Sisic Z, David R, Schmaderer C, Marx N, et al. Modulation of dendritic cells and toll-like receptors by marathon running. Eur J Appl Physiol. (2012) 112(5):1699–708. doi: 10.1007/s00421-011-2140-8
98. Nickel T, Hanssen H, Emslander I, Drexel V, Hertel G, Schmidt-Trucksäss A, et al. Immunomodulatory effects of aerobic training in obesity. Mediat Inflamm. (2011) 2011:308965. doi: 10.1155/2011/308965
99. White AT, Light AR, Hughen RW, Vanhaitsma TA, Light KC. Differences in metabolite-detecting, adrenergic, and immune gene expression after moderate exercise in patients with chronic fatigue syndrome, patients with multiple sclerosis, and healthy controls. Psychosom Med. (2012) 74(1):46–54. doi: 10.1097/PSY.0b013e31824152ed
100. Rodriguez-Miguelez P, Fernandez-Gonzalo R, Almar M, Mejias Y, Rivas A, de Paz JA, et al. Role of toll-like receptor 2 and 4 signaling pathways on the inflammatory response to resistance training in elderly subjects. Age (Dordr). (2014) 36(6):9734. doi: 10.1007/s11357-014-9734-0
101. Prestes J, da Cunha Nascimento D, Tibana RA, Teixeira TG, Vieira DC, Tajra V. Understanding the individual responsiveness to resistance training periodization. Age (Dordr). (2015) 37(3):9793. doi: 10.1007/s11357-015-9793-x
102. Jun JK, Lee WL, Park HG, Lee SK, Jeong SH, Lee YR. Moderate intensity exercise inhibits macrophage infiltration and attenuates adipocyte inflammation in ovariectomized rats. J Exerc Nutrition Biochem. (2014) 18(1):119–27. doi: 10.5717/jenb.2014.18.1.119
103. Zheng Q, Cui G, Chen J, Gao H, Wei Y, Uede T, et al. Regular exercise enhances the immune response against microbial antigens through up-regulation of toll-like receptor signaling pathways. Cell Physiol Biochem. (2015) 37(2):735–46. doi: 10.1159/000430391
104. Holland AM, Hyatt HW, Smuder AJ, Sollanek KJ, Morton AB, Roberts MD, et al. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res Notes. (2015) 8:514. doi: 10.1186/s13104-015-1500-6
105. Oliveira M, Gleeson M. The influence of prolonged cycling on monocyte toll-like receptor 2 and 4 expression in healthy men. Eur J Appl Physiol. (2010) 109(2):251–7. doi: 10.1007/s00421-009-1350-9
106. Simpson RJ, McFarlin BK, McSporran C, Spielmann G, Hartaigh B, Guy K. Toll-like receptor expression on classic and pro-inflammatory blood monocytes after acute exercise in humans. Brain Behav Immun. (2009) 23(2):232–9. doi: 10.1016/j.bbi.2008.09.013
107. Azizbeigi K, Azarbayjani MA, Atashak S, Stannard SR. Effect of moderate and high resistance training intensity on indices of inflammatory and oxidative stress. Res Sports Med. (2015) 23(1):73–87. doi: 10.1080/15438627.2014.975807
108. Mucci P, Anselme-Poujol F, Caillaud C, Couret I, Rossi M, Préfaut C. Basophil releasability in young highly trained and older athletes. Med Sci Sports Exerc. (1999) 31(4):507–13. doi: 10.1097/00005768-199904000-00003
109. Alves MDJ, Silva DDS, Pereira EVM, Pereira DD, de Sousa Fernandes MS, Santos DFC, et al. Changes in cytokines concentration following long-distance running: a systematic review and meta-analysis. Front Physiol. (2022) 13:838069. doi: 10.3389/fphys.2022.838069
110. Bove GM, Harris MY, Zhao H, Barbe MF. Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J Neurol Sci. (2016) 361:168–80. doi: 10.1016/j.jns.2015.12.029
111. Hashemi S, Habibagahi Z, Heidari M, Abdollahpour-Alitappeh M, Karimi MH. Effects of combined aerobic and anaerobic exercise training on cytokine profiles in patients with systemic lupus erythematosus (SLE); a randomized controlled trial. Transpl Immunol. (2022) 70:101516. doi: 10.1016/j.trim.2021.101516
112. Della Gatta PA, Garnham AP, Peake JM, Cameron-Smith D. Effect of exercise training on skeletal muscle cytokine expression in the elderly. Brain Behav Immun. (2014) 39:80–6. doi: 10.1016/j.bbi.2014.01.006
113. Uzeloto JS, De Toledo-Arruda AC, Silva BSA, Braz AMM, De Lima FF, Grigoletto I, et al. Effect of physical training on cytokine expression in CD4+ T lymphocytes in subjects with stable COPD. Ther Adv Respir Dis. (2022) 16:175346662210911. doi: 10.1177/17534666221091179
114. Garneau L, Parsons SA, Smith SR, Mulvihill EE, Sparks LM, Aguer C. Plasma myokine concentrations after acute exercise in non-obese and obese sedentary women. Front Physiol. (2020) 11:18. doi: 10.3389/fphys.2020.00018
115. Celestrin CP, Rocha GZ, Stein AM, Guadagnini D, Tadelle RM, Saad MJA, et al. Effects of a four week detraining period on physical, metabolic, and inflammatory profiles of elderly women who regularly participate in a program of strength training. Eur Rev Aging Phys Act. (2020) 17:12. doi: 10.1186/s11556-020-00244-8
116. Plisak U, Szczepaniak J, Żmigrodzka M, Giercuszkiewicz-Hecold B, Witkowska-Piłaszewicz O. Changes in novel anti-infalmmatory cytokine concetration in the bood of endurance and race horses at different levels of training. Comput Struct Biotechnol J. (2023) 21:418–24. doi: 10.1016/j.csbj.2022.12.016
117. Minari ALA, Avila F, Oyama LM, Thomatieli-Santos RV. Skeletal muscles induce recruitment of Ly6C+ macrophage subtypes and release inflammatory cytokines 3 days after downhill exercise. Am J Physiol Regul Integr Comp Physiol. (2019) 317(4):R597–605. doi: 10.1152/ajpregu.00163.2019
118. Frisbee JC, Samora JB, Peterson J, Bryner R. Exercise training blunts microvascular rarefaction in the metabolic syndrome. Am J Physiol Heart Circ Physiol. (2006) 291(5):H2483–92. doi: 10.1152/ajpheart.00566.2006
119. García JJ, Bote E, Hinchado MD, Ortega E. A single session of intense exercise improves the inflammatory response in healthy sedentary women. J Physiol Biochem. (2011) 67:87–94. doi: 10.1007/s13105-010-0052-4
120. Andersson H, Bøhn SK, Raastad T, Paulsen G, Blomhoff R, Kadi F. Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72-h recovery. Scand J Med Sci Sports. (2010) 20(5):740–7. doi: 10.1111/j.1600-0838.2009.00989.x
121. Alizadeh H, Safarzade A. High intensity intermittent training induces anti-inflammatory cytokine responses and improves body composition in overweight adolescent boys. Horm Mol Biol Clin Investig. (2019) 39(3). /j/hmbci.2019.39.issue-3/hmbci-2019-0004/hmbci-2019-0004.xml.31369392
122. Martins FM, Santagnello SB, De Oliveira Junior GN, De Sousa JDFR, Michelin MA, Nomelini RS, et al. Lower-body resistance training reduces interleukin-1β and transforming growth factor-β1 levels and fatigue and increases physical performance in breast cancer survivors. J Strength Cond Res. (2023) 37:439–51. doi: 10.1519/JSC.0000000000004270
123. Czarkowska-Paczek B, Bartlomiejczyk I, Przybylski J. The serum levels of growth factors: PDGF, TGF-beta and VEGF are increased after strenuous physical exercise. J Physiol Pharmacol. (2006) 57:189–97.16845225
124. Dekker J, Nelson K, Kurgan N, Falk B, Josse A, Klentrou P. Wnt signaling–related osteokines and transforming growth factors before and after a single bout of plyometric exercise in child and adolescent females. Pediatr Exerc Sci. (2017) 29:504–12. doi: 10.1123/pes.2017-0042
125. Kolasa-Trela R, Konieczynska M, Bazanek M, Undas A. Specific changes in circulating cytokines and growth factors induced by exercise stress testing in asymptomatic aortic valve stenosis. PLoS One. (2017) 12:e0173787. doi: 10.1371/journal.pone.0173787
126. Czarkowska-Paczek B, Zendzian-Piotrowska M, Bartlomiejczyk I, Przybylski J, Gorski J. The influence of physical exercise on the generation of TGF-β1, PDGF-AA, and VEGF-A in adipose tissue. Eur J Appl Physiol. (2011) 111:875–81. doi: 10.1007/s00421-010-1693-2
127. Silva VRR, Lenhare L, Katashima CK, Morari J, M-Assis A, Gaspar RS, et al. TGF-β1 downregulation in the hypothalamus of obese mice through acute exercise. J Cell Biochem. (2019) 120(10):18186–92. doi: 10.1002/jcb.29124
128. Hering J, Schulz H, Schatz A. Circulating transforming growth factor β1 (TGFβ1) is elevated by extensive exercise. Eur J Appl Physiol. (2002) 86:406–10. doi: 10.1007/s00421-001-0537-5
129. Han AJ, Alexander LC, Huebner JL, Reed AB, Kraus VB. Increase in free and total plasma TGF-β1 following physical activity. Cartilage. (2021) 13:1741S–8S. doi: 10.1177/1947603520916523
130. Heinemeier K, Langberg H, Kjaer M. Exercise-induced changes in circulating levels of transforming growth factor-β-1 in humans: methodological considerations. Eur J Appl Physiol. (2003) 90:171–7. doi: 10.1007/s00421-003-0881-8
131. Calderone A, Murphy RJL, Lavoie J, Colombo F, Béliveau L. TGF-β 1 and prepro-ANP mRNAs are differentially regulated in exercise-induced cardiac hypertrophy. J Applied Physiology. (2001) 91:771–6. doi: 10.1152/jappl.2001.91.2.771
132. Rosa L, Teixeira A, Lira F, Tufik S, Mello M, Santos R. Moderate acute exercise (70% VO 2 peak) induces TGF-β, α-amylase and IgA in saliva during recovery. Oral Dis. (2014) 20:186–90. doi: 10.1111/odi.12088
133. Touvra A-M, Volaklis K, Spassis A, Zois C, Douda H, Kotsa K, Tokmakidis S. Combined strength and aerobic training increases transforming growth factor-β1 in patients with type 2 diabetes. Hormones (Athens). 2011;10:125–30. doi: 10.14310/horm.2002.1302
134. Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation in women. J Applied Physiology. (2020) 129:1493–504. doi: 10.1152/japplphysiol.00655.2020
135. Kim J, Lee J. Plasma MMP-9, TIMP-1, and TGF-β1 responses to exercise-induced muscle injury. IJERPH. (2020) 17:566. doi: 10.3390/ijerph17020566
136. Eka Widiastuti IA, Arsyad A, Idris I, Patellongi I, Kadriyan H, Buanayuda GW, et al. Exercise adaptations and TGF-β1 levels in recreational cyclists. Ann Med Surg. (2021) 70:102872. doi: 10.1016/j.amsu.2021.102872
137. Cui X, Wang K, Zhang J, Cao Z-B. Aerobic exercise ameliorates myocardial fibrosis via affecting vitamin D receptor and transforming growth factor-β1 signaling in vitamin D-deficient mice. Nutrients. (2023) 15:741. doi: 10.3390/nu15030741
138. Silva VRR, Katashima CK, Lenhare L, Silva CGB, Morari J, Camargo RL, et al. Chronic exercise reduces hypothalamic transforming growth factor-β1 in middle-aged obese mice. Aging (Albany NY). (2017) 9(8):1926–40. doi: 10.18632/aging.101281
139. Baria MR, Miller MM, Borchers J, Desmond S, Onate J, Magnussen R, et al. High intensity interval exercise increases platelet and transforming growth factor-β yield in PLATELET-RICH plasma. PM&R. (2020) 12:1244–50. doi: 10.1002/pmrj.12368
140. Aicher BO, Zhang J, Muratoglu SC, Galisteo R, Arai AL, Gray VL, et al. Moderate aerobic exercise prevents matrix degradation and death in a mouse model of aortic dissection and aneurysm. Am J Physiol Heart Circ Physiol. (2021) 320:H1786–801. doi: 10.1152/ajpheart.00229.2020
141. Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol. (2020) 128:87–99. doi: 10.1152/japplphysiol.00495.2019
142. Ma Y, Kuang Y, Bo W, Liang Q, Zhu W, Cai M, et al. Exercise training alleviates cardiac fibrosis through increasing fibroblast growth factor 21 and regulating TGF-β1-Smad2/3-MMP2/9 signaling in mice with myocardial infarction. IJMS. (2021) 22:12341. doi: 10.3390/ijms222212341
143. Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. (2017) 17:21–9. doi: 10.1038/nri.2016.125
144. Deak T, Quinn M, Cidlowski JA, Victoria NC, Murphy AZ, Sheridan JF. Neuroimmune mechanisms of stress: sex differences, developmental plasticity, and implications for pharmacotherapy of stress-related disease. Stress. (2015) 18:367–80. doi: 10.3109/10253890.2015.1053451
145. Carr EJ, Dooley J, Garcia-Perez JE, Lagou V, Lee JC, Wouters C, et al. The cellular composition of the human immune system isshaped by age and cohabitation. Nat Immunol. (2016) 17:461–8. doi: 10.1038/ni.3371
146. Taishi P, Chen Z, Obál F, Hansen MK, Zhang J, Fang J, et al. Sleep-associated changes in interleukin-1β mRNA in the brain. J Interferon Cytokine Res. (1998) 18:793–8. doi: 10.1089/jir.1998.18.793
147. Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. (2016) 80:40–52. doi: 10.1016/j.biopsych.2015.05.014
148. Wright KP, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. (2015) 47:24–34. doi: 10.1016/j.bbi.2015.01.004
149. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. (2011) 12:5–9. doi: 10.1038/ni0111-5
150. Childs , Calder , Miles . Diet and immune function. Nutrients. (2019) 11:1933. doi: 10.3390/nu11081933
151. Renna ME, O’Toole MS, Spaeth PE, Lekander M, Mennin DS. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress Anxiety. (2018) 35:1081–94. doi: 10.1002/da.22790
152. O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin M-T, O’Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion–biology relationships. Brain Behav Immun. (2010) 24:1074–7. doi: 10.1016/j.bbi.2010.03.003
153. Murphy TM, O’Donovan A, Mullins N, O’Farrelly C, McCann A, Malone K. Anxiety is associated with higher levels of global DNA methylation and altered expression of epigenetic and interleukin-6 genes. Psychiatr Genet. (2015) 25:71–8. doi: 10.1097/YPG.0000000000000055
154. Maydych V. The interplay between stress, inflammation, and emotional attention: relevance for depression. Front Neurosci. (2019) 13:384. doi: 10.3389/fnins.2019.00384
155. Li Y-C, Chou Y-C, Chen H-C, Lu C-C, Chang D-M. Interleukin-6 and interleukin-17 are related to depression in patients with rheumatoid arthritis. Int J Rheum Dis. (2019) 22:980–5. doi: 10.1111/1756-185X.13529
156. McMurray KMJ, Sah R. Neuroimmune mechanisms in fear and panic pathophysiology. Front Psychiatry. (2022) 13:1015349. doi: 10.3389/fpsyt.2022.1015349
157. Anna G-M, Joanna T, Paulina R, Jadwiga S, Jan B. Effect of prior stress on interleukin-1β and HPA axis responses to acute stress. Pharmacol Rep. (2011) 63:1393–403. doi: 10.1016/S1734-1140(11)70703-4
158. Tian R, Hou G, Li D, Yuan T-F. A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health. Sci World J. (2014) 2014:1–8.
159. Pacheco-López G, Engler H, Niemi M-B, Schedlowski M. Expectations and associations that heal: immunomodulatory placebo effects and its neurobiology. Brain Behav Immun. (2006) 20:430–46. doi: 10.1016/j.bbi.2006.05.003
160. Koelsch S, Boehlig A, Hohenadel M, Nitsche I, Bauer K, Sack U. The impact of acute stress on hormones and cytokines and how their recovery is affected by music-evoked positive mood. Sci Rep. (2016) 6:23008. doi: 10.1038/srep23008
161. Clydesdale GJ, Dandie GW, Muller HK. Ultraviolet light induced injury: immunological and inflammatory effects. Immunol Cell Biol. (2001) 79:547–68. doi: 10.1046/j.1440-1711.2001.01047.x
162. Perret J, Bowatte G, Lodge C, Knibbs L, Gurrin L, Kandane-Rathnayake R, et al. The dose–response association between nitrogen dioxide exposure and serum interleukin-6 concentrations. Int J Mol Sci. (2017) 18:1015. doi: 10.3390/ijms18051015
163. Pinho-Ribeiro FA, Verri WA, Chiu IM. Nociceptor sensory neuron–immune interactions in pain and inflammation. Trends Immunol. (2017) 38:5–19. doi: 10.1016/j.it.2016.10.001
164. Wang L, Liu F, Luo Y, Zhu L, Li G. Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats. Biomed Rep. (2015) 3:425–9. doi: 10.3892/br.2015.445
165. Vedova CD, Cathcart S, Dohnalek A, Lee V, Hutchinson MR, Immink MA, et al. Peripheral interleukin-1β levels are elevated in chronic tension-type headache patients. Pain Res Manag. (2013) 18:301–6. doi: 10.1155/2013/796161
166. Kosek E, Altawil R, Kadetoff D, Finn A, Westman M, Le Maître E, et al. Evidence of different mediators of central inflammation in dysfunctional and inflammatory pain — interleukin-8 in fibromyalgia and interleukin-1β in rheumatoid arthritis. J Neuroimmunol. (2015) 280:49–55. doi: 10.1016/j.jneuroim.2015.02.002
167. Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. (2012) 23:85–97. doi: 10.1016/j.cytogfr.2012.04.001
168. Hilker M, Schwachtje J, Baier M, Balazadeh S, Bäurle I, Geiselhardt S, et al. Priming and memory of stress responses in organisms lacking a nervous system: priming and memory of stress responses. Biol Rev. (2016) 91:1118–33. doi: 10.1111/brv.12215
169. Saeidnia S, Manayi A, Abdollahi M. From in vitro experiments to in vivo and clinical studies; pros and cons. Curr Drug Discov Technol. (2016) 12:218–24. doi: 10.2174/1570163813666160114093140
170. Ichihara M, Hara T, Kim H, Murate T, Miyajima A. Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood. (1997) 90:165–73. doi: 10.1182/blood.V90.1.165.165_165_173
171. Molina PE. Neurobiology of the stress response: contributions of the sympathetic nervous system to the neuroimmune axis in traumatic injury. Shock. (2005) 24:3–10. doi: 10.1097/01.shk.0000167112.18871.5c
172. Gomarasca M, Banfi G, Lombardi G. Myokines: the endocrine coupling of skeletal muscle and bone. Adv Clin Chem. (2020) 94:155–218. Elsevier. doi: 10.1016/bs.acc.2019.07.010
173. Tsigos C, Kyrou I, Kassi E, Chrousos GP. Stress: Endocrine Physiology and Pathophysiology. [Updated 2020 Oct 17]. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al., editors. Endotext [Internet]. South Dartmouth, MA: MDText.com, Inc (2000). PMID: 25905226.
174. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. (2019) 19:433–47. doi: 10.1038/s41577-019-0147-2
175. Wehner R, Dietze K, Bachmann M, Schmitz M. The bidirectional crosstalk between human dendritic cells and natural killer cells. J Innate Immun. (2011) 3:258–63. doi: 10.1159/000323923
176. Zhang H, Wu L-M, Wu J. Cross-talk between apolipoprotein E and cytokines. Mediators Inflamm. (2011) 2011:1–10.
177. Newman G, Gonzalez-Perez RR. Leptin–cytokine crosstalk in breast cancer. Mol Cell Endocrinol. (2014) 382:570–82. doi: 10.1016/j.mce.2013.03.025
178. Chiu IM, Von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. (2012) 15:1063–7. doi: 10.1038/nn.3144
179. Kennedy RH, Silver R. Neuroimmune signaling: cytokines and the CNS. In: Pfaff DW, Volkow ND, editors. Neuroscience in the 21st Century. New York, NY: Springer New York (2015). p. 1–41.
Keywords: precision medicine, pain, cytokines, manual therapy, exercise, musculoskeletal
Citation: Cook CE, Keter D, Cade WT, Winkelstein BA and Reed WR (2024) Manual therapy and exercise effects on inflammatory cytokines: a narrative overview. Front. Rehabil. Sci. 5:1305925. doi: 10.3389/fresc.2024.1305925
Received: 29 November 2023; Accepted: 12 April 2024;
Published: 30 April 2024.
Edited by:
Mallikarjuna Korivi, Zhejiang Normal University, ChinaReviewed by:
Elen H. Miyabara, University of São Paulo, BrazilKesava Kovanur Sampath, Waikato Institute of Technology, New Zealand
© 2024 Cook, Keter, Cade, Winkelstein and Reed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chad E. Cook Y2hhZC5jb29rQGR1a2UuZWR1
 Chad E. Cook
Chad E. Cook Damian Keter4
Damian Keter4 William Todd Cade
William Todd Cade William R. Reed
William R. Reed