
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Rehabil. Sci. , 08 January 2024
Sec. Rehabilitation in Neurological Conditions
Volume 4 - 2023 | https://doi.org/10.3389/fresc.2023.1258737
This article is part of the Research Topic Preventive and Therapeutic Potential of Physical Exercise in Neurodegenerative Diseases View all 5 articles
Background and purpose: Impaired sensorimotor function, reduced physical activity and unemployment are common challenges in persons with multiple sclerosis (pwMS), even when disability is low. CoreDISTparticipation is a new, multidisciplinary intervention delivered across healthcare levels systematically addressing these elements. This study primarily aimed to evaluate the feasibility of CoreDISTparticipation in terms of process, resources, management, and scientific outcomes. The secondary aim was to evaluate initial efficacy in terms of possible short-term effects compared with the usual care on barriers to employment, balance, walking, health-related quality of life (HRQoL), and physical activity.
Methods: This assessor-blinded prospective pilot randomized controlled trial included 29 pwMS [Expanded Disability Status Scale (EDSS): 0–3.5] randomly allocated to the intervention group (CoreDISTparticipation) (n = 15) or usual care (n = 14). CoreDISTparticipation consists of three phases: (1) hospital outpatient clinic: MS nurse work-focused session and physiotherapist exploring balance; (2) municipality: a digital meeting with pwMS, employer, MS nurse, and physiotherapist addressing employment and physical activity, 4 weeks indoor CoreDIST balance training (60 min × 2/week); and (3) 4 weeks outdoor CoreDIST balance training and high-intensity running/walking (60 min × 2/week). Assessments were undertaken at baseline and at weeks 6 and 11. Primary feasibility metric outcomes were the reporting of process, resources, management, and scientific outcomes. Efficacy measures included evaluation of the Multiple Sclerosis Work Difficulties Questionnaire-23 Norwegian Version (MSWDQ-23NV) and 6 Minute Walk-test as well as the Trunk Impairment Scale-modified Norwegian Version, Mini-Balance Evaluation Systems Test (Mini-BESTest), Multiple Sclerosis Walking Scale-12, Multiple Sclerosis Impact Scale-29 Norwegian Version (MSIS-29NV), ActiGraph wGT3x-BT monitors, and AccuGait Optimized force platform. The statistical analyses included repeated-measures mixed models performed in IBM SPSS Version 29.
Results: The primary feasibility metric outcomes demonstrated the need for minor adjustments in regard to the content of the intervention and increasing the number of staff. In regard to the efficacy measures, one person attended no postintervention assessments and was excluded, leaving 28 participants (mean EDSS: 1.8, SD: 1). The mean percentage employment was 46.3 (SD: 35.6) and 65.4 (SD: 39.3) in the CoreDISTparticipation and usual care group, respectively. No between-group differences were found. MSWDQ-23NV demonstrated a within-group difference of 5.7 points from baseline to Week 11 (P = 0.004; confidence interval: 2.2–9.3). Mini-BESTest and MSIS-29NV demonstrated within-group differences. The study is registered in ClinicalTrials.gov (Identifier: NCT05057338).
Discussion: The CoreDISTparticipation intervention is feasible to support pwMS when the identified feasibility metric outcomes in regard to process, resource, management, and scientific outcome metrics are adjusted to improve feasibility. Regarding efficacy measures, no between-group differences were detected; however, within-group differences in barriers to employment, balance, and HRQoL were detected for the CoreDISTparticipation group. A larger comparative trial is needed to explore between-group differences and should accurately and precisely define usual care and address the identified limitations of this study.
Multiple sclerosis (MS) is a chronic, neurological disease (1) that affects adults and children at any age, with a mean age of diagnosis of 32 years (2). The disease often follows an unpredictable and fluctuating course with accumulation of sensorimotor disturbances, balance and walking problems, fatigue, and cognitive problems (3–7). All these challenges, which are common even in the early stages, are associated with low levels of physical activity, impaired health-related quality of life (HRQoL), unemployment or reduced positions, and are accompanied by substantial personal burdens and societal costs (8, 9). Of Concern, only approximately half of individuals living with MS and mild disability seem to be meeting the current physical activity guidelines (10), and unemployment is reported in 55%–70% of persons with MS (pwMS) (11–13). Globally, 43% of pwMS leave their jobs within the first 3 years after diagnosis, and 70% quit within 10 years (9). These reports demonstrate that physical activity and employment are of major importance for people with MS (14) and should be monitored and addressed from the very start of the diagnosis (15).
Factors associated with pwMS staying employed are having low levels of fatigue and few mobility-related symptoms (12, 14, 16). Physical activity, physiotherapy, and exercise can reduce fatigue (17, 18), improve balance, walking (19, 20), and HRQoL (18, 21), and may also improve neuromuscular and physical functioning in pwMS (22). It is recommended to start exercising from the start of the diagnosis (23). This early phase, when disability is often mild and neuroplasticity is optimal, is the best window of opportunity to optimize sensorimotor function (22, 24), create good habits with regard to physical activity, and a basis for optimizing sustained employment. Physical activity levels lower than the recommended minimum of 150–300 min of moderate training, exercise, or physical activity per week (23, 25, 26) are common both in the MS population in general (27, 28) and in individuals with mild disability (29). The self-reported dose and intensity of physical activity have even decreased during the COVID-19-pandemic (30).
To meet these complex challenges regarding sensorimotor function, physical activity, and work, we have developed a comprehensive multidisciplinary intervention and pathway delivered across healthcare levels, targeting the promotion of balance, walking, physical activity, and work participation. This new, individualized, group-based intervention, entitled CoreDISTparticipation, is built on GroupCoreDIST (31), which has previously appeared to be feasible (32), effective in improving balance, trunk control (29), and walking (33), and meaningful among ambulant pwMS (34) and physiotherapists (35–37). The GroupCoreDIST intervention focused on prerequisites for postural control and balance and was undertaken in an indoor environment for 60 min, three times per week for 6 weeks. CoreDIST stands for the coordinated relationship between the proximal and distal areas of the body, as core/trunk muscle activation coordinated with activity in the extremities is important for postural control during balance, walking, and daily activities (38). Furthermore, CoreDIST emphasizes elements of importance for motor learning, such as high dose (D), dual task (D) individualization (I), (high) intensity (I) and insights/meaning (I), as well as addressing underlying prerequisites for postural control, such as somatosensory function (S), selective movement (S), muscle length, and advanced balance challenges in a task-oriented training format (T). The new intervention, CoreDISTparticipation, links specialist and municipal healthcare services and includes the person with MS and their employer. The group training has been expanded to include 4 weeks of indoor GroupCoreDIST training followed by 4 weeks of outdoor training. The new elements in CoreDISTparticipation are as follows: (1) an assessment with a physiotherapist at the hospitals MS outpatient (MS-OP) clinic assessing balance and potential for change in sensorimotor function, (2) a work-related session with an MS nurse at the MS-OP clinic, (3) a digital multidisciplinary meeting with the pwMS, their employer, the municipal physiotherapist, and the MS nurse, (4) outdoor CoreDIST sessions integrating balance and high-intensity interval training, and (5) digital home-exercise videos. To the best of our knowledge, follow-ups integrating work, physical activity, and combinations of high-intensity training and specific sensorimotor functions have not been previously explored. Therefore, the primary aim of this study was to evaluate the feasibility of the new intervention, CoreDISTparticipation, in terms of process, resources, management, and scientific outcomes. The secondary aim was to evaluate initial efficacy in terms of possible short-term effects on barriers to work, walking distance, physical activity, balance, and HRQoL. Evaluating various feasibility metrics in preparation for a subsequent large-scale study is in line with current recommendations (39, 40). We posed the following research question: What are the feasibility and short-term preliminary effects of CoreDISTparticipation compared with the usual care on barriers to employment, balance, walking, quality of life, and physical activity in individuals with MS having lower levels of disability?
This two-armed prospective, assessor-blinded pilot feasibility randomized controlled trial (RCT) included 29 individuals with mild to moderate disability due to MS measured by the Expanded Disability Status Scale (41) (EDSS ≤ 3.5).
The study was approved by the Regional Committee for Medical Research Ethics, North Norway (Grant number 174837) and the Local Ethical Committee at the Nordland Hospital Trust (NLSH) (Project number 209). It was registered in ClinicalTrials.gov (Identifier: NCT05057338) and was conducted in accordance with the Helsinki Declaration. All participants provided written informed consent prior to inclusion. The study was funded by the Northern Norwegian Regional Health Authorities (Grant number 174837). The funder played no role in the design, conduct, or reporting of the study. The CONSORT guidelines (42) were followed throughout the study.
The study was conducted between August and November 2021 at NLSH's (regional hospital) MS-OP clinic in cooperation with physiotherapists in two municipalities (50,000 and 10,000 inhabitants, respectively) (Figure 1, flow chart). The project group consisted of three user representatives from the Nordland MS Association, an MS nurse, a neurologist, and three physiotherapists.
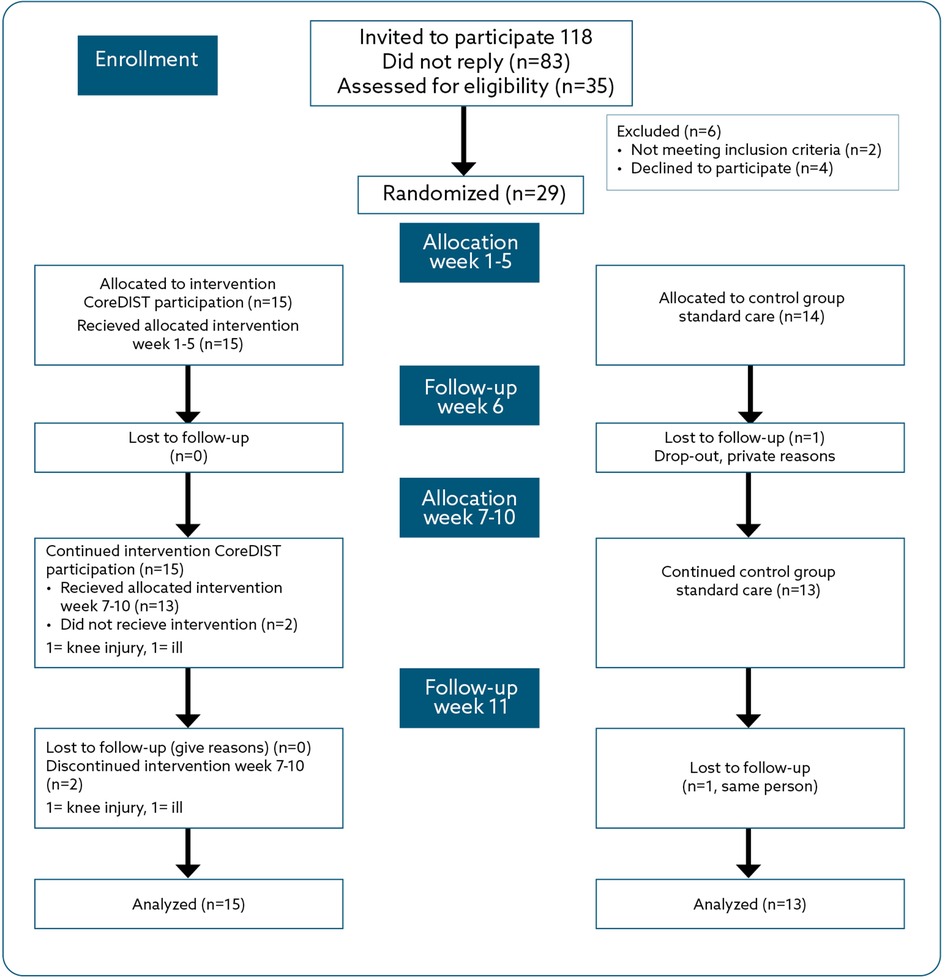
Figure 1. Flow chart for enrolment, allocation, and follow-up for the CoreDISTparticipation and usual care groups.
One physiotherapist, a specialist in neurological physiotherapy, who was adequately trained in the assessment procedures and blinded to group allocation, performed all assessments. One physiotherapist at the MS-OP clinic conducted the first assessment. An experienced MS nurse took part in the digital meetings. Four physiotherapists, two specialists in neurological physiotherapy, and two generalists led the training groups. These followed 2 days of practical and theoretical training and 2 h of digital education in the CoreDISTparticipation program. Guidelines for the first assessment, the MS nurse meeting regarding work, and the multidisciplinary digital meeting were developed by the project group. The user representatives took part in the planning, implementation, discussion of results, and evaluation of the study.
In July 2021, written information regarding the study was sent by post from the MS-OP clinic to the pwMS who were registered at the Nordland Hospital Trust, lived in the two selected municipalities, and had EDSS ≤ 3.5 (n = 118). One reminder was sent to ensure maximum patient enrolment, and 35 individuals replied with signed informed consent. We had limited information regarding how many of the 118 invited persons were employed; therefore, we expected a low response rate. Enrolment started in August 2021, and follow-up assessments were completed in November 2021, organized by the MS-OP clinic. At enrolment, the EDSS values of all participants were recorded from their hospital journals. The inclusion criteria were a diagnosis of MS according to McDonald's criteria (43), EDSS ≤3.5 (no disability/fully ambulatory with moderate disability), 18 years or older, and being employed (10%–100%). The exclusion criteria were pregnancy at enrolment, exacerbation within 2 weeks prior to enrolment, and other serious conditions compromising balance, walking, or work capacity, such as recently surviving a stroke. Of the 35 participants who consented to participate, six were excluded based on the following: four withdrew their consent based on lack of time or other personal reasons, one had an EDSS higher than 3.5, and one turned out not to have MS. This left a sample of 29 pwMS.
After baseline assessments, the participants were randomized to CoreDISTparticipation (intervention group) or usual care (control group) in a 1:1 relationship. We stratified for municipality to ensure possibilities for group training using the Research Electronic Data Capture Tool (performed by ECA), supported by the Clinical Research Department, University Hospital of Northern Norway.
Both groups continued their regular medical treatment and were encouraged to stay employed and to be physically active.
CoreDISTparticipation is a further development of GroupCoreDIST developed by Normann and Arntzen and I-CoreDIST developed by Normann, Sivertsen, and Arntzen (32, 44). Norman and Arntzen further developed the CoreDISTparticipation, and S.S. Haakonsen Dahl contributed to developing the outdoor training. The TIDieR author tool has been used in the development of the new intervention (45). The CoreDISTparticipation consists of three phases and is further described in the Appendix.
The usual care group continued routine consultations at the MS-OP clinic with the usual follow-up in the municipality, including physiotherapy, physical activity, or other follow-up.
The primary feasibility metric outcome measures were registered by the researchers, assessors, clinicians, and participants in the study. These included (1) process: recruitment rates, retention rates, appropriateness of eligibility criteria, participants compliance with protocol, indoor and outdoor sessions, digital meetings with the MS nurse, digital multidisciplinary meetings, and participants reactions to assessment; (2) resources: time from sending out invitations to response, time for clinician to learn the intervention, time for assessment of outcome measures and for patients to fill out questionnaires, budget, intervention sustainability within the proposed setting, staff training needs, and equipment access; (3) management: personnel, data management, resource site capacity, assessment capacity, MS outpatient clinic MS nurse and physiotherapist capacity, including municipal physiotherapists, equipment usage for digital meetings, meeting links sent from the hospital, data processing time, and software appropriateness; and (4) scientific metrics: safety, outcome measures, content of intervention of the indoor and outdoor training, dose and intensity of the intervention, fidelity of the intervention (therapists), patients’ acceptance of the intervention and tolerance to the protocol, and potential participant bias. All metrics were discussed in the research group during and after the study was completed.
At baseline, demographic characteristics were registered: age, gender, marital status, years of education, current employment status, how much the participants wanted to work if the job was perfectly arranged for them, type and duration of MS, EDSS, smoking, and medications. The assessments were undertaken by a blinded, independent tester at baseline, Follow-up 1 (Week 6), and Follow-up 2 (Week 11) (Figure 1, flow chart). The secondary feasibility metrics were the efficacy measures that were considered to be the most relevant for the aim of the captured employment-related barriers through the Multiple Sclerosis Work Difficulties Questionnaire-23 Norwegian Version (MSWDQ-23NV) and walking capacity [6-Min Walk Test (6MWT)]. The MSWDQ-23NV is a 23-item questionnaire that measures how frequently a pwMS perceives psychological/cognitive (11 items), physical (eight items), and external barriers (four items) related to their current or latest job. It is scored on a five-point scale (best score = 0) (46). The MSWDQ-23NV has recently been translated into Norwegian and is currently undergoing research evaluation for validity and responsiveness. The 6MWT is a valid and reliable outcome in pwMS that measures the distance within 6 min of walking in a 25 m long hallway (47). The minimal clinically important difference for pwMS is calculated to be 19.7 m for improvement (48).
The other efficacy outcome measures were the Trunk Impairment Scale-modified Norwegian Version (TIS-modNV), which records dynamic trunk control and sitting balance. It has a 0–16 rating scale, is valid for individuals with stroke, has been frequently used in the MS population, and is currently being validated (ClinicalTrials.gov Identifier: NCT05057338). The Mini-Balance Evaluation Systems Test (Mini-BESTest) measures pro- and reactive balance in sit to stand, standing, and walking. There are 14 items, each scored on a three-point scale with a top score of 28. The Mini-BESTest is valid and reliable for pwMS (49). The AccuGait Optimized force platform measures postural control in the form of symmetry/asymmetry of weight-bearing in standing, postural sway of centre of pressure (COP) as the participant stands on the platform with feet close together and at hip-width with eyes open and closed. Data on COP displacements in centimetres were collected for 30 s with a frequency of 50 Hz in the domains eyes open and eyes closed, and root mean square (RMS) values of the COP displacements were calculated (50). ActiGraph wGT3x-BT monitors measure physical activity levels (inactive, light, moderate, vigorous) and number of steps (51). The activity monitor was worn in a belt around the waist for seven consecutive days after all assessment points. The ActiGraph is an objective measure of community ambulation and physical activity in pwMS (52).
The EQ-5D-3l (European Quality of Life 5-Dimension-3-Level) measures self-perceived HRQoL regarding five domains, each with three items, and a VAS scale (0–100) recording perceived health (53). The MS Impact Scale 29-Norwegian Version (MSIS-29NV) measures self-perceived physical (20 items) and psychological (nine items) impact of MS on HRQoL recorded by a five-point scale (54). The MSIS-29NV is valid and reliable in Norwegian pwMS (55). Eight points is considered a minimal clinically important difference in pwMS (56). The MS Walking Scale-12 (MSWS-12) measures self-perceived limitations in walking due to MS on 12 items with a scale of 1–5. The MSWS-12 is valid and reliable in pwMS (57, 58). A change of −0.7 points from the patient perspective and −10.7 points from the therapist perspective is considered clinically meaningful in pwMS having mild to moderate disability (EDSS ≤ 4) (59).
After the in- and outdoor trainings, the participants scored the Borg scale regarding intensity of the training. During the outdoor training, some participants wore pulse belts and watches (some wore their own, and the municipal physiotherapists lent out three watches) to monitor the intensity of the training. As these watches were neither calibrated nor validated and not worn by all participants, the results were used only to monitor the trainings.
The sample size was based on prior literature where 20–40 participants are recommended for pilot studies to enable estimates of standard deviations to calculate sample size for subsequent large-scale studies (60).
IBM SPSS version 29 (IBM, Armonk, NY, USA) was used, and the intention-to-treat principle was applied for all analyses. Descriptive statistics were used to clarify the clinical and demographic characteristics of the sample, baseline measurements, and recordings at all time-points. Linear regression models were applied to examine the score differences between groups adjusted for baseline score in all outcomes at each follow-up. Linear mixed models were used to examine between-group differences over time and overall between-group differences at follow-up adjusted for baseline scores, where the term overall refers to the mean of the outcome values at Weeks 6 and 11. We used the Bayesian Information Criterion to select the appropriate statistical models. The model assumptions were assessed by residual plots and considered sufficiently fulfilled. All time-points were included in one model. The results from the primary outcomes will serve as a basis for sample size calculations and for adjustments of CoreDISTparticipation when planning a subsequent large-scale study.
The clinical and demographic characteristics for all participants are presented in Table 1. Out of 118 pwMS contacted, 35 responded with informed consent, which is a response rate of 34%. After screening in relation to inclusion and exclusion criteria, a sample of 29 pwMS took part. Regarding trial completion, one person in the control group did not attend any follow-up assessments and hence was excluded, which meant that 28 pwMS completed the whole trial. The primary feasibility metrics in terms of process, resources, and management are presented in Table 2. The attendance was high for indoor GroupCoreDIST, with an 85% attendance rate (mean 6.8 sessions of eight possible sessions per person). The attendance for outdoor sessions was moderate and ranged from 0 to 8 sessions, with a mean of 4.6 sessions per person and altogether 57.3% attendance. There was a 100% attendance for the digital meetings with the MS nurse and the multidisciplinary meetings. There were no reports of new relapses or adverse events during assessments. During high-intensity running/walking, three adverse reactions were reported; a hamstring strain, an ankle strain, and increased dizziness. A fourth person felt unsecure about reaching a high pulse because of heart medications. No new relapses were reported during the study period.
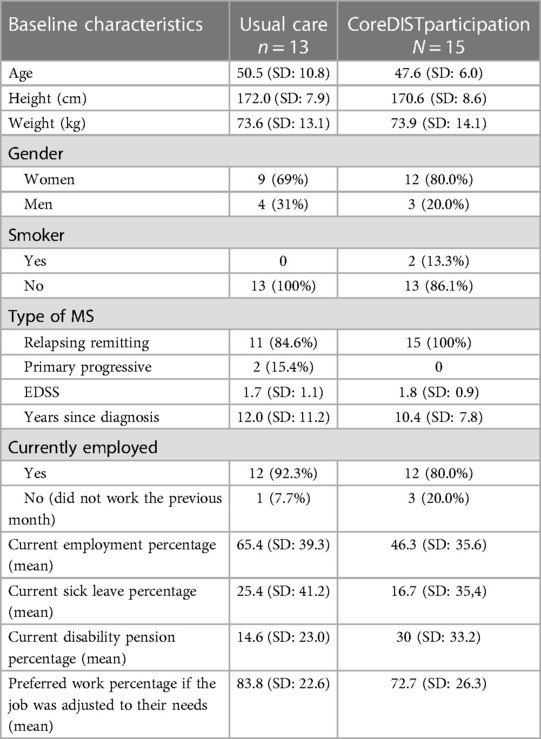
Table 1. Baseline clinical and demographic characteristics of the participants in the CoreDISTparticipation and usual care group as measured by means, mean percentage, and standard deviation.
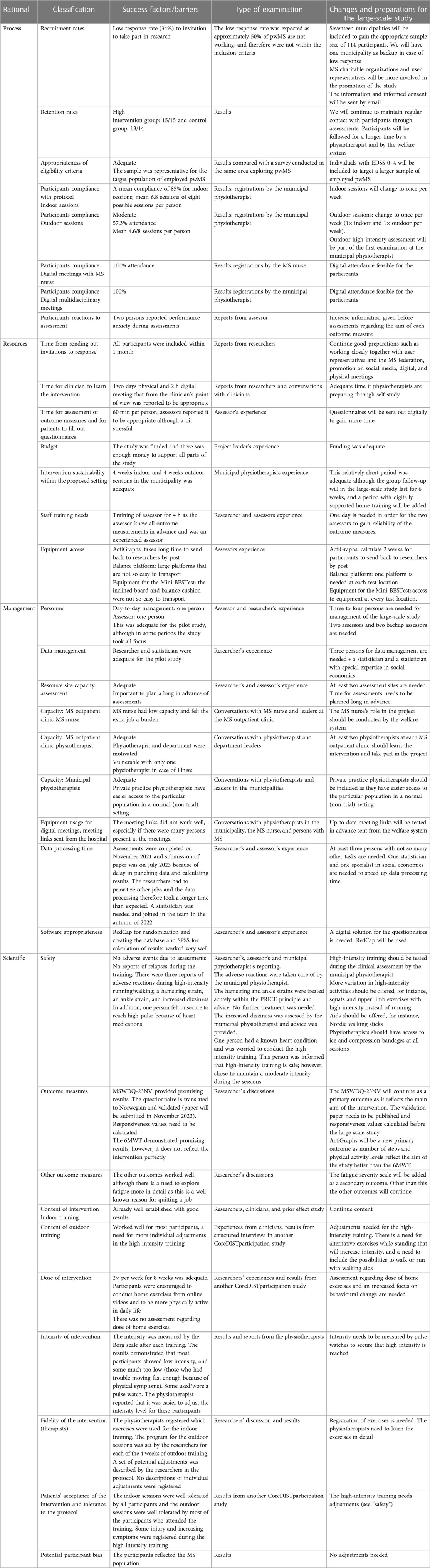
Table 2. Primary feasibility metrics in terms of process, resources, management, and safety with reflection on changes needed to improve for a future trial.
The main findings were that the primary feasibility metrics were adequate. However, there is a need for adequate access to equipment at all test locations and to increase the number of personnel especially with regard to data management. The MS nurse reported a lack of capacity and inadequate software for digital meetings. In addition, the content of the outdoor sessions would benefit from revision. These details are presented in Table 2.
The efficacy outcome measures in regard to short-term effects are presented in Table 3. There were no differences between the CoreDISTparticipation and usual care group with regard to clinical and disease characteristics at baseline. The EDSS levels were low in both groups, with a mean of 1.7 (SD: 1.1) in the intervention group and 1.8 (SD: 0.9) in the control group. The mean percentage of employees was 46.3 (SD: 35.6) in the CoreDISTparticipation group and 65.4 (SD: 39.3) in the usual care group the previous month. When asked about desired work percent the participants would prefer if the job was perfectly arranged for them, the intervention group responded with a mean of 72.7% (SD: 26.3), and the standard care group responded with a mean of 83.8% (SD: 22.6). No significant interaction effects were observed.
At Follow-up one (6 weeks), all the remaining participants attended the assessments. One person only attended one (because of knee injury), and another attended no outdoor training sessions (because of illness). The same two individuals did not complete the 6MWT and the ActiGraph outcome measures at Week 11.
The efficacy measures demonstrated no significant between-group differences between the CoreDISTparticipation and the control group. The results for the MSWDQ-23NV and 6MWT are presented in Figures 2, 3. The CoreDISTparticipation group demonstrated within-group changes in the MSWDQ-23NV [mean difference from baseline to Week 11 of 5.7 points, P = 0.004; confidence interval (CI): 2.2–9.3] and a non-significant tendency for change in the 6MWT from 597.3 m (SD: 72.6) at baseline to 611.9 m (SD: 90.7) at Week 11. Both the intervention (mean 14.5, SD: 1.5) and control group (mean 13.2; SD: 1.9) scored high at baseline on the TIS-modNV, which demonstrated a ceiling effect for this outcome (top score of 16). Mini-BESTest demonstrated within-group differences from baseline to Week 11 in both the CoreDISTparticipation (mean difference 1.5, P = 0.001, CI: −2.3 to −0.7) and the control group (mean difference 1.6, P = 0.003, CI: −2.5 to 0.7). The MSIS-29NV showed a within-group difference from baseline to Week 6 (mean difference 6.3 points, P = 0.006, CI: 2.1–10.5) in the CoreDISTparticipation group. Self-reported walking (MSWS-12), force platform, the EQ-5D-3l, and ActiGraphs demonstrated no changes. ActiGraphs revealed as much as 27.2 (38.6) min of vigorous intensity at baseline and a tendency for improvement, demonstrating 35.8 (35.5) min at Week 6.
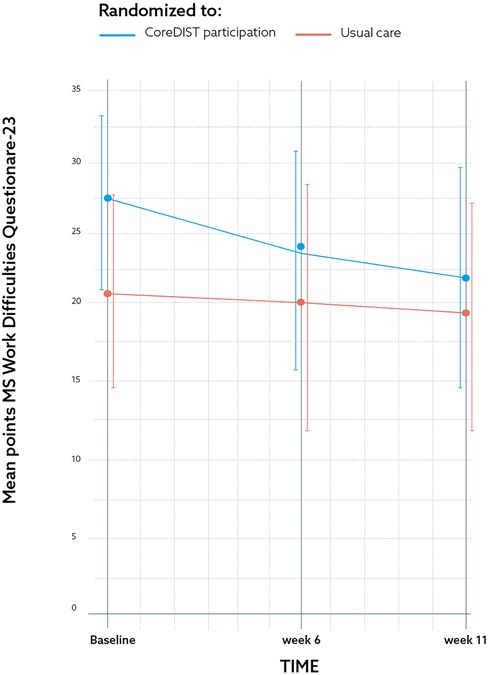
Figure 2. The results for one of the secondary feasibility metrics outcome measures; the Multiple Sclerosis Work Difficulties Questionnaire-23 Norwegian Version at baseline, Week 6, and Week 11 demonstrating CoreDISTparticipation (blue) and usual care groups (red) by mean and CI. The graph demonstrates a significant within-group 5.71-point improvement regarding barriers to work from baseline to Week 11 (p = 0.004) (CI: 2.17–9.25).
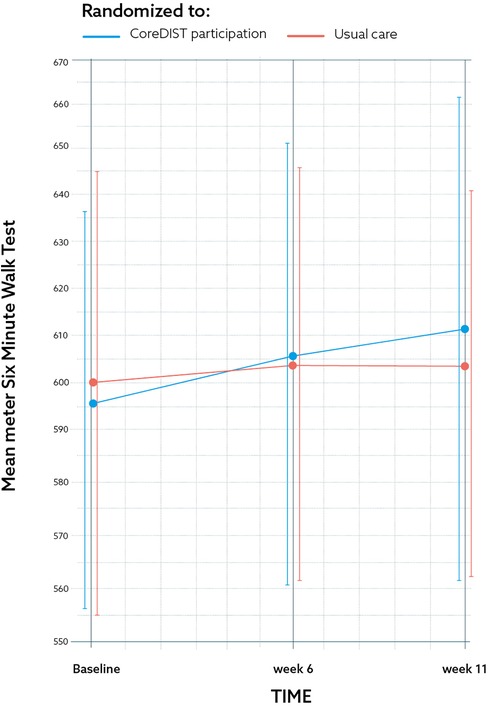
Figure 3. The results for one of the secondary feasibility metrics outcome measures; the 6-min walk test at baseline, 6, and 11 weeks for the CoreDISTparticipation group (blue) and usual care group (red) by mean and CI. The graph demonstrates a non-significant change of 14.6 m for the CoreDISTparticipation group from baseline to the 11-week retest.
Sample size calculation for a large-scale study is based on the mean score change from baseline to post-test at the MSWDQ-23NV (5.9 points for the intervention group and 2.3 for the control group). Assuming a common standard deviation of 6.0 for the score change, and calculating with a power of 0.8 and alpha = 0.05, the large-scale RCT will need to include 114 persons altogether (57 persons in each group) to detect a between-group difference in mean score change, assuming a dropout of 20%.
This study has identified primary feasibility metrics in relation to a new individualized, multidisciplinary intervention, termed CoreDISTparticipation. The study had a response rate to postal invitation of 34%, which is relatively low. There are many potential reasons why 66% of people invited did not respond, which could include: a lack of interest; self-assessment of not meeting the eligibility criteria; and a lack of sufficient time to potentially take part. In relation to employment, there is literature that indicates that 55%–70% of individuals living with MS are not employed (11–13), which may be a factor. The low response rate suggests that it would be potentially useful to consider alternative ways of recruiting potential candidates in future trials, for example, approaching charitable organizations who support people living with MS.
Despite the initial low response rate, after screening, the participant completion rate for the study was high: 100% (15/15) for the intervention group and 92.9% (13/14) for the usual care group. These high numbers demonstrated an interest in attending a comprehensive intervention. The attendance was high for the indoor GroupCoreDIST sessions (85%), which demonstrates feasibility and is in line with our previous CoreDIST studies (29, 33, 61). The moderate attendance in the outdoor sessions (57.3%) indicates needs for adjustments, even though most had good reasons for not attending (knee injury, illness). This was a period of the COVID-19 pandemic (although with no restrictions), and the low attendance is in line with pwMS reporting being less active during the pandemic (10, 30). The cold and rainy autumn weather may be another reason for not attending outdoor sessions. Another study from our research group however demonstrated that for those who attend outdoor training sessions, bad weather may provide a feeling of mastery (62). Further explorations of the outdoor environment may therefore be of interest. Three adverse reactions were reported during the high-intensity outdoor sessions. These are identified in Table 2 and were resolved by the municipal physiotherapist. The hamstring and ankle strains were treated acutely within the protection, rest, ice, compression, and elevation (PRICE) principle and advice. No further treatment was needed. The increased dizziness was assessed by the municipal physiotherapist and advice was provided. One person had a known heart condition and was worried about participating in the high-intensity training. This person was informed that high-intensity training is safe; however, chose to maintain a moderate intensity during the sessions. These are the relevant safety metrics for this trial. High-intensity interval training is reported to be safe and effective for pwMS with mild disability, but some studies do report some injuries (63). To increase safety during high-intensity exercise, aids, such as Nordic walking sticks, could be implemented in the intervention, and more alternatives for high-intensity exercise while standing could be offered. Exploring high-intensity activities in pwMS may be important for sustained function, as increased exercise capacity is indicated to influence neuroprotection and slow the rate of neuronal atrophy for pwMS (22, 24, 25). An intervention such as CoreDISTparticipation, which integrates high-intensity training with prerequisites for balance and walking, may therefore be an interesting contribution to the MS field.
The work-related follow-up was digital and had 100% attendance. Digital support opens new opportunities for the availability of competence and efficiency in communication between specialists and municipal healthcare. It is also a timesaving way of bringing employers into the loop. Good communication with the leader is one key for sustained employment (64). Such a multidisciplinary meeting may be a way of increasing communication, knowledge, and understanding (62). The meetings at the OP clinic with the MS nurse were however time-consuming, needed much logistics, and appear not to be feasible for a large-scale study. Instead, the welfare system will be linked to the intervention to address work in a more detailed manner within the existing system.
The necessary resources for this trial included the time needed for recruitment invitations to be posted, for clinicians to learn about the intervention, and assessors to learn about the selected outcome measures. The grant funding enabled adequate budgeting for the trial in terms of supporting the staff involved and access to the relevant equipment. All these metrics were adequate because of long-term and structured preparations and motivated clinicians and staff. For a potential larger multicentre study, it would be important to consider how to organize access to equipment for assessments at all site locations. With regard to overall trial management, it would be useful to consider increasing the size of the research group to speed up data management and review the equipment used for digital meetings that proved inadequate. Scientific aspects of the trial included evaluation of recordings, intervention content, dose, intensity, and the fidelity of the intervention and are further discussed.
Recordings demonstrated that the participants in both groups had mild disability (1.7 control/1.8 CoreDISTparticipation) and were young (mean 50.5 years in the usual care group/47.6 years in CoreDISTparticipation group), indicating the potential for a high work percentage. The rather low work percentage reported in both groups (65.4% usual care/46.3% CoreDISTparticipation) stands in contrast to their preferred work percentage if the job was perfectly adjusted to their needs, which was much higher (83.8% in usual care and 72.67% in CoreDISTparticipation). These results demonstrate a potential and desire to work more, which emphasizes the need to focus on employment in the follow-up of pwMS.
The results from the efficacy outcome measures demonstrated no between-group differences, as expected in a pilot study. However, there were within-group differences for the primary outcome MSWDQ-23NV, with a mean improvement of 5.9 points reduction of barriers for work at Week 11 in the CoreDISTparticipation group. This indicates that integrating work, physical activity, and sensorimotor function may have a potential impact on barriers to work in pwMS and demonstrates the feasibility of the MSWDQ-23NV as a primary outcome in a future study. The significant within-group change in HRQoL (MSIS-29NV) may support the decreased barriers for work, as some of the items in MSIS-29NV are related to items in the MSWDQ-23NV. However, the changes of over 6 points in the MSIS-29NV did not meet the 8 points needed for a clinically meaningful difference (56). The MSIS-29NV seems suitable for detecting changes in a future large-scale study. Fatigue was only measured through the elements in the mentioned outcomes and should be included in a larger study, as fatigue is a common reason for unemployment and because exercise may reduce fatigue in pwMS (21, 65).
The 6MWT and the physical activity monitors (ActiGraphs) demonstrated no significant change in the distance walked over 6 min, number of steps, or physical activity levels. Interestingly, the participants in the CoreDISTparticipation group walked a high mean number of steps already at baseline [mean 9,269 (SD: 5,304)] and first retest [9,524 (SD: 4,731)]. They also recorded more minutes in vigorous physical activity than expected, with a mean of 27.2 min (SD: 38.6) at baseline and 35.8 min (SD: 35.5) at the 6-week retest. Current physical activity recommendations (23) were, in contrast to most other studies (10, 29, 66, 67), fulfilled in our sample. The high levels of physical activity may indicate a biased sample of very active pwMS, or at least that a few persons who were particularly active biased the results in this small sample. No significant improvements in physical activity are in line with other studies, and it is well documented that changing physical activity habits is a challenge (68). Some studies emphasize the success criteria for sustainability, self-mastery, and long-term behavioural change of physical activity include a long-term follow-up, behavioural change techniques, and activity choices (68–70), and these elements may be considered integrated in a future study. Quite few RCT studies measure physical activity objectively, and studies that measure physical activity are warranted (69).
The within-group changes demonstrated at the Mini-BESTest may be due to the detailed sensorimotor and balance exercises undertaken during the in- and outdoor sessions and the walking/running in various terrains. The indoor sessions address prerequisites for postural control and balance, such as somatosensory function, muscle length, trunk control, larger muscle groups, and selective movements, and the outdoor sessions address the activity itself. Both are of importance for optimizing anticipatory postural adjustments: the minor adjustments in the trunk, hips, and ankle/legs that prepare for predictable perturbations of the COP before and during any movement (71), and compensatory postural adjustments that are used to regain balance after unpredictable perturbations of the COP (72). In an outdoor setting, dual/multiple tasks are additionally needed constantly, which may also be captured through the Mini-BESTest. The TIS-modNV demonstrated a ceiling effect and was considered removed from a large-scale study. However, in a different sample, trunk control may be more prominent, as trunk control may also be affected in those with mild to moderate disability (73). Trunk control is a central element in the CoreDIST intervention and should be assessed in a future study. Furthermore, objective measures of balance through the force platform should be further explored, as standing on two legs with eyes open and closed did not reveal any change. More challenging postural control tasks, such as one-leg standing, may be relevant to add to this mildly disabled population.
A significant limitation to this study is the lack of identification of what usual care was. This means that there can be no clear conclusion or comparisons drawn between the two groups because there is no documented information on what the usual care standard was. In addition, the sample size of the study was small, though adequate in terms of feasibility. For this reason, caution is recommended regarding the interpretation of secondary effects. A further limitation is the higher percentage of women than men participating, though this does correspond to the gender distribution in the MS population (1). To improve the understanding and accuracy of potential future intervention implementation, it would be beneficial for participants to record and document exercise choice and number of repetitions performed within sessions, as well as individualization. The intensity was only partly measured by pulse belts and watches, and these recordings should be conducted in detail in a future study. An 11-week follow-up is relatively short, and a longer-term follow-up would be beneficial.
This study demonstrated that it is feasible and safe to use the CoreDISTparticipation intervention to support pwMS who live in the community. While there were no statistically significant differences in the clinical outcome measurements taken between the two groups at the end of the trial, some within-group effects for the CoreDISTparticipation group regarding barriers for work, HRQoL, and balance were found. It could be interesting to consider a larger future trial with a detailed recording of the usual care, feasibility metrics, and implementation of overall trial reflections and learning.
The datasets presented in this article are not readily available because the dataset is at the moment not available as there is no current permission from the ethical committee to share data. Requests to access the datasets should be directed toZWxsZW4uYy5hcm50emVuQG5vcmQubm8=.
The studies involving humans were approved by the Regional Ethics Committee, Northern Norway. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TB: Data analysis, Writing – review & editing. HF: Data curation, Investigation, Writing – review & editing. BN: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
The study was funded by the Northern Norway Regional Health Authority (Grant no. 174837).
The authors would like to thank all persons with MS who participated in the study and their respective leaders at work. The authors also acknowledge the Nordland Hospital Trust, Bodø, and the participating municipalities for contributing and supporting the clinical physiotherapists and the user representatives.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Aarseth JH, Smedal T, Skår AB, Wergeland S. Annual report 2021 from the Norwegian MS register and biobank. Helse Bergen, Haukeland universitetssjukehus (2022). Available online at: https://www.kvalitetsregistre.no/sites/default/files/2022-06/%C3%85rsrapport%202021%20Norsk%20MS-register%20og%20biobank.pdf. (accessed June 11, 2023).
2. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. (2020) 26(14):1816–21. doi: 10.1177/1352458520970841
3. Clemens L, Langdon D. How does cognition relate to employment in multiple sclerosis? A systematic review. Mult Scler Relat Disord. (2018) 26:183–91. doi: 10.1016/j.msard.2018.09.018
4. Comber L, Galvin R, Coote S. Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. (2017) 51:25–35. doi: 10.1016/j.gaitpost.2016.09.026
5. Comber L, Sosnoff JJ, Galvin R, Coote S. Postural control deficits in people with multiple sclerosis: a systematic review and meta-analysis. Gait Posture. (2018) 61:445–52. doi: 10.1016/j.gaitpost.2018.02.018
6. Coote S, Comber L, Quinn G, Santoyo-Medina C, Kalron A, Gunn H. Falls in people with multiple sclerosis: risk identification, intervention, and future directions. Int J MS Care. (2020) 22(6):247–55. doi: 10.7224/1537-2073.2020-014
7. Kister I, Bacon T, Cutter GR. A longitudinal study of symptom botheration in multiple sclerosis. Mult Scler Relat Disord. (2020) 46:102585. doi: 10.1016/j.msard.2020.102585
8. Gyllensten H, Kavaliunas A, Murley C, Alexanderson K, Hillert J, Tinghög P, et al. Costs of illness progression for different multiple sclerosis phenotypes: a population-based study in Sweden. Mult Scler J Exp Transl Clin. (2019) 5(2):2055217319858383. doi: 10.1177/2055217319858383
9. Global MS employment report 2016. Available online at: https://www.msif.org/resource/global-ms-employment-report/International Multiple Sclerosis Federation. (accessed June 11, 2023).
10. Pedullà L, Santoyo-Medina C, Novotna K, Moumdjian L, Smedal T, Arntzen EC, et al. Physical activity in multiple sclerosis: meeting the guidelines at the time of COVID-19 pandemic. J Neurol Phys Ther. (2023) 47(2):112–21. doi: 10.1097/NPT.0000000000000430
11. Bøe Lunde HM, Telstad W, Grytten N, Kyte L, Aarseth J, Myhr K-M, et al. Employment among patients with multiple sclerosis—a population study. PLoS One. (2014) 9(7):1–7. doi: 10.1371/journal.pone.0103317
12. Glad SB, Nyland H, Aarseth JH, Riise T, Myhr K-M. How long can you keep working with benign multiple sclerosis? J Neurol Neurosurg Psychiatry. (2011) 82(1):78–82. doi: 10.1136/jnnp.2010.210732
13. Svendsen B. High cost of MS to patients in Norway. PharmacoEconomics Outcomes News. (2018) 802:16–5. doi: 10.1007/s40274-018-4907-1
14. Raggi A, Covelli V, Schiavolin S, Scaratti C, Leonardi M, Willems M. Work-related problems in multiple sclerosis: a literature review on its associates and determinants. Disabil Rehabil. (2016) 38(10):936–44. doi: 10.3109/09638288.2015.1070295
15. Sweetland J, Howse E, Playford ED. A systematic review of research undertaken in vocational rehabilitation for people with multiple sclerosis. Disabil Rehabil. (2012) 34(24):2031–8. doi: 10.3109/09638288.2012.669019
16. Simmons RD, Tribe KL, McDonald EA. Living with multiple sclerosis: longitudinal changes in employment and the importance of symptom management. J Neurol. (2010) 257(6):926–36. doi: 10.1007/s00415-009-5441-7
17. Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. (2015) 9:1–115.
18. Razazian N, Kazeminia M, Moayedi H, Daneshkhah A, Shohaimi S, Mohammadi M, et al. The impact of physical exercise on the fatigue symptoms in patients with multiple sclerosis: a systematic review and meta-analysis. BMC Neurol. (2020) 20(1):1–11. doi: 10.1186/s12883-020-01654-y
19. Learmonth YC, Ensari I, Motl RW. Physiotherapy and walking outcomes in adults with multiple sclerosis: systematic review and meta-analysis. Phys Ther Rev. (2016) 21(3–6):160–72. doi: 10.1080/10833196.2016.1263415
20. Khan F, Amatya B. Rehabilitation in multiple sclerosis: a systematic review of systematic reviews. Arch Phys Med Rehabil. (2017) 98(2):353–67. doi: 10.1016/j.apmr.2016.04.016
21. Amatya B, Khan F, Galea M. Rehabilitation for people with multiple sclerosis: an overview of Cochrane reviews. Cochrane Database Syst Rev. (2019) 1:1–40. doi: 10.1002/14651858.24
22. Rooney S, Riemenschneider M, Dalgas U, Jørgensen M-LK, Michelsen A-S, Brønd JC, et al. Physical activity is associated with neuromuscular and physical function in patients with multiple sclerosis independent of disease severity. Disabil Rehabil. (2021) 43(5):632–9. doi: 10.1080/09638288.2019.1634768
23. Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E, et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler. (2020) 26(12):1459–69. doi: 10.1177/1352458520915629
24. Dalgas U, Langeskov-Christensen M, Stenager E, Riemenschneider M, Hvid LG. Exercise as medicine in multiple sclerosis—time for a paradigm shift: preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep. (2019) 19(11):88. doi: 10.1007/s11910-019-1002-3
25. Thrue C, Riemenschneider M, Hvid LG, Stenager E, Dalgas U. Time matters: early-phase multiple sclerosis is accompanied by considerable impairments across multiple domains. Mult Scler. (2021) 27(10):1477–85. doi: 10.1177/1352458520936231
26. Riemenschneider M, Hvid LG, Stenager E, Dalgas U. Is there an overlooked “window of opportunity” in MS exercise therapy? Perspectives for early MS rehabilitation. Mult Scler. (2018) 24(7):886–94. doi: 10.1177/1352458518777377
27. Coulter EH, Bond S, Dalgas U, Paul L. The effectiveness of interventions targeting physical activity and/or sedentary behaviour in people with multiple sclerosis: a systematic review. Disabil Rehabil. (2020) 42(5):594–612. doi: 10.1080/09638288.2018.1503737
28. Learmonth YC, Motl RW. Physical activity and exercise training in multiple sclerosis: a review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil Rehabil. (2016) 38(13):1227–42. doi: 10.3109/09638288.2015.1077397
29. Arntzen EC, Straume BK, Odeh F, Feys P, Zanaboni P, Normann B. Group-based individualized comprehensive core stability intervention improves balance in persons with multiple sclerosis: a randomized controlled trial. Phys Ther. (2019) 99(8):1027–38. doi: 10.1093/ptj/pzz017
30. Moumdjian L, Smedal T, Arntzen EC, van der Linden ML, Learmonth Y, Pedullà L, et al. Impact of the COVID-19 pandemic on physical activity and associated technology use in persons with multiple sclerosis: an international RIMS-SIG mobility survey study. Arch Phys Med Rehabil. (2022) 103(10):2009–15. doi: 10.1016/j.apmr.2022.06.001
31. Normann B, Zanaboni P, Arntzen EC, Øberg GK. Innovative physiotherapy and continuity of care in people with multiple sclerosis: a randomized controlled trial and a qualitative study. J Clin Trials. (2016) 6:1–10. doi: 10.4172/2167-0870.1000282
32. Normann B, Arntzen EC, Sivertsen M. Comprehensive core stability intervention and coordination of care in acute and subacute stroke rehabilitation—a pilot study. Eur J Physiother. (2018) 21:187–96. doi: 10.1080/21679169.2018.1508497
33. Arntzen EC, Straume B, Odeh F, Feys P, Normann B. Group-based, individualized, comprehensive core stability and balance intervention provides immediate and long-term improvements in walking in individuals with multiple sclerosis: a randomized controlled trial. Physiother Res Int. (2020) 25(1):e1798. doi: 10.1002/pri.1798
34. Arntzen EC, Øberg GK, Gallagher S, Normann B. Group-based, individualized exercises can provide perceived bodily changes and strengthen aspects of self in individuals with MS: a qualitative interview study. Physiother Theory Pract. (2021) 37(10):1080–95. doi: 10.1080/09593985.2019.1683923
35. Lahelle AF, Øberg GK, Normann B. A group-based, individualized physiotherapy intervention for people with multiple sclerosis—a qualitative study. Physiother Res Int. (2018) 23(4):e1734. doi: 10.1002/pri.1734
36. Lahelle AF, Øberg GK, Normann B. Group dynamics in a group-based, individualized physiotherapy intervention for people with multiple sclerosis: a qualitative study. Physiother Res Int. (2020) 25(3):e1829. doi: 10.1002/pri.1829
37. Lahelle AF, Øberg GK, Normann B. Physiotherapy assessment of individuals with multiple sclerosis prior to a group intervention—a qualitative observational and interview study. Physiother Theory Pract. (2020) 36(3):386–96. doi: 10.1080/09593985.2018.1488022
38. Kibler WB, Press J, Sciascia A. The role of core stability in athletic function. Sports Med. (2006) 36(3):189–98. doi: 10.2165/00007256-200636030-00001
39. Learmonth YC, Motl RW. Important considerations for feasibility studies in physical activity research involving persons with multiple sclerosis: a scoping systematic review and case study. Pilot Feasibility Stud. (2018) 4:1. doi: 10.1186/s40814-017-0145-8
40. Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. (2010) 10:1–10. doi: 10.1186/1471-2288-10-1
41. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33(11):1444–52. doi: 10.1212/WNL.33.11.1444
42. Butcher NJ, Monsour A, Mew EJ, Chan AW, Moher D, Mayo-Wilson E, et al. Guidelines for reporting outcomes in trial reports: the CONSORT-outcomes 2022 extension. JAMA. (2022) 328(22):2252–64. doi: 10.1001/jama.2022.21022
43. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17(2):162–73. doi: 10.1016/S1474-4422(17)30470-2
44. Sivertsen M, Arntzen E, Alstadhaug K, Normann B. Effect of innovative vs. usual care physical therapy in subacute rehabilitation after stroke. A multicenter randomized controlled trial. Front Rehabil Sci. (2022) 3:1–16. doi: 10.3389/fresc.2022.987601
45. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. Br Med J. (2014) 348:g1687. doi: 10.1136/bmj.g1687
46. Honan CA, Brown RF, Hine DW. The multiple sclerosis work difficulties questionnaire (MSWDQ): development of a shortened scale. Disabil Rehabil. (2014) 36(8):635–41. doi: 10.3109/09638288.2013.805258
47. Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. (2008) 14(3):383–90. doi: 10.1177/1352458507082607
48. Oosterveer DM, van den Berg C, Volker G, Wouda NC, Terluin B, Hoitsma E. Determining the minimal important change of the 6-minute walking test in multiple sclerosis patients using a predictive modelling anchor-based method. Mult Scler Relat Disord. (2022) 57:103438. doi: 10.1016/j.msard.2021.103438
49. Hamre C, Botolfsen P, Tangen GG, Helbostad JL. Interrater and test-retest reliability and validity of the Norwegian version of the BESTest and mini-BESTest in people with increased risk of falling. BMC Geriatr. (2017) 17(1):92. doi: 10.1186/s12877-017-0480-x
50. Bressel E. Innovative analyses of human movement: analytical tools for human movement research. Med Sci Sports Exerc. (2004) 36(10):1834. doi: 10.1249/00005768-200410000-00027
51. Block VA, Pitsch E, Tahir P, Cree BA, Allen DD, Gelfand JM. Remote physical activity monitoring in neurological disease: a systematic review. PLoS One. (2016) 11(4):e0154335. doi: 10.1371/journal.pone.0154335
52. Weikert M, Motl RW, Suh Y, McAuley E, Wynn D. Accelerometry in persons with multiple sclerosis: measurement of physical activity or walking mobility? J Neurol Sci. (2010) 290(1–2):6–11. doi: 10.1016/j.jns.2009.12.021
53. Euroqol. EQ-5D 2023. Available online at: https://euroqol.org/ (accessed July 4, 2023).
54. Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The multiple sclerosis impact scale (MSIS-29): a new patient-based outcome measure. Brain. (2001) 124(5):962–73. doi: 10.1093/brain/124.5.962
55. Smedal T, Johansen HH, Myhr KM, Strand LI. Psychometric properties of a Norwegian version of multiple sclerosis impact scale (MSIS-29). Acta Neurol Scand. (2010) 122(4):244–51. doi: 10.1111/j.1600-0404.2009.01298.x
56. Costelloe L, O'Rourke K, Kearney H, McGuigan C, Gribbin L, Duggan M, et al. The patient knows best: significant change in the physical component of the multiple sclerosis impact scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry. (2007) 78(8):841–4. doi: 10.1136/jnnp.2006.105759
57. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of MS on walking ability: the 12-item MS walking scale (MSWS-12). Neurology. (2003) 60(1):31–6. doi: 10.1212/WNL.60.1.31
58. Kieseier BC, Pozzilli C. Assessing walking disability in multiple sclerosis. Mult Scler. (2012) 18(7):914–24. doi: 10.1177/1352458512444498
59. Baert I, Freeman J, Smedal T, Dalgas U, Romberg A, Kalron A, et al. Responsiveness and clinically meaningful improvement, according to disability level, of five walking measures after rehabilitation in multiple sclerosis: a European multicenter study. Neurorehabil Neural Repair. (2014) 28(7):621–31. doi: 10.1177/1545968314521010
60. Browne RH. On the use of a pilot sample for sample size determination. Stat Med. (1995) 14(17):1933–40. doi: 10.1002/sim.4780141709
61. Normann B, Salvesen R, Christin Arntzen E. Group-based individualized core stability and balance training in ambulant people with multiple sclerosis: a pilot feasibility test–retest study. Eur J Physiother. (2016) 18:173–8. doi: 10.3109/21679169.2016.1170204
62. Fikke HK, Norman B, Sivertsen M, Dahl SSH, Arntzen EC. Utprøving av intervensjon for optimalisering av funksjon, fysisk aktivitet og arbeidsdeltakelse ved multippel sklerose. Fysioterapeuten. (2022) 7:32–42.
63. Campbell E, Coulter EH, Paul L. High intensity interval training for people with multiple sclerosis: a systematic review. Mult Scler Relat Disord. (2018) 24:55–63. doi: 10.1016/j.msard.2018.06.005
64. Roessler RT, Rumrill P, Hennessey ML, Vierstra C, Pugsley E, Pittman A. Perceived strengths and weaknesses in employment policies and services among people with multiple sclerosis: results of a national survey. Work. (2003) 21(1):25–36.12897388
65. Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. (2012) 126(4):256–62. doi: 10.1111/j.1600-0404.2011.01634.x
66. Casey B, Coote S, Galvin R, Donnelly A. Objective physical activity levels in people with multiple sclerosis: meta-analysis. Scand J Med Sci Sports. (2018) 28(9):1960–9. doi: 10.1111/sms.13214
67. Gervasoni E, Anastasi D, Di Giovanni R, Solaro C, Rovaris M, Brichetto G, et al. Physical activity in non-disabled people with early multiple sclerosis: a multicenter cross-sectional study. Mult Scler Relat Disord. (2022) 64:103941. doi: 10.1016/j.msard.2022.103941
68. Casey B, Coote S, Hayes S, Gallagher S. Changing physical activity behavior in people with multiple sclerosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2018) 99(10):2059–75. doi: 10.1016/j.apmr.2017.12.013
69. Arntzen EC, Bidhendi-Yarandi R, Sivertsen M, Knutsen K, Dahl SSH, Hartvedt MG, et al. The effect of exercise and physical activity-interventions on step count and intensity level in individuals with multiple sclerosis: a systematic review and meta-analysis of randomized controlled trials. Front Sports Act Living. (2023) 5:1162278. doi: 10.3389/fspor.2023.1162278
70. Motl RW, Kidwell-Chandler A, Sandroff BM, Pilutti LA, Cutter GR, Aldunate R, et al. Primary results of a phase-III, randomized controlled trial of the behavioral intervention for increasing physical activity in multiple sclerosis project. Mult Scler. (2023) 29(3):415–26. doi: 10.1177/13524585221146430
71. Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett. (2012) 506(2):256–60. doi: 10.1016/j.neulet.2011.11.018
72. Ivanenko Y, Gurfinkel VS. Human postural control. Front Neurosci. (2018) 12:171. doi: 10.3389/fnins.2018.00171
73. Raats J, Arntzen EC, Lamers I, Feys P, Normann B. What is the distribution of trunk impairments and its relationship with disability level in individuals with multiple sclerosis? Mult Scler Relat Disord. (2022) 57:103325. doi: 10.1016/j.msard.2021.103325
Keywords: multiple sclerosis, sensorimotor, feasibility, balance, walking, high-intensity, physical activity, employment
Citation: Arntzen EC, Braaten T, Fikke HK and Normann B (2024) Feasibility of a new intervention addressing group-based balance and high-intensity training, physical activity, and employment in individuals with multiple sclerosis: a pilot randomized controlled trial. Front. Rehabil. Sci. 4:1258737. doi: 10.3389/fresc.2023.1258737
Received: 14 July 2023; Accepted: 8 December 2023;
Published: 8 January 2024.
Edited by:
Luodan Yang, South China Normal University, ChinaReviewed by:
Brice T. Cleland, University of Illinois Chicago, United States© 2024 Arntzen, Braaten, Fikke and Normann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen Christin Arntzen ZWxsZW4uYy5hcm50emVuQG5vcmQubm8=
†ORCID Ellen Christin Arntzen orcid.org/0000-0001-5396-4071
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.