- 1Department of Neurology, Maastricht University Medical Centre+, Maastricht, Netherlands
- 2Research School MHeNS, Maastricht University, Maastricht, Netherlands
- 3Department of Rehabilitation Medicine, Adelante Rehabilitation Centre, Valkenburg, Netherlands
- 4Department of Rehabilitation Medicine, Maastricht University, Maastricht, Netherlands
- 5Research School CAPHRI, Maastricht University, Maastricht, Netherlands
- 6Department of Rehabilitation Medicine, Adelante Centre of Expertise in Rehabilitation and Audiology, Hoensbroek, Netherlands
Background: Objective: To identify and examine neural reorganization of the sensory network in terms of lesion type, somatotopic organization of the primary somatosensory area, and functional connectivity in relation to sensory function in children and young adults with cerebral palsy (CP).
Methods: Design: systematic review, Prospero registration ID 342570. Data sources: PubMed; Cochrane; Web of Science; Embase; CINAHL and PEDro from inception to March 13, 2021. Eligibility criteria: All types of original studies, concerning sensory connectivity in relation to sensory outcome in patients with spastic CP, <30 years of age. No publication status or date restrictions were applied. Data extraction and synthesis: Two authors independently determined the eligibility of studies. Quality assessment was performed by a third author. Neuro-imaging/neurophysiological techniques, sensory outcomes and patient characteristics were extracted.
Results: Children and young adults with periventricular leucomalacia (PVL) lesions have significantly better hand function and sensation scores than patients with cortical-subcortical/middle cerebral artery (MCA) lesions. Ipsilesional reorganization of the S1 (primary somatosensory cortex) area appears to be the primary compensation mechanism after a unilateral early brain lesion, regardless of the timing of the lesion. Interhemispheric reorganization of the sensory system after early brain lesions is rare and, when it occurs, poorly effective. Diffusion tractography shows a positive correlation between the ascending sensory tract (AST) diffusivity metrics of the more affected hemisphere and sensory test outcomes.
Discussion and conclusions: Because of the large variability in study design, patient characteristics, neuroimaging/neurophysiological techniques and parameters as well as sensory assessment methods used, it is difficult to draw definite inferences on the relationship between the reorganization of the sensory network following early brain damage and sensory function in children and young adults with CP. In general, sensory function seems to be worse in cortical as opposed to white matter tract (PVL) lesions. International consensus on a clinically relevant sensory test battery is needed to enhance understanding of the intriguing compensatory mechanisms of sensory network following early brain damage and potential consequences for rehabilitation strategies.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/.
Introduction
Cerebral palsy
Cerebral palsy (CP) is a broad term for disorders of the development of movement and posture, causing activity limitation, which are attributed to non-progressive disturbances in the developing fetal or infant brain (1, 2). CP is one of the most common causes of physical disability in children, and the majority of these children have impaired hand function that makes them experience difficulties in performing daily activities (3, 4). It occurs in 2–3:1,000–1:2,500 live births (5, 6). Depending on the timing of the lesion during fetal development, different types of lesions occur; cortical maldevelopments (first and second trimester of gestation), periventricular white matter (PVL) lesions (early third trimester) or cortical and subcortical lesions/ middle cerebral artery infarctions (MCA) (around term age).
Motor reorganization
The compensatory motor capabilities following early focal brain injury are intriguing, and these are reported to be superior to those of the adult brain. This observation, known as the Kennard principle, is based on a study of recovery following experimental lesions of the motor cortex in monkeys (7–9). Both lesion timing and cortical spinal tract (CST) wiring patterns have been shown to relate to upper limb function in children with unilateral CP (10). However, a large part of the variability in upper limb function still remains unexplained (7, 11).
Sensory impairment
Aside from motor impairment, somatosensory impairment is also observed in children with CP. Somatosensory impairment is a broad term used for tactile deficits as well as for impairments in the processing of sensory information such as vibration, stereognosis, and two-point discrimination. In addition, proprioception can be considered one of the subsystems within the somatosensory system (12, 13). Proprioception consists of kinesthesia and joint position sense. Kinesthesia is the sense of extremity movement without visual input, and position sense is characterized by static limb position (14).
Thalamocortical projections start to reach the somatosensory cortex at the beginning of the third trimester. Conversely to CST wiring, developing thalamocortical somatosensory projections can still bypass even large periventricular brain lesions during this period (10). This tends to lead to sprouting to a broader area in the somatosensory cortex (15). Somatosensory processing is located in the primary somatosensory cortex (S1) in the postcentral gyrus of the parietal lobe and the secondary somatosensory cortex, located on the parietal operculum (16, 17). Median nerve stimulation, as well as object recognition, have been shown to activate the secondary somatosensory cortex (S2) bilaterally, regardless of the hand being stimulated, but only the contralateral S1 (18, 19). According to Auld et al., children with UCP performed worse in sensory tasks with their impaired hand compared to the unimpaired hand. However both hands performed worse than either hand of typically developing children. Over 75% of children with UCP have tactile deficits (20).
Interaction motor and sensory system
Tactile deficits account for approximately 30% of the variance in upper-limb motor function in children with UCP (21). One example of this is the necessary sensory feedback in the modulation of fine motor tasks such as precision grip (15, 22). The majority of existing studies focus on especially motor performance, and there is only limited information about the involvement of the sensory system on functional outcome. However, understanding the extent and impact of sensory function on upper limb motor function is essential to improve rehabilitation approaches and functional outcomes (23).
Aim of the study
In this systematic review we focus on all children and young adults with CP. However, experience shows that most studies on sensorimotor function concentrate on children and young adults with unilateral CP. Therefore, in the current study, we aimed to synthesize information on the consequences of brain damage (white matter characteristics, brain lesion types, functional connectivity) on somatosensory impairment and its impact on upper limb function in children and young adults with CP.
Hypothesis
Based on the current literature, somatosensory impairment seems to be an important factor in upper limb dysfunction in children and young adults with CP (23). Therefore, we hypothesize a potential relation between neuroanatomical lesions, functional connectivity, and somatosensory impairment.
Materials and methods
Design
A systematic review was designed. The protocol has been registered in the National Institute for Health Research (NHS) on PROSPERO (International Prospective Register of Systematic Reviews) database: ID 342570.
Data source and search strategy
A literature search was performed in six online databases: PubMed; Cochrane; Web of Science; Embase; CINAHL, and PEDro, from inception to March 13, 2021. Each search contained three main concepts: CP, MRI (magnetic resonance imaging), and sensation. The following Medical Subject Headings (MESH) terms and text words were used; ((((((Sensation) OR “Somatosensory Disorders”[Mesh]) OR “Sensation”[Mesh]) OR Sensory) OR Sensory function)) AND ((Cerebral palsy) OR Cerebral palsy [MeSH])) AND (((MRI) OR “Magnetic Resonance Imaging”[Mesh]) OR Magnetic Resonance Imaging). All results were uploaded to Rayyan Systems inc. (https://www.rayyan.ai/), an online tool to screen and select articles by multiple reviewers. Indicated duplicates were removed after checking by one of the first authors (CS). First, two review authors (CS, RV) independently screened titles and abstracts to remove irrelevant articles. Dissertation articles and conference abstracts were removed. Second, both authors reviewed the full text of potentially relevant studies to determine their eligibility. Consensus was reached on all articles.
Inclusion criteria
The following inclusion criteria were applied: (1) human participants with spastic CP; (2) mean age of the participants was not older than thirty years of age, since the focus of the study included children and young adults; (3) MRI imaging available; (4) assessment of somatosensory function; (5) published in English, Dutch, French or German; (6) original research papers (exclusion of study protocols, reviews and conference abstracts).
Data collection and data items
Our study objective was to investigate somatosensory deficits in relation to specific MRI abnormalities in children and young adults with spastic CP. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to extract the articles included in the review (24).
Data extracted included (1) first author and year of publication; (2) number and age of participants; (3) type of cerebral lesion; (4) clinical assessment of participants (motor and/or somatosensory assessment or categorization according to the Surveillance of Cerebral Palsy in Europe); (6) whether a control group was involved; (7) additional neuroimaging/neurophysiological method (e.g., electroencephalography, transcranial magnetic stimulation, somatosensory evoked potential, functional magnetic resonance imaging) (8) outcome of the studies.
Quality assessment
Study quality was assessed (YJ) using the Standard quality assessment criteria for evaluating primary research papers from a variety of fields (25).
Results
General results
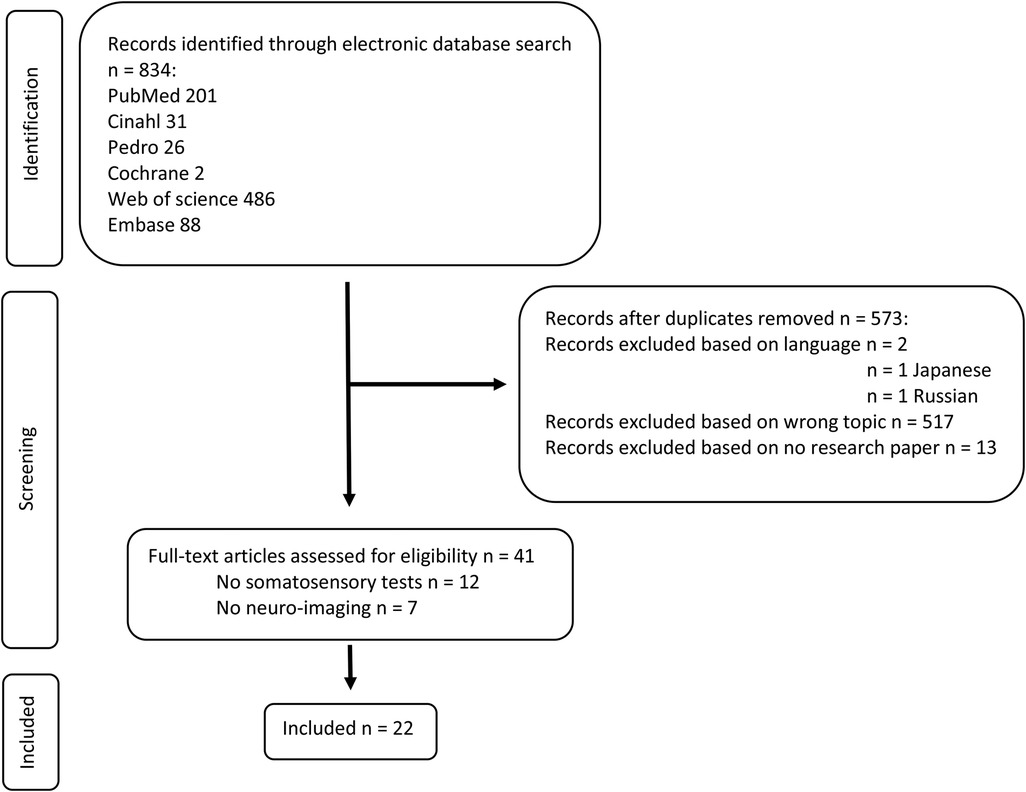
After removing duplicates, the database search (last updated on March 13, 2021) yielded 573 citations. Twenty-two records were eventually included in the review. Figure 1 displays the flowchart of the study selection process according to the PRISMA guidelines (24).
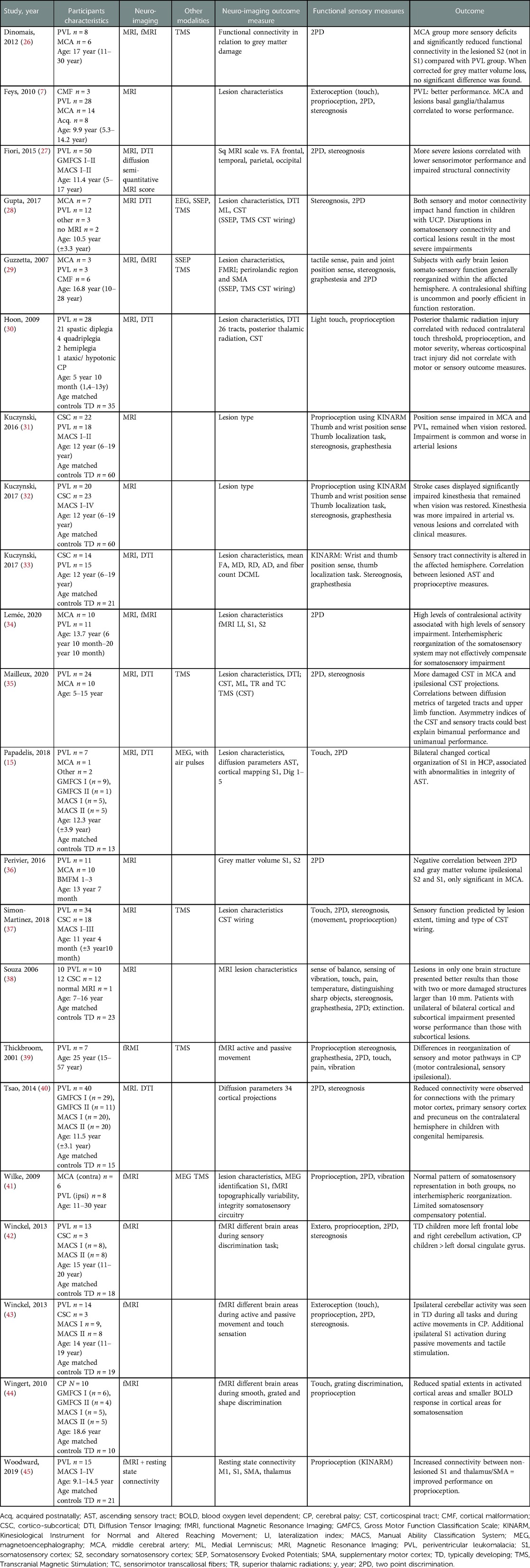
The extracted information from the included articles is summarized in Table 1.
These twenty-two articles reported a total of 905 observations, 388 with a PVL-type lesion, 163 with an MCA-type lesion, 326 typically developing children (TD), and 25 with other lesions (Table 1). Most studies included children and young adults with a clinical unilateral cerebral palsy, except for the study of Wingert et al., which analyzed children with spastic diplegia, and Hoon et al., which analyzed children with mainly spastic diplegia (21/28) and also quadriplegia (4/28), hemiplegia (2/28) and ataxic CP (1/28) (30, 44). Next to MRI, five additional neuro-imaging/neurophysiological techniques were used. Sensory function was assessed in all studies. A combination of thirteen sensory assessments, i.e., tests, protocols as well as evaluation criteria, were used (see Table 1). For instance, stereognosis assessment protocols differed. Some studies used identification of six out of twelve familiar objects; three of six objects matched in pairs (pencil/pen, coin/button, paperclip/safety pin), and three of six differing objects (key, clothespin, marble, comb, spoon, ball) (7, 35, 42, 43). Whereas others used the number of correct responses out of a possible maximum of ten (28), nine (27, 40), five (29), six (37), or three objects(nickel, key, paperclip) (31–33). Two other studies did not include a detailed description of the stereognosis assessment (38, 39).
In almost all studies, motor function was reported, though not all studies reported on GMFCS or MACS. Some of the papers are from the same research groups (26, 31–33, 35, 37, 41–43, 45), potentially resulting in 312 overlapping observations. The results of the reports will be discussed according to sensory function.
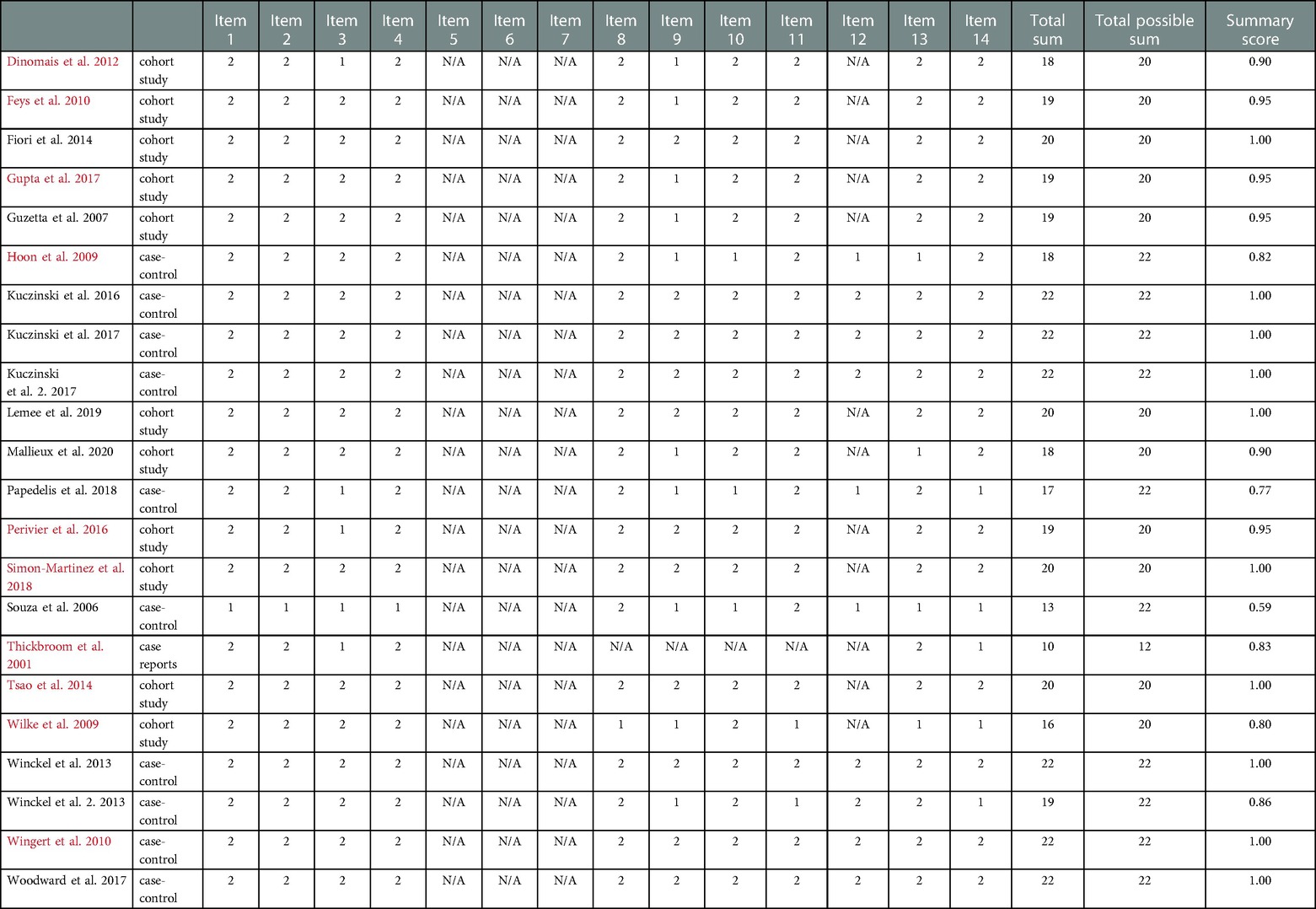
Using the standard quality assessment criteria, most studies were of strong quality, with a total score above 0.80; one study was rated adequate and one good (25). The quality assessment is shown in Table 2.
Tactile perception
Lesion characteristics
Patients with an MCA-type lesion have more severe deficits on two-point discrimination (2PD) test scores (7, 26, 28, 31, 32, 37, 41), stereognosis (7, 28, 31, 32, 37), and on graphesthesia test scores (31, 33), when compared with patients with a PVL-type lesion.
The location of the lesion is also correlated with performance on 2PD and stereognosis tests; Patients with basal ganglia and thalamic lesions (7, 37) have more severe deficits on 2PD and stereognosis tests. Patients with brainstem and temporal lobe lesions have more severe deficits on stereognosis tests. Patients with lesions in the corpus callosum and caudate have a more severe deficit on 2PD tests (27). In addition, larger lesions in these areas correlated with more severe deficits on 2PD and stereognosis tests (37, 38).
Patients with a disrupted CST and an ipsilateral wiring pattern, assessed by diffusion tensor imaging (DTI), have significantly more severe deficits in stereognosis tests (28, 35, 37). The relation between CST and performance on 2PD tests is less clear. Some studies found a correlation between damage and a more severe deficit on 2PD scores (35, 37), whereas others did not find this correlation (28).
Functional connectivity
In unilateral early brain lesions, an ipsilesional reorganization of the S1 area is found, regardless of the timing of the lesion (29, 34). Indeed, even in patients with brain malformations, contralateral shifting of the sensory function to the unaffected hemisphere is uncommon and poorly effective (29). Lemee et al. also showed most frequent contralateral pattern of sensory innervation, using brain activation with passive movement of the hand, suggesting intrahemispheric reorganization (34). Only in about 20% of the participants, an ipsilateral activation pattern was observed, coinciding with severe sensory deficits (34).
Within de S1 region, texture and shape recognition are impaired in damage to Brodmann area (BA)3, texture in BA1, and shape in BA2 (44).
S2 is predominately contralesional activated; however, high contralesional activation was associated with a more severe sensory deficit on 2PD tests (34). Patients with reduced functional connectivity in S2 (but not S1) had more severe sensory deficits on 2PD tests. However, when accounting for grey matter volume loss, this difference disappeared (26). Patients with gray matter volume loss in S1 and S2 had more severe sensory deficits on 2PD tests. This correlation was significant in patients with MCA-type lesions but not in PVL-type lesions (36).
Patients with either MCA-type lesions or PVL-type lesions, assessed with magnetoencephalography (MEG) and diffusion tensor imaging (DTI), showed larger distances between cortical representation areas of digits 1, 3, and 5 in the S1. In addition, in the more affected hemisphere, S1 was shifted anteriorly into the precentral gyrus. These increased distances in S1 digit representations are correlated with a more severe deficit on 2PD tests (15).
Patients with disrupted somatosensory connectivity of the ascending sensory tract (medial lemniscus), tested with Diffusion Tensor Imaging (DTI), have significantly more sensory deficit on 2PD and stereognosis tests as compared to those with intact somatosensory connectivity (15, 28, 33, 35, 40).
Patients with lower Fractional anisotropy of the medial lemniscus and higher mean diffusivity (MD) of the medial lemniscus, have more severe deficits on 2PD (15) and stereognosis tests (33, 35, 40). Patients with lower Fractional anisotropy of the superior thalamic radiations also have more severe deficits on 2PD and stereognosis. Low to no correlations were found between the diffusion metrics of the sensorimotor transcallosal fibers and sensory impairments (35). Patients with MCA-type lesions have more extensive differences in diffusion metrics compared to typically developing children. The differences in diffusion metrics between typically developing children and children with PVL-type lesions are limited (28, 33).
In patients with CP, an abnormal sensorimotor system, both anatomically (DTI) and electrophysiologically (EEG), was strongly correlated with abnormal motor function (Jebsen Taylor hand function test, Box and Blocks) and abnormal sensorimotor function (stereognosis and 2PD). The anatomy of the motor system, tested anatomically (DTI) correlated only weakly with bimanual function. Electrophysiology testing of the motor system (TMS) showed some correlation with the stereognosis (28). Using the lesion type classification, most effects could be explained by the more evident cortical involvement in the MCA-type lesions as compared to the PVL-type lesions.
Despite different motor and sensory reorganization patterns, the cortico-cerebellar circuitry, using functional MRI (fMRI), was well preserved in almost all patients and did not correlate with sensory deficits (26, 41, 43).
Tactile registration
Tactile registration has been studied to a lesser extent. Hence the classification into “lesion characteristics” and “functional connectivity” has been discarded here. Feys et al. reported normal touch sensation in all children with PVL-type lesions, compared to approximately 75% of the children with cortical-subcortical brain lesions (7). Touch sensation could be significantly predicted by lesion timing, lesion location, and lesion extent (37, 38). In patients with PVL-type lesions, injury severity in thalamocortical pathways is related to sensation of touch (30, 37).
Proprioception
Lesion characteristics
Lesion timing is not unambiguously associated to proprioception. In the study of Feys et al. no significant differences between lesion types were found (7), whereas Kuczynski et al. found position sense deficits to be more common and severe in children with MCA-type lesions (31, 32). Guzetta et al. included only one patient with an impaired position sense. This patient had the most severe sensory impairment (29).
Proprioception impairment is correlated with lesion location. Patients with posterior thalamic radiation injuries have more severe contralesional proprioception deficits (30).
Patients with lesions in only one brain structure have a better performance on proprioceptive tests compared to those with two or more damaged structures and lesions larger than 10 mm. Patients with unilateral or bilateral cortical and subcortical impairment have more sensory deficits (position sense, as well as tactile perception and registration tests) than patients with subcortical lesions (38).
Functional connectivity
Patients with MCA-type lesions and PVL-type lesions and lower FA, and higher MD, RD, and AD of the DCML tract, tested with DTI, have a more severe proprioceptive deficit (33). Patients with PVL-type lesions showed a more posteriorly and laterally organization of the AST compared with controls (33).
Patients with CP and decreased functional connectivity, tested with fMRI, between the non-lesioned S1 and thalamus/supplementary motor cortex (SMA) have a more severe position sense deficit. Whereas in typically developing children, position sense is positively correlated with connectivity between the thalamus and bilateral sensorimotor regions; increased connectivity is associated with poorer performance. Overall, the thalamus showed decreased connectivity in children with PVL-type lesions compared to controls (45).
In patients with CP, tested with fMRI, S1 activation is seen for active and passive movements as well as for tactile stimulation. There is additional ipsilateral S1 activation during passive movements and tactile stimulation. Ipsilateral cerebellar activity was observed in TD children during all tasks, but in CP children only during active movements (43). Typically developing children show more left frontal lobe and right cerebellum activation on fMRI during proprioceptive tasks compared to children with CP. Conversely, CP children activated the left dorsal cingulate gyrus to a greater extent than TD children (42).
One patient with a contralesional shift of primary sensory function, tested with SEP, was found; the responses in the ipsilateral hemisphere did not conform to latency and morphology of the response from the unaffected hand. This patient had the most severe position sense deficit (29).
Discussion
General
Reorganization of the sensory system after early brain lesions is a complex and intriguing process. Although information on reorganization of sensory functions in children and young adults with CP is increasing, this reorganization process is still not fully understood. Understanding the pathophysiology of this reorganization process, and its relationship to sensory outcomes, and its relationship to its impact on functional outcomes might ultimately lead to different rehabilitation strategies, as shown schematically in Figure 2.
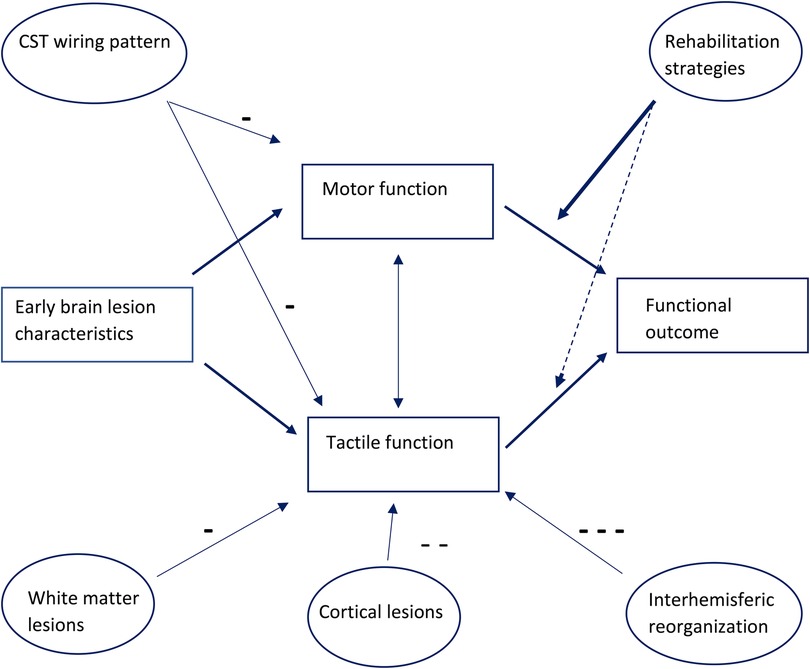
Figure 2. Diagram lesion relating to tactile, motor function and functional outcome, influencing anatomical lesions, rehabilitation strategies so far focused mostly on improvement of motor function.
However, a comprehensive comparison of the evidence in the literature on sensory function in relation to anatomical lesions is difficult because different test batteries, sensory test protocols, and evaluation criteria are used, as well as different outcome measures for neuro-imaging, as shown in Table 1. Moreover, when the available sensory information is associated with lesion timing, lesion location, lesion extent, and functional connectivity, studies tend to focus on one particular tract or lesion type. In addition, we need to take into account that currently used protocols for sensory assessment most likely underestimate the sensory deficits in patients with CP (22). Therefore, international consensus on comprehensive sensory test batteries, protocols, and evaluating criteria is necessary to allow comparison of somatosensory function in relation to brain injury characteristics and ultimately influence functional outcome with personalized rehabilitation strategies.
Most of the literature on sensorimotor function in CP focuses on children and young adults with unilateral CP. The results of our search confirmed this; while the intent was to include all children with CP, most studies included patients with clinical unilateral cerebral palsy and mild impairments. The study of Wingert et al., and Hoon et al., included a large/solely group of participants with spastic diplegia. The results of these studies were included in this systematic review because they were in our intended patient category. Even in the unilateral cerebral palsy groups, especially in case of white matter damage, the abnormalities were often bilateral, meaning even though the patients have a clinical unilateral CP the groups are more similar in lesion type than clinically expected. Further, the results of the study of Hoon et al. are in line with the study of Mailleux et al. and the study of Wingert et al. uses a different study protocol (grating discrimination), making the risk of bias low (30, 35, 44).
There is substantial evidence that patients with PVL-type lesions have significantly less sensory deficits as opposed to patients with cortical-subcortical/MCA-type lesions (7, 27, 28, 31, 32, 34, 37, 38, 41). Lesion extent, type of CST wiring pattern, and lesion location significantly further impact sensory function (7, 37).
In the next paragraphs, we will elaborate on the relationship between specific neuroanatomical abnormalities and specific sensory deficits and the potential consequence for rehabilitation in more detail.
Tactile perception
Damage to cortical and subcortical structures reduces the likelihood of the CST trajectory developing in the typical contralateral pathway. In case of ipsilateral CST wiring, the association between sensory and motor functions is disrupted. This points toward a different mechanism of sensorimotor integration in patients with CP as a probable cause for the association between CST wiring and increased sensory deficits (39). The relation between CST wiring and stereognosis is found across multiple studies (28, 35, 37). Some studies found a correlation between CST wiring and poorer performance of the 2PD test, while others did not find this correlation (35, 37). However, there was a large spread of 2PD test scores in this group, which possibly explains the lack of a statistical significant difference (28).
Ipsilesional reorganization of the S1 area appears to be the primary compensation mechanism after a unilateral early brain lesion, regardless of the timing of the lesion. Developing thalamocortical somatosensory projections can still bypass even large periventricular brain lesions during the third trimester (10). This tends to lead to sprouting to a broader area in the somatosensory cortex, and the wider distances correlate with impaired sensory function (15). Somatosensory deficits in patients with PVL-type lesions are better explained by loss of integrity of these thalamocortical pathways than by loss of grey matter volume in ipsilesional S1 and/or S2. The loss of grey matter volume in S1 and S2 in these patients was not related to sensory outcome (36). In patients with MCA-type lesions and subsequent volume loss of the grey matter in S1 and S2, the reorganization capabilities of these thalamocortical pathways are less, resulting in a more severe deficit. The observed inter-hemispheric reorganization of S2 (34) could be related to the observed bilateral activation of S2 after tactile stimulation, seen in healthy volunteers (16, 19). However, high contralesional activation was associated with a severe impairment of sensory function, making this compensatory mechanism inadequate to say the least (34).
Diffusion tractography shows a positive correlation between the ascending sensory tract (AST) axial diffusivity (AD) of the more affected hemisphere and sensory test outcomes. Increased axial diffusivity may indicate gliosis and structural abnormalities in the integrity of the AST (15, 33, 40). These differences are more extensive in patients with MCA-type lesions compared to patients with PVL-type lesions, implying that damage to the ascending sensory tracts is more extensive in patients with MCA-type lesions (33). This is consistent with the finding that patients with MCA-type lesions have more severe sensory deficits. Posterior thalamic radiation injury also correlates with sensory impairment (30, 35).
Cortico-cerebellar circuits, measured using functional MRI (fMRI), were well preserved in almost all patients. Despite this well-preserved cortico-cerebellar circuitry, no correlations were found with sensory deficits. Thus, this intact circuitry did not compensate for sensory deficits (26, 41, 43).
Proprioception
When assessing proprioceptive deficits in relation to lesion types, this relationship is less clear. In the study of Feys et al. no significant differences in proprioception between lesion types were found (7). In contrast, Kuczynski et al. found deficits in position sense to be more common and also more severe in children with MCA-type lesions (31, 32). Guzetta et al. included only one patient with an impaired position sense; this patient had the most severe sensory impairment (29). It should be noted that in all studies a substantial number of patients with normal proprioception were included, which potentially caused a bias in the conclusions (7, 29, 31–33, 42, 43). Another potential explanation lies in the assessment itself, since sensory deficits are most likely to be underestimated in patients with CP (22).
Functional connectivity between the non-lesioned S1 and thalamus/SMA in patients with cerebral palsy is inversely correlated to position sense; higher functional connectivity is associated with better performance. Whereas in typically developing children, position sense is positively correlated with connectivity between the thalamus and bilateral sensorimotor regions; increased connectivity is associated with poorer performance. Overall, the thalamus showed decreased connectivity in children with PVL-type lesions compared to controls, suggesting that early lesions can disrupt sensory network components, and connectivity between these areas is related to tactile perception deficits (45).
There is limited evidence of interhemispheric reorganization of the somatosensory functions, only two studies reported on patients with an interhemispheric reorganization, and these patients had the most severe sensory deficit. So when an interhemispheric reorganization is observed, this reorganization does not lead to improvement of sensory function (29, 34).
Neurorehabilitation and sensory deficits
In recent years, rehabilitation programs have paid more attention to enhancing sensory functions during CIMT/HABIT(ILE) programs (23, 46–49) and in study protocols (50–52). As in our systematic review, these studies show a large variability in study design, patient characteristics, and sensory assessment methods used. This makes it difficult to compare the findings. No distinction was made based on lesion type across these studies/ in the study protocols. However, all studies found an improvement in one or more sensory domains, making it worthwhile to explore these differences and the effect of lesion characteristics on the ability to achieve these differences (23, 46–49). In a study on adult stroke patients, different altered patterns of cortical activation were observed following touch discrimination training when patients with thalamic/capsular lesions were compared with patients with S1/S2 cortical somatosensory lesions. These changes were different despite common training and similar improvement (53). If the ability to change cortical activation in relation to lesion type and in relation to sensory improvement could be unraveled, a foundation could be laid for a more individualized training program.
Study limitations
There are several limitations to be considered. Due to the large variability in study design, patient characteristics, neuroimaging/neurophysiological techniques and outcome parameters, and sensory assessment methods used, only a partial synthesis of evidence was possible. In addition, some of the papers included in this systematic review used the same study population. Ten of the twenty-two articles included described at least one patient over eighteen. Not wanting to exclude a large portion of the studies and thus missing essential observations, led to the selection of papers including both children and young adults in this systematic review. The populations are by nature small and often heterogeneous. Several papers included children with relatively minor sensory deficits, which potentially caused a bias in the conclusions (29, 42, 43).
When reviewing the literature, another paper was discovered, which should have been included in the original search. This paper by Chu et al. researched the reorganization of hand somatosensory cortex in children with CP (54). Fortunately, we did not miss any essential information because this study shows similar results to the papers included in the original search. Key words in the paper of Chu were “perinatal brain injury” as opposed to “cerebral palsy” used in our original search terms. This might be the reason this paper was not included in the original search.
As we included only original research papers, the reviews of Brun et al. (12) and Poitras et al. (13), in which somatosensory deficits in children with cerebral palsy were discussed, were not selected for this review. Although neural correlates were mentioned, the main focus in these reviews was on different sensory domains. The review of Garberova et al. focused on somatosensory function in relation to fMRI, (functional magnetic resonance imaging), disregarding other modalities (55). This makes our review complementary to these reviews.
Conclusion and recommendation
In conclusion, it is hard to draw definite inferences on the relationship between the reorganization of the sensory network following early brain damage and sensory function in children with CP because of the large variability in study design, patient characteristics, neuroimaging/neurophysiological techniques, and parameters and sensory assessment methods used. In general, lesion timing, lesion location, lesion extent, integrity of the ascending sensory tract, and structural abnormality of the somatosensory areas have an impact on sensory function. In line with these observations, patients with cortical MCA lesions have more severe sensory deficits across all sensory modalities as opposed to patients with white matter (PVL) lesions. Intrahemisferic reorganization is the most common type of reorganization of the sensory system. In addition, an interhemisferic reorganization of the sensory system was associated with poor sensory function. International consensus on a clinically relevant sensory test battery is needed to enhance understanding of the intriguing compensatory mechanisms of sensory network following early brain damage and potential consequences for rehabilitation approaches.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AK and CS these authors share first authorship and contributed equally to writing first draft manuscript. CS and RV selected included articles. YJ quality assessment, revision of manuscript. AD revision of manuscript. RV revision of manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgements
We would like to acknowledge S.M. Koudijs for her involvement at the start of the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bax M, Goldstein M, Rosenbaum P, Leviton A, Paneth N, Dan B, et al. Proposed definition and classification of cerebral palsy, April 2005. Dev Med Child Neurol. (2005) 47(8):571–6. doi: 10.1017/S001216220500112X
2. Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat. (2020) 16:1505–18. doi: 10.2147/NDT.S235165
3. van Eck M, Dallmeijer AJ, van Lith IS, Voorman JM, Becher J. Manual ability and its relationship with daily activities in adolescents with cerebral palsy. J Rehabil Med. (2010) 42(5):493–8. doi: 10.2340/16501977-0543
4. Arner M, Eliasson AC, Nicklasson S, Sommerstein K, Hägglund G. Hand function in cerebral palsy. Report of 367 children in a population-based longitudinal health care program. J Hand Surg Am. (2008) 33(8):1337–47. doi: 10.1016/j.jhsa.2008.02.032
5. Krägeloh-Mann I, Horber V. The role of magnetic resonance imaging in elucidating the pathogenesis of cerebral palsy: a systematic review. Dev Med Child Neurol. (2007) 49(2):144–51. doi: 10.1111/j.1469-8749.2007.00144.x
6. Aisen ML, Kerkovich D, Mast J, Mulroy S, Wren TA, Kay RM, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. (2011) 10(9):844–52. doi: 10.1016/S1474-4422(11)70176-4
7. Feys H, Eyssen M, Jaspers E, Klingels K, Desloovere K, Molenaers G, et al. Relation between neuroradiological findings and upper limb function in hemiplegic cerebral palsy. Eur J Paediatr Neurol. (2010) 14(2):169–77. doi: 10.1016/j.ejpn.2009.01.004
8. Elliott D. The legacy of the kennard principle. J Undergrad Neurosci Educ. (2020) 19(1):R11–14.33880106
9. K MA. Age and other factors in motor recovery from precentral lesions in monkeys. Am J Physiol. (1936) 115:137–46. doi: 10.1152/ajplegacy.1936.115.1.138
10. Staudt M. Reorganization after pre- and perinatal brain lesions. J Anat. (2010) 217(4):469–74. doi: 10.1111/j.1469-7580.2010.01262.x
11. Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krägeloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. (2004) 56(6):854–63. doi: 10.1002/ana.20297
12. Brun C, Traverse É, Granger É, Mercier C. Somatosensory deficits and neural correlates in cerebral palsy: a scoping review. Dev Med Child Neurol. (2021) 63(12):1382–93. doi: 10.1111/dmcn.14963
13. Poitras I, Martinie O, Robert MT, Campeau-Lecours A, Mercier C. Impact of sensory deficits on upper limb motor performance in individuals with cerebral palsy: a systematic review. Brain Sci. (2021) 11(6):744. doi: 10.3390/brainsci11060744
14. Yardımcı-Lokmanoğlu BN, Bingöl H, Mutlu A. The forgotten sixth sense in cerebral palsy: do we have enough evidence for proprioceptive treatment? Disabil Rehabil. (2020) 42(25):3581–90. doi: 10.1080/09638288.2019.1608321
15. Papadelis C, Butler EE, Rubenstein M, Sun L, Zollei L, Nimec D, et al. Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts. Neuroimage Clin. (2018) 17:198–212. doi: 10.1016/j.nicl.2017.10.021
16. Lamp G, Goodin P, Palmer S, Low E, Barutchu A, Carey LM. Activation of bilateral secondary somatosensory Cortex with right hand touch stimulation: a meta-analysis of functional neuroimaging studies. Front Neurol. (2018) 9:1129. doi: 10.3389/fneur.2018.01129
17. Lee SD, Jung Y, Chung YA, Lee W. Neural substrates in secondary somatosensory area for the perception of different tactile sensations. Int J Imaging Syst Technology. (2016) 26:85–91. doi: 10.1002/ima.22160
18. Boakye M, Huckins SC, Szeverenyi NM, Taskey BI, Hodge CJ Jr. Functional magnetic resonance imaging of somatosensory cortex activity produced by electrical stimulation of the median nerve or tactile stimulation of the index finger. J Neurosurg. (2000) 93(5):774–83. doi: 10.3171/jns.2000.93.5.0774
19. Young JP, Herath P, Eickhoff S, Choi J, Grefkes C, Zilles K, et al. Somatotopy and attentional modulation of the human parietal and opercular regions. J Neurosci. (2004) 24(23):5391–9. doi: 10.1523/JNEUROSCI.4030-03.2004
20. Auld ML, Boyd R, Moseley GL, Ware R, Johnston LM. Tactile function in children with unilateral cerebral palsy compared to typically developing children. Disabil Rehabil. (2012) 34(17):1488–94. doi: 10.3109/09638288.2011.650314
21. Auld ML, Boyd R, Moseley GL, Ware R, Johnston LM. Impact of tactile dysfunction on upper-limb motor performance in children with unilateral cerebral palsy. Arch Phys Med Rehabil. (2012) 93(4):696–702. doi: 10.1016/j.apmr.2011.10.025
22. Bleyenheuft Y, Gordon AM. Precision grip control, sensory impairments and their interactions in children with hemiplegic cerebral palsy: a systematic review. Res Dev Disabil. (2013) 34(9):3014–28. doi: 10.1016/j.ridd.2013.05.047
23. Kuo HC, Gordon AM, Henrionnet A, Hautfenne S, Friel KM, Bleyenheuft Y. The effects of intensive bimanual training with and without tactile training on tactile function in children with unilateral spastic cerebral palsy: a pilot study. Res Dev Disabil. (2016) 49–50:129–39. doi: 10.1016/j.ridd.2015.11.024
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Kmet LM, Cook LS, Lee RC. Standard quality assessment criteria for evaluating primary research papers from a variety of fields (2004).
26. Dinomais M, Groeschel S, Staudt M, Krägeloh-Mann I, Wilke M. Relationship between functional connectivity and sensory impairment: red flag or red herring? Hum Brain Mapp. (2012) 33(3):628–38. doi: 10.1002/hbm.21227
27. Fiori S, Guzzetta A, Pannek K, Ware RS, Rossi G, Klingels K, et al. Validity of semi-quantitative scale for brain MRI in unilateral cerebral palsy due to periventricular white matter lesions: relationship with hand sensorimotor function and structural connectivity. Neuroimage Clin. (2015) 8:104–9. doi: 10.1016/j.nicl.2015.04.005
28. Gupta D, Barachant A, Gordon AM, Ferre C, Kuo HC, Carmel JB. Effect of sensory and motor connectivity on hand function in pediatric hemiplegia. Ann Neurol. (2017) 82(5):766–80. doi: 10.1002/ana.25080
29. Guzzetta A, Bonanni P, Biagi L, Tosetti M, Montanaro D, Guerrini R, et al. Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol. (2007) 118(5):1110–21. doi: 10.1016/j.clinph.2007.02.014
30. Hoon AH Jr., Stashinko EE, Nagae LM, Lin DD, Keller J, Bastian A, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. (2009) 51(9):697–704. doi: 10.1111/j.1469-8749.2009.03306.x
31. Kuczynski AM, Dukelow SP, Semrau JA, Kirton A. Robotic quantification of position sense in children with perinatal stroke. Neurorehabil Neural Repair. (2016) 30(8):762–72. doi: 10.1177/1545968315624781
32. Kuczynski AM, Semrau JA, Kirton A, Dukelow SP. Kinesthetic deficits after perinatal stroke: robotic measurement in hemiparetic children. J Neuroeng Rehabil. (2017) 14(1):13. doi: 10.1186/s12984-017-0221-6
33. Kuczynski AM, Carlson HL, Lebel C, Hodge JA, Dukelow SP, Semrau JA, et al. Sensory tractography and robot-quantified proprioception in hemiparetic children with perinatal stroke. Hum Brain Mapp. (2017) 38(5):2424–40. doi: 10.1002/hbm.23530
34. Lemée JM, Chinier E, Ali P, Labriffe M, Ter Minassian A, Dinomais M. (Re)organisation of the somatosensory system after early brain lesion: a lateralization index fMRI study. Ann Phys Rehabil Med. (2020) 63(5):416–21. doi: 10.1016/j.rehab.2019.02.001
35. Mailleux L, Simon-Martinez C, Radwan A, Blommaert J, Gooijers J, Wenderoth N, et al. White matter characteristics of motor, sensory and interhemispheric tracts underlying impaired upper limb function in children with unilateral cerebral palsy. Brain Struct Funct. (2020) 225(5):1495–509. doi: 10.1007/s00429-020-02070-1
36. Perivier M, Delion M, Chinier E, Loustau S, Nguyen S, Ter Minassian A, et al. Relationship between somatosensory deficit and brain somatosensory system after early brain lesion: a morphometric study. Eur J Paediatr Neurol. (2016) 20(3):403–11. doi: 10.1016/j.ejpn.2015.11.013
37. Simon-Martinez C, Jaspers E, Mailleux L, Ortibus E, Klingels K, Wenderoth N, et al. Corticospinal tract wiring and brain lesion characteristics in unilateral cerebral palsy: determinants of upper limb motor and sensory function. Neural Plast. (2018) 2018:2671613. doi: 10.1155/2018/2671613
38. de Souza RT, Ciasca SM, Moura-Ribeiro MV, Zanardi VA. Hemiparetic cerebral palsy: clinical data compared with neuroimaging. Rev Bras Fisioter. (2006) 10(2):143–7. doi: 10.1590/S1413-35552006000200004
39. Thickbroom GW, Byrnes ML, Archer SA, Nagarajan L, Mastaglia FL. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol. (2001) 49(3):320–7. doi: 10.1002/ana.68
40. Tsao H, Pannek K, Fiori S, Boyd RN, Rose S. Reduced integrity of sensorimotor projections traversing the posterior limb of the internal capsule in children with congenital hemiparesis. Res Dev Disabil. (2014) 35(2):250–60. doi: 10.1016/j.ridd.2013.11.001
41. Wilke M, Staudt M, Juenger H, Grodd W, Braun C, Krägeloh-Mann I. Somatosensory system in two types of motor reorganization in congenital hemiparesis: topography and function. Hum Brain Mapp. (2009) 30(3):776–88. doi: 10.1002/hbm.20545
42. Van de Winckel A, Verheyden G, Wenderoth N, Peeters R, Sunaert S, Van Hecke W, et al. Does somatosensory discrimination activate different brain areas in children with unilateral cerebral palsy compared to typically developing children? An fMRI study. Res Dev Disabil. (2013) 34(5):1710–20. doi: 10.1016/j.ridd.2013.02.017
43. Van de Winckel A, Klingels K, Bruyninckx F, Wenderoth N, Peeters R, Sunaert S, et al. How does brain activation differ in children with unilateral cerebral palsy compared to typically developing children, during active and passive movements, and tactile stimulation? An fMRI study. Res Dev Disabil. (2013) 34(1):183–97. doi: 10.1016/j.ridd.2012.07.030
44. Wingert JR, Sinclair RJ, Dixit S, Damiano DL, Burton H. Somatosensory-evoked cortical activity in spastic diplegic cerebral palsy. Hum Brain Mapp. (2010) 31(11):1772–85. doi: 10.1002/hbm.20977
45. Woodward KE, Carlson HL, Kuczynski A, Saunders J, Hodge J, Kirton A. Sensory-motor network functional connectivity in children with unilateral cerebral palsy secondary to perinatal stroke. Neuroimage Clin. (2019) 21:101670. doi: 10.1016/j.nicl.2019.101670
46. Jobst C, D'Souza SJ, Causton N, Master S, Switzer L, Cheyne D, et al. Somatosensory plasticity in hemiplegic cerebral palsy following constraint induced movement therapy. Pediatr Neurol. (2022) 126:80–8. doi: 10.1016/j.pediatrneurol.2021.09.019
47. Matusz PJ, Key AP, Gogliotti S, Pearson J, Auld ML, Murray MM, et al. Somatosensory plasticity in pediatric cerebral palsy following constraint-induced movement therapy. Neural Plast. (2018) 2018:1891978. doi: 10.1155/2018/1891978
48. Saussez G, Van Laethem M, Bleyenheuft Y. Changes in tactile function during intensive bimanual training in children with unilateral spastic cerebral palsy. J Child Neurol. (2018) 33(4):260–8. doi: 10.1177/0883073817753291
49. Maitre NL, Jeanvoine A, Yoder PJ, Key AP, Slaughter JC, Carey H, et al. Kinematic and somatosensory gains in infants with cerebral palsy after a multi-component upper-extremity intervention: a randomized controlled trial. Brain Topogr. (2020) 33(6):751–66. doi: 10.1007/s10548-020-00790-5
50. McLean B, Blakeman M, Carey L, Ward R, Novak I, Valentine J, et al. Discovering the sense of touch: protocol for a randomised controlled trial examining the efficacy of a somatosensory discrimination intervention for children with hemiplegic cerebral palsy. BMC Pediatr. (2018) 18(1):252. doi: 10.1186/s12887-018-1217-5
51. Chorna O, Heathcock J, Key A, Noritz G, Carey H, Hamm E, et al. Early childhood constraint therapy for sensory/motor impairment in cerebral palsy: a randomised clinical trial protocol. BMJ Open. (2015) 5(12):e010212. doi: 10.1136/bmjopen-2015-010212
52. Araneda R, Sizonenko SV, Newman CJ, Dinomais M, Le Gal G, Ebner-Karestinos D, et al. Protocol of changes induced by early hand-arm bimanual intensive therapy including lower extremities (e-HABIT-ILE) in pre-school children with bilateral cerebral palsy: a multisite randomized controlled trial. BMC Neurol. (2020) 20(1):243. doi: 10.1186/s12883-020-01820-2
53. Carey LM, Abbott DF, Lamp G, Puce A, Seitz RJ, Donnan GA. Same intervention-different reorganization: the impact of lesion location on training-facilitated somatosensory recovery after stroke. Neurorehabil Neural Repair. (2016) 30(10):988–1000. doi: 10.1177/1545968316653836
54. Chu D, Huttenlocher PR, Levin DN, Towle VL. Reorganization of the hand somatosensory cortex following perinatal unilateral brain injury. Neuropediatrics. (2000) 31(2):63–9. doi: 10.1055/s-2000-7475
Keywords: cerebral palsy, sensory function, functional connectivity, systematic review, somatosensory representation
Citation: Knijnenburg ACS, Steinbusch CVM, Janssen-Potten YJM, Defesche A and Vermeulen RJ (2023) Neuro-imaging characteristics of sensory impairment in cerebral palsy; a systematic review. Front. Rehabil. Sci. 4:1084746. doi: 10.3389/fresc.2023.1084746
Received: 30 October 2022; Accepted: 28 February 2023;
Published: 17 March 2023.
Edited by:
Hércules Ribeiro Leite, Federal University of Minas Gerais, BrazilReviewed by:
Ana Carolina De Campos, Federal University of São Carlos, BrazilRachel Hawe, University of Minnesota Twin Cities, United States
© 2023 Knijnenburg, Steinbusch, Janssen-Potten, Defesche and Vermeulen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
Specialty Section: This article was submitted to Pediatric Rehabilitation, a section of the journal Frontiers in Rehabilitation Sciences
*Correspondence: A. C. S. Knijnenburg YW5uZW1hcmllLmtuaWpuZW5idXJnQG11bWMubmw=
†These authors have contributed equally to this work
 A. C. S. Knijnenburg
A. C. S. Knijnenburg C. V. M. Steinbusch
C. V. M. Steinbusch Y. J. M. Janssen-Potten3,4,5,6
Y. J. M. Janssen-Potten3,4,5,6 R. J. Vermeulen
R. J. Vermeulen