- 1MetroHealth Rehabilitation Institute, MetroHealth System, Cleveland, OH, United States
- 2Department of Physical Medicine and Rehabilitation, Case Western Reserve University School of Medicine, Cleveland, OH, United States
- 3Department of Physical Medicine and Rehabilitation, McGovern Medical School, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 4The Institute of Rehabilitation / Research (TIRR) Memorial Hermann, Houston, TX, United States
- 5The KITE Research Institute, University Health Network, Toronto, ON, Canada
- 6Department of Physical Therapy, University of Toronto, Toronto, ON, Canada
- 7HealthTech Connex Centre for Neurology Studies/Neuromotion Physiotherapy, Vancouver, BC, Canada
- 8Krembil Research Institute-University Health Network, Toronto, ON, Canada
- 9Institute of Biomedical Engineering, University of Toronto, Toronto, ON, Canada
- 10CRANIA, University Health Network, Toronto, ON, Canada
- 11Department of Health Research Methods, Evidence, and Impact, Faculty of Health Sciences, McMaster University, Hamilton ON, Canada
- 12Biostatistics Unit, St. Joseph’s Healthcare, Hamilton, ON, Canada
- 13Faculty of Health Sciences, University of Johannesburg, Johannesburg, Gauteng, South Africa
Background: Loss of upper extremity function after tetraplegia results in significant disability. Emerging evidence from pilot studies suggests that functional electrical stimulation (FES) therapy may enhance recovery of upper extremity function after tetraplegia. The aim of this trial was to determine the effectiveness of FES therapy delivered by the Myndmove stimulator in people with tetraplegia.
Methods: A multi-center, single-blind, parallel-group, two-arm, randomized controlled trial was conducted comparing FES to conventional therapy in adults (≥18 years) with C4–C7 traumatic incomplete tetraplegia between 4 and 96 months post-injury, and with a baseline spinal cord injury independence measure III -self-care (SCIM III-SC) score of ≤10. Participants were enrolled at four SCI-specialized neurorehabilitation centers in the U.S. and Canada. Participants were stratified by center and randomized in a 1:1 ratio to receive either 40 sessions of FES or conventional therapy targeting upper extremities over a 14-week period. Blinded assessors measured SCIM III, Toronto Rehabilitation Institute Hand Function Test, and Graded Redefined Assessment of Strength, Sensibility, and Prehension at baseline, after 20th session, after 40th session or 14 weeks after 1st session, and at 24 weeks after 1st session. The primary outcome measure was change in SCIM III-SC from baseline to end of the treatment. Based on the primary outcome measure, a sample size of 60 was calculated. Seventeen participants' progress in the study was interrupted due to the COVID-19 lockdown. The protocol was modified for these participants to allow them to complete the study.
Results: Between June 2019 to August 2021, 51 participants were randomized to FES (n = 27) and conventional therapy (n = 24). Both groups gained a mean of 2 points in SCIM-SC scores at the end of treatment, which was a clinically meaningful change. However, there was no statistically significant difference between the groups on any outcomes.
Conclusion: Forty sessions of FES therapy delivered by the MyndMove stimulator are as effective as conventional therapy in producing meaningful functional improvements that persist after therapy is completed. Limitations of this study include the impact of COVID-19 limiting the ability to recruit the target sample size and per-protocol execution of the study in one-third of the participants.
Registration: This trial is registered at www.ClinicalTrials.gov, NCT03439319.
Introduction
Recovery of upper extremity function after spinal cord injury (SCI) is reported as the highest priority by individuals living with tetraplegia, which is essential to improve independence and quality of life after SCI (1). Therefore, it is vital to study and develop interventions to improve the upper extremity motor deficits following SCI. Conventional rehabilitation is the most common approach to restoring motor function after SCI. However, therapeutic interventions beyond conventional therapy are limited in availability. Hence, there is an interest in combining rehabilitation therapy with various interventions including functional electrical stimulation (FES) to enhance the effects of conventional rehabilitation therapy.
Emerging evidence suggests that FES therapy, when combined with conventional rehabilitation interventions, results in greater improvement in motor function after SCI and stroke (2–4). FES provides small electrical pulses to stimulate motor neurons via surface or implanted electrodes to facilitate muscle contraction during a functional activity (5, 6). There are different approaches to using FES therapy to treat individuals living with SCI. One of the techniques is the short-term therapeutic application of FES to improve voluntary hand function. During this type of FES therapy, individuals are asked to perform or attempt functional movements with their paralyzed extremity, combined with electrical stimulation of muscles responsible for producing that movement. This process is repeated and practiced with multiple functional movements during a session. Many FES systems have been developed for this purpose, including the NESS H200, the Bionic Glove and its newer version the HandEstim Wireless Hand Stimulator, the Compex Motion system and its later commercial version the MyndMove stimulator (5–12).
FES therapy delivered by the Compex Motion system was studied in a single-site pilot randomized controlled trial (RCT). The efficacy of 40 h of FES therapy (Compex Motion system) with conventional therapy (n = 9) was compared to conventional therapy alone (n = 12) to improve grasp function in people with subacute traumatic incomplete cervical SCI (13). The key outcomes were changes in Functional Independence Measure (FIM) self-care subscores, Spinal Cord Independence Measure III (SCIM) self-care subscores, and Toronto Rehabilitation Institute Hand Function Test (TRI-HFT) performed at baseline and after the intervention at the end of 8 weeks. The FES therapy group had greater improvement in SCIM self-care and TRI-HFT scores. These findings were consistent with previous studies of FES therapy performed by this group in individuals with stroke (14).
Based on these single-center clinical studies of FES therapy delivered by the Compex Motion system, the commercial version of the stimulator (named MyndMove) was developed for use as a neurorehabilitation intervention tool applicable across a broad range of inpatient and outpatient clinical settings. The MyndMove stimulator is cleared as a non-significant risk device by the U.S. Food and Drug Administration and exempt from an Investigational Device Exemption (IDE; reference file Q131135). The MyndMove stimulator is used to deliver non-invasive FES therapy via surface electrodes to pursue target muscle contractions to facilitate functional movements. It is hypothesized that the massed practice of these FES-facilitated functional movements induces neuroplasticity, restoring the voluntary function of the upper extremities.
As a next step, a large multi-center RCT was conducted to compare the effectiveness of FES therapy (delivered by the MyndMove stimulator) to conventional therapy (C.T.) in improving upper extremity motor function in individuals with incomplete cervical SCI. We hypothesized that 40 h of FES therapy would result in greater improvements in upper extremity function and quality of life in individuals with cervical traumatic incomplete subacute to early chronic SCI compared to an equal dose of conventional therapy.
Materials and methods
Summary of design and participants
This multi-site study was designed as a parallel-group, two-arm, single-blind, RCT comparing FES therapy to C.T. in individuals with incomplete tetraplegia. The detailed protocol was previously published (12). A summary description is provided below.
Eligible participants were individuals age 18 and older, with C4–7 traumatic SCI, ASIA Impairment Scale (AIS) grades B-D, between 4 and 96 months post-injury, and with a baseline SCIM III self-care (SCIM-SC) subscale score ≤10 (full inclusion and exclusion criteria published in Anderson et al. (12). Participants were enrolled at four SCI-specialized neurorehabilitation centers in the U.S. and Canada. A sample size of 60 was calculated based on the hypothesis that the mean difference in SCIM-SC in the FES group is better than the C.T. group after 14 weeks of treatment [full description of sample size calculation described in Anderson et al. (12) as well as rationale for selecting the SCIM-SC as the primary outcome]. Figure 1 depicts the study design and timeline.
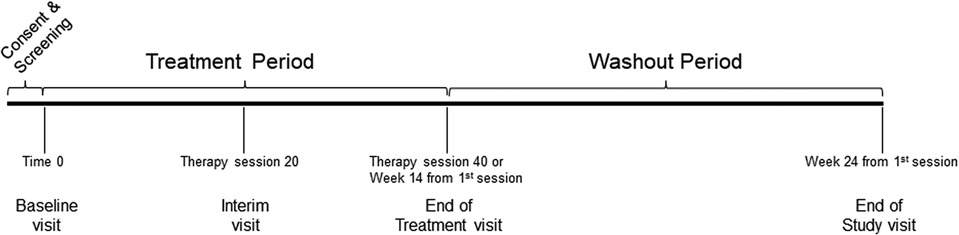
Figure 1. Study design and timeline. The baseline visit was considered Time 0 and randomization occured within three days after the visit. The treatment period included forty therapy sessions that had to be completed in a time window of no more than fourteen weeks from the first therapy session. The washout period was from the last therapy until twenty-four weeks from the first therapy session.
Allocation and intervention
Participants were stratified by study center and randomized in a 1:1 ratio based on permutated blocks of random sizes to one of the two treatment arms below:
1. FES therapy (intervention group)—participants engaged in 36–40 1-hour sessions of FES therapy within a 14-week period.
2. C.T. (active control group)—participants engaged in 36–40 1-hour sessions of upper limb conventional therapy within a 14-week period.
FES therapy was provided using the MyndMove device (MyndTec, Inc., Mississauga, Canada) (12). Based on each participant's clinical presentation and goals, therapists could choose from eight movement patterns during each 1-hour therapy session. The movement patterns were palmar grasp, lateral pinch grasp, pinch grasp, lumbrical grasp, tripod grasp, side reach with finger extension, forward reach and grasp, and hand to mouth [see Anderson et al. (12) for full description]. These preprogrammed movement patterns are intended to facilitate task-specific movements that are practiced in a massed or distributed fashion. The FES therapy group did not receive C.T.
Conventional therapy (C.T.) served as an active control and was intended as a time equivalent to the FES group. Also based on each participant's clinical presentation and goals, therapists could choose from seven categories of conventional upper limb rehabilitation therapy [see Anderson et al. (12) for full description]. The categories were facilitation of reach or prehension movements, bilateral task-specific movements, range of motion and mobilization of joints, splinting, sensorimotor stimulation, electrical stimulation (single muscles for strength, not function), and reduction of edema. The type, frequency, and duration of each category was not prescribed aside from fitting within the 1-hour therapy session time frame to meet the 36–40 sessions within a 14-week period.
For both groups, therapy was delivered in 1-hour sessions with participants engaging in 3–5 sessions each week. Completing 36–40 sessions within a 14-week period was considered a successful completion of the intervention protocol.
Assessments and analyses
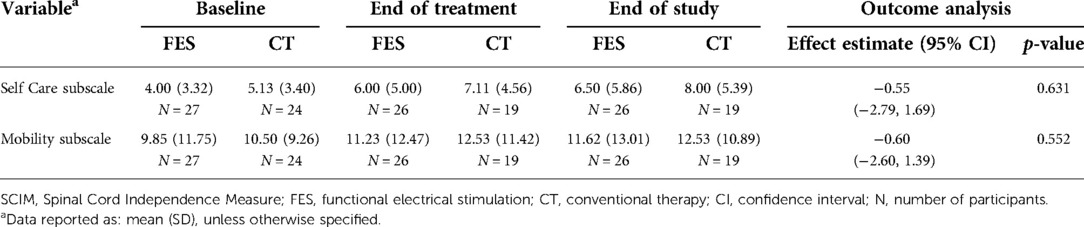
Therapists not involved with treatment performed blinded assessments of three functional outcome measures [SCIM III, TRI-HFT, Graded Redefined Assessment of Strength, Sensibility, and Prehension (GRASSP)] at Baseline, Interim (after 20th session), End of Treatment (after 40th session/14 weeks from 1st session), and End of Study (24 weeks from 1st session). The primary outcome (the change in SCIM-SC subscale score from Baseline to End of Treatment) was derived from the full SCIM III. Secondary analyses of all time points compared to baseline included SCIM-SC and mobility subscores, the TRI-HFT subscores, and the GRASSP subscores.
The SCIM-SC was chosen as the primary outcome based on the results of the pilot study (15) and because it is recommended as the “Supplemental Highly Recommended” outcome measure to use when assessing function in persons with SCI (SCI Common Data Elements). The SCIM, TRI-HFT, and GRASSP were all designed specifically for SCI (Catz et al., 1997 (16); Kapadia et al., 2012 (17); Kalsi-Ryan et al., 2009 (18), and have all been validated in SCI (Itzkovich et al., 2007 (19); Kapadia et al., 2012 (17); Kalsi-Ryan et al., 2012 (20). All components of each outcome measure were used.
Participant demographics and disease characteristics were captured at baseline. Unblinded assessments were obtained on 9 of the 22 Spinal Cord Injury-Quality of Life (SCI-QOL) outcome scales at Baseline, End of Treatment, and End of Study. All adverse events were recorded, graded, and evaluated for relationship to study intervention.
Primary, secondary, and sensitivity analyses were described in detail in the protocol publication (12). The statistician was blind to study group. Briefly, we adopted an intention-to-treat principle to analyze all outcomes and used multiple imputation to handle missing data. We used analysis of covariance adjustiging for baseline score for all outcomes. The criterion for statistical significance was set at alpha = 0.05. The results are reported as estimate of effect, corresponding 95% confidence interval (CI), and associated p-value. All p-values are reported to three decimal places with those less than 0.001 reported as p < 0.001. We performed some sensitivity analyses to assess to robustness of the results on the primary outcome of SCIM-SC subscale score using: (i) per-protocol approach that included only those participants that adhered to the protocol; (ii) last observation carried forward to handle missing data; (iii) adjusting for age, SCIM-SC baseline subscale score. All analyses were performed using SAS 9.4 (Cary, NC).
Unplanned interruption due to COVID-19
Seventeen participants (Supplementary Table S1) whose therapy sessions were paused at the time of the COVID-19 lockdown (March 2020) in the U.S. and Canada were dealt with in the following manner when each resumed activity in the trial:
1. Those who completed less than or equal to 20 sessions at the time of the lockdown (N = 7 of 17) were asked to restart the study with a new baseline assessment of SCIM, TRI-HFT, and GRASSP,
2. Those who completed more than 20 sessions (N = 3 of 17 plus 1 passed away prior to restart) were restarted at the therapy session number they had paused at and completed the remaining sessions (a 2nd interim assessment of SCIM, TRI-HFT, and GRASSP was performed prior to restarting the study in these participants), and
3. Participants who completed 40 sessions at the time of the lockdown but missed their follow-up assessments (N = 6 of 17) had an end of treatment assessment performed when studies were restarted in their respective institutions.
Ethics approval
This study had ethics approval from: MetroHealth System Institutional Review Board (IRB18-0751); University Health Network Research Ethics Board (REB17-6029); University of Texas Health Science Center IRB (HSC-MS-18–0862); Advarra IRB for HealthTech Connex Centre for Neurology Studies (Pro00030094); as well as approval from the US Army Medical Research and Materiel Command, Office of Research Protections and Human Research Protection Office.
Results
Participants
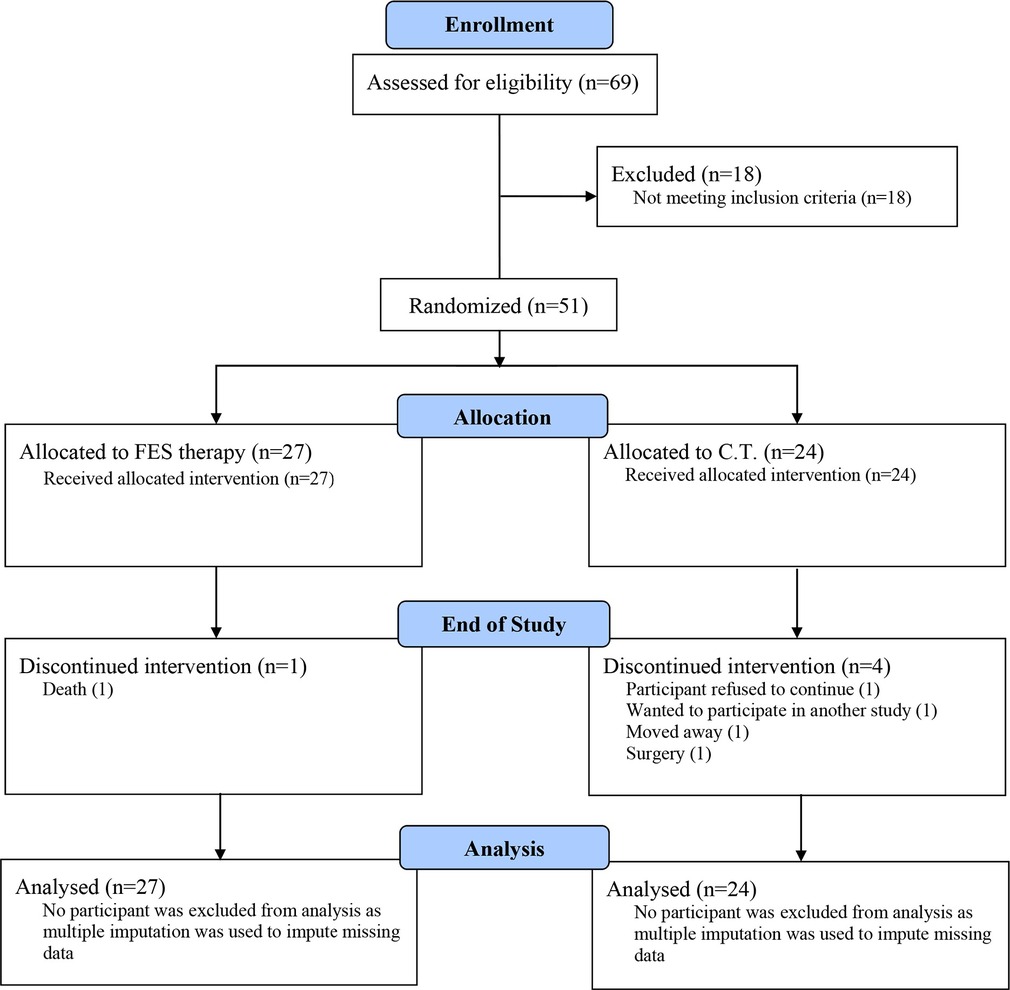
The first participant was enrolled in June 2019 and the final participant was enrolled in August 2021. Table 1 shows the CONSORT flow diagram for the study. Of the 69 individuals assessed, 51 were randomized to a study group. The COVID-19 pandemic did impact enrollment. As a result, the randomization target of 60 was not met.
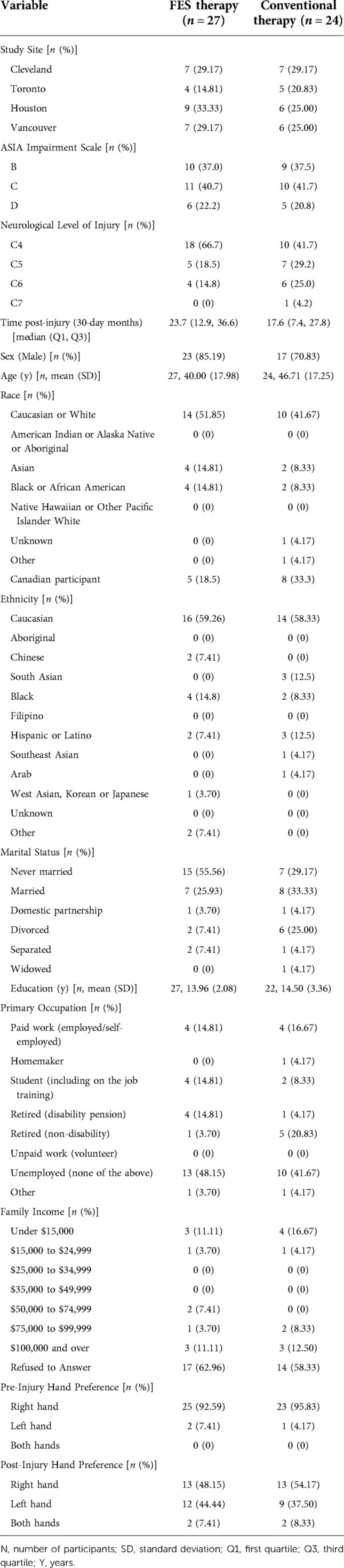
Table 2 contains site distribution, demographic, and injury characteristic data for the 51 randomized participants. Based on these specific parameters, there were no significant differences between the FES group compared to the C.T. group, but there was a trend for individuals in the FES group to have a higher level of injury and to be slightly longer post-injury than those in the C.T. group.
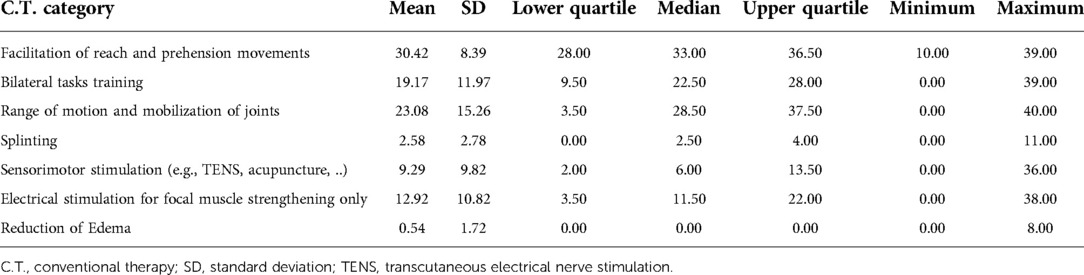
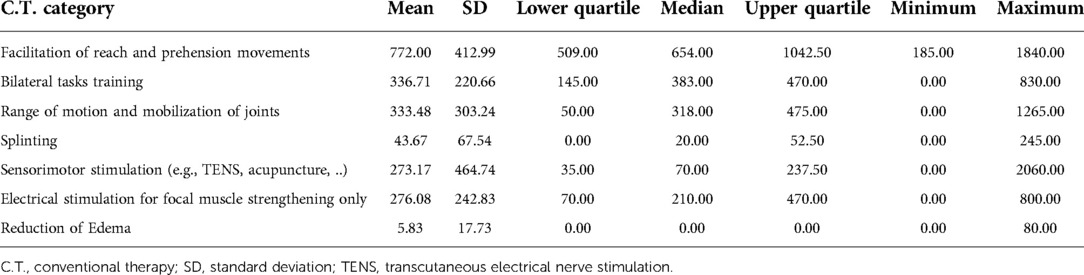
Table 3 provides a summary of the frequency of categories utilized during the C.T. group therapy sessions and Table 4 provides a summary of the minutes spent in each category. The most frequent category used (and for the longest duration) during C.T. therapy sessions was facilitation of reach and prehension movements.
Primary outcomes
The primary outcome for the study is the change in SCIM-SC between baseline and end of treatment, hypothesizing that FES therapy is better than C.T. The SCIM-SC subscale ranges from 0 to 20 points. A change of 1.12 points is considered a small meaningful change and a change of 2.8 points is considered a substantial meaningful change (16). Figure 2A shows the mean, median, and upper and lower interquartile range for both groups. Both groups gained a mean of 2 points at the end of treatment, which persisted at the end of study (secondary outcome). However, there was no statistically significant difference between either group (Table 5).
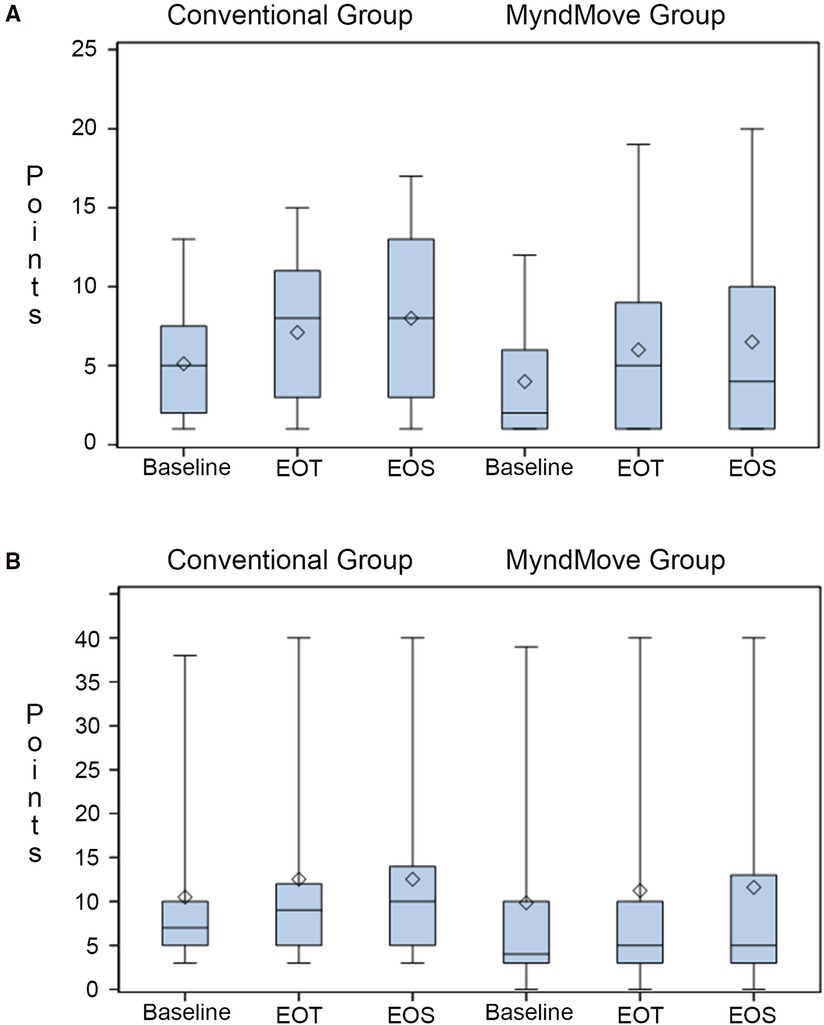
Figure 2. Box plots of spinal cord independence measure (SCIM) scale. (A) SCIM self-care subscale results. (B) SCIM mobility subscale results. Mean (line), median (diamond), quartile 1, and quartile 3 presented in each plot. EOT, end of treatment; EOS, end of study.
Secondary outcomes
The SCIM mobility subscale includes various mobility items involving the upper and lower extremities and the subscore ranges from 0 to 40 points. The change in this subcore between groups across all time points was a secondary outcome. There is not a single meaningful change for the full mobility subscale (16), it is subdivided into the three room/toilet questions [small meaningful change = 0.58, substantial meaningful change = 1.45] and the six indoors/outdoors questions [small meaningful change = 0.78, substantial meaningful change = 1.95]. Figure 2B shows the mean, median, and upper and lower interquartile range for both groups. Both groups gained a mean of 2 points (across the full subscale) at the end of treatment, which persisted at the end of study. However, there was no statistically significant difference between groups (Table 5).
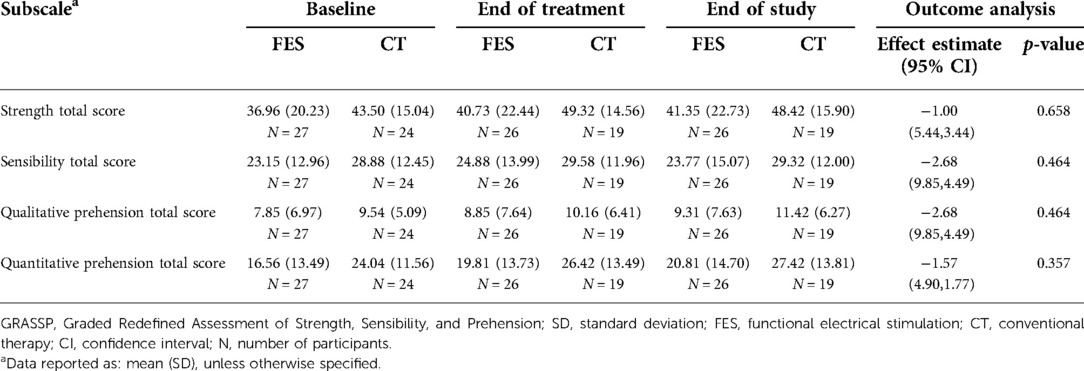
The GRASSP outcome data are presented as the change in total score of each subscale (Table 6) at end of treatment and end of study compared to baseline. The subscales are strength, sensibility, qualitative prehension, and quantitative prehension. The minimal detectable difference (MDD) and the minimal clinically important difference (MCID) are known for the GRASSP quantitative prehension subscale. The MDD is 9.7 points for the combined score and 6.0/5.3 for right/left side scores (17) and the MCID is 6.4 points (18). Though both groups gained points at the end of treatment that persisted at the end of study, the gains in the quantitative prehension subscore did not reach MDD or MCID and the differences were not statistically significant.
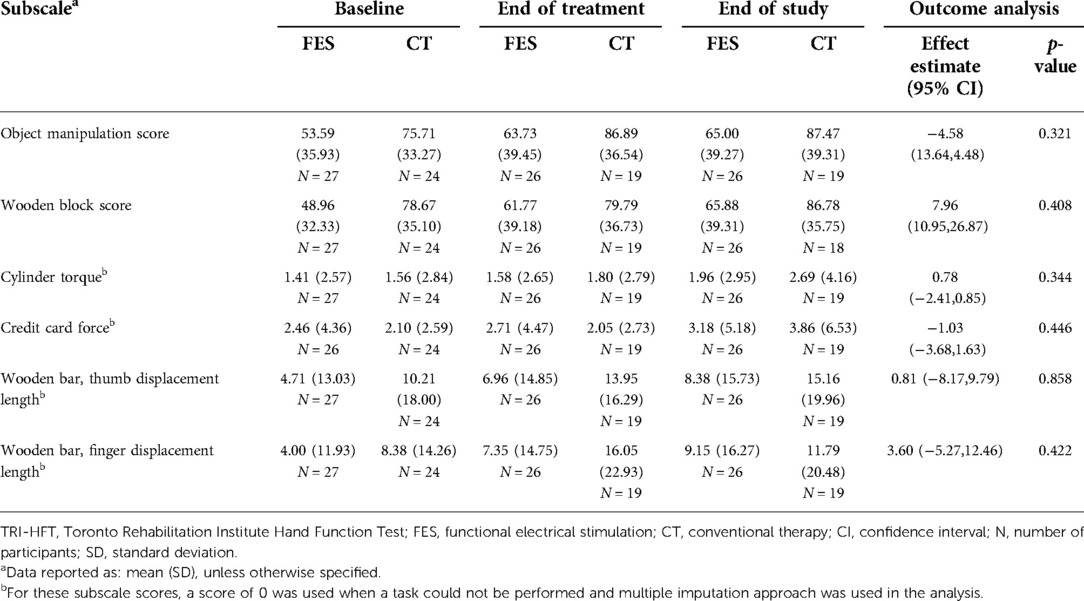
The TRI-HFT outcome data are also presented as the change in total score of each subscale (Table 7) at end of treatment and end of study compared to baseline. The subscales are object manipulation, wooden block, cylinder torque, credit card force, wooden bar thumb displacement length, and wooden bar finger displacement length. The minimal detectable change (MDC) with 95% confidence intervals is 9.1 for the object manipulation subscale and 9.2 for the wooden block subscale (calculated using the formula MDC = 1.96 × SEM × √2 (19), by author ND using a subacute SCI dataset provided by author MP; SEM was calculated using the formula SEM = SD/√N as the ICC of test-retest reliability for the dataset used was not available). The MDC was achieved for the object manipulation subscale in both groups at the end of treatment (object manipulation = 10.14 points in FES group, 11.18 points in C.T. group), but these changes were not statistically significant. For the wooden block subscale, the FES group exceeded the MDC (12.81 points) at the end of treatment while the C.T. group did not (1.12 points).
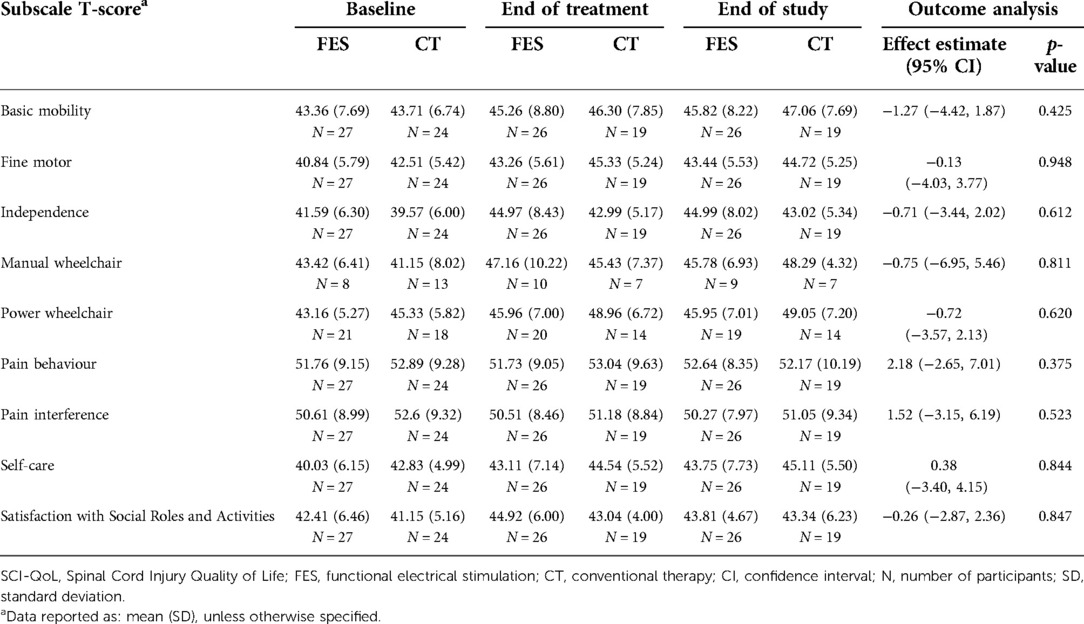
The outcome data for the SCI-QOL basic mobility, fine motor, manual wheelchair, power wheelchair, self-care, independence, pain behavior, pain interference, and satisfaction with social roles and activities subscales are presented in Table 8. There were minimal gains in both groups on several subscales, however the standard deviations were high, and no differences were statistically significant (Table 8).
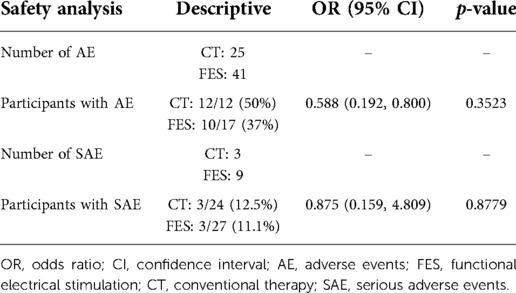
Safety was assessed based on serious and non-serious adverse events in each group. Table 9 shows the total number of adverse events (AE) and serious adverse events (SAE) in each group and the related analysis. There were no statistically significant differences in the proportion of participants having experienced an AE or SAE between either group. The types of SAE that occurred fell into the categories of urinary, respiratory, fever/sepsis, and skin (not related to electrode sites) complications that resulted in overnight hospitalizations. One participant died while enrolled in the trial and the death was not related to study activities. One participant experienced two AE that were definitely related to the FES therapy (redness at electrode site). That same participant experienced two other AE that were possibly related to FES therapy (left dorsum hand swelling, right ventral forearm swelling).
Sensitivity analysis
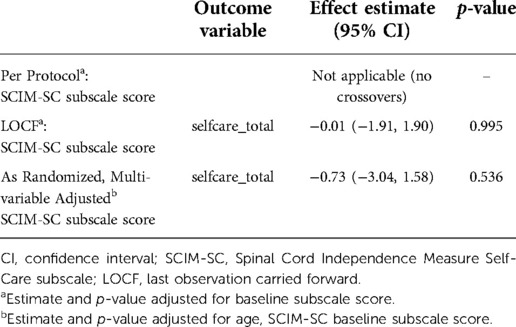
The following sensitivity analyses were conducted to assess the robustness of results: (1) per-protocol, based on participants with complete data, (2) last observation carried forward, for imputed missing data, and (3) adjusted, based on age SCIM-SC baseline subscore. The results are in Table 10 and are consistent with the main analysis results.
COVID interruption
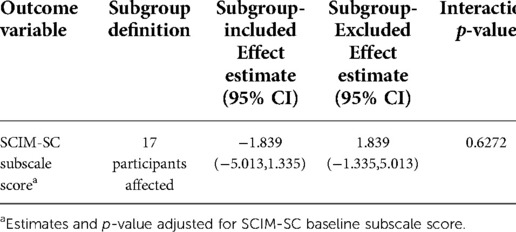
Because the interruption due to the COVID-19 pandemic lockdown impacted 17 of the 51 participants, we analysed whether that interruption impacted the primary outcome (change in SCIM-SC subscale score at the end of treatment). Using multiple imputation analysis with the subgroup included of excluded, there was no significant effect of the primary outcome (Table 11).
Discussion
The key findings from this trial indicate that FES therapy (delivered using the MyndMove stimulator) and conventional therapy both produce more than a small meaningful change in SCIM self-care in individuals with traumatic C4–7 incomplete SCI in the subacute to early chronic time range post-injury. Additionally, FES therapy is safe and has a similar safety profile as conventional therapy.
The results of this study are different from a previous study (13) in which FES therapy using the Compex Motion stimulator (1 h) was compared to C.T. (1 h). That trial also involved individuals with C4–7 AIS B-D SCI, but they were within six months post-injury so all participants also received an additional hour of C.T. as part of their standard clinical care. Each group received 10 h of therapy per week for eight weeks, and in that situation, FES therapy was superior. Time post-injury could contribute to the difference in results in the current study as the FES group was a median of twenty-four months post-injury and the C.T. group was a median of eighteen months post-injury, as opposed to an average of two months post-injury in the previous study (13). It is known that the majority of natural recovery occurs within the first twelve months post-injury (20, 21). However, it is also known that some activity-based therapies as well as conventional therapies can improve upper extremity function in individuals well past twelve months post-injury (22), especially in individuals with incomplete tetraplegia. A pilot study comparing FES therapy (Compex Motion stimulator) to C.T. in persons greater than twenty-four months post-injury suggested more improvement over control (15), justifying including early chronic time points in the current trial. The dose of therapy could also be a contributing factor to the observed differences. In the current study (chronic), participants received 40 h of research therapy over fourteen weeks whereas in the previous study (sub-acute), participants received 40 h of research therapy and 40 h of clinical therapy over eight weeks (due to the inclusion of only sub-acute SCI which coincided with the timing of clinical therapy after an injury). By expanding the time window in the current study to include individuals up to ninety-six months/eight years post-injury, variability was introduced in regard to the amount of clinical therapy individuals may have had access to. It has been demonstrated that the type of therapy may be more important than the dose of therapy in regard to improving hand function in individuals with incomplete tetraplegia (23).
The results of the current trial must also be interpreted through the haze of the COVID-19 pandemic. The lockdown across Canada and the U.S. started ten months after the beginning of enrollment and lasted for varying lengths of time across the four study centers. Two sites were shut down for approximately three months, one site was shut down for approximately five months, and one site was shut down for approximately ten months. As shown in Supplementary Table S1, seventeen participants were paused mid-protocol due to the lockdown. When each site was able to resume in-person research activities, the paused participants were invited to resume the trial based on the plan described in the Methods. However, not each participant resumed immediately (some reasons included hesitancy to be in-person, complications that occurred while on pause, etc.). Hence, there was great variability between each of the seventeen participants related to the number of therapy sessions completed prior to the lockdown, the length of time on pause, the number of sessions repeated upon resuming trial activities, and the timing of outcome assessments relative to the first session. The effect of these variables on outcomes is not known. Additionally, the length of the COVID-19 pandemic impacted our ability to reach the randomization target of 60 participants based on power analysis. Enrollment was expected to be completed by December 2020 but was extended for an additional eight months, at which point enrollment had to be closed due to the funding timeline.
There are several limitations to the current trial. Based on the review of baseline data of SCIM-SC, GRASSP, TRI-HFT, and neurological level, it appears that participants in the FES group were likely a little more severely impaired than those in the C.T. group, which could have affected the trajectory of recovery or capacity for improvement between the two groups. Randomization was stratified by site but not based on baseline function. We suggest stratifying participants with incomplete SCI based on the baseline function of the primary outcome to allow for equal distribution between groups in future studies. Similarly, though the median time post-injury was not statistically different between the two groups, there appears to be a shift of about five months between the groups, with the FES group being slightly longer post-injury.
Categories of C.T. were prescribed for the trial, but the implementation was not standardized between sites as was done for FES therapy. Therefore, the active controls in this cohort may have received different categories of interventions based on the site's standard for C.T.
In conclusion, forty sessions of FES therapy delivered via the MyndMove stimulator are as effective as C.T. in producing meaningful functional improvements that persist after therapy is completed. Future post hoc analyses of this trial's dataset could include a responder analysis, right and left side analysis, or stratification based on baseline function.
Data availability statement
All data from this work will be maintained at the Biostatistics Unit at St. Joseph's Healthcare Hamilton. Data sharing and access to the trial dataset is incorporated into the Clinical Trial Agreements between the MyndTec Inc. and each of the Principal Investigators and Sites according to institutional requirements. Access to the trial dataset by individuals outside the study will be reviewed on a case-by-case basis.
Ethics statement
The studies involving human participants were reviewed and approved by MetroHealth System Institutional Review Board (IRB18-0751); University Health Network Research Ethics Board (REB17-6029); University of Texas Health Science Center IRB (HSC-MS-18–0862); Advarra IRB for HealthTech Connex Centre for Neurology Studies (Pro00030094); as well as approval from the US Army Medical Research and Materiel Command, Office of Research Protections and Human Research Protection Office. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All of the authors made substantial contributions to study design (KDA, RK, KEM, JP, JRW, NY, ND, MRP, LT); KDA, RK, KEM, JP, JRW, NY, and LT were involved in conduct; all authors were involved in analyses and drafting of this manuscript for important intellectual content and gave final approval of the version to be published (KDA, RK, KEM, JP, JRW, NY, ND, MRP, LT). All authors contributed to the article and approved the submitted version.
Funding
This trial is funded in part under the U.S. AMRMC, award number W81XWH-16-1-0790, SC150251. MyndTec Inc. was responsible for training and distribution related to the MyndMove device and resolution of clinical and technical device-related issues throughout the study. MyndTec Inc provided additional funding for costs which were not covered by funding. In addition, funding provided through the grant was administered to the participating sites through MyndTec Inc. A blinded assessor at the Cleveland site was partially supported by the V.A. Rehabilitation R/D Center for Advanced Platform Technology (APT) Center, Grant 2 I50 RX001871-06.
Acknowledgments
The authors would like to thank all of the participants for engaging in this clinical trial despite the onset of an unforeseen, prolonged pandemic. Special thanks to Darshika Mistry for her project management throughout all aspects of this trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2022.995244/full#supplementary-material.
References
1. Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. (2004) 21:1371–83. doi: 10.1089/neu.2004.21.1371
2. Beekhuizen KS, Field-Fote EC. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil. (2008) 89:602–8. doi: 10.1016/j.apmr.2007.11.021
3. Hebert DA, Bowen JM, Ho C, Antunes I, O’Reilly DJ, Bayley M. Examining a new functional electrical stimulation therapy with people with severe upper extremity hemiparesis and chronic stroke: a feasibility study. Br J Occup Ther. (2017) 80(11):651–9. doi: 10.1177/0308022617719807
4. Mangold S, Keller T, Curt A, Dietz V. Transcutaneous functional electrical stimulation for grasping in subjects with cervical spinal cord injury. Spinal Cord. (2005) 43:1–13. doi: 10.1038/sj.sc.3101644
5. Handa Y, Handa T, Ichie M, Murakami H, Hoshimiya N, Ishikawa S, et al. Functional electrical stimulation (FES) systems for restoration of motor function of paralyzed muscles–versatile systems and a portable system. Front Med Biol Eng. (1992) 4:241–55. PMID: 1476953
6. Popovic MR, Thrasher TA, Adams ME, Takes V, Zivanovic V, Tonack MI. Functional electrical therapy: retraining grasping in spinal cord injury. Spinal Cord. (2006) 44:143–51. doi: 10.1038/sj.sc.3101822
7. IJzerman MJ, Stoffers TS, in ‘T Groen FACG, Klatte MAP, Snoek GJ, Vorsteveld JHC, et al. The NESS Handmaster orthosis: restoration of hand function in C5 and stroke patients by means of electrical stimulation. J Rehabil Sci. (1996) 9:86–9.
8. Nathan RH, Ohry A. Upper limb functions regained in quadriplegia: a hybrid computerized neuromuscular stimulation system. Arch Phys Med Rehabil. (1990) 71:415–21. PMID: 2334287
9. Popovic DSA, Pjanovic A, Radosavljevic S, Popovic M, Jovic S, Vulovic D. Clinical evaluation of the bionic glove. Arch Phys Med Rehabil. (1999) 80:299–304. doi: 10.1016/S0003-9993(99)90141-7
10. Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Arch Phys Med Rehabil. (1997) 78:608–14. doi: 10.1016/S0003-9993(97)90426-3
11. Snoek GJ, IJzerman MJ, in ‘t Groen FA, Stoffers TS, Zilvold G. Use of the NESS handmaster to restore handfunction in tetraplegia: clinical experiences in ten patients. Spinal Cord. (2000) 38:244–9. doi: 10.1038/sj.sc.3100980
12. Anderson KD, Wilson JR, Korupolu R, Pierce J, Bowen JM, O’Reilly D, et al. Multicentre, single-blind randomised controlled trial comparing MyndMove neuromodulation therapy with conventional therapy in traumatic spinal cord injury: a protocol study. BMJ Open. (2020) 10:e039650. doi: 10.1136/bmjopen-2020-039650 32988951
13. Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. (2011) 25:433–42. doi: 10.1177/1545968310392924
14. Thrasher TA, Zivanovic V, McIlroy W, Popovic MR. Rehabilitation of reaching and grasping function in severe hemiplegic patients using functional electrical stimulation therapy. Neurorehabil Neural Repair. (2008) 22:706–14. doi: 10.1177/1545968308317436
15. Kapadia N, Zivanovic V, Popovic MR. Restoring voluntary grasping function in individuals with incomplete chronic spinal cord injury: pilot study. Top Spinal Cord Inj Rehabil. (2013) 19:279–87. doi: 10.1310/sci1904-279
16. Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM-spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord. (1997) 35:850–6. doi: 10.1038/sj.sc.3100504
17. Kapadia N, Zivanovic V, Verrier M, Popovic MR. Toronto rehabilitation institute-hand function test: assessment of gross motor function in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. (2012) 18:167–86. doi: 10.1310/sci1802-167
18. Kalsi-Ryan S, Curt A, Fehlings MG, Verrier MC. Assessment of the hand in tetraplegia using the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): impairment versus function. Top Spinal Cord Inj Rehabil. (2009) 14:34–46. doi: 10.1310/sci1404-34
19. Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT, Craven BC, et al. The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil Rehabil. (2007) 29:1926–33. doi: 10.1080/09638280601046302
20. Kalsi-Ryan S, Beaton D, Curt A, Duff S, Popovic MR, Rudhe C, et al. The graded redefined assessment of strength sensibility and prehension: reliability and validity. J Neurotrauma. (2012) 29:905–14. doi: 10.1089/neu.2010.1504
21. Scivoletto G, Tamburella F, Laurenza L, Molinari M. The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil Rehabil. (2013) 35:1808–13. doi: 10.3109/09638288.2012.756942
22. Kalsi-Ryan S, Beaton D, Ahn H, Askes H, Drew B, Curt A, et al. Responsiveness, sensitivity, and minimally detectable difference of the Graded and Redefined Assessment of Strength, Sensibility, and Prehension, Version 1.0. J Neurotrauma. (2016) 33:307–14. doi: 10.1089/neu.2015.4217
23. Marino RJ, Sinko R, Bryden A, Backus D, Chen D, Nemunaitis GA, et al. Comparison of responsiveness and minimal clinically important difference of the Capabilities of Upper Extremity Test (CUE-T) and the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP). Top Spinal Cord Inj Rehabil. (2018) 24:227–38. doi: 10.1310/sci2403-227
24. Stratford PN. Getting more from the literature: estimating the standard error of measurement from reliability studies. Physiother Can. (2004) 56:27–30. doi: 10.2310/6640.2004.15377
25. Curt A, Van Hedel HJA, Klaus D, Dietz V. Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma. (2008) 25:677–85. doi: 10.1089/neu.2007.0468
26. Marino RJ, Burns S, Graves DE, Leiby BE, Kirshblum S, Lammertse DP. Upper- and lower-extremity motor recovery after traumatic cervical spinal cord injury: an update from the national spinal cord injury database. Arch Phys Med Rehabil. (2011) 92:369–75. doi: 10.1016/j.apmr.2010.09.027
27. Quel de Oliveira C, Refshauge K, Middleton J, de Jong L, Davis GM. Effects of activity-based therapy interventions on mobility, independence, and quality of life for people with spinal cord injuries: a systematic review and meta-analysis. J Neurotrauma. (2017) 34:1726–43. doi: 10.1089/neu.2016.4558
Keywords: functional electrical simulation (FES), spinal cord injury, tetraplegia, therapy, rehabilation
Citation: Anderson KD, Korupolu R, Musselman KE, Pierce J, Wilson JR, Yozbatiran N, Desai N, Popovic MR and Thabane L (2022) Multi-center, single-blind randomized controlled trial comparing functional electrical stimulation therapy to conventional therapy in incomplete tetraplegia. Front. Rehabilit. Sci. 3:995244. doi: 10.3389/fresc.2022.995244
Received: 15 July 2022; Accepted: 25 August 2022;
Published: 9 September 2022.
Edited by:
Ashraf S. Gorgey, Hunter Holmes McGuire VA Medical Center, United StatesReviewed by:
Eric Turner Harness, Neuro Ex, Inc, United StatesFeilong Zhu, Beijing Normal University, China
© 2022 Anderson, Korupolu, Musselman, Pierce, Wilson, Yozbatiran, Desai, Popovic and Thabane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kim D. Anderson a3hhMzA0QGNhc2UuZWR1
Specialty Section: This article was submitted to Interventions for Rehabilitation, a section of the journal Frontiers in Rehabilitation Sciences
 Kim D. Anderson
Kim D. Anderson Radha Korupolu
Radha Korupolu Kristin E. Musselman
Kristin E. Musselman Jacqueline Pierce7
Jacqueline Pierce7 Nuray Yozbatiran
Nuray Yozbatiran Naaz Desai
Naaz Desai Lehana Thabane
Lehana Thabane