- 1Department of Veterans Affairs (VA) Rocky Mountain Mental Illness Research Education and Clinical Center (MIRECC) for Veteran Suicide Prevention, Aurora, CO, United States
- 2Department of Physical Medicine and Rehabilitation, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 3Departments of Physical Medicine and Rehabilitation and Psychiatry, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 4Departments of Physical Medicine and Rehabilitation, Psychiatry, and Neurology, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
Background: Sleep problems are common among Veterans with mild traumatic brain injury (mTBI) and may contribute to participation restrictions. However, explanatory mechanisms underlying this relationship are poorly understood. Sleep problems are associated with post-concussive symptoms (e.g., headaches). In turn, post-concussive symptoms contribute to participation restrictions. We hypothesized that post-concussive symptom severity mediates the purported relationship between sleep problems and participation restrictions among Veterans with mTBI.
Materials and Methods: This study was a retrospective analysis of clinical data among 8,733 Veterans with mTBI receiving Veterans Health Administration outpatient care. Sleep problems (yes/no) were identified using the sleep-related item from the Neurobehavioral Symptom Inventory (NSI). Post-concussive symptoms were measured using remaining NSI items. Participation restrictions were measured using the Mayo-Portland Adaptability Inventory Participation Index. We specified a latent variable path model to estimate relationships between: (1) sleep problems and three latent indicators of post-concussive symptoms [vestibular-sensory (e.g., headache)]; mood-behavioral [e.g., anxiety]; cognitive [e.g., forgetfulness]); and, (2) the three latent indicators of post-concussive symptoms and two latent indicators of participation restrictions (social and community participation [e.g., leisure activities]; productivity [e.g., financial management]). We examined the indirect effects of sleep problems upon participation restrictions, as mediated by post-concussive symptoms. Estimates were adjusted for sociodemographic factors (e.g., age), injury characteristics (e.g., blast), and co-morbid conditions (e.g., depression).
Results: 87% of Veterans reported sleep problems. Sleep problems were associated with greater social and community participation restrictions, as mediated by mood-behavioral (β = 0.41, p < 0.001) and cognitive symptoms (β = 0.13, p < 0.001). There was no evidence that vestibular-sensory symptoms mediated this relationship (β = -0.01, p = 0.48). Sleep problems were associated with greater productivity restrictions, as mediated by vestibular-sensory (β = 0.16, p < 0.001) and cognitive symptoms (β = 0.14, p < 0.001). There was no evidence that mood-behavioral symptoms mediated this relationship (β = 0.02, p = 0.37).
Discussion: Findings suggest that evidence-based sleep treatment should occupy a prominent role in the rehabilitation of Veterans with mTBI. Indirect effects of sleep problems differed when considering impact on social and community participation vs. productivity, informing individualized rehabilitative care for Veterans with mTBI.
Introduction
Participation has been defined as involvement in activities which facilitate the fulfillment of socially defined roles (e.g., parent), connection with others, and the elicitation of subjective meanings (e.g., perceived competence) (1). Veterans of Operations Enduring Freedom, Iraqi Freedom, and New Dawn (post-9/11 Veterans) are at substantial risk for mild traumatic brain injury (mTBI) (2–5), which may contribute to participation restrictions (6). Participation restrictions among those with mTBI are in part due to post-concussive symptoms, or neurobehavioral symptoms that arise following the injury, which span across vestibular (e.g., balance), sensory (e.g., headache), behavioral (e.g., irritability), and/or cognitive (e.g., forgetfulness) domains (7–9). While sequalae of mTBI typically resolve within three months of the injury event, a notable subset experience persistent symptoms (10–15), posing ongoing risk for participation challenges among Veterans with mTBI.
Sleep problems are among the most prevalent and disabling post-concussive symptoms among post-9/11 Veterans. A nationwide study of post-9/11 Veterans revealed that 85% of those with clinician-confirmed traumatic brain injury (TBI; i.e., mild, moderate, or severe) experience sleep disturbance (16). Individuals with mTBI experience multi-faceted sleep problems, including greater daytime sleepiness, lesser total sleep time and difficulties with initiating and maintaining sleep (17). Poor sleep quality may prolong recovery from mTBI and exacerbate risk for participation restrictions (18–20). For example, sleep disturbances have been shown to predict decreased function up to a year following a mTBI, even after adjusting for other relevant factors (e.g., psychological distress) (21). However, the unique effect of sleep problems on participation restrictions among Veterans with mTBI is not well understood. Further, there is a need to establish empirical support for explanatory mechanisms underlying the purported relationship between participation restrictions and post-concussive sleep problems.
Post-concussive symptoms may serve as a mediating factor explaining the relationship between sleep problems and participation restrictions among Veterans with mTBI. The link between poor sleep quality and neurophysiological dysfunction is well-documented (22, 23). Likewise, sleep disruption may undermine these same neurophysiological processes and impede post-concussive recovery (18, 20). Indeed, poor sleep quality is linked to more severe post-concussive symptoms across vestibular, sensory, behavioral, and cognitive domains (24, 25). In turn, such post-concussive symptoms are a robust risk factor for participation restrictions among post-9/11 Veterans with TBI (8, 9).
The purpose of this study was to examine whether post-concussive symptoms mediate the relationship between sleep problems and participation restrictions among post-9/11 Veterans with mTBI. There is theoretical and empirical support for relationships between: (1) sleep problems and post-concussive symptoms (24, 25); and, (2) post-concussive symptoms and participation restrictions (8, 9). Thus, we hypothesized that the relationship between sleep problems and participation restrictions would be mediated by post-concussive symptoms. See supplementary material for a conceptual model illustrating hypothesized relationships. Understanding potential mechanisms (i.e., post-concussive symptoms) by which sleep problems and participation restrictions are related may inform strategies to enhance rehabilitative care for Veterans with mTBI.
Materials and methods
Participants and procedures
This study was a retrospective analysis of clinical data among a national sample of Veterans with mTBI who received outpatient care in the Veterans Health Administration (VHA) between 2012 and 2020. Study procedures were approved by the local Institutional Review Board and VA committees. Data was extracted from the Comprehensive TBI Evaluation (CTBIE) database, the National Veterans TBI Health Registry database (26), and the Corporate Data Warehouse, which stores VHA electronic medical record data (e.g., diagnoses). The CTBIE is an extensive clinical interview conducted with Veterans who screen positive for a potential TBI, per the VHA TBI screening protocol (27). The interview includes a thorough examination of the injury event (e.g., severity and mechanism) as well as severity of specific post-concussive symptoms. Starting in 2012, the administration of the Mayo-Portland Adaptability Inventory Participation Index (M2PI) subscale within 30 days of the CTBIE was encouraged (see details below) (8, 28). M2PI results are uploaded to the National Veterans TBI Health Registry database (26).
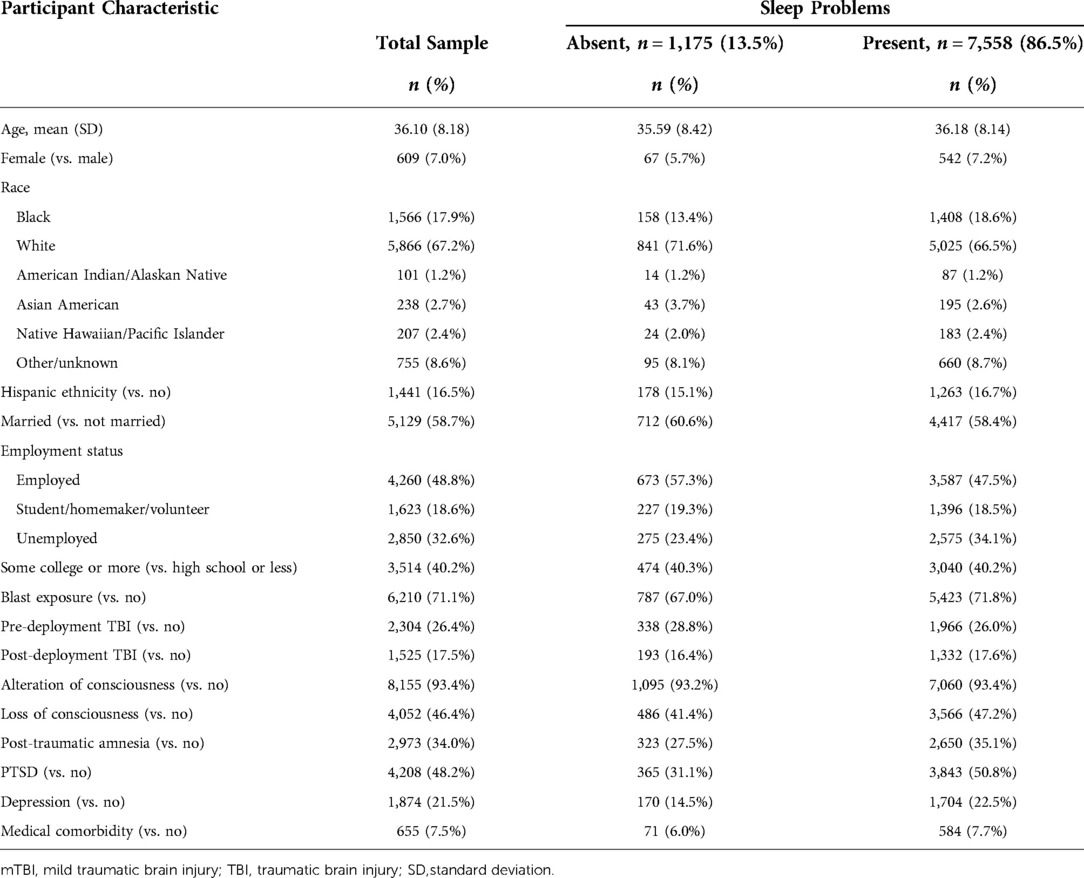
Veterans were included in this study if the following was documented: (1) a complete CTBIE; (2) a clinician-confirmed mTBI, per criteria specified in the VA/DoD Clinical Practice Guideline for mTBI (e.g., loss of consciousness: 0–30 min) (29); and, (3) a complete self-reported M2PI record within 30 days of the CTBIE. Records were excluded for Veterans who had: (1) an inpatient stay which overlapped with the date at which the CTBIE and/or M2PI was administered; and, (2) an M2PI administered by a clinician or significant other, as prior research indicates discrepancies across reporting method (30). For individuals with more than one CTBIE and/or M2PI, the first chronological record was used. We excluded six eligible individuals with incomplete data on covariates. The eligible study cohort for this study included 8,733 Veterans with clinician-confirmed mTBI.
Measures
Sleep problems
Presence of sleep problems (yes vs. no) was determined using the corresponding item from the Neurobehavioral Symptom Inventory (NSI), a 22-item assessment of post-concussive symptoms (31). Participants rated their “difficulty falling or staying asleep” on a scale ranging from 0 (“none”) to 4 (“very severe”). Consistent with past research (8, 16, 32), we dichotomized the sleep item to indicate the presence of sleep problems: “present” (score ≥2 [i.e., moderate to very severe, indicating impact on daily function]) vs. “absent/mild” (score <2 [i.e., no impact on daily function]).
Post-concussive symptoms
The remaining items from the NSI were used to measure post-concussive symptoms (31). Participants rated a variety of vestibular (e.g., loss of balance), sensory (e.g., headaches), behavioral (e.g., feeling anxious), and cognitive (e.g., poor concentration) symptoms on a scale ranging from 0 (“none”) to 4 (“very severe”). The validity and reliability of the NSI, including in post-9/11 Veterans with mTBI, has been documented (7, 33).
Participation restrictions
The 8-item participation index of the Mayo-Portland Adaptability Inventory-4 (M2PI) was used to measure participation (34). Participants rate the following activities on a 5-point scale from 0 (i.e., no participation restriction) to 4 (i.e., severe participation restriction): initiation of activities; social contact; leisure and recreational activities; self-care; residence management (e.g., meal preparation); transportation; employment/other employment; and, financial management. The full Mayo-Portland Adaptability Inventory-4 is valid and reliable among community-based individuals living with TBI, and its subscales (i.e., the M2PI) can be used as standalone assessments (34–36). The M2PI has exhibited adequate psychometric properties when administered to post-9/11 Veterans with TBI (37).
Covariates
We included the following sociodemographic characteristics as covariates: age (in years at the time of the CTBIE); gender (male vs. female); race (Black, American Indian/Alaskan Native, Asian American, Native Hawaiian/Pacific Islander, White, other/unknown); ethnicity (Hispanic vs. non-Hispanic); marital status (married vs. non-married); employment status (unemployed, student/homemaker/volunteer, employed); and, pre-military educational level (high school or less vs. some college or more).
We included the following injury characteristics as covariates: blast exposure (yes vs. no); pre-deployment TBI (yes vs. no); post-deployment TBI (yes vs. no); and, presence of any alteration of consciousness (yes vs. no), any loss of consciousness (yes vs. no), or any post-traumatic amnesia (yes vs. no).
Lastly, we included indicators of co-morbid mental and medical conditions as covariates. We included indicators of co-morbid posttraumatic stress disorder (PTSD; yes vs. no) and depression (yes vs. no) using ICD-9 or −10 codes within the VHA electronic medical record documented between one year prior and 90 days following the CTBIE. Probable PTSD and depression was determined by the presence of corresponding ICD-9 or −10 codes associated with either (1) two outpatient encounters or (2) one inpatient encounter during the above timeframe. Medical comorbidity (yes vs. no) was measured using the Charlson comorbidity index, derived using ICD-9 or -10 codes documented between one year prior and 90 days following the CTBIE (38).
Data analysis
We performed a descriptive analysis for observed and latent variables. Latent variable path analysis with a robust maximum likelihood estimator was used to evaluate hypotheses using Mplus Version 8.6 (39). For the measurement component of the model, consistent with default Mplus procedures, we identified latent variables of post-concussive symptoms and participation restrictions by estimating factor loadings and constraining latent variable means/intercepts to 0 and the factor variance to 1. The measurement (i.e., latent variables and covariance of residuals) and structural components of the model (i.e., inclusion of hypothesized paths amongst observed and latent variables) were evaluated and refined based on widely adopted model fit criteria (i.e., RMSEA ≈ 0.06; CFI ≈ 0.95; TLI ≈ 0.95; SRMR ≈ 0.08) (40) as well as theoretical rationale.
Upon arriving at a measurement model that fit the data reasonably well, we estimated the bivariate correlations between sleep problems and latent variables. We then specified two theoretically plausible latent variable path models. For Model 1, the following paths were estimated: (1) the path from sleep problems to latent indicators of post-concussive symptoms; and, (2) the path from each latent indicator of post-concussive symptoms to latent indicators of participation. For Model 2, we added the paths estimating the relationship between sleep problems and latent indicators of participation. We used BIC and a descriptive analysis of global fit indices (e.g., RMSEA) to compare model fit, thus determining which of the competing models was most consistent with the data.
Hypothesized mediation effects were tested using the product of coefficients method, from which we derived estimates of the indirect effect of sleep problems upon participation restrictions, as mediated by latent indicators of post-concussive symptoms (41). All estimates were adjusted for the aforementioned covariates. Statistical significance for all parameter estimates was evaluated at α = 0.05.
Results
A substantial portion of Veterans in the sample reported sleep problems (87%). The average age in our sample was 36 years, and most Veterans were White (67%), male (93%), and non-Hispanic (84%). Most reported exposure to a blast (71%), with a notable portion of the sample experiencing co-morbid PTSD (48%) and depression (22%). See Table 1.
Measurement component of the model
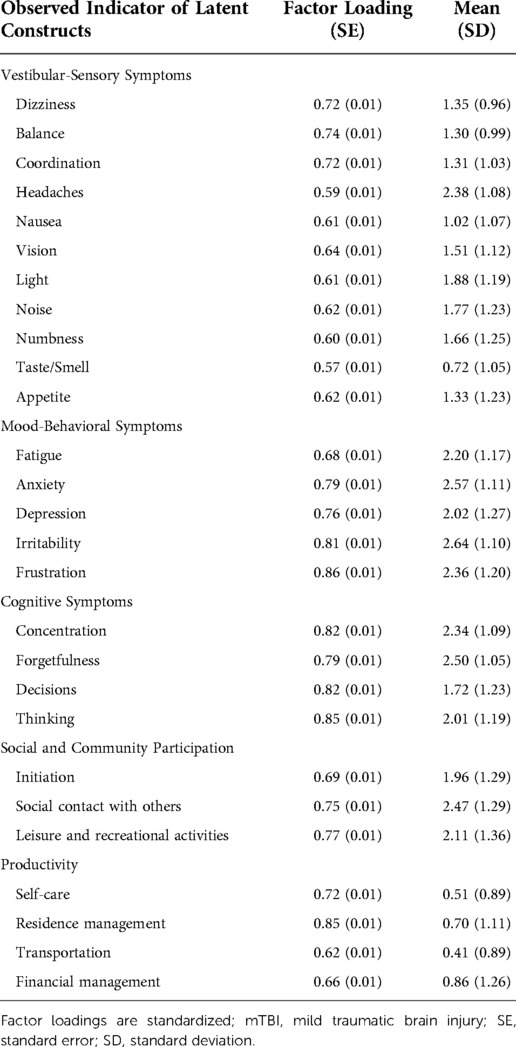
We first specified an unconditional measurement model, identifying three latent indicators of post-concussive symptoms and two latent indicators of participation (five total). All latent variable covariances were freely estimated. The model exhibited reasonable fit per standard global fit indices (RMSEA = 0.06, CFI = 0.92, TLI = 0.91, SRMR = 0.04).
The three latent indicators of post-concussive symptoms represented the following domains, similar to domains empirically derived in a prior study (7): Vestibular-Sensory (11 items; e.g., headache); Mood-Behavioral (5 items; e.g., frustration); and, Cognitive (4 items; e.g., forgetfulness). The hearing item was removed because it is consistent with prior examinations of the factor structure of the NSI and because it exhibited a relatively weak factor loading to the vestibular-sensory latent construct (0.50) (7, 42).
We identified two latent indicators of participation restrictions: (1) Social and community participation (comprised of initiation, social contact, and leisure items); and, (2) Productivity (comprised of self-care, residence management, transportation, and financial management items). We identified two distinct latent indicators of participation because the model with a single latent indicator of participation exhibited inadequate model fit (RMSEA = 0.17, CFI = 0.78, TLI = 0.67, SRMR = 0.08) (40). Further, theory holds that participation is multi-faceted, comprised of varying “types” of participation that can be distinguished based on the nature of activity engagement (e.g., social vs. productivity-based) (43). Consistent with prior studies (37, 44), the employment indicator was excluded due to low factor loadings on latent constructs (<0.40). See Table 2 for a summary of the measurement model.
Structural component of the model
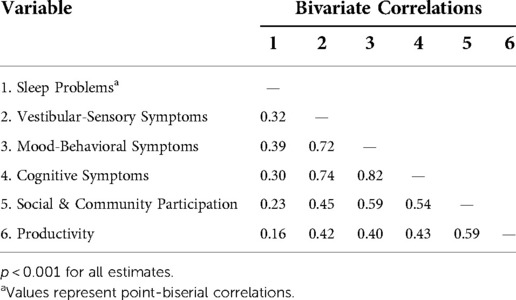
Examination of the bivariate correlations between sleep problems and latent indicators signaled relationships consistent with the hypothesized model. Specifically, those with sleep problems reported more severe post-concussive symptoms across domains, as well as greater restrictions in social and community participation and productivity. Similarly, more severe post-concussive symptoms were associated with greater restrictions in social and community participation and productivity. See Table 3.
We first specified Model 1, estimating the following paths: (1) sleep problems to each latent indicator of post-concussive symptoms (vestibular-sensory; mood-behavioral; cognitive); and, (2) the path from each latent indicator of post-concussive symptoms to each latent indicator of participation (social and community participation; productivity). This model fit the data well (RMSEA = 0.04, CFI = 0.91, TLI = 0.89, SRMR = 0.03) (40).
Subsequently, we specified Model 2, which included the additional paths from sleep problems to each latent indicator of participation, adjusted for post-concussive symptoms. We rejected Model 2 for two reasons. First, the added model complexity needed to specify Model 2 did not result in improved model fit compared to Model 1. Specifically, a descriptive analysis of model fit indices indicated identical model fit for Models 1 and 2 (RMSEA = 0.04, CFI = 0.91, TLI = 0.89, SRMR = 0.03). Further, examination of the BIC values for Model 1 (614066.81) and Model 2 (614077.38) yielded a BIC difference of 10.58, providing “very strong evidence” in favor of the more parsimonious model (Model 1) (45). Second, examination of the parameter estimates for the direct effects revealed the emergence of a negative suppressor effect (46). Specifically, while both theory and observed bivariate relations between sleep problems and productivity support a positive relationship (r = 0.16, p < 0.001), when included in the model with the latent indicators of post-concussive symptoms, a statistically significant and negative relationship between sleep problems and productivity emerged (b = −0.05, p = 0.004). It is best practice to remove one or both variables causing the suppressor effect and retain the more parsimonious model, assuming that doing so is consistent with relevant theory (46). As such, we rejected Model 2 and interpreted Model 1. See Figure 1 for a visual illustration of Model 1.
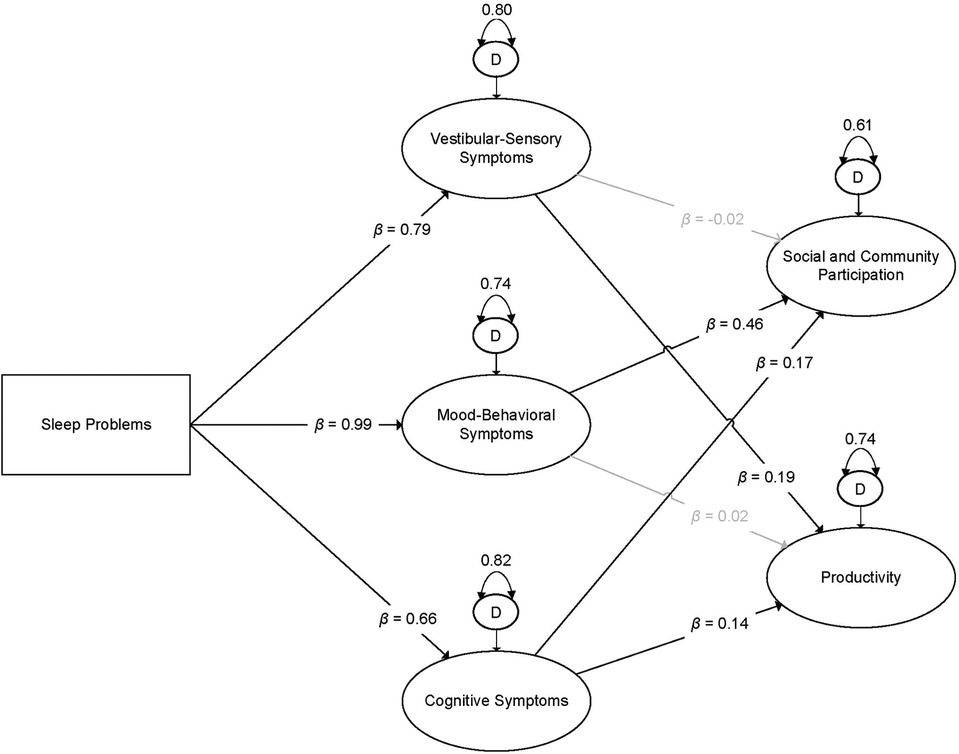
Figure 1. Visual illustration of the final latent variable path model. Boxes indicate observed variables; ovals indicate latent indicators. Dark lines indicate estimates that were statistically significant at α = 0.05; gray lines indicate relationships that were not statistically significant. β = standardized estimate; D = disturbance term (i.e., residual variance of latent indicator). All estimates are adjusted for sociodemographic, injury-related, and co-morbidity co-variates.
Indirect effect of sleep problems upon social and community participation
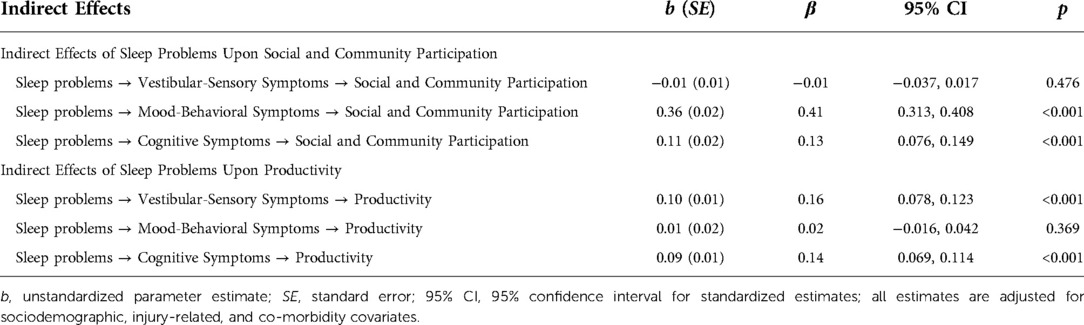
The indirect effects of sleep problems upon restrictions in social and community participation offered mixed support for hypotheses. Sleep problems were associated with greater restrictions in social and community participation, as mediated by mood-behavioral and cognitive symptoms. However, there was no evidence that vestibular-sensory symptoms mediated the relationship between sleep problems and social and community participation. Sleep problems were associated with more severe vestibular-sensory, mood-behavioral, and cognitive post-concussive symptoms. In turn, more severe mood-behavioral and cognitive, but not vestibular-sensory symptoms, were associated with greater restrictions in social and community participation. See Table 4 for indirect effect estimates and Table 5 for specific parameter estimates.
Indirect effect of sleep problems upon productivity
Similarly, we identified mixed support for our hypotheses regarding the indirect effect of sleep problems upon productivity. Sleep problems were associated with greater productivity restrictions, as mediated by vestibular-sensory and cognitive symptoms. There was no evidence that mood-behavioral symptoms mediated the relationship between sleep problems and productivity restrictions. As previously stated, sleep problems were associated with more severe post-concussive symptoms across all domains. More severe vestibular-sensory and cognitive symptoms, but not mood-behavioral symptoms, were associated with greater productivity restrictions.
Discussion
In a national sample of 8,733 Veterans with clinician-confirmed mTBI, we examined whether post-concussive symptoms mediated the relationship between sleep problems and participation restrictions. Generally, our proposed model was supported, but the indirect effect of sleep problems differed when considering distinct types of participation. While sleep problems were associated with more severe post-concussive symptoms across all three domains (vestibular-sensory, mood-behavioral, and cognitive), the impact of such symptoms upon Veterans' participation depended on the specific nature of the activities (social and community participation vs. productivity). Findings underscore the importance of integrating evidence-based sleep treatment in the rehabilitative care of Veterans with mTBI. Further, our findings can inform efforts aimed at tailoring such care to their individualized clinical needs.
Study findings provide empirical support for evidence-based sleep treatment occupying a prominent role in the rehabilitation of Veterans with mTBI. It has been recommended that post-concussive sleep problems be prioritized in those with mTBI because they are amenable to treatment and their reduction contributes to overall post-concussive recovery (18–20, 47). Our findings bolster and extend these claims by offering evidence of relationships which suggest that by prioritizing the treatment of sleep problems, rehabilitation services may also ameliorate participation restrictions in Veterans with mTBI. For instance, providing cognitive behavioral therapy for insomnia (CBT-I), an evidence-based treatment for insomnia, has been linked to both reduced neurobehavioral symptoms (e.g., depression) and enhanced participation in Veterans (48, 49). Enhancing Veterans' participation is an organizing principle of VHA rehabilitative care (28, 50). Our findings indicate that VHA rehabilitative care could achieve this worthy objective by prioritizing the clinical management of sleep problems.
Systematic effort should be devoted to aligning “real-world” rehabilitation for post-concussive sleep problems with evidence-based recommendations. First, we echo previous calls for the screening of sleep problems for individuals with TBI, including in rehabilitation settings (51, 52). Systematically detecting sleep problems is requisite for subsequent clinical management. Second, according to the VA/DoD Clinical Practice Guideline (CPG) for mTBI (29), clinical management of identified post-concussive sleep problems should align with recommendations within the VA/DoD CPG for the management of insomnia and obstructive sleep apnea (OSA; Sleep CPG) (53). The Sleep CPG includes evidence-based recommendations for the assessment and treatment of insomnia and OSA, two sleep conditions for which Veterans with mTBI are at disproportionately high risk (54). However, adherence to such recommendations within clinical practice may be variable. For example, there are documented barriers to the delivery of guideline-recommended care for post-concussive sleep problems in VHA rehabilitation settings (e.g., provider awareness) (55). Further, Veterans with mTBI and associated participation restrictions may experience unique barriers to accessing specialized sleep treatment. For example, sequalae of mTBI (e.g., cognitive impairment) may exacerbate logistical challenges (e.g., transportation) that pose a barrier to accessing sleep treatment among those without mTBI (56). Strategies that systematically target barriers to Veterans' receipt of evidence-based treatment for post-concussive sleep problems should be developed and evaluated (57). By enhancing the quality of care received, such efforts could promote sleep quality and overall post-concussive recovery among Veterans with mTBI.
Cognitive symptoms were the only post-concussive symptom domain observed to mediate the relationship between sleep problems and both indicators of participation, suggesting a potentially broad impact upon Veterans' functioning following mTBI. This finding is consistent with a recent study in a sample of Veterans with mTBI that found participation restrictions were primarily associated with cognitive symptoms, but not other post-concussive symptom domains (9). Our study expands upon these findings by providing evidence that such cognitive symptoms may be downstream from sleep problems, expanding potential treatment targets for rehabilitative care aiming to promote Veterans' participation. Preliminary evidence indicates that sleep treatments may enhance cognitive function in those with TBI (58–60), although additional scientific investment is needed (52). Further, according to the VA/DoD mTBI CPG (29), Veterans with post-concussive cognitive impairment should receive specialized cognitive rehabilitation services (e.g., compensatory cognitive training) due to documented benefits on cognitive function, including among post-9/11 Veterans with mTBI (61).
By examining two distinct indicators of participation, we revealed divergent impacts of vestibular-sensory and mood-behavioral symptoms upon Veterans' functioning. Our study extends prior work which adopted an overall summary score of participation, observing that post-concussive symptoms across domains undermined Veterans' participation (8). Our findings suggest that such an approach may obscure more nuanced relationships between post-concussive symptoms and Veteran participation challenges, an understanding of which can enhance rehabilitative care for those with mTBI. For example, in our study, mood-behavioral symptoms were associated with restrictions in social and community participation (e.g., social contact), but not productivity (e.g., self-care). This may indicate that the interpersonal aspect of social and community participation may be challenging for those with chiefly mood-behavioral post-concussive impairments. Indeed, Veterans with such challenges (e.g., depression) report difficulty with securing healthy social bonds with others (62, 63). These interpersonal challenges may not necessarily translate to participation in productivity-related activities, the performance of which may occur in isolation from others. Adopting such a nuanced perspective on the link between post-concussive symptoms and participation challenges among Veterans with mTBI can inform priority targets of intervention according to clinical presentation, enabling more individualized rehabilitative care.
Studies examining participation in Veterans with mTBI, including the present study, typically emphasize the observable aspects of participation (e.g., independence), overlooking the subjective dimension of this complex construct (1, 64). Future research that examines the inter-relationships between sleep problems, post-concussive symptoms, and participation restrictions should also consider the meaning associated with Veterans' daily participation, or the extent to which daily activity aligns with their values and interests (1, 65). Engagement in meaningful activity is a critical ingredient for Veterans' community reintegration and contributes to their overall wellbeing, making it an important target for the rehabilitation of Veterans with mTBI (66, 67). Further, measuring all dimensions of participation may further elucidate the impact of treating sleep problems and other post-concussive symptoms on the daily lives of Veterans. For example, a recent study evaluating the efficacy of CBT-I in Veterans found improvements in the meaningfulness of daily activity, but not in the observable aspects of participation (i.e., performance) (49). Advancing understanding of such relationships could enable the refinement of rehabilitative care to better meet the individualized needs of Veterans with post-concussive sleep problems.
Study limitations
The cross-sectional nature of this study precludes definitive conclusions regarding the temporal order of observed relationships. We could not identify whether sleep problems contribute to other post-concussive symptoms, or whether the reverse is more consistent with the data. However, the implied temporal order of our proposed model is supported by substantial theoretical and empirical support indicating that improved sleep quality is associated with enhanced overall post-concussive recovery (18–20, 47–49, 52, 68). Nonetheless, longitudinal studies should be conducted to disentangle the temporal ordering of sleep problems, other post-concussive symptoms, and participation restrictions. Further, many Veterans who received the CTBIE did not receive the M2PI, and the extent to which Veterans' receipt of the M2PI systematically varied is unclear. Such systematic variation may threaten the generalizability of findings. In addition, our findings were collected in a sample of Veterans receiving outpatient VHA care, and findings may not generalize to other Veterans or to civilian populations. However, the generalizability of our findings benefits from the national sample of Veterans included in this study. Nonetheless, findings should be replicated in other populations (e.g., civilians with sports-related concussion). In the current study, we did not account for symptom validity using embedded validity scales (e.g., Validity-10) (69) for two primary reasons. First, prior examinations of self-reported NSI scores among Veterans indicate that removing individuals based on embedded validity scales may undermine measurement precision among those with more severe symptoms (7). Second, invalid responding is a complex behavior, the identification of which requires the integration of multiple sources of data (e.g., Mild Brain Injury Atypical Symptoms scale) (70) rather than the use of embedded validity scales in isolation (7, 71). Future research should replicate study results among valid responders identified using multiple sources of data that were unavailable for the current study. Sleep problems were measured using a single self-reported indicator, and future research should attempt to replicate findings using standardized measures of sleep problems (e.g., Pittsburgh Sleep Quality Index) (72). Further, the effects of clinical conditions underlying such sleep problems (e.g., insomnia) upon observed relationships should also be studied. Finally, we were unable to account for all potentially confounding variables in our model. For example, we were unable to adjust for the influence of environmental factors, which are inextricably connected to participation (73). However, we accounted for many theoretically plausible confounding variables (e.g., co-morbid PTSD and depression). Nonetheless, future studies should expand upon the set of covariates used in this study.
Conclusion
In this study, we found that post-concussive symptoms mediated the relationship between sleep problems and participation restrictions in Veterans with mTBI, although the indirect effect of sleep problems varied across different types of participation. Sleep problems were associated with more severe post-concussive symptoms across all domains. However, effects of post-concussive symptoms were conditional on the specific nature of participation (i.e., social and community participation vs. productivity). Findings underscore the importance of integrating evidence-based sleep treatment in the rehabilitative care of Veterans with mTBI and can inform efforts aimed at tailoring rehabilitative care to their individualized needs.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: N/A. Requests to access these datasets should be directed toYWRhbS5raW5uZXlAdmEuZ292.
Ethics statement
The studies involving human participants were reviewed and approved by Colorado Multiple Institutional Review Board and Department of Veterans Affairs committees. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
AK conceptualized the study, completed data analysis, and led manuscript completion. LB, NB, and JF contributed to the conceptualization of the study. XY, AS, and SK facilitated data retrieval, management, and cleaning. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the VA Rocky Mountain MIRECC, but does not necessarily represent the views of the Department of Veterans Affairs or the United States Government. Dr. Brenner reports grants from the VA, DOD, NIH, and the State of Colorado, editorial renumeration from Wolters Kluwer, and royalties from the American Psychological Association and Oxford University Press. In addition, she consults with sports leagues via her university affiliation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cogan AM, Carlson M. Deciphering participation: an interpretive synthesis of its meaning and application in rehabilitation. Disabil Rehabil. (2018) 40(22):2692–703. doi: 10.1080/09638288.2017.1342282
2. Schneiderman AI, Braver ER, Kang HK. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol. (2008) 167(12):1446–52. doi: 10.1093/aje/kwn068
3. Schwab KA, Ivins B, Cramer G, Johnson W, Sluss-Tiller M, Kiley K, et al. Screening for traumatic brain injury in troops returning from deployment in Afghanistan and Iraq: initial investigation of the usefulness of a short screening tool for traumatic brain injury. J Head Trauma Rehabil. (2007) 22(6):377–89. doi: 10.1097/01.HTR.0000300233.98242.87
4. Tanielian T, Haycox LH, Schell TL, Marshall GN, Burnam MA, Eibner C, et al. Invisible wounds of war: summary and recommendations for addressing psychological and cognitive injuries. Santa Monica, CA: RAND Corporation (2008).
5. Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, et al. Traumatic brain injury screening: preliminary findings in a US army brigade combat team. J Head Trauma Rehabil. (2009) 24(1):14–23. doi: 10.1097/HTR.0b013e31819581d8
6. Pugh MJ, Swan AA, Carlson KF, Jaramillo CA, Eapen BC, Dillahunt-Aspillaga C, et al. Traumatic brain injury severity, comorbidity, social support, family functioning, and community reintegration among veterans of the Afghanistan and Iraq wars. Arch Phys Med Rehabil. (2018) 99(2):S40–S9. doi: 10.1016/j.apmr.2017.05.021
7. Bahraini NH, Hostetter TA, Forster JE, Schneider AL, Brenner LA. A rasch analysis of the neurobehavioral symptom inventory in a national cohort of operation enduring and Iraqi freedom veterans with mild traumatic brain injury. Psychol Assess. (2018) 30(8):1013–27. doi: 10.1037/pas0000555
8. Cogan AM, Smith B, Pape TLB, Mallinson T, Eapen BC, Scholten J. Self-reported participation restrictions among male and female veterans with traumatic brain injury in veterans health administration outpatient polytrauma programs. Arch Phys Med Rehabil. (2020) 101(12):2071–79. doi: 10.1016/j.apmr.2020.06.030
9. O'Rourke J, Critchfield E, Soble J, Bain K, Fullen C, Eapen B. The utility of the mayo-portland adaptability inventory participation Index (M2PI) in US military veterans with a history of mild traumatic brain injury. J Head Trauma Rehabil. (2019) 34(1):30–5. doi: 10.1097/HTR.0000000000000405
10. Cassidy JD, Cancelliere C, Carroll LJ, Côté P, Hincapié CA, Holm LW, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. (2014) 95(3):S132–S51. doi: 10.1016/j.apmr.2013.08.299
11. Ferdosi H, Schwab KA, Metti A, Brenner LA, Terrio H, Pazdan RM, et al. Trajectory of postconcussive symptoms 12 months after deployment in soldiers with and without mild traumatic brain injury: warrior strong study. Am J Epidemiol. (2019) 188(1):77–86. doi: 10.1093/aje/kwy199
12. Mac Donald CL, Johnson AM, Wierzechowski L, Kassner E, Stewart T, Nelson EC, et al. Outcome trends after US military concussive traumatic brain injury. J Neurotrauma. (2017) 34(14):2206–19. doi: 10.1089/neu.2016.4434
13. Schwab K, Terrio HP, Brenner LA, Pazdan RM, McMillan HP, MacDonald M, et al. Epidemiology and prognosis of mild traumatic brain injury in returning soldiers: a cohort study. Neurology. (2017) 88(16):1571–9. doi: 10.1212/WNL.0000000000003839
14. Stein MB, Ursano RJ, Campbell-Sills L, Colpe LJ, Fullerton CS, Heeringa SG, et al. Prognostic indicators of persistent post-concussive symptoms after deployment-related mild traumatic brain injury: a prospective longitudinal study in US army soldiers. J Neurotrauma. (2016) 33(23):2125–32. doi: 10.1089/neu.2015.4320
15. Walker WC, Franke LM, Sima AP, Cifu DX. Symptom trajectories after military blast exposure and the influence of mild traumatic brain injury. J Head Trauma Rehabil. (2017) 32(3):E16–26. doi: 10.1097/HTR.0000000000000251
16. Scholten JD, Sayer NA, Vanderploeg RD, Bidelspach DE, Cifu DX. Analysis of US veterans health administration comprehensive evaluations for traumatic brain injury in operation enduring freedom and operation Iraqi freedom veterans. Brain Inj. (2012) 26(10):1177–84. doi: 10.3109/02699052.2012.661914
17. Grima N, Ponsford J, Rajaratnam SM, Mansfield D, Pase MP. Sleep disturbances in traumatic brain injury: a meta-analysis. J Clin Sleep Med. (2016) 12(3):419–28. doi: 10.5664/jcsm.5598
18. Lucke-Wold BP, Smith KE, Nguyen L, Turner RC, Logsdon AF, Jackson GJ, et al. Sleep disruption and the sequelae associated with traumatic brain injury. Neurosci Biobehav Rev. (2015) 55:68–77. doi: 10.1016/j.neubiorev.2015.04.010
19. Sandsmark DK, Elliott JE, Lim MM. Sleep-wake disturbances after traumatic brain injury: synthesis of human and animal studies. Sleep. (2017) 40(5):zsx044. doi: 10.1093/sleep/zsx044
20. Wickwire EM, Williams SG, Roth T, Capaldi VF, Jaffe M, Moline M, et al. Sleep, sleep disorders, and mild traumatic brain injury. What we know and what we need to know: findings from a national working group. Neurotherapeutics. (2016) 13(2):403–17. doi: 10.1007/s13311-016-0429-3
21. Chan LG, Feinstein A. Persistent sleep disturbances independently predict poorer functional and social outcomes 1 year after mild traumatic brain injury. J Head Trauma Rehabil. (2015) 30(6):E67–75. doi: 10.1097/HTR.0000000000000119
22. Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. (2007) 3(5):519–28. doi: 10.5664/jcsm.26918
23. Zielinski MR, McKenna JT, McCarley RW. Functions and mechanisms of sleep. AIMS Neurosci. (2016) 3(1):67. doi: 10.3934/Neuroscience.2016.1.67
24. Lu LH, Reid MW, Cooper DB, Kennedy JE. Sleep problems contribute to post-concussive symptoms in service members with a history of mild traumatic brain injury without posttraumatic stress disorder or major depressive disorder. Neuro Rehabil. (2019) 44(4):511–21. doi: 10.3233/NRE-192702
25. Theadom A, Cropley M, Parmar P, Barker-Collo S, Starkey N, Jones K, et al. Sleep difficulties one year following mild traumatic brain injury in a population-based study. Sleep Med. (2015) 16(8):926–32. doi: 10.1016/j.sleep.2015.04.013
26. Affairs USDoV. Traumatic Brain Injury Veterans Health Registry (Available from: https://www.publichealth.va.gov/epidemiology/reports/oefoifond/health-care-utilization/tbi-registry.asp).
27. Affairs USDoV. VHA Directive 1184: screening and evaluation of post-9/11 veterans for deployment-related traumatic brain injury. Washington, D.C.: U.S. Department of Veterans Affairs (2022).
28. Affairs USDoV. VHA Directive 1172.01: polytrauma system of care. Washington, D.C.: U.S. Department of Veterans Affairs (2019).
29. VA/DoD. VA/DoD Clinical Practice Guideline for Management and Rehabilitation of Post-Acute Mild Traumatic Brain Injury (mTBI). (2021).
30. McCulloch K, Pastorek NJ, Miller BI, Romesser J, Linck J, Sim AH, et al. Clinician versus veteran ratings on the mayo-portland participation index in veterans with a history of mild traumatic brain injury. J Head Trauma Rehabil. (2015) 30(1):38–46. doi: 10.1097/HTR.0000000000000041
31. Cicerone KD, Kalmar K. Persistent postconcussion syndrome: the structure of subjective complaints after mild traumatic brain injury. J Head Trauma Rehabil. (1995) 10(3):1–17. doi: 10.1097/00001199-199510030-00002
32. Baldassarre M, Smith B, Harp J, Herrold A, High WM Jr, Babcock-Parziale J, et al. Exploring the relationship between mild traumatic brain injury exposure and the presence and severity of postconcussive symptoms among veterans deployed to Iraq and Afghanistan. PM/R. (2015) 7(8):845–58. doi: 10.1016/j.pmrj.2015.03.003
33. Silva MA. Review of the neurobehavioral symptom inventory. Rehabil Psychol. (2020). 66(2): 170–82. doi: 10.1037/rep0000367
34. Malec JF. The mayo-portland participation Index: a brief and psychometrically sound measure of brain injury outcome. Arch Phys Med Rehabil. (2004) 85(12):1989–96. doi: 10.1016/j.apmr.2004.01.032
35. Malec JF, Lezak MD. Manual for the Mayo-Portland Adaptability Inventory (MPAI-4) for adults, children and adolescents (Available from: http://www.tbims.org/combi/mpai/manual.pdf).
36. Kean J, Malec JF, Altman IM, Swick S. Rasch measurement analysis of the mayo-portland adaptability inventory (MPAI-4) in a community-based rehabilitation sample. J Neurotrauma. (2011) 28(5):745–53. doi: 10.1089/neu.2010.1573
37. Cogan AM, Weaver JA, Scholten J, Pape TB, Mallinson T. Psychometric properties and sex differences on the mayo-portland adaptability inventory participation subscale (M2PI) in veterans with traumatic brain injury. Arch Phys Med Rehabil. (2021) 102(11):2193–200. e3. doi: 10.1016/j.apmr.2021.06.003
38. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. (2005) 43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
39. Muthén LK, Muthén BO. Mplus user's Guide (8th ed.). Los Angeles, CA: Muthén / Muthén (1998-2017).
40. Lt H, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. (1999) 6(1):1–55. doi: 10.1080/10705519909540118
41. Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: theory, methods, and prescriptions. Multivariate Behav Res. (2007) 42(1):185–227. doi: 10.1080/00273170701341316
42. Vanderploeg RD, Silva MA, Soble JR, Curtiss G, Belanger HG, Donnell AJ, et al. The structure of postconcussion symptoms on the neurobehavioral symptom inventory: a comparison of alternative models. J Head Trauma Rehabil. (2015) 30(1):1–11. doi: 10.1097/HTR.0000000000000009
43. Chang F-H, Coster WJ. Conceptualizing the construct of participation in adults with disabilities. Arch Phys Med Rehabil. (2014) 95(9):1791–8. doi: 10.1016/j.apmr.2014.05.008
44. Waid-Ebbs JK, Wen P-S, Graham DP, Leroux AJ, O’Connor MK, Helmer DA. Measurement properties of the MPAI-4 in veterans with mTBI. Arch Phys Med Rehabil. (2020) 101(5):789–96. doi: 10.1016/j.apmr.2019.10.191
45. Raftery AE. Bayesian Model selection in social research. Sociol Methodol. (1995) 25:111–63. doi: 10.2307/271063
46. Maassen GH, Bakker AB. Suppressor variables in path models: definitions and interpretations. Sociol Methods Res. (2001) 30(2):241–70. doi: 10.1177/0049124101030002004
47. Silverberg ND, Iaccarino MA, Panenka WJ, Iverson GL, McCulloch KL, Dams-O’Connor K, et al. Management of concussion and mild traumatic brain injury: a synthesis of practice guidelines. Arch Phys Med Rehabil. (2020) 101(2):382–93. doi: 10.1016/j.apmr.2019.10.179
48. Eakman AM, Schmid AA, Henry KL, Rolle NR, Schelly C, Pott CE, et al. Restoring effective sleep tranquility (REST): a feasibility and pilot study. Br J Occup Ther. (2017) 80(6):350–60. doi: 10.1177/0308022617691538
49. Eakman AM, Schmid AA, Rolle NR, Kinney AR, Henry KL. Follow-up analyses from a wait-list controlled trial of occupational therapist–delivered cognitive–behavioral therapy for insomnia among veterans with chronic insomnia. Am J Occup Ther. (2022) 76(2). doi: 10.5014/ajot.2022.045682
50. Malec JF. Polytrauma transitional rehabilitation in the Veterans Administration: implementing the principles of person-centered, participation-oriented rehabilitation. J Head Trauma Rehabil. (2019) 34(3):135–40. doi: 10.1097/HTR.0000000000000456
51. Mollayeva T, Colantonio A, Mollayeva S, Shapiro CM. Screening for sleep dysfunction after traumatic brain injury. Sleep Med. (2013) 14(12):1235–46. doi: 10.1016/j.sleep.2013.07.009
52. Ouellet M-C, Beaulieu-Bonneau S, Morin CM. Sleep-wake disturbances after traumatic brain injury. Lancet Neurol. (2015) 14(7):746–57. doi: 10.1016/S1474-4422(15)00068-X
53. VA/DoD. VA/Dod clinical practice guideline for the management of chronic insomnia disorder and obstructive sleep apnea. Washington, DC: The Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea Work Group. (2019).
54. Leng Y, Byers AL, Barnes DE, Peltz CB, Li Y, Yaffe K. Traumatic brain injury and incidence risk of sleep disorders in nearly 200,000 US veterans. Neurology. (2021) 96(13):e1792–e9. doi: 10.1212/WNL.0000000000011656
55. Kinney AR, Bahraini N, Forster JE, Brenner LA. Factors influencing the implementation of guideline-recommended practices for postconcussive sleep disturbance and headache in the veterans health administration: a mixed methods study. Arch Phys Med Rehabil. (2022) doi: 10.1016/j.apmr.2022.01.164
56. Koffel E, Bramoweth AD, Ulmer CS. Increasing access to and utilization of cognitive behavioral therapy for insomnia (CBT-I): a narrative review. J Gen Intern Med. (2018) 33(6):955–62. doi: 10.1007/s11606-018-4390-1
57. Kinney AR, Bahraini N, Forster JE, Brenner LA. Factors influencing the implementation of guideline-recommended practices for post-concussive sleep disturbance and headache in the veterans health administration (1004151). American Congress of rehabilitation medicine annual conference; 2021, September
58. Ruff RL, Ruff SS, Wang X-F. Improving sleep: initial headache treatment in OIF/OEF veterans with blast-induced mild traumatic brain injury. J Rehabilitation Res Dev. (2009) 46(9):1071–84. doi: 10.1682/JRRD.2009.05.0062
59. Wiseman-Hakes C, Murray B, Moineddin R, Rochon E, Cullen N, Gargaro J, et al. Evaluating the impact of treatment for sleep/wake disorders on recovery of cognition and communication in adults with chronic TBI. Brain Inj. (2013) 27(12):1364–76. doi: 10.3109/02699052.2013.823663
60. Wiseman-Hakes C, Victor JC, Brandys C, Murray BJ. Impact of post-traumatic hypersomnia on functional recovery of cognition and communication. Brain Inj. (2011) 25(12):1256–65. doi: 10.3109/02699052.2011.608215
61. Storzbach D, Twamley EW, Roost MS, Golshan S, Williams RM, O'Neil M, et al. Compensatory cognitive training for operation enduring freedom/operation Iraqi freedom/operation new dawn veterans with mild traumatic brain injury. J Head Trauma Rehabil. (2017) 32(1):16–24. doi: 10.1097/HTR.0000000000000228
62. Daggett VS, Bakas T, Buelow J, Habermann B, Murray LL. Needs and concerns of male combat veterans with mild traumatic brain injury. J Rehabil Res Dev. (2013) 50(3):327–40. doi: 10.1682/JRRD.2011.09.0168
63. Resnik LJ, Allen SM. Using international classification of functioning, disability and health to understand challenges in community reintegration of injured veterans. J Rehabil Res Dev. (2007) 44(7): 991–1006. doi: 10.1682/JRRD.2007.05.0071
64. Kinney AR, Stephenson RO, Cogan AM, Forster JE, Gerber HR, Brenner LA. Participation mediates the relationship between postconcussive symptoms and suicidal ideation among veterans. Am J Occup Ther. (2022) 76(3): 7603205020. doi: 10.5014/ajot.2022.048561
65. Eakman AM, Atler KE, Rumble M, Gee BM, Romriell B, Hardy N. A qualitative research synthesis of positive subjective experiences in occupation from the journal of occupational science (1993–2010). J Occup Sci. (2018) 25(3):346–67. doi: 10.1080/14427591.2018.1492958
66. Eakman AM, Kinney AR, Reinhardt R. Participation, meaningful activity, and social support among US student service members/veterans. OTJR: occupation. Participation Health. (2019) 39(4):222–31. doi: 10.1177/1539449219833351
67. Kinney AR, Graham JE, Eakman AM. Participation is associated with well-being among community-based veterans: an investigation of coping ability, meaningful activity, and social support as mediating mechanisms. Am J Occup Ther. (2020) 74(5):7405205010p1-p11. doi: 10.5014/ajot.2020.037119
68. Spencer RJ, Collings AS, Bloor LE. Cognitive behavioral therapy for insomnia as treatment for post-concussive symptoms. Med Res. (2019) 1(1):1–7. doi: 10.6913/MRHK.201912_1(1).0001
69. Vanderploeg RD, Cooper DB, Belanger HG, Donnell AJ, Kennedy JE, Hopewell CA, et al. Screening for postdeployment conditions: development and cross-validation of an embedded validity scale in the neurobehavioral symptom inventory. J Head Trauma Rehabil. (2014) 29(1):1–10. doi: 10.1097/HTR.0b013e318281966e
70. Cooper DB, Nelson L, Armistead-Jehle P, Bowles AO. Utility of the mild brain injury atypical symptoms scale as a screening measure for symptom over-reporting in operation enduring freedom/operation Iraqi freedom service members with post-concussive complaints. Arch Clin Neuropsychol. (2011) 26(8):718–27. doi: 10.1093/arclin/acr070
71. Paulson D, Horner MD, Bachman D. A comparison of four embedded validity indices for the RBANS in a memory disorders clinic. Arch Clin Neuropsychol. (2015) 30(3):207–16. doi: 10.1093/arclin/acv009
72. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28(2):193–213. doi: 10.1016/0165-1781(89)90047-4
Keywords: concussion, veterans, sleep, participation, postconcussive symptoms, neurobehavioral symptoms, mild traumatic brain injury (mTBI)
Citation: Kinney AR, Yan X-D, Schneider AL, King S, Forster JE, Bahraini N and Brenner LA (2022) Post-concussive symptoms mediate the relationship between sleep problems and participation restrictions among veterans with mild traumatic brain injury. Front. Rehabilit. Sci. 3:964420. doi: 10.3389/fresc.2022.964420
Received: 8 June 2022; Accepted: 14 September 2022;
Published: 12 October 2022.
Edited by:
Dagmara Dimitriou, University College London, United KingdomReviewed by:
Amy A Herrold, Northwestern University, United StatesAnthony P Salvatore, University of Louisiana at Lafayette, United States
This work is authored by Adam R. Kinney, Xiang-Dong Yan, Alexandra Lindsay Schneider, Samuel King, Jeri E. Forster, Nazanin Bahraini and Lisa Anne Brenner on behalf of the U.S. Government and as regards Dr. Kinney, Dr. Yan, Dr. Schneider, Dr. King, Dr. Forster, Dr. Bahraini and Dr. Brenner, and the U.S. Government, is not subject to copyright protection in the United States. Foreign and other copyrights may apply. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam R. Kinney YWRhbS5raW5uZXlAdmEuZ292
Specialty Section: This article was submitted to Human Functioning, a section of the journal Frontiers in Rehabilitation Sciences
 Adam R. Kinney
Adam R. Kinney Xiang-Dong Yan
Xiang-Dong Yan Alexandra L. Schneider
Alexandra L. Schneider Samuel King1
Samuel King1 Jeri E. Forster
Jeri E. Forster Nazanin Bahraini
Nazanin Bahraini Lisa A. Brenner
Lisa A. Brenner