- 1Department of Occupational Therapy, Kinugasa Hospital, Yokosuka, Japan
- 2Department of Occupational Therapy, Kanagawa University of Human Services, Yokosuka, Japan
- 3Department of Occupational Therapy, Tokyo University of Technology, Tokyo, Japan
- 4Department of Occupational Therapy, Yokohama Brain and Spine Center, Yokohama, Japan
Background and purpose: The effects of therapy and patient characteristics on rehabilitation outcomes in patients with acute stroke are unclear. We investigated the effects of intensive occupational therapy (OT) on patients with acute stroke.
Methods: We performed a retrospective cohort study using the 2005–2016 Japan Rehabilitation Database, from which we identified patients with stroke (n = 10,270) who were admitted to acute care hospitals (n = 37). We defined active OT (AOT) and non-AOT as OT intervention times (total intervention time/length of hospital stay) longer or shorter than the daily physical therapy intervention time, respectively. The outcomes assessed were the Functional Independence Measure (FIM) and National Institutes of Health Stroke Scale (NIHSS) scores, duration of hospitalization, and rate of discharge. Propensity scores and inverse probability of treatment weighting analyses adjusted for patient characteristics were performed to investigate the effects of AOT on patient outcomes.
Results: We enrolled 3,501 patients (1,938 and 1,563 patients in the AOT and non-AOT groups, respectively) in the study. After inverse probability of treatment weighting, the AOT group had a shorter length of hospitalization (95% confidence interval: −3.7, −1.3, p < 0.001), and the FIM (95% confidence interval: 2.0, 5.7, p < 0.001) and NIHSS (95% confidence interval; 0.3, 1.1, p < 0.001) scores improved significantly. Subgroup analysis showed that lower NHISS scores for aphasia, gaze, and neglect and lower overall NIHSS and FIM scores on admission led to a greater increase in FIM scores in the AOT group.
Conclusions: AOT improved the limitations in performing activities of daily living (ADL) and physical function in patients with acute stroke and reduced the length of hospitalization. Additionally, subgroup analysis suggested that the increase in FIM score was greater in patients with severe limitations in performing ADLs and worse cognitive impairment, such as neglect, on admission.
Introduction
Stroke is associated with a significant burden of care worldwide; 43% of elderly patients with stroke have moderate to severe neurological deficits (1, 2). Rehabilitation after stroke is associated with a reduced incidence of disability and mortality, and several guidelines recommend initiation of rehabilitation during the acute phase of stroke (3, 4). Additionally, recent meta-analyses have reported that early rehabilitation after stroke improves the limitations in performing activities of daily living (ADLs) (5).
Researchers have previously investigated the intensity and timing of the initiation of rehabilitation for patients with acute stroke (6–11). Performing at least 3 h of high-intensity rehabilitation between 24 and 48 h after stroke onset can reportedly improve the modified Rankin (mRankin) score 3 months after onset (9). In these studies, the interventions (1) commenced within 24 h of stroke onset, (2) focused on out-of-bed activities, such as sitting, and (3) provided at least three out-of-bed activities in addition to usual care (6–9). These interventions were provided by a physical therapist or nurse, but some studies did not differentiate between physical and occupational therapists. Furthermore, the interventions did not include direct ADLs (e.g., dressing exercises) performed by occupational therapists. Therefore, fundamental questions regarding the type of therapy and patient characteristics on rehabilitation outcomes remain unanswered (9).
In clinical practice, physical therapy, occupational therapy (OT), and speech and language therapy are often provided to patients with acute stroke. In a recent systematic review, OT during the acute phase of stroke effectively improved limitations in performing ADLs, reduced symptoms of delirium, and improved cognitive function, although with limitations (12). Further, the role of occupational therapists in the intensive care unit is not well established, behooving occupational therapists to expand their role and lead original research (13). Although most patients received physical therapy, OT was not widely implemented for patients with acute stroke (14). However, we believe that OT may effectively improve the quality of life of patients with acute stroke. Thus, we hypothesized that active and high intensity OT would improve the limitations in performing ADLs in patients with stroke.
The purpose of this study was to investigate the effects of active OT (AOT) on patients with acute stroke. Additionally, we conducted a subgroup analysis to determine which patient characteristics were associated with the efficacy of AOT.
Materials and methods
We performed a retrospective cohort study using information from the Japan Rehabilitation Database (15, 16). The need for informed consent was waived because all data were de-identified. The study was approved by the ethics committee of the Kanagawa University of Human Services (No. 7-20-30).
Data source
We retrospectively obtained data from the Japan Rehabilitation Database, which included voluntary sampling data collected from patients admitted to participating hospitals between January 2005 and March 2016 (15–17). The data are divided into various sections depending on the diagnosis and stroke phase such as stroke in the medical ward, stroke in the convalescent rehabilitation ward, and other conditions. The stroke database includes patient characteristics, such as age, stroke type, and severity based on the National Institutes of Health Stroke Scale (NIHSS) and Functional Independence Measure (FIM) scores, type of rehabilitation, and rehabilitation time provided. As of 2016, 80 hospitals were participating and data from 33,657 patients had been accumulated. In this study, we used all data collected between the time of admission and discharge of patients with stroke admitted to acute care hospitals (37 hospitals, N = 10,270).
Patients
Patients who were included in the study were as follows: those with a first episode of stroke, those who independently performed ADLs before stroke onset (mRankin score of 0 or 1), those hospitalized directly due to stroke (onset did not occur during hospitalization), those living at home before stroke onset, and those hospitalized within 7 days following stroke onset. The exclusion criteria were as follows: those who died during hospitalization, those whose duration of hospitalization could not be confirmed, those who did not receive confirmed rehabilitation during hospitalization, those who were hospitalized for over 180 days, and those who received over 180 min of OT, physical therapy, or speech therapy individually.
Intervention
The patients were divided into the AOT and non-AOT groups. AOT and non-AOT were defined as daily OT intervention times (total intervention time/length of hospital stay) longer or shorter than the daily physical therapy intervention times, respectively. Reportedly, occupational therapy places emphasis on increasing upper-extremity control and improving performance of basic ADLs (18). Additionally, acute phase occupational therapy is provided on an individualized basis and addresses training and re-education in ADLs, assessment of assistive devices, training in use, and support for discharge (12). Supplemental interventions include cognitive stimulation, multi-sensory stimulation, and positioning techniques, along with family and/or primary caregiver education or visits to the patient's home with subsequent environmental assessments (12). Therefore, it was expected that more of these interventions would be provided in the AOT group.
Outcomes
The primary outcome was an increase in the FIM score (FIM score at discharge minus FIM score at admission) as a measure of improvement in performing ADLs before and after the intervention. The FIM is widely used to assesses limitations in performing ADLs based on the amount of assistance required to perform basic physical and cognitive activity functions. It consists of 18 items that assess motor (13) and cognitive (5) functions. The total score ranges from 18 to 126, with higher scores indicating better functional status (19, 20).
The secondary outcomes were an improvement in the NIHSS scores (NIHSS value at admission minus NIHSS value at discharge), length of hospitalization (days), and rate of discharge to home. The NIHSS is a reliable tool that is widely used to determine stroke severity in emergency departments (21, 22). It consists of 15 items that assess the following: level of consciousness, eye movements, integrity of visual fields, facial movements, upper and lower extremity strength, sensation, coordination, language, speech, and neglect. Each impairment is rated based on an ordinal scale ranging from 0 to 2, 3, or 4. Scores for each item are added to obtain a total score ranging from 0 to 42, with higher scores indicating greater stroke severity.
Multiple imputation
We used the multiple imputation method to replace variables with missing values (including the outcome variables) (23). We created 20 imputed datasets using multivariate imputation by chained equations and the “mi impute chained” syntax in Stata (24). The variables used to estimate the substitution value were as follows: age, total FIM scores on admission and at discharge, FIM score on admission, mRankin scale scores on admission and at discharge, Glasgow coma scale (GCS) scores on admission and at discharge, NIHSS scores on admission and at discharge; time before initiating rehabilitation after admission (days); and severity of aphasia, gaze, and neglect. Propensity scores and treatment effects were estimated for each of the 20 datasets.
Inverse probability of treatment weighting (IPTW)
To reduce the chance of confounding due to non-random assignment to the treatment group, propensity scores were used to balance the distribution of patient characteristics of all the treatment groups at baseline (25, 26). We estimated the propensity scores for all participants in the intervention and control groups using logistic regression analysis within each multiple-imputed dataset (27). When estimating the propensity score, we identified potential confounding factors that may influence the main outcome (FIM score), secondary outcome (NIHSS score), length of hospitalization, and discharge rate based on clinical experience (28). The covariates used to estimate the propensity score were as follows: sex, age, time from stroke onset to admission (days), time to initiation of rehabilitation after admission (days), mRankin, Glasgow coma scale, and NIHSS scores, total FIM score on admission, type of stroke, treatment with recombinant tissue plasminogen activator (yes or no), surgery after hemorrhagic stroke (yes or no), surgery after subarachnoid hemorrhage (yes or no), type of anticoagulant therapy, number of caregivers, affected sides, severity of aphasia, gaze, and neglect according to the NIHSS, and speech and language therapy (yes or no). Additionally, the covariates used to estimate the propensity score must consist solely of pre-treatment covariates (29); therefore, those that occurred after interventions, such as daily rehabilitation time, could not be included in the propensity score estimation. However, when the covariates were unbalanced among the groups, they were adjusted for multiple regression analysis.
We calculated the stabilized IPTW of the observed group using the estimated propensity score to reduce variability in each group and reduce the influence of outliers (30). Each patient was weighted as follows: AOT group, proportion of the AOT group*1/propensity score; non-AOT group, proportion of the non-AOT group*1/(1 – propensity score). We assessed the balance of covariates between the AOT and non-AOT groups by calculating the standardized differences, where a value of <0.1 indicated good balance (31). However, when calculating the propensity score using the variables listed above, we found that the absolute value of the standardized differences for the total FIM score on admission was 0.136. Therefore, based on the literature, we chose a standardized difference of 0.15 rather than 0.1 before conducting our final analyses (32–34).
Statistical analysis
We compared the baseline characteristics of the eligible patients using two-tailed independent t-tests for continuous data and χ2 tests for categorical data before performing multiple imputation. We then summarized the pre- and post-IPTW baseline characteristics of the patients in both groups by calculating the standardized differences.
For the outcome assessment, we first calculated the mean and standard error of each variable before and after IPTW. Next, the outcomes were compared using multiple regression analysis after IPTW adjustment for unbalanced factors (variables with a standardized difference >0.1 after IPTW were excluded from the propensity score calculation). We conducted a subgroup analysis and examined interactions after IPTW to explore the patient characteristics associated with effective AOT. We unified the pooling of treatment effects for all analyses by averaging the dataset values and estimating standard error based on Ruben's rule and using the “mi estimate: bin- reg” syntax in Stata (27, 28, 35). Stata15.1 (Stata Corp, College Station, TX, United States) was used for all analyses, including the calculation of propensity score, and the significance level was set at p < 0.05.
Results
Baseline patient characteristics
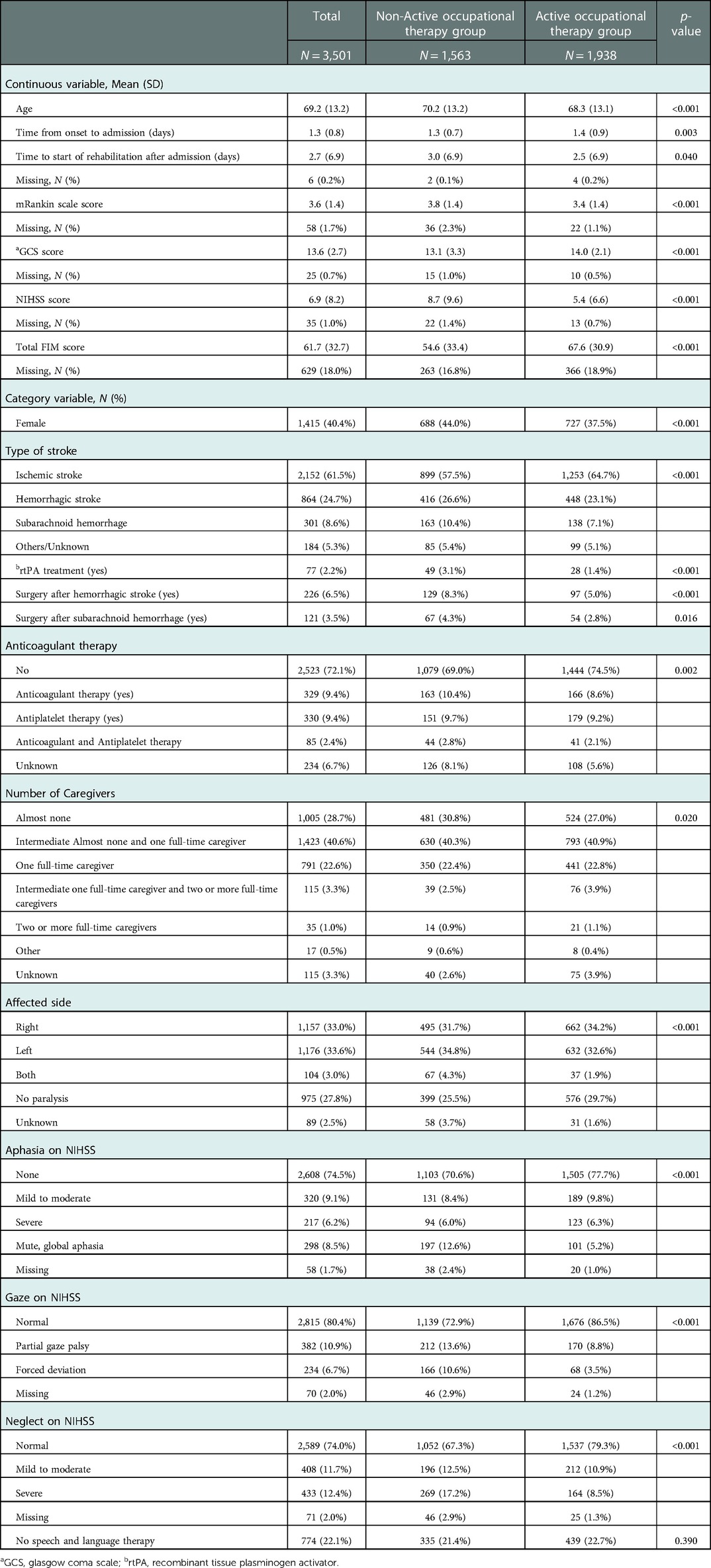
After applying the inclusion and exclusion criteria of this study, 3,501 participants were included in the analysis (Figure 1), with 1,563 assigned to the AOT group and 1,938 to the non-AOT group. Table 1 shows the baseline characteristics of the eligible patients before multiple imputation. The variable with the most missing values was the total FIM score on admission (18.0%). There were significant differences between the AOT and non-AOT groups at almost all baseline variables. Table 2 presents the baseline characteristics before and after IPTW after multiple imputations. Before IPTW, the standardized difference of both groups was >0.15 for several variables, indicating significant differences in clinical characteristics, demographics, functional level, and stroke severity. After IPTW, all standardized differences in the weighted comparisons were <0.15, indicating a similar distribution of baseline characteristics between the two groups (Table 2 and Supplementary Figures S1, S2). However, to maintain robustness, a standardized difference of >0.1 for the total FIM score was used as an adjustment factor in the final analysis.
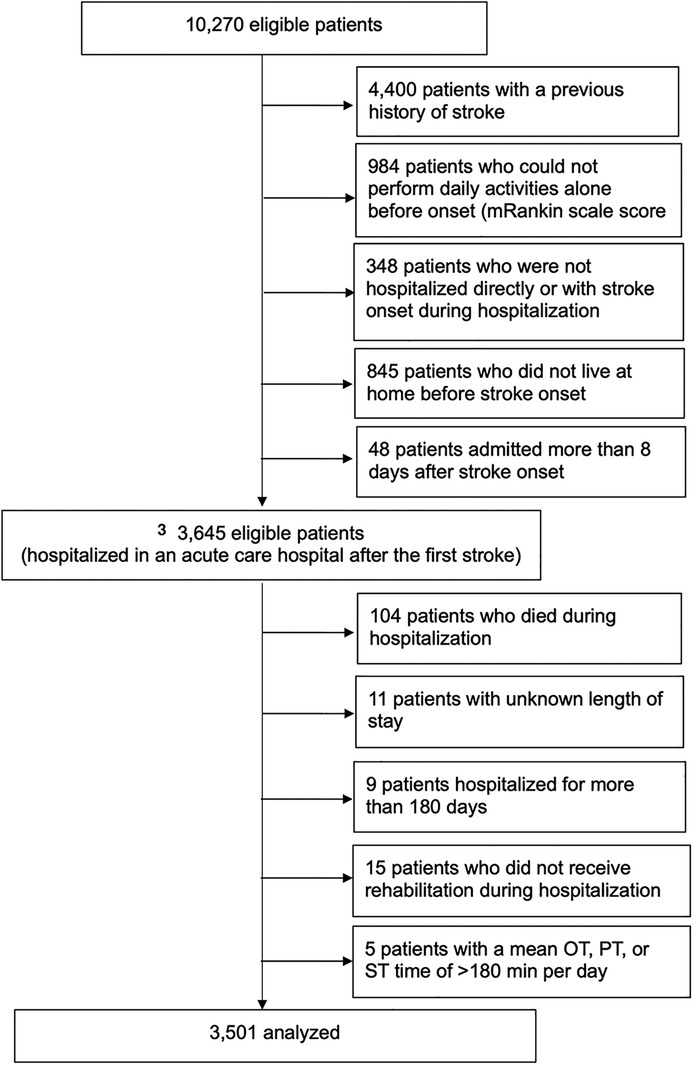
Figure 1. Study flowchart and patient selection. OT, occupational therapy; PT, physical therapy; ST, speech therapy. Final analysis patients were divided into the AOT and non-AOT groups. AOT and non-AOT were defined as daily OT intervention times (total intervention time/length of hospital stay) longer or shorter than the daily physical therapy intervention times, respectively.

Table 2. Characteristics of eligible patients before and after inverse probability of treatment weighting (IPTW).
Outcomes and subgroup analysis
Table 3 shows the primary and secondary outcomes, total rehabilitation time, mean daily rehabilitation time, and mean daily time for each type of therapy. There was a significant difference in the mean daily rehabilitation time and daily speech and language therapy time between the AOT and non-AOT groups. Additionally, the total FIM score on admission indicated an absolute standardized difference of >0.1 after IPTW. Therefore, these variables were adjusted for in the final outcomes and subgroup analyses because they were potential confounding factors. The results of the multiple regression analysis adjusted for these variables showed that the AOT group had a significantly greater improvement in FIM and NIHSS scores and a significantly shorter length of hospitalization than the non-OT group (p < 0.001 for all). Meanwhile, the rate of discharge between the two groups did not differ significantly (p = 0.201).
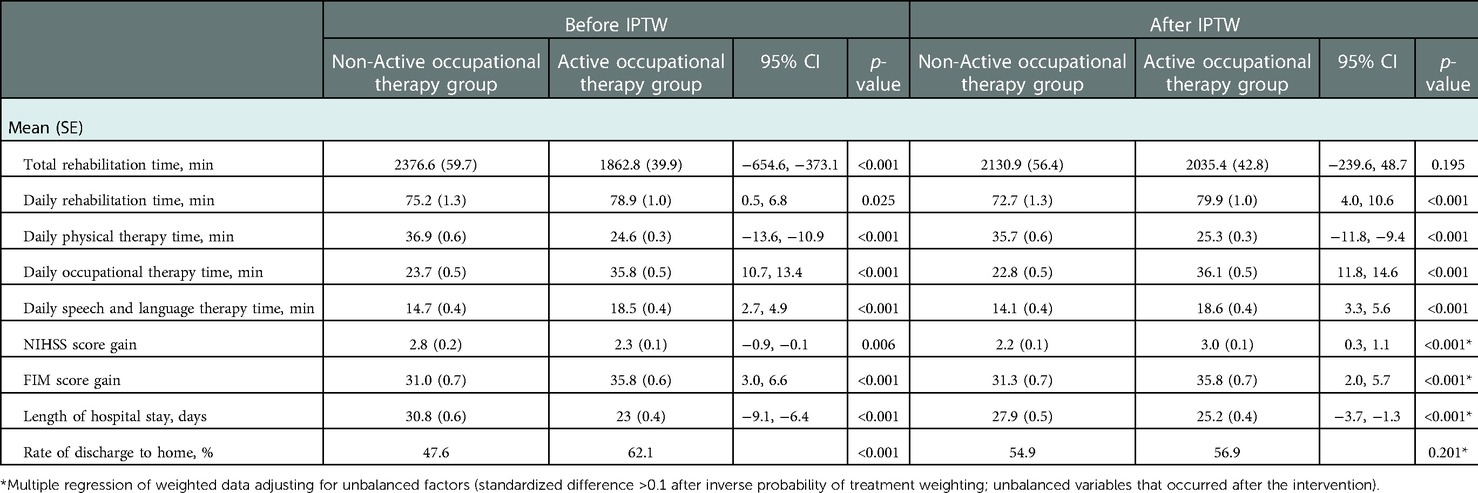
Table 3. Intensity of rehabilitation and outcomes before and after inverse probability of treatment weighting.
Table 4 shows the effects of AOT on the FIM scores according to the patients' baseline characteristics after IPTW. We observed significant interactions for increased FIM scores of consciousness, stroke severity (NIHSS score on admission), ADLs (FIM score on admission), and aphasia, gaze, and neglect on the NIHSS. In terms of stroke severity, the more severe the limitation in performing ADLs and the worse the NIHSS scores for aphasia, gaze, and neglect, the higher the increase in FIM scores in the AOT group (p = 0.001–0.040). Regarding consciousness, the increase in FIM scores in the AOT group was significantly lower in patients with lower levels of consciousness (p = 0.004; GCS score ≥16 vs. <9).
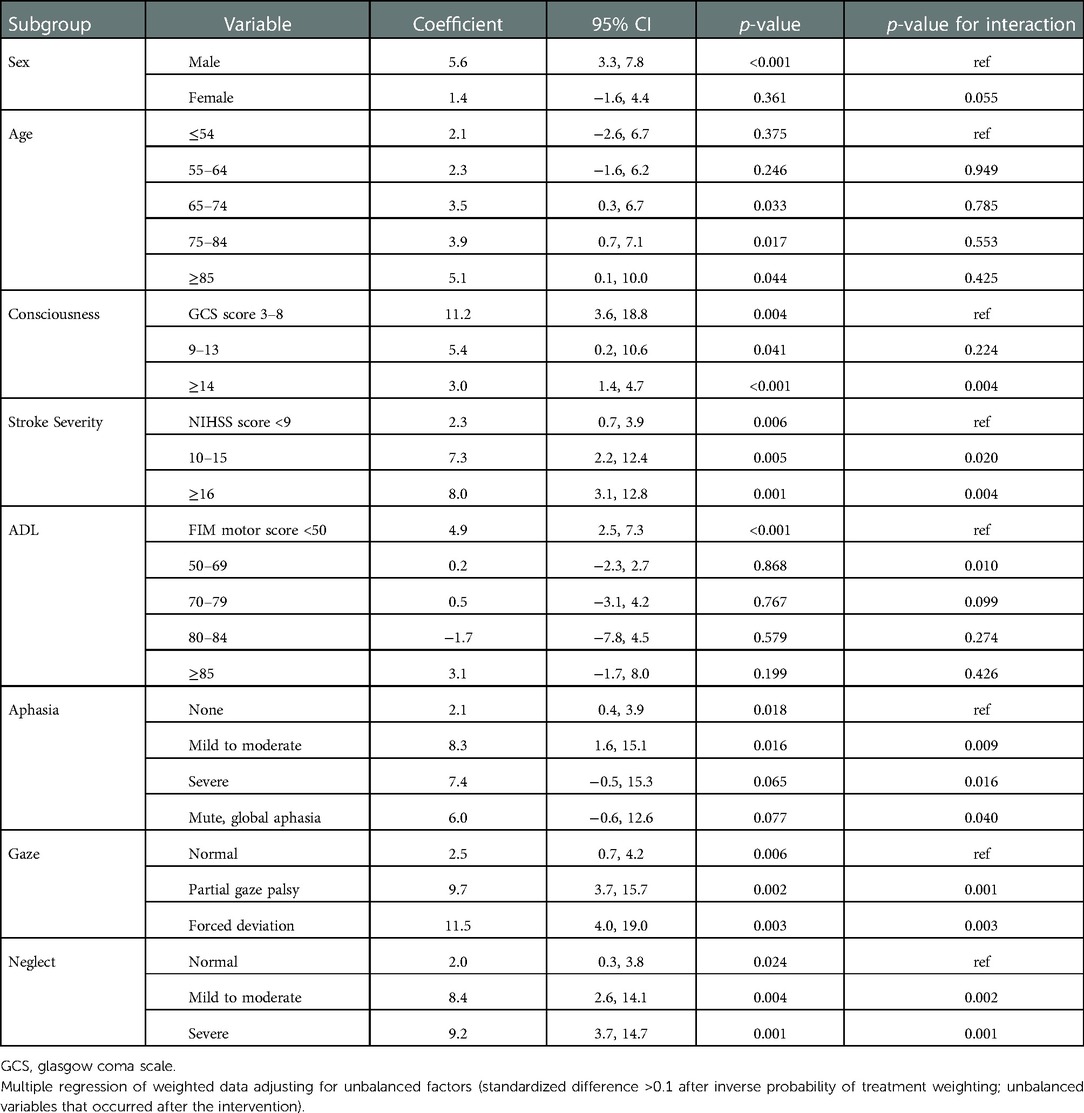
Table 4. Adjusted coefficients [95% confidence intervals (CI)] and interactions of the active occupational therapy group in terms of the gain in functional independence measures (FIM) score according to baseline patient characteristics after inverse probability of treatment weighting.
Discussion
This retrospective cohort study investigated how AOT affected patients with acute stroke while replacing missing values using multiple imputation and IPTW to adjust for confounding factors. Additionally, we conducted a subgroup analysis using the data after IPTW to identify patient characteristics associated with effective AOT. The results suggested that AOT was more effective than non-AOT in improving the limitations in performing ADLs and reducing the length of hospitalization of patients with acute stroke. The subgroup analysis results also indicated that AOT was more effective in patients with severe limitations in performing ADLs and cognitive impairment, such as neglect, on admission.
A previous study reported that the total duration of OT significantly influenced the FIM scores of patients with stroke in a rehabilitation unit (36). Furthermore, OT resulted, for patients with stroke and those with other illnesses, in reduced readmission rates (37, 38). Occupational therapists primarily engage patients in activities targeted at improving their ability to perform ADLs using an approach aimed at reducing impairment and improving function (18). The results of this study confirmed those of previous studies and were also novel in that they revealed the effects of intensive OT during the acute phase of stroke; however, OT is administered less commonly than physical therapy during this phase, with only 61% of patients receiving both physical therapy and OT (39). We believe that intervention with OT in the acute phase of stroke to improve limitations in performing ADLs may increase the FIM scores and reduce the length of hospitalization in patients, as revealed in this study. AOT is widely available and, according to our results, should be recommended for patients with acute stroke.
There are several limitations to this study. First, the duration of daily rehabilitation and speech and language therapies differed between the two groups; however, the difference in the daily rehabilitation times between groups was only approximately 7 min, which is unlikely to have a substantial effect. Furthermore, the final analysis involved adjustment for this covariate. Second, the difference in outcome scores between the two groups was small (4.5 points for the FIM gain score and 0.8 points for the NIHSS score) and should be interpreted with caution; however, compared with those of previous studies, the observed differences in these scores would be considered sufficient (17, 36). Furthermore, in the subgroup analysis, there was a difference of nearly 10 points between the two groups in FIM gain score, suggesting that AOT should be provided according to patient characteristics. Finally, although we adjusted for 18 potential confounders affecting the main outcome, including those related to the patients' age, level of consciousness, and stroke severity on admission, unmeasured confounders could have remained in the multivariate logistic regression calculation of the propensity score. In the future, randomized controlled trials that strictly adjust for confounding factors should be conducted to definitively determine the efficacy of AOT in treating patients with acute stroke.
The results of this study indicate that AOT improved the limitations in performing ADLs and physical function as well as reduced the length of hospitalization of patients with acute stroke. Additionally, the results of the subgroup analysis suggested that AOT was more effective for patients with severe limitations in performing ADLs and cognitive impairment, such as neglect, on admission. OT should be widely recommended for patients with acute stroke.
Data availability statement
The datasets presented in this article are not readily available because of the confidential data provided by the Japan Association of Rehabilitation Database. Therefore, this data could not be shared. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The ethics committee of the Kanagawa University of Human Services (No. 7-20-30). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SY, HN, KT and AN: conceived of the study and planned the research design. SY, HN: analyzed and interpreted the data. HN, KT: contributed reagents/materials/analysis tools. SY and HN: drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by JSPS KAKENHI Grant Number JP20H03914.
Acknowledgments
We thank the Rehabilitation Patient Database developed by the Japan Association of Rehabilitation Database for providing the data for this study. The results and conclusions of this study are the views of the authors, not the official views of the Japanese Association for Rehabilitation Medicine. Finally, we are particularly grateful to Taisei Takeda and Natsuki Inoue, who are members of Nagayama laboratory, for their encouragement, without which this paper would not have materialized.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2022.1045231/full#supplementary-material.
Abbreviations
AOT, active occupational therapy; CI, confidence interval; FIM, functional independence measure; IPTW, inverse probability of treatment weighting; mRankin scale, modified rankin scale; NIHSS, national institutes of health stroke scale; Non-AOT, non-active occupational therapy group.
References
1. Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. (2003) 12:119–26. doi: 10.1016/S1052-3057(03)00042-9
2. Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. (2003) 2:43–53. doi: 10.1016/s1474-4422(03)00266-7
3. Winstein CJ, Stein J, Arena R, Bates B, Cherney LR, Cramer SC, et al. Guidelines for adult stroke rehabilitation and recovery: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2016) 47:e98–e169. doi: 10.1161/STR.0000000000000098
4. Gittler M, Davis AM. Guidelines for adult stroke rehabilitation and recovery. JAMA. (2018) 319:820–1. doi: 10.1001/jama.2017.22036
5. Li Z, Zhang X, Wang K, Wen J. Effects of early mobilization after acute stroke: a meta-analysis of randomized control trials. J Stroke Cerebrovasc Dis. (2018) 27:1326–37. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.021
6. Cumming TB, Churilov L, Collier J, Donnan G, Ellery F, Dewey H, et al. Early mobilization and quality of life after stroke: findings from AVERT. Neurology. (2019) 93:e717–28. doi: 10.1212/WNL.0000000000007937
7. Bernhardt J, Langhorne P, Lindley RI, Thrift AG, Ellery F, Collier J, et al. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. (2015) 386:46–55. doi: 10.1016/S0140-6736(15)60690-0
8. Langhorne P, Wu O, Rodgers H, Ashburn A, Bernhardt J. A very early rehabilitation trial after stroke (AVERT): a phase III, multicentre, randomised controlled trial. Health Technol Assess. (2017) 21:1–20. doi: 10.3310/hta21540
9. Tong Y, Cheng Z, Rajah GB, Honglian D, Cai L, Zhang N, et al. High intensity physical rehabilitation later than 24 h post stroke is beneficial in patients: a pilot randomized controlled trial (RCT) study in mild to moderate ischemic stroke. Front Neurol. (2019) 10:113. doi: 10.3389/fneur.2019.00113
10. Matsui H, Hashimoto H, Horiguchi H, Yasunaga H, Matsuda S. An exploration of the association between very early rehabilitation and outcome for the patients with acute ischaemic stroke in Japan: a nationwide retrospective cohort survey. BMC Health Serv Res. (2010) 10:213. doi: 10.1186/1472-6963-10-213
11. Yagi M, Yasunaga H, Matsui H, Morita K, Fushimi K, Fujimoto M, et al. Impact of rehabilitation on outcomes in patients with ischemic stroke: a nationwide retrospective cohort study in Japan. Stroke. (2017) 48:740–6. doi: 10.1161/STROKEAHA.116.015147
12. Cuevas-Lara C, Izquierdo M, Gutierrez-Valencia M, Marin-Epelde I, Zambom-Ferraresi F, Contreras-Escamez B, et al. Effectiveness of occupational therapy interventions in acute geriatric wards: a systematic review. Maturitas. (2019) 127:43–50. doi: 10.1016/j.maturitas.2019.06.005
13. Costigan FA, Duffett M, Harris JE, Baptiste S, Kho ME. Occupational therapy in the ICU: a scoping review of 221 documents. Crit Care Med. (2019) 47:e1014–21. doi: 10.1097/CCM.0000000000003999
14. Reuter B, Gumbinger C, Sauer T, Wiethölter H, Bruder I, Diehm C, et al. Access, timing and frequency of very early stroke rehabilitation - insights from the Baden-Wuerttemberg stroke registry. BMC Neurol. (2016) 16:222. doi: 10.1186/s12883-016-0744-7
15. Kinoshita S, Kakuda W, Momosaki R, Yamada N, Sugawara H, Watanabe S, et al. Clinical management provided by board-certificated physiatrists in early rehabilitation is a significant determinant of functional improvement in acute stroke patients: a retrospective analysis of Japan rehabilitation database. J Stroke Cerebrovasc Dis. (2015) 24:1019–24. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.026
16. Jeong S, Kondo K, Shiraishi N, Inoue Y. An evaluation of the quality of post-stroke rehabilitation in Japan. Clinical Audit. (2010) 2:59–66. doi: 10.2147/CA.S7970
17. Kamo T, Momosaki R, Suzuki K, Asahi R, Azami M, Ogihara H, et al. Effectiveness of intensive rehabilitation therapy on functional outcomes after stroke: a propensity score analysis based on Japan rehabilitation database. J Stroke Cerebrovasc Dis. (2019) 28:2537–42. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.007
18. Latham NK, Jette DU, Coster W, Richards L, Smout RJ, James RA, et al. Occupational therapy activities and intervention techniques for clients with stroke in six rehabilitation hospitals. Am J Occup Ther. (2006) 60:369–78. doi: 10.5014/ajot.60.4.369
19. Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. (1996) 77:1226–32. doi: 10.1016/s0003-9993(96)90184-7
20. Chumney D, Nollinger K, Shesko K, Skop K, Spencer M, Newton RA. Ability of Functional Independence Measure to accurately predict functional outcome of stroke-specific population: systematic review. J Rehabil Res Dev. (2010) 47:17–30. doi: 10.1682/jrrd.2009.08.0140
21. Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.str.20.7.864
22. Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS). J Physiother. (2014) 60:61. doi: 10.1016/j.jphys.2013.12.012
23. Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. (1991) 10:585–98. doi: 10.1002/sim.4780100410
24. Eulenburg C, Suling A, Neuser P, Reuss A, Canzler U, Fehm T, et al. Propensity scoring after multiple imputation in a retrospective study on adjuvant radiation therapy in lymph-node positive vulvar cancer. PLoS One. (2016) 11:e0165705. doi: 10.1371/journal.pone.0165705
25. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. (1984) 79:516–24. doi: 10.1080/01621459.1984.10478078
26. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
27. Leyrat C, Seaman SR, White IR, Douglas I, Smeeth L, Kim J, et al. Propensity score analysis with partially observed covariates: how should multiple imputation be used? Stat Methods Med Res. (2019) 28:3–19. doi: 10.1177/0962280217713032
28. Ali MS, Prieto-Alhambra D, Lopes LC, Ramos D, Bispo N, Ichihara MY, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol. (2019) 10:973. doi: 10.3389/fphar.2019.00973
29. Jackson JW, Schmid I, Stuart EA. Propensity scores in pharmacoepidemiology: beyond the horizon. Curr Epidemiol Rep. (2017) 4:271–80. doi: 10.1007/s40471-017-0131-y
30. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. Br Med J. (2019) 367:l5657. doi: 10.1136/bmj.l5657
31. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. (2009) 38:1228–34. doi: 10.1080/03610910902859574
32. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. (2010) 25:1–21. doi: 10.1214/09-STS313
33. Izawa J, Komukai S, Gibo K, Okubo M, Kiyohara K, Nishiyama C, et al. Pre-hospital advanced airway management for adults with out-of-hospital cardiac arrest: nationwide cohort study. Br Med J. (2019) 364:l430. doi: 10.1136/bmj.l430
34. Onwubiko U, Wall K, Sales RM, Holland DP. Using directly observed therapy (dot) for latent tuberculosis treatment - a hit or a miss? A propensity score analysis of treatment completion among 274 homeless adults in Fulton County, GA. PLoS One. (2019) 14:e0218373. doi: 10.1371/journal.pone.0218373
35. Aloisio KM, Micali N, Swanson SA, Field A, Horton NJ. Analysis of partially observed clustered data using generalized estimating equations and multiple imputation. Stata J. (2014) 14:863–83. doi: 10.1177/1536867X1401400410
36. Foley N, McClure JA, Meyer M, Salter K, Bureau Y, Teasell R. Inpatient rehabilitation following stroke: amount of therapy received and associations with functional recovery. Disabil Rehabil. (2012) 34:2132–8. doi: 10.3109/09638288.2012.676145
37. Rogers AT, Bai G, Lavin RA, Anderson GF. Higher hospital spending on occupational therapy is associated with lower readmission rates. Med Care Res Rev. (2017) 74:668–86. doi: 10.1177/1077558716666981
38. Burke JF, Skolarus LE, Adelman EE, Reeves MJ, Brown DL. Influence of hospital-level practices on readmission after ischemic stroke. Neurology. (2014) 82:2196–204. doi: 10.1212/WNL.0000000000000514
Keywords: occupational therapy (OT), stroke rehabilitaiton, recovery of function/prognosis, acute care, physical therapy (PT)
Citation: Yamakawa S, Nagayama H, Tomori K, Ikeda K and Niimi A (2023) Effectiveness of active occupational therapy in patients with acute stroke: A propensity score-weighted retrospective study. Front. Rehabilit. Sci. 3:1045231. doi: 10.3389/fresc.2022.1045231
Received: 15 September 2022; Accepted: 9 December 2022;
Published: 5 January 2023.
Edited by:
Masachika Niimi, Nihon University, JapanReviewed by:
Ljubica Konstantinovic, University of Belgrade, SerbiaArchana Hinduja, The Ohio State University, United States
© 2023 Yamakawa, Nagayama, Tomori, Ikeda and Niimi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirofumi Nagayama aGlyb2Z1bWluYWdheWFtYUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Medical and Surgical Rehabilitation, a section of the journal Frontiers in Rehabilitation Sciences
 Shiori Yamakawa1,†
Shiori Yamakawa1,† Hirofumi Nagayama
Hirofumi Nagayama Kounosuke Tomori
Kounosuke Tomori Kohei Ikeda
Kohei Ikeda