- 1Department of Human Movement Sciences, Faculty of Behavioural and Movement Sciences, Vrije Universiteit Amsterdam, Amsterdam, Netherlands
- 2Sport Science College, Beijing Sport University, Beijing, China
- 3Institute of Brain and Behavior Amsterdam, Amsterdam, Netherlands
- 4Biomechanics Laboratory, Fujian Medical University, Quanzhou, China
Our aim was to evaluate differences in gait acceleration intensity, variability, and stability of feet and trunk between older females (OF) and young females (YF) using inertial sensors. Twenty OF (mean age 68.4, SD 4.1 years) and 18 YF (mean age 22.3, SD 1.7 years) were asked to walk straight for 100 meters at their preferred speed, while wearing inertial sensors on their heels and lower back. We calculated spatiotemporal measures, foot and trunk acceleration characteristics, their variability, and trunk stability using the local divergence exponent (LDE). Two-way ANOVA (such as the factors foot and age), Student's t-test and Mann–Whitney U test were used to compare statistical differences of measures between groups. Cohen's d effects were calculated for each variable. Foot maximum vertical (VT) acceleration and amplitude, trunk-foot VT acceleration attenuation, and their variability were significantly smaller in OF than in YF. In contrast, trunk mediolateral (ML) acceleration amplitude, maximum VT acceleration, amplitude, and their variability were significantly larger in OF than in YF. Moreover, OF showed lower stability (i.e., higher LDE values) in ML acceleration, ML, and VT angular velocity of the trunk. Even though we measured healthy OF, these participants showed lower VT foot accelerations with higher VT trunk acceleration, lower trunk-foot VT acceleration attenuation, less gait stability, and more variability of the trunk, and hence, were more likely to fall. These findings suggest that instrumented gait measurements may help for early detection of changes or impairments in gait performance, even before this can be observed by clinical eye or gait speed.
Introduction
Falls among older adults are the leading indirect cause of disability and death (1). Epidemiological studies have shown that the 30% of people aged 65 years and older fall, with an increase in incidence to 40% in people over 80 years (2). This is due to poorer physiological function and control of stability with aging (3). In China, 53% of falls occur while walking (4), and hence, it is particularly important to pay attention to the gait performance of older adults for early identification of stability problems to prevent falls. Moreover, many studies have shown that among people over 60 years, females were more likely to fall (5–7), as about 65% of women and 44% of men fell in their usual place of residence (8). Therefore, we focused on the gait stability of females in our study.
There are several ways to evaluate gait, such as clinical function tests, questionnaires, and measurements in a biomechanics laboratory (9). Questionnaires and clinical tests cannot reflect gait performance outside the laboratory, and sometimes have poor objectivity (10). Gait assessment in a biomechanical laboratory has the advantage of capturing whole-body kinematics which is accurate but also costly, time-consuming, and limited to space and time (11). Nowadays, the feasibility of inertial sensors to quantify the whole-body gait kinematics has been demonstrated (12), and they can be used to collect gait data in people's own environment by a single sensor on either the trunk or foot (13, 14).
Gait stability reflects the ability to keep walking in the face of perturbations (15, 16). Dynamical systems and non-linear time series analysis can be used to evaluate gait stability by quantifying the complex and chaotic characteristics of the human body (17). One of these measures, the local divergence exponent (LDE), has been shown to have good reliability and validity (18–20). The LDE quantifies the average exponential rate of divergence of neighboring trajectories in state space and provides a direct measure of the sensitivity of a system to small perturbations (21).
Internal perturbations of the human body cause variability and randomness in gait (22). If gait is within a stable range, people would not need to correct this variability. Increased variability likely reflects a less automatic gait pattern, instability, and increased susceptibility to falls (14). Studies also confirmed that variability in some gait characteristics (such as stride length, stride width, and stride time) is highly related to the risk of falling (23, 24). However, some studies suggested that variability is not equal to stability, as the level of variability was not necessarily negatively related to the level of stability (25, 26).
As the control of stability in gait declines with aging, we aimed to use inertial sensors to assess differences in gait stability and variability between healthy young (YF) and older females (OF). In doing so, we focused on data obtained from the trunk and foot sensors and calculated acceleration intensity, stability, and variability measures. We hypothesized that OF have lower gait stability and increased variability on trunk accelerations compared with YF.
Materials and Methods
Participants
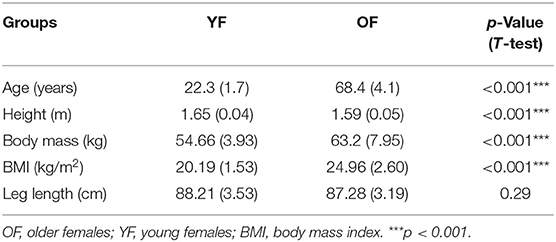
A total of 20 healthy OF and 18 YF were recruited from the campus of Beijing Sport university, China (Table 1). None of our participants had any orthopedic or neurological disorders, acute pain, or other complaints that might have affected gait and they were all able to walk independently without a walking aid. All participants were informed about the research procedures, and the protocol was approved by the Ethics Committee of Sports Science Experiment of Beijing Sport University (approval number: 2021010H).
Data Acquisition
Participants wore three inertial sensors (Xsens MTw Awinda, the Netherland) on the heels and the lumbar region of the trunk, using the supplied elastic belt. These sensors had a sample rate of 100 samples and a range of −160 m/s2 and +160 m/s2. Data collection was synchronized between sensors. All participants wore the same model of shoes. They were asked to walk 100 meters on a straight running track at a self-selected speed, since gait variability is expected to be minimal at this speed for healthy people (27). In addition, although clinical gait tests are usually 4 or 10 meters, these tests do not represent daily-life gait very well (28). Therefore, 100 meters used in this study can well reflect the natural gait at a comfortable speed without participants being exhausted.
Gait Measures
MATLAB (R2019b, MathWorks Inc, Natick, MA, USA) was used to analyze data without the first and last steps. Each gait cycle was identified from the sagittal plane angular velocity of foot sensors with three gait events: heel-strike (Theel_strike), toe-off (Ttoe_off), and foot-flat (Tfoot_flat) (29). Stride time was defined as the duration between two consecutive Theel_strike. Combined with the gait events of both feet, we got the initial double support (IDS) period and the terminal double support (TDS) period.
For the trunk sensor, sensor data were realigned to a coordinate system based on the accelerometer's orientation with respect to gravity (vertical, VT, axis) and optimization of left-right symmetry (mediolateral, ML, axis) (10, 30).
For the foot sensors, initial displacements were calculated by integrating linear accelerations twice for each gait cycle (in the global coordinate system), using the zero-velocity-update method to eliminate drift, assuming linearity of the drift (31). The hence obtained direction of displacement was not necessary along the x- or y-axis of the global coordinate system. To obtain meaningful stride lengths, we thus rotated the obtained positions, the acceleration, and angular velocity of the feet to a coordinate system that was aligned with the direction of walking (i.e., end position minus starting position), with the VT axis being VT. Then, walking speed was obtained by dividing the distance of the walking direction by the time.
For acceleration measures, maximum VT acceleration of feet and trunk was calculated to reflect the intensity of ground contact (32). It has been suggested that people stabilize their heads during walking (33). Although the trunk segment plays a key role in damping gait-related oscillations (33), the damping of oscillations by the trunk in the VT direction has been suggested to be minor (34, 35). Hence, such accelerations must be attenuated by the lower limbs. Thus, we calculated trunk-foot VT acceleration attenuation, which was used in our study, and was calculated by the difference in maximum VT acceleration between trunk and foot, which represents the impact absorption of the lower limbs. Acceleration amplitude (in the coordinate system prescribed by the walking direction, see above) for each direction [anteroposterior direction (AP), ML, and VT] was calculated as the range of acceleration in a gait cycle.
For the above measures of each person, after getting the mean and SD over all cycles (see Tables 2, 3), we obtained the coefficient of variation (CV) by dividing the SD by the mean (36) (see Supplementary Table 1).
We calculated the LDE of acceleration and angular velocity of each dimension separately (in the coordinate system prescribed by the walking direction, see above). The time series of 50 gait cycles was normalized into 5,000 samples, with an average of 100 samples per cycle. From these data, state spaces were reconstructed using the method of correlation integral (C-C method), which not only can determine both embedding dimension and delay time but also has good robustness to the noise in a small amount of data (37) (see Supplementary Tables 2, 3 for dimension and delay values). LDE was expressed as the mean logarithmic rate of divergence per stride using Rosenstein's method (38). Higher values of the LDE indicate lower local stability.
Statistical Analysis
Normality was assessed using the Kolmogorov–Smirnov test. For measures of the left and right feet, differences were tested using two-way ANOVAs, with within-subject factor Foot (left and right) and between-subject factor Group (YF and OF). For other measures, we used Student's t-tests to compare between age groups. For LDE, which appeared not distributed normally, we compared between groups using the Mann–Whitney U test. For all measures, p < 0.05 was considered as a significant effect. Cohen's d effects were calculated for each variable as the difference between group means divided by the group pooled SD. Magnitudes of d = 0.01, 0.20, 0,50, 0.80, 1.20, and 2.0 were considered very small, small, medium, large, very large, and huge, separately (39–41).
Results
Descriptive characteristics of the participants are summarized in Table 1. OF were significantly older, shorter, and had a higher weight and BMI than YF. The mean age of OF and YF was 68.4 and 22.3, respectively.
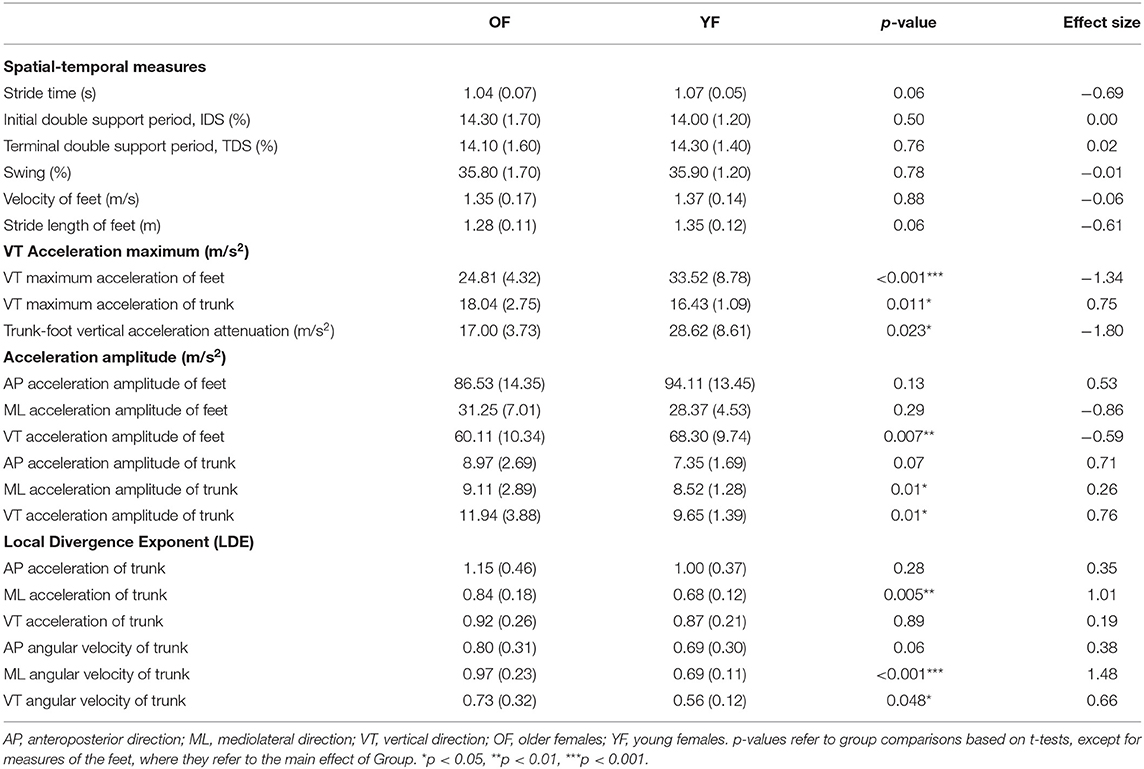
Table 2 shows the mean values for all measures. We found no interaction between Foot and Group for any of the outcome measures and no significant effect of Foot. Hence, all variables that were calculated for both feet are displayed as averages over both feet. OF had a higher maximum VT acceleration of the trunk than YF, with a medium effect size (0.75), but a smaller maximum VT acceleration of the feet than YF, with a very large effect size (1.34). As a result, OF had significantly smaller trunk-foot VT acceleration attenuation, with a very large effect size of 1.8. In addition, VT accelerations of OF amplitude of the feet were significantly smaller than YF, with a medium effect size (−0.59). For the trunk, OF's ML and VT acceleration amplitudes were significantly larger than YF, and the effect size of the latter was the largest (0.76). The LDE of trunk from ML acceleration and from ML and VT angular velocity was significantly larger (less stable) for OF than for YF, with large (1.01), very large (1.48), and medium effect size (0.66), respectively.
Table 3 shows the variability of all measures. No significant differences in variability of spatial-temporal gait measures were found between groups. The variability of maximum VT acceleration of the feet was significantly smaller for OF than YF, and its effect size was 1.70. While for the trunk, the variability of the maximum VT acceleration was significantly larger for the OF (medium effect size 0.72). The variability of trunk-foot VT acceleration attenuation was smaller in OF than in YF (effect size very large, 1.87). OF had significantly smaller variability of acceleration amplitude of the feet in three directions than YF, with huge effect size in ML direction (2.53) and large effect size in the VT direction (1.01). For the trunk, variability of acceleration amplitude of OF was significantly larger than YF in ML and VT direction, with effect sizes of 0.41 and 0.86, respectively. The CV of gait measures showed largely the same pattern as the SD (see Supplementary Table 1).
Discussion
Mean Gait Measures
In this study, we used inertial sensors to evaluate differences in acceleration intensity, variability, and stability of feet and trunk during gait between healthy YF and OF. Although older adults generally were suggested to walk slower due to physical limitations, such as muscle weakness or loss of flexibility (42), the OF in our study walked at a similar preferred speed and stride length as the YF.
We found a reduction in foot VT maximum acceleration in OF, which probably reflected a reduction of peak ground reaction forces. Such a reduction of ground reaction forces could result from a crouch-like gait, which has been shown in young adults to lead to a reduction of the peak ground reaction force (43, 44). Such a crouch-like gait may increase the metabolic cost of locomotion in the elderly (45). Although the trunk segment plays a key role in damping gait-related oscillations (33), the damping of oscillations by the trunk in the VT direction has been suggested to be minor (34, 35). In our study, we found a lower trunk-foot VT acceleration attenuation and a higher trunk acceleration amplitude in OF, which implies decreased cushioning (impact absorption) and hence less preservation of the stability of the head (46). Even though foot (VT) accelerations were lower in OF, suggesting less impact, the OF were not able to attenuate the higher accelerations in the trunk. This reduction in impact absorption may be caused by age-related neuromuscular changes, such as a reduced muscle strength of the triceps surae and quadriceps femoris (47), degraded stiffness and elastic modulus of the tendons (48), muscle co-contraction, and degraded absorption of the intervertebral disc (49). Considering that two-thirds of the weight of the human body is in the upper body, such higher trunk accelerations may be destabilizing, which may cause falls (50).
For stability, LDE calculated from trunk time-series data has been shown to better reflect differences in gait stability due to age than LDE calculated from data of other segments (51). In our study, OF showed significantly lower local dynamic stability (higher LDE) in ML acceleration, ML, and VT angular velocity. Among these, the LDE calculated from trunk ML angular velocity had the largest effect size. As stability in the ML direction needs more control than stability in the AP direction during gait (52, 53), decreased LDE of trunk angular velocity in ML direction could be an early indicator of gait stability problems.
Variability Measures
All participants in this study walked under the same environmental conditions. Thus, any between-subject differences in variability arose from differences in (internal) neuromotor noise and not (external) environmental noise. No differences were found in the variability of spatiotemporal measures, which was consistent with a previous study showing that temporal gait variability of older non-fallers was not significantly different from young adults in terms of SD and CV (27).
Our OF walked with less variability of maximum VT acceleration of feet variability than YF (Table 3, Supplementary Table 1). However, the variability of ML and VT acceleration amplitude of the trunk was larger for the OF, which could suggest OF are at a higher risk of balance loss and falling (36).
All in all, our findings suggest that stability of the trunk might be a more sensitive indicator of locomotor impairment and potential future risk of falls than changes in variability of the trunk, as the LDE had higher effect sizes (54). Measures of the variability of acceleration of the feet showed even higher effect sizes and might thus be even more useful. However, here, it should be noted that these effects were opposite from theoretically expected, with the OF having lower (means and variability) acceleration of the foot.
Limitations
All tests in our study were aimed at testing the same hypothesis, that is, OF are less stable and more variable than YF, hence, we did not use a correction for multiple testing. Nonetheless, not correcting may lead to Type I errors, and thus, some caution is warranted. Furthermore, the older participants in our study were quite fit and additional studies are needed to further investigate the applicability of acceleration attenuation when studying older adults. Future research can expand the sample size and conduct a multi-center study to obtain more representative results. Although we used only trunk and feet sensors for practical usefulness, the underlying mechanisms for the alterations in gait in the older women remain unclear and would require more detailed assessments of, e.g., whole-body kinematics and muscle activity.
Conclusions
Although healthy OF had similar walking speeds and spatiotemporal parameters as YF during steady-state walking, they showed lower VT foot accelerations and higher VT trunk accelerations, suggesting less impact and less absorption of the impact. In addition, lower gait stability and higher variability of trunk movements for OF also indicated they were more likely to fall. The measures derived from the accelerations of the trunk were sensitive to reflect the gait instability as expected, especially trunk-foot VT acceleration attenuation and its variability. While the variability of foot acceleration amplitudes was also sensitive to age, these differences were opposite from expected, making it harder to draw any conclusion as to their usefulness for fall prediction. These findings suggest that instrumented gait measurements may help for early detection of changes or impairments in gait performance, even before this can be observed by clinical eye or gait speed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sports Science Experiment of Beijing Sport University (approval number: 2021010H). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YZ: methodology, software, formal analysis, investigation, data curation, writing—original draft, writing—review and editing, and visualization. XZ: conceptualization and resources. MP: formal analysis, writing review and editing, supervision, and funding acquisition. SB: methodology, software, formal analysis, writing—review and editing, supervision, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
YZ was funded by a CSC Scholarship Council (CSC) fellowship (202009110145). MP was funded by a VIDI (Grant No. 91714344) from the Dutch Organization for Scientific Research (NWO). SB was funded by a VIDI Grant (016.Vidi.178.014) from the Dutch Organization for Scientific Research (NWO).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2021.763309/full#supplementary-material
References
1. Tang Y, Guo X, Qiao Z, Qiu P. Analysis on prevalence and risk factors for falls among the elderly in communities of Beijing and Shanghai. Chin J Dis Control Prev. (2017) 21:72–6. doi: 10.16462/j.cnki.zhjbkz.2017.01.017
2. Weber M, Van Ancum JM, Bergquist R, Taraldsen K, Gordt K, Mikolaizak AS, et al. Concurrent validity and reliability of the Community Balance and Mobility scale in young-older adults. BMC Geriatr. (2018) 18:1–10. doi: 10.1186/s12877-018-0845-9
3. Winter DA. Human balance and posture control during standing and walking. Gait and posture. (1995) 3:193–214. doi: 10.1016/0966-6362(96)82849-9
4. Xia Q, Jiang Y, Tang C, Niu C. Study on the epidemiologic characteristics and medical burden of falls among adults in community. Chin J Dis Control Prev. (2010) 14:647–9.
5. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. (1988) 319:1701–7. doi: 10.1056/NEJM198812293192604
6. Daley MJ, Spinks WL. Exercise, mobility and aging. Sports Med. (2000) 29:1–12. doi: 10.2165/00007256-200029010-00001
7. De Rekeneire N, Visser M, Peila R, Nevitt MC, Cauley JA, Tylavsky FA, et al. Is a fall just a fall: correlates of falling in healthy older persons. the health, aging and body composition study. J Am Geriatr Soc. (2003) 51:841–6. doi: 10.1046/j.1365-2389.2003.51267.x
8. Masud T, Morris RO. Epidemiology of falls. Age Ageing. (2001) 30:3–7. doi: 10.1093/ageing/30.suppl_4.3
9. Hamacher D, Singh N, Van Dieën JH, Heller M, Taylor WR. Kinematic measures for assessing gait stability in elderly individuals: a systematic review. J R Soc Interface. (2011) 8:1682–98. doi: 10.1098/rsif.2011.0416
10. Van Schooten KS, Pijnappels M, Rispens SM, Elders PJ, Lips P, Van Dieen JH. Ambulatory fall-risk assessment: amount and quality of daily-life gait predict falls in older adults. J Gerontol A Biol Sci Med Sci. (2015) 70:608–15. doi: 10.1093/gerona/glu225
11. Terrier P, Deriaz O. Kinematic variability, fractal dynamics and local dynamic stability of treadmill walking. J Neuroeng Rehabil. (2011) 8:12–12. doi: 10.1186/1743-0003-8-12
12. Tao W, Liu T, Zheng R, Feng H. Gait analysis using wearable sensors. Sensors. (2012) 12:2255–83. doi: 10.3390/s120202255
13. Zhu S, Anderson H, Wang Y. “A real-time on-chip algorithm for IMU-Based gait measurement.” In: Proceedings of Pacific-Rim Conference on Multimedia. Berlin; Heidelberg: Springer (2012). p. 93–104. doi: 10.1007/978-3-642-34778-8_9
14. Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, et al. Does the evaluation of gait quality during daily life provide insight into fall risk? a novel approach using 3-day accelerometer recordings. Neurorehabil Neural Repair. (2013) 27:742–52. doi: 10.1177/1545968313491004
15. Pai Y-C, Bhatt TS. Repeated-slip training: an emerging paradigm for prevention of slip-related falls among older adults. Phys Ther. (2007) 87:1478–91. doi: 10.2522/ptj.20060326
16. Bruijn SM, Meijer O, Beek P, Van Dieen JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface. (2013) 10:20120999. doi: 10.1098/rsif.2012.0999
17. Bressel E. Innovative analyses of human movement: analytical tools for human movement research. Med Sci Sports Exerc. (2004) 36:1834. doi: 10.1249/00005768-200410000-00027
18. England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait Posture. (2007) 25:172–8. doi: 10.1016/j.gaitpost.2006.03.003
19. Son K, Park J, Park S. Variability analysis of lower extremity joint kinematics during walking in healthy young adults. Med Eng Phys. (2009) 31:784–92. doi: 10.1016/j.medengphy.2009.02.009
20. Hu F, Gu D, Dai K, An B, Chen J, Wu Y. Nonlinear time series analysis of gait stability during walking. J Med Biomech. (2012) 27:51–7.
21. Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. J Biomech. (2006) 39:444–52. doi: 10.1016/j.jbiomech.2004.12.014
22. Zhang B, Jiang S, Yan K, Wei D, Smigorski K. Human walking analysis, evaluation and classification based on motion capture system. Heal. Manag.–Differ. Approaches Solut. (2011)0.361–398. doi: 10.5772/21387
23. O'loughlin JL, Boivin J-F, Robitaille Y, Suissa S. Falls among the elderly: distinguishing indoor and outdoor risk factors in Canada. J Epidemiol Community Health. (1994) 48:488. doi: 10.1136/jech.48.5.488
24. Chau T, Young S, Redekop S. Managing variability in the summary and comparison of gait data. J Neuroeng Rehabil. (2005) 2:22. doi: 10.1186/1743-0003-2-22
25. Li L, Haddad JM, Hamill J. Stability and variability may respond differently to changes in walking speed. Hum Mov Sci. (2005) 24:257–67. doi: 10.1016/j.humov.2005.03.003
26. Van Emmerik RE, Ducharme SW, Amado AC, Hamill J. Comparing dynamical systems concepts and techniques for biomechanical analysis. J Sport Health Sci. (2016) 5:3–13. doi: 10.1016/j.jshs.2016.01.013
27. Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. (1997) 78:278–83. doi: 10.1016/S0003-9993(97)90034-4
28. Van Ancum JM, Van Schooten KS, Jonkman NH, Huijben B, Van Lummel RC, Meskers CG, et al. Gait speed assessed by a 4-m walk test is not representative of daily-life gait speed in community-dwelling adults. Maturitas. (2019) 121:28–34. doi: 10.1016/j.maturitas.2018.12.008
29. Mariani B, Hoskovec C, Rochat S, Bula C, Penders J, Aminian K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J Biomech. (2010) 43:2999–3006. doi: 10.1016/j.jbiomech.2010.07.003
30. Rispens SM, Van Schooten KS, Pijnappels M, Daffertshofer A, Beek PJ, Van Dieen JH. Identification of fall risk predictors in daily life measurements: gait characteristics' reliability and association with self-reported fall history. Neurorehabil Neural Repair. (2015) 29:54–61. doi: 10.1177/1545968314532031
31. Skog I, Handel P, Nilsson J-O, Rantakokko J. Zero-velocity detection—an algorithm evaluation. IEEE Trans Biomed Eng. (2010) 57:2657–66. doi: 10.1109/TBME.2010.2060723
32. Gill H, O'connor J. Heelstrike and the pathomechanics of osteoarthrosis: a pilot gait study. J Biomech. (2003) 36:1625–31. doi: 10.1016/S0021-9290(03)00189-1
33. Kavanagh J, Barrett R, Morrison S. The role of the neck and trunk in facilitating head stability during walking. Exp Brain Res. (2006) 172:454. doi: 10.1007/s00221-006-0353-6
34. Prince F, Winter DA, Stergiou P, Walt SE. Anticipatory control of upper body balance during human locomotion. Gait Posture. (1994) 2:19–25. doi: 10.1016/0966-6362(94)90013-2
35. Kavanagh JJ, Barrett RS, Morrison S. Upper body accelerations during walking in healthy young and elderly men. Gait Posture. (2004) 20:291–8. doi: 10.1016/j.gaitpost.2003.10.004
36. Kavanagh JJ, Menz HB. Accelerometry: a technique for quantifying movement patterns during walking. Gait Posture. (2008) 28:1–15. doi: 10.1016/j.gaitpost.2007.10.010
37. Kim HS, Eykholt R, Salas J. Nonlinear dynamics, delay times, and embedding windows. Physica D. (1999) 127:48–60. doi: 10.1016/S0167-2789(98)00240-1
38. Rosenstein MT, Collins JJ, De Luca CJ. A practical method for calculating largest Lyapunov exponents from small data sets. Physica D. (1993) 65:117–34. doi: 10.1016/0167-2789(93)90009-P
39. Sawilowsky SS. New effect size rules of thumb. J Mod Appl Stat Methods. (2009) 8:26. doi: 10.22237/jmasm/1257035100
40. Charach A, Dashti B, Carson P, Booker L, Lim CG, Lillie E, et al. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis, and Treatment. Rockville, MD: Agency for Healthcare Research and Quality (2011).
41. Cohen J. Statistical Power Analysis For the Behavioral Sciences. Academic press (2013). doi: 10.4324/9780203771587
42. Hamacher D, Hamacher D, Schega L. Towards the importance of minimum toe clearance in level ground walking in a healthy elderly population. Gait Posture. (2014) 40:727–9. doi: 10.1016/j.gaitpost.2014.07.016
43. Li Y, Crompton R, Alexander RM, Günther M, Wang W. Characteristics of ground reaction forces in normal and chimpanzee-like bipedal walking by humans. Folia Primatologica. (1996) 66:137–59. doi: 10.1159/000157191
44. Grasso R, Zago M, Lacquaniti F. Interactions between posture and locomotion: motor patterns in humans walking with bent posture versus erect posture. J Neurophysiol. (2000) 83:288–300. doi: 10.1152/jn.2000.83.1.288
45. Carey TS, Crompton RH. The metabolic costs of ‘bent-hip, bent-knee’ walking in humans. J Hum Evol. (2005) 48:25–44. doi: 10.1016/j.jhevol.2004.10.001
46. Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. (2003) 18:35–46. doi: 10.1016/S0966-6362(02)00159-5
47. Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol. (2006) 91:483–98. doi: 10.1113/expphysiol.2005.032896
48. Mccrum C, Leow P, Epro G, König M, Meijer K, Karamanidis K. Alterations in leg extensor muscle-tendon unit biomechanical properties with ageing and mechanical loading. Front Physiol. (2018) 9:150. doi: 10.3389/fphys.2018.00150
49. Brzuszkiewicz-Kuzmicka G, Szczegielniak J, Baczkowicz D. Age-related changes in shock absorption capacity of the human spinal column. Clin Interv Aging. (2018) 13:987. doi: 10.2147/CIA.S156298
50. Woollacott MH, Tang P-F. Balance Control During Walking in the Older Adult: Research and Its Implications. Physical Therapy. (1997) 77:646–60. doi: 10.1093/ptj/77.6.646
51. Punt M, Bruijn SM, Wittink H, Van Dieën JH. Effect of arm swing strategy on local dynamic stability of human gait. Gait Posture. (2015) 41:504–9. doi: 10.1016/j.gaitpost.2014.12.002
52. Bruijn SM, Van Dieën JH, Meijer OG, Beek PJ. Is slow walking more stable? J Biomech. (2009) 42:1506–12. doi: 10.1016/j.jbiomech.2009.03.047
53. O'connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. (2009) 102:1411–9. doi: 10.1152/jn.00131.2009
Keywords: walking, aging, wearable system, motor control, balance
Citation: Zhang Y, Zhou X, Pijnappels M and Bruijn SM (2021) Differences in Gait Stability and Acceleration Characteristics Between Healthy Young and Older Females. Front. Rehabilit. Sci. 2:763309. doi: 10.3389/fresc.2021.763309
Received: 23 August 2021; Accepted: 06 October 2021;
Published: 17 November 2021.
Edited by:
Rosie Morris, Northumbria University, United KingdomReviewed by:
Naoya Hasegawa, Hokkaido University, JapanJannis Papathanasiou, Medical University-Sofia, Bulgaria
Copyright © 2021 Zhang, Zhou, Pijnappels and Bruijn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sjoerd M. Bruijn, cy5tLmJydWlqbkBnbWFpbC5jb20=
 Yuge Zhang
Yuge Zhang Xinglong Zhou2
Xinglong Zhou2 Mirjam Pijnappels
Mirjam Pijnappels Sjoerd M. Bruijn
Sjoerd M. Bruijn