- 1Assistive Robot Center, National Center for Geriatrics and Gerontology, Obu, Japan
- 2Department of Rehabilitation Medicine, Kariya Toyota General Hospital, Kariya, Japan
- 3Department of Rehabilitation Medicine I, School of Medicine, Fujita Health University, Toyoake, Japan
Background: A self-monitoring approach utilizing fitness trackers that provide feedback regarding physical activities has been recently applied to rehabilitation patients to promote voluntary walking activities. Although this approach has been proven to increase physical activity, it is uncertain whether the intervention improves walking ability.
Aim: This review investigated whether the additional self-monitoring approach using activity trackers would improve walking ability in any type of rehabilitation setting.
Methods: A systematic search was performed in four databases [PubMed (MEDLINE), The Cochrane Library, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature] to identify studies that examined the self-monitoring approach combined with rehabilitative intervention vs. the same rehabilitative intervention only in participants with any unhealthy conditions. Two review authors independently assessed the eligibility of all the retrieved English literature published from 2009 to 2019, then discussed the final inclusion. The risk of bias was assessed referring to the criteria of the Cochrane Risk of Bias tool. The key findings were synthesized using narrative synthesis. In addition, a quantitative synthesis was conducted when more than two studies investigating the same disease were identified.
Results: Eleven randomized controlled trials satisfied the eligibility criteria, nine of which had a lower risk of bias. The types of diseases included stroke, chronic obstructive pulmonary disease (COPD), cancer, Parkinson's disease, hemophilia, peripheral artery disease, post-total knee arthroplasty, and geriatric rehabilitation. Eight studies reported measures of walking endurance and four reported measures of gait speed. In the quantitative synthesis of two studies investigating COPD, there was a significant between-group difference in terms of changes in the 6-min walking distance from the baseline, which was favorable to the additional self-monitoring intervention group (mean difference: 13.1 m; 95% confidence interval, 1.8–24.5; 2 studies, 124 participants; p = 0.02; I2 = 0%). Other available data revealed no consistent evidence regarding effectiveness of the intervention.
Conclusions: The findings indicate that there is little evidence suggesting the effectiveness of the self-monitoring approach in improving walking ability in rehabilitation settings. However, a weak recommendation for patients with stable COPD was implicated in the quantitative synthesis. Further research would be required to explore the best indications for this self-monitoring approach.
Systematic Review Registration: CRD 42020157695.
Introduction
According to research in motor skill learning, an increase in the amount of training leads to greater efficacy (1). When this theory is applied in rehabilitation medicine for achieving better walking ability, it is essential to increase the dose of walking activity (2, 3). However, in clinical practice, the number of steps taken per day by those living with disabilities are extremely low (4, 5), and only training sessions provided by physiotherapists are often inadequate to increase the real-world walking activity in daily life (6, 7). Therefore, effective techniques are required to motivate behavioral change in various rehabilitation settings (8).
Recently, a self-monitoring approach using wearable fitness trackers or activity monitors (pedometers or accelerometers) has been applied to promote voluntary walking activity as a part of rehabilitative intervention. This strategy aims to encourage patients to increase their physical activity by displaying their current activity/inactivity and providing specific information for subsequent improvement (9). Additionally, this kind of approach is getting more inexpensive and practical as many types of high-quality consumer-based pedometers have been made available recently (9). In cardiac rehabilitation, some studies involving the use of pedometers reported significant increase in the physical activity in patients after discharge or with low motivation (10, 11). Several systematic reviews have also revealed significant increase in the physical activity of some populations such as chronic obstructive pulmonary disease (COPD), cardiovascular diseases, and people with sedentary habits (12–15). Regarding stroke, a Cochrane review (16) confirmed that some studies reported the effectiveness of activity monitoring in increasing physical activity. However, these previous reviews raise the following questions. First, it remains unclear whether the increase in physical activity gained by self-monitoring is sufficient for improving walking ability, which is the ultimate goal of gait rehabilitation. Second, many of the included studies provided not just self-monitoring but a comprehensive program as intervention or did not provide any rehabilitative approach for the control groups, if any. Consequently, the pure effectiveness of a self-monitoring approach alone has yet to be proved when added to the usual rehabilitation.
Therefore, this review summarized the available evidence regarding the effectiveness of the self-monitoring approach using fitness trackers for actually improving walking ability, not merely increasing physical activity, in rehabilitation settings. Particularly, we reviewed the studies that compared the effects of the self-monitoring approach in addition to rehabilitation with the same rehabilitation alone.
Materials and Methods
The study adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (17, 18) and the checklist. The protocol was registered with the International Prospective Register of Systematic Reviews (CRD 42020157695).
Review Question
The research question was as follows: Does an additional self-monitoring approach using wearable activity trackers result in improved walking ability compared to rehabilitative intervention alone?
Data Sources
A systematic search of the literature was performed using four different electronic databases [PubMed (MEDLINE), The Cochrane Library, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL)]. We developed individualized search strategies for each database (Appendix A).
Study Selection
Types of Study Design
Randomized controlled trials (RCTs) and non-RCTs were included.
Types of Participants
We included studies with participants who underwent rehabilitative intervention due to older age, frailty, various disorders, or any unhealthy conditions, regardless of the disease type. Studies regarding children, adolescents, or healthy adults (<65 years of age with no need for rehabilitative intervention) were excluded.
Types of Intervention
We included all trials that examined the self-monitoring approach combined with rehabilitative intervention vs. the same rehabilitative intervention only. The self-monitoring approach is defined as a method designed to promote physical activity by wearing any type of pedometer, activity monitor, fitness tracker, or accelerometer. These devices were intended to encourage participants to be more active by recording and displaying their daily activities—counting the number of steps walked daily, recording the time spent in moderate-to-vigorous intensity physical activity, or calculating the calories expended. Based on these records, participants receive various types of feedback, including daily records, weekly summaries, or sometimes individualized tasks, instructions, or goals based on a specific protocol. Therefore, the devices adopted in this intervention had to possess the capability to retain data for the preceding several days in stored memory, thereby enabling the average or total count for a certain period to be evaluated. Some of these devices have functions to display cumulative step counts for each day so that the user could see the step counts immediately and daily, but this type of function was not considered necessary for providing regular feedback.
Types of Comparators
We included studies in which participants in the control group had the same health conditions as the intervention group and the same rehabilitative intervention was provided for both groups, except for the self-monitoring approach.
Types of Outcome Measures
We defined the primary outcome domain of interest as the quantified walking ability measured by the gait speed or walking endurance (timed walking distance) at the end of the intervention. Moreover, we adopted any physical functions related to walking ability by measuring the balance (Berg Balance Scale, Timed Up and Go Test), aerobic capacity (maximum oxygen uptake), muscle strength or power (handgrip strength, quantified knee extension muscle strength, and 30-s chair stand test) at the end of the intervention as additional outcomes of interest. It was considered eligible if the outcome analysis included any of the final values or changes from baseline. Whether the outcomes of interest were the primary outcomes of the study was not considered.
Study Selection Procedure
After eliminating irrelevant studies by examining the titles and abstracts, the full texts of all remaining studies were obtained, and two independent review authors (EO and YO) assessed the eligibility of all the manuscripts. After disagreements between individual judgments were discussed, consensus was gained and the decision of the studies to be ultimately included was finalized.
Data Extraction
Two independent review authors (EO and YO) performed the data extraction. Study investigators were contacted for unreported data if necessary to complete the description of the included studies.
Assessing Risk of Bias
The risk of bias was assessed by collaboration of the two review authors (EO and YO), and disagreements were resolved by discussion. The criteria of the Cochrane risk of bias tool (19) were used to evaluate the methodological quality. We also inspected funnel plots to assess the risk of publication bias.
Data Synthesis
At the registry, we did not intend to conduct a meta-analysis because we did not anticipate that there would be ≥2 studies with similar participant populations, interventions, and outcome measures of interest. However, we identified a small number of similar studies that evaluated the effect of the self-monitoring approach for the same disease; we therefore partially conducted a quantitative synthesis by pooling the data using Review Manager 5.3 (Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We undertook both a fixed-effect model and a random-effects model in synthesizing the studies and obtained the same results in all cases. In addition, we used the mean difference and 95% confidence interval (CI) to summarize studies using the same measurement scales in the analyses, and standardized mean difference and 95% CIs when the studies adopted different scales to assess the same outcome. I2 statistics were used to assess the heterogeneity. Regarding the remaining included studies, we provided a narrative synthesis as planned a priori, giving priority to studies with a lower risk of bias.
Results
Flow Diagram of the Studies Retrieved for Review
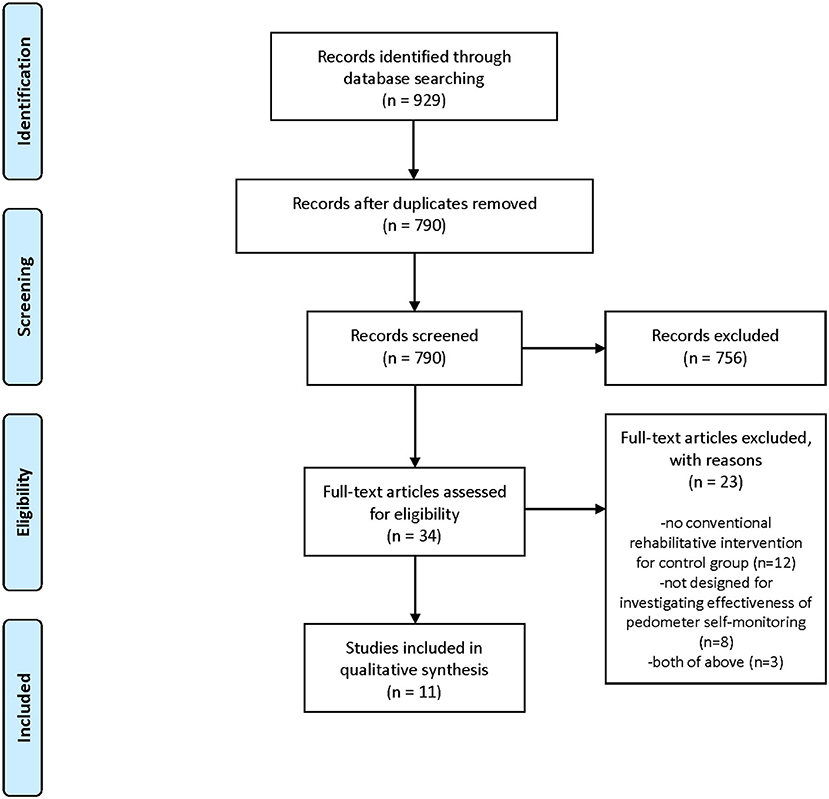
Figure 1 depicts a flow diagram of the study selection for this review. We identified 929 references from our search. Of these, we removed 139 duplications, and eliminated 756 by screening the titles and abstracts. From the 34 remaining articles, we excluded 23 and finally included 11 studies in the review. Appendix B provides the excluded studies at the full-text stage and the reasons for exclusion. Exclusion from the final selection was because no rehabilitative intervention was provided for the control group (15 studies), or because the study was not designed to investigate the effectiveness of a solely self-monitoring approach, that is, the investigated intervention was a comprehensive program including a self-monitoring approach as well as dietary or psychological approaches that were not provided for the control group (10 studies).
Characteristics of Included Studies
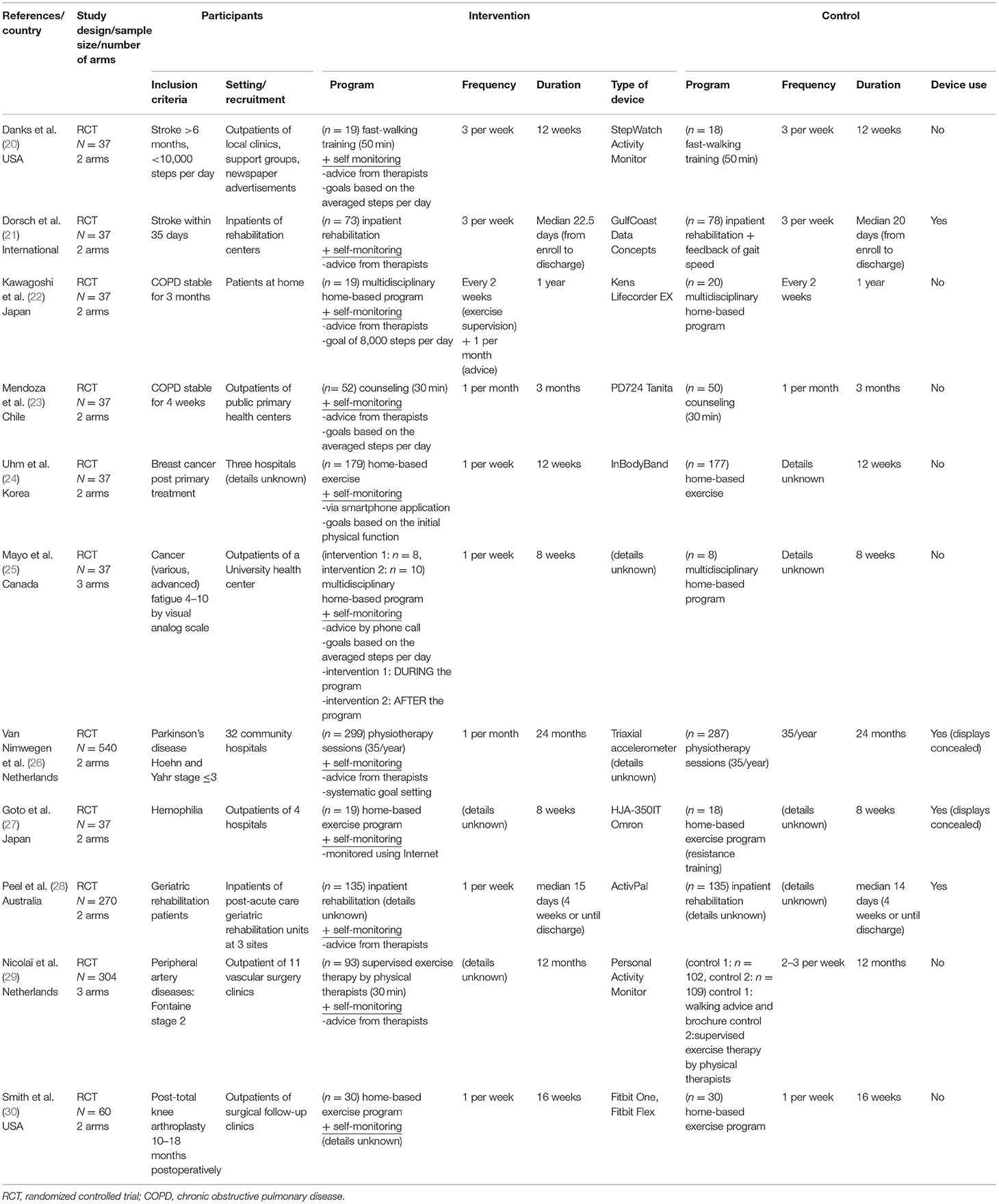
A detailed description of the 11 studies included is presented in Tables 1, 2. The study participants in these studies had a wide variety of diseases, including stroke (20, 21), COPD (22, 23), cancer (24, 25), Parkinson's disease (26), hemophilia (27), peripheral artery disease (29), post-total knee arthroplasty (30), and geriatric rehabilitation in various diseases (28). Regarding study settings, two studies (21, 28) recruited inpatients during rehabilitation and others targeted outpatients. Consequently, the duration of study intervention varied from within 1 month (21, 28) to 24 months (26).
The self-monitoring approach in the present review utilized various methods to show the monitored activity data and provide feedback to encourage increased activity. Most of the included studies provided data of activities directly from the physiotherapists during face-to-face appointments (20–23, 26, 28, 29) or phone calls (25), whereas two studies (24, 27) provided information using an Internet application, not accompanying face-to-face sessions with therapists. In addition, some studies had specific goals to be achieved, such as step goals based on the average steps per day (23), 8,000-step goal (22), weekly goal and achievement rate provided automatically (24), and systematic goal setting in physiotherapy sessions (26, 28).
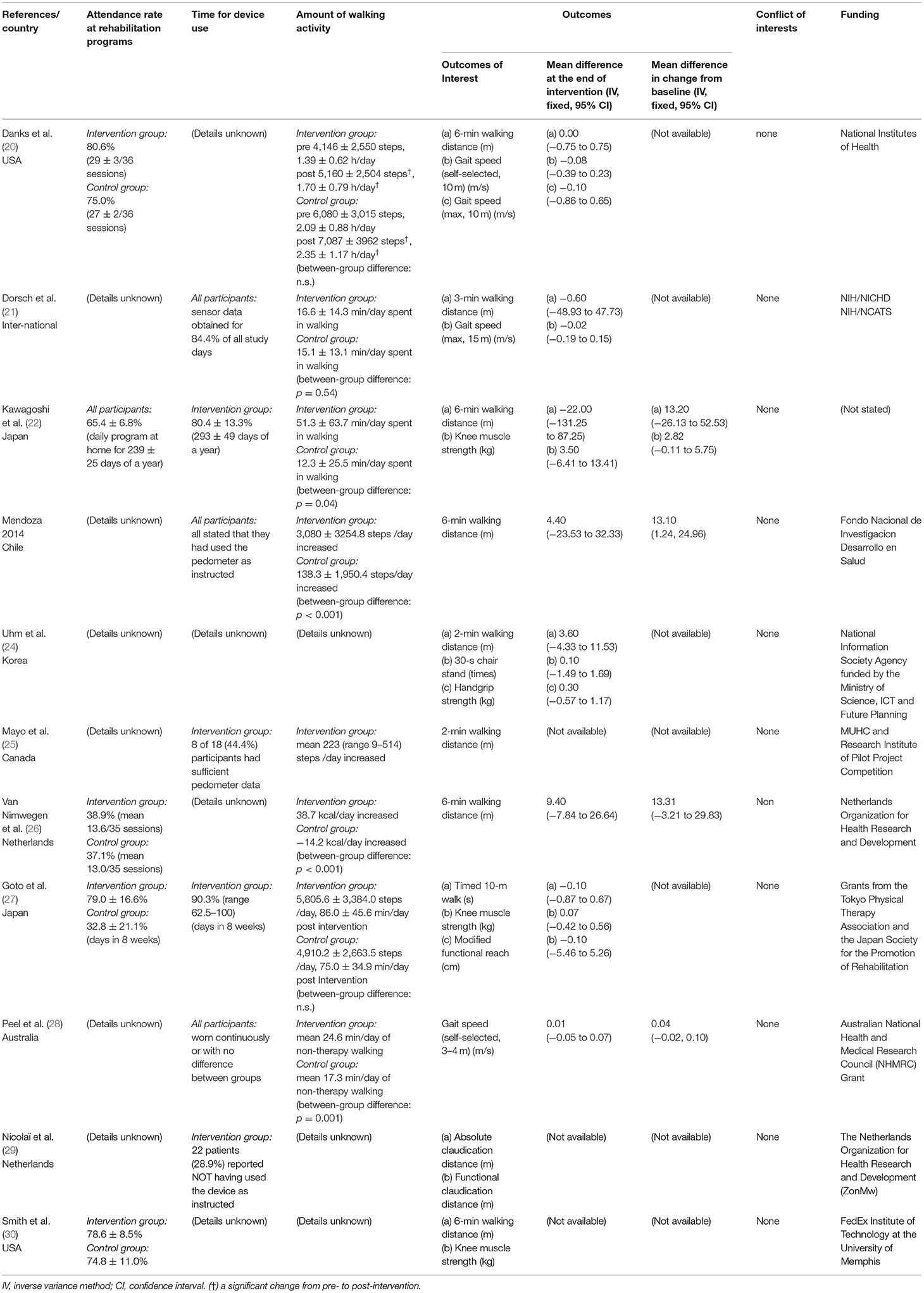
For demonstrating the extent to which the interventions were delivered and practiced as intended, attendance rate for the rehabilitation program, time for device use, and amount of walking activity is shown in Table 2. These aspects are known to be important in the implementation of the study in real-world settings (31, 32). Attendance rate for rehabilitation program was reported in five studies (20, 22, 26, 27, 30) and varied from 30 to 80%. Regarding time for device use, only two studies reported precise data of the intervention groups (22, 27), in both of which participants wore pedometers for more than 80% of the days the intervention was held. One study (21) reported that the data of the accelerometers in all the participants (including both the intervention and the control groups) was obtained for more than 80% of the study days. Two studies (23, 28) simply described that the participants wore pedometers as intended, and two other studies (25, 29) stated that the participants had not used pedometers sufficiently. Regarding the amount of walking activity, seven studies (20–23, 26–28) reported that the walking activity increased more in the intervention group than in the control group.
Eight studies reported measurement of walking endurance (20, 22–26, 29, 30), most of which were 6-min walking distances (20, 22, 23, 30), and four reported measurement of gait speed (20, 21, 27, 28). For the secondary outcomes of interest, three studies (22, 27, 30) reported knee extension muscle strength. Handgrip strength (24), 30-s chair stand test (24) as a measure of muscle power, and Modified Functional Reach Test (27) as a measure of balance function were each found in single studies.
Risk of Bias Assessment
Figure 2 summarizes the trials regarding risk of bias, which consists of five domains. The most prevalent risk of bias was in the area of Domain 2, deviations from the intended interventions, in which only 2 out of 11 studies were judged as having low risk. All the included studies failed to blind participants and therapists because the objective of the intervention was to make them aware of the participants' activity through use of the devices. The two studies with a low risk of bias reported information suggesting that deviations from the intended intervention that arose because of the trial context were unlikely to occur. In addition, six studies were judged as having some concerns in Domain 5, selection of the reported result, because we were unable to find protocols registered or published in advance.
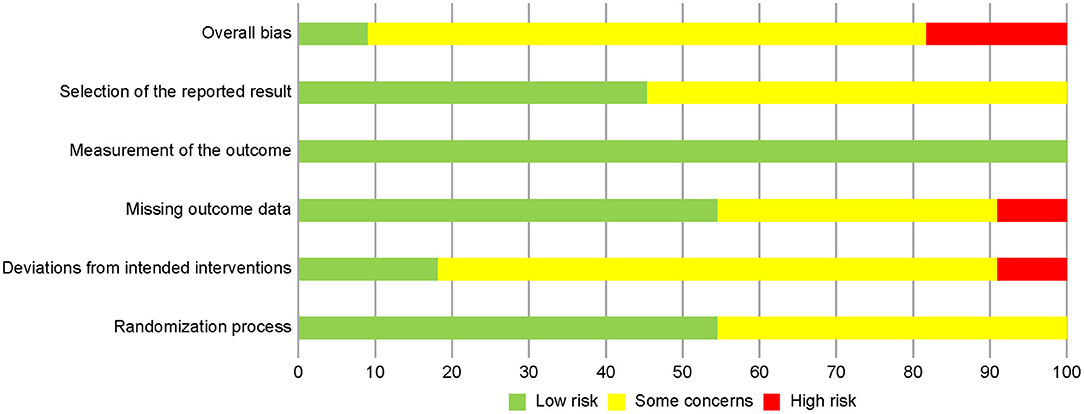
Figure 2. Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Figure 3 shows the risk of bias in each of the included studies. Only one study was judged as having a low risk of bias, and eight studies were judged to have some concerns. One study had a high risk in Domain 3, missing outcome data, because exacerbated pain caused dropouts in the intervention group only (20). Another study described no information regarding blindness of assessors and was therefore considered to have a high risk in Domain 2, deviations from the intended interventions (30).
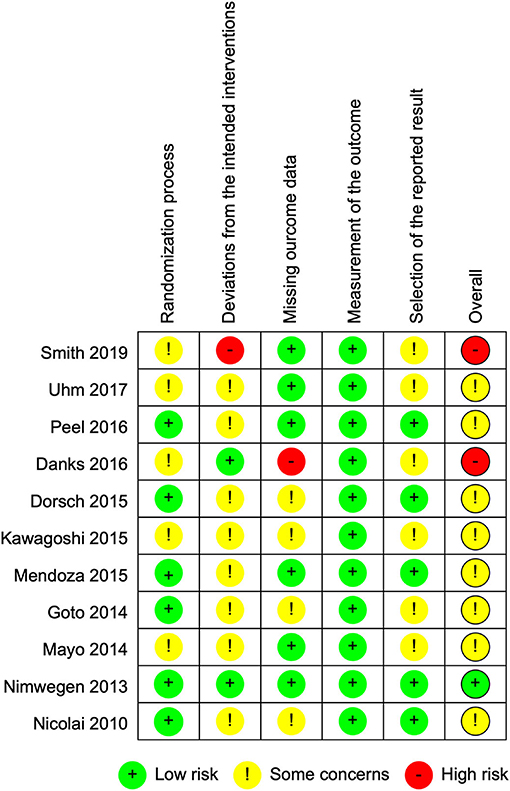
Figure 3. Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Synthesis of Key Findings—Walking Ability (Primary Outcome of Interest)
The included studies were categorized according to the types of diseases investigated as follows:
Stroke
One international study (21) investigated 151 inpatients with subacute stroke 1–3 months from onset. During the inpatient rehabilitation period, the effect of the additional provision of walking activity recorded by wireless sensors compared to the walking speed feedback only, was investigated. The other study (20) recruited 37 chronic outpatients more than 6 months post-stroke to examine the effect of a step activity monitoring program in addition to fast-walking training, which was perceived as having a high risk of bias, as mentioned above.
Although time spent walking was longer in the intervention group, the post-intervention values in the study with a lower risk of bias revealed no between-group differences (Figures 4A,B). This trend was consistent with that observed when we conducted a quantitative synthesis combined with the study with a high risk of bias (6-min walking distance: standard mean difference, fixed-effect model: −0.00, 95% CI −0.31 to 0.31; 2 studies, 162 participants; p = 0.98; I2 = 0%, gait speed: mean difference, fixed-effect model: −0.03, 95% CI −0.18 to 0.13; 2 studies, 162 participants; p = 0.75; I2 = 0%) (Figures 4C,D).
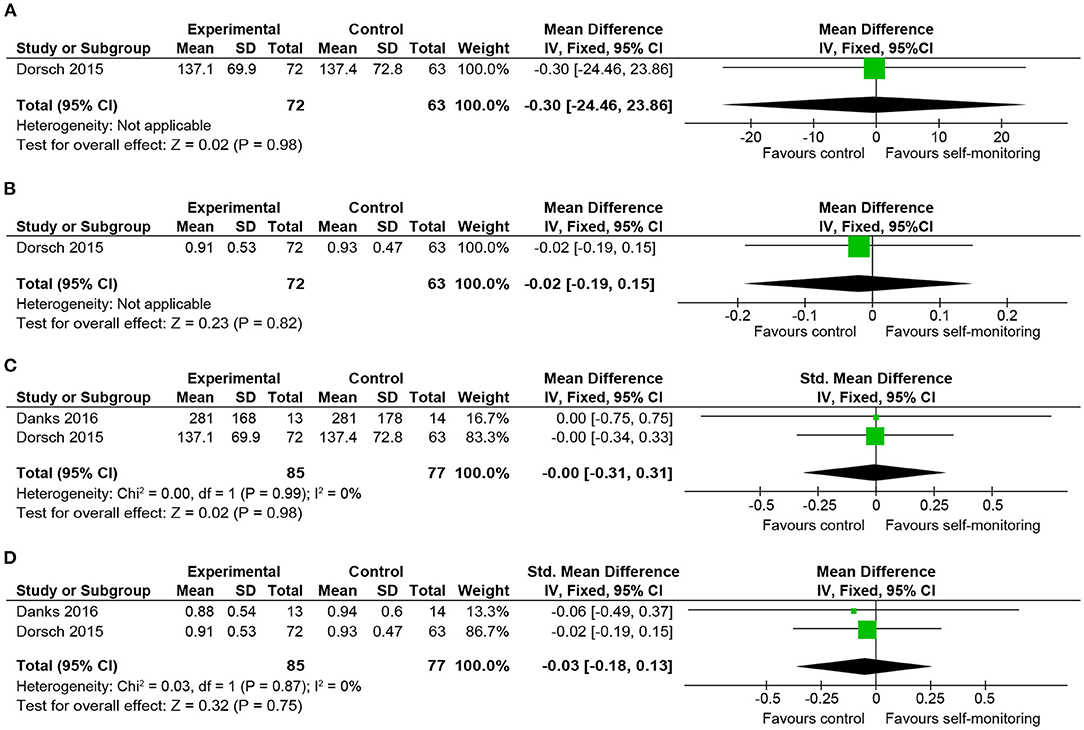
Figure 4. Comparison of walking ability in patients with stroke. (A) Walking endurance (3-min walking distance, m) post-intervention. (B) Gait speed (maximal, m/s) post-intervention. (C) Walking endurance (timed walking distance, m) post-intervention including a study at high risk of bias. (D) Gait speed (maximal, m/s) post-intervention including a study at high risk of bias. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
COPD
Both included studies examined outpatients who were recently stable and evaluated the 6-min walking distance. One RCT (23) with 102 participants examined the effect of an additional self-monitoring approach with goals based on the averaged steps per day compared to monthly 30-min rehabilitative counseling with provision of a diary only. The intervention group showed significantly greater benefit upon changing the 6-min walking distance, although there was no significant between-group difference in the final value of the 6-min walking distance. Another RCT with 42 participants (22) provided additional feedback regarding physical activity with goal-setting of steps for outpatients undergoing a pulmonary rehabilitation program.
As a result of quantitative synthesis of the two studies, which showed significant increase in the amount of walking activity in the intervention groups, there was a between-group difference in the changes in 6-min walking distance, which was favorable to the intervention group and statistically significant (Figures 5A,B) (mean difference: 13.1 m; 95% CI, 1.8–24.4; 2 studies, 124 participants; p = 0.02; I2 = 0%). However, the mean difference in the changes from the baseline (13.1 m) was smaller than the minimal clinically important difference (MCID) reported in COPD (25–54 m) (33, 34).
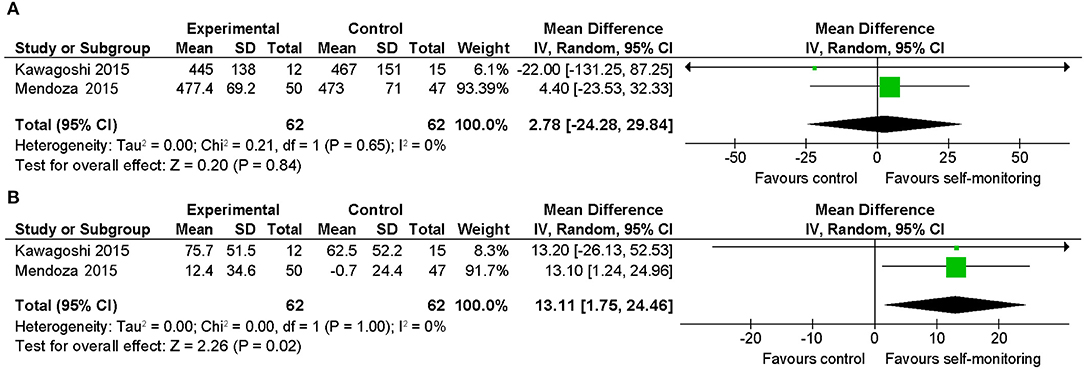
Figure 5. Comparison of walking ability in patients with stable COPD. (A) Walking endurance (6-min walking distance, m) post-intervention. (B) Walking endurance (6-min walking distance, m) change from baseline. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
Cancer
One RCT (24) recruited 356 patients with breast cancer who completed primary cancer treatment. The post-intervention (12 weeks) value of 2-min walking distance was favorable to the intervention group (Figure 6). However, the difference was not statistically significant and the mean difference of post-intervention values (3.60 m) was smaller than the MCID reported in older adults (12.2–14.7 m) (35).
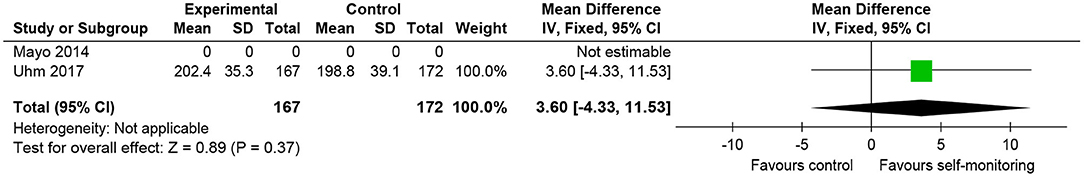
Figure 6. Comparison of walking ability in patients with cancer. Walking endurance (2-min walking distance, m) post-intervention. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
The other RCT (25) investigated two patterns of intervention for advanced cancer patients: self-monitoring during and after a home-based program, compared to a home-based program only. Although the outcomes included a 2-min walking distance, the data was not suitable for analyzing according to our protocol.
Parkinson's Disease
The effect of additional self-monitoring with systematic goal setting compared to physiotherapy sessions only was examined in 540 sedentary patients with Parkinson's disease (26). Daily energy expenditure due to walking activity significantly increased in the intervention group. Both the post-intervention values and the change from baseline of the 6-min walking distance were favorable to the intervention group (Figures 7A,B). However, between-group differences in these indices were not statistically significant, and the mean differences of post-intervention values (9.40 m) and of changes from baseline (13.31 m) were smaller than the MCID reported in Parkinson's disease (82 m) (36).
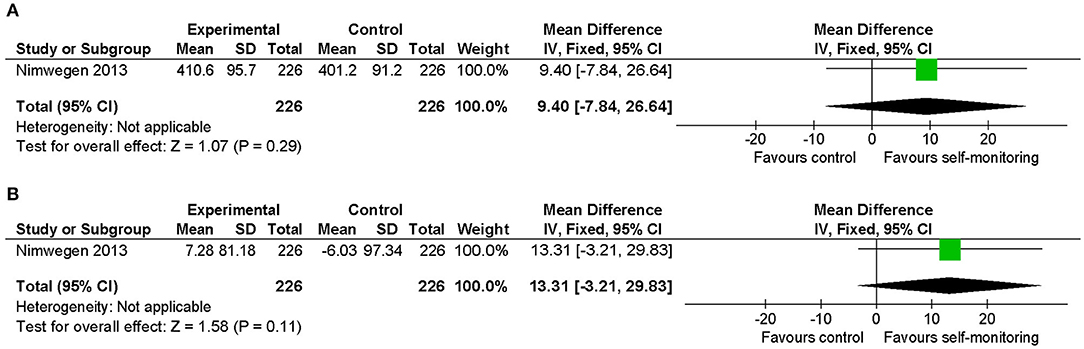
Figure 7. Comparison of walking ability in patients with Parkinson's disease. (A) Walking endurance (6-min walking distance, m) post-intervention. (B) Walking endurance (6-min walking distance, m) change from baseline. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
Hemophilia
One RCT investigated the effect of self-monitoring in addition to a home-based exercise program in 37 patients with hemophilia (27). The final value of the timed 10-m walk was favorable to the intervention group (Figure 8). However, the difference was not statistically significant and the mean difference (0.1-s) was very small when considered clinically.
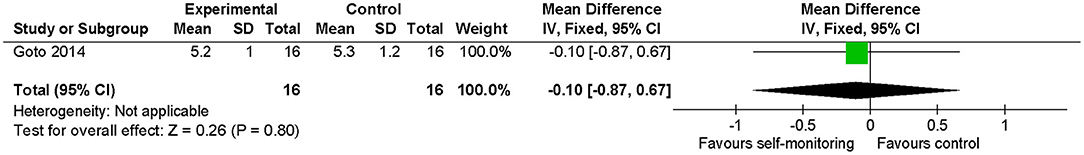
Figure 8. Comparison of walking ability in patients with hemophilia. Gait speed (timed 10-m walk, s) post-intervention. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
Geriatric Rehabilitation
We included one RCT in this category, which recruited 270 inpatients of post-acute geriatric rehabilitation units (28) and provided accelerometer data in addition to usual inpatient rehabilitation for the intervention group. The primary diagnoses included fractures, infections, neurological diseases, and cardiopulmonary diseases. Time spent walking was longer in the intervention group, and the change in gait speed from baseline was favorable to the intervention group. However, these differences were not statistically significant (Figures 9A,B). The mean difference of changes from baseline (0.04 m/s) was smaller than the MCID reported in heart failure (0.05 m/s) (37) or after hip fracture (0.10 m/s) (38).
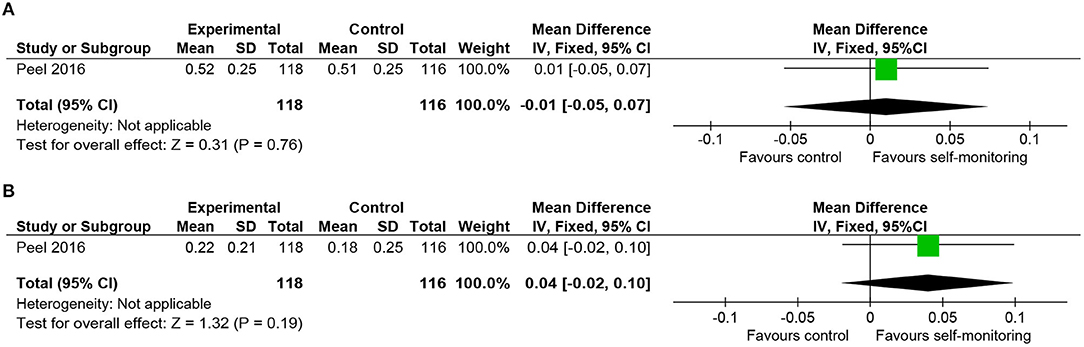
Figure 9. Comparison of walking ability in rehabilitation of geriatric patients. (A) Gait speed (self-selected, m/s) post-intervention. (B) Gait speed (self-selected, m/s) change from baseline. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
Others
Two other studies that recruited patients with diseases in different categories [peripheral artery diseases (29) and post-total knee arthroplasty (30)] were included, but failed to obtain sufficient information to evaluate the effect of self-monitoring.
Finally, we inspected a funnel plot for the 6-min walking distance in patients with various diseases for reference. Publication bias was not graphically evident, although the number of included studies was small (Appendix C).
Synthesis of Key Findings—Physical Functions Related to Walking Ability (Secondary Outcomes of Interest)
Regarding knee muscle strength, no significant difference was observed between the intervention and control groups in the data for stable COPD (Figures 10A,B) and hemophilia (Figure 10C). In addition, a chair-stand test (Figure 10D) and handgrip strength (Figure 10E) in patients with cancer and Modified Functional Reach Test (Figure 10F) in patients with hemophilia showed no between-group differences.
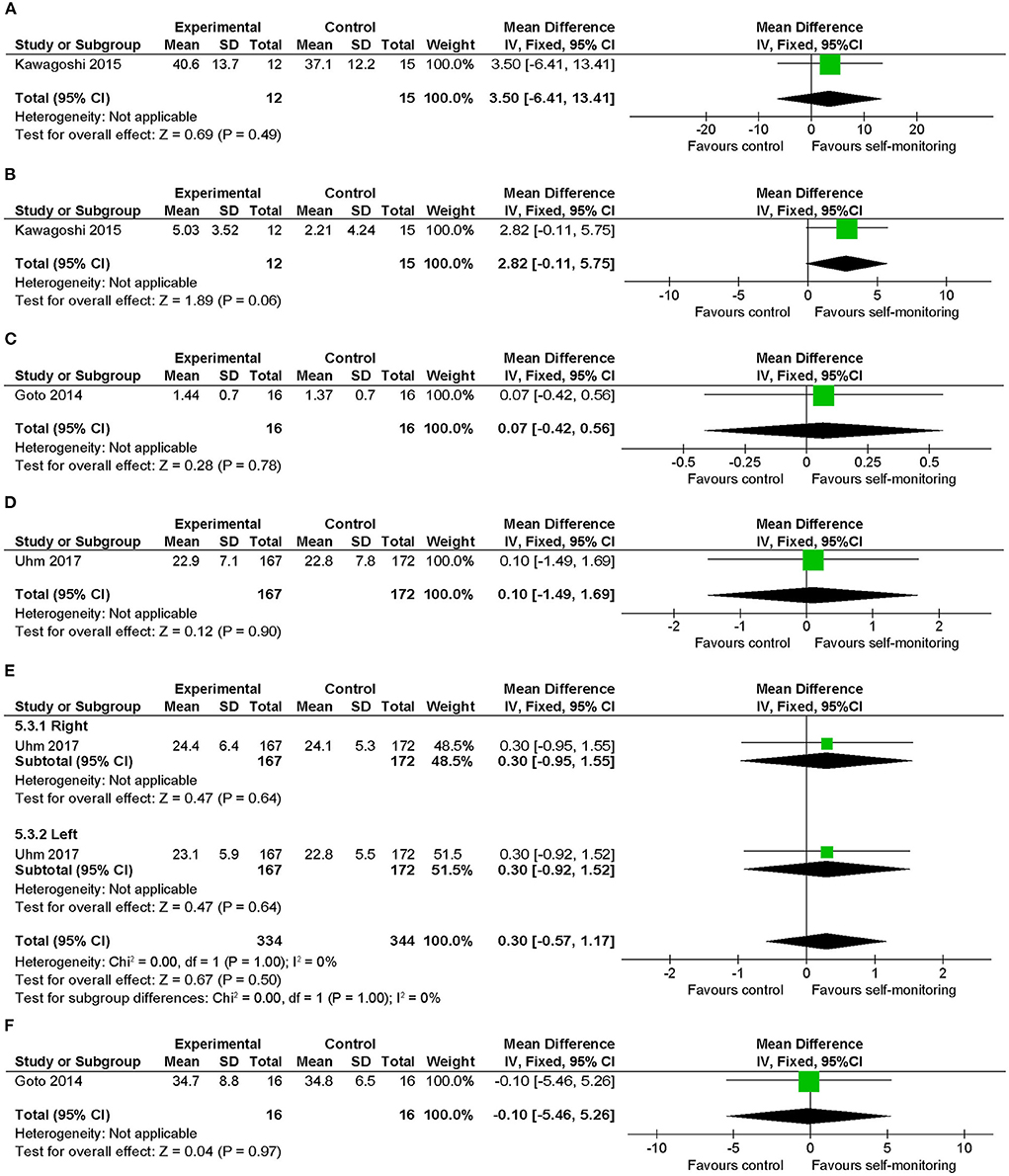
Figure 10. Comparison of physical functions related to walking ability (secondary outcomes of interest) (17). (A) Knee muscle strength (kg) post intervention in patients with stable COPD, (B) knee muscle strength (kg) change from baseline in patients with stable COPD, (C) knee muscle strength (kg) post intervention in patients with hemophilia, (D) 30-s chair-stand test (no.) post intervention in patients with cancer, (E) handgrip strength (kg) post intervention in cancer, (F) Modified Functional Reach Test (cm) post intervention in patients with hemophilia. SD, standard deviation; IV, inverse variance method; CI, confidence interval.
Discussion
Summary of Main Findings
This systematic review aimed to investigate the effectiveness of an additional self-monitoring approach using fitness trackers to improve walking ability when compared to rehabilitative intervention alone. We identified 11 studies that fulfilled the inclusion criteria and eight different types of diseases in these studies.
Among these, the most consistent evidence identified was a significant change from baseline in the 6-min walking distance in the self-monitoring group in patients with stable COPD. However, the mean effect of intervention did not achieve the MCID. In Parkinson's disease and post-acute geriatric rehabilitation conditions, the self-monitoring approach resulted in a significant increase in the amount of walking activity, and improvement in the walking ability was observed, but it was smaller than the MCID.
Indication for the Self-Monitoring Approach
Overall, this review detected no specific type of disease with evident indications for self-monitoring, except for weak recommendations for patients with stable COPD. Some of the results in single RCTs favored the intervention, yet these changes were of little clinical importance, regardless of whether they were significant or not. Given these results, the effect of the self-monitoring approach might be relatively weak or non-existent, compared with the effect of rehabilitative intervention itself, which is customized for the targeted medical conditions.
Four studies (22, 23, 26, 28) reported increased physical activity with statistical significance and favorable results by self-monitoring, although three of the results were not statistically significant. The effectiveness of self-monitoring in increasing activity was consistent with previous studies (12–15). In addition, these results suggest that the self-monitoring approach can lead to an increase in the walking activity followed by improvement in the walking ability, which is substantially supportive of the theory that a higher dose of walking activity is necessary in improving walking ability in rehabilitation.
The most promising result was shown in the studies with stable COPD. The pathophysiology lies in deconditioning due to decreased activity and is considered to be a major cause of limb muscle dysfunction and subsequent decreased walking ability in COPD (39); however, there are no problems in the motor system of the gait. Therefore, the self-monitoring approach to motivate increasing activity might be a direct and effective solution for the walking ability in cases of COPD. Contrarily, neurological diseases such as stroke or Parkinson's disease have primary problems in the motor system of the gait, and thus there might be difficulty in obtaining the desired effect by simply motivating voluntary walking activity.
Another perspective is the indication according to the baseline physical function. Generally, pre-intervention walking ability or activity may play an important role in the effectiveness of walking interventions. The findings in patients with stable COPD in this review, in which a significant between-group difference was observed in the change from baseline, but not in the final value, suggest that populations with lower walking ability than their potential may benefit from this intervention to a larger extent. Moreover, in a study on chronic stroke (20), regression analysis and a sub-comparison involving participants with lower baseline values revealed that the self-monitoring approach was substantially more effective in participants with limited baseline walking activity and low baseline 6-min walking distance. Thus, the self-monitoring approach may be more effective for those with lower initial abilities or activities. Further studies are required to confirm the most appropriate indications for the self-monitoring approach based on the baseline walking ability or activity regardless of the type of disease.
Although patients with stroke are believed to experience benefits from increased walking activity to gain functional recovery and for secondary stroke prevention, this review revealed little evidence of the benefit of the self-monitoring approach for enhancing walking ability in stroke rehabilitation. However, the results were obtained from single studies, each from different stages. One study (21) recruited stroke participants within 35 days from onset and the other (20) recruited stroke participants whose onset was more than 6 months previously, which are categorized as the “early subacute” and “chronic” phases, respectively, according to the framework recommended by the Stroke Recovery and Rehabilitation Roundtable (40). Since the time post-stroke is extremely important in planning stroke rehabilitation, more studies in each of the phases would be necessary to determine the optimal use of activity trackers for patients with stroke.
Regarding the setting of the studies, only two (21, 28) recruited inpatients during rehabilitation. Inpatients have the potential to experience substantial gains in walking ability, although they may experience difficulties in increasing walking distance in closed and monotonous environments compared to the outpatients targeted in most of the included studies. Comparing the amount of walking (time or steps per day) in inpatients and outpatients are required to discuss this issue.
Specific Means of Self-Monitoring
Most studies adopted the means of providing activity data and feedback directly from physiotherapists face-to-face. However, the provision of information using Internet applications was also performed in some studies. A slight advantage might exist for providing tailored advice or appraisal beneficial in encouraging walking activities from the rehabilitation staff, although this review obtained inadequate findings to draw any concrete conclusions. This should be evaluated in further studies considering the cost-effectiveness of the intervention.
Regarding setting goals, the self-monitoring approach with any trend in favor of intervention groups were showing specific goals to be achieved. Setting appropriate goals might be an important factor in performing the self-monitoring approach as part of rehabilitation (41).
Limitations
As limitations, we described the studies with some concerns regarding the risk of bias in the results as well as the study with an absolutely low risk of bias. The synthesized key findings should thus be interpreted with caution when applied to clinical settings. However, as mentioned in the assessment of risk of bias, the most prevalent risk was due to the nature of the intervention, which aimed to manifest the amount of activity and considered to have fewer concerns than in usual medical interventions assessed by the Cochrane Risk of Bias tool. Finally, there was publication restriction due to the exclusive inclusion of English literature.
Conclusions
This systematic review identified 11 studies on patients with eight different disease types that investigated the effectiveness of an additional self-monitoring approach using fitness trackers to improve walking ability. The findings indicated that there was little, if any, evidence of the effectiveness of the self-monitoring approach using fitness trackers in rehabilitation settings as a whole, whereas there was weak recommendation for patients with stable COPD implied in a quantitative synthesis. Nevertheless, the self-monitoring approach could be an alternative intervention for motivating rehabilitation patients when an appropriate population is selected, as this method using a consumer-based pedometer is relatively inexpensive and practical (42).
Despite the limitations listed above, this review provides insight into the impact of the self-monitoring approach in clinical rehabilitation settings. Moreover, considering the findings in this review, future research strategies are recommended to explore the best indications for this self-monitoring approach, including the types of diseases and baseline physical function or physical activities.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
EO and YO determined the concept and design, performed study selection and data extraction, analyzed and interpreted the data, and edited the manuscript. KO participated in concept determination, data interpretation, manuscript editing, and critical revisions of the manuscript. IK participated in data interpretation and critical revisions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the members of the Department of Rehabilitation at Kariya Toyota General Hospital for inspiring the concept of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fresc.2021.752727/full#supplementary-material
References
1. Schmidt RA, Lee TD. Conditions of Practice. In: Motor Control and Learning: A Behavioral Emphasis. 4th ed. Champaign, IL: Human Kinetics (2005). p. 321–63.
2. Klassen TD, Dukelow SP, Bayley MT, Benavente O, Hill MD, Krassioukov A, et al. Higher doses improve walking recovery during stroke inpatient rehabilitation. Stroke. (2020) 51:2639–48. doi: 10.1161/STROKEAHA.120.029245
3. Said CM, McGinley JL, Szoeke C, Workman B, Hill KD, Wittwer JE, et al. Factors associated with improved walking in older people during hospital rehabilitation: secondary analysis of a randomized controlled trial. BMC Geriatrics. (2021) 21:90. doi: 10.1186/s12877-021-02016-0
4. Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. (2011) 8:80. doi: 10.1186/1479-5868-8-80
5. Tudor-Locke C, Washington TL, Hart TL. Expected values for steps/day in special populations. Prev Med. (2009) 49:3–11. doi: 10.1016/j.ypmed.2009.04.012
6. Mudge S, Barber PA, Stott NS. Circuit-based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomized controlled trial. Arch Phys Med Rehabil. (2009) 90:1989–96. doi: 10.1016/j.apmr.2009.07.015
7. Mador MJ, Patel AN, Nadler J. Effects of pulmonary rehabilitation on activity levels in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. (2011) 31:52–9. doi: 10.1097/HCR.0b013e3181ebf2ef
8. Peek K, Sanson-Fisher R, Mackenzie L, Carey M. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: a systematic review. Physiotherapy. (2016) 102:127–35. doi: 10.1016/j.physio.2015.10.003
9. Treacy D, Hassett L, Schurr K, Chagpar S, Paul SS, Sherrington C. Validity of different activity monitors to count steps in an inpatient rehabilitation setting. Phys Ther. (2017) 97:581–8. doi: 10.1093/ptj/pzx010
10. Izawa KP, Watanabe S, Hiraki K, Morio Y, Kasahara Y, Takeichi N, et al. Determination of the effectiveness of accelerometer use in the promotion of physical activity in cardiac patients: a randomized controlled trial. Arch Phys Med Rehabil. (2012) 93:1896–902. doi: 10.1016/j.apmr.2012.06.015
11. Furber S, Butler L, Phongsavan P, Mark A, Bauman A. Randomised controlled trial of a pedometer-based telephone intervention to increase physical activity among cardiac patients not attending cardiac rehabilitation. Patient Educ Couns. (2010) 80:212–8. doi: 10.1016/j.pec.2009.11.012
12. Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. (2007) 298:2296–304. doi: 10.1001/jama.298.19.2296
13. Albergoni A, Hettinga FJ, La Torre A, Bonato M, Sartor F. The role of technology in adherence to physical activity programs in patients with chronic diseases experiencing fatigue: a systematic review. Sports Med Open. (2019) 5:41. doi: 10.1186/s40798-019-0214-z
14. Hannan AL, Harders MP, Hing W, Climstein M, Coombes JS, Furness J. Impact of wearable physical activity monitoring devices with exercise prescription or advice in the maintenance phase of cardiac rehabilitation: systematic review and meta-analysis. BMC Sports Sci Med Rehabil. (2019) 11:14. doi: 10.1186/s13102-019-0126-8
15. Larsen RT, Christensen J, Juhl CB, Andersen HB, Langberg H. Physical activity monitors to enhance amount of physical activity in older adults - a systematic review and meta-analysis. Eur Rev Aging Phys Act. (2019) 16:7. doi: 10.1186/s11556-019-0213-6
16. Lynch EA, Jones TM, Simpson DB, Fini NA, Kuys SS, Borschmann K, et al. Activity monitors for increasing physical activity in adult stroke survivors. Cochrane Database Syst Rev. (2018) 7:Cd012543. doi: 10.1002/14651858.CD012543.pub2
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
20. Danks KA, Pohlig R, Reisman DS. Combining fast-walking training and a step activity monitoring program to improve daily walking activity after stroke: a preliminary study. Arch Phys Med Rehabil. (2016) 97:S185–93. doi: 10.1016/j.apmr.2016.01.039
21. Dorsch AK, Thomas S, Xu X, Kaiser W, Dobkin BH. SIRRACT: an international randomized clinical trial of activity feedback during inpatient stroke rehabilitation enabled by wireless sensing. Neurorehabil Neural Repair. (2015) 29:407–15. doi: 10.1177/1545968314550369
22. Kawagoshi A, Kiyokawa N, Sugawara K, Takahashi H, Sakata S, Satake M, et al. Effects of low-intensity exercise and home-based pulmonary rehabilitation with pedometer feedback on physical activity in elderly patients with chronic obstructive pulmonary disease. Respir Med. (2015) 109:364–71. doi: 10.1016/j.rmed.2015.01.008
23. Mendoza L, Horta P, Espinoza J, Aguilera M, Balmaceda N, Castro A, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. (2015) 45:347–54. doi: 10.1183/09031936.00084514
24. Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. (2017) 161:443–52. doi: 10.1007/s10549-016-4065-8
25. Mayo NE, Moriello C, Scott SC, Dawes D, Auais M, Chasen M. Pedometer-facilitated walking intervention shows promising effectiveness for reducing cancer fatigue: a pilot randomized trial. Clin Rehabil. (2014) 28:1198–209. doi: 10.1177/0269215514536209
26. van Nimwegen M, Speelman AD, Overeem S, van de Warrenburg BP, Smulders K, Dontje ML, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ. (2013) 346:f576. doi: 10.1136/bmj.f576
27. Goto M, Takedani H, Haga N, Kubota M, Ishiyama M, Ito S, et al. Self-monitoring has potential for home exercise programmes in patients with haemophilia. Haemophilia. (2014) 20:e121–7. doi: 10.1111/hae.12355
28. Peel NM, Paul SK, Cameron ID, Crotty M, Kurrle SE, Gray LC. Promoting activity in geriatric rehabilitation: a randomized controlled trial of accelerometry. PLoS ONE. (2016) 11:e0160906. doi: 10.1371/journal.pone.0160906
29. Nicolaï SP, Teijink JA, Prins MH. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. J Vas Surg. (2010) 52:348–55. doi: 10.1016/j.jvs.2010.02.022
30. Smith WA, Zucker-Levin A, Mihalko WM, Williams M, Loftin M, Gurney JG. A randomized study of exercise and fitness trackers in obese patients after total knee arthroplasty. Orthop Clin North Am. (2019) 50:35–45. doi: 10.1016/j.ocl.2018.08.002
31. Lambert JD, Greaves CJ, Farrand P, Cross R, Haase AM, Taylor AH. Assessment of fidelity in individual level behaviour change interventions promoting physical activity among adults: a systematic review. BMC Public Health. (2017) 17:765. doi: 10.1186/s12889-017-4778-6
32. Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. (2011) 71:S52–63. doi: 10.1111/j.1752-7325.2011.00233.x
33. Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. (2010) 91:221–5. doi: 10.1016/j.apmr.2009.10.017
34. Rasekaba T, Lee AL, Naughton MT, Williams TJ, Holland AE. The six-minute walk test: a useful metric for the cardiopulmonary patient. Intern Med J. (2009) 39:495–501. doi: 10.1111/j.1445-5994.2008.01880.x
35. Connelly DM, Thomas BK, Cliffe SJ, Perry WM, Smith RE. Clinical utility of the 2-minute walk test for older adults living in long-term care. Physiother Can. (2009) 61:78–87. doi: 10.3138/physio.61.2.78
36. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther. (2008) 88:733–46. doi: 10.2522/ptj.20070214
37. Pulignano G, Del Sindaco D, Di Lenarda A, Alunni G, Senni M, Tarantini L, et al. Incremental value of gait speed in predicting prognosis of older adults with heart failure: insights from the IMAGE-HF study. JACC Heart Fail. (2016) 4:289–98. doi: 10.1016/j.jchf.2015.12.017
38. Palombaro KM, Craik RL, Mangione KK, Tomlinson JD. Determining meaningful changes in gait speed after hip fracture. Phys Ther. (2006) 86:809–16. doi: 10.1093/ptj/86.6.809
39. Kim SH, Shin MJ, Shin YB, Kim KU. Sarcopenia associated with chronic obstructive pulmonary disease. J Bone Metab. (2019) 26:65–74. doi: 10.11005/jbm.2019.26.2.65
40. Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the stroke recovery and rehabilitation roundtable taskforce. Int J Stroke. (2017) 12:444–50. doi: 10.1177/1747493017711816
41. Dekker J, de Groot V, Ter Steeg AM, Vloothuis J, Holla J, Collette E, et al. Setting meaningful goals in rehabilitation: rationale and practical tool. Clin Rehabil. (2020) 34:3–12. doi: 10.1177/0269215519876299
Keywords: activity monitors, motivation, pedometers, rehabilitation, self-monitoring, wearable devices, behavioral change, walking ability
Citation: Otaka E, Oguchi K, Kondo I and Otaka Y (2021) Effectiveness of Self-Monitoring Approach Using Fitness Trackers to Improve Walking Ability in Rehabilitation Settings: A Systematic Review. Front. Rehabilit. Sci. 2:752727. doi: 10.3389/fresc.2021.752727
Received: 03 August 2021; Accepted: 28 October 2021;
Published: 02 December 2021.
Edited by:
Laurie A. King, Oregon Health and Science University, United StatesReviewed by:
Mohammad Sami Al-Wardat, Aqaba University of Technology, JordanRikke Helene Moe, Diakonhjemmet Hospital, Norway
Copyright © 2021 Otaka, Oguchi, Kondo and Otaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yohei Otaka, b3Rha2ExMTlAbWFjLmNvbQ==
 Eri Otaka
Eri Otaka Kazuyo Oguchi2
Kazuyo Oguchi2 Yohei Otaka
Yohei Otaka