
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Radiol., 11 March 2025
Sec. Cardiothoracic Imaging
Volume 5 - 2025 | https://doi.org/10.3389/fradi.2025.1552644
 Giacomo Sica1*
Giacomo Sica1* Gaetano Rea1
Gaetano Rea1 Roberta Lieto1
Roberta Lieto1 Mariano Scaglione2
Mariano Scaglione2 Ahmad Abu-Omar3
Ahmad Abu-Omar3 Giorgio Bocchini1
Giorgio Bocchini1 Federica Romano1
Federica Romano1 Salvatore Masala2
Salvatore Masala2 Stefania Tamburrini4
Stefania Tamburrini4 Salvatore Guarino1
Salvatore Guarino1 Candida Massimo1
Candida Massimo1 Tullio Valente1
Tullio Valente1
Acute aortic intramural hematoma (IMH) is a relatively uncommon but potentially life-threatening aortic disease that can occur primarily in hypertensive and atherosclerotic patients. The course of IMH varies widely, with the condition either regressing, remaining stable, or progressing until it leads to outward rupture or intimal layer disruption, eventually resulting in overt aortic dissection. Therefore, poor prognostic computed tomography (CT) features must be promptly recognized and reported by the radiologist. In emergency departments, readily accessible non-invasive CT angiography is crucial for achieving a rapid and accurate diagnosis essential for appropriate management. For Type A and B aortic dissection, surgery is typically recommended in Western countries for patients with Stanford Type A IMH and those experiencing irrepressible pain. For Stanford Type B IMH patients without complications or incessant pain, medical treatment is suggested but with imaging follow-up. In complicated Stanford Type B situations, thoracic endovascular aortic repair (TEVAR) is currently indicated. This review aims to present pathophysiology, CT diagnosis, and IMH fate and provide the reader CT image-based review of the CT diagnostic criteria, complications, and associated critical prognostic findings of this rather rare aortic disease.
Acute aortic syndrome (AAS) encompasses overlapping, interchangeable, and life-threatening diseases, such as acute intramural hematoma (IMH). IMH is characterized by a small hematoma (≥5 mm) within the aortic wall's media, without a visible intimal tear or intimomedial flap (1–3). IMH constitutes 5%–27% of AAS cases and can develop spontaneously as an isolated event (90%) or be associated with penetrating atherosclerotic ulcer (PAU, 5%) or result from post-traumatic or iatrogenic aortic injury (2, 4–6). Asian cohorts have shown a greater incidence of IMH compared to the International Registry of Acute Aortic Dissection series, with rates of 28.3% vs. 3.6%, respectively. Additionally, these groups have higher rates of early medical treatment and lower overall mortality (6–12). Sudden tearing chest or back pain and chronic hypertension are common clinical presentations associated with AAS entities. However, in IMH, these symptoms and typical findings may be absent or subtle, depending on the extent of the anatomic lesion and the involvement of adjacent vascular structures.
A clinically high index of suspicion is essential to identify the disease and request appropriate imaging studies for prompt diagnosis, which can provide crucial life-saving indications for timely treatment. Indeed, IMH continues to be frequently misdiagnosed today, with mortality rates ranging from 10% to 50% and progression to overt aortic dissection (AD) in over 40% of patients (13). The most common location of IMH is the descending thoracic aorta (Type B IMH, 60%–70%) followed by the ascending aorta and aortic arch (Type A IMH, 30% and 10%, respectively) (2, 13). Rapid and non-invasive computed tomography (CT) angiography allows for correct diagnosis in most cases, with sensitivity and negative predictive value approaching 99% (2, 14–16). The evolution of IMH involves a hyperacute phase (<24 h), an acute phase (2 weeks from pain onset), a subacute phase (2–6 weeks), or a chronic phase (>6 weeks) (10, 15, 17). Usually, treatment and diagnosis for IMH are similar to those recommended for AD based on the segment of the aorta involved (Stanford classification) (2, 7, 17, 18). However, given the highly variable and often unpredictable natural history of IMH, the prognosis and management largely depend on several key morphologic features that can be identified through CT imaging.
The etiology of IMH remains controversial, with no single recognized cause identified to date. In 1920, Krukemberg was the first to propose rupture of the vasa vasorum as the initial event of the AD process (19). Later, Gore proposed that underlying degeneration of the media could be a predisposing factor for vasa vasorum hemorrhage and IMH (20). Historically, IMH was believed to emerge from rupture or rhexis of the vasa vasorum within the aortic media, linked to the atherosclerotic process and systemic hypertension, where increased stress on the aortic wall contributes to medial hemorrhage without intimal disruption (20–22).
However, the development of increasingly high-performance CT scanners and the significant increase in their spatial resolution, associated with ever-increasing clinical experience, have allowed us to focus our attention on the possible progressive focal rupture of the intima, called ulcer-like projection (ULP) found by imaging tools in 20%–50% of IMH and considered the “primum movens” that leads to the formation of IMH over time (6, 8, 22–28).
Conversely, other explanations suggest that ULP may develop over time from IMH, as intimal disruption can occur progressively in the acute or subacute phase, leading to the appearance of ULP after focal intimal disruption by the IMH. ULP corresponds to a focal contrast enhancement in the thickness of the IMH communicating with the aortic lumen, in the absence of signs of atherosclerosis or calcification (8, 29).
Currently, the belief that IMH is an AD without intimal rupture is increasingly strong and that therefore this can be considered a variant or precursor of AD. Moreover, some patients may present with both lesions in different segments of the aorta simultaneously. Essentially, in IMH, a thrombus separates and fills the layers of the aortic wall (probably because IMH has an entry tear without re-entry) rather than free-flowing blood as seen in typical AD. Therefore, IMH cases are essentially cases of acute AD or AD with an acutely closed and thrombosed false lumen, representing a non-communicating type of AD (24–26). Several studies also report a coexisting penetrating atherosclerotic ulcer (PAU) in 50%–90% of cases in acute IMH (28, 30–34).
On histological analysis, IMH is predominantly intramedial, although in certain segments of the aorta, it may be subadventitial (between the media and the adventitia), indicating a contained rupture of the aortic wall. Less commonly, IMH can result from a PAU involving the internal elastic lamina (21, 22, 34) or from blunt or iatrogenic traumatic injuries to the aortic wall, such as during percutaneous catheterization, angioplasty, placement of a balloon pump, or left side catheter ablation (Figure 1).
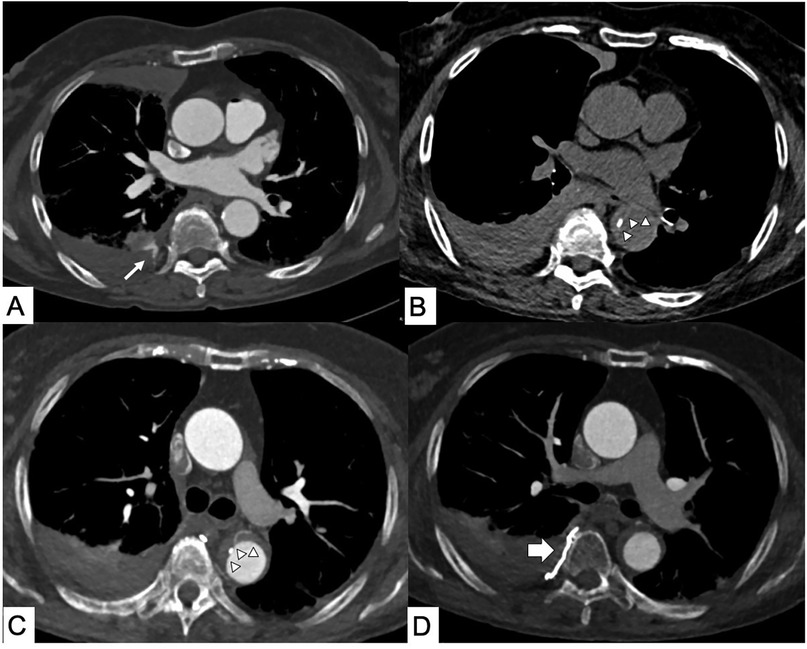
Figure 1. Iatrogenic intramural hematoma (IMH). Computed tomography (CT) axial scans. (A) Right intercostal active bleeding after lung biopsy (thin arrow). (B) Axial CT performed for chest pain 1 day after the intercostal artery transcatheter embolization procedure shows the appearance of hyperattenuated aortic wall thickening from IMH with (C) displacement of intimal calcification and bronchial artery in the contest of the Type B IMH. (D) No more active bleeding in the site of embolization (thick arrow).
In the recent Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ (7), authors reiterated minimum technical standards capable of guaranteeing diagnostic reliability: an isotropic resolution of 1 mm or less, ECG gating (for the study of the aortic root and ascending aorta), and fast acquisition techniques to reduce pulsation artifacts increasing measurement accuracy for any surgical planning.
The latest dual-energy CT scanners, such as photon-counting CT scanners, enable image acquisition with an isotropic spatial resolution of 0.2 mm, a high temporal resolution of 66 ms, and a coverage of 2 m in <3 s. These advancements promise to significantly enhance the visibility of the arterial wall in CT images and improve diagnostic quality, while also reducing the volume of contrast agent needed through low-keV virtual monoenergetic reconstructions, and minimizing the number of acquisitions and radiation exposure by using virtual non-contrast imaging derived from a contrast-enhanced acquisition (35–42).
In cases of suspected AAS, our institution utilizes a CT protocol that begins with a helical high-pitch non-contrast scan. This scan is crucial for distinguishing aortic wall thickening in IMH from that due to other causes, and it helps identify hemopericardium or newly thrombosed blood, as well as surgical materials in patients with a history of cardiac surgery (Figure 2). Following this, a high-pitch prospective ECG-gated arterial-phase scan is performed to obtain motion-free images not only of the aortic root and thoracic aorta but also of the coronary arteries, thus allowing the evaluation of the health status of the coronary arteries without subjecting eventually the patient to invasive coronary angiography (7, 35). A subsequent portal venous phase can help assess malperfusion of the abdominal organs. A high iodine concentration of contrast medium (CM) (370–400 mgI/ml) can improve contrast enhancement and image quality to allow the identification of even the tiniest intimal disruptions possibly associated with IMH (43–45).
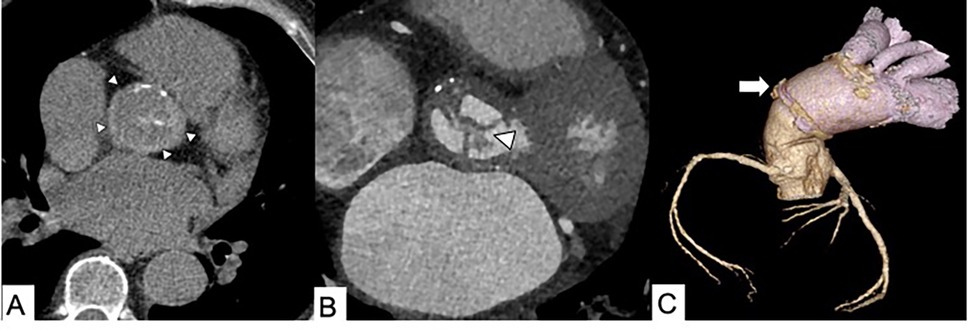
Figure 2. Infected endocarditis in a 65-year-old male patient with aortic bioprosthetic valved conduit. (A) A non-enhanced axial CT scan shows a hyperattenuating aortic prosthetic wall (arrowheads). (B) Thickening of prosthetic valvular leaflets with endocarditis vegetation (arrowhead) and (C) a 3D volume rendering reconstruction showing distal graft aortic anastomosis (arrow).
Getting high intravascular attenuation is essential for having high-quality diagnostic images. Several factors influence the attenuation of vascular structures, including patient size and circulation time, but above all, a good contrast medium injection protocol is influenced by the concentration and volume of the contrast medium as well as the injection rate. Vascular enhancement is in fact directly dependent on the iodine concentration of the contrast medium and the injection rate, represented as the iodine delivery rate (IDR). IDR represents the iodine load administrated per unit of time and can be evaluated by applying the following formula IDR = (I/1,000) × FR where I is the contrast medium (CM) iodine concentration (mgI/ml) and FR is the CM injection flow rate (ml/s). It is possible to increase IDR either using high-concentration CM or increasing the FR.
The total amount of contrast depends on the protocol adopted. In the case of only the angiographic phase, we calculate it with the formula Vcm = (Scan duration + delay) × FR. In the case of suspected organ malperfusion, to be also studied with a portal phase, the total amount of contrast media needs to be calculated based on the body weight of patients and the concentration of contrast media. At our institution, we follow the recommendations of our scientific society, the Italian Society of Medical Radiology, maintaining an IDR between 1.6 and 2.2 grI/s. Furthermore, intravascular attenuation can be increased by using tube potentials of 100 kVp, lowering it up to 80 kVp in small patients, combined with an iterative reconstruction algorithm, thereby reducing the need for higher concentrations and volumes of iodinated contrast medium (46–48).
CT has become the preferred modality for evaluating most patients with suspected AAS due to its wide availability, its quickness to perform, and its panoramic view capable of evaluating the entire aorta and any signs of malperfusion, pericardial or pleural effusion, and periaortic or mediastinal hematoma. Nevertheless, at our institution, AAS initial diagnosis is always constituted by an integration between transesophageal echocardiography (TEE) if transthoracic echocardiography (TTE) is suspicious and CT angiography. In general, echocardiography is an advantageous method because it can be performed at the patient's bedside in whatever department he is located and even if he is in an unstable hemodynamic situation. While TTE should be used routinely in clinical practice, TEE requires greater knowledge and experience. Echocardiographic IMH features include an intramural echo-free space, a crescentic or circular wall thickness of >7 mm, and an aortic lumen or intimal calcification displacement caused by media intramural hematoma that is useful for the differential diagnosis; the inner margin of IMH is smooth and aortic thickening occurs beneath the bright echo-dense intima. However, differentiating an IMH from an AD with a thrombosed false lumen can be very challenging. Echocardiographic artifacts yield a significant number of false positive results, particularly in the ascending aorta (49, 50).
Normal aortic wall thickness is <3 mm. Evaluation of IMH thickness is usually based on axial measurements or perpendicular to the longitudinal axis of the aorta, measured between the outer walls of the vessel.
Unenhanced CT images play a crucial role in establishing the diagnostic hallmark, characterized by circular or crescent-shaped, non-spiral, hyperattenuating (60 ± 15 HU) wall thickening of ≥5 mm (7, 45, 51). This may include centripetal displacement of intimal calcification (Figure 3) and a certain degree of narrowing of the aortic lumen, although the luminal-wall interface remains smooth. By definition, both an intimal flap and double channel intraluminal flow are absent in IMH. Post-contrast images alone can result in false-negative interpretations due to masking from the high-attenuation contrast in the adjacent vascular lumen. Additionally, the hematoma appears similar on contrast CT, with no enhancement of the thickened wall. IMH can be distinguished from AD not only by the absence of an intimal flap and tear but also by its relatively constant circumferential relationship with the wall, as opposed to the spiral configuration commonly observed in AD (Figure 4).
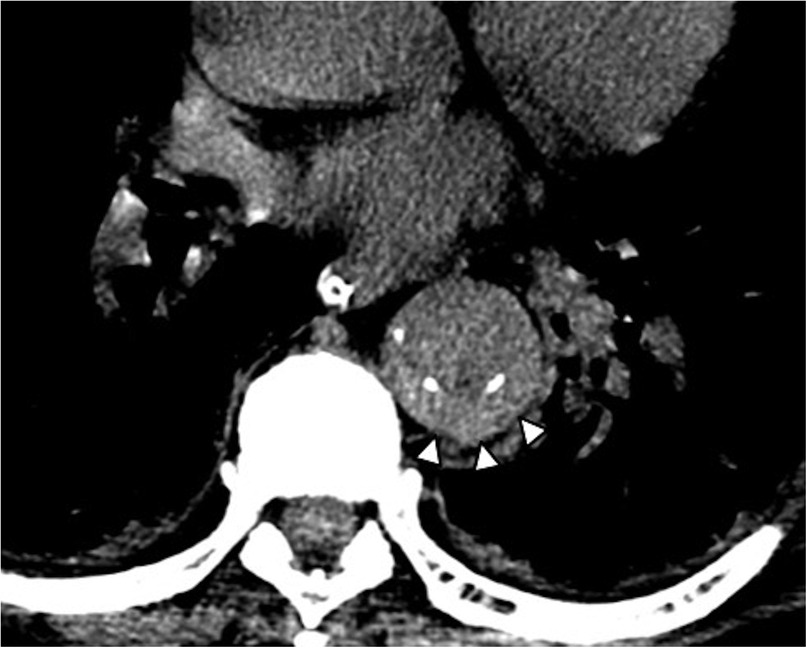
Figure 3. Non-contrast CT axial image in a 73-year-old female patient shows the diagnostic hallmark of IMH: displacement of intimal calcifications and hyperattenuating crescentic thickening of the aortic wall (arrowheads).

Figure 4. CT scan in a 62-year-old male patient with acute chest pain and Type B IMH. (A) Axial non-enhanced CT image shows hyperattenuating crescentic thickening of the aortic wall (arrow). (B) Axial contrast-enhanced CT scan shows a reduced diameter of the aortic lumen and the slick interface between the lumen and wall. (C) Contrast-enhanced sagittal multiplanar reformation CT image shows a reduced diameter of the aortic lumen and its constant circumferential relationship with the wall.
The longitudinal extent of IMH can vary greatly, ranging from very short (∼1 cm) to extending the entire aorta. In the presence of Type A IMH, the space between the aortic lumen and the right atrial appendage, typically closely contiguous structures, increases due to the presence of aortic wall thickening (52, 53). To monitor the possible progression of the disease, it is essential to report at the time of diagnosis the minimum and maximum axial diameters of the aorta involved in the hematoma (54, 55).
MRI techniques allow for the assessment of the age of the hematoma by detecting methemoglobin formation within the IMH, leading to increased signal intensity on T1-weighted images in subacute IMH (56, 57).
The widefield view provided by axial imaging, aided by multiplanar reformations (MPR), is crucial for accurately defining the extension of the hematoma and periaortic bleeding. Common findings in IMH also include extravasation of fluid, mediastinal hemorrhage, and pleuropericardial effusion (58).
Acute Type A IMH involving the ascending aorta can be a life-threatening condition, with a high in-hospital mortality rate reaching 40%. In contrast, Type B IMH is less frequently associated with poor outcomes, showing an in-hospital mortality risk of <10% (2, 3, 5, 16).
Compared to AD, malperfusion syndrome is less common in patients with IMH, while periaortic hematoma and pericardial effusion are much more frequent (5). After initial detection and management of IMH, regular follow-up imaging studies with CT or MRI are performed to monitor resolution, stability, or progression.
IMH has an unpredictable evolution without treatment compared to AD. IMH may partially resolve or completely regress after the first 30 days or within 1 year and in some cases even 5 years from initial presentation. Resolution occurs in approximately 19% of Western patients and over 60% of Asian patients (18, 57–60). More frequently, conversion of IMH to AD may occur within the first 6–8 days (3); in 33%–40% of Type A IMH patients at hospital admission, the condition progresses to Type A AD while up to 18% are complicated by rupture (7), compared to Type B IMH which has a certainly more benign course even if not free from complications which can occur in approximately 2.5% of patients (Figure 5) (60).
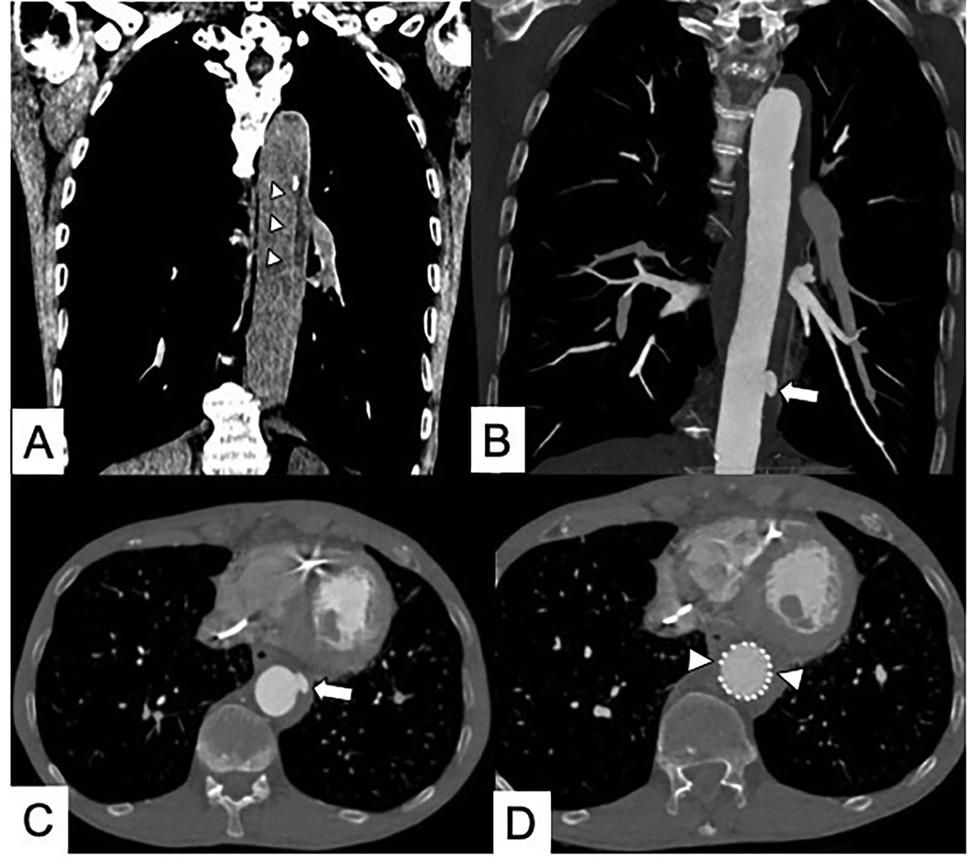
Figure 5. (A) Coronal multiplanar reformation CT in subacute Type B IMH (arrowheads). (B,C) One-month CT follow-up shows the appearance of an ulcer-like projection (ULP) treated (D) with thoracic endovascular aortic repair (TEVAR) (arrowheads).
Development of an aneurysm (saccular or fusiform) indicates a progressive weakening of all three layers of the aortic wall. In particular, structural weakness of the media determines the formation of the most common long-term complication of IMH, the fusiform aneurysm, typically during the subacute or chronic stages of the disease (60–63).
These mutable natural progression patterns and remodeling processes in all phases of the disease highlight the increased vulnerability of the “aortic organ” and dynamic condition of IMH underscoring the critical need for close imaging surveillance to prevent progressive dilation or rupture, particularly within the first 30–60 days (Figure 6) (59–62, 64). Therefore, therapeutic decisions heavily rely on imaging, and for this reason, it is essential for the radiologist to recognize the CT high-risk features in time.
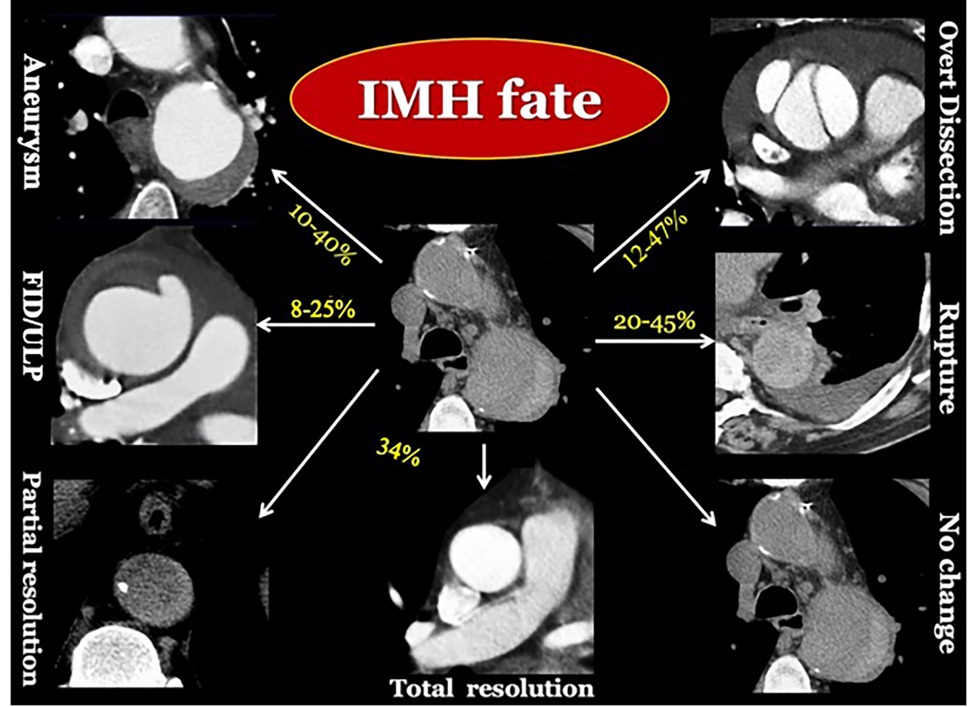
Figure 6. Longitudinal CT imaging of IMH evolving patterns. IMH is a vulnerable dynamic condition, with unpredictable late fate (16, 18, 57, 61).
The identification of some imaging prognostic features, as listed in Table 1 and below, can determine the cases of IMH at higher risk for complications or progression (61–65):
- The differentiation according to Stanford classification has prognostic implications. Approximately 30%–35% of cases are Stanford Type A IMH, which carries a higher risk of pleural or/and pericardial effusion, AD, aneurysmatic evolution, and death (53, 54, 62, 63).
- According to Laplace’s law and vascular wall stress, the maximum aortic diameter is an independent risk factor for complications, including rupture and death. Previous studies have stratified increased risk of complications based on the Stanford classification (48–55 mm for Type A IMH; 40–41 mm for Type B IMH). However, the recent European Association for Cardio-Thoracic Surgery/Society of Thoracic Surgeons Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ, as suggested by Czerny et al., propose a cutoff diameter >45 mm, irrespective of the location (7, 65–67). Mazzolai et al., in 2024 ESC guidelines for the management of peripheral arterial and aortic diseases, consider a high-risk feature of a slightly larger maximum aortic diameter in IMH Type B (>47 mm) (18).
- The maximum hematoma thickness (>10 mm) decreases the chance of complete resorption, increasing the probability of progression (Figure 7) (7, 68–72).
- A focal intimal disruption characterized by a small pouch (<3 mm) filled with contrast material and projecting outside the opacified aortic lumen should always be identified on the baseline CT because it can rapidly develop into an ulcer-like projection (ULP) until a frank double-barrel dissection (Figure 8). A ULP is a new or already existing intimal disruption associated with a poorer prognosis (Figure 9) (72). Its incidence ranges from 20% to 60% (28, 73, 74) and can develop after the acute event from 2.4 to 17.8 months (67, 74). As a focal intimal disruption, ULP is a contrast material-filled pouch that projects outside the opacified aortic lumen and communicates with it through an orifice of the intimal layer >3 mm (28). ULP is differentiated from PAU because it is usually not visible on the initial CT scan but appears during follow-up imaging. Additionally, atherosclerotic disease is not associated with ULP. The detection of ULP already in the acute phase affects the prognosis making it poor especially if located in the ascending aorta or aortic arch and if the diameter exceeds 20 mm and depth reaches 10 mm. ULP often progresses to complications such as AD, saccular pseudoaneurysm, or rupture (67, 74). Indeed, the risk of adverse aortic events in the presence of ULP is reported to be 2.76 times higher compared to cases without ULP, and this risk increases to 3.84 times in patients with newly developed ULP (71, 72, 75). The simultaneous overlapping of different types of AAS (aortic dissection, IMH, ULP, and PAU) in an individual is classified as a mixed-type aortic lesion, which poses additional challenges in interpretation.
- Intramural blood pool (IBP) or aortic branch artery pseudoaneurysms are focal areas of contrast pooling within the hematoma that communicates with the lumen of the aorta through the ostia of intercostal or lumbar arteries that have been disrupted by the hematoma. These are typically found exclusively along the non-pleural circumference of the descending aorta, at the origin of aortic side branches such as bronchial, intercostal, intercostobronchial, pericardial, or lumbar arteries. Communication with the aortic lumen is usually very small (<2 mm) or imperceptible. IBP are generally considered benign features even if their prognostic significance is uncertain and currently the studies performed are limited (Figure 10). IBP occurs due to damage from IMH extending across the origin of the aortic branch artery, resulting in partial or complete tear. IMH with multiple IBP at various levels is reported as the “Chinese ring sword sign” on CT coronal reconstructions of the descending aorta (76). IBPs larger than 10 mm or that are enlarging may require endovascular treatment (77).
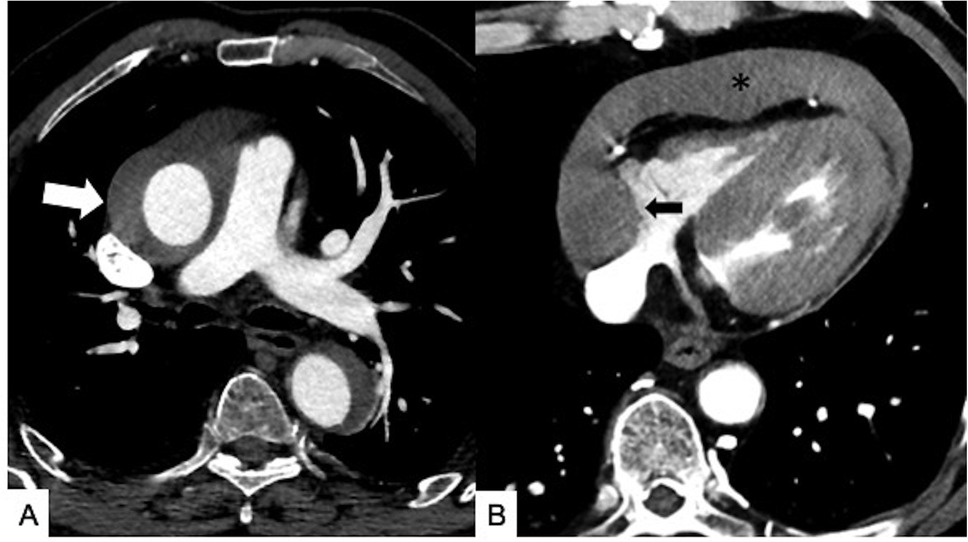
Figure 7. Maximal IMH thickness and pericardial effusion as poor prognostic factors in a 68-year-old male patient with acute back pain and dyspnea. (A) Axial contrast-enhanced CT scan shows a Stanford Type A IMH with hematoma thickness >10 mm (white arrow). (B) Axial contrast-enhanced CT image shows hemorrhagic pericardial effusion (asterisk) and mass effect (black arrow) on the right atrium suggesting impending cardiac tamponade.
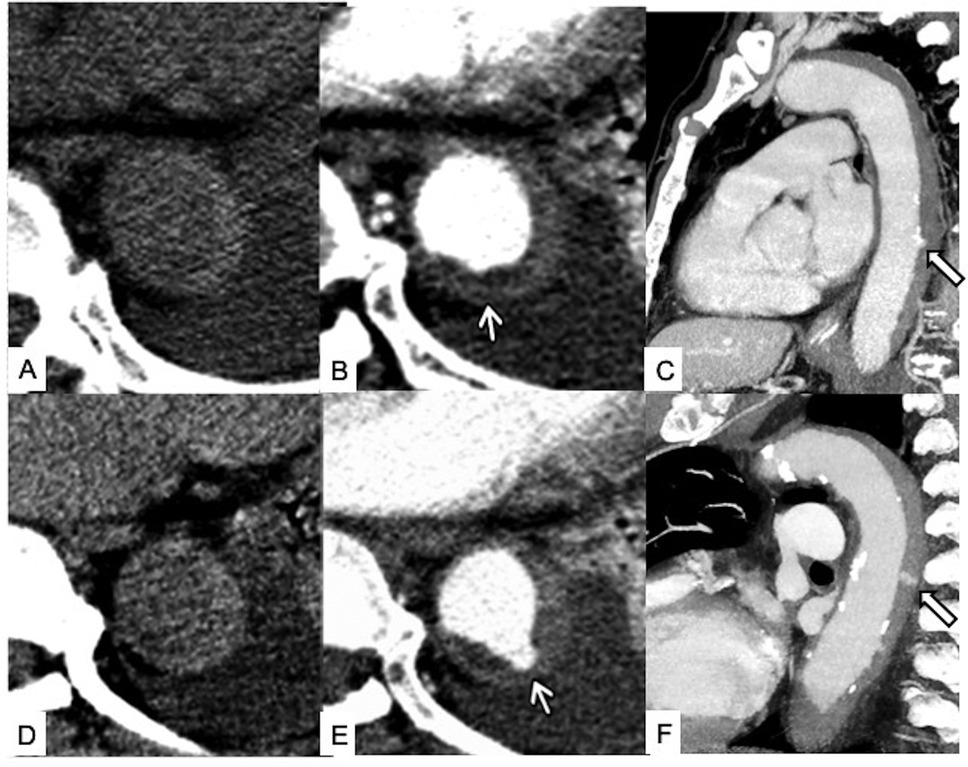
Figure 8. Temporal evolution of unstable Type B IMH. Initial unenhanced scan (A) and contrast-enhanced axial (B) and sagittal (C) CT images showing a Type B IMH with tiny intimal erosion of the descending aorta (white arrows). Four-day follow-up unenhanced scan (D) and contrast-enhanced axial (E) and sagittal (F) CT images at the same level showing enlargement of the intimal defect and enhancing ULP with neck >3 mm from the aortic lumen into the hematoma (white arrows), indicative of overt intimal tear and impending dissection.
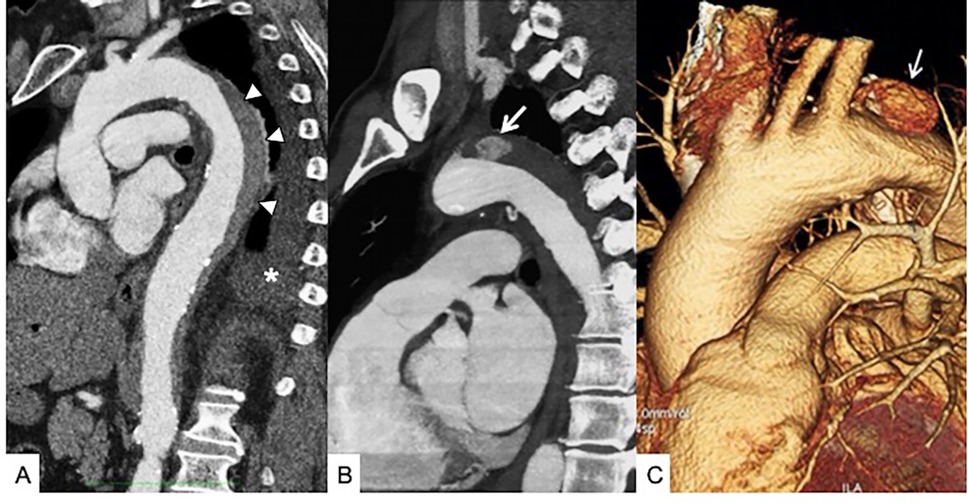
Figure 9. Unstable Type B IMH. (A) Sagittal contrast-enhanced baseline CT shows Type B IMH (arrowheads) in a 68-year-old male patient with hypertension peak and persistent thoracoabdominal pain; pleural effusion is present (asterisk). (B) Six-day follow-up contrast-enhanced CT sagittal multiplanar reformation shows the appearance of an ULP close to the aortic isthmus (arrow). (C) Sagittal oblique 3D volume rendering (VR) reconstruction confirms ULP (arrow). The patient undergoes TEVAR.
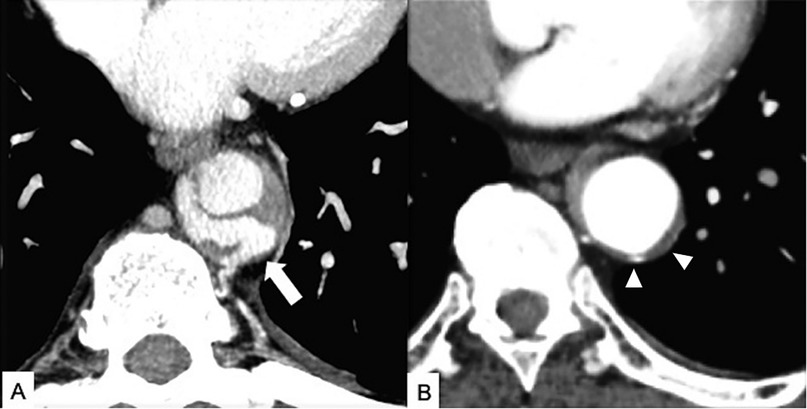
Figure 10. Intramural blood pool (IBP) in a 61-year-old patient with systemic hypertension and Type B thoracoabdominal IMH and acute back pain. (A) Axial CT maximum intensity projection (MIP) reconstruction image shows a T9 level intercostal artery pseudoaneurysm (arrow) in the contest of the Type B IMH with absent communication with the true aortic lumen. (B) Enhanced CT axial image obtained 3 months later shows a partial IBP and IMH regression (arrowheads).
Additional high-risk features for progression and mortality include age over 70 years, pleural or pericardial effusion based on Hounsfield units (values >40–60 Hounsfeld units suggest acute/subacute hemorrhage) (78), and periaortic hematoma (7, 67). Furthermore, some previous studies have indicated that a luminal compression ratio (the minimum to maximum transverse luminal diameters at the site of maximal hematoma thickness determined by three-dimensional double-oblique MPR reconstruction) that is <0.75 is also predictive of IMH progression in aortic dissection (79, 80).
The treatment of IMH has always been controversial. Upon diagnosis, all patients receive immediate medical treatment focused on minimizing stress on the aortic wall, mainly using β-blockers to manage heart rate, ventricular contractility, and systemic blood pressure, as well as pain control. Subsequent treatment strategies depend on the affected aortic segment as determined by the Stanford classification. Over the years, Type A IMH has always been considered a surgical emergency, based on evidence showing a lower mortality rate with early surgical intervention compared to a 40%–80% mortality rate in patients who did not undergo surgery. Although some reports from Asian cohorts have considered initial non-surgical treatment for this condition, reserving surgical treatment only to patients who experience complications (60, 65, 66, 73), and some surgeons advocate for urgent rather than emergent surgery—assuming that delayed treatment can reduce inflammation making the aortic tissue more manageable for repair—this strategy appears to offer no clinical advantage (81).
The recent Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ recommend surgical intervention for all patients exhibiting high-risk type A IMH features, as emergency surgical repair has shown superior outcomes compared to medical therapy alone (7).
For patients lacking high-risk features or who are elderly or have significant comorbidities, a “wait-and-watch strategy” involving non-operative management with pain control and frequent imaging follow-up may be considered a viable option (7, 82, 83).
On the contrary, the initial choice for Type B IMH is non-operative management, reserving thoracic endovascular aortic repair (TEVAR) to the complicated acute Type B IMH or in cases with CT high-risk features (e.g., enlargement of aortic diameter, appearance of ULP, hematoma progression to frank dissection, or visceral ischemia) and would include coverage of the entire diseased segment. Surveillance imaging in patients with non-surgically treated Type B IMH or surgically treated IMH is similar to that in patients with classic Type B AD (13).
In IMH, both progression and regression are possible over the first 30 days to 1 year or as long as 5 years after the onset (59), so a CT or MR evaluation at discharge and at 1, 3, 6, and 12 months after the acute event is necessary and, if not resolved, annually for the next 5 years in which it is possible to have a further aortic event (5, 16, 64).
IMH is easily distinguished from classical AD by the absence of an intimomedial tear or flap in the acute phase and the lack of direct flow communication. Diagnosis can often be challenging if the AD false lumen is completely thrombosed, and, in these cases, the direct identification of the entry tear may be possible only after surgery or autopsy. Furthermore, while IMH maintains a constant circumferential relationship with the aortic wall, the dissection describes a spiral longitudinally (23). In incomplete AD (Svensson Class III of AAS) in which in the presence of an intimomedial rupture the separation of the medial layers does not occur (2, 84), typical features are an eccentric bulge near the tear and concomitant aortic dilation (1, 2, 13, 17, 84).
The presence of atherosclerotic changes or aneurysmal dilation with mural thrombi also makes the differential diagnosis particularly challenging. The presence of non-hyperattenuating wall thickening on non-enhanced CT, an irregular internal contour in the arterial phase, and irregular, thick calcifications should guide the radiologist toward diagnosing atheromatous changes rather than IMH, which typically presents with smooth inner margins and thin, curvilinear intimal calcification displacement (1, 7, 17, 21, 33, 34).
Infectious and non-infectious aortitis (e.g., giant cell arteritis or Takayasu’s arteritis) can present with uniform and hyperattenuating wall thickening on non-contrast CT scans, posing a challenge in differentiating from IMH. Aortitis often manifests with gradual narrowing of the distal aortic segment, creating a characteristic “rat-tail” sign. Additionally, the aortic wall typically enhances in the portal venous phase following contrast medium administration and may exhibit features such as transmural calcification or a double-ring enhancement pattern. This pattern consists of a poorly enhanced inner ring (representing the swollen intima) and an enhanced outer ring (indicating active inflammation in the medial and adventitial layers) (85). In case of aortitis and in non-emergency settings, diffuse [18F]-fluorodeoxyglucose (FDG) uptake in areas of active inflammation in the aortic wall will be present on FDG positron emission tomography (PET) allowing not only to distinguish AAS from inflammatory and infectious aortitis but also to evaluate the effectiveness of the therapy (Figure 11) (86). Furthermore, in IMH, the aortic lumen is slightly narrowed, and a definite transition between the involved and normal aorta is clearly appreciable, a feature not typically seen in aortitis and thrombus (67, 87). Lastly, the absence of acute chest pain should help distinguish inflammation from intramural thrombus or IMH which typically presents with marked painful symptoms.
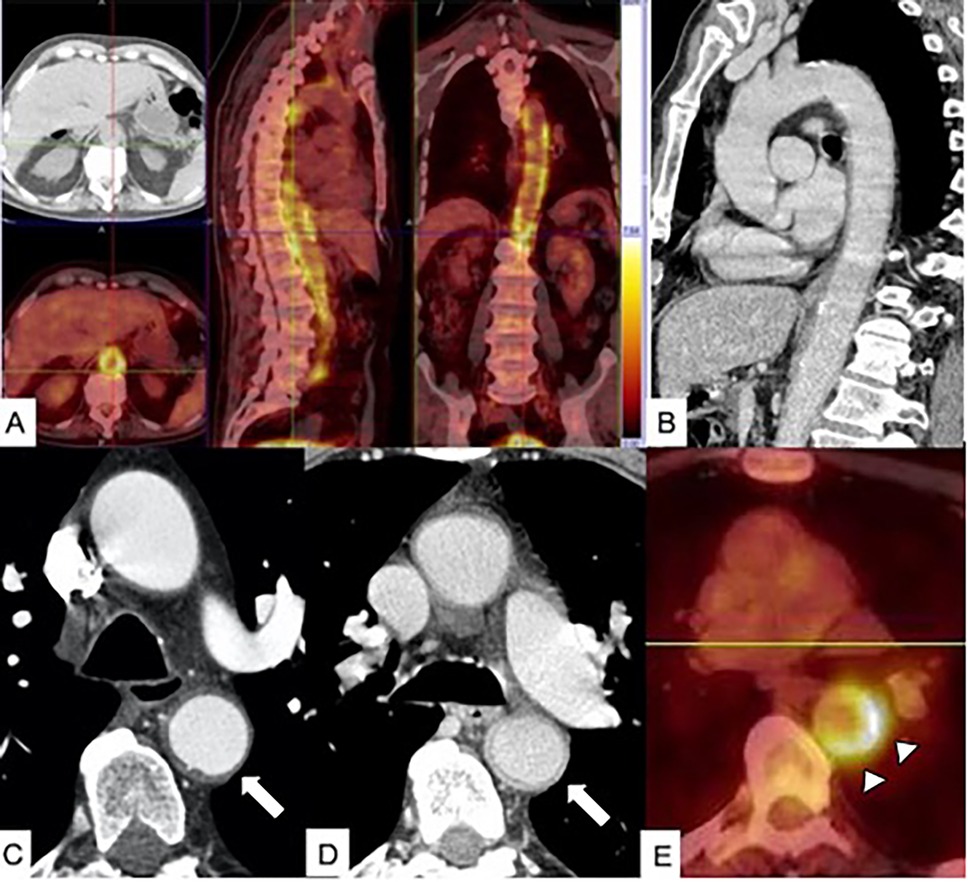
Figure 11. PET/CT in aortitis. (A) Axial PET/CT fusion image in a 68-year-old male patient with diffuse aortitis showing an [18F]-fluorodeoxyglucose (FDG) uptake in the thoracoabdominal aortic wall (arrow). (B) Sagittal multiplanar reconstruction CT image acquired in the portal phase shows thickened aortic wall enhancement. In a different 70-year-old female patient with giant cell arteritis, arterial-phase CT scan (C) shows thickening of the descending aortic wall mimicking an IMH (arrow), and venous phase CT image (D) shows enhancement of thickened aortic wall (arrow). (E) Axial PET/CT fusion image in the same patient shows FDG uptake of the descending aorta wall (arrowheads).
Acute contained rupture of an abdominal or thoracic aortic aneurysm may mimic acute IMH, as both conditions exhibit a high-attenuation crescent sign. However, while IMH begins in the aortic media, in acute contained rupture, high attenuation and CM infiltration begin from the aneurysm lumen through the intramural thrombus and may later penetrate the wall. In the case of chronic contained rupture, CT features can include discontinuity of the rim of calcification in the aneurysm wall, well-defined soft tissue density adjacent to the aorta, psoas muscle involvement, displaced abdominal structures, and no appearance of contrast material in the hematoma (88). Furthermore, the association with the erosion of the vertebral bodies is frequent as recently reported by Parillo et al. (89). In both acute and chronic aortic contained rupture, the “draped aorta sign” can be present in which the aortic posterior wall is not identifiable from adjacent structures or molds the contour of adjacent vertebral bodies; identifying this sign is also useful for differentiating aortic contained rupture from extra aortic pathologies, such as retroperitoneal tumors and osteomyelitis (90, 91).
Aortic sarcoma, a very rare disease with intimal or mural origin, presents with CT features similar to those of IMH. In particular, it can present crescent-shaped thickening of the thoracic aorta wall with the same attenuation of the aortic lumen and displacement of intimal calcification; however, in the presence of an irregular polypoid mass within the aortic lumen with lobulated contour, extravascular tissue growth, and neoplastic enhancement, sarcoma is an eventuality to keep in mind. Embolization of distant organs is common. Diagnosis is almost always by postoperative histopathology and immunohistochemical markers.
The main differential diagnoses of IMH are listed in Table 2.
Today, IMH remains a frequently challenging and potentially life-threatening diagnosis. Therefore, the aim of this review is not only to describe its CT features but also to emphasize the concept that the aortic organ should be considered as an independent “functional unit” where every single component of its structure (vasa vasorum/adventitia, media, or intima) can be affected by different pathologies, often interconnected, overlapping, or even existing simultaneously, but in most cases benefiting from similar management approaches. Despite the ongoing controversy surrounding IMH pathogenesis, an emergency radiologist, especially one who works in small community hospitals without highly specialized cardiothoracic departments and who suspects AAS, must promptly identify high-risk CT features of IMH and centralize patients in a reference “aorta center” to ensure that they receive the most suitable treatment. In acute settings, non-invasive CT scans have contributed to a better understanding of AAS as well as IMH and its evolving nature that therefore requires serial control CT scans with dose-saving techniques both during hospitalization and after discharge. Decision-making should be individualized depending on IMH location, appearance, dimensions, and associated pathology; moreover, case discussion by a multidisciplinary team is mandatory to reduce early mortality and avoid reoperations improving long-term outcomes.
GS: Conceptualization, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GR: Visualization, Writing – review & editing. RL: Visualization, Writing – review & editing. MS: Supervision, Visualization, Writing – review & editing. AA-O: Writing – review & editing. GB: Visualization, Writing – review & editing. FR: Visualization, Writing – review & editing. SM: Visualization, Writing – review & editing. ST: Visualization, Writing – review & editing. SG: Visualization, Writing – review & editing. CM: Writing – review & editing, Visualization. TV: Writing – review & editing, Conceptualization, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vilacosta I, San Roman JA. Acute aortic syndrome. Heart. (2001) 85:365–8. doi: 10.1136/heart.85.4.365
2. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. (2014) 35(41):2873–926. doi: 10.1093/eurheartj/ehu281
3. Valente T, Sica G, Bocchini G, Romano F, Lassandro F, Rea G, et al. MDCT imaging of non-traumatic thoracic aortic emergencies and its impact on diagnosis and management-A reappraisal. Tomography. (2022) 8(1):200–28. doi: 10.3390/tomography8010017
4. Nienaber CA, Sievers HH. Intramural hematoma in acute aortic syndrome—more than one variant of dissection? Circulation. (2002) 106:284–5. doi: 10.1161/01.CIR.0000023453.90533.82
5. Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, et al. International registry of aortic dissection (IRAD) investigators. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. (2005) 111(8):1063–70. doi: 10.1161/01.CIR.0000156444.26393.80
6. Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, et al. Acute aortic intramural hematoma: an analysis from the international registry of acute aortic dissection. Circulation. (2012) 126(11 Suppl 1):S91–6. doi: 10.1161/CIRCULATIONAHA.111.084541
7. Czerny M, Grabenwöger M, Berger T, Aboyans V, Della Corte A, Chen EP, et al. EACTS/STS guidelines for diagnosing and treating acute and chronic syndromes of the aortic organ. Eur J Cardiothorac Surg. (2024) 65(2):ezad426. doi: 10.1093/ejcts/ezad426 Erratum in: Eur J Cardiothorac Surg. 2024 Jun 3;65(6):ezae235. doi: 10.1093/ejcts/ezae235.38408364
8. Shimizu H, Yoshino H, Udagawa H, Watanuki A, Yano K, Ide H, et al. Prognosis of aortic intramural hemorrhage compared with classic aortic dissection. Am J Cardiol. (2000) 85(6):792–5. doi: 10.1016/s0002-9149(99)00867-x
9. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. (2000) 283(7):897–903. doi: 10.1001/jama.283.7.897
10. Song JK, Yim JH, Ahn JM, Kim DH, Kang JW, Lee TY, et al. Outcomes of patients with acute type a aortic intramural hematoma. Circulation. (2009) 120(21):2046–52. doi: 10.1161/CIRCULATIONAHA.109.879783
11. O’Gara PT, DeSanctis RW. Acute aortic dissection and its variants. Toward a common diagnostic and therapeutic approach. Circulation. (1995) 92:1376–8. doi: 10.1161/01.CIR.92.6.1376
12. DeMartino RR, Sen I, Huang Y, Bower TC, Oderich GS, Pochettino A, et al. Population-based assessment of the incidence of aortic dissection, intramural hematoma, and penetrating ulcer, and its associated mortality from 1995 to 2015. Circ Cardiovasc Qual Outcomes. (2018) 11(8):e004689. doi: 10.1161/CIRCOUTCOMES.118.004689
13. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines, American association for thoracic surgery, American college of radiology, American stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons, and society for vascular medicine. Catheter Cardiovasc Interv. (2010) 76(2):E43–86. doi: 10.1002/ccd.22537
14. Mussa FF, Horton JD, Moridzadeh R, Nicholson J, Trimarchi S, Eagle KA. Acute aortic dissection and intramural hematoma: a systematic review. JAMA. (2016) 316(7):754–63. doi: 10.1001/jama.2016.10026
15. Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. (1970) 10(3):237–47. doi: 10.1016/s0003-4975(10)65594-4
16. Moral S, Cuéllar H, Avegliano G, Ballesteros E, Salcedo MT, Ferreira-González I, et al. Clinical implications of focal intimal disruption in patients with type B intramural hematoma. J Am Coll Cardiol. (2017) 69(1):28–39. doi: 10.1016/j.jacc.2016.10.045
17. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease. A report of the American College of Cardiology foundation/American Heart Association task force on practice guidelines, American association for thoracic surgery, American college of radiology, American stroke association, society of cardiovascular anesthesiologists, society for cardiovascular angiography and interventions, society of interventional radiology, society of thoracic surgeons,and society for vascular medicine. J Am Coll Cardiol. (2010) 55(14):e27–e129. doi: 10.1016/j.jacc.2010.02.015
18. Mazzolai L, Teixido-Tura G, Lanzi S, Boc V, Bossone E, Brodmann M, et al. 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J. (2024) 45(36):3538–700. doi: 10.1093/eurheartj/ehae179
19. Krukenberg E. Beitrage zur frage des aneurisms dissecans. Beitr Pathol Anat Allg Pathol. (1920) 67:329–51.
20. Gore I. Pathogenesis of dissecting aneurysm of the aorta. AMA Arch Pathol. (1952) 53:142–53.14884831
21. Macura KJ, Corl FM, Fishman EK, Bluemke DA. Pathogenesis in acute aortic syndromes: aortic dissection, intramural hematoma, and penetrating atherosclerotic aortic ulcer. AJR Am J Roentgenol. (2003) 181:309–16. doi: 10.2214/ajr.181.2.1810309
22. Coady MA, Rizzo JA, Elefteriades JA. Pathologic variants of thoracic aortic dissections. Penetrating atherosclerotic ulcers and intramural hematomas. Cardiol Clin. (1999) 17(4):637–57. doi: 10.1016/S0733-8651(05)70106-5
23. Maddu KK, Shuaib W, Telleria J, Johnson JO, Khosa F. Nontraumatic acute aortic emergencies: part 1, acute aortic syndrome. AJR Am J Roentgenol. (2014) 202(3):656–65. doi: 10.2214/AJR.13.11437
24. Park KH, Lim C, Choi JH, Sung K, Kim K, Lee YT, et al. Prevalence of aortic intimal defect in surgically treated acute type A intramural hematoma. Ann Thorac Surg. (2008) 86(5):1494–500. doi: 10.1016/j.athoracsur.2008.06.061
25. Uchida K, Imoto K, Karube N, Minami T, Cho T, Goda M, et al. Intramural haematoma should be referred to as thrombosed-type aortic dissection. Eur J Cardiothorac Surg. (2013) 44(2):366–9. discussion 369 doi: 10.1093/ejcts/ezt040
26. Kitai T, Kaji S, Yamamuro A, Tani T, Kinoshita M, Ehara N, et al. Detection of intimal defect by 64-row multidetector computed tomography in patients with acute aortic intramural hematoma. Circulation. (2011) 124(11 Suppl):S174–8. doi: 10.1161/CIRCULATIONAHA.111.037416
27. Ferrera C, Vilacosta I, Gómez-Polo JC, Villanueva-Medina S, Cabeza B, Ortega L, et al. Evolution and prognosis of intramural aortic hematoma. Insights from a midterm cohort study. Int J Cardiol. (2017) 249:410–3. doi: 10.1016/j.ijcard.2017.09.170
28. Evangelista A, Moral S, Ballesteros E, Castillo-Gandía A. Beyond the term penetrating aortic ulcer: a morphologic descriptor covering a constellation of entities with different prognoses. Prog Cardiovasc Dis. (2020) 63:488–95. doi: 10.1016/j.pcad.2020.05.006
29. Yamada T, Takamiya M, Naito H, Kozuka T, Nakajima N. Diagnosis of aortic dissection without intimal rupture by x-ray computed tomography. Nippon Acta Radio1. (1985) 45:699–710.
30. Eggebrecht H, Herold U, Schmermund A, Lind AY, Kuhnt O, Martini S, et al. Endovascular stent-graft treatment of penetrating aortic ulcer: results over a median follow-up of 27 months. Am Heart J. (2006) 151(2):530–6. doi: 10.1016/j.ahj.2005.05.020
31. Hirst AE Jr, Barbour BH. Dissecting aneurysm with hemopericardium; report of a case with healing. N Engl J Med. (1958) 258:116–20. doi: 10.1056/NEJM195801162580303
32. Braverman AC. Penetrating atherosclerotic ulcers of the aorta. Curr Opin Cardiol. (1994) 9:591–7. doi: 10.1097/00001573-199409000-00014
33. Kazerooni EA, Bree RL, Williams DM. Penetrating atherosclerotic ulcers of the descending thoracic aorta: evaluation with CT and distinction from aortic dissection. Radiology. (1992) 183:759–65. doi: 10.1148/radiology.183.3.1584933
34. Hayashi H, Matsuoka Y, Sakamoto I, Sueyoshi E, Okimoto T, Hayashi K, et al. Penetrating atherosclerotic ulcer of the aorta: imaging features and disease concept. Radiographics. (2000) 20(4):995–1005. doi: 10.1148/radiographics.20.4.g00jl01995
35. Rajiah P. Updates in vascular computed tomography. Radiol Clin N Am. (2020) 58:671–91. doi: 10.1016/j.rcl.2020.02.011
36. Rajendran K, Petersilka M, Henning A, Shanblatt ER, Schmidt B, Flohr TG, et al. First clinical photon-counting detector CT system: technical evaluation. Radiology. (2022) 303(1):130–8. doi: 10.1148/radiol.212579
37. Euler A, Higashigaito K, Mergen V, Sartoretti T, Zanini B, Schmidt B, et al. High-pitch photon-counting detector computed tomography angiography of the aorta: intraindividual comparison to energy-integrating detector computed tomography at equal radiation dose. Invest Radiol. (2022) 57(2):115–21. doi: 10.1097/RLI.0000000000000816
38. Sica G, Rea G, Scaglione M. Editorial for the special issue “cardiothoracic imaging: recent techniques and applications in diagnostics”. Diagnostics. (2024) 14(5):461. doi: 10.3390/diagnostics14050461
39. Abu-Omar A, Murray N, Ali IT, Khosa F, Barrett S, Sheikh A, et al. Utility of dual-energy computed tomography in clinical conundra. Diagnostics (Basel). (2024) 14(7):775. doi: 10.3390/diagnostics14070775
40. Si-Mohamed S, Dupuis N, Tatard-Leitman V, Rotzinger D, Boccalini S, Dion M, et al. Virtual versus true non-contrast dual-energy CT imaging for the diagnosis of aortic intramural hematoma. Eur Radiol. (2019) 29(12):6762–71. doi: 10.1007/s00330-019-06322-5
41. Rotzinger DC, Si-Mohamed SA, Shapira N, Douek PC, Meuli RA, Boussel L. “Dark-blood” dual-energy computed tomography angiography for thoracic aortic wall imaging. Eur Radiol. (2020) 30(1):425–31. doi: 10.1007/s00330-019-06336-z
42. Valente T, Sica G, Romano F, Rea G, Lieto R, De Feo M, et al. Non-A non-B acute aortic dissection: is there some confusion in the radiologist’s mind? Tomography. (2023) 9(6):2247–60. doi: 10.3390/tomography9060174
43. Scaglione M, Iaselli F, Sica G, Feragalli B, Nicola R. Errors in imaging of traumatic injuries. Abdom Imaging. (2015) 40(7):2091–8. doi: 10.1007/s00261-015-0494-9
44. Sica G, Guida F, Bocchini G, Codella U, Mainenti PP, Tanga M, et al. Errors in imaging assessment of polytrauma patients. Semin Ultrasound CT MR. (2012) 33(4):337–46. doi: 10.1053/j.sult.2012.01.012
45. Valente T, Rossi G, Lassandro F, Rea G, Marino M, Muto M, et al. MDCT evaluation of acute aortic syndrome (AAS). Br J Radiol. (2016) 89(1061):20150825. doi: 10.1259/bjr.20150825
46. Ko JP, Goldstein JM, Jr LL, Azour L, Gozansky EK, Moore W, et al. Chest CT angiography for acute aortic pathologic conditions: pearls and pitfalls. Radiographics. (2021) 41(2):399–424. doi: 10.1148/rg.2021200055
47. Rengo M, Dharampal A, Lubbers M, Kock M, Wildberger JE, Das M, et al. Impact of iodine concentration and iodine delivery rate on contrast enhancement in coronary CT angiography: a randomized multicenter trial (CT-CON). Eur Radiol. (2019) 29(11):6109–18. doi: 10.1007/s00330-019-06196-7
48. Available online at: https://sirm.org/2022/10/03/protocolli-di-tomografia-computerizzata-per-indicazione-clinica/
49. Mansour M, Berkery W, Kozlowski L, Widell J, Obeid A. Mild thickening of the aortic wall: subtle intramural hematoma? Echocardiography. (2001) 18(6):519–22. doi: 10.1046/j.1540-8175.2001.00519.x
50. Evangelista A, Maldonado G, Gruosso D, Gutiérrez L, Granato C, Villalva N, et al. The current role of echocardiography in acute aortic syndrome. Echo Res Pract. (2019) 6(2):R53–63. doi: 10.1530/ERP-18-0058
51. Rubin GD. Helical CT angiography of the thoracic aorta. J Thorac Imaging. (1997) 12:128–49. doi: 10.1097/00005382-199704000-00011
52. Maslow A, Atalay MK, Sodha N. Intramural hematoma. J Cardiothorac Vasc Anesth. (2018) 32:1341–62. doi: 10.1053/j.jvca.2018.01.025
53. Siriapisith T, Wasinrat J, Slisatkorn W. Computed tomography of aortic intramural hematoma and thrombosed dissection. Asian Cardiovasc Thorac Ann. (2010) 18:456–63. doi: 10.1177/0218492310380473
54. Valente T, Rossi G, Lassandro F, Rea G, Marino M, Urciuolo S, et al. MDCT distinguishing features of focal aortic projections (FAP) in acute clinical settings. Radiol Med. (2015) 120(1):50–72. doi: 10.1007/s11547-014-0459-z
55. Yamada T, Tada S, Harada J. Aortic dissection without intimal rupture: diagnosis with MR imaging and CT. Radiology. (1988) 168:347–52. doi: 10.1148/radiology.168.2.3393653
56. Nienaber CA, von Kodolitsch Y, Petersen B, Loose R, Helmchen U, Haverich A, et al. Intramural hemorrhage of the thoracic aorta. Diagnostic and therapeutic implications. Circulation. (1995) 92(6):1465–72. doi: 10.1161/01.cir.92.6.1465
57. Evangelista A, Dominguez R, Sebastia C, Salas A, Permanyer-Miralda G, Avegliano G, et al. Prognostic value of clinical and morphologic findings in short-term evolution of aortic intramural haematoma. Therapeutic implications. Eur Heart J. (2004) 25(1):81–7. doi: 10.1016/j.ehj.2003.10.011
58. Lansman SL, Saunders PC, Malekan R, Spielvogel D. Acute aortic syndrome. J Thorac Cardiovasc Surg. (2010) 140:S92–7. doi: 10.1016/j.jtcvs.2010.07.062
59. Vilacosta I, San Román JA, Ferreirós J, Aragoncillo P, Méndez R, Castillo JA, et al. Natural history and serial morphology of aortic intramural hematoma: a novel variant of aortic dissection. Am Heart J. (1997) 134(3):495–507. doi: 10.1016/s0002-8703(97)70087-5
60. Ahn JM, Kim H, Kwon O, Om SY, Heo R, Lee S, et al. Differential clinical features and long-term prognosis of acute aortic syndrome according to disease entity. Eur Heart J. (2019) 40(32):2727–36. doi: 10.1093/eurheartj/ehz153
61. Abbas A, Brown IW, Peebles CR, Harden SP, Shambrook JS. The role of multidetector-row CT in the diagnosis, classification and management of acute aortic syndrome. Br J Radiol. (2014) 87(1042):20140354. doi: 10.1259/bjr.20140354
62. Nienaber CA, Powell JT. Management of acute aortic syndromes. Eur Heart J. (2012) 33:26–35. doi: 10.1093/eurheartj/ehr186
63. Sueyoshi E, Matsuoka Y, Sakamoto I, Uetani M, Hayashi K, Narimatsu M. Fate of intramural hematoma of the aorta: CT evaluation. J Comput Assist Tomogr. (1997) 21(6):931–8. doi: 10.1097/00004728-199711000-00016
64. Eagle KA, Bossone E. Intramural hematoma: when does a sheep become a wolf? J Am Coll Cardiol. (2017) 69(1):40–2. doi: 10.1016/j.jacc.2016.07.793
65. Evangelista A, Czerny M, Nienaber C, Schepens M, Rousseau H, Cao P, et al. Interdisciplinary expert consensus on management of type B intramural haematoma and penetrating aortic ulcer. Eur J Cardiothorac Surg. (2015) 47(2):209–17. doi: 10.1093/ejcts/ezu386
66. Lee YK, Seo JB, Jang YM, Do KH, Kim SS, Lee JS, et al. Acute and chronic complications of aortic intramural hematoma on follow-up computed tomography: incidence and predictor analysis. J Comput Assist Tomogr. (2007) 31(3):435–40. doi: 10.1097/01.rct.0000250112.87585.8e
67. Gutschow SE, Walker CM, Martínez-Jiménez S, Rosado-de-Christenson ML, Stowell J, Kunin JR. Emerging concepts in intramural hematoma imaging. Radiographics. (2016) 36(3):660–74. doi: 10.1148/rg.2016150094
68. Park GM, Ahn JM, Kim DH, Kang JW, Song JM, Kang DH, et al. Distal aortic intramural hematoma: clinical importance of focal contrast enhancement on CT images. Radiology. (2011) 259(1):100–8. doi: 10.1148/radiol.11101557
69. Schlatter T, Auriol J, Marcheix B, Lebbadi M, Marachet MA, Dang-Tran KD, et al. Type B intramural hematoma of the aorta: evolution and prognostic value of intimal erosion. J Vasc Interv Radiol. (2011) 22(4):533–41. doi: 10.1016/j.jvir.2010.10.028
70. Sawaki S, Hirate Y, Ashida S, Takanohashi A, Yagami K, Usui M. Clinical outcomes of medical treatment of acute type A intramural hematoma. Asian Cardiovasc Thorac Ann. (2010) 18(4):354–9. doi: 10.1177/0218492310375855
71. Song JM, Kim HS, Song JK, Kang DH, Hong MK, Kim JJ, et al. Usefulness of the initial noninvasive imaging study to predict the adverse outcomes in the medical treatment of acute type A aortic intramural hematoma. Circulation. (2003) 108(Suppl 1):II324–8. doi: 10.1161/01.cir.0000087651.30078.38
72. Li Z, Lu B, Chen Y, Hou Z, Chen B, Zhang Y, et al. Acute type B aortic intramural hematoma: the added prognostic value of a follow-up CT. Eur Radiol. (2019) 29(12):6571–80. doi: 10.1007/s00330-019-06254-0
73. Kitai T, Kaji S, Yamamuro A, Tani T, Kinoshita M, Ehara N, et al. Impact of new development of ulcer-like projection on clinical outcomes in patients with type B aortic dissection with closed and thrombosed false lumen. Circulation. (2010) 122(11 Suppl):S74–80. doi: 10.1161/CIRCULATIONAHA.109.927517
74. Sueyoshi E, Matsuoka Y, Imada T, Okimoto T, Sakamoto I, Hayashi K. New development of an ulcerlike projection in aortic intramural hematoma: CT evaluation. Radiology. (2002) 224(2):536–41. doi: 10.1148/radiol.2242011009
75. Sahu KK, Lal A, Mishra AK. Additional insights regarding aortic intramural hematoma. Can J Anaesth. (2020) 67:382–3. doi: 10.1007/s12630-019-01490-w
76. Wu MT, Wu TH, Lee D. Multislice computed tomography of aortic intramural hematoma with progressive intercostal artery tears: the Chinese ring-sword sign. Circulation. (2005) 111:e92–3. doi: 10.1161/01.CIR.0000154547.65893.45
77. Ferro C, Rossi UG, Seitun S, Scarano F, Passerone G, Williams DM. Aortic branch artery pseudoaneurysms associated with intramural hematoma: when and how to do endovascular embolization. Cardiovasc Intervent Radiol. (2013) 36(2):422–32. doi: 10.1007/s00270-012-0512-z
78. Valente T, Pignatiello M, Sica G, Bocchini G, Rea G, Cappabianca S, et al. Hemopericardium in the acute clinical setting: are we ready for a tailored management approach on the basis of MDCT findings? Radiol Med. (2021) 126(4):527–43. doi: 10.1007/s11547-020-01303-x
79. Choi SH, Choi SJ, Kim JH, Bae SJ, Lee JS, Song KS, et al. Useful CT findings for predicting the progression of aortic intramural hematoma to overt aortic dissection. J Comput Assist Tomogr. (2001) 25(2):295–9. doi: 10.1097/00004728-200103000-00025
80. Wei C, Li J, Du E, Miao Y, Li P, Guan W. Clinical and imaging differences between Stanford type B intramural hematoma-like lesions and classic aortic dissection. BMC Cardiovasc Disord. (2023) 23(1):378. doi: 10.1186/s12872-023-03413-6
81. Estrera AL, Sandhu HK, Leake SS, Charlton-Ouw KM, Afifi RO, Miller CC 3rd, et al. Early and late outcomes of acute type A aortic dissection with intramural hematoma. J Thorac Cardiovasc Surg. (2015) 149(1):137–42. doi: 10.1016/j.jtcvs.2014.10.028
82. Chou AS, Ziganshin BA, Charilaou P, Tranquilli M, Rizzo JA, Elefteriades JA. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. (2016) 151(2):361–72. 373.e1 doi: 10.1016/j.jtcvs.2015.09.012
83. Wee I, Varughese RS, Syn N, Choong A. Non-operative management of type A acute aortic syndromes: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. (2019) 58:41–51. doi: 10.1016/j.ejvs.2018.10.015
84. Svensson LG, Labib SB, Eisenhauer AC, Butterly JR. Intimal tear without hematoma: an important variant of aortic dissection that can elude current imaging techniques. Circulation. (1999) 99:1331–6. doi: 10.1161/01.CIR.99.10.1331
85. Matsunaga N, Hayashi K, Sakamoto I, Ogawa Y, Matsumoto T. Takayasu arteritis: protean radiologic manifestations and diagnosis. Radiographics. (1997) 17(3):579–94. doi: 10.1148/radiographics.17.3.9153698
86. Hartlage GR, Palios J, Barron BJ, Stillman AE, Bossone E, Clements SD, et al. Multimodality imaging of aortitis. JACC Cardiovasc Imaging. (2014) 7(6):605–19. doi: 10.1016/j.jcmg.2014.04.002
87. Restrepo CS, Ocazionez D, Suri R, Vargas D. Aortitis: imaging spectrum of the infectious and inflammatory conditions of the aorta. RadioGraphics. (2011) 31:435–51. doi: 10.1148/rg.312105069
88. Yuksekkaya R, Koner AE, Celikyay F, Beyhan M, Almus F, Acu B. Multidetector computed tomography angiography findings of chronic-contained thoracoabdominal aortic aneurysm rupture with severe thoracal vertebral body erosion. Case Rep Radiol. (2013) 2013:596517. doi: 10.1155/2013/596517
89. Parillo M, Vaccarino F, Beomonte Zobel B, Quattrocchi CC. A rare case of contained chronic rupture of abdominal aortic aneurysm associated with vertebral erosion: pre- and post-operative findings on computed tomography and a narrative review. Vasc Endovascular Surg. (2022):15385744221108040. doi: 10.1538/5744221108040
90. Halliday KE, al-Kutoubi A. Draped aorta: CT sign of contained leak of aortic aneurysms. Radiology. (1996) 199(1):41–3. doi: 10.1148/radiology.199.1.8633170
Keywords: aortic intramural hematoma, computed tomography angiography (CTA), acute aortic syndrome (AAS), penetrating atherosclerotic ulcer (PAU), aortic dissection (AD), diagnosis
Citation: Sica G, Rea G, Lieto R, Scaglione M, Abu-Omar A, Bocchini G, Romano F, Masala S, Tamburrini S, Guarino S, Massimo C and Valente T (2025) CT diagnosis and destiny of acute aortic intramural hematoma. Front. Radiol. 5:1552644. doi: 10.3389/fradi.2025.1552644
Received: 20 January 2025; Accepted: 13 February 2025;
Published: 11 March 2025.
Edited by:
Guglielmo M. Trovato, European Medical Association (EMA), BelgiumReviewed by:
Marco Sperandeo, Home for Relief of Suffering (IRCCS), ItalyCopyright: © 2025 Sica, Rea, Lieto, Scaglione, Abu-Omar, Bocchini, Romano, Masala, Tamburrini, Guarino, Massimo and Valente. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacomo Sica, Z2lhY29tby5zaWNhQG9zcGVkYWxpZGVpY29sbGkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.