
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Radiol. , 20 February 2025
Sec. Neuroradiology
Volume 5 - 2025 | https://doi.org/10.3389/fradi.2025.1546069
This article is part of the Research Topic Current Challenges and Future Perspectives in Neuro-Oncological Imaging View all articles
 Simone Cataldi1
Simone Cataldi1 Paola Feraco2
Paola Feraco2 Maurizio Marrale3
Maurizio Marrale3 Pierpaolo Alongi1,4
Pierpaolo Alongi1,4 Laura Geraci5
Laura Geraci5 Ludovico La Grutta6
Ludovico La Grutta6 Giuseppe Caruso1
Giuseppe Caruso1 Tommaso Vincenzo Bartolotta1
Tommaso Vincenzo Bartolotta1 Massimo Midiri1
Massimo Midiri1 Cesare Gagliardo1,7*
Cesare Gagliardo1,7*
Nowadays, the genetic and biomolecular profile of neoplasms—related with their biological behaviour—have become a key issue in oncology, as they influence many aspects of both diagnosis and treatment. In the neuro-oncology field, neuroradiological research has recently explored the potential of non-invasively predicting the molecular phenotype of primary brain neoplasms, particularly gliomas, based on magnetic resonance imaging (MRI), using both conventional and advanced imaging techniques. Among these, diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI), MR spectroscopy (MRS) and susceptibility-weighted imaging (SWI) and have been used to explore various aspects of glioma biology, including predicting treatment response and understanding treatment-related changes during follow-up imaging. Recently, intratumoral susceptibility signals (ITSSs)—visible on SWI—have been recognised as an important new imaging tool in the evaluation of brain gliomas, as they offer a fast and simple non-invasive window into their microenvironment. These intratumoral hypointensities reflect critical pathological features such as microhemorrhages, calcifications, necrosis and vascularization. Therefore, ITSSs can provide neuroradiologists with more biological information for glioma differential diagnosis, grading and subtype differentiation, providing significant clinical support in prognosis assessment, therapeutic management and treatment response evaluation. This review summarizes recent advances in ITSS applications in glioma assessment, emphasizing both its potential and limitations while referencing key studies in the field.
Genetic and biomolecular analyses of neoplasms have become increasingly critical in the era of precision and personalized medicine, as they reflect biologic behaviour of tumours. Consequently, biomolecular analyses have increasingly complemented traditional pathological assessments for both diagnosis and management in oncology. This principle is equally relevant in neuro-oncology, as reflected in the last editions (2016 and 2021) of World Health Organization (WHO) classification of central nervous system (CNS) tumors, which have progressively emphasized the role of genetic and biomolecular data in brain gliomas evaluation. For example, the newest classification of diffuse gliomas—the most common primary malignant brain neoplasms—is primary based on specific genetic alterations, such as isocitrate dehydrogenase (IDH) genes mutations and 1p/19q codeletion (1–3).
Currently, diagnosis and characterization of gliomas require histopathological analysis of a sample obtained through surgical biopsy. Therefore, neuroradiologists play a critical role in identifying imaging features—primarily through magnetic resonance imaging (MRI) with both conventional and advanced sequences—that may suggest the underlying nature of a primary brain tumor before biomolecular and pathological analysis (4, 5).
Over the last decades, Susceptibility-Weighted Imaging (SWI) has emerged as improved substitute of conventional T2*-weighted Gradient Echo (GRE) imaging and has become a standard component of MRI protocols (6, 7). SWI exploits magnetic susceptibility differences in brain tissues, enabling visualization of paramagnetic and diamagnetic substances such as calcium, blood products and iron.
This review explores the role of intratumoral susceptibility signal (ITSS), an imaging parameter defined as low-signal-intensity fine-linear or dot-like structures, with or without conglomeration, visible within tumors on SWI. These ITSSs are variably associated with intratumoral microhemorrhage, necrosis, vascular proliferation, and calcifications, providing a unique insight into glioma biology (8).
The 2016 and the latter 2021 WHO Classification of CNS tumors marked a significant shift by integrating molecular profiling into the characterization of gliomas. This updated framework moves beyond traditional histopathological evaluation to incorporate genetic and molecular alterations, providing a more precise and clinically relevant understanding of gliomas (1–3). Nowadays the most important molecular markers are isocitrate dehydrogenase (IDH) mutation status, 1p/19q co-deletion, genetic alterations in the promoter region of the telomerase reverse transcriptase (TERT) gene, O6-Methylguanine-DNA Methyltransferase (MGMT) promoter methylation status, Ki-67 nuclear protein proliferation index and X-linked alpha thalassaemia intellectual disability syndrome (ATRX) gene mutations. All these play pivotal roles in defining glioma subtypes and their prognostic implications.
For example, gliomas are now classified into IDH-mutant or IDH-wildtype categories, with IDH mutation generally associated with better outcomes (9–11). Additionally, the identification of 1p/19q co-deletion distinguishes oligodendrogliomas from astrocytomas, further refining diagnosis and guiding therapeutic decisions (12).
TERT promoter mutations are found predominantly in IDH-wildtype glioblastomas and oligodendrogliomas with 1p/19q co-deletion, while they are rare in IDH-mutant astrocytomas, highlighting their diagnostic relevance in differentiating glioma subtypes (13–15).
MGMT is a DNA repair enzyme that removes alkyl groups from the O6 position of guanine, a site commonly damaged by alkylating chemotherapeutic agents like temozolomide (TMZ) so gliomas with a methylated MGMT promoter, the reduced repair capability increases the efficacy of alkylating agents, making chemotherapy more effective (16–19).
Ki-67 is a nuclear protein assessed through immunohistochemistry that is widely used as a marker of cellular proliferation in cancer diagnostics, including in brain gliomas (20). It is expressed during active phases of the cell cycle (G1, S, G2, and mitosis) but is absent in quiescent cells (G0). A high Ki-67 proliferation index indicates rapid cell division and aggressive tumor behavior, which is common in high-grade gliomas like glioblastomas while lower Ki-67 indices are typically associated with less aggressive, low-grade gliomas (21).
Mutations or loss of ATRX function are commonly observed in IDH-mutant gliomas and are associated with a specific alternative lengthening of telomeres (ALT) mechanism. This allows tumor cells to maintain telomere length independently of telomerase activation, enabling continued cell division (22–24).
SWI, a high-resolution 3D GRE sequence, visualizes brain tissues on the basis of differences in their magnetic susceptibility. This susceptibility is an intrinsic magnetic property of substances and determines their response to an external magnetic field: substances with negative susceptibility, such as calcium (i.e., diamagnetic substances), oppose the applied field, while those with positive susceptibility, such as blood, iron, and deoxyhemoglobin (i.e., paramagnetic substances), enhance the field (6, 25).
SWI images are generated by separately acquiring magnitude and phase images. The magnitude images, combined with a phase mask derived from phase data, enhance the visibility of both diamagnetic and paramagnetic substances, appearing as areas of signal loss. An important consideration is how vendors present phase information on SWI phase images. In a left-handed system (i.e., Canon and Siemens) phase increases positively in a clockwise direction. Conversely, in a right-handed system (i.e., the more common configuration used on GE Healthcare, Philips and United Neusoft), phase increases positively in a counterclockwise direction-aligned with the way the fingers of your right-hand curl when forming a fist. Thus, choice between left-handed and right-handed systems affects the appearance of the resulting images. In clinical practice phase data are particularly useful for differentiating between calcium and blood: in calcifications, phase images exhibit low contrast in a left-handed system, while blood products show low contrast in a right-handed system. However, in routine practice, distinguishing microbleeds from microcalcifications can be challenging and sometimes inconclusive, particularly in larger or more geometrically complex lesions, which may produce intricate signal patterns on phase images (6, 25).
In glioma microenvironment (Figure 1), ITSSs correspond to both microcalcifications and microhemorrhages or necrosis (visible as dot-like hypointensities), as well as to aberrant vascular proliferation (visible as fine-linear hypointensities) (8).
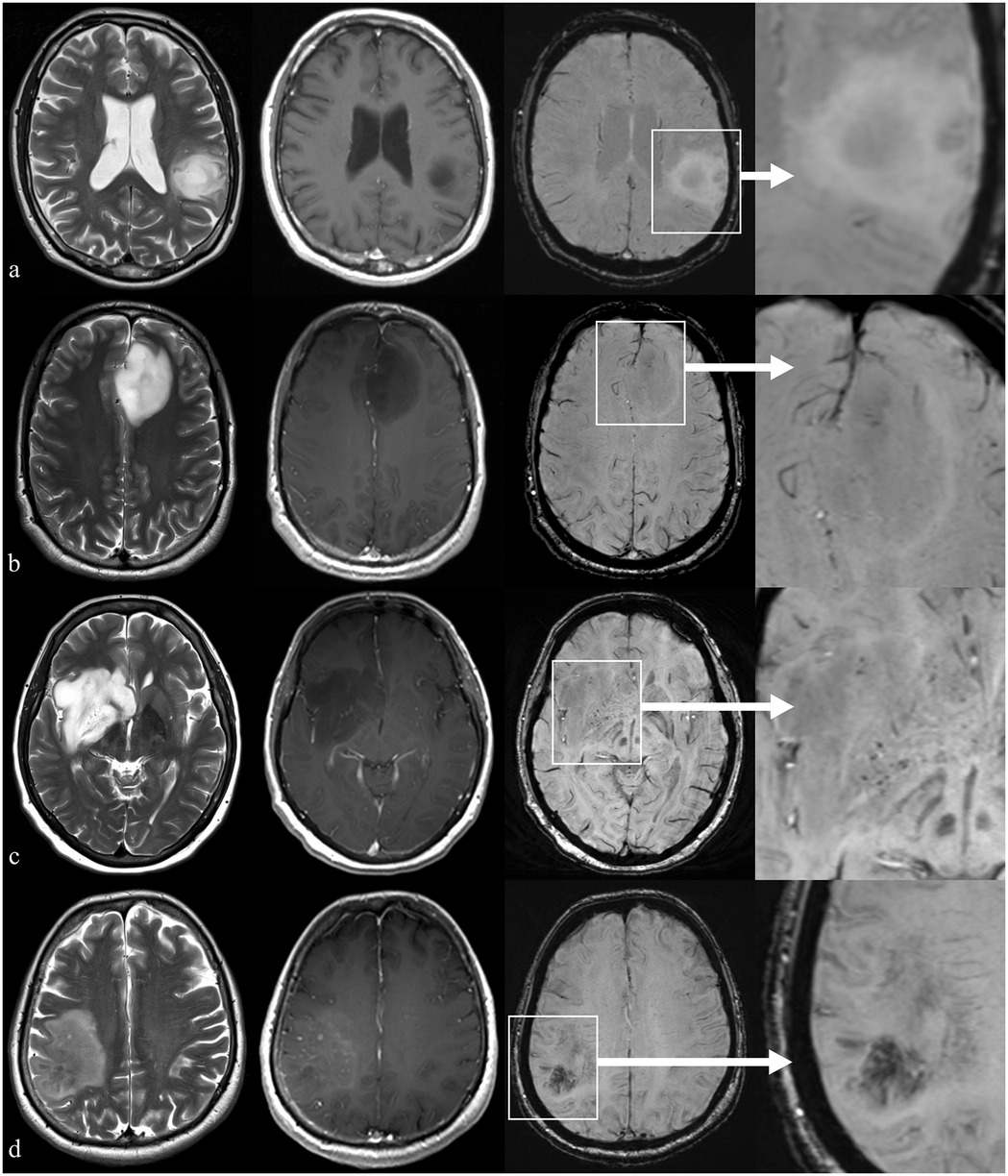
Figure 1. Example of patients with brain glioma presenting with different ITSS grades but similar findings on conventional imaging. First column: axial T2-weighted images; second column: axial T1-weighted images acquired after i.v. injection of gadolinium-based contrast medium; third column: axial 3D susceptibility-weighted images (SWI); last column: a magnified view of the area of interest on SWI. Row (a) parieto-temporal glioma infiltrating the left supramarginal and angular gyri in a 47 y.o. right-handed male showing no relevant findings on SWI (ITSS grade 0); row (b) left frontal glioma infiltrating the anterior cingulate gyrus in a 36 y.o. male with a cluster of (<5) tiny hypointense black dots in SWI (ITSS grade 1); row (c) right basal ganglia and insular glioma in a 55 y.o. female showing multiple (<10) millimetric intralesional hypointense spots on SWI (ITSS grade 2); row (d) right parietal glioma glioma in a 72 y.o. male showing diffuse hypointense subcortical deposits on SWI (ITSS grade 3).
High-grade gliomas (HGGs) exhibit more prominent ITSSs due to their aggressive nature. These tumors invade fragile and abnormal blood vessels, leading to microbleeds, as well as in regions of micro- and macro-necrosis (26).
While rare in other glioma subtypes, intratumoral calcifications, better detected on SWI compared to GRE, are more common in IDH mutant, 1p/19q codeleted astrocytomas (oligodendrogliomas) (27, 28). In some cases, phase images can help distinguish between calcifications and microbleeds, offering a valid tool in differentiation of this subtype of glioma (25). Other potential causes of intratumoral calcification include products of previous intratumoral bleeding or treatment-related changes (29).
The first attempt to grade ITSS in gliomas was proposed by Park et al. which proposed a semi-quantitative approach consisting of counting ITSSs on the slice where they are most conspicuous: grade 0, no ITSS; grade 1, 1–5 ITSSs; grade 2, 6–10 ITSSs; and grade 3, ≥11 ITSSs (Table 1) (8).
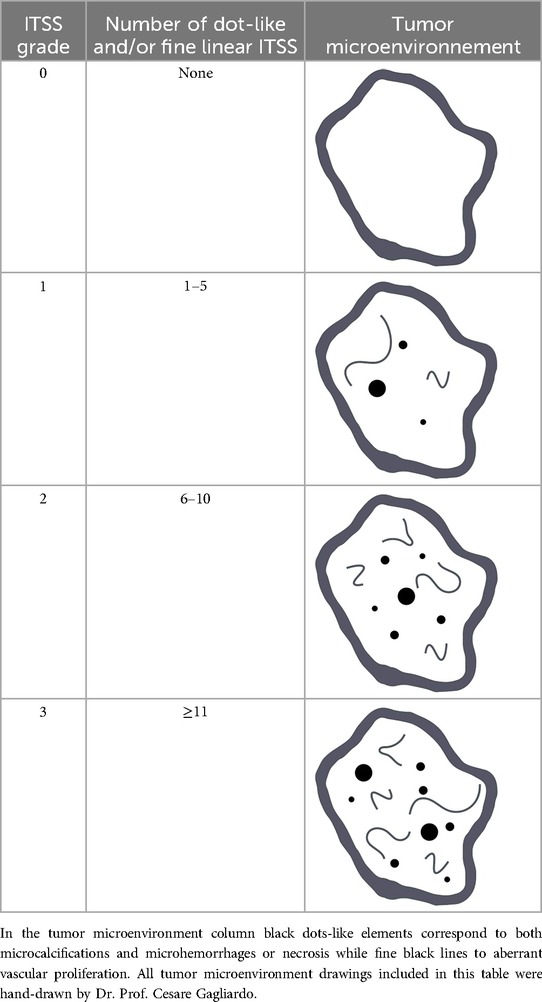
Table 1. ITSS grading scale (0–3) according to Park et al. (8).
Several studies have highlighted the potential of SWI as an accurate tool for differentiating solitary expansive brain lesions (30, 31). Particularly, HGGs exhibit a higher grade of ITSSs compared to primary CNS lymphoma (PCNSL) (30, 32, 33) and other non-neoplastic lesions (e.g., brain abscesses or tumefactive demyelinating lesions). Metastases demonstrate intermediate ITSSs grades, lower than HGGs but higher than those of PCNSL (34–36).
Focusing on gliomas, the number and patterns of ITSSs vary according to tumor grade. HGGs exhibit more prominent vascular structures (fine-linear ITSSs, often with a conglomerated pattern), microhemorrhages (dot-like structures) and areas of necrosis, compared to low-grade gliomas (LGGs), reflecting the more aggressive biological behaviour of these tumors (37–40). Hori et al. showed that the ratio between total ITSSs volume and tumor volume differentiates HGGs (grades 3 and 4) from LGGs (grades 1 and 2) (41). Yang et al. applied this approach to IDH-mutant astrocytomas, finding a greater number of ITSSs in grade 4 IDH-mutant astrocytomas compared to grade 2 and/or 3 (42). Bhattacharjee et al. attempt to discriminate between vascular and micro hemorrhagic components of ITSSs using R2* values. They found that the ITSS vasculature volume (IVV) could significantly differentiate not only HGGs from LGGs but also distinguish between different degrees of malignancy (grades 2 vs. 3, 2 vs. 4, and 3 vs. 4) (43). Thus, in adult patients, the quantity and heterogeneity of ITSSs increase with tumor grade, allowing differentiation between LGGs (with minimal dot-like and sparse linear ITSS) and HGGs (characterized by abundant ITSSs, often with a conglomerated pattern). However, this relationship is less consistent in the pediatric population. Gaudino et al. demonstrated that the absence of ITSSs often correlated with low-grade tumors in children. Conversely, the presence of ITSSs was not always indicative of high-grade tumors due to the extreme histological heterogeneity of pediatric brain tumors (44). Kong et al. demonstrated that ITSS levels are significantly higher in HGGs compared to LGGs. Furthermore, gliomas with IDH1 mutations exhibit lower ITSS grades than their IDH wild-type counterparts. Additionally, MGMT-methylated gliomas display lower ITSS grades than MGMT-unmethylated ones. However, no significant differences in ITSS grade were observed based on 1p/19q co-deletion status in the same study (45). Moreover, higher ITSS levels correlate with an elevated Ki-67 labeling index (Ki-67 LI) and ATRX gene wild-type status, both of which are markers of poorer prognosis in IDH-mutant astrocytomas (46).
As mentioned earlier, ITSS correlates with several biomolecular markers, providing a valuable prognostic imaging tool in glioma evaluation.
Highly vascularised gliomas are more responsive to antiangiogenic drugs compared to those with less vascularization or those that are necrotic (47). Thus, high levels of ITSSs—reflecting tumor aggressiveness and vascularity—may help in assessing the potential response to chemotherapy or radiotherapy in gliomas, in combination with other advanced MRI techniques such as DWI and PWI (48).
Lupo et al. demonstrated that the percentage of hypointense signals within contrast-enhancing lesions on susceptibility-weighted imaging can predict response to a treatment regimen combining anti-angiogenic therapy (enzastaurin), cytotoxic agents (temozolamide), and radiation therapy in gliomas (49).
Additionally, evaluating ITSS during follow-up imaging may provide valuable insights into therapeutic effects of chemotherapy and radiotherapy. A reduction in ITSS can signal a positive response, while an increase in ITSS may indicate tumor recurrence or progression, as well as treatment-related changes such as necrosis and calcifications. Furthermore, ITSS evaluation helps monitor vascular structural changes in glioblastomas, tracking the effects of anti-angiogenic therapy, cytotoxic chemotherapy, and radiation therapy in vivo, making it an effective tool for neuroradiological follow-up, as indicated by Grabner et al. (50).
However, Martucci et al. reported that, while the angiogenic profile of primary glioblastomas, measured using perfusion weighted imaging, may serve as an MRI biomarker for regorafenib response in recurrent glioblastomas but no significant correlation was observed between ITSS grade and regorafenib response was reported (51).
Recently, even neurosurgical research has explored the potential role of ITSS in planning brain tumor biopsies. Specifically, a significant correlation has been observed between high ITSS grades (particularly grade 3) and the risk of significant hemorrhage following stereotactic biopsy (STB) (52, 53).
Okamoto et al. suggested that ITSS grade (in combination with tumor volume) influences intraoperative blood loss during the surgical resection of pediatric posterior-fossa tumors (54). These findings support the routine use of SWI in pre-biopsy planning to perform safer biopsy techniques (open or endoscopic) and more accurate hemostasis in tumors with higher ITSS grades. Additionally, when multiple candidate biopsy sites are available, prioritizing areas with lower ITSS grades may offer a safer approach.
Nowadays, ITSS has become a well-recognized biomarker in neuro-oncology, playing a crucial role for glioma evaluation. However, several challenges remain in its application in routine clinical practice, primarily the lack of a standardized quantification method.
The semi-quantitative approach introduced by Park et al. was the first attempt to standardize ITSS evaluation. This method involves manually counting ITSSs on the slice where they are most conspicuous (8). While this approach is straightforward and easy to use, it has notable limitations, including a lack of comprehensive visualization of the tumor and significant operator dependency, which implicates subjectivity to the measurements.
To include comprehensive evaluation of the tumor, Hori et al. proposed a grading system based on the ratio of total SWI hypointense volume to overall tumor volume (41).
Radbruch et al. aimed to reduce operator dependence by proposing a percentage-wise quantification method for ITSS using automated post-processing techniques. This method has proven particularly valuable in differentiating between specific types of brain metastases (55, 56).
Moreover, Quantitative Susceptibility Mapping (QSM) has emerged as a promising tool to overcome the limitations of semi-quantitative methods. By providing quantitative measures of magnetic susceptibility, QSM eliminates operator dependency and can differentiate between distinct sources of ITSS, such as hemorrhage, calcification, and other susceptibility effects (25, 55, 57, 58).
As mentioned earlier, Bhattacharjee et al. proposed a quantitative approach that calculates the ITSS vasculature volume (IVV) within tumors. This technique leverages R2* values to filter out hemorrhagic contributions, offering a more precise evaluation of intratumoral microvasculature (43).
Recently, the fractal dimension (FD), introduced by Di Ieva et al., represents another innovative parameter derived from computational fractal-based analyses. Initially developed on a 7 T MRI scanner, this technique provides detailed reconstructions of the geometric architecture of SWI hypointensities. The FD offers an “architectural fingerprint” of gliomas, useful for both diagnostic and follow-up purposes (59, 60). Indeed, higher FD values correlate with higher glioma grades, reflecting increased complexity and vascular density. Furthermore, FD may be used to assess therapeutic effects, particularly in antiangiogenic therapies, by indicating favorable effects (such as decreased intratumoral microvasculature) or unfavorable results (such as increased microvasculature) (60).
ITSS patterns in gliomas necessitate careful interpretation, as the low signal intensity, particularly in dot-shaped lesions, may be attributable to microhemorrhages or calcifications on conventional magnitude images.
SWI parameters, including field strength and sequence settings, directly influence ITSS visibility as magnetic field strength increases, the number of identified ITSSs consequently rises. Therefore, it is recommended to use the same MRI system for follow-up studies, particularly for the same patients, to ensure consistency and reliability of results.
Protocol standardization is crucial, and the development of standardized or automated methods is necessary to improve the reproducibility of ITSS assessment. In this regard, artificial intelligence (AI)-based approaches, particularly deep learning algorithms for image acquisition, are being integrated into nearly all new MRI systems, helping reduce acquisition times and improve image quality (61, 62).
Longitudinal multicenter studies conducted with equipment from different vendors but with homogeneous protocols are essential to validate ITSS as a biomarker for glioma progression and treatment response. Advances in imaging technologies, such as ultra-high-field MRI, may further enhance ITSS detection. Although, on the other hand, the possibility of identifying ITSS even with scanners operating at 1.5 T, routinely used in the clinical setting outside of research and academic scenarios, makes this topic worthy of further research in differential diagnosis, grading, and molecular profiling of brain gliomas. Moreover, the implementation of ITSS paves the way for the possibility of a non-contrast brain tumor imaging protocol for patients who either have contraindications to contrast agents or are unable to tolerate bolus injections (8).
Additionally, integrating ITSS analysis with molecular and genetic profiling will significantly improve personalized management strategies for glioma patients especially if we consider modern and emerging new theranostics approaches like for instance transcranial focused ultrasound that offers non-invasive way to target tumors and enhancing drug delivery by modulating the blood-brain barrier (63).
Furthermore, nuclear medicine, combined with the evaluation of Intra-Tumoral Susceptibility Signals (ITSS), could significantly advance the diagnosis and treatment of brain gliomas. Nuclear imaging modalities such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT) can provide functional and metabolic insights into tumor behavior, while ITSS analysis offers detailed structural and vascular information. Integrating these approaches could enhance tumor characterization, enabling precise localization, grading, and differentiation of gliomas. Furthermore, radiotheranostics could leverage ITSS data to optimize targeted delivery of therapeutic isotopes, maximizing treatment efficacy and minimizing damage to healthy tissue. This synergy holds promise for a more personalized and effective approach to managing brain gliomas (64).
Finally, calling into question a very hot topic in recent years, radiomics and artificial intelligence (AI) could revolutionize tumor diagnosis by enhancing the evaluation of Intra-Tumoral Susceptibility Signals (ITSS). Radiomics extracts quantitative features from medical images, such as geometrical features, textures, and intensities, that may not be visible to the human eye, enabling a deeper analysis of ITSS characteristics like different vascular abnormalities or iron deposition. Machine learning as well as deep learning algorithms can process this data to identify complex patterns, classify tumor subtypes, and predict aggressiveness or response to treatment with high accuracy. Together, radiomics and AI offer a powerful, non-invasive approach to improve diagnostic precision, facilitate personalized treatment planning, and advance our understanding of tumor heterogeneity (65–67).
ITSS can be seamlessly integrated into standard MRI protocols for both pre- and post-treatment evaluation of gliomas, providing a distinctive insight into tumor biology that correlates with key pathological features and clinical outcomes. It serves as a valuable biomarker, complementing findings from conventional and advanced neuroimaging techniques.
ITSS supports glioma differential diagnosis, grading, subtype differentiation, and treatment management, thereby contributing to precision and personalized medicine. Although challenges remain—particularly related to technical limitations and the lack of a unified model for ITSSs quantification—ongoing advancements in imaging technology and computational analysis, as well as longitudinal studies in this field, are expected to fully unlock the potential of ITSS in glioma management. Although the sensitivity in identifying ITSS increases with magnetic field intensity, the possibility of identifying such findings even with MRI scanners routinely used in clinical settings operating at 1.5 T, makes this imaging biomarker potentially increasingly important during neuroradiological evaluations and it is hoped that future studies on larger samples may further define the role of ITSS in the differential diagnosis, grading, and molecular profiling of brain gliomas.
SC: Methodology, Writing – original draft, Writing – review & editing, Conceptualization. PF: Methodology, Writing – review & editing. MauM: Writing – review & editing. PA: Writing – review & editing. LG: Writing – review & editing. LL: Writing – review & editing. GC: Writing – review & editing. TB: Writing – review & editing. MasM: Writing – review & editing. CG: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that two of them (PF and PA) were editorial board members of Frontiers at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Osborn AG, Louis DN, Poussaint TY, Linscott LL, Salzman KL. The 2021 world health organization classification of tumors of the central nervous system: what neuroradiologists need to know. AJNR Am J Neuroradiol. (2022) 43(7):928–37. doi: 10.3174/ajnr.A7462
2. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
3. Gritsch S, Batchelor TT, Gonzalez Castro LN. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer. (2022) 128(1):47–58. doi: 10.1002/cncr.33918
4. Yang X, Lin Y, Xing Z, She D, Su Y, Cao D. Predicting 1p/19q codeletion status using diffusion-, susceptibility-, perfusion-weighted, and conventional MRI in IDH-mutant lower-grade gliomas. Acta Radiol. (2021) 62(12):1657–65. doi: 10.1177/0284185120973624
5. Halefoglu AM, Camurcuoglu E, Tanik C, Kizilkaya O, Yilmaz A. Predictive role of magnetic resonance imaging in the distinction of isocitrate dehydrogenase (IDH) mutant grade 4 astrocytomas versus glioblastomas. Acta Radiol. (2023) 64(6):2074–86. doi: 10.1177/02841851231165282
6. Haacke EM, Mittal S, Wu Z, Neelavalli J, Cheng YC. Susceptibility-weighted imaging: technical aspects and clinical applications, part 1. AJNR Am J Neuroradiol. (2009) 30(1):19–30. doi: 10.3174/ajnr.A1400
7. Cheng AL, Batool S, McCreary CR, Lauzon ML, Frayne R, Goyal M, et al. Susceptibility-weighted imaging is more reliable than T2*-weighted gradient-recalled echo MRI for detecting microbleeds. Stroke. (2013) 44(10):2782–6. doi: 10.1161/STROKEAHA.113.002267
8. Park MJ, Kim HS, Jahng GH, Ryu CW, Park SM, Kim SY. Semiquantitative assessment of intratumoral susceptibility signals using non-contrast-enhanced high-field high-resolution susceptibility-weighted imaging in patients with gliomas: comparison with MR perfusion imaging. AJNR Am J Neuroradiol. (2009) 30(7):1402–8. doi: 10.3174/ajnr.A1593
9. Han S, Liu Y, Cai SJ, Qian M, Ding J, Larion M, et al. IDH Mutation in glioma: molecular mechanisms and potential therapeutic targets. Br J Cancer. (2020) 122(11):1580–9. doi: 10.1038/s41416-020-0814-x
10. Liu Y, Lang F, Chou FJ, Zaghloul KA, Yang C. Isocitrate dehydrogenase mutations in glioma: genetics, biochemistry, and clinical indications. Biomedicines. (2020) 8(9):294. doi: 10.3390/biomedicines8090294
11. Kotecha R, Schiff D, Chakravarti A, Fleming JL, Brown PD, Puduvalli VK, et al. Multidisciplinary management of isocitrate dehydrogenase-mutated gliomas in a contemporary molecularly defined era. J Clin Oncol. (2024) 42(21):2588–98. doi: 10.1200/JCO.23.02195
12. Sasaki H, Kitamura Y, Toda M, Hirose Y, Oligodendroglioma YK. IDH-mutant and 1p/19q-codeleted-prognostic factors, standard of care and chemotherapy, and future perspectives with neoadjuvant strategy. Brain Tumor Pathol. (2024) 41(2):43–9. doi: 10.1007/s10014-024-00480-1
13. Bell RJ, Rube HT, Xavier-Magalhães A, Costa BM, Mancini A, Song JS, et al. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. (2016) 14(4):315–23. doi: 10.1158/1541-7786.MCR-16-0003
14. Hua R, Li Q, Gao H, Wang B, He C, Wang Y, et al. Association of human telomerase reverse transcriptase promoter mutation with unfavorable prognosis in glioma: a systematic review and meta-analysis. J Res Med Sci. (2023) 28:47. doi: 10.4103/jrms.jrms_371_22
15. Powter B, Jeffreys SA, Sareen H, Cooper A, Brungs D, Po J, et al. Human TERT promoter mutations as a prognostic biomarker in glioma. J Cancer Res Clin Oncol. (2021) 147(4):1007–17. doi: 10.1007/s00432-021-03536-3
16. Mansouri A, Hachem LD, Mansouri S, Nassiri F, Laperriere NJ, Xia D, et al. MGMT Promoter methylation status testing to guide therapy for glioblastoma: refining the approach based on emerging evidence and current challenges. Neuro Oncol. (2019) 21(2):167–78. doi: 10.1093/neuonc/noy132
17. Philteos J, Karmur BS, Mansouri A. MGMT testing in glioblastomas: pitfalls and opportunities. Am J Clin Oncol. (2019) 42(2):117–22. doi: 10.1097/COC.0000000000000490
18. Butler M, Pongor L, Su YT, Xi L, Raffeld M, Quezado M, et al. MGMT status as a clinical biomarker in glioblastoma. Trends Cancer. (2020) 6(5):380–91. doi: 10.1016/j.trecan.2020.02.010
19. McAleenan A, Kelly C, Spiga F, Kernohan A, Cheng HY, Dawson S, et al. Prognostic value of test(s) for O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation for predicting overall survival in people with glioblastoma treated with temozolomide. Cochrane Database Syst Rev. (2021) 3(3):CD013316. doi: 10.1002/14651858.CD013316.pub2
20. Andrés-Sánchez N, Fisher D, Krasinska L. Physiological functions and roles in cancer of the proliferation marker Ki-67. J Cell Sci. (2022) 135(11):jcs258932. doi: 10.1242/jcs.258932
21. Chen WJ, He DS, Tang RX, Ren FH, Chen G. Ki-67 is a valuable prognostic factor in gliomas: evidence from a systematic review and meta-analysis. Asian Pac J Cancer Prev. (2015) 16(2):411–20. doi: 10.7314/apjcp.2015.16.2.411
22. Nandakumar P, Mansouri A, Das S. The role of ATRX in glioma biology. Front Oncol. (2017) 7:236. doi: 10.3389/fonc.2017.00236
23. Haase S, Garcia-Fabiani MB, Carney S, Altshuler D, Núñez FJ, Méndez FM, et al. Mutant ATRX: uncovering a new therapeutic target for glioma. Expert Opin Ther Targets. (2018) 22(7):599–613. doi: 10.1080/14728222.2018.1487953
24. Ohba S, Kuwahara K, Yamada S, Abe M, Hirose Y. Correlation between IDH, ATRX, and TERT promoter mutations in glioma. Brain Tumor Pathol. (2020) 37(2):33–40. doi: 10.1007/s10014-020-00360-4
25. Haller S, Haacke EM, Thurnher MM, Barkhof F. Susceptibility-weighted imaging: technical essentials and clinical neurologic applications. Radiology. (2021) 299(1):3–26. doi: 10.1148/radiol.2021203071
26. Mohammed W, Xunning H, Haibin S, Jingzhi M. Clinical applications of susceptibility-weighted imaging in detecting and grading intracranial gliomas: a review. Cancer Imaging. (2013) 13(2):186–95. doi: 10.1102/1470-7330.2013.0020
27. Lin Y, Xing Z, She D, Yang X, Zheng Y, Xiao Z, et al. IDH Mutant and 1p/19q co-deleted oligodendrogliomas: tumor grade stratification using diffusion-, susceptibility-, and perfusion-weighted MRI. Neuroradiology. (2017) 59(6):555–62. doi: 10.1007/s00234-017-1839-6
28. Zulfiqar M, Dumrongpisutikul N, Intrapiromkul J, Yousem DM. Detection of intratumoral calcification in oligodendrogliomas by susceptibility-weighted MR imaging. AJNR Am J Neuroradiol. (2012) 33(5):858–64. doi: 10.3174/ajnr.A2862
29. Qin D, Yang G, Jing H, Tan Y, Zhao B, Zhang H. Tumor progression and treatment-related changes: radiological diagnosis challenges for the evaluation of post treated glioma. Cancers (Basel). (2022) 14(15):3771. doi: 10.3390/cancers14153771
30. Peters S, Knöß N, Wodarg F, Cnyrim C, Jansen O. Glioblastomas vs. Lymphomas: more diagnostic certainty by using susceptibility-weighted imaging (SWI). Rofo. (2012) 184(8):713–8. doi: 10.1055/s-0032-1312862
31. Kim HS, Jahng GH, Ryu CW, Kim SY. Added value and diagnostic performance of intratumoral susceptibility signals in the differential diagnosis of solitary enhancing brain lesions: preliminary study. AJNR Am J Neuroradiol. (2009) 30(8):1574–9. doi: 10.3174/ajnr.A1635
32. Saini J, Kumar Gupta P, Awasthi A, Pandey CM, Singh A, Patir R, et al. Multiparametric imaging-based differentiation of lymphoma and glioblastoma: using T1-perfusion, diffusion, and susceptibility-weighted MRI. Clin Radiol. (2018) 73(11):986.e7–e15. doi: 10.1016/j.crad.2018.07.107
33. Sakata A, Okada T, Yamamoto A, Kanagaki M, Fushimi Y, Dodo T, et al. Primary central nervous system lymphoma: is absence of intratumoral hemorrhage a characteristic finding on MRI? Radiol Oncol. (2015) 49(2):128–34. doi: 10.1515/raon-2015-0007
34. Fu JH, Chuang TC, Chung HW, Chang HC, Lin HS, Hsu SS, et al. Discriminating pyogenic brain abscesses, necrotic glioblastomas, and necrotic metastatic brain tumors by means of susceptibility-weighted imaging. Eur Radiol. (2015) 25(5):1413–20. doi: 10.1007/s00330-014-3518-x
35. Lai PH, Chung HW, Chang HC, Fu JH, Wang PC, Hsu SH, et al. Susceptibility-weighted imaging provides complementary value to diffusion-weighted imaging in the differentiation between pyogenic brain abscesses, necrotic glioblastomas, and necrotic metastatic brain tumors. Eur J Radiol. (2019) 117:56–61. doi: 10.1016/j.ejrad.2019.05.021
36. Toh CH, Wei KC, Chang CN, Hsu PW, Wong HF, Ng SH, et al. Differentiation of pyogenic brain abscesses from necrotic glioblastomas with use of susceptibility-weighted imaging. AJNR Am J Neuroradiol. (2012) 33(8):1534–8. doi: 10.3174/ajnr.A2986
37. Zhang H, Tan Y, Wang XC, Qing JB, Wang L, Wu XF, et al. Susceptibility-weighted imaging: the value in cerebral astrocytomas grading. Neurol India. (2013) 61(4):389–95. doi: 10.4103/0028-3886.117617
38. Li C, Ai B, Li Y, Qi H, Wu L. Susceptibility-weighted imaging in grading brain astrocytomas. Eur J Radiol. (2010) 75(1):e81–5. doi: 10.1016/j.ejrad.2009.08.003
39. Tavakoli MB, Khorasani A, Jalilian M. Improvement grading brain glioma using T2 relaxation times and susceptibility-weighted images in MRI. Inform Med Unlocked. (2023) 37(5):101201. doi: 10.1016/j.imu.2023.101201
40. Martín-Noguerol T, Santos-Armentia E, Ramos A, Luna A. An update on susceptibility-weighted imaging in brain gliomas. Eur Radiol. (2024) 34(10):6763–75. doi: 10.1007/s00330-024-10703-w
41. Hori M, Mori H, Aoki S, Abe O, Masumoto T, Kunimatsu S, et al. Three-dimensional susceptibility-weighted imaging at 3 T using various image analysis methods in the estimation of grading intracranial gliomas. Magn Reson Imaging. (2010) 28(4):594–8. doi: 10.1016/j.mri.2010.01.002
42. Yang X, Xing Z, She D, Lin Y, Zhang H, Su Y, et al. Grading of IDH-mutant astrocytoma using diffusion, susceptibility and perfusion-weighted imaging. BMC Med Imaging. (2022) 22(1):105. doi: 10.1186/s12880-022-00832-3
43. Bhattacharjee R, Gupta RK, Patir R, Vaishya S, Ahlawat S, Singh A. Quantitative vs. semiquantitative assessment of intratumoral susceptibility signals in patients with different grades of glioma. J Magn Reson Imaging. (2020) 51(1):225–33. doi: 10.1002/jmri.26786
44. Gaudino S, Marziali G, Pezzullo G, Guadalupi P, Giordano C, Infante A, et al. Role of susceptibility-weighted imaging and intratumoral susceptibility signals in grading and differentiating pediatric brain tumors at 1.5 T: a preliminary study. Neuroradiology. (2020) 62(6):705–13. doi: 10.1007/s00234-020-02386-z
45. Kong LW, Chen J, Zhao H, Yao K, Fang SY, Wang Z, et al. Intratumoral susceptibility signals reflect biomarker status in gliomas. Sci Rep. (2019) 9(1):17080. doi: 10.1038/s41598-019-53629-w
46. Yang X, Hu C, Xing Z, Lin Y, Su Y, Wang X, et al. Prediction of Ki-67 labeling index, ATRX mutation, and MGMT promoter methylation status in IDH-mutant astrocytoma by morphological MRI, SWI, DWI, and DSC-PWI. Eur Radiol. (2023) 33(10):7003–14. doi: 10.1007/s00330-023-09695-w
47. Kong Z, Yan C, Zhu R, Wang J, Wang Y, Wang Y, et al. Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas. Neuroimage Clin. (2018) 20:51–60. doi: 10.1016/j.nicl.2018.07.001
48. Thust SC, Heiland S, Falini A, Jäger HR, Waldman AD, Sundgren PC, et al. Glioma imaging in Europe: a survey of 220 centres and recommendations for best clinical practice. Eur Radiol. (2018) 28(8):3306–17. doi: 10.1007/s00330-018-5314-5
49. Lupo JM, Essock-Burns E, Molinaro AM, Cha S, Chang SM, Butowski N, et al. Using susceptibility-weighted imaging to determine response to combined anti-angiogenic, cytotoxic, and radiation therapy in patients with glioblastoma multiforme. Neuro Oncol. (2013) 15(4):480–9. doi: 10.1093/neuonc/nos325
50. Grabner G, Nöbauer I, Elandt K, Kronnerwetter C, Woehrer A, Marosi C, et al. Longitudinal brain imaging of five malignant glioma patients treated with bevacizumab using susceptibility-weighted magnetic resonance imaging at 7T. Magn Reson Imaging. (2012) 30(1):139–47. doi: 10.1016/j.mri.2011.08.004
51. Martucci M, Ferranti AM, Schimperna F, Infante A, Magnani F, Olivi A, et al. Magnetic resonance imaging-derived parameters to predict response to regorafenib in recurrent glioblastoma. Neuroradiology. (2023) 65(10):1439–45. doi: 10.1007/s00234-023-03169-y
52. Tanji M, Mineharu Y, Sakata A, Okuchi S, Fushimi Y, Oishi M, et al. High intratumoral susceptibility signal grade on susceptibility-weighted imaging: a risk factor for hemorrhage after stereotactic biopsy. J Neurosurg. (2023) 138(1):120–7. doi: 10.3171/2022.4.JNS212505
53. Sugii N, Matsuda M, Tsurubuchi T, Ishikawa E. Hemorrhagic complications after brain tumor biopsy: risk-reduction strategies based on safer biopsy targets and techniques. World Neurosurg. (2023) 176:e254–64. doi: 10.1016/j.wneu.2023.05.046
54. Okamoto T, Yamanaka T, Takeuchi H, Takahashi Y, Tanigawa S, Nakasho T, et al. Prediction of intraoperative blood loss in pediatric posterior fossa tumors by neuroradiological evaluation: preliminary study. Neurochirurgie. (2024) 70(6):101592. doi: 10.1016/j.neuchi.2024.101592
55. Reichenbach JR, Schweser F, Serres B, Deistung A. Quantitative susceptibility mapping: concepts and applications. Clin Neuroradiol. (2015) 25(Suppl 2):225–30. doi: 10.1007/s00062-015-0432-9
56. Radbruch A, Graf M, Kramp L, Wiestler B, Floca R, Bäumer P, et al. Differentiation of brain metastases by percentagewise quantification of intratumoral-susceptibility-signals at 3Tesla. Eur J Radiol. (2012) 81(12):4064–8. doi: 10.1016/j.ejrad.2012.06.016
57. Deistung A, Schweser F, Wiestler B, Abello M, Roethke M, Sahm F, et al. Quantitative susceptibility mapping differentiates between blood depositions and calcifications in patients with glioblastoma. PLoS One. (2013) 8(3):e57924. doi: 10.1371/journal.pone.0057924
58. Zeng S, Ma H, Xie D, Huang Y, Wang M, Zeng W, et al. Quantitative susceptibility mapping evaluation of glioma. Eur Radiol. (2023) 33(10):6636–47. doi: 10.1007/s00330-023-09647-4
59. Di Ieva A, Göd S, Grabner G, Grizzi F, Sherif C, Matula C, et al. Three-dimensional susceptibility-weighted imaging at 7T using fractal-based quantitative analysis to grade gliomas. Neuroradiology. (2013) 55(1):35–40. doi: 10.1007/s00234-012-1081-1
60. Di Ieva A. Computational fractal-based analysis of MR susceptibility-weighted imaging (SWI) in neuro-oncology and neurotraumatology. Adv Neurobiol. (2024) 36:445–68. doi: 10.1007/978-3-031-47606-8_23
61. Kames C, Doucette J, Birkl C, Rauscher A. Recovering SWI-filtered phase data using deep learning. Magn Reson Med. (2022) 87(2):948–59. doi: 10.1002/mrm.29013
62. Genc O, Morrison MA, Villanueva-Meyer JE, Burns B, Hess CP, Banerjee S, et al. DeepSWI: using deep learning to enhance susceptibility contrast on T2*-weighted MRI. J Magn Reson Imaging. (2023) 58(4):1200–10. doi: 10.1002/jmri.28622
63. Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep. (2019) 9(1):321. doi: 10.1038/s41598-018-36340-0.30674905
64. Tolboom N, Verger A, Albert NL, Brendel M, Cecchin D, Fernandez PA, et al. EANM Position paper: theranostics in brain tumours-the present and the future. Eur J Nucl Med Mol Imaging. (2023) 51(1):202–5. doi: 10.1007/s00259-023-06425-8
65. Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. (2014) 5:4006. doi: 10.1038/ncomms5006
66. Sotoudeh H, Shafaat O, Bernstock JD, Brooks MD, Elsayed GA, Chen JA, et al. Artificial intelligence in the management of glioma: era of personalized medicine. Front Oncol. (2019) 9:768. doi: 10.3389/fonc.2019.00768
Keywords: magnetic resonance imaging, susceptibility-weighted imaging, intra-tumoral susceptibility signals, brain, glioma
Citation: Cataldi S, Feraco P, Marrale M, Alongi P, Geraci L, La Grutta L, Caruso G, Bartolotta TV, Midiri M and Gagliardo C (2025) Intra-tumoral susceptibility signals in brain gliomas: where do we stand? Front. Radiol. 5:1546069. doi: 10.3389/fradi.2025.1546069
Received: 16 December 2024; Accepted: 28 January 2025;
Published: 20 February 2025.
Edited by:
Federico Bruno, San Salvatore Hospital, ItalyReviewed by:
Fabio Martino Doniselli, IRCCS Carlo Besta Neurological Institute Foundation, ItalyCopyright: © 2025 Cataldi, Feraco, Marrale, Alongi, Geraci, La Grutta, Caruso, Bartolotta, Midiri and Gagliardo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cesare Gagliardo, Y2VzYXJlLmdhZ2xpYXJkb0B1bmlwYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.