
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Radiol., 20 February 2024
Sec. Interventional Radiology
Volume 4 - 2024 | https://doi.org/10.3389/fradi.2024.1346550
 Julia Wagenpfeil1,2*†
Julia Wagenpfeil1,2*† Patrick Arthur Kupczyk1,2
Patrick Arthur Kupczyk1,2 Philipp Bruners2,3
Philipp Bruners2,3 Robert Siepmann2,3
Robert Siepmann2,3 Emelie Guendel1,2
Emelie Guendel1,2 Julian Alexander Luetkens1,2
Julian Alexander Luetkens1,2 Alexander Isaak1,2
Alexander Isaak1,2 Carsten Meyer1,2
Carsten Meyer1,2 Fabian Kuetting2
Fabian Kuetting2 Claus Christian Pieper1,2
Claus Christian Pieper1,2 Ulrike Irmgard Attenberger1,2
Ulrike Irmgard Attenberger1,2 Daniel Kuetting1,2
Daniel Kuetting1,2
Purpose: Due to a lack of data, there is an ongoing debate regarding the optimal frontline interventional therapy for unresectable hepatocellular carcinoma (HCC). The aim of the study is to compare the results of transarterial radioembolization (TARE) as the first-line therapy and as a subsequent therapy following prior transarterial chemoembolization (TACE) in these patients.
Methods: A total of 83 patients were evaluated, with 38 patients having undergone at least one TACE session prior to TARE [27 male; mean age 67.2 years; 68.4% stage Barcelona clinic liver cancer (BCLC) B, 31.6% BCLC C]; 45 patients underwent primary TARE (33 male; mean age 69.9 years; 40% BCLC B, 58% BCLC C). Clinical [age, gender, BCLC stage, activity in gigabecquerel (GBq), Child–Pugh status, portal vein thrombosis, tumor volume] and procedural [overall survival (OS), local tumor control (LTC), and progression-free survival (PFS)] data were compared. A regression analysis was performed to evaluate OS, LTC, and PFS.
Results: No differences were found in OS (95% CI: 1.12, P = 0.289), LTC (95% CI: 0.003, P = 0.95), and PFS (95% CI: 0.4, P = 0.525). The regression analysis revealed a relationship between Child–Pugh score (P = 0.005), size of HCC lesions (>10 cm) (P = 0.022), and OS; neither prior TACE (Child–Pugh B patients; 95% CI: 0.120, P = 0.729) nor number of lesions (>10; 95% CI: 2.930, P = 0.087) correlated with OS.
Conclusion: Prior TACE does not affect the outcome of TARE in unresectable HCC.
Hepatocellular carcinoma (HCC), the third leading cause of cancer-related mortality, constitutes 75%–85% of primary liver malignancies (1, 2). The main risk factors for HCC vary geographically but generally include chronic hepatitis B virus (HBV) and C virus (HCV), as well as alcohol-associated cirrhosis and non-alcoholic steatohepatitis (2–4). The type of therapy depends on several factors, e.g., HCC size and number of lesions, location, portal vein infiltration, and liver function.
Based on the individual Barcelona clinic liver cancer (BCLC) stage, the treatment of choice varies from resection/local ablation to chemotherapy/immunotherapy.
However, in most cases, HCC is diagnosed in advanced stages, highlighting the need for effective systemic therapies.
Patients with locally advanced tumor disease with vascular infiltration, especially in the presence of extrahepatic manifestations, have shown significant progress in treatment. One example is the use of oral multikinase inhibitors such as sorafenib, which inhibits multiple receptor tyrosine kinases in addition to intracellular kinases (Raf1/B-Raf); its efficacy and safety have been demonstrated in Phase II/III studies (5).
In recent years in particular, rapid progress has also been achieved in the field of immunotherapy with the approval of checkpoint inhibitors for the treatment of advanced HCC. Particularly noteworthy results were achieved via a combination therapy of a PDL-1 inhibitor and a VEGF antibody (atezolizumab and bevacizumab) (6).
For the intermediate-stage (BCLC B) patients, transarterial chemoembolization (TACE) is the preferred option (7, 8). Transarterial radioembolization (TARE), frequently performed as a second-line therapy in case of TACE failure, is preferred by some institutions as the primary treatment in BCLC B (9, 10). Although both TACE and TARE in HCC have been extensively studied, there is a dearth of data regarding the potential impact of prior TACE therapy on the outcome of TARE treatment.
The present study aims to compare the outcome of TARE treatment in patients with and without prior TACE.
In this multicenter retrospective study, patients with unresectable, non-metastasized hepatocellular carcinoma treated with TACE and TARE (Group A) or solely TARE (Group B) between February 2011 and July 2019 were included. The inclusion criteria were non-metastatic HCC with or without portal vein thrombosis, Child–Pugh stage A/B, BCLC stages A–C, Eastern Cooperative Oncology Group (ECOG) Stage 0, no prior intra-arterial treatment, and availability of procedural, clinical, and follow-up data. Patients who had received TACE therapy after TARE therapy were excluded from the study (Table 1).
A total of 199 patients were reviewed, and 116 patients were excluded. Overall, 83 patients were eligible. Thirty-eight consecutive patients initially underwent one or more TACE sessions before receiving TARE therapy; the control group consisted of 45 patients who received TARE without receiving prior chemoembolization treatment. Clinical [age, gender, BCLC stage, activity in gigabecquerel (GBq), Child–Pugh status, portal vein thrombosis, and tumor volume] and procedural [overall survival (OS), local tumor control (LTC), and progression-free survival (PFS)] data were analyzed and compared between the two groups as previously described (9). The therapy indication was confirmed by an interdisciplinary tumor board. The institutional ethics committee approved data analysis with a waiver for additional informed patient consent.
The baseline was established from the most recent computed tomography (CT) or magnetic resonance imaging (MRI) conducted prior to TARE. All patients underwent continuous follow-up, including clinical visits, PET CT, and liver MRI.
Tumor response assessment was defined using the Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (11). LTC was defined as the time until the progression of any tumor lesion within a treated segment following TARE. PFS was defined as the time between TARE and intra- or extrahepatic tumor progression. OS was determined as the time period from the first treatment date to either the date of death or the last date of follow-up.
OS, LTC, and PFS were assessed in all patients and compared between the two groups using the Chi-square test. Furthermore, subgroup analyses for OS and LTC were performed for BCLC stage B patients and compared between the two groups. Patients lost to follow-up were censored (contingency tables for proportional distribution). P-values < 0.05 were considered significant. A Cox regression analysis was conducted with covariates including age, BCLC stage, Child–Pugh score, activity in GBq, previous TACE seasons (number of TACE), and the size and number of HCC lesions to evaluate the impact on the outcome.
A total of 83 patients were included, and 38 patients received at least one TACE session prior to TARE therapy (Group A; 11 female, 27 male; mean age 67.2 years). Ten patients underwent one TACE therapy prior to TARE, 10 patients received two TACE treatments, seven patients received three treatments, five patients underwent four treatments, five patients received five treatments, and one patient underwent seven cycles of TACE. Partial portal vein thrombosis was detected in four patients (10.5%), and bilobar HCC manifestation was identified in 34 patients (89.5%). A total of 29 patients (73.3%) were classified as Child–Pugh A, while nine patients (23.7%) were classified as Child–Pugh B. None of the patients fell under the classification of Child–Pugh C. A total of 26 patients (68.4%) were graded stage BCLC B, and 12 patients (31.6%) were graded stage BCLC C (see Table 2 for details).
The control group consisted of 45 patients who received TARE therapy without prior TACE (Group B; 12 female, 33 male; mean age 69.9 years). Portal vein thrombosis was detected in 20 patients (44.4%), and bilobar HCC manifestation was observed in 33 patients (73.3%). Sixteen patients (35.6%) were classified as Child–Pugh A, 21 patients (46.7%) were classified as Child–Pugh B, and none of the patients were categorized as Child–Pugh C. Laboratory results were incomplete in eight patients (17.8%). Eighteen patients (40%) were graded as stage BCLC B, 26 patients (58%) were graded as stage BCLC C, and BCLC stage could not be defined (2%) in one patient (see Table 2 for details).
In the entire collective, the median OS was 375.7 ± 34 days (95% CI = 308–442). In Group A, the median OS was 421.6 ± 49.7 days (95% CI = 324–518). In Group B, the median OS was 334.8 ± 45.9 days (95% CI = 244–424); χ² = 1.126; P = 0.289 (Figure 1).
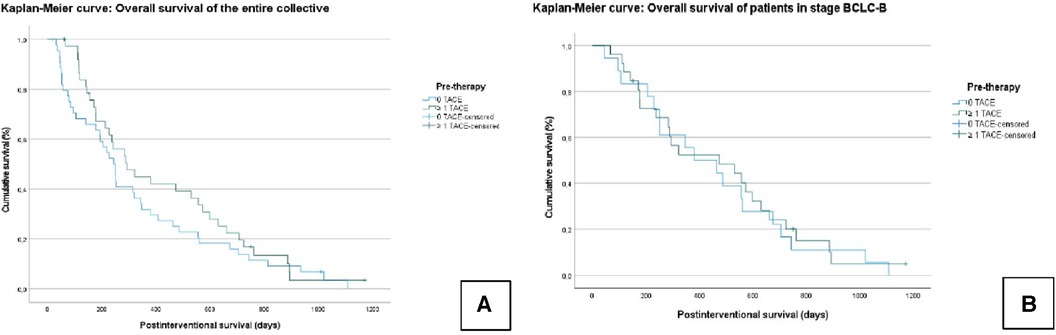
Figure 1. Kaplan–Meier curves: overall survival (OS) of the entire collective (A); overall survival of patients who graduated stage B (BCLC) (B). No significant difference is seen between the TACE/TARE and TARE-only groups.
In the subgroup analysis, the median OS in all BCLC B patients was 468.2 ± 46 days (95% CI = 377–558). In Group A, the median OS was 473.9 ± 60.6 days (95% CI = 355–592), and in Group B, the median OS was 457.3 ± 72.9 days (95% CI = 315–598); χ² = 0.123; P = 0.726 (Figure 1).
The LTC in the entire collective was 201.4 ± 26.2 days (95% CI = 150–252). In Group A, the LTC was 195.6 ± 32.3 days [95% confidence interval (CI) = 132–258], and in Group B, the LTC was 208 ± 43 days (95% CI = 123–292); χ² = 0.003; P = 0.956 (Figure 2).
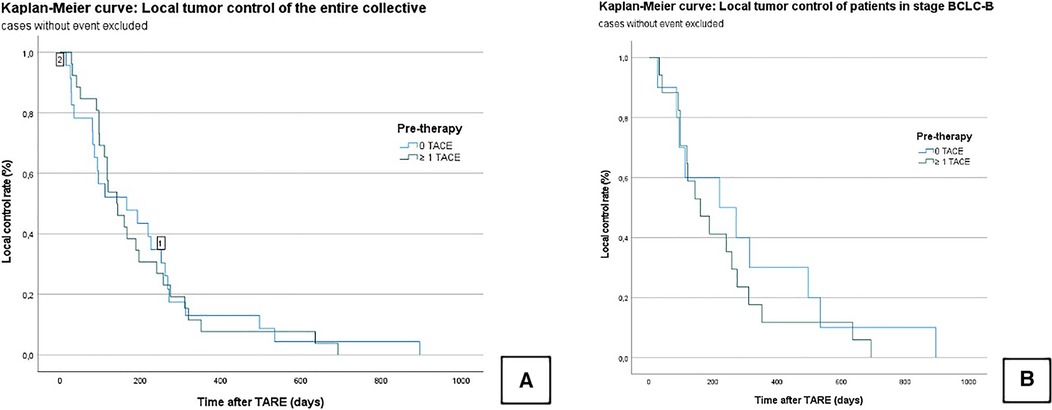
Figure 2. Kaplan–Meier curves: local tumor control of the censored entire collective (A); local tumor control of censored patients who graduated stage B (BCLC) (B). No significant difference is seen between the TACE/TARE and TARE-only group.
In the subgroup analysis, the median LTC in all BCLC B patients was 255.7 ± 42.5 days (95% CI = 172–338). In Group A, the median LTC was 305.1 ± 85.3 days (95% CI = 137–472), and in Group B, it was 226.6 ± 45.8 days (95% CI = 136–316); χ² = 0.568; P = 0.451 (Figure 2).
In the entire collective, the median PFS was 154.9 ± 19 days (95% CI = 117–192). In Group A, the median PFS was 134.3 ± 21.7 days (95% CI = 92–177), and in Group B, it was 172.8 ± 30.4 days (95% CI = 113–232); χ² = 0.404; P = 0.525 (Figure 3).
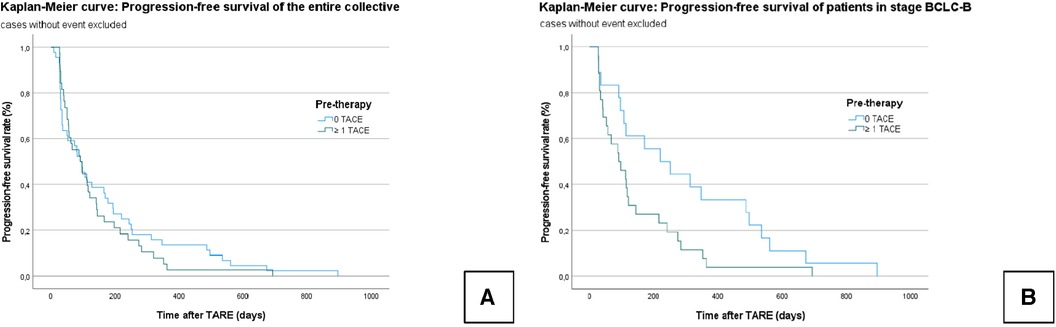
Figure 3. Kaplan–Meier curves: progression-free survival of the censored entire collective (A): no significant difference is seen between the TACE/TARE and TARE-only groups. Progression-free survival of censored patients who graduated stage B (BCLC) (B): A significant difference is seen between the TACE/TARE and TARE-only groups.
In the subgroup analysis, the median PFS in all BCLC B patients was 208.7 ± 32 days (95% CI = 146–271); Group A: 143.7 ± 29.8 days (95% CI = 82–202); Group B: 302.4 ± 59.9 days (95% CI = 185–420); χ² = 4.680; P = 0.031) (Figure 3).
Cox regression analysis for age (P = 0.736), activity in GBq (P = 0.805), number of treatments (P = 0.308), number of lesions (P = 0.916), and lesion size <5 cm (P = 0.072) and <10 cm (P = 0.257) referred to overall survival did not reach statistical significance. The Child–Pugh score (P = 0.005) and size of lesions >10 cm (P = 0.022) showed hazard ratios of 2,717 (Child–Pugh score) and 2,505 (size of lesion).
In patients classified as Child–Pugh B, the median OS was 236 ± 58.4 days (95% CI = 121.6–350); Group A: 199 ± 65.4 days (95% CI = 70–327); Group B: 263 ± 91 days (95% CI = 85–443); χ² = 0.120; P = 0.729 (Figure 4A).
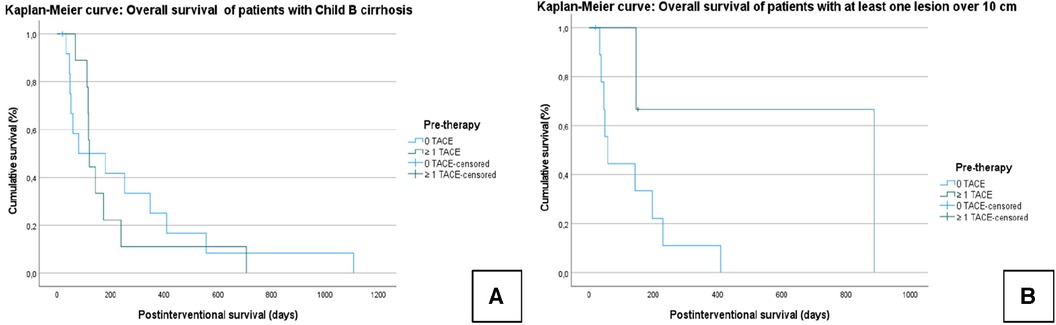
Figure 4. Kaplan–Meier curves: the median overall survival of patients with Child B cirrhosis (A) and with at least one lesion over 10 cm (B). For both TACE and TARE, the Child–Pugh score and lesion size (>10 cm) had an effect on the outcome; a reduced OS was found in patients with higher tumor burden and reduced liver function. However, the OS in this subgroup did not differ depending on whether a prior TACE had been performed.
In patients with lesions >10 cm, the median OS was 221.6 ± 81 days (95% CI = 62–380); Group A: 639.7 ± 283.6 days (95% CI = 80–1199); Group B: 133 ± 42.4 days (95% CI = 50–216); χ² = 2.930; P = 0.087 (Figure 4B).
No differences in OS, LTC, and PFS were found between patients receiving TACE before TARE and those receiving only TARE.
The Child–Pugh score and the size of the HCC lesions (>10 cm) correlated with OS; neither previous TACE nor the number of lesions correlated with OS.
This study examined the outcome of TARE in patients with prior TACE treatment and compared it with those without prior embolic therapy for unresectable HCC. The main findings are that the outcomes of TARE do not differ between patients who received TACE prior to radioembolization and those who only received radioembolization in a patient collective with mainly advanced HCC.
For HCC patients who are not eligible for transplantation, local ablation, or resection due to tumor location and/or several tumor lesions (i.e., BCLC stage B patients), transarterial chemoembolization is a validated treatment option (7); TARE is a suitable treatment alternative for unresectable intermediate-stage HCC and even offers further advantages in cases where ablative radioembolization/radiation segmentectomy is possible (12, 13).
Nonetheless, TACE remains the standard of care in intermediate well-defined HCC, due to the lack of randomized controlled trial data proving the superiority of TARE, as well as its substantially higher procedural costs of TARE (9, 14–19).
Thus, in a clinical setting, it is common for patients to receive TARE only after initial TACE failure. Until now, the efficacy of TARE has not been evaluated in patients with prior chemoembolic treatment.
A possible downside of initial TACE may result from macro- and microvascular damage caused by repetitive embolization, potentially reducing the effects of a second-line TARE therapy. However, HCC progression is commonly based on neo-angiogenesis; thus, in a growing or de novo HCC lesion, new or recanalized feeding vessels are to be expected to facilitate further embolic therapy (16, 20). This is supported by the current results, indicating that prior TACE does not have an impact on sequential TARE therapy. The regression analysis found no relationship between the number of prior TACE treatments and outcome in this patient cohort receiving up to seven sessions of chemoembolization.
In particular, the fact that TACE can be repeated several times before employing TARE as a sequential escalating therapy option in the event of tumor progression may be seen as an advantage of initial TACE therapy (15). On average, 60% of HCC patients treated with TACE receive multiple treatment sessions compared with 70% of TARE patients receiving only a single treatment (18, 21–23); in the current study, 73% of patients in the TACE/TARE group received multiple prior chemoembolization. TARE therapy was repeated once in 12 patients: twice in two patients in the TARE-only group and once in eight patients in the TACE/TARE group. The number of TARE sessions is limited by more extensive collateral damage to residual liver tissue during treatment, depending on the type of TARE execution (i.e., lobar vs. segmental).
Although encouraging data are available from smaller retrospective studies regarding combination therapies in large HCC lesions for both TACE and TARE (24–27), the current results support the established concept that the Child–Pugh score and the lesion size (>10 cm) have an effect on the outcome (28); lower OS was found in patients with higher tumor burden and reduced liver function. On the contrary, no differences were found in OS between the TACE/TARE and the TARE-only subgroup. In contrast to previous studies evaluating the impact of tumor radiation dose in TARE, the applied radiation dose did not have an impact on OS in the current study, mostly including patients receiving lobar therapy (29).
The main limitation of this study is the retrospective design with a relatively small, heterogeneous patient cohort with multifocal/advanced HCC. This study was not conceptualized to investigate the therapeutic potential of TARE or TACE with regard to OS. In fact, due to the retrospective nature of this study, patients were included (e.g., portal-venous infiltration) who, according to the current guidelines, are not considered primary candidates for TACE or TARE (30). OS analysis is further limited by an uneven distribution of Child status within Group A and Group B. Thus, the cohort composition may be seen as an explanation for the comparably low OS rates when compared with the current data (31–33). Nonetheless, LTC and PFS did not differ in the cohorts, underlining the technical feasibility of sequencing the procedures.
The tumor-absorbed dose could not be calculated for all patients; therefore, the total applied radiation dose was investigated as a dosimetry parameter. Advanced strategies such as personalized TARE, including radiation segmentectomy/lobectomy (34) or ethanol embolization (35), were not investigated.
As there are currently few data available on sequential therapy of TACE and TARE, our results provide preliminary evidence that prior TACE does not impair the therapeutic effect of TARE in multifocal, unresectable HCC treatment. However, further studies involving larger and controlled patient cohorts in this area are needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the institutional review board of the Medical Faculty of the Rheinische Friedrich-Wilhelms University of Bonn, and hence all methods were performed in compliance with the ethical standards set in the 1964 Declaration of Helsinki as well as its later amendments. Written informed consent for retrospective data analysis was waived. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from clinical, procedural, and follow-up data collected retrospectively from the electronic patient files. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JW: Conceptualization, Writing – original draft. PK: Writing – review & editing. PB: Conceptualization, Supervision, Writing – review & editing. RS: Data curation, Writing – review & editing. EG: Data curation, Writing – review & editing. JL: Writing – review & editing. AI: Writing – review & editing. CM: Writing – review & editing. FK: Writing – review & editing. CP: Writing – review & editing. UA: Writing – review & editing. DK: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang B, Liang J, Qu Z, Yang F, Liao Z, Gou H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: a systematic review. PLoS One. (2020) 15:1–19. doi: 10.1371/journal.pone.0227475
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. European Association for the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
4. Orci LA, Sanduzzi-Zamparelli M, Caballol B, Sapena V, Colucci N, Torres F, et al. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. Clin Gastroenterol Hepatol. (2022) 20:283–92. doi: 10.1016/j.cgh.2021.05.002
5. Sacco R, Faggioni L, Bargellini I, Ginanni B, Battaglia V, Romano A, et al. Assessment of response to sorafenib in advanced hepatocellular carcinoma using perfusion computed tomography: results of a pilot study. Dig Liver Dis. (2013) 45:776–881. doi: 10.1016/j.dld.2013.03.004
6. Gamboa AC, Kooby DA, Maithel SK, Gamblin TC. Immune checkpoint inhibitors in hepatocellular carcinoma: a review of current clinical trials. J Surg Oncol. (2024) 129:63–70. doi: 10.1002/jso.27545
7. Eggert T, Greten TF. Current standard and future perspectives in non-surgical therapy for hepatocellular carcinoma. Digestion. (2017) 96:1–4. doi: 10.1159/000464282
8. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. (2015) 21:10327–35. doi: 10.3748/wjg.v21.i36.10327
9. Auer TA, Jonczyk M, Collettini F, Marth A, Wieners G, Hamm B, et al. Trans-arterial chemoembolization with degradable starch microspheres (DSM-TACE) versus selective internal radiation therapy (SIRT) in multifocal hepatocellular carcinoma. Acta Radiol. (2021) 62:313–21. doi: 10.1177/0284185120926474
10. Pitton MB, Kloeckner R, Ruckes C, Wirth GM, Eichhorn W, Wörns MA, et al. Randomized comparison of selective internal radiotherapy (SIRT) versus drug-eluting bead transarterial chemoembolization (DEB-TACE) for the treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2015) 38:352–60. doi: 10.1007/s00270-014-1012-0
11. Lee JS, Choi HJ, Kim BK, Park JY, Kim DY, Ahn SH, et al. The modified response evaluation criteria in solid tumors (RECIST) yield a more accurate prognoses than the RECIST 1.1 in hepatocellular carcinoma treated with transarterial radioembolization. Gut Liver. (2020) 6:765–74. doi: 10.5009/gnl19197
12. Fidelman N, Kerlan RK Jr. Transarterial chemoembolization and (90)Y radioembolization for hepatocellular carcinoma: review of current applications beyond intermediate-stage disease. AJR Am J Roentgenol. (2015) 205:742–52. doi: 10.2214/AJR.15.14802
13. Kolligs FT, Bilbao JI, Jakobs T, Iñarrairaegui M, Nagel JM, Rodriguez M, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int. (2015) 35:1715–21. doi: 10.1111/liv.12750
14. Moreno-Luna LE, Yang JD, Sanchez W, Paz-Fumagalli R, Harnois DM, Mettler TA, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. (2013) 36:714–23. doi: 10.1007/s00270-012-0481-2
15. Guiu B, Garin E, Allimant C, Edeline J, Salem R. TARE in hepatocellular carcinoma: from the right to the left of BCLC. Cardiovasc Intervent Radiol. (2022) 45:1599–1607. doi: 10.1007/s00270-022-03072-8
16. Casadei Gardini A, Tamburini E, Iñarrairaegui M, Frassineti GL, Sangro B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: a meta-analysis of randomized trials. Onco Targets Ther. (2018) 11:7315–21. doi: 10.2147/OTT.S175715
17. Kishore SA, Bajwa R, Madoff DC. Embolotherapeutic strategies for hepatocellular carcinoma: 2020 update. Cancers (Basel). (2020) 12:791. doi: 10.3390/cancers12040791
18. Lance C, McLennan G, Obuchowski N, Cheah G, Levitin A, Sands M, et al. Comparative analysis of the safety and efficacy of transcatheter arterial chemoembolization and yttrium-90 radioembolization in patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. (2011) 22:1697–705. doi: 10.1016/j.jvir.2011.08.013
19. Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. (2011) 140:497–507. doi: 10.1053/j.gastro.2010.10.049
20. Moawad AW, Szklaruk J, Lall C, Blair KJ, Kaseb AO, Kamath A, et al. Angiogenesis in hepatocellular carcinoma; pathophysiology, targeted therapy, and role of imaging. J Hepatocell Carcinoma. (2020) 7:77–89. doi: 10.2147/JHC.S224471
21. Salem R, Gilbertsen M, Butt Z, Memon K, Vouche M, Hickey R, et al. Increased quality of life among hepatocellular carcinoma patients treated with radioembolization, compared with chemoembolization. Clin Gastroenterol Hepatol. (2013) 11:1358–65. doi: 10.1016/j.cgh.2013.04.028
22. Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional therapy approaches for hepatocellular carcinoma: recent advances and management strategies. Cancers (Basel). (2020) 12:1914. doi: 10.3390/cancers12071914
23. Van Thai N, Thinh NT, Ky TD, Bang MH, Giang DT, Ha LN, et al. Efficacy and safety of selective internal radiation therapy with yttrium-90 for the treatment of unresectable hepatocellular carcinoma. BMC Gastroenterol. (2021) 21:216. doi: 10.1186/s12876-021-01805-6
24. Hu H, Chen GF, Yuan W, Wang JH, Zhai B. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperthermia. (2018) 34:1351–8. doi: 10.1080/02656736.2018.1462536
25. Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, et al. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. (2014) 20:17483–90. doi: 10.3748/wjg.v20.i46.17483
26. Liu C, Liang P, Liu F, Wang Y, Li X, Han Z, et al. MWA combined with TACE as a combined therapy for unresectable large-sized hepatocellular carcinoma. Int J Hyperthermia. (2011) 27:654–62. doi: 10.3109/02656736.2011.605099
27. Chen B, Wei W, Ma L, Yang B, Gill RM, Chua MS, et al. Computational discovery of niclosamide ethanolamine, a repurposed drug candidate that reduces growth of hepatocellular carcinoma cells in vitro and in mice by inhibiting cell division cycle 37 signaling. Gastroenterology. (2017) 152:2022–36. doi: 10.1053/j.gastro.2017.02.039
28. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. (2008) 100:698–711. doi: 10.1093/jnci/djn134
29. Hermann AL, Dieudonné A, Ronot M, Sanchez M, Pereira H, Chatellier G, et al. Relationship of tumor radiation-absorbed dose to survival and response in hepatocellular carcinoma treated with transarterial radioembolization with 90Y in the SARAH study. Radiology. (2020) 296:673–84. doi: 10.1148/radiol.2020191606
30. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. (2022) 76:681–93. doi: 10.1016/j.jhep.2021.11.018
31. Vogel A, Saborowski A. Current strategies for the treatment of intermediate and advanced hepatocellular carcinoma. Cancer Treat Rev. (2020) 82:101946. doi: 10.1016/j.ctrv.2019.101946
32. Ricke J, Klümpen HJ, Amthauer H, Bargellini I, Bartenstein P, de Toni EN, et al. Impact of combined selective internal radiation therapy and sorafenib on survival in advanced hepatocellular carcinoma. J Hepatol. (2019) 71:1164–74. doi: 10.1016/j.jhep.2019.08.006
33. Kolligs F, Arnold D, Golfieri R, Pech M, Peynircioglu B, Pfammatter T, et al. Factors impacting survival after transarterial radioembolization in patients with hepatocellular carcinoma: results from the prospective CIRT study. JHEP Rep. (2022) 5:100633. doi: 10.1016/j.jhepr.2022.100633
34. Miller FH, Lopes Vendrami C, Gabr A, Horowitz JM, Kelahan LC, Riaz A, et al. Evolution of radioembolization in treatment of hepatocellular carcinoma: a pictorial review. Radiographics. (2021) 41:1802–18. doi: 10.1148/rg.2021210014
Keywords: hepatocellular carcinoma, transarterial radioembolization, transarterial chemoembolization, interventional therapy, Barcelona clinic liver cancer staging system
Citation: Wagenpfeil J, Kupczyk PA, Bruners P, Siepmann R, Guendel E, Luetkens JA, Isaak A, Meyer C, Kuetting F, Pieper CC, Attenberger UI and Kuetting D (2024) Outcome of transarterial radioembolization in patients with hepatocellular carcinoma as a first-line interventional therapy and after a previous transarterial chemoembolization. Front. Radiol. 4:1346550. doi: 10.3389/fradi.2024.1346550
Received: 29 November 2023; Accepted: 23 January 2024;
Published: 20 February 2024.
Edited by:
Cinzia Pettinato, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyReviewed by:
Elias Kehayas, University Hospital of Heraklion, Greece© 2024 Wagenpfeil, Kupczyk, Bruners, Siepmann, Guendel, Luetkens, Isaak, Meyer, Kuetting, Pieper, Attenberger and Kuetting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julia Wagenpfeil anVsaWEud2FnZW5wZmVpbEB1a2Jvbm4uZGU=
Abbreviations HCC, hepatocellular carcinoma; TARE, transarterial radioembolization; TACE, transarterial chemoembolization; BCLC, Barcelona clinic liver cancer staging system; PFS, progression-free survival; OS, overall survival; LTC, local tumor control.
†ORCID Julia Wagenpfeil orcid.org/0000-0002-8530-3523
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.