- 1Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milan, Italy
- 2Department of Perioperative Cardiology and Cardiovascular Imaging, Centro Cardiologico Monzino IRCCS, Milan, Italy
- 3Department of Biomedical, Surgical and Dental Sciences, University of Milan, Milan, Italy
Introduction: Cardiac amyloidosis (CA) shares similar clinical and imaging characteristics (e.g., hypertrophic phenotype) with aortic stenosis (AS), but its prognosis is generally worse than severe AS alone. Recent studies suggest that the presence of CA is frequent (1 out of 8 patients) in patients with severe AS. The coexistence of the two diseases complicates the prognosis and therapeutic management of both conditions. Thus, there is an urgent need to standardize and optimize the diagnostic process of CA and AS. The aim of this study is to develop a robust and reliable radiomics-based pipeline to differentiate the two pathologies.
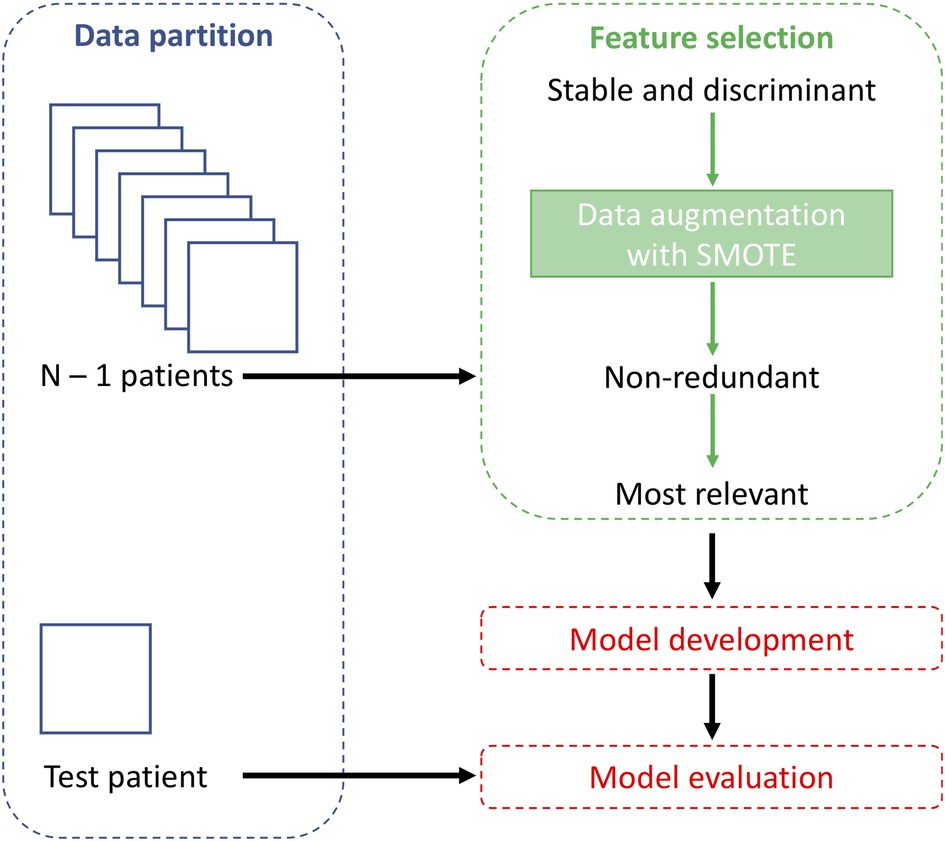
Methods: Thirty patients were included in the study, equally divided between CA and AS. For each patient, a cardiac computed tomography (CCT) was analyzed by extracting 107 radiomics features from the LV wall. Feature robustness was evaluated by means of geometrical transformations to the ROIs and intra-class correlation coefficient (ICC) computation. Various correlation thresholds (0.80, 0.85, 0.90, 0.95, 1), feature selection methods [p-value, least absolute shrinkage and selection operator (LASSO), semi-supervised LASSO, principal component analysis (PCA), semi-supervised PCA, sequential forwards selection] and machine learning classifiers (k-nearest neighbors, support vector machine, decision tree, logistic regression and gradient boosting) were assessed using a leave-one-out cross-validation. Data augmentation was performed using the synthetic minority oversampling technique. Finally, explainability analysis was performed by using the SHapley Additive exPlanations (SHAP) method.
Results: Ninety-two radiomic features were selected as robust and used in the further steps. Best performances of classification were obtained using a correlation threshold of 0.95, PCA (keeping 95% of the variance, corresponding to 9 PCs) and support vector machine classifier reaching an accuracy, sensitivity and specificity of 0.93. Four PCs were found to be mainly dependent on textural features, two on first-order statistics and three on shape and size features.
Conclusion: These preliminary results show that radiomics might be used as non-invasive tool able to differentiate CA from AS using clinical routine available images.
1. Introduction
Cardiac amyloidosis (CA) is an increasingly diagnosed condition caused by conformational changes in amyloidogenic proteins leading to amyloid fibril deposition in the heart (1). The two predominant amyloid proteins found in the heart are immunoglobulin light chain (AL) and transthyretin (ATTR) (2). ATTR-CA occurs in two most common forms: wild-type, or senile, transthyretin (wtATTR), more prevalent in elderly people, and hereditary or variable TTR (vATTR), genetic autosomal dominant disease (3). Clinical presentation associated to CA shows an increased biventricular wall thickness, myocardial stiffening and restrictive physiology of the left and right ventricles (2) caused by amyloid fibril deposition in the extracellular myocardial space. Similar myocardial remodeling processes affect the heart of patients with aortic stenosis (AS). AS is the most common cause of valvular heart disease (4). It is characterized by a progressive aortic valve narrowing leading to an increase in afterload and wall stress, compensated with a gradual hypertrophy of left ventricle (LV) (5). Therefore, as CA shares several common phenotypical features with AS, and considering the high prevalence of subclinical CA among AS undergoing transcatheter aortic valve implantation (TAVI), the differential diagnosis of these two entities has important prognostic and therapeutic implications. Patients affected by both CA and AS have, indeed, worsen prognosis and are less likely to benefit from aortic valve replacement (AVR) surgery with respect to lone AS patients. In addition, the recent introduction of new effective therapies for improving the prognosis of patients with CA makes early recognition of this pathology even more relevant than in the past.
In this framework, radiomics can be a non-invasive tool useful to perform differential diagnosis starting from medical images such as cardiac computed tomography (CCT), usually used for interventional planning of AS patients undergoing TAVI. Radiomics is an emerging research field aimed to improve diagnosis, characterization, and prognosis using quantitative features extracted from medical images. Radiomics is widely employed in oncology for tumors characterization (6, 7), treatment response (8–10), and overall survival analysis (11, 12). Recently, it has been proposed in the cardiovascular field to improve diagnostic accuracy, patients cardiac risk prediction and stratification (13–22).
As radiomics generates hundreds of features, a crucial step of radiomic workflow is selecting features according to their relevance with respect to the clinical question of interest. Assessing features robustness is a necessary preliminary step in the process of feature selection (23). The most applied techniques to evaluate features reliability are test-retest and multiple delineations of the ROI (15, 17, 18), methods affected by a time-consuming intrinsic limit: test-retest requires multiple scan acquisitions, whereas multiple delineations require several ROI segmentations. To overcome these problems, in the current study, for the first time in CCT, features robustness was assessed performing geometrical transformations of the ROIs (23). To mimic multiple manual delineations, small ROI transformations are applied to simulate errors due to manual delineation thus assessing feature stability. In addition, large ROI transformations are performed to assess feature discrimination capacity. The underlying hypothesis is that robust features need to be stable, i.e., similar for small transformations, and discriminative, i.e., different for large transformation. Studies on feature robustness already exist in oncological field (23–25), whereas a very limited research is available in cardiovascular radiomics (15, 26).
In this study, we aim to develop a robust and reliable pipeline to differentiate between hypertrophic phenotype due to CA versus AS using radiomics and machine learning techniques. The final goal is to provide early detection of CA patients to administer specific treatment and improve their prognosis. Radiomic features will be extracted from the LV muscle in CCT, and different methods of feature selection and machine learning algorithm will be tested.
2. Methods
2.1. Study population and baseline characteristics
Thirty patients were included in the study: 15 with CA and 15 with AS. CA was defined by the presence of ATTR amyloid in a myocardial biopsy (Congo red and immunohistochemical staining) or positive DPD scintigraphy, while AL amyloidosis was proven with biopsies from non-cardiac tissues. In AS patients, the concomitant presence of CA was excluded by bone scintigraphy and/or cardiac magnetic resonance (CMR).
All patients underwent comprehensive evaluation with transthoracic echocardiography using commercially available equipment (iE33 or Epiq, Philips Medical System, or Vivid-9, GE Healthcare) measuring LV end-diastolic (LVEDV) and end-systolic (LVESV) volumes indexed for body surface area, LV ejection fraction (LVEF) and intraventricular septum thickness (IVS). Clinical characteristics are shown in Table 1.
The institutional Ethical Committee approved the study, and all the patients signed the informed consent.
2.2. CCT scan protocol
CCT examinations were performed using Revolution CT (GE Healthcare, Milwaukee, WI) or Aquilion ONE VisionTM (Canon Medical Systems Corp., Tokyo, Japan). Details of the CT image acquisition parameter are reported in Supplementary Table S1.
No premedication with beta-blockers or nitrates was added before CT acquisition. Patients received a fixed dose of 50 ml bolus of contrast medium (400 mg of iodine per milliliter, Iomeprol; Bracco, Milan, Italy) despite the BMI via an antecubital vein at an infusion rate of 5 ml s−1 followed by 50 ml of saline solution at 5 ml·s−1.
2.3. Images segmentation, preprocessing and radiomics features extraction
For each patient, the LV wall was manually segmented by a unique operator, and all the segmentations were revised by a different, blinded, level III, European Association of Cardiovascular Imaging certified operator (27).
Image preprocessing was performed to reduce the imaging-related variability: a 3D Gaussian filter with a 3 × 3 × 3 voxel kernel and σ = 0.5 was used to denoise the images. Then, voxel size resampling to an isotropic resolution of 2 mm [as in (28)] was performed with B-spline interpolation.
One-hundred and seven radiomic features were extracted using Pyradiomics 3.0 (29). The features belong to the following classes: shape and size (SS, 14 features), first order statistics (FOS, 18 features) and textural features (75 features). See Pyradiomics documentation for more details (29). A fixed-bin width histogram discretization (0.5 Hounsfield units per bin) was used prior to features extraction. Since features were extracted with Pyradiomics, they were compatible with the Image Biomarker Standardization Initiative (30).
2.4. Feature selection
All the feature selection steps were performed on the training set and then applied on the test set.
2.4.1. Selection of stable and discriminant features
Stability and discrimination capacity were assessed using geometrical transformations (translations) of the ROIs as in (23). The entire workflow is implemented in MATLAB 2017a (Mathworks, Natick, MA, USA) and applied to FOS and textural features, as SS features do not change with a ROI translation.
Two entity translations were applied to the ROIs, along both the x (medial-lateral) and y (antero-posterior) directions. The minimal entity translation was ±0.5% the length of the bounding box surrounding the ROI in the direction of interest (Figure 1A), and the maximal entity translation was ±30% (Figure 1B). Radiomic features were computed on each translated ROI and compared to the ones obtained from the original ROI by expert cardiac radiologists. Briefly, for each feature calculated from a single ROI, 4 intraclass correlation coefficients (ICCs) with their mean (ICCmean) were calculated. Following the general guidelines (31), two ICC threshold values were identified to select robust features: ICC = 0.75, which indicates good agreement between data, and ICC = 0.5, which reflects poor similarity. Thus, a feature is considered stable if the ICCmean for the minimal entity translation is higher than 0.75 and discriminative if the ICCmean for the maximal entity translation is lower than 0.5. For each translation, the percentage of overlapping volume was also computed to assess whether most of the original ROI was part of the transformed ROI. After this step, the features were z-scored.
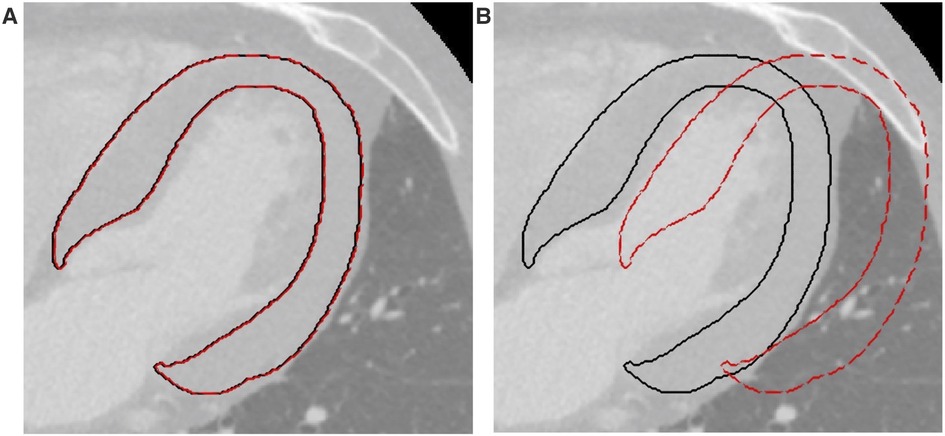
Figure 1. Example of translations applied to the ROIs. (A) Minimal entity translation: dashed red line represents a translation of 0.5% in the x direction. (B) Maximal entity translation: dashed red line represents a translation of 30% in the x direction. In both figures, continuous black lines represent the contours of the original ROIs. ROI, region of interest.
2.4.2. Selection of non-redundant features
The second step consisted of a correlation-based feature selection, performed to ensure a set of features with low internal redundancy. When a pair of features had an absolute Spearman correlation coefficient above a fixed threshold only one of the two was kept. In particular, the one with lower mean correlation with all the other n-2 features was selected. Obviously, the higher the threshold, the fewer features were removed. Five thresholds were considered (0.80, 0.85, 0.90, 0.95, 1), where a threshold equal to 1 means no features are removed in this step.
2.4.3. Selection of more relevant features
Six feature selection methods were tested (32), namely: p-value, least absolute shrinkage and selection operator (LASSO), semi-supervised LASSO (ssLASSO), principal component analysis (PCA), semi-supervised PCA (ssPCA) and sequential forwards selection (SFS). Briefly, the p-value method performs a Wilcoxon rank-sum test on each feature to identify the ones significantly different between the two unpaired groups of patients; LASSO shrinks the coefficients of less important features to zero to keep only the most important features by mean of penalty factor lambda; ssLASSO employs statistically significant features (selected by the p-value method) as input for LASSO algorithm; the PCA transforms the high-dimensional dataset into a lower-dimensional space by identifying the most important principal components capturing the maximum variance in the data, while minimizing the loss of information; ssPCA employs statistically significant features (selected by the p-value method) as input for PCA; SFS iteratively adds the most relevant features to the model, one at a time, based on their individual contribution to the model's performance (until the score improvement exceeds a threshold, here fixed to 0.02).
2.5. Classification model development
The dataset was divided into training and test sets using a leave-one-out (LOO) cross-validation method, as a result, for each iteration, the test set contained only one observation, while the others were used as training. Since the dataset was balanced but small, data augmentation was performed on the training set, that was doubled in size (maintaining the class balance) using the synthetic minority oversampling technique (SMOTE) algorithm (33). SMOTE over-samples the minority class by generating synthetic examples. This is done by selecting each sample from the minority class and creating new samples along the line segments that connect the sample to its Q nearest neighbors within the minority class. For every minority class sample, we find the Q closest neighbors belonging to the same class and select one of them randomly. The synthetic sample is then positioned at a random point along the line that connects the two original samples. In this study, the value of Q was set to 5. By applying SMOTE, both classes end up having an equal number of instances available for classification. As in this study the classes were balanced, the samples of a single class were first duplicated and subsequently, SMOTE was applied: this procedure was performed separately for both classes and the new samples were added to the original dataset. As a result, the number of training samples was increased from 29 to 58 patients.
To find the best classification model, five machine learning models were trained: k-nearest neighbors (kNN), support vector machine (SVM), decision tree (DT), logistic regression (LR), and gradient boosting (GB). Parameters values set for each classification model are shown in Supplementary Table S2. The p-value feature selection method was used to select the best-performing model. Model performance was evaluated based on sensitivity, specificity and diagnostic accuracy, being the latter used to identify the best model. The best pair of feature selection method and correlation threshold was also selected.
The entire workflow is shown in Figure 2. The feature selection and classification are implemented in Scikit-Learn Python library.
2.6. Explainability analysis
After selecting the best correlation threshold, feature selection method and machine learning model, the final model was built by training on the whole original dataset. As during model development, the training set was oversampled using SMOTE to double the total number of samples.
To interpret the machine learning model and its prediction, the SHapley Additive exPlanations (SHAP) method (34) was used on the best performing model. The SHAP method provides a way to determine the importance of different features in making a prediction by measuring how much each feature contributes to the final prediction. This allows to gain insight into how the model is making its predictions and which features are most influential.
The SHAP method is based on the concept of Shapley values, i.e., the average expected marginal contribution of each feature to the overall prediction. The Shapley values can be visualized using a variety of plots, such as a summary plot or a force plot for single instance, which can help to interpret the results and identify which features are most important in making a prediction.
3. Results
3.1. Feature selection
The first step of feature selection identified 92 stable and discriminant features: 6 and 9 features were excluded as they do not satisfy the criterium for the minimal and maximal entity translation, as shown in Figure 3.
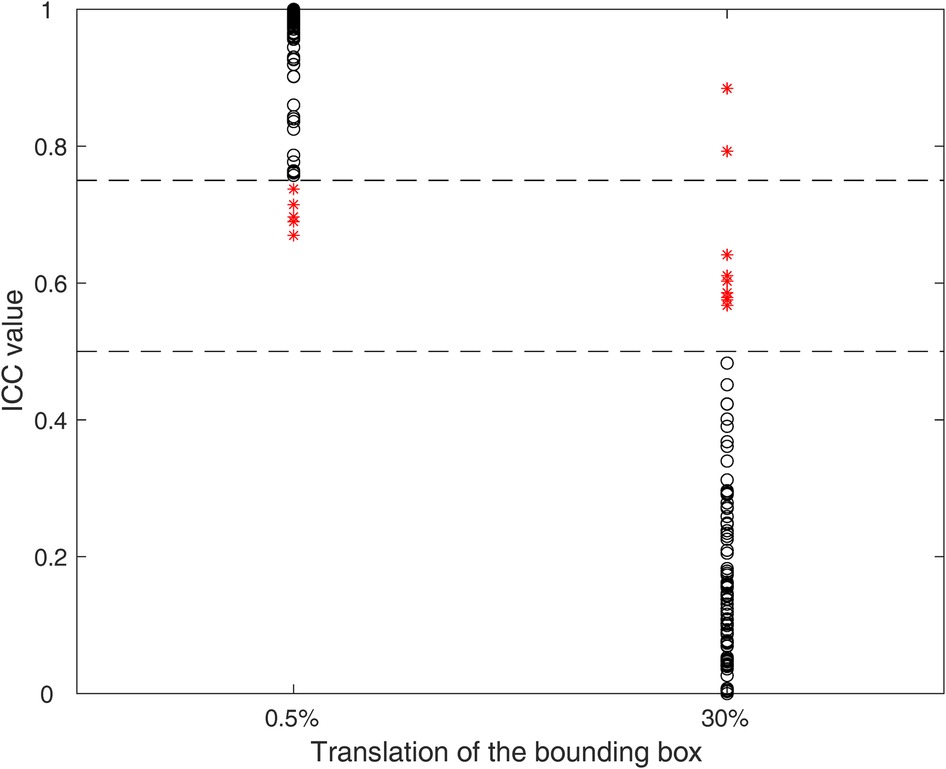
Figure 3. ICCs for the minimal (0.5%) and maximal (30%) entity translation. Each black circle represents a feature satisfying the criterium, while red asterisks represent features not satisfying the criteria. The dashed lines represent the threshold values (0.75 and 0.5) for minimal and maximal entity translations, respectively.
The correlation-based feature selection identified on average 23 ± 1, 28 ± 1, 35 ± 1, 47 ± 1 and 92 ± 0 features for the five correlation thresholds (0.80, 0.85, 0.90, 0.95, 1), respectively.
The different feature selection methods selected a number of features ranging from 7 to 58, as shown in Table 2, again depending both on the method itself and the correlation threshold.
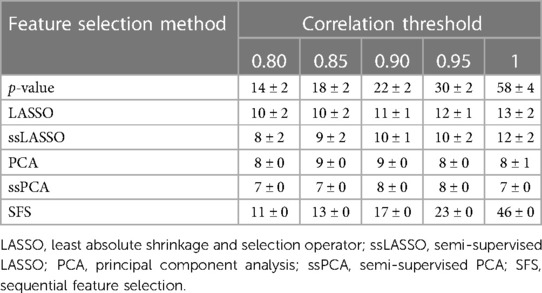
Table 2. Number of features selected by the different feature selection methods as a function of the used correlation threshold (mean ± standard deviation).
3.2. Influence of the model
The performance of the five models (namely, kNN, SVM, DT, LR, and GB) in terms of accuracy (averaging the results obtained from the different correlation thresholds) is shown in Figure 4, with respect to the feature sets selected by the p-value method. The analogous figures for the other feature selection methods are shown in Supplementary Figure S1, while the other performance metrics are shown in Supplementary Figures S2, S3 for all the feature selection methods. SVM presents the highest median accuracy for any feature selection method and a relatively stable behavior over the correlation thresholds, as highlighted by the small interquartile range (IQR), outperforming all the other models. Considering that SVM model achieved the best performances among different machine learning models with all the feature selection methods, it was selected as the best model to evaluate the influence of the correlation thresholds and feature selection methods. Thus, with the data at disposal, SVM was indicated as the optimal model, trading off complexity and performance.
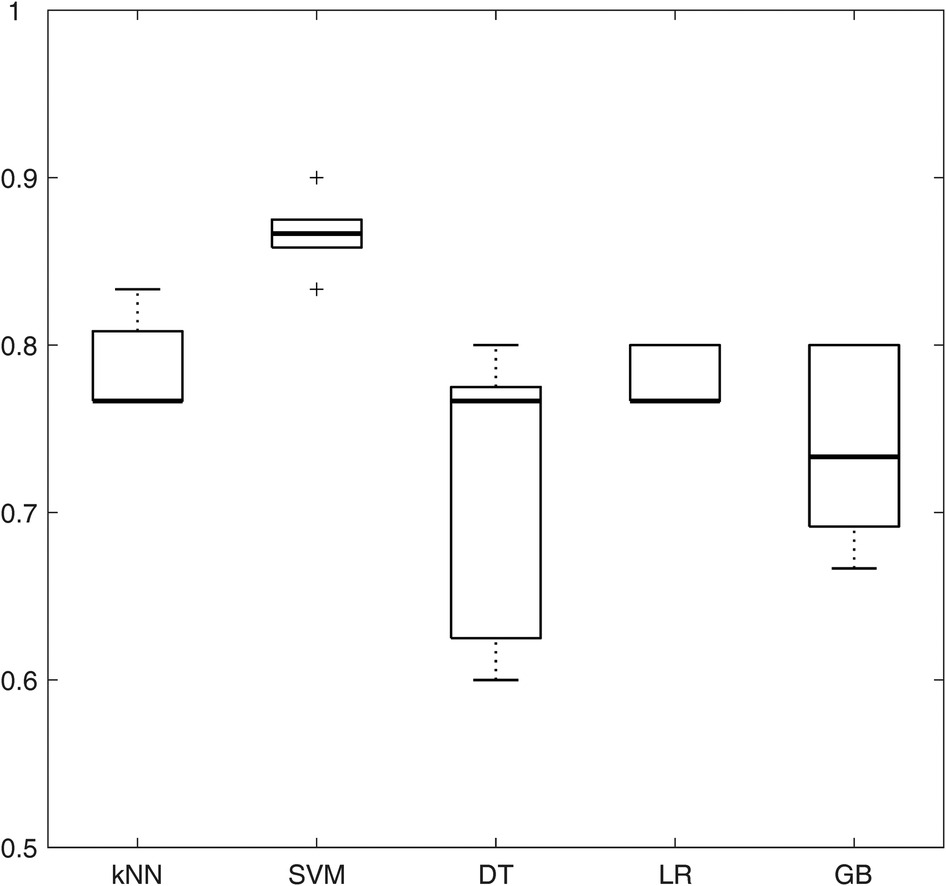
Figure 4. Boxplots representing the average accuracy of the five machine learning models, obtained averaging all the correlation thresholds and p-value as feature selection method. kNN, k-nearest neighbors; SVM, support vector machine; DT, decision tree; LR, logistic regression; GB, gradient boosting.
3.3. Influence of the correlation threshold and feature selection
The impact of the correlation threshold and feature selection method on accuracy is depicted in Figure 5. In the figure, the rows represent different feature selection methods while the columns represent varying correlation thresholds. The bright colors in the color map indicate higher accuracy, while darker colors represent lower accuracy. It is evident that PCA-based methods (both PCA and ssPCA) have higher accuracy across all correlation thresholds, ranging from 0.83 to 0.93 vs. 0.77 to 0.90 for the other feature selection methods. The best performances were observed using the PCA feature selection method which reached an accuracy of 0.90, with no correlation (threshold = 1), and an accuracy of 0.90 and 0.93 with a correlation threshold of 0.90 and 0.95, respectively. The corresponding sensitivity and specificity are shown in Supplementary Tables S3, S4.
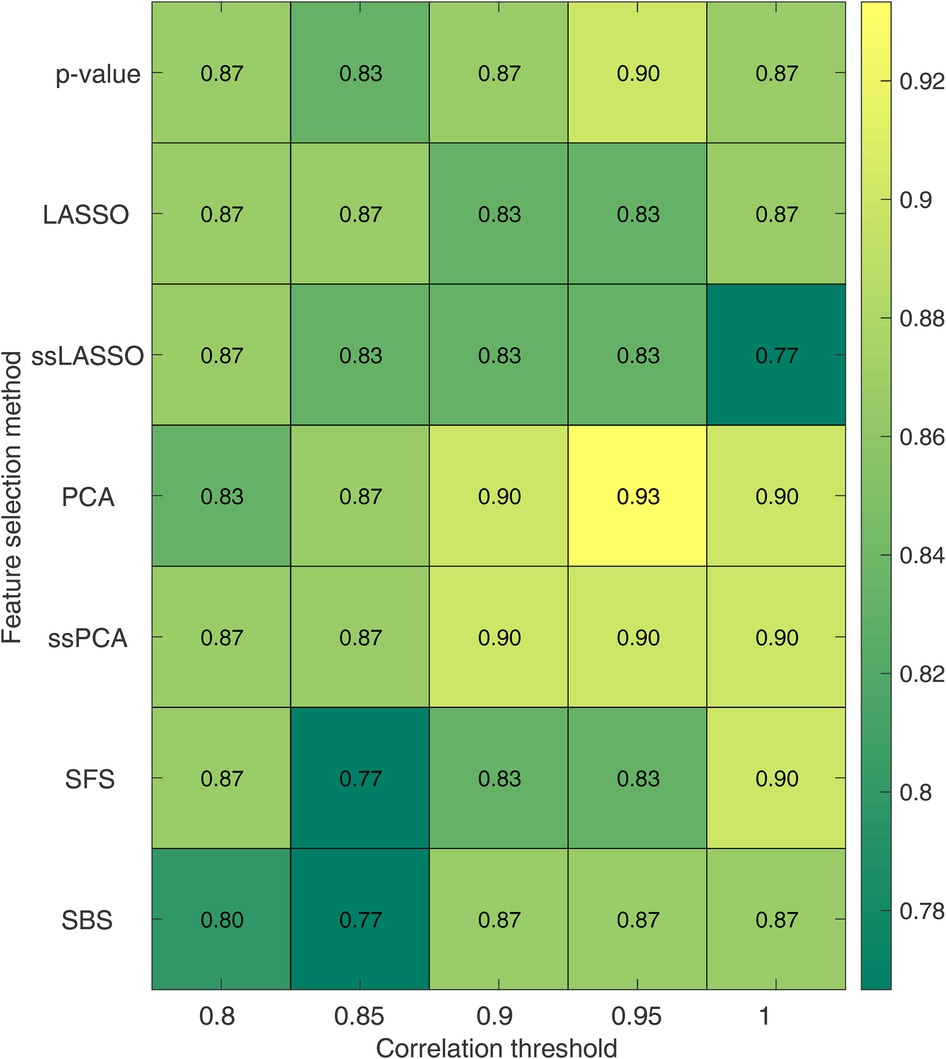
Figure 5. Accuracy for the SVM model as a function of the correlation threshold and the feature selection method. Each row represents a feature selection method, while each column represents a correlation threshold. LASSO, least absolute shrinkage and selection operator; ssLASSO, semi-supervised LASSO; PCA, principal component analysis; ssPCA, semi-supervised PCA; SFS, sequential feature selection.
3.4. Final model and explainability
The final model was built using the model and parameters which were the best performing ones in the LOO cross-validation: SVM, PCA, a correlation threshold of 0.95 and SMOTE. For this model, the explainability by using SHAP analysis was performed, and results are shown in Figures 6, 7. Figure 6A shows the SHAP feature importance for the 9 principal components of the final model. Among them, the first principal component (PC) contributed the most to the model (0.25 mean absolute SHAP value), the next highest-ranking features were the PC 4, 3, 2, 6 and 7, whereas PC1, 5, 8 and 9 contributed less to the model (0.1 mean absolute SHAP value). Figure 6B shows the overall correlation and directionality between features and the SHAP value during model training. Each dot represents one patient, and the color reflects the high and low values of each feature, with the red color indicating a higher value and the blue color indicating a lower value. The x-axis of the graph represents the SHAP value, and a positive SHAP value indicates that it contributes positively to predicting CA and vice versa.
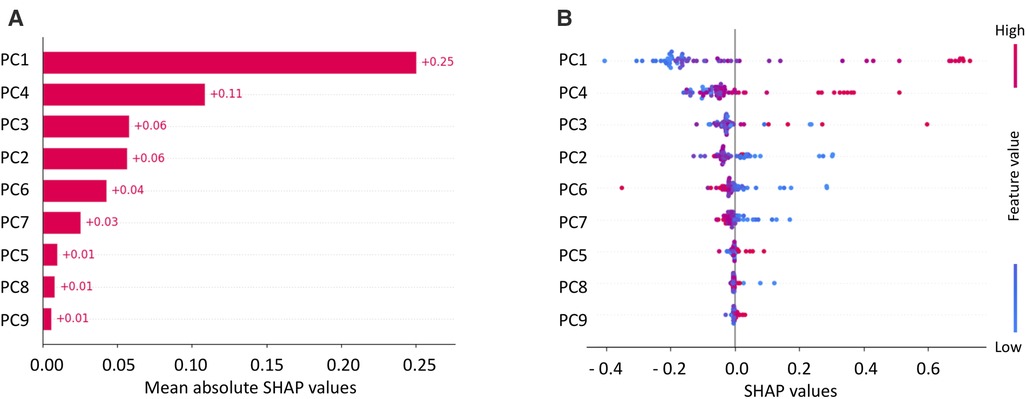
Figure 6. Mean SHAP values for principal components (PCs) of SVM (A) and SHAP values with feature values (B).
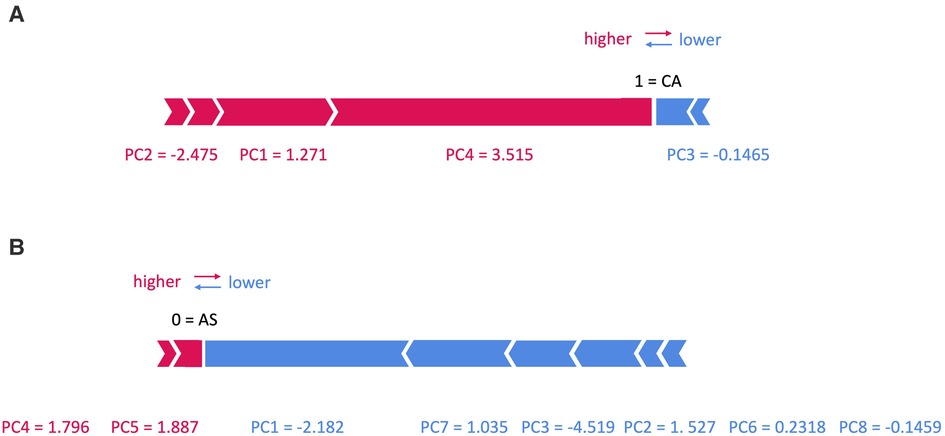
Figure 7. SHAP force plots for two patients with (A) cardiac amyloidosis (CA) and (B) aortic stenosis (AS).
In Figure 7, two examples are shown: in panel (A) an example of a patient with CA is shown, with a prediction equal to 1, corresponding to CA class. In particular, the values of PC1, 2 and 4 increase its rating, while those of PC3 decreases it. On the contrary, in panel (B) an example of a patient with AS is shown, with a prediction equal to 0, corresponding to AS class: here only the value of PC4 and 5 increases its rating, while that of PC1, 2, 3, 6, 7 and 8 decreases it.
To conclude the explainability analysis, the class of the most important features in the different PCs was evaluated. Loadings of each PC, i.e., the coefficients describing the importance of the independent variables in each PC were considered and squared. Squared values, higher than half of the maximum squared loading, were included in the analysis and divided according to the feature class and finally summed up. Table 3 reports the sums of the considered squared PC loadings for each feature class. It can be noted that the first three PCs are mainly dependent on textural features while PC4 and 6 on FOS and the last three PCs on SS features.
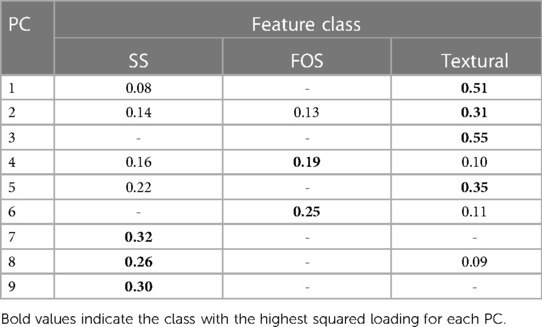
Table 3. Relevance of the different feature class in the principal components (PCs): PCs loadings were squared and summed if the individual squared value is higher than half of the maximum squared loading.
4. Discussion
In the present study a robust and reliable radiomic-based pipeline was developed based on a dataset composed of patients with CA or AS, to differentiate LV hypertrophy due either of the two diseases. The pipeline included three steps of feature selection to obtain a set of (i) stable and discriminative, (ii) non-redundant and (iii) more relevant features, followed by the classification model development. A systematic analysis was performed in which, the influence of (i) multiple machine learning algorithms (kNN, SVM, DT, LR and GB), (ii) correlation thresholds (0.80, 0.85, 0.90, 0.95 and 1) and (iii) feature selection methods (p-value, LASSO, ssLASSO, PCA, ssPCA, SFS) were assessed. Specifically, with the data at disposal, the SVM algorithm, combined with a correlation threshold of 0.95 and PCA feature reduction method, outperformed all the other machine learning models.
Finally, an explainability analysis was conducted in order to gain insight in the trained model. In particular, the analysis revealed that the most impactful features were textural features, as observed from Table 3, which might reflect the differences existing between the hypertrophic phenotype caused by CA and AS. The increase in LV wall thickness caused by CA, is the result of an extracellular deposit of amyloid fibrils within the myocardium while the hypertrophy characterizing AS patients is given by the pressure overload on the LV. This difference seems to be captured by the radiomics analysis CA and AS differences in the hypertrophic structural pattern also appear to be partly detected by SS radiomic features.
From a clinical point of view, the main finding of the study is that radiomics can be used to differentiate CA from AS starting from CCT image scans whose acquisition is comprised in the interventional planning of AS patients undergoing TAVI. The gold standard for CA diagnosis is endomyocardial biopsy. However, endomyocardial biopsy is invasive, and some risks are associated with this technique. Nowadays, it is widely recognized that bone scintigraphy represents a reliable diagnostic tool for CA, in particular for the ATTR variant, avoiding endomyocardial biopsy. However, this diagnostic tool does not appear to be an optimal approach from logistic and economic standpoints (35). Regarding other imaging techniques, echocardiographic appearances seen in the advanced stages of CA are fairly pathognomonic (36) but most echocardiographic parameters do not provide CA diagnosis in the early stages of disease and do not to allow to distinguish CA from other restrictive or hypertrophic cardiomyopathy (37). CMR imaging offers a high-definition structural imaging and tissue characterization that are often incremental to information obtained on echocardiography. Some CMR markers, although pathognomonic in patients with biopsy-proven CA, are not specific for CA and can be elevated in other forms of cardiovascular disease, including reactive or replacement fibrosis and inflammation (38). Very recently, it has been shown that CMR-derived right ventricular global strains and various regional longitudinal strains provide discriminative radiological features for CA and hypertrophic cardiomyopathy differentiation (39). However, CMR is expensive and contraindicated in a substantial proportion of patients (e.g., patients with atrial fibrillation, advanced renal dysfunction or non-compatible intracardiac devices) (38).
Goto et al. (40) developed two deep learning algorithms using electrocardiogram (ECG) and echocardiography data coming from respectively 3 and 5 academic medical centers. The ECG-based model reached a mean C-statistic of 0.86 in differentiating 587 CA patients from 8,612 controls while the echocardiography-based model achieved better performances with a mean C-statistics of 0.95 in distinguishing 609 CA patients from 303 controls. Also, CMR imaging was employed in different studies: Zhou et al. (41) classified 139 patients (79 CA positive vs. 60 controls) by employing CMR radiomics-based machine learning algorithm with a mean accuracy of 80%, and Martini et al. (42) developed a deep learning model to diagnose CA in 206 patients achieving an accuracy of 88%.
As compared with the previous experience, several points of strength could be considered in our study. First, our results based on CCT radiomics obtained an accuracy of 93%, significantly higher as compared previous studies. Second, to the best of our knowledge, this is the first study to employing CCT for amyloidosis identification. This has several clinical implications. Indeed, several studies showed that CA is frequent (11.8%) (43) in patients with severe AS referred for TAVI and the challenge, in this context, is to differentiate a wooden horse (lone AS) from a Trojan horse (AS with CA) (35). Nitsche et al. (43) developed in this setting the RAISE (remodelling, age, injury, system, and electrical) score, to predict the presence of CA in patients with severe AS, obtaining a sensitivity and specificity of 84% and 94%, respectively. Alternatively, to RAISE score, Oda et al. (44) proposed the measurement of extracellular volume by using CCT dataset. However, this approach requires triple scan acquisition (unenhanced scan arterial phase acquisition and late scan) and higher volume of contrast agent. On the contrary our approach is easily performed by using the single arterial phase CCT dataset acquired during the usual diagnostic work-up of these patients.
There are a few limitations to this study that should be considered. Firstly, the population size was small, which means that these findings should be viewed as preliminary and confirmed with a larger dataset. A very different approach could be based on deep learning, however to the best of our knowledge, no pre-trained 3D deep learning model is available to consider the LV volume. Moreover the limited size of the study population does not allow appropriate transfer learning, necessary to adapt the pre-trained network. Secondly, the patients included in the study were randomly selected from a cohort of patients with AS and CA who were referred for CCT. Lastly, the study examined CA and AS separately and did not include patients who were affected by both conditions. Thus, these preliminary results have shown the radiomic features potential to distinguish CA from AS patients, but future studies will be needed both to confirm the results on larger dataset and to investigate the differences between lone AS and its coexistence with CA.
5. Conclusion
In this study, a radiomic-based machine learning model able to differentiate CA and AS patients was developed. The analysis investigated the effect that key choices, as the features selection pipeline and the machine learning algorithm, may have on the classification performance. These preliminary results show that radiomics might help in differentiating CA from AS using clinical routine available images.
Developing a computer-based application able to differentiate hypertrophic cardiac phenotypes given by diseases such as AS, versus those from CA, is clinically relevant as CA plays an important prognostic role and may adversely affect the prognosis of patients who are undergoing AVR surgery (either with traditional or transcatheter surgery, TAVI).
In addition, the current availability of drugs that improve the prognosis of patients with CA makes the correct and early detection of this clinical condition, which is often underdiagnosed and confused with other forms of cardiac hypertrophy, even more important.
Finally, once automatized the process of LV segmentation, this radiomic application has the potential to routinely detect sub-clinical AM from CT scans regularly acquired in clinical practise for TAVI planning.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by IRCCS Centro Cardiologico Monzino ethical committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
FL: radiomic features extraction, prognostic model training and validation, manuscript writing; VC: data analysis, manuscript writing; RM: image segmentation, image data collection and cleaning, manuscript revision; GP: study design, supervision, manuscript revision. All authors contributed to the article and approved the submitted version.
Conflict of interest
GP declares the following conflict of interest: Honorarium as speaker/consultant and/or research grant from GE Healthcare, Bracco, Heartflow, Boheringher.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors GP, VC declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fradi.2023.1193046/full#supplementary-material
References
1. Baker KR, Rice L. The amyloidoses: clinical features, diagnosis and treatment. Methodist Debakey Cardiovasc J. (2012) 8:3. doi: 10.14797/mdcj-8-3-3
2. Bonelli A, Paris S, Nardi M, Henein MY, Agricola E, Troise G, et al. Aortic valve stenosis and cardiac amyloidosis: a misleading association. J Clin Med. (2021) 10:4234. doi: 10.3390/jcm10184234
3. Gherasim L. Amyloid cardiopathy and aortic stenosis. Maedica. (2021) 16:473–81. doi: 10.26574/maedica.2020.16.3.473
4. Elmariah S. Patterns of left ventricular remodeling in aortic stenosis: therapeutic implications. Curr Treat Options Cardio Med. (2015) 17:31. doi: 10.1007/s11936-015-0391-0
5. Shah ASV, Chin CWL, Vassiliou V, Cowell SJ, Doris M, Kwok TC, et al. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. (2014) 130:1607–16. doi: 10.1161/CIRCULATIONAHA.114.011085
6. La Greca Saint-Esteven A, Vuong D, Tschanz F, van Timmeren JE, Dal Bello R, Waller V, et al. Systematic review on the association of radiomics with tumor biological endpoints. Cancers. (2021) 13:3015. doi: 10.3390/cancers13123015
7. Corino VDA, Montin E, Messina A, Casali PG, Gronchi A, Marchianò A, et al. Radiomic analysis of soft tissues sarcomas can distinguish intermediate from high-grade lesions. J Magn Reson Imaging. (2018) 47:829–40. doi: 10.1002/jmri.25791
8. Kothari G. Role of radiomics in predicting immunotherapy response. J Med Imaging Radiat Oncol. (2022) 66:575–91. doi: 10.1111/1754-9485.13426
9. Bologna M, Calareso G, Resteghini C, Sdao S, Montin E, Corino V, et al. Relevance of apparent diffusion coefficient features for a radiomics-based prediction of response to induction chemotherapy in sinonasal cancer. NMR Biomed (2022) 35: e4265. doi: 10.1002/nbm.4265
10. Zhang B, Ouyang F, Gu D, Dong Y, Zhang L, Mo X, et al. Advanced nasopharyngeal carcinoma: pre-treatment prediction of progression based on multi-parametric MRI radiomics. Oncotarget. (2017) 8:72457–65. doi: 10.18632/oncotarget.19799
11. Bologna M, Corino V, Calareso G, Tenconi C, Alfieri S, Iacovelli NA, et al. Baseline MRI-radiomics can predict overall survival in non-endemic EBV-related nasopharyngeal carcinoma patients. Cancers. (2020) 12:2958. doi: 10.3390/cancers12102958
12. Zhao L, Gong J, Xi Y, Xu M, Li C, Kang X, et al. MRI-based radiomics nomogram may predict the response to induction chemotherapy and survival in locally advanced nasopharyngeal carcinoma. Eur Radiol. (2020) 30:537–46. doi: 10.1007/s00330-019-06211-x
13. Ponsiglione A, Stanzione A, Cuocolo R, Ascione R, Gambardella M, De Giorgi M, et al. Cardiac CT and MRI radiomics: systematic review of the literature and radiomics quality score assessment. Eur Radiol. (2022) 32:2629–38. doi: 10.1007/s00330-021-08375-x
14. Shang J, Guo Y, Ma Y, Hou Y. Cardiac computed tomography radiomics: a narrative review of current status and future directions. Quant Imaging Med Surg. (2022) 12:3436–53. doi: 10.21037/qims-21-1022
15. Hu W, Wu X, Dong D, Cui L-B, Jiang M, Zhang J, et al. Novel radiomics features from CCTA images for the functional evaluation of significant ischaemic lesions based on the coronary fractional flow reserve score. Int J Cardiovasc Imaging. (2020) 36:2039–50. doi: 10.1007/s10554-020-01896-4
16. Lin A, Kolossváry M, Yuvaraj J, Cadet S, McElhinney PA, Jiang C, et al. Myocardial infarction associates with a distinct pericoronary adipose tissue radiomic phenotype. JACC Cardiovasc Imaging. (2020) 13:2371–83. doi: 10.1016/j.jcmg.2020.06.033
17. Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J. (2019) 40:3529–43. doi: 10.1093/eurheartj/ehz592
18. Shang J, Ma S, Guo Y, Yang L, Zhang Q, Xie F, et al. Prediction of acute coronary syndrome within 3 years using radiomics signature of pericoronary adipose tissue based on coronary computed tomography angiography. Eur Radiol. (2022) 32:1256–66. doi: 10.1007/s00330-021-08109-z
19. Nam K, Suh YJ, Han K, Park SJ, Kim YJ, Choi BW. Value of computed tomography radiomic features for differentiation of periprosthetic mass in patients with suspected prosthetic valve obstruction. Circ Cardiovasc Imaging. (2019) 12:e009496. doi: 10.1161/CIRCIMAGING.119.009496
20. Raisi-Estabragh Z, Jaggi A, Gkontra P, McCracken C, Aung N, Munroe PB, et al. Cardiac magnetic resonance radiomics reveal differential impact of sex, age, and vascular risk factors on cardiac structure and myocardial tissue. Front Cardiovasc Med. (2021) 8:763361. doi: 10.3389/fcvm.2021.763361
21. Izquierdo C, Casas G, Martin-Isla C, Campello VM, Guala A, Gkontra P, et al. Radiomics-based classification of left ventricular non-compaction, hypertrophic cardiomyopathy, and dilated cardiomyopathy in cardiovascular magnetic resonance. Front Cardiovasc Med. (2021) 8:764312. doi: 10.3389/fcvm.2021.764312
22. Rauseo E, Izquierdo Morcillo C, Raisi-Estabragh Z, Gkontra P, Aung N, Lekadir K, et al. New imaging signatures of cardiac alterations in ischaemic heart disease and cerebrovascular disease using CMR radiomics. Front Cardiovasc Med. (2021) 8:716577. doi: 10.3389/fcvm.2021.716577
23. Bologna M, Corino VDA, Montin E, Messina A, Calareso G, Greco FG, et al. Assessment of stability and discrimination capacity of radiomic features on apparent diffusion coefficient images. J Digit Imaging. (2018) 31:879–94. doi: 10.1007/s10278-018-0092-9
24. Gevaert O, Mitchell LA, Achrol AS, Xu J, Echegaray S, Steinberg GK, et al. Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology. (2014) 273:168–74. doi: 10.1148/radiol.14131731
25. Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. (2014) 5:4006. doi: 10.1038/ncomms5006
26. Raisi-Estabragh Z, Gkontra P, Jaggi A, Cooper J, Augusto J, Bhuva AN, et al. Repeatability of cardiac magnetic resonance radiomics: a multi-centre multi-vendor test-retest study. Front Cardiovasc Med. (2020) 7:586236. doi: 10.3389/fcvm.2020.586236
27. Pontone G, Moharem-Elgamal S, Maurovich-Horvat P, Gaemperli O, Pugliese F, Document reviewers, et al. Training in cardiac computed tomography: eACVI certification process. Eur Heart J Cardiovasc Imaging. (2018) 19:123–6. doi: 10.1093/ehjci/jex310
28. Leijenaar RT, Bogowicz M, Jochems A, Hoebers FJ, Wesseling FW, Huang SH, et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: a multicenter study. Br J Radiol. (2018) 91:20170498. doi: 10.1259/bjr.20170498
29. van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. (2017) 77:e104–7. doi: 10.1158/0008-5472.CAN-17-0339
30. Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. (2020) 295:328–38. doi: 10.1148/radiol.2020191145
31. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15:155–63. doi: 10.1016/j.jcm.2016.02.012
32. James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning: With applications in R. New York: Springer (2013). 426.
33. Blagus R, Lusa L. SMOTE for high-dimensional class-imbalanced data. BMC Bioinform. (2013) 14:106. doi: 10.1186/1471-2105-14-106
34. Molnar C. A guide for making black box models explainable, 2nd ed. (2022) Available at: https://christophm.github.io/interpretable-ml-book/
35. Pibarot P, Lancellotti P, Narula J. Concomitant cardiac amyloidosis in severe aortic stenosis: the Trojan horse? J Am Coll Cardiol. (2021) 77:140–3. doi: 10.1016/j.jacc.2020.11.007
36. Falk RH, Quarta CC. Echocardiography in cardiac amyloidosis. Heart Fail Rev. (2015) 20:125–31. doi: 10.1007/s10741-014-9466-3
37. Jung M-H, Chang S, Han EJ, Youn J-C. Multimodal imaging and biomarkers in cardiac amyloidosis. Diagnostics. (2022) 12:627. doi: 10.3390/diagnostics12030627
38. Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging. (2020) 13:1368–83. doi: 10.1016/j.jcmg.2019.07.015
39. Eckstein J, Körperich H, Weise Valdés E, Sciacca V, Paluszkiewicz L, Burchert W, et al. CMR-based right ventricular strain analysis in cardiac amyloidosis and its potential as a supportive diagnostic feature. IJC Heart Vasc. (2023) 44:101167. doi: 10.1016/j.ijcha.2022.101167
40. Goto S, Mahara K, Beussink-Nelson L, Ikura H, Katsumata Y, Endo J, et al. Artificial intelligence-enabled fully automated detection of cardiac amyloidosis using electrocardiograms and echocardiograms. Nat Commun. (2021) 12:2726. doi: 10.1038/s41467-021-22877-8
41. Zhou XY, Tang CX, Guo YK, Tao XW, Chen WC, Guo JZ, et al. Diagnosis of cardiac amyloidosis using a radiomics approach applied to late gadolinium-enhanced cardiac magnetic resonance images: a retrospective, multicohort, diagnostic study. Front Cardiovasc Med. (2022) 9:818957. doi: 10.3389/fcvm.2022.818957
42. Martini N, Aimo A, Barison A, Della Latta D, Vergaro G, Aquaro GD, et al. Deep learning to diagnose cardiac amyloidosis from cardiovascular magnetic resonance. J Cardiovasc Magn Reson. (2020) 22:84. doi: 10.1186/s12968-020-00690-4
43. Nitsche C, Scully PR, Patel KP, Kammerlander AA, Koschutnik M, Dona C, et al. Prevalence and outcomes of concomitant aortic stenosis and cardiac amyloidosis. J Am Coll Cardiol. (2021) 77:128–39. doi: 10.1016/j.jacc.2020.11.006
44. Oda S, Kidoh M, Takashio S, Inoue T, Nagayama Y, Nakaura T, et al. Quantification of myocardial extracellular volume with planning computed tomography for transcatheter aortic valve replacement to identify occult cardiac amyloidosis in patients with severe aortic stenosis. Circ Cardiovasc Imaging. (2020) 13:e010358. doi: 10.1161/CIRCIMAGING.119.010358
Keywords: cardiac amyloidosis, aortic stenosis, radiomics, radiomic feature stability, cardiac computed tomography
Citation: Lo Iacono F, Maragna R, Pontone G and Corino VDA (2023) A robust radiomic-based machine learning approach to detect cardiac amyloidosis using cardiac computed tomography. Front. Radiol. 3:1193046. doi: 10.3389/fradi.2023.1193046
Received: 24 March 2023; Accepted: 26 May 2023;
Published: 16 June 2023.
Edited by:
Mingchen Gao, University at Buffalo, United States© 2023 Lo Iacono, Maragna, Pontone and Corino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina D. A. Corino dmFsZW50aW5hLmNvcmlub0Bwb2xpbWkuaXQ=
 Francesca Lo Iacono
Francesca Lo Iacono Riccardo Maragna2
Riccardo Maragna2 Gianluca Pontone
Gianluca Pontone Valentina D. A. Corino
Valentina D. A. Corino