- 1Department of Health Administration and Management, Faculty of Health Sciences, College of Medicine, University of Nigeria, Enugu, Nigeria
- 2Health Policy Research Group, College of Medicine, University of Nigeria, Enugu, Nigeria
- 3Department of Psychology, Faculty of Social Sciences, University of Nigeria, Nsukka, Nigeria
- 4Department of Community Medicine, Institute of Public Health, College of Medicine, University of Nigeria, Enugu, Nigeria
- 5Leeds Institute of Health Sciences, University of Leeds, Leeds, United Kingdom
Background: Despite the availability of effective interventions, malaria and diarrhea continue to be leading causes of disease burden in Nigeria. Informal healthcare providers (IHPs) account for a significant proportion of health service providers in urban slums and may pose a challenge to service quality if they are untrained and unregulated. This study assessed IHPs' knowledge of the diagnosis and treatment of malaria and diarrhea.
Methodology: A cross-sectional quantitative study was conducted in eight urban informal settlements (slums) in southeast Nigeria. Data were collected from 235 informal health providers using an interviewer-administered questionnaire.
Results: The mean overall knowledge scores for malaria and diarrhea were 5.2 (95% CI: 4.3–6.1) and 5.4 (95% CI: 4.1–6.7), respectively, among the different IHPs. However, private medicine vendors (PMVs) and traditional birth attendants (TBAs) showed higher knowledge of treating malaria and diarrhea. Having more than 8 years of formal education and receiving on-the-job training had a statistically significant effect on adequate knowledge of malaria and diarrhea treatment.
Conclusion: Institutionalizing and strengthening service delivery through appropriate training and support for IHPs can improve the quality of health service delivery in urban slums.
Introduction
Communicable diseases, including malaria and diarrheal diseases, pose a significant challenge in many low- and middle-income countries (LMICs), such as Nigeria, as they disproportionately affect the poorest populations. Therefore, eliminating these diseases as public health concerns through appropriate prevention and control measures would greatly improve the health of the population.
Malaria remains a public health concern in Nigeria, with an estimated 68 million cases of malaria and ~194,000 malaria-related deaths occurring in 2021 (1). Furthermore, Nigeria is among the countries with the highest rates of malaria, with an estimated 25% of the global burden, leading households to incur catastrophic health expenditures due to the cost of treatment (2).
Similarly, diarrhea is a leading cause of childhood mortality and morbidity in developing countries such as Nigeria. The National Demographic Health Survey (30) reported that the prevalence of diarrhea in households with poor drinking water sources was slightly higher (16%) than in households with improved drinking water sources (12%) (2).
There has been rapid urbanization in Nigeria, which has led to increases in risk factors for many communicable diseases. In addition, rapid urbanization increases competition for scarce urban resources and underlines the need for policies that promote equitable access to resources (3). Poor sanitation and inadequate water supply predispose urban slum residents to diarrhea and create an environment conducive to breeding vectors for infectious diseases such as malaria. This problem is compounded by weak healthcare systems that lack sufficient infrastructure to cope with these burdens (4).
The growth rate of the urban population in Nigeria outpacing its economic growth, surpassing the capacity of the country's formal health system to adequately provide care, especially in urban slums (5). Therefore, it has become evident that the public sector alone cannot achieve the United Nations' Sustainable Development Goal 3 (SDG3) and universal health coverage. Collaboration and partnerships with private sector actors, including both formal and informal healthcare providers, are essential (6).
Informal healthcare providers (patent medicine vendors—PMVs and traditional birth attendants—TBAs, traditional bone setters, and herbal medicine practitioners) account for a significant proportion of healthcare providers in Nigeria, delivering care, especially in under-resourced communities (7). They are often the first point of care for common childhood and adult illnesses for those living in urban informal settlements in Nigeria (8). In communities with non-functional or inaccessible formal health systems, informal healthcare providers (IHPs) bridge the gap in access to healthcare services, even for households that would typically prefer to use formal (public) healthcare facilities (9, 10).
Widely available IHPs present opportunities to improve access to appropriate essential health services in underserved urban areas in many LMICs (7). However, IHPs lack the necessary formal training, proper regulation, and oversight (11, 12), which can compromise the quality and safety of the healthcare services they offer. While the contributions to healthcare delivery by IHPs in informal settlements are indisputable, the overarching concern of public health experts hinges on issues surrounding their knowledge and the quality of healthcare services they render.
Training IHPs to correctly recognize and manage common communicable diseases in urban slums (such as malaria and diarrheal diseases) will contribute to better healthcare for their clients. Several studies have highlighted the lack of understanding of malaria and diarrheal case management among informal providers (13–15). However, there is a paucity of evidence regarding the level of knowledge among informal health providers on the diagnosis and treatment of both malaria and diarrhea in urban informal settlements.
This is part of a larger study that involved informal providers in selected urban slums and formal providers in primary health centers serving these slums. This study provides new knowledge into the level of knowledge among informal health providers in slums regarding the diagnosis and treatment of communicable diseases, with a particular focus on malaria and diarrheal diseases, as well as the factors influencing such knowledge.
Methods
Study area
The study was conducted in eight urban slums informal settlements, each in Enugu and Anambra states in southeastern Nigeria. Enugu state had a population of ~4.4 million in 2019, while Anambra state had a population of 5.6 million in 2019 with an estimated 2.83% annual growth rate (1). Administratively, Enugu and Anambra states have 17 and 21 local government areas, respectively. Urban dwellers in both states are mostly civil servants, traders, transporters, or artisans.
It was a quantitative cross-sectional study. The study population was informal healthcare providers working in the purposively selected informal settlements. The eight informal settlements were purposively selected based on the size of the settlement, availability of different IHPs, and a functional primary health center close to the informal settlement. Areas with security challenges were excluded from the study. Enugu urban informal settlements included in the study were Afia Nine, Ugbo Oghe, Ngenevu, and Ikilike, while in Anambra state, the informal settlements sampled were Okpoko 4, Okpoko 5, Ibollo, and Prison Marine.
Data were collected from the heads of informal health facilities operating within the informal settlements using a pre-tested structured questionnaire. However, in their absence, any senior health provider in the facility was surveyed. Pre-testing of the instrument was performed in two slums not included in the study to assess the clarity and simplicity of the questionnaire items. The questionnaire was also reviewed by content and program experts to ensure the appropriateness of wordings and correct placement of items in the questionnaire. The survey was carried out between October and December 2022, with a computed minimum sample size of 256 providers including formal healthcare providers. The sample size was computed using the guidelines outlined in the Demographic and Health Survey (DHS) listing manual (32). A total of 235 informal health providers, including patent medicine vendors (PMVs), traditional birth attendants (TBAs), bonesetters, traditional healers, and herbal medicine dealers, participated in the survey, and their data were included in this study. Data from formal health providers, however, were excluded from this study.
The questionnaire was structured into the following seven sections: (1) background of the respondent (facility type, status in the facility, and training status); (2) knowledge, attitude, and practice (malaria and diarrhea); (3) treatment provision history; (4) motives and incentives; (5) challenges; (6) solutions for improving health provisions; and (7) determining the quality of malaria treatment as a case study, among others.
Data analysis
Using SPSS (version 25), we calculated the frequency distributions of the key variables, including background characteristics of respondents and knowledge, diagnosis, and treatment of malaria and diarrhea. Using R statistical software (version 3.5.2), we calculated inferential statistics in the form of point estimates and 95% confidence intervals to draw conclusions from responses to the relevant questions. These statistics were presented as means and their associated 95% confidence intervals, as the questions had numerical responses. The relationship between the knowledge score and respondents' characteristics was analyzed.
Knowledge was summarized as composite scores, and two categories (adequate and inadequate knowledge) were generated. These two categories of knowledge are supported by the literature (16). The scores were added up to create a knowledge ranking for the aforementioned categories. The pooled scores of questions were classified into inadequate and adequate knowledge using median (50%) score values. Inadequate knowledge was labeled as ‘1', and adequate knowledge was labeled as ‘0'.
Ethical consideration
Ethical approval for the study was obtained from the research ethics committees of the University of Nigeria Teaching Hospital, Enugu, and the University of Leeds. All the respondents gave informed verbal consent and were assured of total anonymity.
Results
Demographic characteristics of respondents
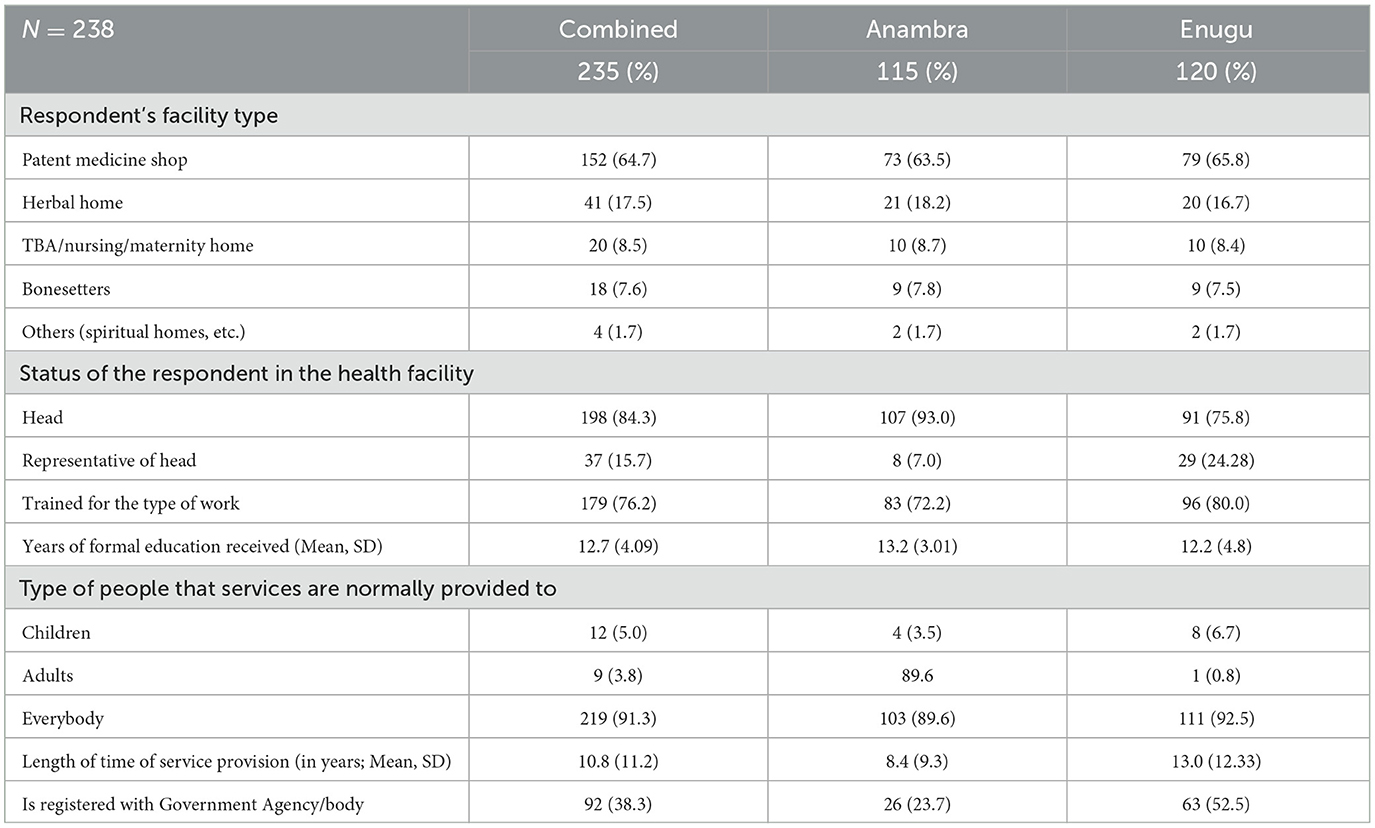
As shown in Table 1, patent medicine vendors were the most common informal health providers in the informal settlements, making up 73% and 72% of those surveyed in Anambra and Enugu states, respectively. It was found that 84.7% of the respondents were heads of the unit. On average, the respondents had up to 13.4 years of formal education and 76.2% received training for the job. More than 90% of the respondents provide services for both adults and children and have been providing healthcare services for more than 19 years. However, only 38% of the facilities are registered with the government agency/body (Table 1).
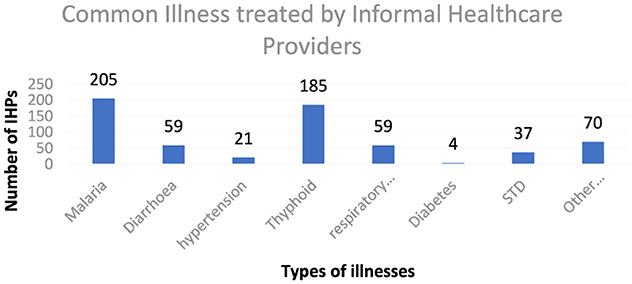
According to the findings in Figure 1, malaria accounted for the most common illness treated by informal providers, with over 90% of the IHPs identifying it as the most common illness they treated in their facilities. This is followed by typhoid, bone setting, respiratory tract infection, diarrhea, hypertension, and others. Across the two states, these common illnesses were also identified by the respondents as the same ones they treat in their facilities.
Knowledge, diagnosis, and treatment of malaria
Figure 2A shows that 211 individuals (87.6% of the respondents) had the knowledge that malaria was caused by mosquitoes, and 131 individuals (52.4% of the respondents) attributed the increase in the number of malaria cases to poor sanitation. However, 20 persons (8.0% of the respondents) were not sure of what caused malaria. Figure 2B shows that for the diagnosis of malaria, 182 respondents (75.7%) were diagnosed by recognizing the symptoms and 112 respondents (47.7%) were diagnosed using physical examination. Only 27 (10.5%) and 15 (6%) of the respondents were diagnosed with malaria using a rapid diagnostic test or microscopic examination of blood slides, respectively. Figure 2C shows that ~204 (85%) and 177 (73.5%) respondents recognized fever and headache as key symptoms of malaria. As shown in Figure 2D, the majority of the respondents, 180 (74.8%), identified Artemisinin Combination Therapy as a treatment for malaria; however, 8.5% of the respondents either did not know or were unsure about the appropriate treatment.
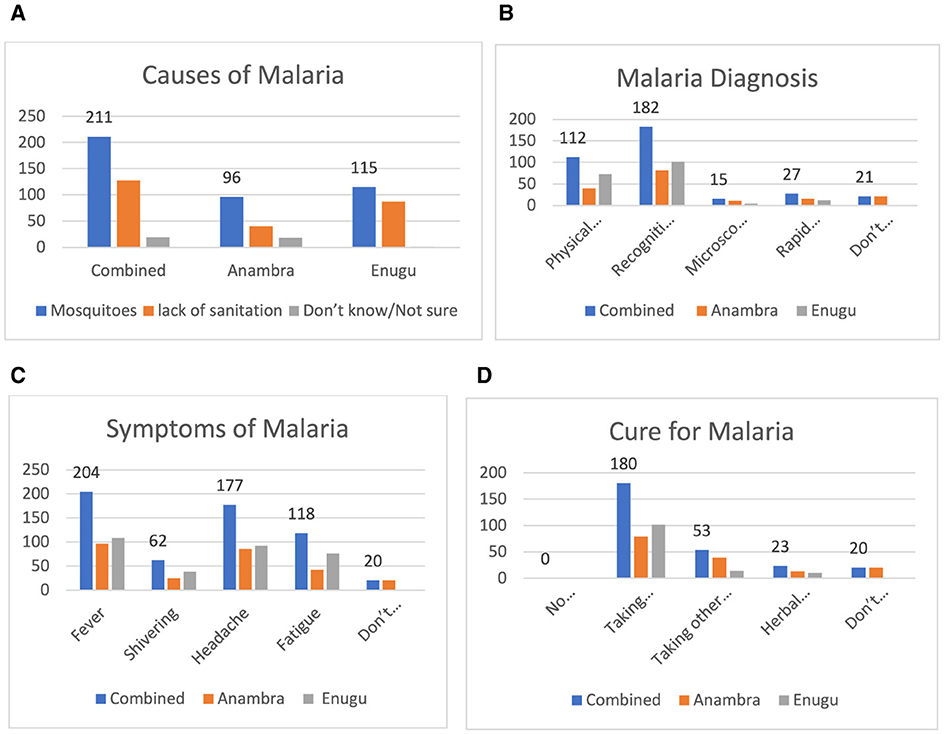
Figure 2. (A) Causes of malaria. (B) How malaria is diagnosed. (C) Responses for symptoms of malaria. (D) Responses on cure for malaria.
Knowledge, diagnosis, and treatment of diarrhea
Figure 3A shows that 146 (61.3%) and 117 respondents (50.8%) recognized that the causes of diarrhea were contaminated food and poor sanitation, respectively. However, 24 persons (10.0% of the respondents) were not sure of what caused diarrhea. Figure 3B shows that for the diagnosis of diarrhea, the majority of individuals, 193 (80.7%), were diagnosed by recognizing the symptoms, and 85 individuals (47.7%) were diagnosed using physical examination. Only 12 individuals (5% of the respondents) were diagnosed with diarrhea using microscopic examination of stools. Figure 3C shows that 167 (68.5%0 and 165 (68.3%) of the respondents identified the symptoms of diarrhea as loose stool and running stomach, respectively. Figure 3D shows that antibiotics and oral rehydration therapy (ORT) were identified as the treatment for diarrhea by 158 (65.5%) and 139 (57.6%) of the respondents, respectively.
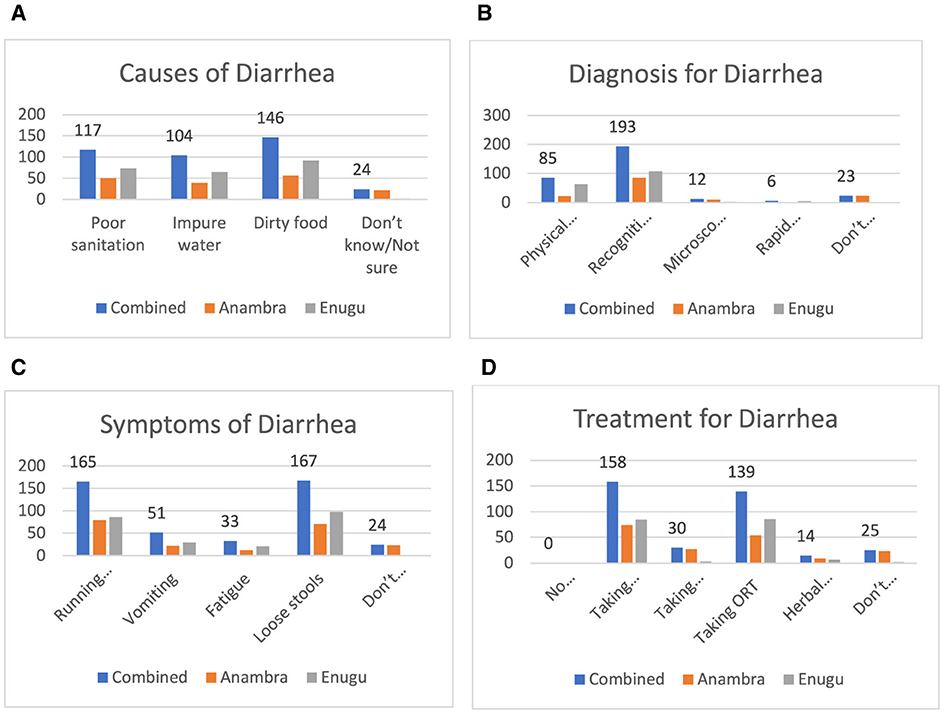
Figure 3. (A) Causes of diarrhea. (B) How diarrhea is diagnosed. (C) Symptoms of diarrhea. (D) Responses on cure for diarrhea.
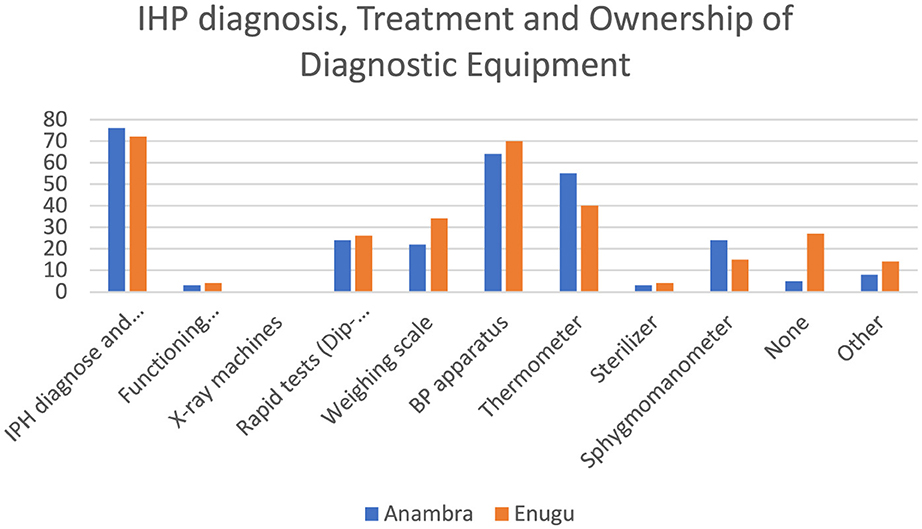
Figure 4 shows that more than half of the respondents were involved in both diagnosing illnesses and providing treatment. In terms of minimum standard equipment for health providers across both states, only ~50% had a blood pressure apparatus and 43% had a thermometer in their facilities. Less than 3% of the respondents had a functional microscope.
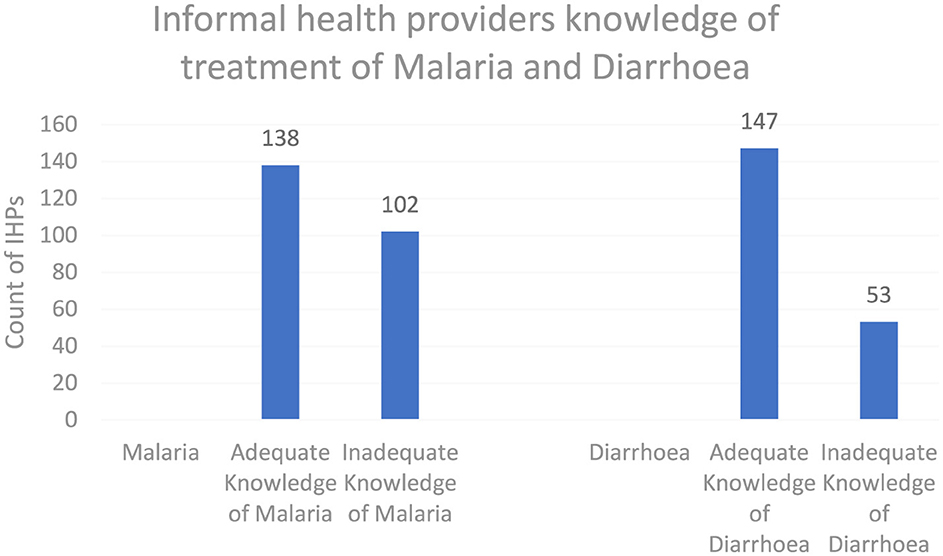
As shown in Figure 5, the knowledge score results, when categorized into adequate and inadequate knowledge levels for both malaria and diarrhea, showed that 57.5 and 61.2% of informal healthcare providers had adequate knowledge, respectively.
As shown in Table 2, a simple linear regression model estimated the mean knowledge scores for malaria and diarrhea at 95% CI to be 5.2 (4.3–6.1) and 5.4 (4.1–6.7), respectively. For these outcome variables, the model did not include any independent variable, as it only describes the central tendency of the knowledge scores and therefore does not provide any insights into the relationship between knowledge scores and other predictors.
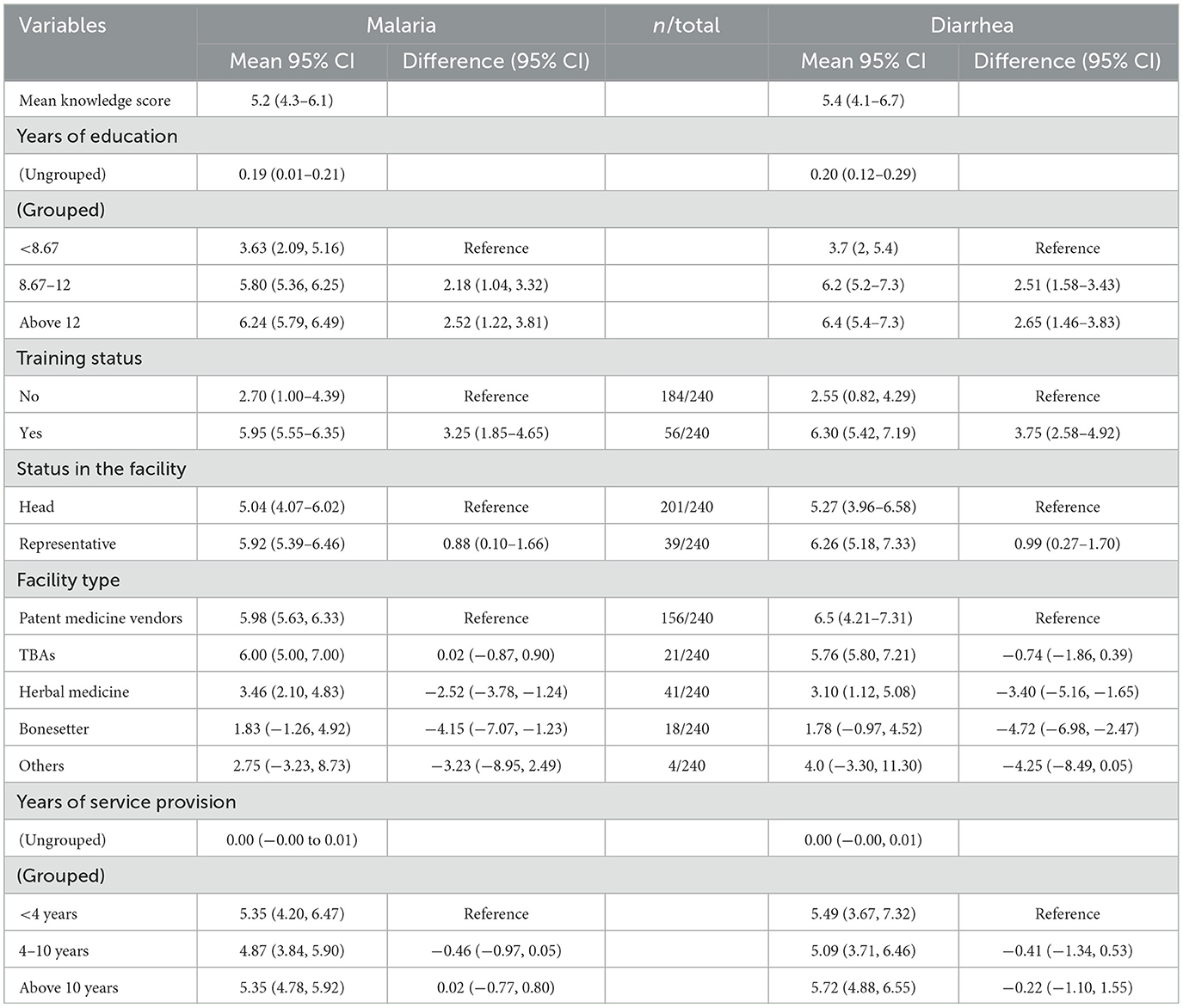
Table 2. Association between knowledge score (for malaria and diarrhea) and demographic characteristics.
Respondents with more than 8 years of education had higher knowledge scores on malaria than those with fewer years of education. Specifically, those with 8.67–12 years of education had a mean score of 5.80 (95% CI: 5.36, 6.25), which was 2.18 points higher (95% CI: 1.04, 3.32) than the reference group with < 8.67 years of education. Respondents with more than 12 years of education had an even higher mean score of 6.24 (95% CI: 5.79, 6.49), showing a difference of 2.52 points (95% CI: 1.22, 3.81) compared to the reference group.
Training status also significantly influenced malaria knowledge scores. Respondents without training had a mean score of 2.70 (95% CI: 1.00, 4.39), while those with training had a mean score of 5.95 (95% CI: 5.55, 6.35), indicating a substantial increase of 3.25 points (95% CI: 1.85, 4.65).
In terms of facility status, heads of facilities had a mean score of 5.04 (95% CI: 4.07, 6.02), whereas representatives had a mean score of 5.92 (95% CI: 5.39, 6.46), with a difference of 0.88 points (95% CI: 0.10, 1.66).
Facility type significantly impacted the knowledge scores. Patent medicine vendors had a mean score of 5.98 (95% CI: 5.63, 6.33), while those practicing herbal medicine and bone setting had lower mean scores of 3.46 (95% CI: 2.10, 4.83) and 1.83 (95% CI: −1.26, 4.92), respectively. These differences were −2.52 points (95% CI: −3.78, −1.24) and −4.15 points (95% CI: −7.07, −1.23) when compared to patent medicine vendors.
Years of service provision, whether grouped or ungrouped, showed minimal impact on knowledge scores. Respondents with < 4 years of service had a mean score of 5.35 (95% CI: 4.20, 6.47), while those with 4–10 years had a mean score of 4.87 (95% CI: 3.84, 5.90), with a difference of −0.46 points (95% CI: −0.97, 0.05). Respondents with more than 10 years of service had a mean score of 5.35 (95% CI: 4.78, 5.92), showing a negligible difference of 0.02 points (95% CI: −0.77, 0.80).
Diarrhea
Respondents' knowledge scores on diarrhea were also influenced by their years of education. Those with 8.67–12 years of education had a mean score of 6.2 (95% CI: 5.2, 7.3), which was 2.51 points higher (95% CI: 1.58, 3.43) than the reference group with < 8.67 years of education. Similarly, respondents with more than 12 years of education had a mean score of 6.4 (95% CI: 5.4, 7.3), showing a difference of 2.65 points (95% CI: 1.46, 3.83) compared to the reference group.
Training status had a marked effect on knowledge scores for diarrhea. Respondents without training had a mean score of 2.55 (95% CI: 0.82, 4.29), while those with training had a mean score of 6.30 (95% CI: 5.42, 7.19), reflecting an increase of 3.75 points (95% CI: 2.58, 4.92).
Facility status showed that heads of facilities had a mean score of 5.27 (95% CI: 3.96, 6.58), while representatives had a mean score of 6.26 (95% CI: 5.18, 7.33), with a difference of 0.99 points (95% CI: 0.27, 1.70).
Knowledge scores varied significantly with the type of facility. Patent medicine vendors had a mean score of 6.5 (95% CI: 4.21, 7.31). In contrast, those practicing herbal medicine and bone setting had lower mean scores of 3.10 (95% CI: 1.12, 5.08) and 1.78 (95% CI: −0.97, 4.52), with differences of −3.40 points (95% CI: −5.16, −1.65) and −4.72 points (95% CI: −6.98, −2.47) compared to patent medicine vendors.
Years of service provision, whether grouped or ungrouped, had a minimal effect on diarrhea knowledge scores. Respondents with < 4 years of service had a mean score of 5.49 (95% CI: 3.67, 7.32), while those with 4–10 years had a mean score of 5.09 (95% CI: 3.71, 6.46), with a difference of −0.41 points (95% CI: −1.34, 0.53). Respondents with more than 10 years of service had a mean score of 5.72 (95% CI: 4.88, 6.55), with a difference of −0.22 points (95% CI: −1.10, 1.55).
Discussion
The findings that informal health providers deliver healthcare for common illnesses for both adults and children were expected from the large numbers in the informal settlements. Our findings are consistent with the evidence from other studies that informal health providers are a crucial source of healthcare for urban poor populations and communicable diseases, which include malaria and diarrhea (17).
The findings on the availability of diagnostic equipment showed that the majority of IHPs lack basic equipment for providing care in their facilities. Therefore, they rely mostly on patient history and symptoms to provide care. The findings are similar to evidence from Bangladesh that showed that in addition to knowledge gaps, IHPs lacked diagnostic tools and medicines to deliver services effectively (18). The implications are that they may provide inaccurate diagnoses and delayed treatments, impacting the quality of care.
The findings on the method of diagnosis showed that the primary way informal providers diagnose malaria is by recognition of symptoms. This is similar to the findings by Ayandipo et al. (31) in Nigeria, where over 85% of health providers exhibited very poor malaria diagnostic practices. This differs from the WHO case management protocol for malaria, which recommends microscopy, RDTs, or both for the diagnosis of malaria. In this study conducted in Enugu and Anambra states in Nigeria, only a few IHPs diagnosed malaria using a rapid diagnostic test or microscopic examination of blood slides. It implies that they either do not recognize the need or that they lack the competencies and equipment for correct diagnosis (19). Whichever is the case, improper diagnosis of malaria can lead to antimalarial drug resistance arising from inappropriate treatment.
Furthermore, antimalarial drug resistance could be a result of inappropriate treatment and the use of substandard drugs. The use of inappropriate treatment and substandard drugs may be an important contributor to the emergence and spread of antimicrobial resistance (AMR) (20, 21). AMR is a global public health problem, with resistance to antibiotics causing an estimated 1.3 million deaths in 2019 (22).
Training on the job, years of education, and the type of facility had a significant impact on knowledge regarding the treatment of malaria and diarrhea. Furthermore, those who received on-the-job training, had more years of education, and worked as PMV or TBAs demonstrated better knowledge of treating malaria and diarrhea. This could imply that they may have an innate ability to understand and internalize the information received during training, which may translate into improved access to quality care and better patient outcomes. These findings are consistent with a previously published study that showed a positive association between training and knowledge of non-communicable diseases among IHPs (23).
However, evidence shows that deploying training alone may have only a modest impact on performance (24). Therefore, it is recommended that supportive supervision, job aides, and both financial and non-financial incentives be incorporated to encourage good practices. These combined strategies have been found to be effective in improving the performance of IHPs (25).
There may be an urgent need to provide support and appropriate training strategies for informal health providers to ensure quality healthcare, especially for communicable diseases. Training and providing support has been found to be one of the ways of improving knowledge of diagnosis and treatment of any disease (26). We therefore recommend the use of appropriate training, job aides, and support for informal health providers to improve their diagnosis and treatment of malaria and diarrhea.
Furthermore, although IHPs have different educational backgrounds and practices, the availability of mobile technologies could allow for context-specific information (in the local language) to be made accessible to them. This would provide up-to-date knowledge of malaria and other common ailments. Digital technologies have been found to promote health literacy and empowerment by providing clear, reliable, and accessible information (27, 28) in a fun and effective way (29). This could facilitate improvements in the provision of quality health services by IHPs.
The study used a small sample size and therefore may not be generalizable; however, the inclusion of different types of informal providers may have made the study more robust. The use of only quantitative methods is also a limitation as there could be bias in the respondent's answer, and it cannot be used to infer certain factors. However, the study has provided valuable information on informal health providers.
Informal health providers' knowledge of the diagnosis and treatment of malaria and diarrhea is inadequate. Strengthening service delivery through appropriate training and provision of health literacy tools for the different types of informal health providers has the potential to improve health service delivery in urban informal settlements. Ensuring quality service delivery through intervention is essential for the achievement of SDG3 (good health and wellbeing) and SDG4 (quality education). Providing quality education will, in turn, lead to improved quality of service delivery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was obtained from the Health Research Ethics Committee of University of Nigeria Teaching Hospital (NHREC/05/01/2008B-FWA00002458-IRB00002323) and the University of Leeds (MREC 21-009 Amd). Informed consent was obtained from all participants after explaining the study objectives, benefits and risks of participating in the study, and their rights to voluntary participation and confidentiality of data. Written consent was obtained from all the participants before they were interviewed.
Author contributions
IA: Writing – original draft. JO: Writing – original draft. BO: Writing – review & editing. CM: Writing – review & editing. OO: Writing – review & editing. BE: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research leading to these results was funded by UK aid from the British people through the Community-led Responsive and Effective Urban Health Systems (CHORUS) research project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
3. Onwujekwe O, Mbachu CO, Ajaero C, Uzochukwu B, Agwu P, Onuh J, et al. Analysis of equity and social inclusiveness of national urban development policies and strategies through the lenses of health and nutrition. Int J Equity Health. (2021) 20:101. doi: 10.1186/s12939-021-01439-w
4. Oleribe OO, Momoh J, Uzochukwu BS, Mbofana F, Adebiyi A, Barbera T, et al. Identifying key challenges facing healthcare systems in Africa and potential solutions. Int J Gen Med. (2019) 12:395–403. doi: 10.2147/IJGM.S223882
5. Aliyu AA, Amadu L. Urbanization, cities, and health: the challenges to Nigeria - a review. Ann Afr Med. (2017) 16:149–58. doi: 10.4103/aam.aam_1_17
6. WHO. Engaging the Private Health Service Delivery Sector through Governance in Mixed Health Systems: Strategy Report of the WHO Advisory Group on the Governance of the Private Sector for Universal Health Coverage. Geneva: WHO (2020).
7. Onwujekwe O, Mbachu C, Onyebueke V, Ogbozor P, Arize I, Okeke C, et al. Stakeholders' perspectives and willingness to institutionalize linkages between the formal health system and informal healthcare providers in urban slums in Southeast, Nigeria. BMC Health Serv Res. (2022) 22:583. doi: 10.1186/s12913-022-08005-2
8. Onyeonoro UU, Ogah OS, Ukegbu AU, Chukwuonye II, Madukwe OO, Moses AO, et al. urban-rural differences in health-care-seeking pattern of residents of Abia State, Nigeria, and the implication in the control of NCDs. Health Services Insights. (2016) 9:29–36. doi: 10.4137/HSI.S31865
9. Bloom G, Standing H, Lucas H, Bhuiya A, Oladepo O, Peters DH, et al. Making health markets work better for poor people: the case of informal providers. Health Policy Plann. (2011) 26(Suppl. 1):i45–52. doi: 10.1093/heapol/czr025
10. Oleffe A, Sako B, Paul E, Mahieu C. Formal and informal medicine retailers in sub-Saharan Africa: a scoping review of research trends. Int J Pharm Pract. (2022) 30:315–25. doi: 10.1093/ijpp/riac038
11. Odii A, Arize I, Agwu P, Mbachu C, Onwujekwe O. To what extent are informal healthcare providers in slums linked to the formal health system in providing services in Sub-Sahara Africa? A 12-year scoping review. J Urban Health. (2024) 101:1248–58. doi: 10.1007/s11524-024-00885-5
12. Sudhinaraset M, Ingram M, Lofthouse HK, Montagu D. What is the role of informal healthcare providers in developing countries? A systematic review. PLoS ONE. (2013) 8:e54978. doi: 10.1371/journal.pone.0054978
13. Awasthi KR, Jancey J, Clements ACA, Leavy JE. A qualitative study of knowledge, attitudes and perceptions towards malaria prevention among people living in rural Upper River Valleys of Nepal. PLoS ONE. (2022) 17:e0265561. doi: 10.1371/journal.pone.0265561
14. Liheluka EA, Massawe IS, Chiduo MG, Mandara CI, Chacky F, Ndekuka L, et al. Community knowledge, attitude, practices and beliefs associated with persistence of malaria transmission in north-western and southern regions of Tanzania. Malar J. (2023) 22:304. doi: 10.1186/s12936-023-04738-5
15. Ravi N, Holsted E, Kadiebwe B, Salthouse A, Sattar A. A malaria knowledge, attitudes and practice survey in a rural community in Guinea. Malar J. (2022) 21:329. doi: 10.1186/s12936-022-04357-6
16. Fatmi Z, Mahmood S, Hameed W, Qazi I, Siddiqui M, Dhanwani A, et al. Knowledge, attitudes and practices towards COVID-19 among Pakistani residents: information access and low literacy vulnerabilities. East Mediterr Health J. (2020) 26:1446–55. doi: 10.26719/emhj.20.133
17. Arize I, Ogbuabor D, Mbachu C, Etiaba E, Uzochukwu B, Onwujekwe O, et al. Stakeholders' perspectives on the unmet needs and health priorities of the urban poor in south-east Nigeria. Community Health Equity Res Policy. (2023) 43:389–98. doi: 10.1177/0272684X211033441
18. Sizear MMI, Nababan HY, Siddique MKB, Islam S, Paul S, Paul AK, et al. Perceptions of appropriate treatment among the informal allopathic providers: insights from a qualitative study in two Peri-Urban Areas in Bangladesh. BMC Health Serv Res. (2019) 19:424. doi: 10.1186/s12913-019-4254-3
19. Sudhinaraset M, Briegleb C, Aung M, Khin HSS, Aung T. Motivation and challenges for use of malaria rapid diagnostic tests among informal providers in myanmar: a qualitative study. Malar J. (2015) 14:61. doi: 10.1186/s12936-015-0585-7
20. Cavany S, Nanyonga S, Hauk C, Lim C, Tarning J, Sartorius B, et al. The uncertain role of substandard and falsified medicines in the emergence and spread of antimicrobial resistance. Nat Commun. (2023) 14:6153. doi: 10.1038/s41467-023-41542-w
21. Holmes AH, Moore LSP, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. (2016) 387:176–87. doi: 10.1016/S0140-6736(15)00473-0
22. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
23. Mbachu CO, Arize I, Obi C, Ebenso B, Elsey H, Onwujekwe O, et al. Assessing knowledge of hypertension and diabetes mellitus among informal healthcare providers in urban slums in Southeastern Nigeria. Discover Public Health. (2024) 21:21. doi: 10.1186/s12982-024-00143-8
24. Shah NM, Brieger WR, Peters DH. Can interventions improve health services from informal private providers in low and middle-income countries?: a comprehensive review of the literature. Health Policy Plann. (2011) 26:275–87. doi: 10.1093/heapol/czq074
25. Gautham M, Shyamprasad KM, Singh R, Zachariah A, Singh R, Bloom G, et al. Informal rural healthcare providers in North and South India. Health Policy Plan. (2014) 29(Suppl 1):i20–29. doi: 10.1093/heapol/czt050
26. Uzochukwu BSC, Ossai EN, Okeke CC, Ndu AC, Onwujekwe OE. Malaria knowledge and treatment practices in enugu state, nigeria: a qualitative study. Int J Health Policy Manag. (2018) 7:859–66. doi: 10.15171/ijhpm.2018.41
27. Shekoni O, Iversen S, Diaz GJ, Aune A, Ubuane PO, Imam Z, et al. Healthcare Workers' perceptions about the use of mobile health technologies in public health facilities in Lagos, Nigeria. SAGE Open Med. (2024) 12:20503121231224568. doi: 10.1177/20503121231224568
28. Soares S, Hoffmeister LV, Fernandes MDF, Henriques A, Costa A. The use of digital technologies in the promotion of health literacy and empowerment of informal caregivers: scoping review. JMIR Aging. (2024) 7:e54913. doi: 10.2196/54913
29. Zaman SB, Khan RK, Evans RG, Thrift AG, Maddison R, Shariful Islam SM, et al. Exploring barriers to and enablers of the adoption of information and communication technology for the care of older adults with chronic diseases: scoping review. JMIR Aging. (2022) 5:e25251. doi: 10.2196/25251
30. National Population Commission (NPC) [Nigeria] ICF Macro. Nigeria Demographic and Health Survey 2018. Abuja; Rockville, MD: NPC and ICF (2019).
31. Ayandipo EO, Babatunde D, Afolayan O, Kalejaye O, Obembe T. Assessing the knowledge and practices of primary healthcare workers on malaria diagnosis and related challenges in view of COVID-19 outbreak in a Nigerian Southwestern metropolis. PLoS Glob Public Health. (2023) 3:e0000625. doi: 10.1371/journal.pgph.0000625
32. USAID. Sampling and Household Listing Manuel: Demographic and Health Surveys Methodology. United States Agency for International Development (USAID) (2012). Available online at: https://dhsprogram.com/pubs/pdf/DHSM4/DHS6_Sampling_Manual_Sept2012_DHSM4.pdf
Keywords: malaria, diarrhea, informal health providers, urban slums, health services
Citation: Arize I, Ozughalu J, Okechi B, Mbachu C, Onwujekwe O and Ebenso B (2025) Assessing informal healthcare providers' knowledge of diagnosis and treatment of malaria and diarrhea: evidence from urban informal settlements in Southeast Nigeria. Front. Public Health 13:1556996. doi: 10.3389/fpubh.2025.1556996
Received: 07 January 2025; Accepted: 19 February 2025;
Published: 12 March 2025.
Edited by:
Nyi Nyi Naing, Sultan Zainal Abidin University, MalaysiaReviewed by:
George Grant, Independent Researcher, Aberdeen, United KingdomJithin Surendran, Kasturba Medical College, Mangalore, India
Copyright © 2025 Arize, Ozughalu, Okechi, Mbachu, Onwujekwe and Ebenso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ifeyinwa Arize, aWZleWlud2EuYXJpemVAdW5uLmVkdS5uZw==
 Ifeyinwa Arize
Ifeyinwa Arize Joy Ozughalu2
Joy Ozughalu2 Chinyere Mbachu
Chinyere Mbachu Obinna Onwujekwe
Obinna Onwujekwe Bassey Ebenso
Bassey Ebenso