- 1Department of Dermatology, Peking University Third Hospital, Beijing, China
- 2Department of Immunology, School of Basic Medical Sciences, NHC Key Laboratory of Medical Immunology, Peking University, Beijing, China
Background: Organophosphate pesticides (OPPs) are widely used environmental chemicals with potential health impacts, but their relationship with atopic dermatitis (AD) remains unclear.
Methods: Using data from the National Health and Nutrition Examination Survey (NHANES) 1999–2007, we investigated associations between urinary OPP metabolites and AD in 4,258 adults. Six dialkyl phosphate (DAP) metabolites were measured, and weighted quantile sum (WQS) regression was used to assess mixture effects.
Results: Both DMP (odds ratio [OR] = 1.17, 95% confidence interval [CI]: 1.05–1.31) and DMDTP (OR = 2.23, 95%CI: 1.08–4.60) showed significant positive associations with AD in fully adjusted models. WQS regression revealed significant associations between mixed OPP exposure and AD (OR = 1.25, 95%CI: 1.04–1.50), with DMP contributing most (45.8%) to the mixture effect. Stratified analyses indicated stronger associations in males, younger adults (<60 years), and smokers.
Conclusion: Our findings suggest that OPP exposure, particularly DMP, may be associated with increased AD risk in adults. These results provide new insights into environmental risk factors for AD.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin condition characterized by intense itching, skin barrier dysfunction and immune dysregulation (1). It represents a significant global health challenge, affecting approximately 2 billion people worldwide with a prevalence of 2.62% (2). The condition demonstrates notable demographic variations, with higher prevalence in children (3.96%) compared to adults (1.95%), and females (2.80%) versus males (2.44%) (3). Epidemiological studies show that the one-year prevalence ranges from 1.2 to 17.1% in adults and 0.96 to 22.6% in children (3). Particularly in developing regions, pediatric AD cases increased significantly between 1990 and 2019, with economically disadvantaged regions experiencing a 96.77% increase (4). Beyond its physical manifestations, AD significantly impacts patients’ quality of life, causing sleep disturbance, psychological distress, and social isolation, which substantially affects both patients and their families (5). This burden is further complicated by healthcare access disparities, with significant barriers in treatment accessibility, including registration challenges, costs, and insurance coverage issues (6).
The etiology of AD involves multiple factors, characterized by complex interactions between genetic and environmental factors. In terms of genetics, studies have identified over 30 genetic loci associated with AD, primarily involving skin barrier function and immune regulation, with loss-of-function mutations in the filaggrin gene representing the strongest known genetic risk factor (7, 8). Additionally, research has shown that parental history of atopic disease significantly increases the risk of AD in offspring (9). Beyond genetic factors, environmental factors also play crucial roles in both pathogenesis and clinical outcomes, including hygiene conditions, microbiota, endotoxin exposure, animal exposure, pollution, weather conditions, and dietary factors (10). Among various environmental exposures, organophosphorus pesticides (OPPs) have emerged as a subject of particular concern due to their widespread use and potential health implications. These compounds, commonly used in agricultural practices, demonstrate remarkable persistence in both terrestrial and aqueous environments (11).
The potential threat of OPPs to human health warrants significant attention, primarily due to their ability to modulate immune system function through multiple mechanisms (12, 13). These compounds mainly regulate inflammatory responses through the cholinergic pathway by interfering with cholinesterase activity, thereby affecting cytokine production and the expression of pro-inflammatory molecules (14). Experimental studies have demonstrated that OPP exposure can induce oxidative stress responses, leading to adverse effects on various biological systems, including immune function (15). Monitoring of human biological samples including urine has revealed the widespread presence of these compounds, indicating that populations are commonly exposed to OPPs (16). More importantly, research has found that both acute and chronic exposure to OPPs may induce chronic degenerative pathological changes and persistent inflammatory conditions (14), and this immune system dysregulation further highlights the potential health risks of OPP exposure.
Epidemiological studies have confirmed associations between pesticide exposure and various immune-mediated diseases. Research shows that pesticide exposure is significantly associated with increased risks of autoimmune diseases such as rheumatoid arthritis (17) and systemic lupus erythematosus (18). As an important class of pesticides, the relationship between OPPs and AD remains largely unexplored. This knowledge gap is particularly concerning given several critical factors. First, the increasing global use of OPPs, especially in developing countries, raises concerns about their potential contribution to the rising prevalence of AD (19). Second, while acute toxicity of OPPs is well documented, their chronic effects on immune-mediated diseases remain poorly understood (20). Third, identifying environmental risk factors for AD is crucial for developing prevention strategies (21), particularly in populations with high pesticide exposure. The potential impact of OPPs on allergic diseases represents a significant public health concern that requires urgent investigation (22).
Therefore, this study is dedicated to elucidating this potential connection. Our primary research questions were: (1) Is there an association between OPP exposure and AD risk in the general adult population? (2) Do different OPP metabolites contribute differently to AD risk? (3) Are there specific population subgroups more susceptible to OPP-associated AD risk? Based on previous evidence showing OPPs’ effects on immune function and inflammatory responses, we hypothesized that increased OPP exposure would be associated with higher AD risk, and this association might vary across different population subgroups due to variations in metabolic capacity. This study aims to provide new clinical evidence for optimizing prevention strategies and treatment approaches for AD.
Methods
Study population and data source
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative survey conducted by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC), designed to assess the health and nutritional status of the U.S. population. NHANES employs a complex multistage stratified probability sampling method, with biennial cycles, selecting representative samples from counties across the United States and conducting comprehensive health and nutrition assessments. Based on the concurrent availability of data on OPP metabolites and AD, this study utilized data from four survey cycles: 1999–2000, 2001–2002, 2003–2004, and 2005–2007. These data are publicly accessible through the CDC website.
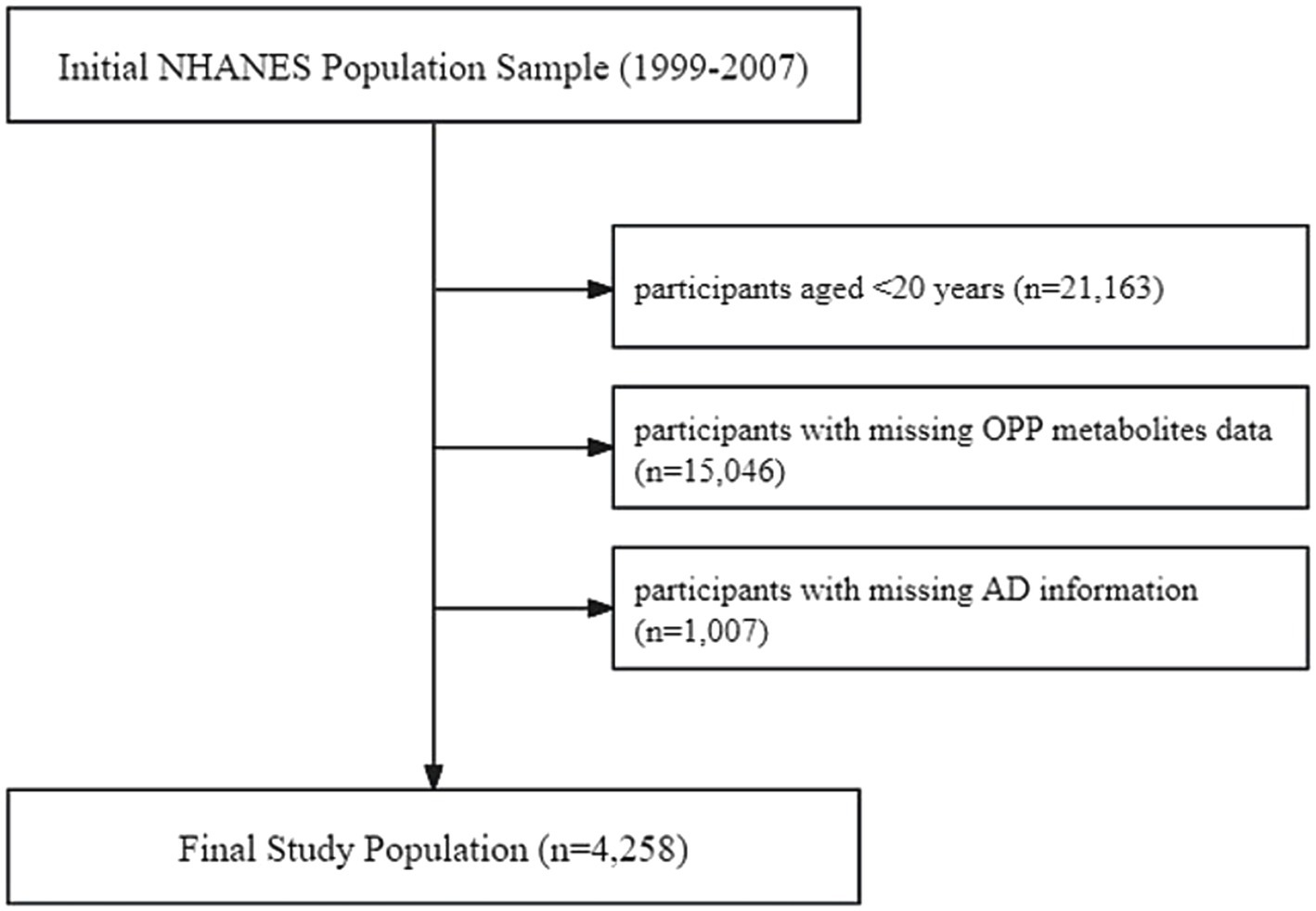
The exclusion criteria for study subjects, as shown in Figure 1, included: (1) age < 20 years (n = 21,163); (2) missing data on OPP metabolites (n = 15,046); and (3) missing information on AD (n = 1,007). A total of 4,258 participants were ultimately included in the analysis. All participants provided written informed consent, and the study protocol was approved by the NCHS Research Ethics Review Board.
Definition of AD
Atopic dermatitis was identified based on self-reported data from the NHANES questionnaires. Three questions were used from the Allergy and Dermatology Questionnaires: “Has a doctor or other health professional ever told you that you have eczema?,” “Do you have dermatitis or eczema today?,” and “During the past 12 months, have you had dermatitis, eczema, or any other type of red, inflamed skin rash?.” Participants who answered “yes” to any of these questions were defined as having AD. All questions were derived from standardized NHANES questionnaire modules that underwent rigorous pretesting and validation.
Measurement of OPPs
Urinary concentrations of six dialkyl phosphate (DAP) metabolites were measured as biomarkers of OPP exposure. These metabolites included dimethylphosphate (DMP), diethylphosphate (DEP), dimethylthiophosphate (DMTP), diethylthiophosphate (DETP), dimethyldithiophosphate (DMDTP), and diethyldithiophosphate (DEDTP). Urine samples were collected from participants as single spot samples and analyzed at the National Center for Environmental Health (NCEH), CDC (Atlanta, Georgia). While spot urine samples are commonly used in large epidemiological studies for practical and feasibility reasons (23). The analysis was performed using solid phase extraction coupled with ultrahigh-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS). For values below the lower limit of detection (LOD), LOD divided by the square root of two was used as a replacement, following standard practice in environmental exposure assessment (24). All analyses were performed following standardized NHANES laboratory procedures and quality control protocols.
Covariates
Potential confounding factors included demographic characteristics (age, sex, race, education level, and poverty income ratio), lifestyle factors (smoking status, alcohol consumption, and physical activity), chronic diseases (hypertension and diabetes), and dietary inflammatory index (DII). Race was categorized into Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other Race. Education level was classified into five categories from lowest to highest: less than 9th grade, 9-11th grade, high school graduate, some college, and college graduate or above. Smokers were defined as those who had smoked at least 100 cigarettes in their lifetime, and alcohol users were defined as those who had at least 12 drinks in the past year. Participants were considered to have hypertension if they had a doctor’s diagnosis, used antihypertensive medication, or had blood pressure ≥ 140/90 mmHg. Diabetes was defined by a doctor’s diagnosis, HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L, random glucose ≥11.1 mmol/L, 2-h OGTT glucose ≥11.1 mmol/L, or use of antidiabetic medication or insulin. Physical activity level was calculated based on the frequency and duration of moderate and vigorous activities related to work, recreation, and transportation from NHANES questionnaires, following previously validated methodology (25). All analyses were adjusted for log-transformed urinary creatinine.
Statistical analysis
To account for NHANES’ representativeness, appropriate sample weights, strata, and cluster variables were employed according to its complex multistage sampling design. Eight-year weights were calculated according to NHANES guidelines as the data spanned four survey cycles (1999–2007). Due to substantial right-skewed distributions, urinary creatinine-corrected concentrations of six OPP metabolites were natural log (ln) transformed and categorized into quartiles (Q1–Q4). Continuous variables were presented as weighted means ± standard errors, and categorical variables as counts with weighted percentages. Weighted t-tests and chi-square tests were used to compare baseline characteristics between AD and non-AD groups.
For correlation analysis, Pearson correlation coefficients were used to assess the strength of associations among six DAPs (|r| < 0.3, 0.3–0.7, and ≥0.7 indicating weak, moderate, and strong correlations, respectively). The main analysis employed survey-weighted generalized linear regression models (SWGLMs) to evaluate associations between OPP metabolites (as both continuous variables and quartiles) and AD, with P for trend calculated for quartile analyses. Three progressively adjusted models were constructed: Model 1 adjusted for ln-transformed urinary creatinine; Model 2 further adjusted for demographic characteristics (age, sex, race, poverty, and education); Model 3 additionally adjusted for lifestyle and health conditions (smoking, alcohol consumption, hypertension, diabetes, DII, and physical activity). Furthermore, to explore potential non-linear associations between OPP metabolites and AD, smoothing curve fitting analysis was performed using generalized additive models (GAM) with adjustment for all covariates in Model 3.
To assess the combined effects of OPP exposure on AD, we employed the Weighted Quantile Sum (WQS) regression model, a statistical method specifically designed for evaluating health outcomes associated with multiple chemical exposures (26). This approach generates a weighted index to assess overall effects while determining each compound’s relative contribution. The original dataset was randomly split into training and validation sets (40: 60 ratio), with 1,000 bootstrap iterations to ensure result stability. Weights were optimized in the training set and validated in the test set. The WQS model adjusted for all covariates from Model 3, including ln-transformed urinary creatinine, demographic characteristics, lifestyle factors, and chronic conditions. Each OPP metabolite was assigned a weight between 0 and 1, with the sum of weights equaling 1, where higher weights indicated greater contributions to AD risk. This analysis enabled simultaneous evaluation of the six OPP metabolites’ mixed exposure effects and identification of key metabolites contributing most significantly to AD risk.
Finally, subgroup analyses and interaction tests were performed for OPP metabolites significantly associated with AD. Analyses were stratified by sex, age (≥60 or <60 years), smoking status, alcohol consumption, hypertension, and diabetes, adjusting for all covariates in Model 3.
All statistical analyses were performed using R version 4.3.2, with the “survey” package for weighted analysis and “gWQS” for WQS regression. Two-sided p < 0.05 was considered statistically significant.
Results
Baseline characteristics of study population
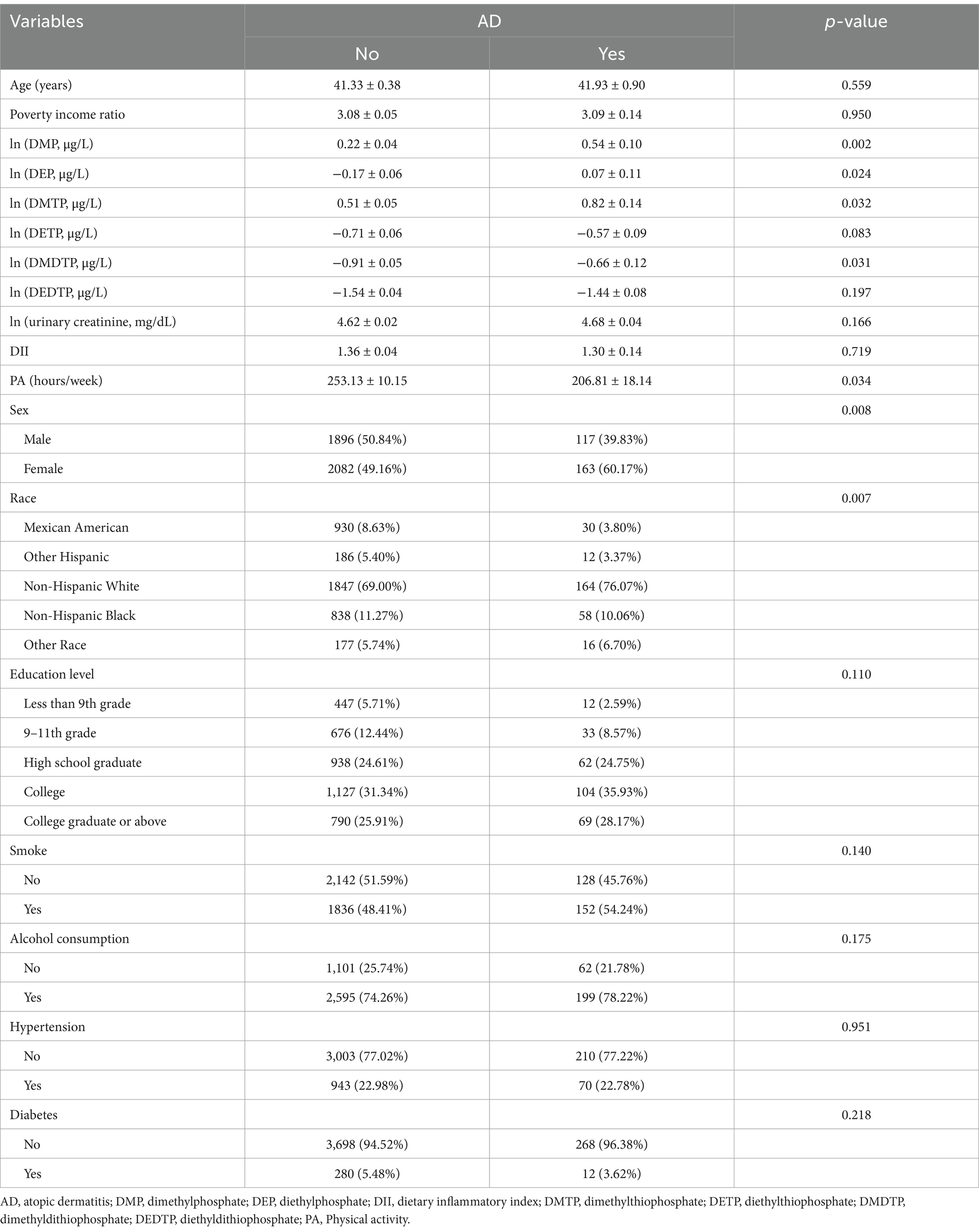
A total of 4,258 participants were included in the final analysis, with 280 (6.6%) diagnosed with AD. Compared to the non-AD group, AD patients showed a significantly higher proportion of females (60.17% vs. 49.16%, p = 0.008) and were predominantly Non-Hispanic White (76.07%). No significant differences were observed in age (41.93 ± 0.90 vs. 41.33 ± 0.38 years) or poverty income ratio between groups (Table 1). AD patients reported significantly lower physical activity levels (206.81 ± 18.14 vs. 253.13 ± 10.15 h/week, p = 0.034), while dietary inflammatory index showed no significant difference between groups (1.30 ± 0.14 vs. 1.36 ± 0.04, p = 0.719). Regarding OPP metabolite levels, the AD group exhibited significantly higher levels of DMP (0.54 ± 0.10 vs. 0.22 ± 0.04, p = 0.002), DEP (0.07 ± 0.11 vs. −0.17 ± 0.06, p = 0.024), DMTP (0.82 ± 0.14 vs. 0.51 ± 0.05, p = 0.032), and DMDTP (−0.66 ± 0.12 vs. −0.91 ± 0.05, p = 0.031). Correlation analysis revealed moderate correlations (0.3 ≤ |r| < 0.7) among all six DAP metabolites, with the strongest correlations observed between DMTP and DMDTP (r = 0.51), followed by DMP and DMTP (r = 0.44), and DETP and DEDTP (r = 0.43) (Figure 2).

Figure 2. Correlation matrix of DAP metabolites among study participants. DMP, dimethylphosphate; DEP, diethylphosphate; DMTP, dimethylthiophosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DEDTP, diethyldithiophosphate.
Association between individual DAP metabolites and AD
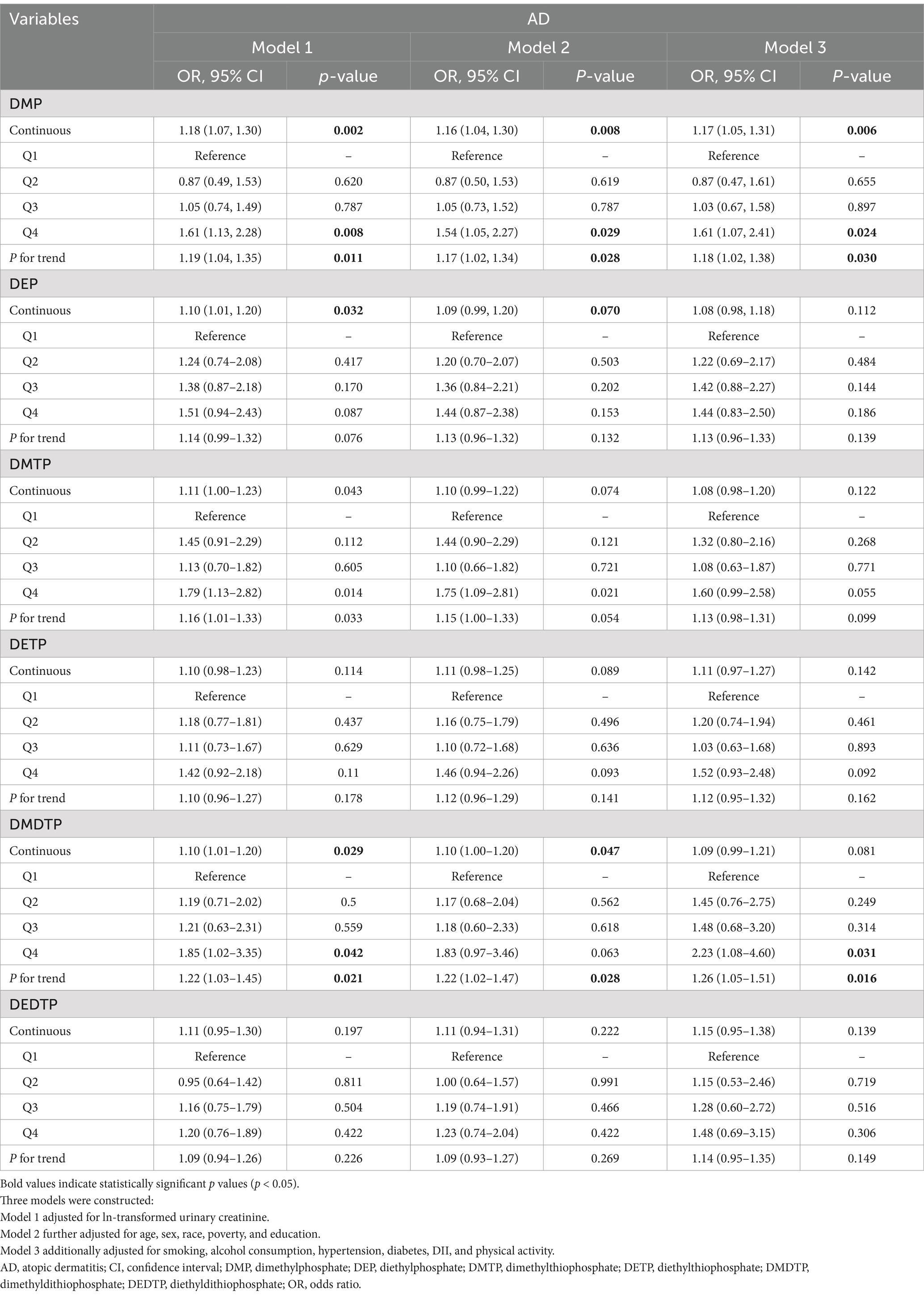
The SWGLMs were used to analyze associations between individual metabolites and AD. For DMP, significant associations were observed in Model 1 for both continuous exposure (OR = 1.18, 95%CI: 1.07–1.30, p = 0.002; P for trend = 0.011) and highest quartile (Q4 vs. Q1: OR = 1.61, 95%CI: 1.13–2.28, p = 0.008). These associations remained consistent in Model 2 (continuous: OR = 1.16, 95%CI: 1.04–1.30, p = 0.008; Q4 vs. Q1: OR = 1.54, 95%CI: 1.05–2.27, p = 0.029; P for trend = 0.028) and Model 3 (continuous: OR = 1.17, 95%CI: 1.05–1.31, p = 0.006; Q4 vs. Q1: OR = 1.61, 95%CI: 1.07–2.41, p = 0.024; P for trend = 0.030). DMDTP showed significant associations in Model 1 (continuous: OR = 1.10, 95%CI: 1.01–1.20, p = 0.029; Q4 vs. Q1: OR = 1.85, 95%CI: 1.02–3.35, p = 0.042; P for trend = 0.021), slightly attenuated in Model 2 (P for trend = 0.028), but regained significance in Model 3’s highest quartile (OR = 2.23, 95%CI: 1.08–4.60, p = 0.031; P for trend = 0.016). No significant associations were found between other metabolites (DEP, DMTP, DETP, and DEDTP) and AD risk (Table 2).
To further explore the association patterns between DAP metabolites and AD, we performed smoothing curve fitting analysis (Figure 3). After adjusting for all potential confounding factors, DMP and DMDTP showed notable positive associations with AD risk, with AD risk increasing gradually as metabolite levels increased. The associations between other metabolites (DEP, DMTP, DETP, and DEDTP) and AD were relatively weak.

Figure 3. Smoothing plots of the associations between DAP metabolites and AD after adjustment for covariates. AD, atopic dermatitis; DMP, dimethylphosphate; DEP, diethylphosphate; DMTP, dimethylthiophosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DEDTP, diethyldithiophosphate.
Combined exposure effects of OPP metabolites
Weighted quantile sum regression analysis adjusted for variables in Model 3 revealed a significant association between mixed exposure and AD risk (OR = 1.25, 95%CI: 1.04–1.50, p = 0.019). In the relative contribution analysis of six metabolites, DMP showed the highest weight (45.8%), followed by DEP (18.5%) and DMDTP (18.2%), while DMTP (10.5%) and DEDTP (6.9%) contributed relatively less, and DETP showed no contribution (0%) (Figure 4).

Figure 4. Weighted contribution of individual DAP metabolites in the WQS regression analysis. DMP, dimethylphosphate; DEP, diethylphosphate; DMTP, dimethylthiophosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DEDTP, diethyldithiophosphate.
Stratified and interaction analyses of DAP metabolites and AD
Dimethylphosphate and DMDTP were analyzed across subgroups of sex, age, smoking status, alcohol consumption, hypertension, and diabetes (Table 3). DMP showed significant positive associations with AD among males (OR = 1.22, 95%CI: 1.06–1.39, p = 0.004), age < 60 years (OR = 1.16, 95%CI: 1.05–1.27, p = 0.003), smokers (OR = 1.22, 95%CI: 1.08–1.38, p = 0.002), and alcohol consumers (OR = 1.15, 95%CI: 1.04–1.28, p = 0.008). No significant associations were found among females (OR = 1.01, 95%CI: 0.89–1.14, p = 0.899) and non-smokers (OR = 0.98, 95%CI: 0.85–1.12, p = 0.744), while a significant negative association was observed in age ≥ 60 years group (OR = 0.69, 95%CI: 0.48–1.00, p = 0.048). Significant interactions were found for sex (P for interaction = 0.045), age (P for interaction = 0.003), and smoking status (P for interaction = 0.017), indicating stronger associations in males versus females, positive association in younger (<60 years) versus negative in older (≥60 years) adults, and stronger association in smokers versus non-smokers. DMDTP showed no significant associations across subgroups and no significant interactions.

Table 3. Stratified and interaction analyses of associations between DMP, DMDTP, and AD according to participant characteristics.
Discussion
This study investigated the associations between six urinary OPP metabolites (DMP, DEP, DMTP, DETP, DMDTP, and DEDTP) and AD in the general adult population using cross-sectional data from NHANES 1999–2007. Our analysis revealed a positive correlation between DMP levels and the risk of AD. Based on multivariable-adjusted WQS regression analysis, exposure to the mixture of OPP metabolites was significantly associated with increased risk of AD, suggesting that OPP exposure might be a potential environmental risk factor for AD.
Previous studies have established various health effects of OPPs, primarily focusing on three key areas: endocrine disruption, metabolic effects, and neurotoxicity. Case reports have documented direct dermatological effects, with occupational exposure cases reporting contact dermatitis and skin irritation among agricultural workers handling OPPs (27). Studies examining chronic OPP exposure have also associated it with various skin manifestations (28). Research has shown that OPPs can disrupt endocrine function through multiple pathways, including effects on hormone biosynthesis, transport, receptor binding, decreased CRH expression with enhanced negative feedback, leading to HPA axis dysfunction and cortisol secretion abnormalities (29), and estrogen receptor activation resulting in adipogenesis (30–32). Furthermore, epidemiological studies have found that OPP exposure is significantly associated with metabolic disorders including hypertension, hyperglycemia, and dyslipidemia, and can cause gut microbiota dysbiosis, such as enrichment of pathogenic bacteria like Paraprevotella and Helicobacter while reducing beneficial flora, potentially contributing to the development of obesity and type 2 diabetes (33, 34). Additionally, research has demonstrated that OPP exposure, particularly during gestation, can induce both acute cholinesterase inhibition and chronic cognitive dysfunction, resulting in symptoms such as memory decline and anxiety-like behavior (35). More importantly, studies have found that OPPs can affect immune system function by inhibiting CD4+ and CD8+ T cell proliferation and reducing IL-2 secretion and CD25 expression (36). Despite these well-documented effects, there has been a notable gap in understanding the relationship between OPP exposure and allergic conditions, particularly AD, in the general population, which motivated our current study.
Atopic dermatitis is characterized by a complex pathophysiological mechanism involving three interconnected primary components: skin barrier dysfunction manifested as impaired stratum corneum integrity and increased transepidermal water loss, immune dysregulation encompassing both innate and adaptive immune response abnormalities, and pruritus closely associated with neuroimmune interactions (37). At the molecular level, AD pathogenesis involves multiple key immune pathways: Th2 cell-mediated IL-4 and IL-13 signaling triggering inflammation and IgE production (38), Langerhans cell-mediated responses through antigen presentation and cytokine secretion (39), autoimmune responses including IgE autoantibody production and autoreactive T cell activation (40), and Th2/Th17 cytokine imbalance mediated through JAK signaling (41). These immune alterations, together with genetic susceptibility, epidermal barrier defects, and skin microbiome dysbiosis, form the pathogenic basis of AD (42). The relationship between OPP metabolites and AD may be mediated through multiple mechanisms. Through rat experiments, Yang et al. found that DEP exposure affects thyroid hormone levels and increases inflammatory factors including TNF-α and IL-6, suggesting its influence on inflammatory responses through the endocrine-immune axis. Animal studies have shown that DEP exposure elevates Th2-type cytokines IL-4 and IL-13, changes associated with AD-like skin inflammation (43). Additionally, as reviewed by Freyre et al. (44), OPP metabolite-induced oxidative stress can activate inflammatory pathways, potentially promoting allergic diseases. Furthermore, OPPs may also directly affect skin health through dermal contact, as studies have shown that occupational or environmental dermal exposure to OPPs can cause direct skin irritation and inflammation (45), potentially contributing to various skin conditions including contact dermatitis (46). This direct irritant effect on the skin barrier might work synergistically with their systemic effects through immune and inflammatory pathways to increase AD risk (47).
The unique contribution of this study lies in using mixture analysis to evaluate the combined effects of six OPP metabolites, not only quantifying the individual impact strength of each metabolite but also revealing for the first time their synergistic effects on AD development. Our identification of DMP’s predominant role in the association between OPPs and AD provides a new perspective for understanding specific OPP metabolites in allergic conditions, extending beyond traditional research focused on acute toxicity and endocrine disruption. DMP is a common metabolite of several widely used organophosphate pesticides, including malathion, methyl parathion, and dichlorvos, which undergo biotransformation through demethylation processes in the human body (16). Notably, recent studies have successively found elevated DMP levels associated with various health issues: increased risk of depression (48), abnormal thyroid autoimmune indicators (49), and close correlation with asthma development (50). This evidence collectively suggests that DMP may play a central role in the pathogenesis of immune-mediated and endocrine diseases through its effects on inflammatory pathways and immune regulation. Given the widespread presence of OPPs in the environment and their significant biological effects, these findings have important public health implications for understanding prevention and intervention strategies for environment-related diseases.
Subgroup analyses revealed several important findings. Males showed higher DMP susceptibility than females, possibly due to sex differences in detoxifying enzyme activities like CYP450 and PON1 regulated by sex hormones (51). Detailed analysis of gender-specific lifestyle patterns revealed distinct differences in alcohol consumption between male and female AD patients (Supplementary Table 1). Female AD patients showed significantly lower rates of alcohol consumption (71.90% vs. 87.21% in males, p = 0.007), while no significant gender difference was observed in smoking status (52.37% vs. 57.06% in males, p = 0.441). These differences in alcohol consumption patterns might partially contribute to the gender-specific susceptibility patterns observed. The biological basis for such gender differences has been well documented in previous studies. Research has shown that androgens can significantly increase the expression of CYP4F2 and CYP4F3 through androgen receptor (AR) in prostate cancer cells (52), while PON1 activity is modulated by estrogen through HDL metabolism pathways (53). Furthermore, animal studies have demonstrated that male subjects show higher metabolic activation and sensitivity to organophosphate compounds (54), which may be attributed to the higher expression of male-specific CYP450 enzymes such as CYP2C11 and CYP3A1 (55). The significant positive correlation observed in younger populations (<60 years) likely reflects their higher metabolic enzyme activity and more efficient detoxification capacity, contrasting with age-related metabolic decline (56). This age-related difference in xenobiotic metabolism has been well documented, as aging is associated with reduced liver blood flow, decreased hepatic mass (57), and declined expression of specific CYP enzymes involved in xenobiotic metabolism (58). The enhanced effect in smokers relates to cigarette smoke polycyclic aromatic hydrocarbons activating AhR, increasing toxic metabolite production and exacerbating DNA damage and oxidative stress (59). Given the widespread environmental presence of OPPs and their significant biological effects, these findings have important public health implications for identifying high-risk populations and developing targeted prevention strategies.
However, several limitations should be acknowledged. The cross-sectional study design precludes the establishment of causal relationships between OPP exposure and AD. The reliance on single-point urinary OPP metabolite measurements represents another important limitation. Given that OPPs have relatively short biological half-lives ranging from hours to days (60), and their exposure patterns can vary substantially due to dietary choices and behavioral changes (61), single spot urine samples may not accurately reflect long-term exposure patterns. It is recognized that they may not fully capture the temporal variability in OPP exposure (62). Another important limitation is the reliance on self-reported questionnaires for AD diagnosis, which may introduce misclassification bias. Although the questionnaires used in NHANES underwent rigorous validation, the lack of clinical verification might lead to either over- or under-reporting of AD cases. Furthermore, we were unable to assess certain environmental risk factors such as occupational exposure to pesticides or other workplace-related chemical exposures, which might contribute to both OPP exposure levels and AD risk. While potential mechanisms involving inflammatory responses and immune modulation were proposed, the study did not directly investigate these biological pathways. Additionally, despite the comprehensive analysis, there might be unmeasured confounding factors that could influence the observed associations. Furthermore, no corrections for multiple comparisons were applied in our stratified analyses, which might increase the risk of Type I error. These limitations suggest the need for future longitudinal studies with repeated exposure measurements, as multiple urine samples collected over time would provide more reliable estimates of chronic exposure (63), along with mechanistic investigations to further validate these findings.
Conclusion
In conclusion, this study demonstrates that OPP exposure, especially DMP, is associated with increased AD risk in adults, with stronger associations in males, younger individuals, and smokers. While mechanistic studies are needed, these findings highlight the role of environmental factors in AD development and suggest targeted prevention strategies for susceptible populations.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: data are accessible in a public, open access repository. Open access data can be found on the NHANES website: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The study utilized publicly available data from the National Health and Nutrition Examination Survey (NHANES), a program conducted by the National Center for Health Statistics (NCHS), which is part of the Centers for Disease Control and Prevention (CDC). All participants provided written informed consent, and the study protocol was approved by the NCHS Research Ethics Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Resources, Supervision, Writing – original draft. YW: Investigation, Methodology, Writing – review & editing. WW: Validation, Visualization, Writing – review & editing. MC: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Peking University Medicine Seed Fund for Interdisciplinary Research supported by “the Fundamental Research Funds for the Central Universities” [grant No. BMU2021MX021], and the Peking University Third Hospital 2022 Innovation Transformation Fund (grant No. BYSYZHKC2022120).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1555731/full#supplementary-material
References
1. Furue, M, Chiba, T, Tsuji, G, Ulzii, D, Kido-Nakahara, M, Nakahara, T, et al. Atopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int. (2017) 66:398–403. doi: 10.1016/j.alit.2016.12.002
2. Tian, J, Zhang, D, Yang, Y, Huang, Y, Wang, L, Yao, X, et al. Global epidemiology of atopic dermatitis: a comprehensive systematic analysis and modelling study. Br J Dermatol. (2023) 190:55–61. doi: 10.1093/bjd/ljad339
3. Bylund, S, Kobyletzki, LB, Svalstedt, M, and Svensson, Å. Prevalence and incidence of atopic dermatitis: a systematic review. Acta Derm Venereol. (2020) 100:adv00160. doi: 10.2340/00015555-3510
4. Cao, X, Wang, M, Zhou, M, Mi, Y, Guo, Q, Fan, Y, et al. Global, regional and National Burden of Paediatric atopic dermatitis: a trend and geographic inequalities analysis. Clin Exp Allergy. (2024) 54:747–59. doi: 10.1111/cea.14558
5. Xu, X, van Galen, LS, Koh, MJA, Bajpai, R, Thng, S, Yew, YW, et al. Factors influencing quality of life in children with atopic dermatitis and their caregivers: a cross-sectional study. Sci Rep. (2019) 9:15990. doi: 10.1038/s41598-019-51129-5
6. Mosam, A, and Todd, G. Global epidemiology and disparities in atopic dermatitis. Br J Dermatol. (2023) 188:726–37. doi: 10.1093/bjd/ljad042
7. Loset, M, Brown, SJ, Saunes, M, and Hveem, K. Genetics of atopic dermatitis: from DNA sequence to clinical relevance. Dermatology. (2019) 235:355–64. doi: 10.1159/000500402
8. Brown, SJ, Elias, MS, and Bradley, M. Genetics in atopic dermatitis: historical perspective and future prospects. Acta Derm Venereol. (2020) 100:adv00163. doi: 10.2340/00015555-3513
9. Ravn, NH, Halling, AS, Berkowitz, AG, Rinnov, MR, Silverberg, JI, Egeberg, A, et al. How does parental history of atopic disease predict the risk of atopic dermatitis in a child? A systematic review and meta-analysis. J Allergy Clin Immunol. (2020) 145:1182–93. doi: 10.1016/j.jaci.2019.12.899
10. Bonamonte, D, Filoni, A, Vestita, M, Romita, P, Foti, C, and Angelini, G. The role of the environmental risk factors in the pathogenesis and clinical outcome of atopic dermatitis. Biomed Res Int. (2019) 2019:1–11. doi: 10.1155/2019/2450605
11. Fu, H, Tan, P, Wang, R, Li, S, Liu, H, Yang, Y, et al. Advances in organophosphorus pesticides pollution: current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J Hazard Mater. (2022) 424:127494. doi: 10.1016/j.jhazmat.2021.127494
12. Bernal-Gonzalez, KG, Covantes-Rosales, CE, Camacho-Perez, MR, Mercado-Salgado, U, Barajas-Carrillo, VW, Giron-Perez, DA, et al. Organophosphate-pesticide-mediated immune response modulation in invertebrates and vertebrates. Int J Mol Sci. (2023) 24:5360. doi: 10.3390/ijms24065360
13. Galloway, T, and Handy, R. Immunotoxicity of organophosphorous pesticides. Ecotoxicology. (2003) 12:345–63. doi: 10.1023/A:1022579416322
14. Camacho-Perez, MR, Covantes-Rosales, CE, Toledo-Ibarra, KJG, Mercado-Salgado, U, Ponce-Regalado, MD, Diaz-Resendiz, KJG, et al. Organophosphorus pesticides as modulating substances of inflammation through the cholinergic pathway. Int J Mol Sci. (2022) 23:4523. doi: 10.3390/ijms23094523
15. Tang, J, Wang, W, Jiang, Y, and Chu, W. Diazinon exposure produces histological damage, oxidative stress, immune disorders and gut microbiota dysbiosis in crucian carp (Carassius auratus gibelio). Environ Pollut. (2021) 269:116129. doi: 10.1016/j.envpol.2020.116129
16. Kavvalakis, MP, and Tsatsakis, AM. The atlas of dialkylphosphates; assessment of cumulative human organophosphorus pesticides' exposure. Forensic Sci Int. (2012) 218:111–22. doi: 10.1016/j.forsciint.2011.10.019
17. Chittrakul, J, Sapbamrer, R, and Sirikul, W. Pesticide exposure and risk of rheumatoid arthritis: a systematic review and Meta-analysis. Toxics. (2022) 10:207. doi: 10.3390/toxics10050207
18. Pan, Q, Guo, Y, Guo, L, Liao, S, Zhao, C, Wang, S, et al. Mechanistic insights of chemicals and drugs as risk factors for systemic lupus erythematosus. Curr Med Chem. (2020) 27:5175–88. doi: 10.2174/0929867326666190404140658
19. Sharma, A, Shukla, A, Attri, K, Kumar, M, Kumar, P, Suttee, A, et al. Global trends in pesticides: a looming threat and viable alternatives. Ecotoxicol Environ Saf. (2020) 201:110812. doi: 10.1016/j.ecoenv.2020.110812
20. Costa, LG. Organophosphorus compounds at 80: some old and new issues. Toxicol Sci. (2018) 162:24–35. doi: 10.1093/toxsci/kfx266
21. Tasker, F, Brown, A, Grindlay, D, Rogers, N, and Harman, K. What’s new in atopic eczema? An analysis of systematic reviews published in 2018. Part 1: prevention and topical therapies. Clin Exp Dermatol. (2020) 45:974–9. doi: 10.1111/ced.14303
22. Corsini, E, Sokooti, M, Galli, C, Moretto, A, and Colosio, C. Pesticide induced immunotoxicity in humans: a comprehensive review of the existing evidence. Toxicology. (2013) 307:123–35. doi: 10.1016/j.tox.2012.10.009
23. Barr, DB, Olsson, AO, Wong, LY, Udunka, S, Baker, SE, Whitehead, RD Jr, et al. Urinary concentrations of metabolites of pyrethroid insecticides in the general U.S. population: National Health and nutrition examination survey 1999-2002. Environ Health Perspect. (2010) 118:742–8. doi: 10.1289/ehp.0901275
24. Nan, W, Peng, Z, Yi, T, Ouyang, M, and Hu, J. Association between exposure to organophosphate esters metabolites and sarcopenia prevalence: a cross-sectional study using NHANES data. Ecotoxicol Environ Saf. (2024) 285:117041. doi: 10.1016/j.ecoenv.2024.117041
25. Ji, J, Hou, Y, Li, Z, Zhou, Y, Xue, H, Wen, T, et al. Association between physical activity and bone mineral density in postmenopausal women: a cross-sectional study from the NHANES 2007-2018. J Orthop Surg Res. (2023) 18:501. doi: 10.1186/s13018-023-03976-2
26. Carrico, C, Gennings, C, Wheeler, DC, and Factor-Litvak, P. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. (2015) 20:100–20. doi: 10.1007/s13253-014-0180-3
27. Spiewak, R. Pesticides as a cause of occupational skin diseases in farmers. Ann Agric Environ Med. (2001) 8:1–5.
28. Hernandez, AF, Parron, T, Tsatsakis, AM, Requena, M, Alarcon, R, and Lopez-Guarnido, O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. (2013) 307:136–45. doi: 10.1016/j.tox.2012.06.009
29. Rezg, R, Mornagui, B, Benahmed, M, Gharsalla Chouchane, S, Belhajhmida, N, Abdeladhim, M, et al. Malathion exposure modulates hypothalamic gene expression and induces dyslipedemia in Wistar rats. Food Chem Toxicol. (2010) 48:1473–7. doi: 10.1016/j.fct.2010.03.013
30. Yang, FW, Li, YX, Ren, FZ, Luo, J, and Pang, GF. Assessment of the endocrine-disrupting effects of organophosphorus pesticide triazophos and its metabolites on endocrine hormones biosynthesis, transport and receptor binding in silico. Food Chem Toxicol. (2019) 133:110759. doi: 10.1016/j.fct.2019.110759
31. Yang, FW, Fang, B, Pang, GF, and Ren, FZ. Organophosphorus pesticide triazophos: a new endocrine disruptor chemical of hypothalamus-pituitary-adrenal axis. Pestic Biochem Physiol. (2019) 159:91–7. doi: 10.1016/j.pestbp.2019.05.021
32. Kim, JT, Lee, HJ, and Lee, HS. Organophosphorus pesticides exert estrogen receptor agonistic effect determined using Organization for Economic Cooperation and Development PBTG455, and induce estrogen receptor-dependent adipogenesis of 3T3-L1 adipocytes. Environ Pollut. (2021) 283:117090. doi: 10.1016/j.envpol.2021.117090
33. Czajka, M, Matysiak-Kucharek, M, Jodlowska-Jedrych, B, Sawicki, K, Fal, B, Drop, B, et al. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ Res. (2019) 178:108685. doi: 10.1016/j.envres.2019.108685
34. Yang, F, Li, J, Pang, G, Ren, F, and Fang, B. Effects of diethyl phosphate, a non-specific metabolite of organophosphorus pesticides, on serum lipid, hormones, inflammation, and gut microbiota. Molecules. (2019) 24:2003. doi: 10.3390/molecules24102003
35. Todd, SW, Lumsden, EW, Aracava, Y, Mamczarz, J, Albuquerque, EX, and Pereira, EFR. Gestational exposures to organophosphorus insecticides: from acute poisoning to developmental neurotoxicity. Neuropharmacology. (2020) 180:108271. doi: 10.1016/j.neuropharm.2020.108271
36. Eddleston, M. Novel clinical toxicology and pharmacology of organophosphorus insecticide self-poisoning. Annu Rev Pharmacol Toxicol. (2019) 59:341–60. doi: 10.1146/annurev-pharmtox-010818-021842
37. Fujii, M. Current understanding of pathophysiological mechanisms of atopic dermatitis: interactions among skin barrier dysfunction, immune abnormalities and pruritus. Biol Pharm Bull. (2020) 43:12–9. doi: 10.1248/bpb.b19-00088
38. Dubin, C, Del Duca, E, and Guttman-Yassky, E. The IL-4, IL-13 and IL-31 pathways in atopic dermatitis. Expert Rev Clin Immunol. (2021) 17:835–52. doi: 10.1080/1744666X.2021.1940962
39. Pan, Y, Hochgerner, M, Cichon, MA, Benezeder, T, Bieber, T, and Wolf, P. Langerhans cells: central players in the pathophysiology of atopic dermatitis. J Eur Acad Dermatol Venereol. (2024) 39:278–89. doi: 10.1111/jdv.20291
40. Badloe, FMS, De Vriese, S, Coolens, K, Schmidt-Weber, CB, Ring, J, Gutermuth, J, et al. IgE autoantibodies and autoreactive T cells and their role in children and adults with atopic dermatitis. Clin Transl Allergy. (2020) 10:34. doi: 10.1186/s13601-020-00338-7
41. Bieber, T, Paller, AS, Kabashima, K, Feely, M, Rueda, MJ, Ross Terres, JA, et al. Atopic dermatitis: pathomechanisms and lessons learned from novel systemic therapeutic options. J Eur Acad Dermatol Venereol. (2022) 36:1432–49. doi: 10.1111/jdv.18225
42. Sroka-Tomaszewska, J, and Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. (2021) 22:4130. doi: 10.3390/ijms22084130
43. Savinko, T, Matikainen, S, Saarialho-Kere, U, Lehto, M, Wang, G, Lehtimäki, S, et al. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J Invest Dermatol. (2012) 132:1392–400. doi: 10.1038/jid.2011.446
44. Freyre, EO, Valencia, AT, Guzman, DD, Maldonado, IC, Ledezma, LEB, and Carrillo, MFZ. Oxidative stress as a molecular mechanism of exposure to organophosphorus pesticides: a review. Curr Protein Pept Sci. (2021) 22:890–7. doi: 10.2174/1389203722666211122092309
45. Anderson, SE, and Meade, BJ. Potential health effects associated with dermal exposure to occupational chemicals. Environ Health Insights. (2014) 8:51–62. doi: 10.4137/EHI.S15258
46. Hoppin, JA, Umbach, DM, Long, S, London, SJ, Henneberger, PK, Blair, A, et al. Pesticides are associated with allergic and non-allergic wheeze among male farmers. Environ Health Perspect. (2017) 125:535–43. doi: 10.1289/EHP315
47. Mostafalou, S, and Abdollahi, M. Pesticides: an update of human exposure and toxicity. Arch Toxicol. (2017) 91:549–99. doi: 10.1007/s00204-016-1849-x
48. Wu, Y, Song, J, Zhang, Q, Yan, S, Sun, X, Yi, W, et al. Association between organophosphorus pesticide exposure and depression risk in adults: a cross-sectional study with NHANES data. Environ Pollut. (2023) 316:120445. doi: 10.1016/j.envpol.2022.120445
49. Diawara, MO, Li, S, Zhang, M, Bigambo, FM, Yang, X, Wang, X, et al. Evaluation of multiple organophosphate insecticide exposure in relation to altered thyroid hormones in NHANES 2007-2008 adult population. Ecotoxicol Environ Saf. (2024) 273:116139. doi: 10.1016/j.ecoenv.2024.116139
50. Liang, JH, Liu, ML, Pu, YQ, Huang, S, Jiang, N, Huang, SY, et al. Biomarkers of organophosphate insecticides exposure and asthma in general US adults: findings from NHANES 1999-2018 data. Environ Sci Pollut Res Int. (2023) 30:92295–305. doi: 10.1007/s11356-023-28740-1
51. Vignaux, PA, Shriwas, P, Revnew, A, Agarwal, G, Lane, TR, McElroy, CA, et al. Human CYP2C19 substrate and inhibitor characterization of organophosphate pesticides. Chem Res Toxicol. (2023) 36:1451–5. doi: 10.1021/acs.chemrestox.3c00188
52. Wu, C-C, Liu, Y, Chen, J, Gotlinger, KH, Ramu, E, Falck, JR, et al. Abstract LB-160: CYP4F isoform expression and 20-HETE synthesis in prostate cancer cells are regulated by androgen and contribute to growth. Cancer Res. (2012) 72:LB-160. doi: 10.1158/1538-7445.AM2012-LB-160
53. Ikhlef, S, Berrougui, H, and Khalil, A. Paraoxonase 1 (PON1) promotes the cholesterol efflux and regulates the reverse cholesterol transport (RCT). Arterioscler Thromb Vasc Biol. (2015) 35:A316–6. doi: 10.1161/atvb.35.suppl_1.316
54. Singh, T, Ramakrishnan, S, Wu, X, and Reddy, DS. Sex differences in organophosphate model of benzodiazepine-refractory status epilepticus and neuronal damage. J Pharmacol Exp Ther. (2024) 388:313–24. doi: 10.1124/jpet.123.001747
55. Chang, TKH, and Waxman, DJ. Sex differences in drug metabolism. In: Encyclopedia of drug metabolism and interactions. Wiley (2012) 1–19.
56. Tarrago, MG, Chini, CCS, Kanamori, KS, Warner, GM, Caride, A, de Oliveira, GC, et al. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD(+) decline. Cell Metab. (2018) 27:1081–1095.e10. doi: 10.1016/j.cmet.2018.03.016
57. McLean, AJ, and Le Couteur, DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. (2004) 56:163–84. doi: 10.1124/pr.56.2.4
58. Wauthier, V, Verbeeck, RK, and Calderon, PB. The effect of ageing on cytochrome p 450 enzymes: consequences for drug biotransformation in the elderly. Curr Med Chem. (2007) 14:745–57. doi: 10.2174/092986707780090981
59. Hughes, D, Guttenplan, JB, Marcus, CB, Subbaramaiah, K, and Dannenberg, AJ. Heat shock protein 90 inhibitors suppress aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and DNA adduct formation. Cancer Prev Res. (2008) 1:485–93. doi: 10.1158/1940-6207.CAPR-08-0149
60. Needham, LL. Assessing exposure to organophosphorus pesticides by biomonitoring in epidemiologic studies of birth outcomes. Environ Health Perspect. (2005) 113:494–8. doi: 10.1289/ehp.7490
61. Lu, C, Barr, DB, Pearson, MA, and Waller, LA. Dietary intake and its contribution to longitudinal organophosphorus pesticide exposure in urban/suburban children. Environ Health Perspect. (2008) 116:537–42. doi: 10.1289/ehp.10912
62. Bradman, A, Kogut, K, Eisen, EA, Jewell, NP, Quirós-Alcalá, L, Castorina, R, et al. Variability of organophosphorous pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ Health Perspect. (2013) 121:118–24. doi: 10.1289/ehp.1104808
Keywords: organophosphate pesticides, atopic dermatitis, NHANES, environmental exposure, dialkyl phosphate metabolites, epidemiology
Citation: Men Y, Wang Y, Wu W and Chu M (2025) Association between organophosphate pesticide exposure and atopic dermatitis: a cross-sectional study based on NHANES 1999–2007. Front. Public Health. 13:1555731. doi: 10.3389/fpubh.2025.1555731
Edited by:
Liana Fattore, CNR Neuroscience Institute (IN), ItalyReviewed by:
Iqbal Ansari, Vel Tech Rangarajan Dr. Sagunthala R&D Institute of Science and Technology, IndiaAnindya Panja, Vidyasagar University, India
Bin Zhang, Capital Medical University, China
A. P. Neves, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2025 Men, Wang, Wu and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: YueHua Men, bWVueXVlaHVhQDEyNi5jb20=; Ming Chu, ZmFtb3VzQGJqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 YueHua Men
YueHua Men YiMeng Wang
YiMeng Wang WenTing Wu1
WenTing Wu1 Ming Chu
Ming Chu