- 1Department of Nursing, Xi’an Jiaotong University Health Science Center, Xi’an, China
- 2Medical School, Xi’an Peihua University, Xi’an, China
- 3Department of Radiotherapy, Shaanxi Provincial Cancer Hospital, Xi’an, China
- 4School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, China
- 5The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
Objectives: Evidence on the combined effect of sleep duration and activities of daily living (ADL) on depressive symptoms is scarce. This study aimed to explore the interaction effects between sleep duration and ADL limitations on depressive symptoms among Chinese individuals aged ≥45 years.
Methods: Data were extracted from the China Health and Retirement Longitudinal Study (CHARLS) wave 2020. Sleep duration was self-reported. The Center for Epidemiological Studies Depression Scale and a 12-item scale were employed to estimate depressive symptoms and ADL limitations, respectively. Logistic regression analysis was conducted to examine the interaction effects between sleep duration and ADL limitations on depressive symptoms.
Results: Logistic regression found that short sleep (OR = 1.69, 95% CI: 1.57–1.83), long sleep (OR = 0.87, 95% CI: 0.79–0.95), and ADL limitations [basic activities of daily living (BADL), OR = 1.82, 95% CI: 1.66–2.01; instrumental activities of daily living (IADL), OR = 1.88, 95% CI: 1.71–2.07] were associated with depressive symptoms. Furthermore, synergistic interaction effects on the depressive symptoms risk were identified between short sleep and IADL limitations (RERI = 1.08, 95% CI: 0.57–1.59) or BADL limitations (RERI = 1.13, 95% CI: 0.60–1.65). Conversely, antagonistic interaction effects were observed between long sleep and IADL limitations (RERI = 0.88, 95% CI: 0.39–1.38) or BADL limitations (RERI = 0.76, 95% CI: 0.25–1.27) on depressive symptoms.
Conclusion: The study revealed significant interactions between sleep duration and ADL limitations on depressive symptoms, suggesting that enhancing ADL’s function and ensuring adequate sleep duration could effectively prevent depressive symptoms.
1 Introduction
Depression is a common mental disorder, characterized by a low mood or the loss of pleasure in activities for a long period (1). Globally, approximately 280 million individuals suffer from depression (1), with an estimated 41.01 million affected in China, ranking as the second highest number worldwide (2). It is projected that depression will be the leading global disease burden by 2030 (3). Depression can interfere with daily life (1), lead to illnesses (e.g., diabetes, cardiovascular disease, disability, etc.) (4, 5), and even increase the premature mortality risk (6). Depressive symptoms, as an early sign of depression (7), encompass a range of emotional and physical symptoms that may not meet the full criteria for diagnosing depression (8). Current research has shown that the prevalence of depressive symptoms among Chinese middle-aged and older individuals has escalated to 37.62% (9), posing a tremendous burden on families and society. Thus, early identification of risk factors for depressive symptoms is imperative for preventing and delaying depression (10). Recent systematic review (11) and meta-analysis (12) have highlighted several modifiable risk factors associated with depressive symptoms, including lifestyle behaviors (e.g., physical activity), physical health-related conditions (e.g., chronic disease and activities of daily living), and psychosocial factors (e.g., social support). Among these, activities of daily living have been recognized as a crucial factor, garnering considerable attention from scholars (11, 12).
Activities of daily living (ADL) refer to an individual’s daily self-care activities, categorized into basic activities of daily living (BADL) and instrumental activities of daily living (IADL) (13). BADL includes fundamental skills necessary for managing one’s basic physical needs (e.g., dressing, bathing, and eating); whereas IADL involves more complex tasks in the community (e.g., doing housework and cooking) (13). In recent years, accumulating longitudinal studies have examined the association of ADL with depressive symptoms, finding that more severe ADL limitations predicted a higher risk of depressive symptoms (14, 15). This suggests that ADL has become a significant predictor of depressive symptoms (16). A plausible explanation is that ADL limitations act as psychological stressors by restricting individuals’ independence in self-care (15) and social relationships (14, 15), ultimately resulting in the onset of depressive symptoms (15, 17). Furthermore, depressive symptoms can, in turn, aggravate ADL limitations (18), potentially creating a vicious circle.
Sleep is a fundamental behavior essential for physical health and cognitive function, characterized by specific brain electrical activity that undergoes predictable changes across the lifespan (19). Considerable evidence has demonstrated that sleep duration was correlated to numerous health problems, such as metabolic syndrome (20), cancer (21), and suicidal behavior (22). However, the relationship between sleep duration and depressive symptoms remains unclear, with numerous studies yielding inconsistent results (23–27). Some studies have indicated that both shorter and longer sleep increased the risk of depressive symptoms among middle-aged and older individuals, showing a U-shaped relationship (23, 24). Others have shown that only short sleep is significantly associated with depressive symptoms (25, 26). However, a 4-year longitudinal study found that long sleep could reduce the risk of depressive symptoms (27). In view of this, the relationship between long sleep and depressive symptoms warrants further exploration.
In summary, mounting studies have delved into the independent associations of sleep duration and ADL with depressive symptoms. Few studies have examined the combined effects of sleep duration and ADL limitations on depressive symptoms and their potential mechanisms. To date, one cross-sectional study identified a multiplicative interaction between sleep duration and ADL limitations on depressive symptoms (28). However, it did not address the additive interaction, which could provide a different perspective on the cumulative effects of these factors on depressive symptoms. Given this gap in the literature, the present study aimed to investigate the additive interaction effects of sleep duration and ADL limitations on depressive symptoms, potentially providing valuable insights into prevention and intervention strategies.
2 Materials and methods
2.1 Study population
This study used data from the China Health and Retirement Longitudinal Study (CHARLS). The CHARLS is a nationally representative longitudinal study targeting individuals aged ≥ 45 years in China, aimed at establishing a high-quality public database. The data collected covers various dimensions relevant to aging research, including socioeconomic status, health conditions, and other aspects. The baseline national survey was conducted in 2011, followed by regular follow-up survey in 2013, 2015, 2018, and 2020. The retention rates in each follow-up survey were above 86%. For a detailed description of CHARLS, additional information can be found on the project’s official website: http://charls.pku.edu.cn/.
The present study is based on the latest data collected in 2020, which included 19,395 participants in total. After excluding participants with missing data on basic demographic information (n = 692), depressive symptoms (n = 3,016), and sleep duration (n = 176), a final sample of 15,511 participants was included in the analysis.
2.2 Assessment of depressive symptoms
Depressive symptoms were estimated using the 10-item Center for Epidemiological Studies Depression Scale (CES-D). This tool is designed to assess a person’s mood and behavioral patterns related to depressive symptoms in the past week, which has demonstrated good reliability and validity in China (29). Each item is scored on a four-point scale ranging from 0 (rarely or none of the time) to 3 (mostly or all the time), with a total score ranging from 0 to 30 points. A higher score indicates more severe depressive symptoms. According to previous studies (30), participants with a score of 10 or higher were classified as exhibiting depressive symptoms in this study.
2.3 Assessment of ADL
The assessment of ADL was conducted using a 12-item scale, including six items of BADL (i.e., dressing, bathing, eating, getting into and out of bed, toileting, and controlling urination and defecation), and six items of IADL (i.e., doing housework, cooking, shopping for groceries, making phone calls, taking medicines, and managing finances). Each item offers four options: (1) no difficulty, (2) difficulty but can still complete, (3) difficulty and need help, and (4) unable to complete. Participants who had difficulty or were unable to perform one or more of the six items of BADL were classified as BADL limitations in this study. The same criteria were applied to determine IADL limitations.
2.4 Assessment of sleep duration
Sleep duration was assessed using a self-reported questionnaire that included the following sleep-related questions: “During the past month, how many hours of actual sleep did you get at night (average hours per night)? This may be shorter than the number of hours you spend in bed.” Based on prior studies (26, 31, 32), a daily sleep duration of 6 to 8 h per night is recommended for Chinese middle-aged and older individuals. Therefore, sleep duration was categorized into three groups: short sleep (<6 h), normal sleep (≥6 h and < 8 h), and long sleep (≥8 h).
2.5 Covariates assessment
The covariates encompassed demographic data, such as age (45–59 years, ≥60 years), gender (male, female), marital status (married, others including divorced, widowed, separated, and never married), residence (urban, rural), education level (primary school or below, secondary school, senior high school or above). Additionally, behavioral factors were collected, including smoking status (never smoked, currently smoking, former smoker), alcohol consumption frequency (>1 time per month, <1 time per month, no consumption), and the number of chronic diseases reported (0, 1, 2, and ≥ 3). The current study controlled potential confounders in the analysis. Model 1 was a crude model. Model 2 adjusted for age, gender, residence, marital status, education level, smoking history, alcohol consumption, and number of chronic diseases.
2.6 Statistical analysis
Statistical analyses were conducted using SPSS 25.0 software, with a significant level of p < 0.05. Categorical variables were represented by frequencies and percentages. The baseline characteristics of participants were assessed using χ2 tests, categorized by the presence of depressive symptoms. To evaluate the associations of sleep duration, ADL limitations, and depressive symptoms, binary logistic regression was performed. Additionally, both multiplicative and additive interactions between sleep duration (short and long sleep) and ADL limitations (including IADL and BADL limitations) were examined in relation to depressive symptoms. Multiplicative interactions were quantified through the ratio of odds ratios (ORs) for the product terms, while additive interactions were assessed using the Excel calculation table, compiled by Andersson et al. (33), which calculated the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP), the synergy index (S), ORs, and their corresponding 95% confidence intervals (95% CIs). An additive interaction was indicated when 95% CIs of RERI and AP excluded 0, and 95% CI of S excluded 1. Specifically, a synergistic interaction was suggested when RERI >0, AP > 0, and S > 1. Conversely, an antagonistic interaction was indicated when RERI <0, AP < 0, and S < 1. Subgroup analyses were performed based on age and gender. Finally, sensitivity analysis was performed regarding “long sleep” (≥ 9 h), defined in other literature (34, 35), to ensure the robustness of the results.
3 Results
3.1 Characteristics of all participants
As shown in Table 1, a total of 15,511 participants were included in this study, of whom 7,017 (45.2%) experienced depressive symptoms, 5,474 (35.3%) reported short sleep, and 3,364 (21.7%) reported long sleep. Additionally, 3,122 (20.1%) had BADL limitations, and 3,196 (20.6%) had IADL limitations. Chi-square tests revealed that compared with participants without depressive symptoms, those with depressive symptoms exhibited significant differences in terms of gender, residence, marital status, education level, smoking status, alcohol consumption frequency, sleep duration, BADL limitations, IADL limitations, and number of chronic diseases (all p < 0.001).
3.2 Independent associations of sleep duration and ADL limitations with depressive symptoms
Table 2 showed that in crude Model 1, compared with participants with normal sleep, short sleep (OR = 2.09, 95% CI: 1.94–2.25) was significantly associated with an increased risk of depressive symptoms, whereas long sleep (OR = 0.86, 95% CI: 0.79–0.93) linked to a decreased risk of depressive symptoms. BADL limitations (OR = 3.00, 95% CI: 2.76–3.26) and IADL limitations (OR = 2.98, 95% CI: 2.75–3.23) both increased the risk of depressive symptoms. In Model 2, adjusting for age, gender residence, marital status, education level, smoking status, alcohol consumption, and number of chronic diseases, the associations between short sleep (OR = 1.78, 95% CI: 1.65–1.92), long sleep (OR = 0.87, 95% CI: 0.79–0.95), BADL limitations (OR = 2.03, 95% CI: 1.85–2.23), and IADL limitations (OR = 2.05, 95% CI: 1.87–2.25) remained significant with depressive symptoms.
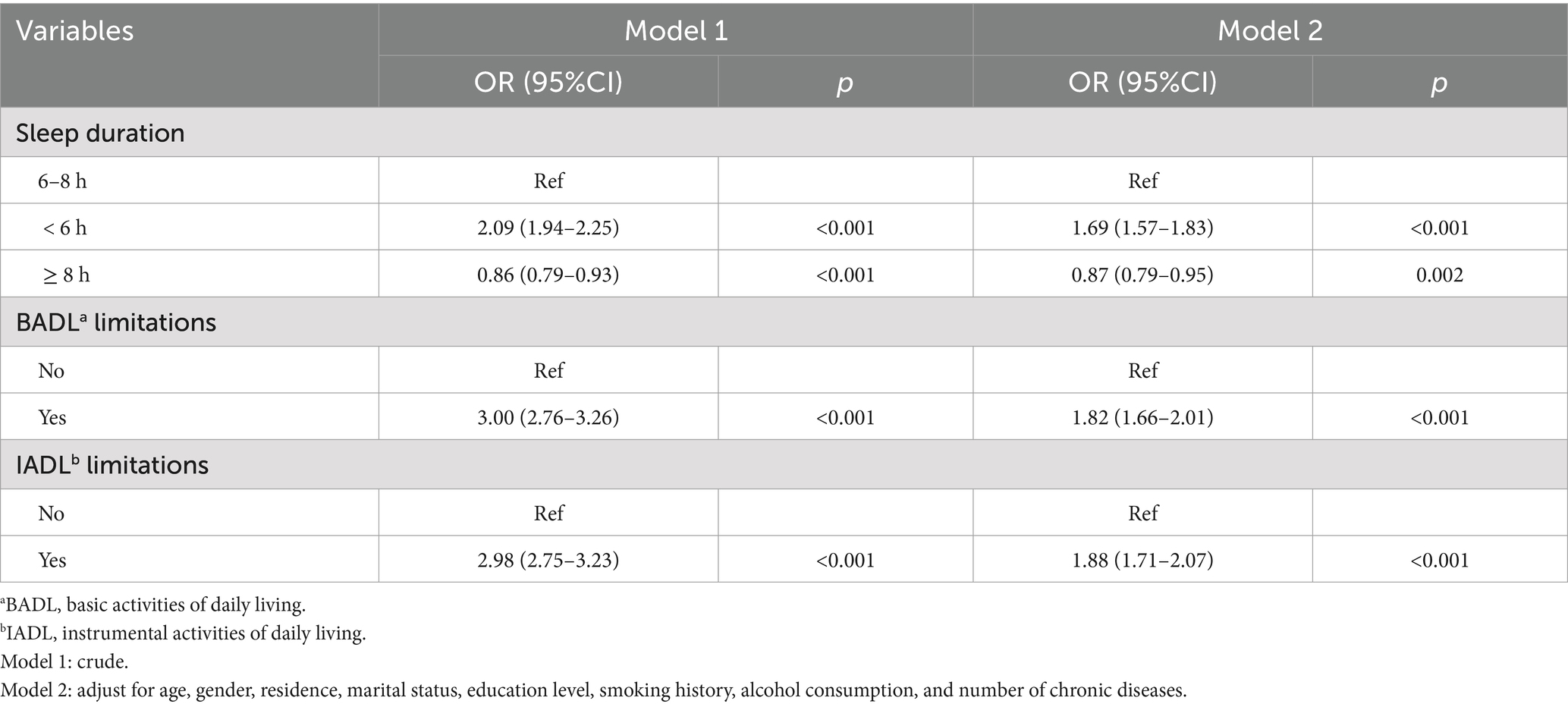
Table 2. Binary logistic regression of ADL limitations and sleep duration for participants with depressive symptoms.
3.3 The interaction effects between sleep duration and ADL limitations on depressive symptoms
3.3.1 The interaction effects between short sleep and IADL or BADL limitations on depressive symptoms
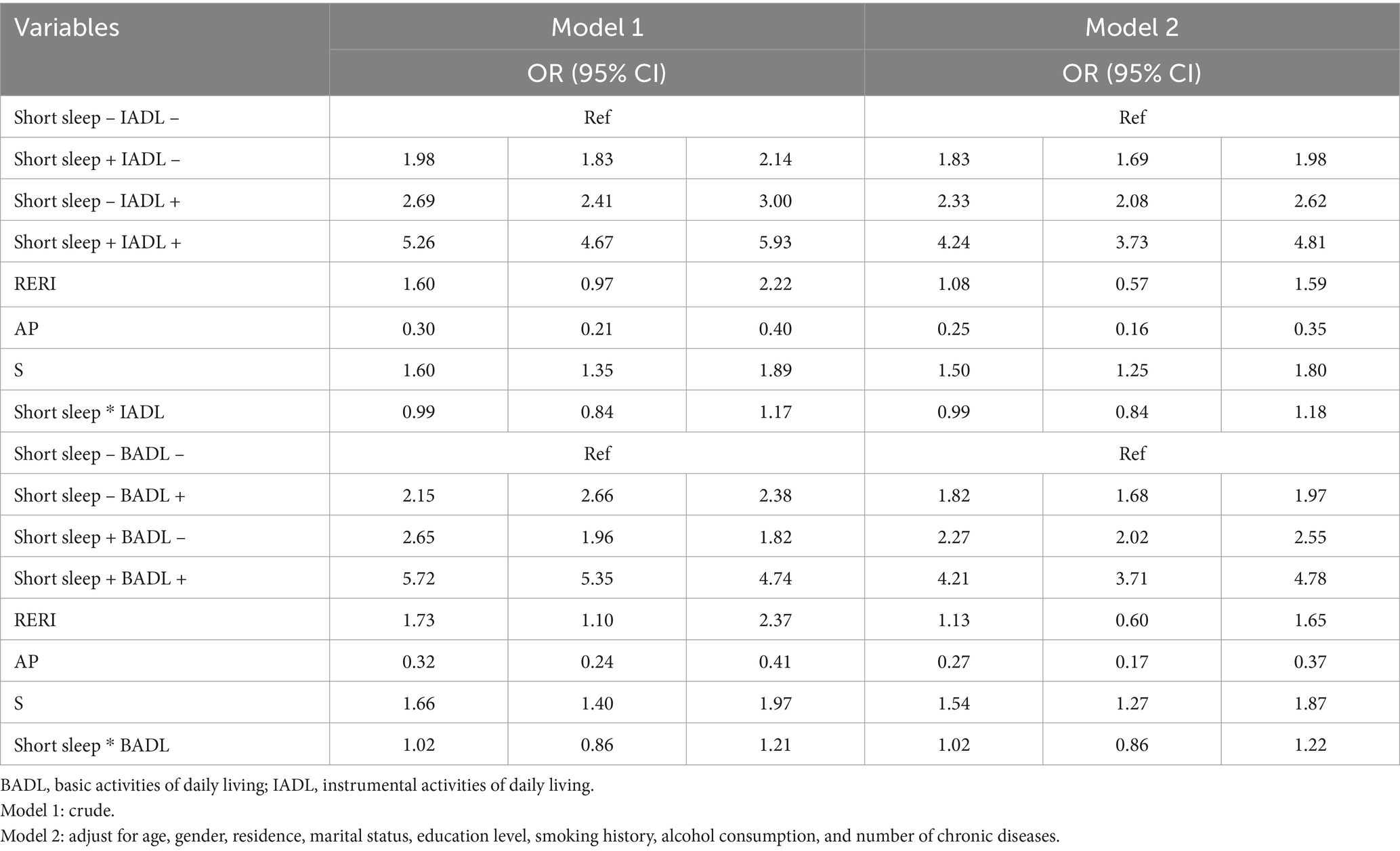
Compared with the reference group (non-short sleep and independent IADL), participants with both short sleep and IADL limitations had a significantly higher risk of depressive symptoms (adjusted OR 4.24, 95% CI: 3.73–4.81), as shown in Table 3. Additive interaction analysis revealed a statistically significant positive interaction between short sleep and IADL limitations on depressive symptoms in the total population, with a RERI of 1.08 (95% CI, 0.57–1.59), an AP of 0.25 (95% CI, 0.16–0.35), and a S of 1.50 (95% CI, 1.25–1.80), indicating that the combined effect of short sleep and IADL limitations on depressive symptoms was greater than their individual effect. Similarly, a significant additive interaction between short sleep and BADL limitations was observed (adjusted RERI = 1.13, 95% CI: 0.60–1.65; AP = 0.27, 95% CI: 0.17–0.37; S = 1.54, 95% CI: 1.27–1.87). In age and gender subgroup analyses, the synergistic interaction effects were consistent with the overall population (Supplementary Tables S1, S2). However, no multiplicative interactions were observed between short sleep and IADL or BADL limitations on depressive symptoms.
3.3.2 The interaction effects between long sleep and IADL or BADL limitations on depressive symptoms
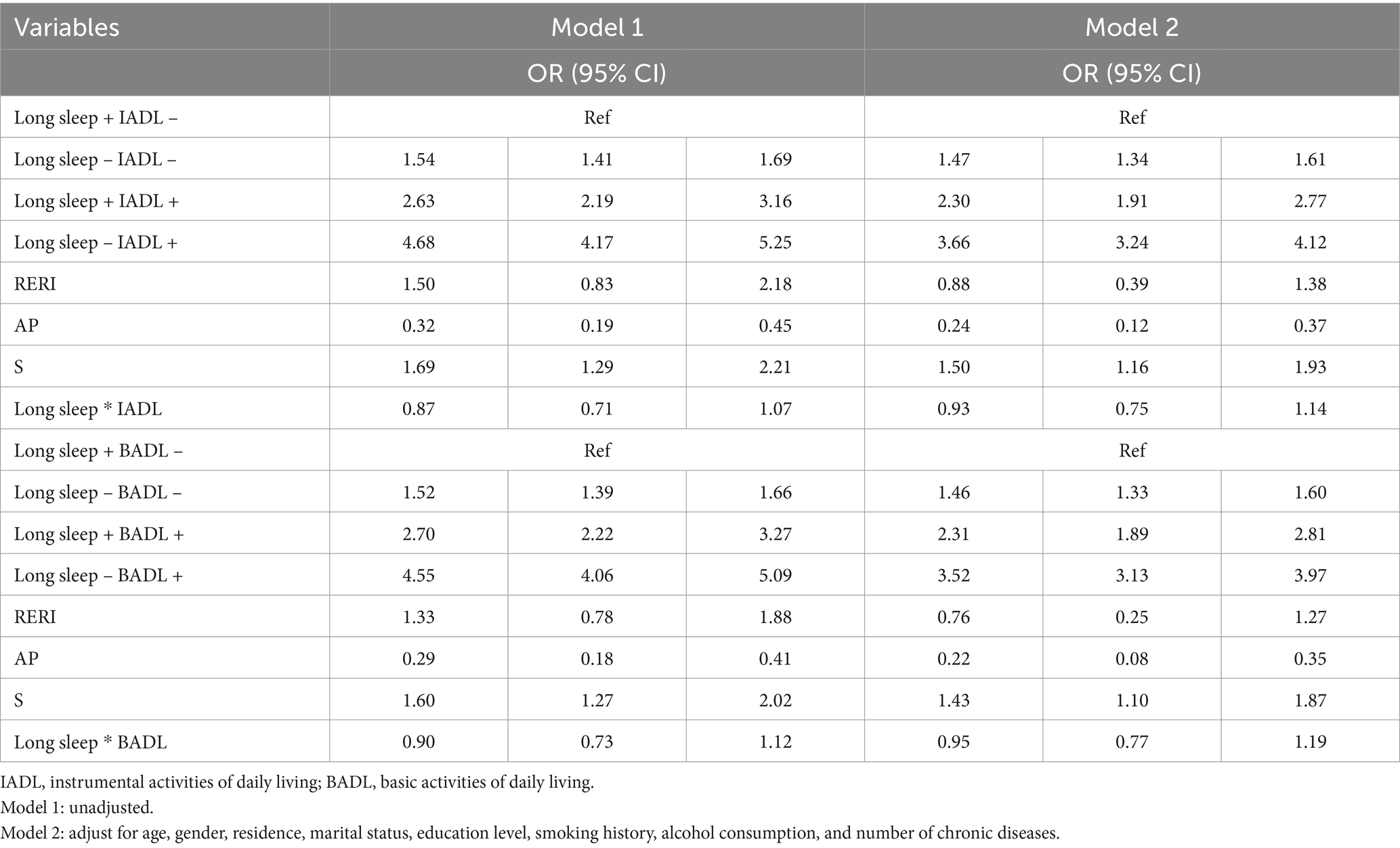
In Table 4, this study used participants with both long sleep and independent IADL as the reference group, which had the lowest risk of depressive symptoms due to the protective effect of long sleep, compared to the other three combinations. Compared with the reference group, participants with both IADL limitations and lack of long sleep had a 3.66 times higher risk on depressive symptoms (adjusted OR 3.66, 95% CI: 3.24–4.12). The additive interaction between IADL limitations and long sleep was significant (adjusted RERI = 0.88, 95% CI: 0.39–1.38; AP = 0.24, 95% CI: 0.12–0.37; S = 1.50, 95% CI: 1.16–1.93). Similarly, compared with participants with independent BADL and long sleep, those with BADL limitations and lack of sleep had 3.52 times higher risk (adjusted OR = 3.52, 95% CI 3.13–3.97), with RERI = 0.76 (95% CI: 0.25–1.27), AP = 0.22 (95% CI: 0.08–0.35), S = 1.43 (95% CI, 1.10–1.80), indicating an antagonistic effect between long sleep and BADL limitations on depressive symptoms. The sensitivity analysis further supported the above results (Supplementary Table S5). Notably, in the subgroup analysis regarding age and gender, the additive interaction effects were significant in the population aged ≥60 years and men (Supplementary Tables S3, S4).
4 Discussion
To the best of our knowledge, this is the first study exploring the potential additive interaction effects between sleep duration (categorized as short and long sleep) and ADL limitations (BADL and IADL limitations) on depressive symptoms utilizing data from the CHARLS. This study demonstrated that in the overall population, there were synergistic interaction effects between short sleep and IADL or BADL limitations on the depressive symptoms risk. In contrast, antagonistic interaction effects were identified between long sleep and IADL or BADL limitations on the depressive symptoms risk.
The present study found the additive interaction effects between sleep duration and ADL limitations on depressive symptoms. Similarly, a hospital-based cross-sectional study investigated the relationship between frailty and depressive symptoms, revealing a multiplicative interaction between sleep duration and ADL on depressive symptoms (28). However, unlike multiplicative interactions that primarily inform biological mechanisms, the additive interactions explored in the present study offer practice value for public health decision-making, particularly in guiding the prioritization of prevention strategies and resource allocation to alleviate the burden of depressive symptoms (36). Compared to the hospital-based study, which was restricted to a single hospital in China and included a relatively small sample size (n = 1,574), the present study utilized a nationally representative sample (n = 15,511), thereby strengthening the generalizability of its findings. Additionally, two studies focused on the mediating relationships among these three factors (37, 38). One 7-year prospective study demonstrated that depression partially mediated the relationship between sleep duration and IADL disability, accounting for 64.8% of the total effect of short nighttime sleep on IADL disability (38). Another cross-sectional study revealed that ADL had a mediating effect on the association between insomnia and depressive symptoms in older adults aged 65 and above (37). While these studies primarily focused on causal pathways, the present study highlighted the combined effect of sleep duration and ADL limitations on depressive symptoms, providing a new perspective on how these factors influence depressive symptoms.
It is worth emphasizing that short sleep and ADL limitations exhibited a positive additive interaction effect on the depressive symptoms risk in middle-aged and older individuals. The interpretation of these findings likely involves the following two reasons. On the one hand, short sleep itself is one of the diagnostic criteria for depression (8). It may lead to ADL limitations by impairing cognitive function (39) and increasing the risk of sarcopenia (40, 41). Meanwhile, ADL limitations may provoke depressive symptoms, perhaps because physical limitations not only hinder participation in social activities (42), thereby reducing social support (43), but also cause the loss of independence and increased reliance on others (15), both of which amplify negative emotions (e.g., shame, guilt, and depressive symptoms) (14, 18, 44). On the other hand, these interactions may be explained by underlying biological mechanisms. Firstly, short sleep can elevate pro-inflammatory cytokines (e.g., C-reactive protein and interleukin-6) by disrupting circadian rhythms and activating the hypothalamic–pituitary–adrenal (HPA) axis (45, 46). Meanwhile, ADL limitations may exacerbate chronic low-grade inflammation due to reduced physical activity (47) and the presence of concomitant chronic diseases (48). These inflammatory factors can impair the function of neurons and glial cells in brain regions involved in emotional processing, thus increasing the risk of depressive symptoms (49). Secondly, short sleep and chronic stress linked to ADL limitations stimulate HPA axis hyperactivity, resulting in elevated cortisol levels (45, 50), which are not only directly related to somatic depressive symptoms (51), but also further worsen ADL impairment (52). Finally, depressive symptoms could shorten sleep duration (53), thereby triggering a vicious cycle.
Notably, the present study found that long sleep and ADL limitations exhibited antagonistic interactions on depressive symptoms in the total population, somewhat aligning with previous evidence that nocturnal sleep of 8 to 10 h can mitigate ADL-related depressive symptoms risks (28). In other words, long sleep may weaken the association between ADL limitations and depressive symptoms. Additionally, in the age subgroup analysis, antagonistic interaction effects were observed in individuals aged 60 and older. Emerging evidence reported that 8 to 10 h of sleep can reduce the risk of ADL limitations in individuals over 90 years (54), which is associated with a decreased risk of depressive symptoms (18). This may be explained by age-related declines in melatonin levels, leading to poor sleep quality (55) and decreased sleep duration (56). Hence, longer sleep duration is necessary to facilitate energy sustainment and functional recovery (28, 54), thereby reducing the risk of depressive symptoms (15). In the gender-stratified subgroup analysis, the antagonistic interaction was observed between long sleep and BADL limitations on depressive symptoms only in men. Sex-specific inflammatory responses to long sleep may underlie this disparity. A longitudinal study demonstrated that long sleep correlates with elevated C-reactive protein (CRP) levels in adult women but not men (57), and heightened CRP can increase the risk of depressive symptoms (e.g., decreased energy and feelings of worthlessness) (58). Moreover, consistent with prior evidence (59), women (60.5%) were more likely to experience depressive symptoms than men (39.5%) in the present study, which may be attributed to gender differences in stress-coping strategies (60), roles and responsibilities (e.g., childcare and caregiving) (59), and susceptibility to depression-related genes (61). However, the underlying mechanisms regarding the relationships among long sleep, ADL limitations, and depressive symptoms remain unclear and warrant further exploration in future studies.
The present study presents several notable strengths. First, this study is the first to assess the additive interaction effects between sleep duration and ADL limitations on the depressive symptoms risk, thereby providing a new perspective for prioritizing resource allocation in prevention efforts. Second, this study is based on data from a national survey, ensuring that results are representative of Chinese middle-aged and older adult individuals. However, several limitations should be acknowledged. The data relied on self-reports, which may introduce measurement errors and misclassification. Additionally, the cross-sectional design limits causal relationships between sleep duration, ADL limitations, and depressive symptoms.
5 Conclusion
The present study highlights the interaction effects of sleep duration and ADL limitations on depressive symptoms among middle-aged and older individuals. If confirmed in future studies, these results will assist in providing valuable insights into prevention and intervention strategies for depression.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: http://charls.pku.edu.cn/.
Ethics statement
The studies involving humans were approved by the Biomedical Ethics Review Committee of Peking University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
TW: Writing – original draft, Writing – review & editing, Formal analysis. WH: Conceptualization, Software, Writing – review & editing. CW: Data curation, Formal analysis, Methodology, Writing – review & editing. YK: Formal analysis, Methodology, Software, Writing – review & editing. YW: Formal analysis, Software, Writing – review & editing. SL: Writing – review & editing. NL: Conceptualization, Writing – review & editing, Supervision, Funding acquisition. ZH: Writing – review & editing. XW: Conceptualization, Supervision, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Natural Science Foundation of Shaanxi Province (No. 2023-YBSF-027).
Acknowledgments
This study used data from the China Health and Retirement Longitudinal Study (CHARLS). The authors sincerely thank the CHARLS research team, the field team, and every respondent for their contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1547329/full#supplementary-material
Abbreviations
ADL, Activities of daily living; BADL, Basic activities of daily living; IADL, Instrumental activities of daily living; CHARLS, China Health and Retirement Longitudinal Study; CEDS, Center for Epidemiological Studies Depression Scale; RERI, Relative excess risk due to interaction; AP, Attributable proportion due to interaction; S, Synergy index; ORs, Odds ratios; 95% CI, 95% confidence interval; HPA, Hypothalamic–pituitary–adrenal.
References
1. World Health Organization. (2023). Available online at: https://www.who.int/health-topics/depression (Accessed September 5, 2024).
2. Xu, Y, Li, R, Hu, C, He, Y, Zhang, X, and Jin, L. Global, regional, and national incidence trends of depressive disorder, 1990–2019: An age-period-cohort analysis based on the global burden of disease 2019 study. Gen Hosp Psychiatry. (2024) 88:51–60. doi: 10.1016/j.genhosppsych.2024.03.003
3. Santomauro, DF, Mantilla Herrera, AM, Shadid, J, Zheng, P, Ashbaugh, C, Pigott, DM, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
4. Li, Q, Cen, W, Yang, T, and Tao, S. Association between depressive symptoms and sarcopenia among middle-aged and elderly individuals in China: the mediation effect of activities of daily living (ADL) disability. BMC Psychiatry. (2024) 24:432. doi: 10.1186/s12888-024-05885-y
5. Alberti, A, Araujo Coelho, DR, Vieira, WF, Moehlecke Iser, B, Lampert, RMF, Traebert, E, et al. Factors associated with the development of depression and the influence of obesity on depressive disorders: a narrative review. Biomedicines. (2024) 12:1994. doi: 10.3390/biomedicines12091994
6. Zhu, L, Wang, Y, Li, J, Zhou, H, Li, N, and Wang, Y. Depressive symptoms and all-cause mortality among middle-aged and older people in China and associations with chronic diseases. Front Public Health. (2024) 12:1381273. doi: 10.3389/fpubh.2024.1381273
7. Tang, L, Yin, R, Hu, Q, Fan, Z, and Zhang, F. The effect of childhood socioeconomic status on depressive symptoms in middle-old age: the mediating role of life satisfaction. BMC Psychiatry. (2022) 22:398. doi: 10.1186/s12888-022-04046-3
8. American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th ed.).
9. Lu, Q, Hu, P, Lian, C, and Chen, X. The association between hobby engagement and depressive symptoms among Chinese middle-aged and older adults: evidence from the China health and retirement longitudinal study. Front Public Health. (2024) 12:1450358. doi: 10.3389/fpubh.2024.1450358
10. Chan, CK, Soldan, A, Pettigrew, C, Wang, M-C, Wang, J, Albert, MS, et al. Depressive symptoms in relation to clinical symptom onset of mild cognitive impairment. Int Psychogeriatr. (2019) 31:561–9. doi: 10.1017/S1041610218001138
11. Maier, A, Riedel-Heller, SG, Pabst, A, and Luppa, M. Risk factors and protective factors of depression in older people 65+. A systematic review. PLoS One. (2021) 16:e0251326. doi: 10.1371/journal.pone.0251326
12. Risk factors for depressive symptoms among older Chinese adults: a meta-analysis. J Affect Disord. (2020) 277:341–6. doi: 10.1016/j.jad.2020.08.036
13. Edemekong, PF, Bomgaars, DL, Sukumaran, S, and Schoo, C. “Activities of daily living.” Stat pearls. Treasure Island (FL): Stat Pearls Publishing (2024). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK470404/ (Accessed September 18, 2024).
14. Zhu, X, Wang, Y, Luo, Y, Ding, R, Shi, Z, and He, P. Bidirectional, longitudinal associations between depressive symptoms and IADL/ADL disability in older adults in China: a national cohort study. BMC Geriatr. (2024) 24:659. doi: 10.1186/s12877-024-05248-y
15. Wang, J, Luo, N, Sun, Y, Bai, R, Li, X, Liu, L, et al. Exploring the reciprocal relationship between activities of daily living disability and depressive symptoms among middle-aged and older Chinese people: a four-wave, cross-lagged model. BMC Public Health. (2023) 23:1180. doi: 10.1186/s12889-023-16100-0
16. Sun, W, Yang, Y, Ding, L, and Wang, L. Association between cognitive function and depressive symptoms in Chinese older adults: the mediating role of activities of daily living. Geriatr Nurs. (2024) 60:258–64. doi: 10.1016/j.gerinurse.2024.09.009
17. Xu, W, Sun, H, Zhu, B, Yu, X, Niu, Y, Kou, C, et al. The prevalence of depressive symptoms and its determinants among adults in mainland China: results from a national household survey. J Affect Disord. (2021) 281:220–7. doi: 10.1016/j.jad.2020.12.009
18. Zhou, L, Wang, W, and Ma, X. The bidirectional association between the disability in activities of daily living and depression: a longitudinal study in Chinese middle-aged and older adults. BMC Public Health. (2024) 24:1884. doi: 10.1186/s12889-024-19421-w
19. Colrain, IM. Sleep and the brain. Neuropsychol Rev. (2011) 21:1–4. doi: 10.1007/s11065-011-9156-z
20. Xie, J, Li, Y, Zhang, Y, Vgontzas, AN, Basta, M, Chen, B, et al. Sleep duration and metabolic syndrome: an updated systematic review and meta-analysis. Sleep Med Rev. (2021) 59:101451. doi: 10.1016/j.smrv.2021.101451
21. Jiang, Y, Gu, X, Yang, X, Sun, A, and Sun, H. Exploring the association between sleep duration and cancer risk in middle-aged and older Chinese adults: observations from a representative cohort study (2011-2020). BMC Public Health. (2024) 24:1819. doi: 10.1186/s12889-024-19313-z
22. Porras-Segovia, A, Pérez-Rodríguez, MM, López-Esteban, P, Courtet, P, Barrigón, MML, López-Castromán, J, et al. Contribution of sleep deprivation to suicidal behaviour: a systematic review. Sleep Med Rev. (2019) 44:37–47. doi: 10.1016/j.smrv.2018.12.005
23. Li, X-L, Wei, J, Zhang, X, Meng, Z, and Zhu, W. Relationship between night-sleep duration and risk for depression among middle-aged and older people: a dose-response meta-analysis. Front Physiol. (2023) 14:1085091. doi: 10.3389/fphys.2023.1085091
24. Zhang, XF, Liu, F, Liu, WP, Ye, XM, Cui, BY, and Wang, HJ. Relationship between sleep duration and depressive symptoms in middle-aged and elderly people in four provinces of China. Chin J Epidemiol. (2021) 42:1955–61. doi: 10.3760/cma.j.cn112338-20200930-01210
25. Sun, Y, Shi, L, Bao, Y, Sun, Y, Shi, J, and Lu, L. The bidirectional relationship between sleep duration and depression in community-dwelling middle-aged and elderly individuals: evidence from a longitudinal study. Sleep Med. (2018) 52:221–9. doi: 10.1016/j.sleep.2018.03.011
26. Chen, R, Chen, Q, Lu, G, Zhang, M, Zhang, M, Yang, H, et al. Sleep duration and depressive symptoms in Chinese middle-aged and older adults: the moderating effects of grip strength. J Affect Disord. (2023) 339:348–54. doi: 10.1016/j.jad.2023.07.059
27. Jing, R, Xu, T, Rong, H, Lai, X, and Fang, H. Longitudinal association between sleep duration and depressive symptoms in Chinese elderly. Nat Sci Sleep. (2020) 12:737–47. doi: 10.2147/NSS.S269992
28. Song, W, Liu, M, Ye, T, Wang, D, Yuan, Q, Li, F, et al. Relationship between frailty and depressive symptoms in older adults: role of activities of daily living and sleep duration. Front Med (Lausanne). (2024) 11:1416173. doi: 10.3389/fmed.2024.1416173
29. Chen, H, and Mui, AC. Factorial validity of the center for epidemiologic studies depression scale short form in older population in China. Int Psychogeriatr. (2014) 26:49–57. doi: 10.1017/S1041610213001701
30. Jin, Y, Luo, Y, and He, P. Hypertension, socioeconomic status and depressive symptoms in Chinese middle-aged and older adults: findings from the China health and retirement longitudinal study. J Affect Disord. (2019) 252:237–44. doi: 10.1016/j.jad.2019.04.002
31. Song, Y, Liu, H, and Liu, Y. The association between nap time, nighttime sleep and depression in Chinese older adults: a cross-sectional study. PLoS One. (2024) 19:e0302939. doi: 10.1371/journal.pone.0302939
32. Chen, W, and Wang, X. Longitudinal associations between sleep duration and cognitive impairment in Chinese elderly. Front Aging Neurosci. (2022) 14:1037650. doi: 10.3389/fnagi.2022.1037650
33. Andersson, T, Alfredsson, L, Källberg, H, Zdravkovic, S, and Ahlbom, A. Calculating measures of biological interaction. Eur J Epidemiol. (2005) 20:575–9. doi: 10.1007/s10654-005-7835-x
34. Dong, J, Huang, J, Parisi, JM, Zhou, ZE, Li, M, Calderon, R, et al. Depressive symptoms among middle-aged and older adults in China: the interaction of physical activity and sleep duration. Sleep Health. (2025) S2352-7218:00268–7. doi: 10.1016/j.sleh.2024.12.004
35. Wang, M, Zeng, X, Liu, Q, Yang, Z, and Li, J. The association between sleep duration and cognitive function in the U.S. elderly from NHANES 2011–2014: a mediation analysis for inflammatory biomarkers. J Affect Disord. (2025) 375:465–71. doi: 10.1016/j.jad.2025.01.154
36. Sun, J, Wang, X, Terry, PD, Ren, X, Hui, Z, Lei, S, et al. Interaction effect between overweight/obesity and alcohol consumption on hypertension risk in China: a longitudinal study. BMJ Open. (2022) 12:e061261. doi: 10.1136/bmjopen-2022-061261
37. Webb, CA, Cui, R, Titus, C, Fiske, A, and Nadorff, MR. Sleep disturbance, activities of daily living, and depressive symptoms among older adults. Clin Gerontol. (2018) 41:172–80. doi: 10.1080/07317115.2017.1408733
38. Wu, Y, Li, S, Han, D, Zhang, M, Zhao, J, Liao, H, et al. The mediating role of depression in association between total sleep time and instrumental activities of daily living in China. Int J Public Health. (2023) 68:1605678. doi: 10.3389/ijph.2023.1605678
39. Liu, M, Du, X, Sun, Y, Zhou, A, Sun, S, and Wu, Y. The mediating role of cognition in the relationship between sleep duration and instrumental activities of daily living disability among middle-aged and older Chinese. Arch Gerontol Geriatr. (2021) 94:104369. doi: 10.1016/j.archger.2021.104369
40. Pourmotabbed, A, Ghaedi, E, Babaei, A, Mohammadi, H, Khazaie, H, Jalili, C, et al. Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep Breath. (2020) 24:1267–78. doi: 10.1007/s11325-019-01965-6
41. Zhou, H, Ding, X, and Luo, M. The association between sarcopenia and functional disability in older adults. J Nutr Health Aging. (2024) 28:100016. doi: 10.1016/j.jnha.2023.100016
42. Vallée, J, Cadot, E, Roustit, C, Parizot, I, and Chauvin, P. The role of daily mobility in mental health inequalities: the interactive influence of activity space and neighbourhood of residence on depression. Soc Sci Med. (2011) 73:1133–44. doi: 10.1016/j.socscimed.2011.08.009
43. Tengku Mohd, TAM, Yunus, RM, Hairi, F, Hairi, NN, and Choo, WY. Social support and depression among community dwelling older adults in asia: a systematic review. BMJ Open. (2019) 9:e026667. doi: 10.1136/bmjopen-2018-026667
44. Steptoe, A, and Di Gessa, G. Mental health and social interactions of older people with physical disabilities in England during the COVID-19 pandemic: a longitudinal cohort study. Lancet Public Health. (2021) 6:e365–73. doi: 10.1016/S2468-2667(21)00069-4
45. Xing, C, Zhou, Y, Xu, H, Ding, M, Zhang, Y, Zhang, M, et al. Sleep disturbance induces depressive behaviors and neuroinflammation by altering the circadian oscillations of clock genes in rats. Neurosci Res. (2021) 171:124–32. doi: 10.1016/j.neures.2021.03.006
46. Pandi-Perumal, SR, Monti, JM, Burman, D, Karthikeyan, R, BaHammam, AS, Spence, DW, et al. Clarifying the role of sleep in depression: a narrative review. Psychiatry Res. (2020) 291:113239. doi: 10.1016/j.psychres.2020.113239
47. Kim, S-D, and Yeun, Y-R. Effects of resistance training on C-reactive protein and inflammatory cytokines in elderly adults: a systematic review and meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:3434. doi: 10.3390/ijerph19063434
48. Rajendran, P, Chen, Y, Chen, Y, Chung, L, Tamilselvi, S, Shen, C, et al. The multifaceted link between inflammation and human diseases. J Cell Physiol. (2018) 233:6458–71. doi: 10.1002/jcp.26479
49. Ge, H, Yang, S, Su, W, Guan, W, Dong, S, Chang, W, et al. The relationship between sarcopenia and mental health status in Chinese older adults: the mediating role of activities of daily living. BMC Geriatr. (2025) 25:64. doi: 10.1186/s12877-025-05723-0
50. Sharan, P, and Vellapandian, C. Hypothalamic-pituitary-adrenal (HPA) axis: unveiling the potential mechanisms involved in stress-induced alzheimer’s disease and depression. Cureus. (2024) 16:e67595. doi: 10.7759/cureus.67595
51. Iob, E, Kirschbaum, C, and Steptoe, A. Persistent depressive symptoms, HPA-axis hyperactivity, and inflammation: the role of cognitive-affective and somatic symptoms. Mol Psychiatry. (2020) 25:1130–40. doi: 10.1038/s41380-019-0501-6
52. Shindel, C, Holland, JM, and Gallagher-Thompson, D. The link between activities of daily living and cortisol in late-life depression. Clin Gerontol. (2020) 43:430–40. doi: 10.1080/07317115.2018.1561581
53. Ji, S, Wang, J, Wang, W, and Liao, F. Longer depressive duration reduces sleep duration more: a longitudinal study in the middle-aged and elderly Chinese. J Affect Disord. (2022) 317:185–92. doi: 10.1016/j.jad.2022.08.051
54. Wang, Z, Ni, X, Gao, D, Fang, S, Huang, X, Jiang, M, et al. The relationship between sleep duration and activities of daily living (ADL) disability in the Chinese oldest-old: a cross-sectional study. PeerJ. (2023) 11:e14856. doi: 10.7717/peerj.14856
55. Alizadeh Feremi, K, Alipoor, L, and Esmaeili, R. Effect of melatonin on the sleep quality: a systematic review. Rev Clin Med. (2021) 8:60–8. doi: 10.22038/rcm.2021.55887.1356
56. Duffy, JF, Wang, W, Ronda, JM, and Czeisler, CA. High dose melatonin increases sleep duration during nighttime and daytime sleep episodes in older adults. J Pineal Res. (2022) 73:e12801. doi: 10.1111/jpi.12801
57. Bakour, C, Schwartz, S, O’Rourke, K, Wang, W, Sappenfield, W, Couluris, M, et al. Sleep duration trajectories and systemic inflammation in young adults: results from the national longitudinal study of adolescent to adult health (add health). Sleep. (2017) 40. doi: 10.1093/sleep/zsx156
58. Wang, D, Xu, J, Liang, N, Xue, Z, Yang, X, Lu, J, et al. Network analysis of depressive symptoms and C-reactive protein levels in major depressive disorder. J Affect Disord. (2024) 367:788–94. doi: 10.1016/j.jad.2024.08.152
59. Guo, L, Fang, M, Wang, L, Liu, L, He, C, Zhou, X, et al. Gender differences in geriatric depressive symptoms in urban China: the role of ADL and sensory and communication abilities. Front Psych. (2024) 15:1344785. doi: 10.3389/fpsyt.2024.1344785
60. Niu, Y, Sun, Y, Xie, Y, and Yu, S. Association between sleep patterns and depression in older adults: a cross-sectional study using data from the national health and nutrition examination survey 2007-2014. BMC Geriatr. (2025) 25:56. doi: 10.1186/s12877-024-05633-7
Keywords: depressive symptoms, sleep duration, activities of daily living, interaction, CHARLS
Citation: Wang T, Han W, Wang C, Kang Y, Wang Y, Lei S, Hui Z, Li N and Wang X (2025) Interaction effects of sleep duration and activities of daily living on depressive symptoms among Chinese middle-aged and older adult individuals: evidence from the CHARLS. Front. Public Health. 13:1547329. doi: 10.3389/fpubh.2025.1547329
Edited by:
Esther-Lee Marcus, Herzog Hospital, IsraelReviewed by:
Hui Liew, University of Nebraska at Kearney, United StatesHuanhuan Huang, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2025 Wang, Han, Wang, Kang, Wang, Lei, Hui, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Li, bGluaW5nNzJAeGp0dS5lZHUuY24=; Xiaoqin Wang, d2FuZ3hpYW9xaW5AeGp0dS5lZHUuY24=
 Tianmeng Wang
Tianmeng Wang Wenjin Han
Wenjin Han Caihua Wang
Caihua Wang Yanqing Kang
Yanqing Kang Yaping Wang1
Yaping Wang1 Shuangyan Lei
Shuangyan Lei Zhaozhao Hui
Zhaozhao Hui Xiaoqin Wang
Xiaoqin Wang