
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 09 April 2025
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1544165
 Athanase Munyaneza1*
Athanase Munyaneza1* Hae-Young Kim2
Hae-Young Kim2 Qiuhu Shi3
Qiuhu Shi3 Ellen Brazier4
Ellen Brazier4 Jonathan Ross5
Jonathan Ross5 Benjamin Muhoza1
Benjamin Muhoza1 Faustin Kanyabwisha1
Faustin Kanyabwisha1 Gallican Kubwimana1
Gallican Kubwimana1 Gad Murenzi1
Gad Murenzi1 Denis Nash4
Denis Nash4 Kathryn Anastos5
Kathryn Anastos5 Marcel Yotebieng5
Marcel Yotebieng5Introduction: Achieving and maintaining HIV viral load suppression (VLS) in pregnant and postpartum women living with HIV (WLWH) is critical for their health and to prevent mother-to-child transmission. However, data on VLS in this population are limited. This study aimed to evaluate the prevalence and factors associated with VLS among pregnant and postpartum WLWH in Rwanda within 12 months of enrolling in antenatal care.
Methods: An open observational cohort study was conducted using routine clinical data from 10 Rwandan HIV clinics in Kigali City. Data from WLWH on ART who became pregnant and were referred to PMTCT services between 2012 and 2020 were analyzed. The primary outcomes were the proportion of WLWH achieving VLS (viral load (VL) <1,000 or <200 copies/mL) within 12 months of antenatal registration. Logistic regression models assessed associations of VLS with socio-demographic and clinical characteristics.
Results: Among 1,002 WLWH, 532 (53%) had documented VL results. The mean age was 30.4 years, with 60% aged 25–34 years. Most (83.7%) were primigravida, and 67% initiated ART before pregnancy. At antenatal care enrollment, 58% had a CD4 count ≥500 cells/uL. Within 12 months, 92% had VL <1,000 copies/mL and 87% had a VL <200 copies/mL. Advanced HIV disease (WHO stage 3 and 4) and lower CD4 counts were associated with lower odds of VLS.
Conclusion: Among those with a recorded VL results, nine out of 10 had a VLS, particularly those without advanced HIV disease. The findings underscore the need for targeted interventions for WLWH with advanced HIV entering antenatal care.
Achieving and maintaining viral load suppression (VLS) in pregnant and postpartum women living with HIV (WLWH) remains a global public health priority (1). The Joint United Nations Programme on HIV/AIDS (UNAIDS) VLS target for ending the epidemic is for 95% of pregnant and postpartum WLWH on antiretroviral treatment (ART) to achieve and sustain VLS both before delivery throughout and beyond the postpartum period (2, 3). While there has been substantial progress in the scale up of ART among pregnant and postpartum WLWH through the prevention of mother-to-child transmission (PMTCT) programs around the globe (4), studies indicate persistent challenges in achieving and sustaining VLS among this population (5, 6). Without adequate viral suppression (VS), pregnant women face higher risks of HIV-related morbidity, mortality, and increased HIV transmission to the infant, which can result in early infant mortality and long-term health issues due to mother-to-child transmission (MTCT) (7). Although high rates of VS are observed during pregnancy, the postpartum period presents greater challenges due to factors like healthcare access, adherence, and breastfeeding, particularly in Southern Africa. This period increases the risk of HIV transmission to the infant and poses additional health risks to the mother (8). Existing VLS data are primarily focused on WLWH in general, with only 76% achieving VLS by 2022 among those on ART (9).
In sub-Saharan Africa (SSA), where the HIV burden is highest, there is little data on VLS among pregnant and postpartum WLWH because the scale up of routine viral load monitoring has been slow, and pregnant and postpartum WLWH are not sufficiently recognized as a priority population (5). Data on VLS among this population primarily comes from research studies rather than routine care programs, and estimates vary widely, depending on the thresholds used for VLS (5, 10–13).
Available data suggest that even with conservative cut-offs (i.e., <1,000 copies/mL), VLS among this population is far below UNAIDS targets. A cross sectional analysis of Population-Based HIV Impact Assessment surveys across 10 SSA countries using data collected between 2015 and 2018 found that, of 1,685 pregnant or breastfeeding WLHIV regardless of their ART status, only 63.8% WLWH had HIV viral load <1,000 copies/mL at the study visit (6). Considerable variation by region, ranging from 40.1% in Central and West Africa to 66.2% in East Africa and 76.2% in Southern Africa (6). Similarly, a population-representative cross sectional study conducted in South Africa (SA) found that, Of 10 052 of pregnant WLWH attending antenatal care on ART, only 79.5% were virally suppressed using a threshold of viral load ≤ 1,000 copies/mL, with only 56.2% being virally suppressed using a lower threshold of 50 copies/mL (10).
As of 2022, Rwanda was one of only five countries meeting UNAIDS targets for VLS among all people living with HIV on ART (4). However, disparities persist among subpopulations. Few data are available on VLS among pregnant and postpartum Rwandan WLWH as neither UNAIDS nor national HIV program data disaggregate VLS data by pregnancy or postpartum status. To our knowledge, the only estimate of VLS among this population are from a 2013 to 2014 observational prospective cohort study that assessed 24-months HIV-free survival among infants born to 608 WLWH enrolled in PMTCT programs (14). This study found that 85% had VLS using a threshold of <1,000 copies/mL, but only half had an undetectable viral load ( ≤ 20 copies/mL) at enrollment (14). In the current study, we aimed to estimate the prevalence of VLS at 12-months after the initial documentation of a pregnancy among WLWH in Rwanda and to explore factors associated with VLS among this population.
We conducted an open observational cohort study using routine clinical data sourced from HIV care clinics in Rwanda that participate in the Central Africa International epidemiology Databases to Evaluate AIDS (CA-IeDEA) (15). In Rwanda, CA-IeDEA collects routine care data from 12 HIV care clinics, including ten that provide PMTCT services. Of these, eight are urban clinics located in Kigali City and two are in rural areas in the Eastern and Northern Provinces of Rwanda. The data for this study were extracted from the PMTCT module of OpenMRS, an electronic medical record system used in all HIV care clinics in Rwanda. The Rwanda Ministry of Health introduced the PMTCT module in early 2018, and data were entered retrospectively for WLWH entering PMTCT care. Non-HIV care data including socio-demographic and obstetric information was manually extracted from antenatal, delivery, and postpartum care registries for women who tested HIV-positive.
We considered data from WLWH on ART who registered for PMTCT services (including antenatal care, delivery, or postpartum care) at IeDEA sites between April 2012, when Rwanda launched Option B+, a World Health Organization (WHO) guidelines for pregnant WLHIV, and June 2020. A period that extends both before and after the implementation of universal test and teat (UTT) policy in the country. To be included in the analysis, WLWH had to have a recorded viral load results between the initial documentation of pregnancy and 12-months after.
The main outcome of interest was VLS within 12 months of the antenatal care registration at the site (12-months VLS). For individuals with multiple recorded viral load results during the evaluation period, the most recent one was used to assess suppression. We used two cut-offs for defining VLS, including VL <1,000 copies/mL in accordance with UNAIDS standards (3) and VL <200 copies/mL in accordance with Rwanda's 2018 National Guidelines for the Prevention and Management of HIV and STIs which also recommends that pregnant WLHIV have a VL test 3 months after ART initiation, then every 6 months thereafter (16). Independent variables included: (1) socio-demographic information at the time of entry into PMTCT, including age (categorized as 15–24 years, 25–34 years, and ≥35 years), marital status (married vs. not married), body mass index (BMI) in kg/m2 (categorized as underweight: < 18.5, normal: 18.5–24.9, overweight: 25–29.9, and obese ≥30); (2) HIV clinical information, including HIV disclosure status, WHO stage (categorized as stages 1 or 2 vs. stages 3 or 4), timing of ART initiation (before vs. after pregnancy), and CD4 count (categorized as < 200, 200–500 and ≥500 cells/μl); and (3) Obstetric and antenatal information, including gravida and gestational week of pregnancy at PMTCT registration. We used the date of ART initiation and date of the last menstrual period (LMP) to determine the timing of ART start as before vs. after pregnancy, and we determined gestational age based on LMP date and the date of PMTCT registration. Before and after the implementation of (UTT) policy in the country was defined as pregnancy registration before date or after.
We summarized individual characteristics using means, standard deviations (SD) or medians and interquartile ranges (IQR) for continuous variables and count and percentages for categorical variables. Logistic regression models were used to examine associations between characteristics of WLWH at PMTCT registration and VLS by 12-months after registration. Variables found to be statistically associated (p < 0.05) with VLS in bivariate analyses were included in a multivariable logistic regression model to estimate adjusted Odds ratios (aOR) and 95% confidence intervals (95%CI). All analyses were conducted using IBM SPSS statistical software, version 21.
The CA-IeDEA study was reviewed and approved by both the Rwanda National Ethics Committee (RNEC) with reference number 355/RNEC/2018 and the Albert Einstein College of Medicine Institutional Review Board. A waiver for informed consent was granted for the analysis of de-identified, routinely collected data.
Data were available in OpenMRS for 1,002 WLWH enrolling in PMTCT from 2012 to 2020 at the 10 CA-IeDEA sites included in the study. Of those, 470 (47%) had no recorded VL results between their registration and 12 months later and were excluded from further analyses. Among those with available VL results, 277 (52.1%) had their pregnancy registration completed before the implementation of UTT, while 253 (47.6%) completed it after the UTT implementation and 2 (0.4%) had a missing registration date (Figure 1). The demographic characteristics of those with and without recorded VL result were similar.
Among 532 pregnant WLWH with available VL results, mean age at the time of PMTCT registration was 30.4 years (SD: 5.5), with 60% (n = 319) aged 25–34 years and 21% (n = 112) aged 24 years or younger (Table 1). The majority of WLWH (71%, n = 228) were married, and most (58%, n = 270) had a normal BMI (18.5–24.9 kg/m2). The majority of WLWH (84%, n = 436) were experiencing their first pregnancy, and most (62%, n = 296) had enrolled in PMTCT between the 14th and 28th weeks of pregnancy. Two-thirds (67%, n = 356) had initiated treatment prior to their current pregnancy. Of those with HIV disease staging recorded (n = 520), 90% (n = 468) had early-stage disease (WHO staging 1 or 2). Of those with a CD4 cell count recorded (n = 526), the median CD4 count was 542, IQR: (374–722) cells/μl and more than half (58%, n=303), had a CD4 count of 500 cells/μl or higher.
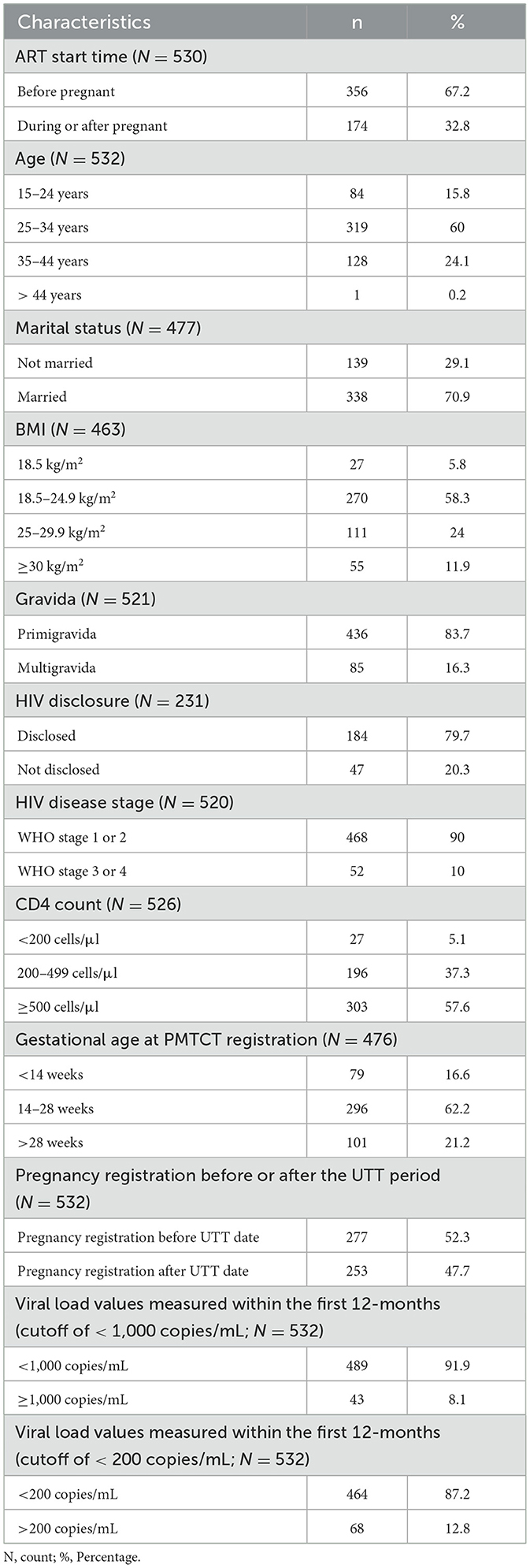
Table 1. Characteristics of WLWH enrolling in PMTCT at 10 health centers at Rwanda IeDEA sites between 2012 and 2020.
Within 12 months of entering PMTCT program, 92% (n = 489) of WLWH with a viral load had a VL <1,000 copies/mL, and 87% (n = 464) had VL <200 copies/mL (Table 1).
In bivariate analyses, using a suppression threshold of <1,000 copies/ml, we did not observe any statistically significant differences in 12-months VLS between those who initiated ART after pregnancy compared with those who initiated ART before (OR 1.04, 95% CI: 052, 1.97; p = 0.96).
Participants with WHO stage 3 or 4 were less likely to be suppressed by 12 months compared with those with WHO stage 1 or 2 (OR 0.37, 95% CI: 0.16, 0.83; p = 0.02; Table 2). Similarly, compared to those with a CD4 count ≥500 cells/μl, participants with a CD4 count <200 cells/μl and those with a CD4 count of 200–499 cells/μl also had lower odds of 12 months VL <1,000 copies/mL (OR 0.22, 95% CI: 0.07, 0.68; p = 0.01 and OR 0.41, 95% CI: 0.20, 0.81; p = 0.01, respectively).
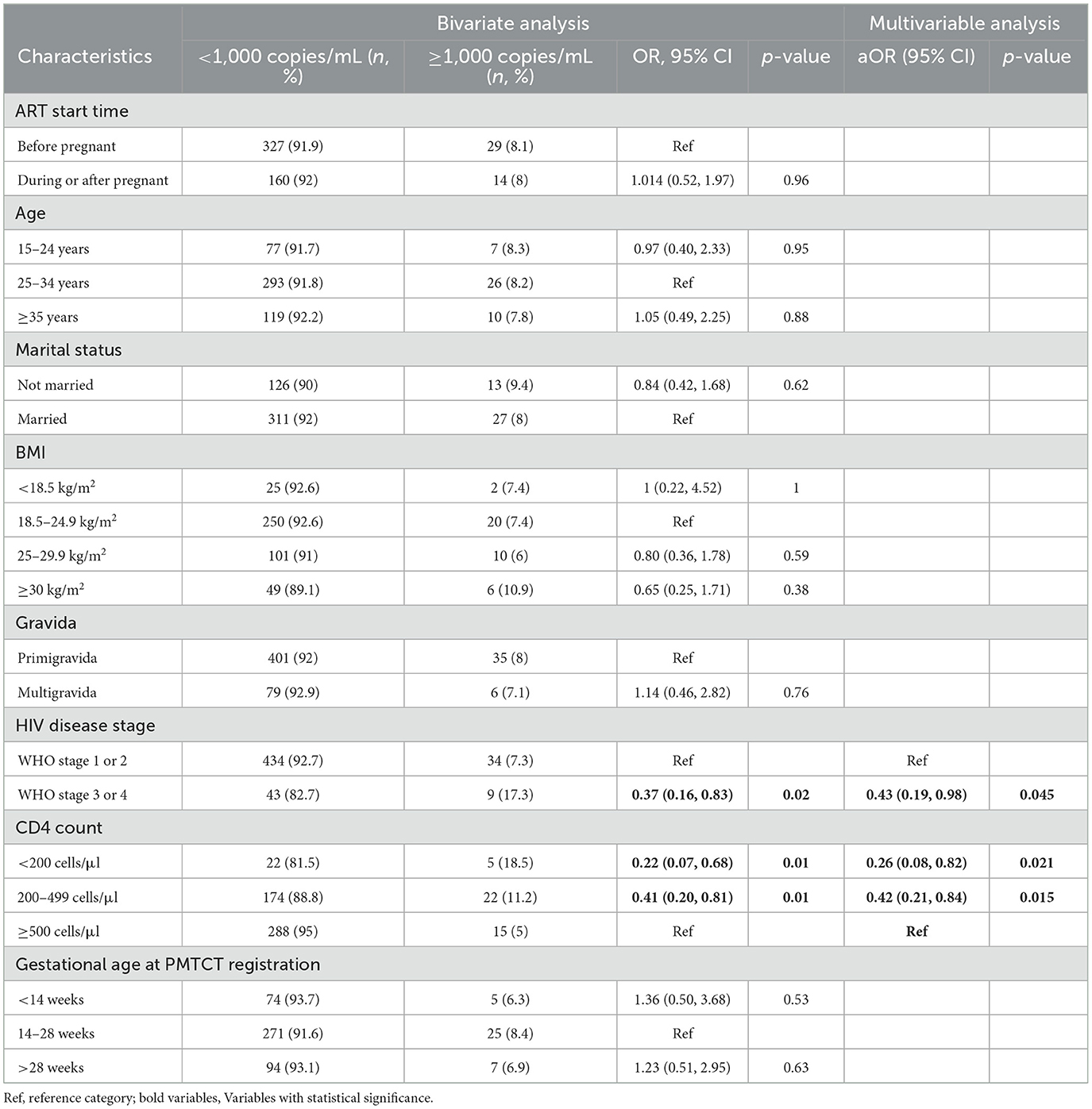
Table 2. Factors associated with VLS (cutoff of <1,000 copies/mL) among pregnant and postpartum WLWH at Rwanda IeDEA site.
Compared with those with WHO stage 1 and 2, those with stage 3 and 4 had lower odds of VLS within 12-months (aOR 0.43, 95% CI: 0.19, 0.98). Compared with those with a CD4 count ≥500 cells/μl, those with a CD4 count ≤ 200 cells/μl and a CD4 count of 200–499 cells/μl also had reduced odds of 12-months VL < 1,000 copies/mL (aOR: 0.26, 95% CI: 0.08, 0.82 and aOR 0.42, 95% CI: 0.21, 0.98, respectively). Results were not sensitive to the threshold used for VLS, as unadjusted and adjusted ORs were nearly identical when using the lower VLS threshold of ≤ 200 copies/mL (Table 3).
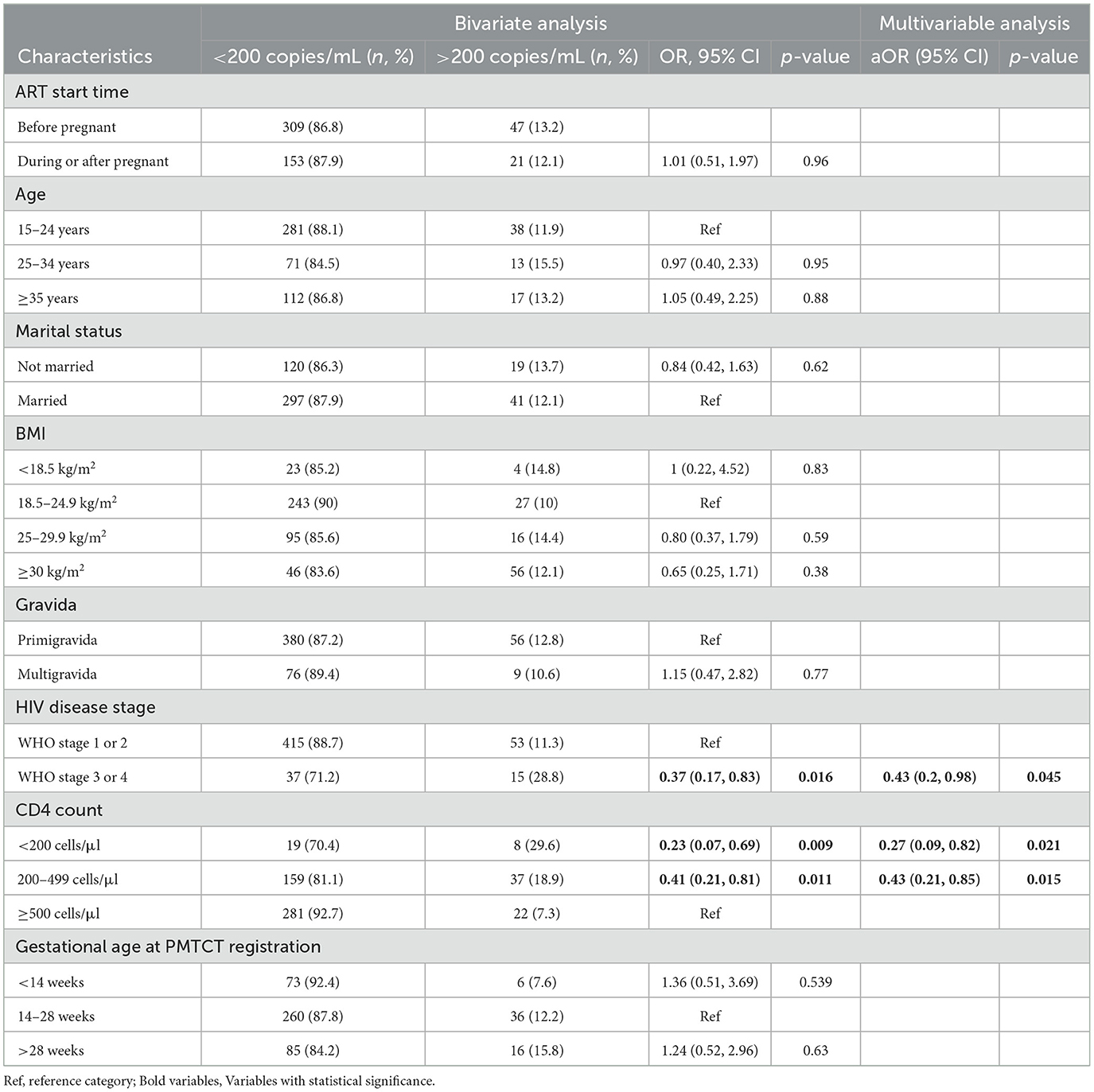
Table 3. Factors associated with VLS (cutoff of <200 copies/mL) among pregnant and postpartum WLWH at Rwanda IeDEA site.
Addressing the scarcity of data on VLS among pregnant and postpartum Rwandan WLWH, this study estimated the prevalence of VLS within 12-months of PMTCT entry at 10 HIV clinics in Rwanda participating in the CA-IeDEA, along with factors associated with VLS. We found that viral load is not routinely recorded in OpenMRS among this key group with just over half of women with at least one viral load result between PMTCT registration and 12-months after. Among those with at least one viral load results during the evaluation period, 92% had a VL <1,000 copies/mL, and 87% <200 copies/mL.
While data on VLS among pregnant and postpartum WLWH in Rwanda are limited, our findings align with a prior study in Rwanda, which found that 85% of pregnant and early postpartum WLWH had a VLS (<1,000 copies/mL) (14). Our findings are also consistent with data on VLS in the general population of Rwanda, including national data from the Rwanda Ministry of Health and other studies reporting virological suppression rates of 90% to 91% among all patients on ART (17, 18) and 93% among the general population of WLWH and 78% among WLWH of reproductive age (19). Although the VLS prevalence observed in our study is slightly below UNAIDS's target of 95% (2, 3), it is considerably higher than those from studies in SSA among pregnant and postpartum WLWH in general, that reported a VLS of 64% (6). Additionally, our findings exhibit slight variations when compared to a study conducted in the Democratic Republic of Congo (DRC), which reported a VLS prevalence of 67% (<1,000 copies/mL) among pregnant and postpartum WLWH on ART up to 12 months post-delivery (11) as well as a South African study indicating a VLS of 56% (< 50 copies/mL) (10).
These disparities in VLS rates could be attributed to: (1) differences in study designs (our study used a 12-months period prevalence while those other studies evaluated point prevalence), (2) differences in ART delivery performance (Rwanda is one of the five countries to have reached the UNAIDS targets of 95/95/95 in general population), routine monitoring, adherence support, and integration of HIV care with maternal health services, which may contribute to the high VLS rates observed, (3) to selection bias (only 53% of eligible WLWH had a recorded viral load measure). While our results indicate that VLS among this population is high, which is promising for efforts to reduce vertical transmission of HIV in Rwanda, understanding these distinctions is crucial for contextualizing and interpreting VLS outcomes.
Interestingly, we found no difference in 12-months VLS between WLWH who initiated treatment before vs. after pregnancy (p = 0.96). This aligns with findings from studies in Kenya and Zimbabwe where VLS did not differ between WLWH initiating ART before pregnancy and those initiating ART during pregnancy (20, 21). This contrasts with findings from SA (10, 22) and the DRC (11), highlighting the complex relationship between treatment duration and VLS. It emphasizes the critical role of additional support beyond the time to ART initiation in achieving VLS among pregnant and postpartum WLWH.
In our study, the only strong factor associated with VLS was advanced HIV disease at PMTCT registration, whether measured by WHO stage or CD4 count. While advanced disease is linked to lower VLS, it is likely a consequence of delayed HIV diagnosis and treatment rather than a direct predictor. This aligns with similar findings from SA (10), Ethiopia (23) and Tanzania (24), reinforcing important of early diagnosis of HIV particularly among women of reproductive age. Healthcare providers should pay particular attention to individuals with advanced HIV disease, and as recommended by WHO (25), tailored interventions and close monitoring should be implemented for optimal ART outcomes.
Several limitations of our study are worth noting. Our data came solely from primary health facilities most of which were located in Kigali, and none of the sites in our study were private clinics, district hospitals or referral-level facilities. While primary-level health centers are the primary providers of routine HIV care in Rwanda, our findings may not be generalizable to WLWH who are in care at other types of clinics. Additionally, most clinics in our study were located in Kigali City, and it is possible that the services and support provided to WLWH during pregnancy and the postpartum period including ART delivery, routine VL monitoring, community-based adherence support, and integrated PMTCT services in Kigali is not representative of care throughout the country. Additionally, while previous studies have identified associations between younger maternal age, higher gravidity, and non-suppression, our study did not observe these relationships. Several factors could explain these discrepancies, including differences in healthcare access, adherence support, or the characteristics of our sample. These limitations suggest the need for further research to explore these findings in greater depth and clarify the potential contributing factors. In additional, a key limitation of our study is the missing VL data for almost half of the participants, which may have affected the VLS analysis. Additionally, the lack of data on variables like adherence, maternal education, and socio-economic status further limits the findings. Lastly, our study data were collected before dolutegravir became the standard of care in Rwanda, so its impact on viral suppression was not assessed. Future research should include dolutegravir to determine its potential effects on VLS among pregnant and postpartum women living with HIV.
These limitations notwithstanding, our study also had several important strengths. Utilizing real-world service delivery data. Our study provides important insights into the prevalence of VLS among pregnant and postpartum WLWH in Rwanda, as well as factors associated with VLS in this population. We utilized data from 10 HIV clinics, over an 8-year period when Option B+ was in effect in Rwanda, encompassing both the pre and post implementation of the UTT. Lastly, the consistency of our results with other studies in various countries and populations, which focus on advanced clinical stages of HIV and lower CD4 counts as predictors of VLS among pregnant and postpartum WLWH, strengthens the reliability and generalizability of these findings.
This study provides valuable information on the prevalence of VLS among pregnant and postpartum WLWH at Rwanda IeDEA sites. It highlights a substantial proportion of pregnant and postpartum WLWH achieving VLS in their first year in the PMTCT care. It also highlights the sub-optimal viral load suppression among those presenting with advanced HIV disease, a group that should be prioritized for support if the UNAIDS targets of 95% VLS among pregnant and postpartum WLWH on ART is to be reached.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Rwanda National Ethics Committee and Albert Einstein College of Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
AM: Methodology, Writing – original draft, Writing – review & editing. H-YK: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. QS: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. EB: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. JR: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. BM: Data curation, Investigation, Writing – original draft, Writing – review & editing. FK: Methodology, Visualization, Writing – original draft, Writing – review & editing. GK: Conceptualization, Resources, Writing – original draft, Writing – review & editing. GM: Conceptualization, Writing – original draft, Writing – review & editing. DN: Conceptualization, Funding acquisition, Methodology, Resources, Writing – original draft, Writing – review & editing. KA: Funding acquisition, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. MY: Conceptualization, Formal analysis, Funding acquisition, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Central Africa IeDEA which is also supported by the National Institutes of Health's National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), the National Heart, Lung, and Blood Institute (NHLBI), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the Fogarty International Center (FIC), the National Library of Medicine (NLM), and the Office of the Director (OD) under Award Number U01AI096299 (Central Africa-IeDEA).
We express our sincere gratitude to the Rwanda IeDEA data entry staff for their invaluable contribution to the extraction of data from individual registries into OpenMRS. We also thank the dedicated staff of the health centers collaborating with Rwanda IeDEA's research, whose support was instrumental to the success of this study. Special thanks go to the CA-IeDEA Multiple Principal Investigators (MPIs) for selecting me as a mentee and for providing resources similar to those of the Fogarty-IeDEA Program (FIMP). My thanks to the CA-IeDEA management team of mentors. Finally, we express our sincere gratitude to the Rwanda Biomedical Center (RBC) for their support in providing and overseeing training on the electronic medical records system using the OpenMRS PMTCT module platform.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Patel M, Tedaldi E, Armon C, Nesheim S, Lampe M, Palella F, et al. HIV RNA suppression during and after pregnancy among women in the HIV outpatient study, 1996 to 2015. J Int Assoc Provid AIDS Care. (2018) 17:1–9. doi: 10.1177/2325957417752259
2. Joint United Nations Programme on HIV/AIDS (UNAIDS). Progress towards the Start Free, Stay Free, AIDS Free targets (2020). 2020 Report. p. 1–36. Available online at: http://www.unaids.org/sites/default/files/media_asset/JC2869_BeFreeBooklet_A4.pdf (accessed November 25, 2023).
3. World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach−2nd ed. WHO (2016). p. 316. Available online at: https://iris.who.int/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1 (accessed November 25, 2023).
4. UNAIDS. Global HIV & AIDS statistics—Fact sheet. UNAIDS (2023). Available online at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed December 19, 2023).
5. Abuogi LL, Humphrey JM, Mpody C, Yotebieng M, Murnane PM, Clouse K, et al. Achieving UNAIDS 90-90-90 targets for pregnant and postpartum women in sub-Saharan Africa: progress, gaps and research needs. J virus Erad. (2018) 4:33–9. doi: 10.1016/S2055-6640(20)30343-5
6. Schrubbe LA, Stöckl H, Hatcher AM, Marston M, Kuchukhidze S, Calvert C. Prevalence and risk factors of unsuppressed viral load among pregnant and breastfeeding women in sub-Saharan Africa: analysis from population-based surveys. AIDS. (2023) 37:659–69. doi: 10.1097/QAD.0000000000003459
7. Leplingard F, Borne S, Martinelli C, Leclère C, Lopez T, Guérin J, et al. FWM-assisted raman laser for second-order raman pumping. In: Optics InfoBase Conference Papers. Washington, DC: Optical Society of America (2003). p. 431–432. doi: 10.1109/OFC.2003.315935
8. Hatcher AM, Brittain K, Phillips TK, Zerbe A, Abrams EJ, Myer L. Longitudinal association between intimate partner violence and viral suppression during pregnancy and postpartum in South African women. AIDS. (2021) 35:791–9. doi: 10.1097/QAD.0000000000002796
9. UNAIDS. The Path that Ends AIDS: UNAIDS Global AIDS Update 2023. Joint United Nations Programme on HIV/AIDS: Geneva (2023). Available online at: https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/2023_report.pdf (accessed Feburary 27, 2024).
10. Woldesenbet SA, Kufa T, Barron P, Chirombo BC, Cheyip M, Ayalew K, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. Aids. (2020) 34:589–97. doi: 10.1097/QAD.0000000000002457
11. Yotebieng M, Mpody C, Ravelomanana NLR, Tabala M, Malongo F, Kawende B, et al. HIV viral suppression among pregnant and breastfeeding women in routine care in the Kinshasa province: a baseline evaluation of participants in CQI-PMTCT study. J Int AIDS Soc. (2019) 22:1–12. doi: 10.1002/jia2.25376
12. Koss CA, Natureeba P, Kwarisiima D, Ogena M, Clark TD, Olwoch P, et al. Viral suppression and retention in care up to 5 years after initiation of lifelong art during pregnancy (option b+) in rural Uganda. J Acquir Immune Defic Syndr. (2017) 74:279–84. doi: 10.1097/QAI.0000000000001228
13. Ngandu NK, Lombard CJ, Mbira TE, Puren A, Waitt C, Prendergast AJ, et al. HIV viral load non-suppression and associated factors among pregnant and postpartum women in rural northeastern South Africa: a cross-sectional survey. BMJ Open. (2022) 12:e058347. doi: 10.1136/bmjopen-2021-058347
14. Gill MM, Hoffman HJ, Bobrow EA, Mugwaneza P, Ndatimana D, Ndayisaba GF, et al. Detectable viral load in late pregnancy among women in the Rwanda option B+ PMTCT program: enrollment results from the Kabeho Study. PLoS ONE. (2016) 11:1–14. doi: 10.1371/journal.pone.0168671
15. CA-IeDEA. Central Africa International Epidemiology Databases to Evaluate AIDS (CA-IeDEA). International epidemiology Databases to Evaluate AIDS (2023). Available online at: https://www.iedea.org/ (accessed December 14, 2023).
16. Ministry Ministry of Health, Rwanda Rwanda Biomedical center: “National Guidelines for Prevention of HIV” Edition 2018: Circular of key changes in HIV prevention and management guidelines, effective 1st, July (2018). Available online at: http://www.rbc.gov.rw/fileadmin/user_upload/gui (accessed November 25, 2023).
17. UNAIDS. UNAIDS DATA 2023. UNAIDS (2023). p. 151. Available online at: https://www.unaids.org/sites/default/files/media_asset/data-book-2023_en.pdf (accessed December 20, 2023).
18. Ross J, Ribakare M, Remera E, Murenzi G, Munyaneza A, Hoover DR, et al. High levels of viral load monitoring and viral suppression under treat All in Rwanda—a cross-sectional study. J Int AIDS Soc. (2020) 23:1–6. doi: 10.1002/jia2.25543
19. Rwanda Biomedical Center (RBC). Rwanda Population-Based HIV Impact Assessment (RPHIA) 2018–2019: Final Report. Kigali: RBC (2020). Available online at: https://phia.icap.columbia.edu/wp-content/uploads/2020/11/RPHIA-Final-Report_Web.pdf (accessed November 26, 2023).
20. Humphrey JM, Songok J, Ofner S, Musick B, Alera M, Kipchumba B, et al. Retention in care and viral suppression in the PMTCT continuum at a large referral facility in western Kenya. AIDS Behav. (2022) 26:3494–505. doi: 10.1007/s10461-022-03666-w
21. Musanhu CCC, Takarinda KC, Shea J, Chitsike I, Eley B. Viral load testing among pregnant women living with HIV in Mutare district of Manicaland province, Zimbabwe. AIDS Res Ther. (2022) 19:52. doi: 10.1186/s12981-022-00480-1
22. Myer L, Phillips TK, McIntyre JA, Hsiao NY, Petro G, Zerbe A, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med. (2017) 18:80–8. doi: 10.1111/hiv.12397
23. Endalamaw Alamneh D, Shiferaw MB, Getachew Demissie M, Emiru MA, Zemene Kassie T, Endaylalu Lakew K, et al. Virological outcomes among pregnant women receiving antiretroviral treatment in the Amhara region, North West Ethiopia. HIV AIDS. (2023) 15:209–16. doi: 10.2147/HIV.S389506
24. Lyatuu GW, Mwashemele SZ, Urrio R, Naburi H, Kashmir N, Machumi L, et al. Long-term virological outcomes in women who started option B+ care during pregnancy for prevention of mother-to-child transmission of HIV in Dar es Salaam, Tanzania: a cohort study. lancet HIV. (2021) 8:e256–65. doi: 10.1016/S2352-3018(20)30308-8
25. WHO. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. World Health Organisation (2021). p. 1. Available online at: https://www.who.int/publications/i/item/9789240031593 (accessed March 1, 2025).
Keywords: pregnant and postpartum women, HIV, viral load suppression, IeDEA, Kigali, Rwanda
Citation: Munyaneza A, Kim H-Y, Shi Q, Brazier E, Ross J, Muhoza B, Kanyabwisha F, Kubwimana G, Murenzi G, Nash D, Anastos K and Yotebieng M (2025) Factors associated with viral load suppression in pregnant and postpartum women living with HIV in Rwanda: an open-observational cohort study. Front. Public Health 13:1544165. doi: 10.3389/fpubh.2025.1544165
Received: 12 December 2024; Accepted: 24 March 2025;
Published: 09 April 2025.
Edited by:
Marcelo A. Soares, National Cancer Institute (INCA), BrazilReviewed by:
Claire Thorne, University College London, United KingdomCopyright © 2025 Munyaneza, Kim, Shi, Brazier, Ross, Muhoza, Kanyabwisha, Kubwimana, Murenzi, Nash, Anastos and Yotebieng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Athanase Munyaneza, bXVueWFuZXphMjAwOEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.