- 1Department of Health Promotion and Health Education, College of Education, National Taiwan Normal University, Taipei, Taiwan
- 2Graduate Institute of Sport, Leisure and Hospitality Management, National Taiwan Normal University, Taipei, Taiwan
- 3Faculty of Sport Sciences, Waseda University, Tokorozawa, Japan
- 4Department of Convergence Medicine, Pusan National University School of Medicine, Yangsan, Republic of Korea
- 5Graduate Institute of Sport Pedagogy, University of Taipei, Taipei, Taiwan
- 6Department of Clinical Bio-Convergence, Graduate School of Convergence in Biomedical Science, Pusan National University School of Medicine, Yangsan, Republic of Korea
- 7Convergence Medical Institute of Technology, Pusan National University Hospital, Busan, Republic of Korea
Background: Depression affects the global burden of disability among older adults. Although dynapenia is related to disability and potential psychological health outcomes, its association with depressive symptoms remains uncertain. Accordingly, the objective of the current study was to investigate this association in older Taiwanese adults, applying the 2019 Asian Working Group for Sarcopenia (AWGS) classification of dynapenia.
Methods: Our research utilized a cross-sectional design implemented from September 2020 to December 2021, enrolling older adults aged over 65 years through National Taiwan University Hospital. The participants underwent standard assessments, including handgrip dynamometry for muscle strength, bioelectrical impedance analysis for muscle mass, and a 6-meter walk test for physical performance, to confirm the classification of dynapenia. The 15-item Geriatric Depression Scale (GDS-15) served as the tool to evaluate whether participants were at risk of potential depressive symptoms. The correlation between dynapenia and the risk of geriatric depressive symptoms was assessed through unadjusted and adjusted binary logistic regression analyses.
Results: In total, 197 older adults (mean age was 80.5 ± 7.0 years; 52.8% female; 17.3% at risk of depressive symptoms; 55.8% with dynapenia) were included. Regardless of the different models, dynapenia remained significantly and positively related to the risk of geriatric depressive symptoms (OR [odds ratio]: 2.67; 95% CI [confidence interval]: 1.01–7.05; p = 0.048) after adjusting for potential confounders.
Conclusion: Our findings highlighted a significant association between dynapenia, as classified by the 2019 AWGS criteria, and a higher risk of depressive symptoms in older Taiwanese adults. Public health professionals and practitioners should screen individuals with dynapenia for depressive symptoms to facilitate the early detection of depression. Future research should investigate the complex physiological and psychological mechanisms underlying this association.
1 Introduction
Depression is acknowledged as the most widespread mental health disorder worldwide (1). Globally, 280 million people were estimated to have depressive disorder in 2019, with the highest prevalence rates observed among older individuals aged 50–69 years (5.8%) and 70 years and above (5.4%) (2). As a major contributor to global years lived with disability (3), depressive disorders constitute a significant burden, impairing healthy aging by reducing the functional capacity among older adults (4). Depression in older adults is particularly concerning as it is associated with increased frailty, cognitive decline, suicidal ideation, and mortality (5–7). Similar to worldwide trends, an estimated 18.9 to 23.7% of older adults exhibit geriatric depressive symptoms in Taiwan (5, 8, 9). This high prevalence of depressive symptoms highlights a critical public health challenge in aging populations, emphasizing the need for early detection and targeted interventions to mitigate the adverse effects of late-life depression. Furthermore, previous longitudinal research revealed that older Taiwanese adults with worsening physical disability status had an association with depressive symptoms (10). This underscores the critical interplay between physical function decline and mental health deterioration in aging populations. Therefore, the factors related to age-associated decline in muscle strength or physical function that could affect geriatric depressive symptoms need to be understood better to promote healthy aging through the early detection and prevention of depression.
Dynapenia, characterized as low muscle strength due to aging (11, 12), is increasingly recognized as a critical public health issue (13–15). In Taiwan, the prevalence of dynapenia among older adults is estimated to range between 28.6 and 31.3% (16, 17), highlighting a substantial proportion of the aging population at risk for adverse health outcomes. Multiple studies have highlighted the physiological consequences of dynapenia in older populations (18), including increased risk of the locomotive syndrome (19, 20), reduced physical ability (21), and mortality (22, 23). However, existing studies on the psychological outcomes of dynapenia have primarily focused on cognitive function (17, 24, 25), and less attention has been given to its potential impact on other mental health outcomes such as depression (26, 27). Due to muscle weakness, dynapenia may exacerbate psychological distress by impairing mobility and increasing dependency on Instrumental Activities of Daily Living (21), which are well-established risk factors for depression (28, 29). However, despite evidence linking low muscle strength with an increased risk of depression (30, 31), the relationship between dynapenia, particularly as classified by the 2019 Asian Working Group for Sarcopenia (AWGS), and depressive symptoms remains unclear owing to the scarcity of research. Therefore, given the significant personal and societal burden of late-life depression, along with the high prevalence of dynapenia and geriatric depressive symptoms among older adults in Taiwan, a rigorous investigation of this association is crucial for informing early detection and intervention strategies to prevent further muscle decline and associated mental health risks.
We aimed to assess the association between dynapenia and the risk of depressive symptoms among older Taiwanese adults. Our hypothesis was that older adults with dynapenia had higher odds of depressive symptoms.
2 Materials and methods
2.1 Research design and recruitment
The current study employed a cross-sectional research design and was implemented from September 2020 to December 2021. A total of 299 participants aged 65 years and above with independent mobility were recruited through convenience sampling. The recruitment process followed two pathways. One approach involved the screening of potential participants who had completed their annual routine health examinations at National Taiwan University Hospital (NTUH) while the other relied on outpatient physicians from the Department of Geriatrics and Gerontology (DGG) at NTNU to identify eligible individuals. After the initial screening, eligible individuals were invited to participate, with eight refusing to consent. We ensured that the rights of all willing participants were protected and obtained a signed informed consent document from all of them before proceeding.
All participants completed a series of self-administered questionnaires during face-to-face interviews to evaluate their health status, health behaviors, and sociodemographic information, and the 15-item Geriatric Depression Scale (GDS-15) was employed to measure their risk of depressive symptoms. Subsequently, they underwent standardized procedures to evaluate muscle mass, strength, and physical performance to confirm whether their condition met the criteria of dynapenia based on the 2019 AWGS. Finally, to ensure accurate measurement of moderate-to-vigorous physical activity (MVPA), we required participants to wear a triaxial accelerometer for a continuous period of 7 days. After completing all procedures, participants were provided with a US$7 convenience store gift voucher in appreciation of their involvement. Approval and monitoring of this study were conducted by the Research Ethics Committee of the National Taiwan University Hospital (REC number: 202008046RINC).
In total, 291 individuals agreed to participate and were further screened based on the following exclusion criteria: (1) failure to complete the questionnaire or to be identified as at risk of cognitive dysfunction, as identified through Mini-Mental State Examination (MMSE) screening (n = 42); (2) inability to finish the standard procedures for assessing dynapenia or being classified as having potential sarcopenia (n = 22); and (3) uncooperative in wearing the triaxial accelerometer or the presence of invalid data (n = 30). The final analysis included 197 participants (Figure 1).
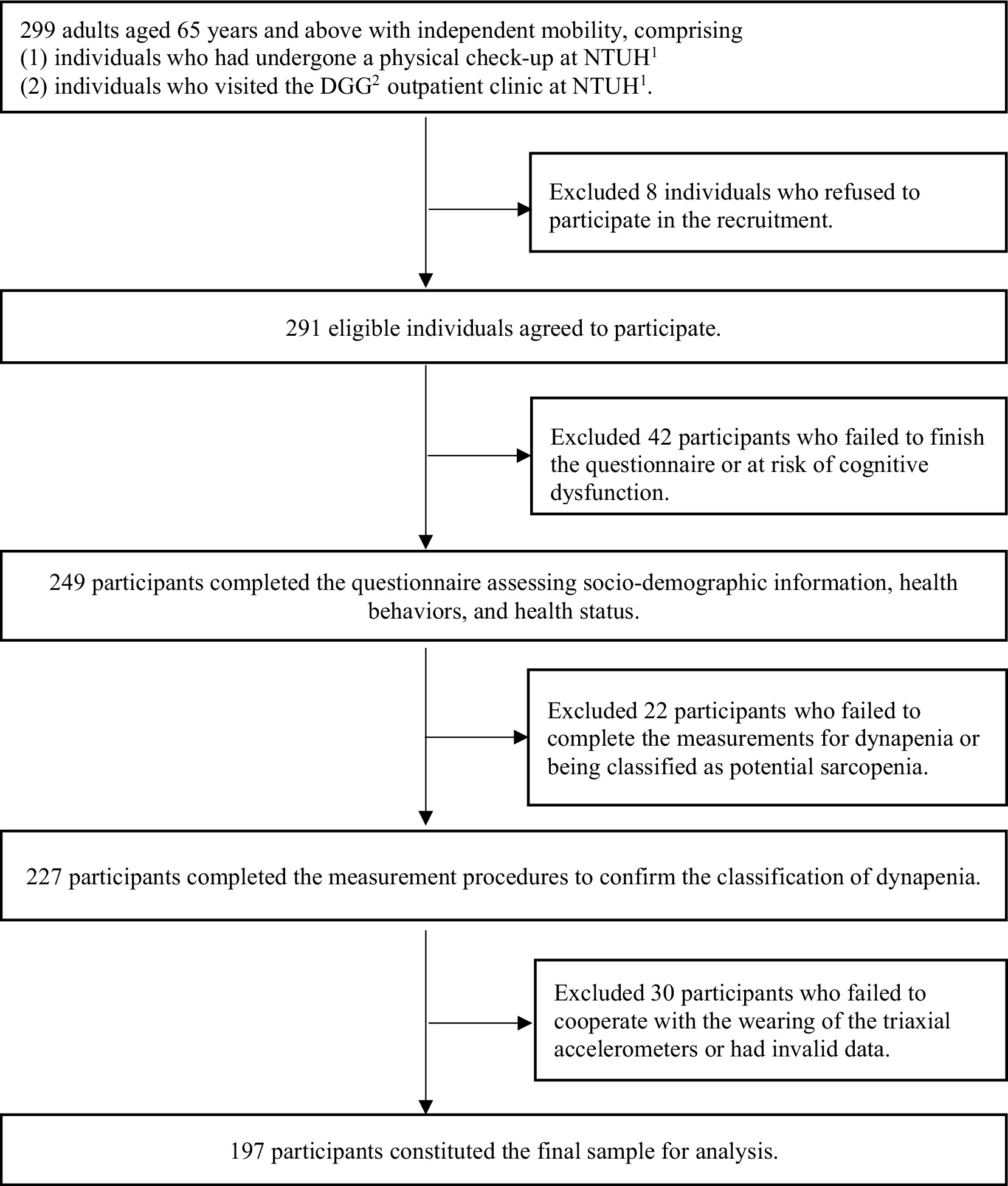
Figure 1. Process flow diagram for this study. NTUH1, National Taiwan University Hospital. DGG2, Department of Geriatrics and Gerontology.
2.2 Measures
2.2.1 Geriatric depressive symptoms
We utilized the GDS-15 scale to measure older adults’ risk of depressive symptoms (32, 33). The GDS-15 assessed the emotional state of participants, focusing on their experiences over the previous week. Responses of “yes” or “no” were recorded for each item. The range of GDS-15 scores was from 0 to 15, with a score of ≥5 suggesting participants at risk of depressive symptoms (34).
2.2.2 Dynapenia
Dynapenia was defined as low muscle strength and/or poor physical performance with normal muscle mass (35, 36). Based on the 2019 AWGS criteria, standardized procedures were conducted, and the established cutoffs were applied to classify dynapenia by measuring participants’ muscle strength, physical performance, and muscle mass. Muscle strength was quantified using handgrip measurements with a hydraulic handheld dynamometer (Jamar Plus + Digital Hand Dynamometer, Lafayette Instrument Company, United States). Participants were directed to perform with maximum strength using their dominant hand while standing, and the process was repeated twice. A one-minute interval between the two attempts was allowed, and the attempt with the highest kilogram value was recorded. The highest handgrip strength data were recorded for analysis.
The walking speed of participants, measured via the 6-meter walk test, served as an assessment tool for physical performance. Each participant performed two walking trials, and the shortest duration was recorded for analysis. Professionals, adhering to standardized procedures, walked alongside the participants to ensure accuracy and recorded the time in seconds.
Bioelectrical impedance analysis was utilized in this study to quantify muscle mass. Participants were required to stand barefoot on a bioelectrical impedance analyzer (DC-430MA, TANITA, Tokyo, Japan) and maintain their grip on the measurement device with both hands until the measurement process was completed to ensure accurate readings.
Participants were categorized as having dynapenia if their handgrip strength was measured at <28.0 kg for men or <18.0 kg for women (indicating low muscle strength) or if their gait speed of <1.0 m/s for both sexes (indicating poor physical performance), while normal muscle mass, defined as ≥7.0 kg/m2 for men and ≥5.7 kg/m2 for women. To distinguish between the classifications of dynapenia and sarcopenia, participants with low muscle mass, potentially classifiable as sarcopenia (13), were excluded from the analysis to avoid overlap between these two conditions. Participants were classified as not having dynapenia if they exhibited normal muscle strength, physical performance, and muscle mass.
2.2.3 Covariates
Sociodemographic data, such as age (categorized as 65 to 74 years or over 75 years), sex (female or male), and educational level (lower than university or university and above) was collected. Health behavior assessments focused on the patterns of substance use, specifically smoking and alcohol consumption. Alcohol consumption and smoking habits in the past year were categorized as “yes” or “no.” In health status assessments, chronic diseases were classified as fewer than 4 or 4 or more based on diagnoses by a physician. The calculation of body mass index (BMI) involved dividing body weight (kg) by height squared (m2). The Mini Nutritional Assessment Short-Form (MNA-SF) was employed to screen for nutritional risk among participants, with classifications of “at risk” for MNA-SF scores <12 (37–39). MVPA was objectively measured using a triaxial accelerometer (GT3X+ ActiGraph, Pensacola, Florida, United States), which participants wore for a week to precisely evaluate the average minutes per week of MVPA (40). In accordance with the WHO recommendation, the cutoff point for MVPA was set at a minimum of 150 min per week for older adults (41).
Previous studies have indicated that being a woman (42, 43), older age (44, 45), low educational attainment (46, 47), and BMI categories such as underweight (BMI < 18.5 kg/m2) (48), overweight (25 kg/m2 ≤ BMI < 30 kg/m2), and obesity (BMI ≥ 30 kg/m2) (49), as well as a high number of chronic diseases (50–52), tobacco use (current use of tobacco products), and alcohol consumption (alcohol consumption ≥1 time per week and ≥2 drinks per time) (53, 54) are associated with a higher risk of depressive symptoms in older populations. Furthermore, a relationship has been observed between the risk of nutrition (MNA-SF scores <12), evaluated using the MNA-SF, and depressive symptoms (55, 56). Moreover, older adults achieving ≥150 min of MVPA weekly demonstrated lower depressive symptoms (57). Based on these findings, the covariates included for analysis in this study were age, sex, educational level, BMI, smoking, alcohol drinking, chronic diseases status, nutritional status, and MVPA, all of which were adjusted in the logistic regression models.
2.3 Statistical analysis
Statistical analyses were conducted using IBM SPSS version 23.0 (SPSS Inc., Chicago, IL, United States). To summarize participant characteristics, descriptive statistics (frequency, percentage, and mean) were calculated, encompassing sociodemographic (age, sex, and educational level), health behaviors (smoking, alcohol drinking, and MVPA), and health status (BMI, chronic diseases, nutritional status, depressive symptoms, and dynapenia classified based on AWGS criteria). Chi-square (χ2) tests were utilized to analyze the correlations between categorical covariates and depressive symptoms. Analyses were performed using adjusted and unadjusted binary logistic regression models to examine the association between dynapenia and the risk of geriatric depressive symptoms. The independent variable was dynapenia (presence or absence), while the dependent variable was depressive symptoms (presence or absence). Three models were constructed to assess this association: Model 1 was an unadjusted model used to evaluate the crude association; Model 2 was adjusted for age and sex; and Model 3 was further adjusted, in addition to age and sex, for additional potential covariates, including educational level, BMI, smoking, alcohol consumption, chronic disease status, nutritional status, and MVPA. The results were described in the form of odds ratios (OR) and 95% confidence intervals (CI). A threshold of p < 0.05 was employed to define the statistical significance.
3 Results
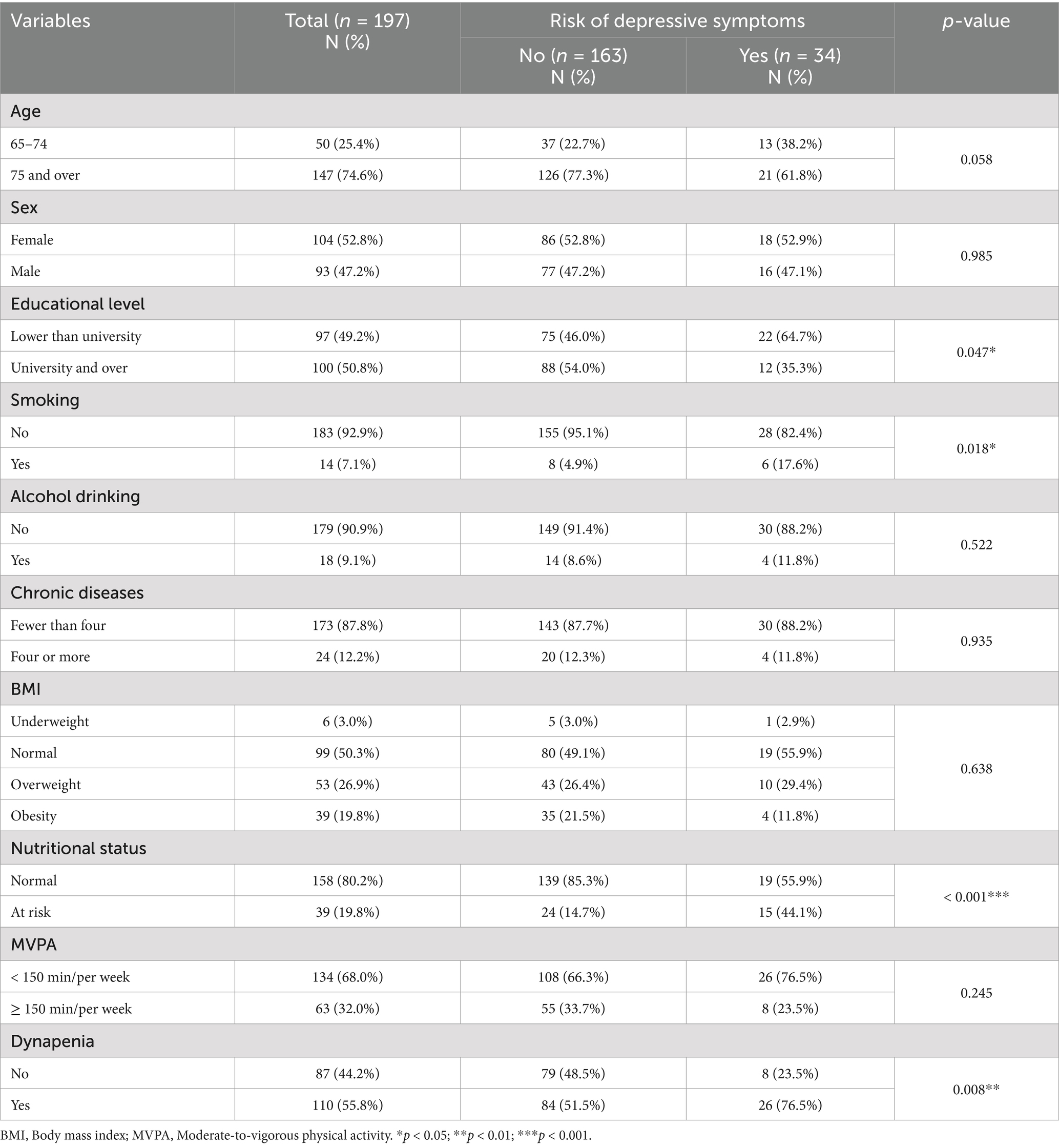
In total, 197 participants were analyzed, with Table 1 presenting their sociodemographic, health behavior, and health status variables. Participants included 52.8% women, with the mean age of 80.5 ± 7.0 years. Approximately half had university-level education or higher (50.8%), and a normal BMI (50.3%). The majority of participants did not use tobacco (92.9%) or alcohol (90.9%). 32.0% of participants achieved at least 150 min/week of MVPA. Most participants had normal nutritional status (80.2%), and fewer than four chronic diseases (87.8%). A total of 110 (55.8%) participants had dynapenia based on the 2019 AWGS definition, whereas 34 (17.3%) were identified as being at risk for depressive symptoms. Significant associations between educational level, smoking status, nutritional status, and dynapenia with the risk of geriatric depressive symptoms were identified in the chi-square test. The group at risk of depressive symptoms had a higher proportion of participants with dynapenia.
In Table 2, three different logistic regression models present the assocaitaion between dynapenia and the odds of depressive symptoms. Model 1 represented the unadjusted analysis. Model 2 accounted for age and sex, while model 3 included adjustments for age, sex, educational level, BMI, chronic disease status, smoking, alcohol consumption, nutritional status, and MVPA. Dynapenia demonstrated a significant association with the risk of geriatric depressive symptoms across model 1 (OR: 3.06; 95% CI: 1.31–7.15; p = 0.010), model 2 (OR: 3.12; 95% CI: 1.32–7.36; p = 0.009), and model 3 (OR: 2.67; 95% CI: 1.01–7.05; p = 0.048). Notably, nutritional risk (OR: 4.60; 95% CI: 1.77–11.95; p = 0.002) was also significantly associated with the risk of geriatric depressive symptoms in model 3.
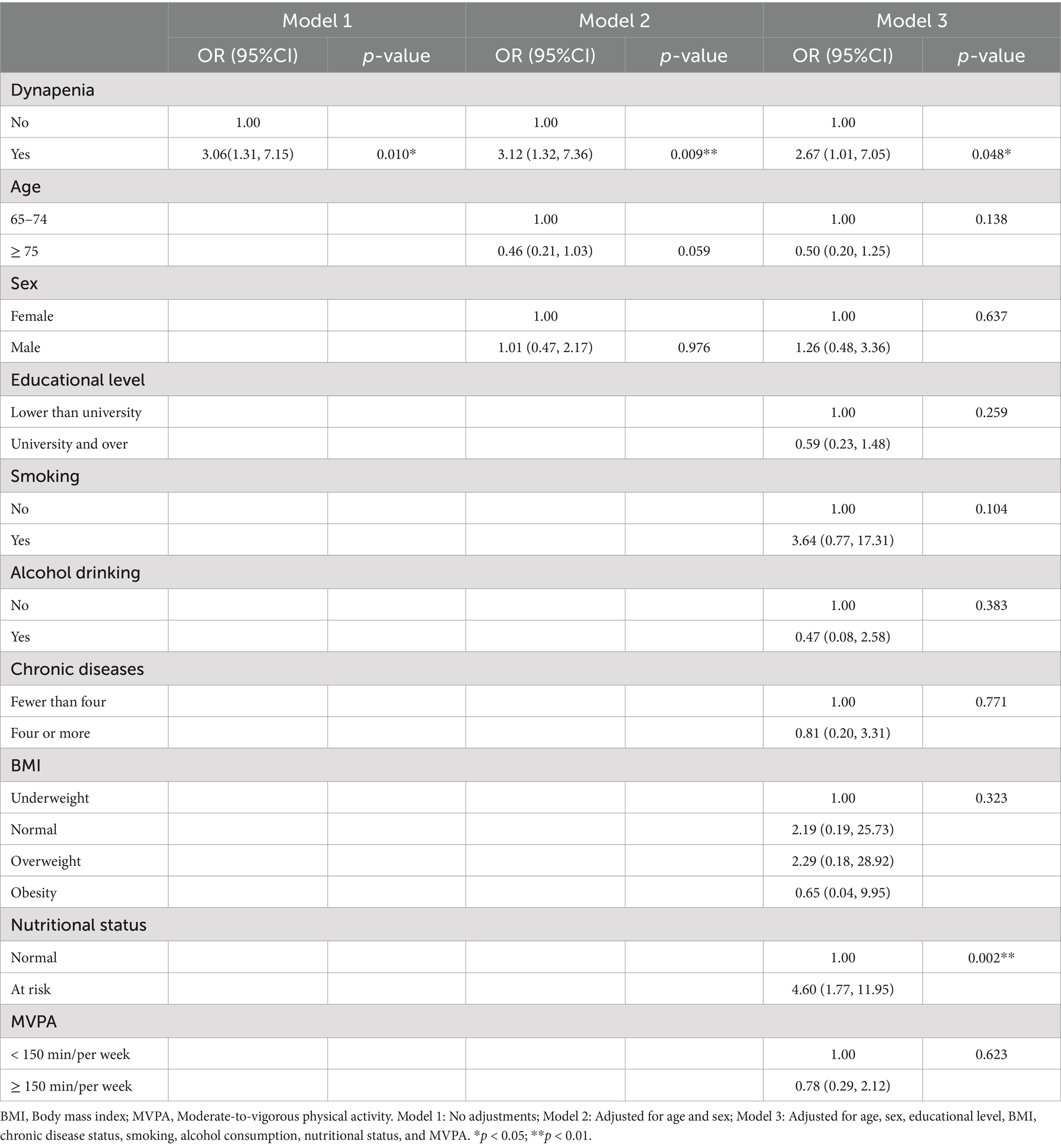
Table 2. Logistic regression analysis of the association of dynapenia with depressive symptoms (n = 197).
4 Discussion
Based on the available evidence, the current study was the first to explore dynapenia, classified strictly by the 2019 AWGS criteria, and its association with the risk of depressive symptoms among older adults in Taiwan. Our main finding revealed a significant positive association between dynapenia and higher risk of geriatric depressive symptoms, following adjustments for several potential confounders. These results could be valuable for public health professionals and practitioners, suggesting strategies for the early screening and prevention of depressive symptoms in the older population.
Consistent with prior studies, this research highlights a connection between dynapenia and depressed mood among older adults (26, 27) and further reports that, even when the more rigorous 2019 AWGS criteria were applied, dynapenia remained related to a higher risk of geriatric depressive symptoms in an Asian population. The Irish study demonstrated a significant association between dynapenia and higher odds of both incident and persistent depressive symptoms among community-dwelling older adults aged over 50 years (26). Similarly, the Korean study reported that dynapenia was associated with an increased risk of depressive symptoms among older adults, particularly in men (27). Our results are in line with these international research and emphasize the community-dwelling older adult population. As the aging population continues to expand, community-dwelling older adults represent a broader at-risk group, highlighting the public health importance of identifying and managing dynapenia as a modifiable risk factor for geriatric depressive symptoms.
There are several possible underlying explanations for the observed relationship between dynapenia and depressive symptoms. First, dynapenia, defined as age-related muscle weakness, substantially impairs older adults’ physical ability (11, 15) to be involved in fundamental and instrumental tasks of daily living (21, 58), leading to a decreased sense of independence and increased frustration, possibly contributing to the onset and exacerbation of depressive symptoms (28, 59). Second, dynapenia is associated with age-associated physiological changes, including a reduction in motor cortex neurotrophic factors and diminished neurotransmitter levels, such as dopamine and serotonin (15), both of which could lead to the worsening of depressive symptoms (60, 61). Third, beyond physical impairment, older adults with dynapenia are at an increased risk of social challenges. Limitations in mobility and daily activities restrict their ability to engage in social interactions, reducing social participation and interpersonal communication. This heightens loneliness and the experience of social isolation (62) and may ultimately increase depressive symptoms with age (63, 64). Since dynapenia may be related to geriatric depressive symptoms through physical, physiological, and social pathways, understanding the relationship between dynapenia and depression would be important. Future studies should explore the mechanisms linking dynapenia to depression.
Furthermore, in addition to dynapenia, we also observed a significant association between nutritional risk and geriatric depressive symptoms, which is consistent with previous studies (55, 56). Moreover, a randomized controlled trial demonstrated that a combined resistance training and diet intervention significantly improved depressive symptoms among older adults (65). These findings further emphasize the need for targeted physical activity and nutritional interventions to mitigate the risk of late-life depression in older populations.
This study was the first to investigate the relationship between dynapenia and the risk of depressive symptoms among the older population by strictly adhering to the 2019 AWGS standard criteria in Taiwan. Previous studies have demonstrated that weaker handgrip strength (66–68) and slower gait speed (69, 70) are associated with depressive symptoms. However, variability in the diagnostic standards employed across these studies might affect the comparability and interpretation of the findings. Hence, we used the definition of dynapenia, according to the AWGS 2019 guidelines, to align our methodologies with the global research standards. In addition to being specifically tailored to Asian populations, this approach ensured that our findings are robust, internationally comparable, and applicable to geriatric health.
This study has some limitations should be acknowledged. First, the cross-sectional research design restricted the ability to establish causality. It remains unclear whether dynapenia contributes to the development of depressive symptoms or if depressive symptoms lead to reduced physical activity, resulting in muscle weakness. Therefore, longitudinal studies are needed to clarify the directionality of this relationship and investigate the underlying mechanisms. Second, participation in routine health checkups was voluntary, which may have resulted in a selection bias toward individuals who were more attentive to their health. Additionally, the recruitment of participants from a single medical center limits the generalizability of the findings to broader aging populations. Future research should aim to incorporate more diverse sample from various community settings and healthcare institutions, to enhance the external validity of the findings. Third, high scores on the GDS-15 do not necessarily imply a diagnosis of clinical depression; therefore, interpretation of the results should consider diagnostic limitations. Future research should incorporate clinical diagnostic assessments to enhance validity. Finally, some potential confounders, such as chronic inflammation, stress (71–73), and sleep disturbances (74–76), commonly associated with aging, may contribute to the relationship between dynapenia and depression. Future studies should account for these confounders to ensure more accurate interpretation.
Despite these limitations, the findings of this study have important public health implications for addressing the intersection of muscle strength decline and mental health in aging populations. Given the increasing prevalence of both dynapenia and geriatric depressive symptoms, early detection and intervention strategies are essential to mitigate the burden of late-life depression. Public health initiatives should integrate muscle function screening into routine geriatric health assessments, particularly in community settings, to identify individuals with dynapenia before they develop severe physical or psychological consequences. Community-based interventions promoting physical activity and nutrition status should be emphasized to prevent the risk of depressive symptoms in older adults.
5 Conclusion
Dynapenia demonstrated a significant relationship with a higher risk of depressive symptoms in older Taiwanese adults. Public health professionals and practitioners should screen older individuals with reduced muscle strength or poor physical performance for depressive symptoms to enable early detection of their depression. Further research should investigate the physiological and psychological pathways through which dynapenia influences geriatric depressive symptoms.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the National Taiwan University Hospital (REC number: 202008046RINC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
C-CC: Conceptualization, Formal analysis, Writing – original draft. YL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. JC: Formal analysis, Writing – review & editing. T-FL: Formal analysis, Writing – review & editing. M-CH: Funding acquisition, Writing – review & editing. J-HP: Funding acquisition, Supervision, Writing – review & editing. Y-JC: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this study included a personal grant awarded to Yung Liao by the Ministry of Science and Technology of Taiwan (MOST 111-2628-H-003-006-MY3). This work was financially supported by the National Taiwan Normal University (NTNU) within the framework of the Higher Education Sprout Project of the Ministry of Education (MOE) in Taiwan. This work was also supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government(MSIT) (No.2022-0-00501, Development of Wearable Device Assessment technology). This research was also funded by National Science and Technology Council, TAIWAN (MOST 109-2410-H-845-037-MY2). This work was also supported by the University of Taipei within the framework of the Competition Program (No. UT-11304) by Research and Development Office of University of Taipei.
Acknowledgments
The authors appreciate all the participants of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Depression and other common mental disorders: Global Health estimates. Geneva: World Health Organization (2017).
2. World Health Organization. World mental health report: Transforming mental health for all. Geneva: World Health Organization (2022).
3. Ferrari, AJ, Santomauro, DF, Aali, A, Abate, YH, Abbafati, C, Abbastabar, H, et al. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
4. World Health Organization. World report on ageing and health. Geneva: World Health Organization (2015).
5. Tsai, SJ, Hsiao, YH, Liao, MY, and Lee, MC. The influence of depressive mood on mortality in elderly with different health status: evidence from the Taiwan longitudinal study on aging (TLSA). Int J Environ Res Public Health. (2022) 19:6922. doi: 10.3390/ijerph19116922
6. Muhammad, T, and Meher, T. Association of late-life depression with cognitive impairment: evidence from a cross-sectional study among older adults in India. BMC Geriatr. (2021) 21:364. doi: 10.1186/s12877-021-02314-7
7. Bickford, D, Morin, RT, Woodworth, C, Verduzco, E, Khan, M, Burns, E, et al. The relationship of frailty and disability with suicidal ideation in late life depression. Aging Ment Health. (2021) 25:439–44. doi: 10.1080/13607863.2019.1698514
8. Chang, K-F, and Weng, L-J. Screening for depressive symptoms among older adults in Taiwan: cutoff of a short form of the Center for Epidemiologic Studies Depression Scale. Health. (2013) 5:588–94. doi: 10.4236/health.2013.53A078
9. Teng, P-R, Yeh, C-J, Lee, M-C, Lin, H-S, and Lai, T-J. Depressive symptoms as an independent risk factor for mortality in elderly persons: results of a national longitudinal study. Aging Ment Health. (2013) 17:470–8. doi: 10.1080/13607863.2012.747081
10. Huang, J-F, Wong, R-H, Chen, C-C, Mao, IF, Huang, C-C, Chang, W-H, et al. Trajectory of depression symptoms and related factors in later life — A population based study. J Affect Disord. (2011) 133:499–508. doi: 10.1016/j.jad.2011.04.048
11. Clark, BC, and Manini, TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. (2008) 63:829–34. doi: 10.1093/gerona/63.8.829
12. Clark, BC, and Manini, TM. What is dynapenia? Nutrition. (2012) 28:495–503. doi: 10.1016/j.nut.2011.12.002
13. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
14. Ko, CH, Chuang, HY, Wu, SJ, Yu, SC, Chang, YF, Chang, CS, et al. Changes of sarcopenia case finding by different Asian working Group for Sarcopenia in community indwelling middle-aged and old people. Front Med (Lausanne). (2022) 9:1041186. doi: 10.3389/fmed.2022.1041186
15. Manini, TM, and Clark, BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. (2012) 67:28–40. doi: 10.1093/gerona/glr010
16. Kao, TW, Peng, TC, Chen, WL, Han, DS, Chen, CL, and Yang, WS. Impact of adiposity on muscle function and clinical events among elders with dynapenia, presarcopenia and sarcopenia: a community-based cross-sectional study. Aging (Albany NY). (2021) 13:7247–58. doi: 10.18632/aging.202581
17. Huang, C-Y, Hwang, A-C, Liu, L-K, Lee, W-J, Chen, L-Y, Peng, L-N, et al. Association of Dynapenia, sarcopenia, and cognitive impairment among community-dwelling older Taiwanese. Rejuvenation Res. (2016) 19:71–8. doi: 10.1089/rej.2015.1710
18. Clark, BC, and Manini, TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. (2010) 13:271–6. doi: 10.1097/MCO.0b013e328337819e
19. Jung, H, Tanaka, S, Kataoka, S, and Tanaka, R. Association of sarcopenia, pre-sarcopenia, and dynapenia with the onset and progression of locomotive syndrome in Japanese older adults: a cross-sectional study. J Physiol Anthropol. (2023) 42:16. doi: 10.1186/s40101-023-00334-3
20. Momoki, C, Habu, D, Ogura, J, Tada, A, Hasei, A, Sakurai, K, et al. Relationships between sarcopenia and household status and locomotive syndrome in a community-dwelling elderly women in Japan. Geriatr Gerontol Int. (2017) 17:54–60. doi: 10.1111/ggi.12674
21. Iwamura, M, and Kanauchi, M. A cross-sectional study of the association between dynapenia and higher-level functional capacity in daily living in community-dwelling older adults in Japan. BMC Geriatr. (2017) 17:1. doi: 10.1186/s12877-016-0400-5
22. Alexandre Tda, S, Duarte, YA, Santos, JL, Wong, R, and Lebrão, ML. Sarcopenia according to the European working group on sarcopenia in older people (EWGSOP) versus dynapenia as a risk factor for mortality in the elderly. J Nutr Health Aging. (2014) 18:751–6. doi: 10.1007/s12603-014-0540-2
23. Lee, WJ, Peng, LN, Liang, CK, Loh, CH, and Chen, LK. Cognitive frailty predicting all-cause mortality among community-living older adults in Taiwan: A 4-year nationwide population-based cohort study. PLoS One. (2018) 13:e0200447. doi: 10.1371/journal.pone.0200447
24. Chou, YY, Lin, CF, Lee, YS, Weng, SC, Kuo, FH, Hsu, CY, et al. The associations of osteoporosis and possible sarcopenia with disability, nutrition, and cognition in community-dwelling older adults. BMC Geriatr. (2023) 23:730. doi: 10.1186/s12877-023-04431-x
25. Hatanaka, S, Sasai, H, Shida, T, Osuka, Y, Kojima, N, Ohta, T, et al. Association between dynapenia and cognitive decline in community-dwelling older Japanese adults: the IRIDE cohort study. Geriatr Gerontol Int. (2024) 24:123–9. doi: 10.1111/ggi.14749
26. Carvalho, AF, Maes, M, Solmi, M, Brunoni, AR, Lange, S, Husain, MI, et al. Is dynapenia associated with the onset and persistence of depressive and anxiety symptoms among older adults? Findings from the Irish longitudinal study on ageing. Aging Ment Health. (2021) 25:468–75. doi: 10.1080/13607863.2019.1699021
27. Noh, HM, and Park, YS. Handgrip strength, dynapenia, and mental health in older Koreans. Sci Rep. (2020) 10:4004. doi: 10.1038/s41598-020-60835-4
28. Chen, CM, Mullan, J, Su, YY, Griffiths, D, Kreis, IA, and Chiu, HC. The longitudinal relationship between depressive symptoms and disability for older adults: a population-based study. J Gerontol A Biol Sci Med Sci. (2012) 67:1059–67. doi: 10.1093/gerona/gls074
29. Maier, A, Riedel-Heller, SG, Pabst, A, and Luppa, M. Risk factors and protective factors of depression in older people 65+. A systematic review. PLoS One. (2021) 16:e0251326. doi: 10.1371/journal.pone.0251326
30. Lian, Y, Wang, GP, Chen, GQ, and Jia, CX. Bidirectional associations between handgrip strength and depressive symptoms: A longitudinal cohort study. J Am Med Dir Assoc. (2021) 22:1744–50.e1. doi: 10.1016/j.jamda.2021.04.006
31. Nazari, T, Moodi, M, Fakhrzadeh, H, Khodabakhshi, H, Khorashadizadeh, M, Arzaghi, SM, et al. The association of depressive symptoms with handgrip strength and gait speed in community-dwelling older adults: data from the baseline phase of Birjand longitudinal aging study. BMC Geriatr. (2024) 24:393. doi: 10.1186/s12877-024-04944-z
32. Shin, C, Park, MH, Lee, SH, Ko, YH, Kim, YK, Han, KM, et al. Usefulness of the 15-item geriatric depression scale (GDS-15) for classifying minor and major depressive disorders among community-dwelling elders. J Affect Disord. (2019) 259:370–5. doi: 10.1016/j.jad.2019.08.053
33. Smarr, KL, and Keefer, AL. Measures of depression and depressive symptoms: Beck depression inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), geriatric depression scale (GDS), hospital anxiety and depression scale (HADS), and patient health Questionnaire-9 (PHQ-9). Arthritis Care Res. (2011) 63:S454–66. doi: 10.1002/acr.20556
34. Nyunt, MS, Fones, C, Niti, M, and Ng, TP. Criterion-based validity and reliability of the geriatric depression screening scale (GDS-15) in a large validation sample of community-living Asian older adults. Aging Ment Health. (2009) 13:376–82. doi: 10.1080/13607860902861027
35. Kobayashi, K, Imagama, S, Ando, K, Nakashima, H, Machino, M, Morozumi, M, et al. Dynapenia and physical performance in community-dwelling elderly people in Japan. Nagoya J Med Sci. (2020) 82:415–24. doi: 10.18999/nagjms.82.3.415
36. Yamada, M, Kimura, Y, Ishiyama, D, Nishio, N, Abe, Y, Kakehi, T, et al. Differential characteristics of skeletal muscle in community-dwelling older adults. J Am Med Dir Assoc. (2017) 18:807.e9–807.e16. doi: 10.1016/j.jamda.2017.05.011
37. Vellas, B, Guigoz, Y, Garry, PJ, Nourhashemi, F, Bennahum, D, Lauque, S, et al. The Mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. (1999) 15:116–22. doi: 10.1016/S0899-9007(98)00171-3
38. Rubenstein, LZ, Harker, JO, Salvà, A, Guigoz, Y, and Vellas, B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci. (2001) 56:M366–72. doi: 10.1093/gerona/56.6.M366
39. Tsai, AC, Yang, SF, and Wang, JY. Validation of population-specific Mini-nutritional assessment with its long-term mortality-predicting ability: results of a population-based longitudinal 4-year study in Taiwan. Br J Nutr. (2010) 104:93–9. doi: 10.1017/S0007114510000188
40. Troiano, RP, Berrigan, D, Dodd, KW, Mâsse, LC, Tilert, T, and McDowell, M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. (2008) 40:181–8. doi: 10.1249/mss.0b013e31815a51b3
41. World Health Organization. WHO guidelines on physical activity and sedentary behaviour: At a glance. Geneva: World Health Organization (2020).
42. Kiely, KM, Brady, B, and Byles, J. Gender, mental health and ageing. Maturitas. (2019) 129:76–84. doi: 10.1016/j.maturitas.2019.09.004
43. Conde-Sala, JL, Garre-Olmo, J, Calvó-Perxas, L, Turró-Garriga, O, and Vilalta-Franch, J. Course of depressive symptoms and associated factors in people aged 65+ in Europe: A two-year follow-up. J Affect Disord. (2019) 245:440–50. doi: 10.1016/j.jad.2018.10.358
44. Weyerer, S, Eifflaender-Gorfer, S, Wiese, B, Luppa, M, Pentzek, M, Bickel, H, et al. Incidence and predictors of depression in non-demented primary care attenders aged 75 years and older: results from a 3-year follow-up study. Age Ageing. (2013) 42:173–80. doi: 10.1093/ageing/afs184
45. Luppa, M, Pabst, A, Löbner, M, Mallon, T, Brettschneider, C, Hajek, A, et al. Age-specific risk factors of depression among the oldest-old - evidence from the multicenter AgeCoDe-AgeQualiDe study. Front Psychol. (2024) 15:1367225. doi: 10.3389/fpsyt.2024.1367225
46. Zhao, R, Wang, J, Lou, J, Liu, M, Deng, J, Huang, D, et al. The effect of education level on depressive symptoms in Chinese older adults-parallel mediating effects of economic security level and subjective memory ability. BMC Geriatr. (2024) 24:635. doi: 10.1186/s12877-024-05233-5
47. Chlapecka, A, Kagstrom, A, and Cermakova, P. Educational attainment inequalities in depressive symptoms in more than 100,000 individuals in Europe. Eur Psychiatry. (2020) 63:e97. doi: 10.1192/j.eurpsy.2020.100
48. Qiao, Z, Wang, Z, Qiu, J, Zhang, J, and Cao, W. Analysis of the effect of BMI on depression and anxiety among older adults in China: the mediating role of ADL and IADL. Front Public Health. (2024) 12:1387550. doi: 10.3389/fpubh.2024.1387550
49. Xiang, X, and An, R. Obesity and onset of depression among U.S. middle-aged and older adults. J Psychosom Res. (2015) 78:242–8. doi: 10.1016/j.jpsychores.2014.12.008
50. Pengpid, S, Peltzer, K, Hajek, A, Anantanasuwong, D, and Kaewchankha, W. Determinants of depressive symptoms among persons 80 years and older: longitudinal national evidence from the health, aging, and retirement study in Thailand, 2015-2022. BMC Geriatr. (2024) 24:880. doi: 10.1186/s12877-024-05479-z
51. Chao, G, Zhang, L, Zhan, Z, and Bao, Y. Effect of multimorbidity on depressive status in older Chinese adults: evidence from the China health and retirement longitudinal study (CHARLS). BMJ Open. (2024) 14:e081776. doi: 10.1136/bmjopen-2023-081776
52. Shon, C, and Kim, J. The factors related to depressive symptoms in urban older adults in South Korea: a study based on the Seoul aging survey. BMC Geriatr. (2024) 24:644. doi: 10.1186/s12877-024-05241-5
53. Quittschalle, J, Pabst, A, Löbner, M, Luppa, M, Heser, K, Wagner, M, et al. Association of alcohol and tobacco consumption with depression severity in the oldest old. Results from the age different old age cohort platform. Int J Environ Res Public Health. (2021) 18:7959. doi: 10.3390/ijerph18157959
54. Tsai, AC, Chi, SH, and Wang, JY. Cross-sectional and longitudinal associations of lifestyle factors with depressive symptoms in ≥ 53-year old Taiwanese - results of an 8-year cohort study. Prev Med. (2013) 57:92–7. doi: 10.1016/j.ypmed.2013.04.021
55. Tasnim, T, Sadiq, MZA, and Karim, KMR. Depression level, nutritional status, and dietary nutrient intake of the older adult at the community level in a selected area of Bangladesh. Heliyon. (2023) 9:e18199. doi: 10.1016/j.heliyon.2023.e18199
56. Chen, CT, Tung, HH, Chen, YC, Lee, HF, Wang, CJ, and Lin, WH. Depressive symptoms and nutritional status in the frail older adults. Arch Gerontol Geriatr. (2019) 83:96–100. doi: 10.1016/j.archger.2019.03.023
57. Jefferis, BJ, Sartini, C, Lee, IM, Choi, M, Amuzu, A, Gutierrez, C, et al. Adherence to physical activity guidelines in older adults, using objectively measured physical activity in a population-based study. BMC Public Health. (2014) 14:382. doi: 10.1186/1471-2458-14-382
58. Yang, M, Ding, X, Luo, L, Hao, Q, and Dong, B. Disability associated with obesity, dynapenia and dynapenic-obesity in Chinese older adults. J Am Med Dir Assoc. (2014) 15:150.e11–6. doi: 10.1016/j.jamda.2013.10.009
59. Zhu, X, Wang, Y, Luo, Y, Ding, R, Shi, Z, and He, P. Bidirectional, longitudinal associations between depressive symptoms and IADL/ADL disability in older adults in China: a national cohort study. BMC Geriatr. (2024) 24:659. doi: 10.1186/s12877-024-05248-y
60. Artin, H, Zisook, S, and Ramanathan, D. How do serotonergic psychedelics treat depression: the potential role of neuroplasticity. World J Psychiatry. (2021) 11:201–14. doi: 10.5498/wjp.v11.i6.201
61. Kadriu, B, Greenwald, M, Henter, ID, Gilbert, JR, Kraus, C, Park, LT, et al. Ketamine and serotonergic psychedelics: common mechanisms underlying the effects of rapid-acting antidepressants. Int J Neuropsychopharmacol. (2021) 24:8–21. doi: 10.1093/ijnp/pyaa087
62. Tomida, K, Shimoda, T, Nakajima, C, Kawakami, A, and Shimada, H. Social isolation/loneliness and mobility disability among older adults. Curr Geriatr Rep. (2024) 13:86–92. doi: 10.1007/s13670-024-00414-x
63. Noguchi, T, Saito, M, Aida, J, Cable, N, Tsuji, T, Koyama, S, et al. Association between social isolation and depression onset among older adults: a cross-national longitudinal study in England and Japan. BMJ Open. (2021) 11:e045834. doi: 10.1136/bmjopen-2020-045834
64. Li, K, Tang, F, Albert, SM, Rauktis, ME, and Ohmer, ML. Social isolation, loneliness, and depressive symptoms among older adults: the moderating effect of resilience. The Gerontologist. (2024) 64:gnae056. doi: 10.1093/geront/gnae056
65. Carcelén-Fraile, MDC, Déniz-Ramírez, NDP, Sabina-Campos, J, Aibar-Almazán, A, Rivas-Campo, Y, González-Martín, AM, et al. Exercise and nutrition in the mental health of the older adult population: A randomized controlled clinical trial. Nutrients. (2024) 16:1741. doi: 10.3390/nu16111741
66. Ashdown-Franks, G, Stubbs, B, Koyanagi, A, Schuch, F, Firth, J, Veronese, N, et al. Handgrip strength and depression among 34,129 adults aged 50 years and older in six low- and middle-income countries. J Affect Disord. (2019) 243:448–54. doi: 10.1016/j.jad.2018.09.036
67. Fukumori, N, Yamamoto, Y, Takegami, M, Yamazaki, S, Onishi, Y, Sekiguchi, M, et al. Association between hand-grip strength and depressive symptoms: locomotive syndrome and health outcomes in Aizu cohort study (LOHAS). Age Ageing. (2015) 44:592–8. doi: 10.1093/ageing/afv013
68. van Milligen, BA, Vogelzangs, N, Smit, JH, and Penninx, BW. Physical function as predictor for the persistence of depressive and anxiety disorders. J Affect Disord. (2012) 136:828–32. doi: 10.1016/j.jad.2011.09.030
69. Demakakos, P, Cooper, R, Hamer, M, de Oliveira, C, Hardy, R, and Breeze, E. The bidirectional association between depressive symptoms and gait speed: evidence from the English longitudinal study of ageing (ELSA). PLoS One. (2013) 8:e68632. doi: 10.1371/journal.pone.0068632
70. Lavie, I, Schnaider Beeri, M, Schwartz, Y, Soleimani, L, Heymann, A, Azuri, J, et al. Decrease in gait speed over time is associated with increase in number of depression symptoms in older adults with type 2 diabetes. J Gerontol: Series A. (2023) 78:1504–12. doi: 10.1093/gerona/glad008
71. Hassamal, S. Chronic stress, neuroinflammation, and depression: an overview of pathophysiological mechanisms and emerging anti-inflammatories. Front Psychol. (2023) 14:1130989. doi: 10.3389/fpsyt.2023.1130989
72. Nascimento, CMC, Cardoso, JFZ, de Jesus, ITM, de Souza, OF, Costa-Guarisco, LP, Gomes, GAO, et al. Are body fat and inflammatory markers independently associated with age-related muscle changes? Clin Nutr. (2021) 40:2009–15. doi: 10.1016/j.clnu.2020.09.021
73. Smith, KJ, Au, B, Ollis, L, and Schmitz, N. The association between C-reactive protein, Interleukin-6 and depression among older adults in the community: A systematic review and meta-analysis. Exp Gerontol. (2018) 102:109–32. doi: 10.1016/j.exger.2017.12.005
74. Fang, H, Tu, S, Sheng, J, and Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. (2019) 23:2324–32. doi: 10.1111/jcmm.14170
75. Lin, TH, Chang, SF, Liao, MT, Chen, YH, and Tsai, HC. The relationships between physical function, nutrition, cognitive function, depression, and sleep quality for facility-dwelling older adults with dynapenia. BMC Geriatr. (2023) 23:278. doi: 10.1186/s12877-023-03847-9
Keywords: handgrip strength, muscle weakness, sarcopenia, mental health, aging
Citation: Chang C-C, Liao Y, Chen J, Lai T-F, Hsueh M-C, Park J-H and Chang Y-J (2025) Dynapenia is associated with a higher risk of depressive symptoms among older adults. Front. Public Health. 13:1533973. doi: 10.3389/fpubh.2025.1533973
Edited by:
Takuma Inagawa, National Center of Neurology and Psychiatry, JapanReviewed by:
Orazio Valerio Giannico, Local Health Authority of Taranto, ItalyKornanong Yuenyongchaiwat, Thammasat University, Thailand
Copyright © 2025 Chang, Liao, Chen, Lai, Hsueh, Park and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong-Hwan Park, cGFya2pAcHVzYW4uYWMua3I=; Yen-Jung Chang, eWpjaGFuZzIwMTJAZ21haWwuY29t
 Chih-Ching Chang
Chih-Ching Chang Yung Liao
Yung Liao Jiaren Chen2
Jiaren Chen2 Ting-Fu Lai
Ting-Fu Lai Jong-Hwan Park
Jong-Hwan Park