
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 25 February 2025
Sec. Public Mental Health
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1531953
This article is part of the Research TopicNutritional psychiatry: a new approach in mental health supportView all 4 articles
Background: Middle-aged and older adult men are at a heightened risk of depression. Green tea, as a popular beverage, has drawn widespread attention for its health benefits. However, there remains controversy over the effects of green tea on combating depression and regulating hormones.
Objective: This study aimed to investigate the effects of long-term green tea consumption on depression levels, hormones, and brain structure in, middle-aged and older adult men.
Methods: A total of 280 volunteers participated in the study, divided into a tea-drinking group and a control group. Basic demographic information and biological marker data, as well as MRI data from some of the volunteers, were collected. A controlled study was conducted to explore the effects of long-term tea drinking on them.
Results: BMI (p = 0.002), depression level (p = 0.003), insomnia severity (p = 0.008), and systemic inflammation index (p = 0.009) were significantly lower in the tea drinking group, and their testosterone levels were significantly higher than those in the control group (p = 0.001). Moreover, GM volume in the right precuneus in the control group was significantly reduced compared with that in the tea drinking group.
Conclusion: Long-term tea consumption helps reduce BMI and increase testosterone levels in middle-aged and older adult men, and it can also reduce their risk of depression by lowering inflammation and improving sleep quality. Additionally, long-term tea consumption may have the potential to delay brain aging in middle-aged and older adult men.
Depression is a common mental disorder that seriously affects people’s social functioning and quality of life, with over half of all suicides being related to depression. The World Health Organization categorized severe depression as the third leading cause of global disease burden in 2008, predicting it to become the first by 2030 (1, 2). Middle-aged and older adult individuals are more susceptible to depression due to facing more diseases and neurological changes associated with aging. Among adults aged 55 and above, 2% suffer from severe depression, and 10–15% of the older adult exhibit significant depressive symptoms. Moreover, the prevalence of depression increases with age (3), with women having a higher risk but men experiencing more severe consequences (4), with a significantly higher risk of suicide than women (5). There is growing attention on how to reduce the risk of depression in middle-aged and older adult men and avoid such dire outcomes. Research suggests that physical activity can effectively improve cognitive function in middle-aged and older adult populations (6), and reduce the risk of depression through various pathways (7, 8). However, in the past 20 years, the amount of physical activity among older adult individuals in China, especially men, has significantly decreased, with more time spent on activities like watching TV and playing mahjong (9). This trend poses a risk for the onset and progression of depression. Consequently, there is an urgent need to identify and implement additional strategies to lower depression risk in this population. One such strategy is the consumption of green tea, a widely consumed, affordable, and healthy beverage, especially popular among middle-aged and older adult men in China (10, 11). Its health benefits have garnered significant attention.
Green tea contains abundant natural bioactive compounds that have been shown to improve physical function and promote metabolism (12–15). Numerous studies have also indicated that green tea may help reduce the risk of depression (16–18). However, there is still no consensus on this conclusion (19, 20), possibly due to significant differences in the observed indicators [such as the amount, concentration, and frequency of green tea consumption (21–23)] among researchers. In recent years, some animal studies have suggested that the effects of tea bioactive compounds on endocrine function may be influenced by the duration of use (24, 25). However, few studies have focused on the long-term effects of the habit of drinking tea on the human body. As a daily beverage, its long-term accumulation’s impact on the body cannot be ignored. Therefore, this study primarily aims to explore the effects of long-term green tea consumption on the levels of depression, hormones, and brain structure in middle-aged and older adult men through a comparative study.
The study was conducted from June 2023 to March 2024 in a rural area of Hubei Province, China, known for the production of green tea (Simian tea), a non-fermented tea rich in a variety of natural active ingredients, mainly tea polyphenols, which is widely and long-term used by local residents. We randomly selected three natural villages in the area and surveyed all males in the villages. The recruitment of research subjects was overseen by the local village leaders, and questionnaire interviews and sample collection were conducted by trained psychiatric doctors and nurses. The questionnaire covered basic demographic information, levels of insomnia, and depression. Individuals who consumed green tea at least 6 days a week, with at least 1 cup (500 mL) per day, for at least 20 years, were defined as the long-term tea-drinking group, while the control group consisted of individuals who never or rarely consumed green tea (<1 time/half a year). Those meeting the inclusion criteria were required to sign an informed consent form and have blood samples collected after completing the questionnaire survey. Subsequently, a stratified random sampling method was used to select volunteers from both groups for MRI data collection. To determine the appropriate sample size for the study, we used G*Power (version 3.1), a widely recognized software tool for conducting power analyses. The calculation was based on an independent-samples t-test, given by the formula.
Where the significance level (α) was set to 0.05, statistical power () was set to 0.90, and effect size (Cohen’s d) was set to = 0.5. A required sample size of 86 participants per group, with a total of 172 participants for both groups combined. This sample size provides 90% power to detect a significant difference between the groups at the specified effect size and significance level. Finally, 280 people were enrolled in the study and 71 completed MRI data collection.
Inclusion criteria were as follows: (1) meeting the corresponding inclusion criteria for the study groups; (2) voluntary participation in the research and ability to provide written informed consent; (3) participants capable of understanding the questionnaire’s questions and completing it; (4) cooperation with sample and MRI data collection; and (5) male participants aged 45 and above.
Exclusion criteria were as follows: (1) a history of psychiatric illness or a family history of psychiatric disorders; (2) recent experience of major traumatic events; (3) have serious physical illness, or have systemic lupus erythematosus, thyroid disease and other diseases that may affect hormones; (4) current or past use of psychiatric medications or hormonal drugs; and (5) recent use of coffee or energy drinks that may have an effect on hormones.
The basic information includes age, height, weight, heart rate, blood pressure, marital status (married, unmarried, widowed), ethnicity, usual residence (urban or rural), education level (illiterate, primary school, junior high school and above), family annual income classified according to the Chinese household income level table into: below 10,000, 10,000–29,999, 30,000–79,999, above 80,000, religious beliefs, whether the participant is an only child, and whether they live alone. The level of social support was assessed using the Perceived Social Support Scale (PSSS), which consists of three subscales with a total of 12 items. A higher score indicates higher levels of social support, with good reliability and validity (26). The beverage consumption habits of volunteers were investigated by professional staff and recorded, including the type, frequency, time, etc.
The Insomnia Severity Index (ISI) was used to screen the severity of participants’ insomnia. The scale consists of 7 items, with each item rated on a scale of 0–4, totaling 28 points. A higher score indicates a higher degree of insomnia. This scale has good validity and reliability for assessing insomnia severity (27, 28). As the above questionnaire cannot reflect the duration of sleep each day, we also separately recorded the volunteers’ sleep duration.
Participants were asked to fast, and blood samples for further analysis were collected every morning (7–9 a.m.). Blood samples were collected using standard venipuncture in the antecubital vein, and 10 mL of blood samples from each participant were drawn into two 5 mL Vacutainer tubes for routine blood analysis and hormone assay. Platelet count, lymphocyte count, and white blood cell count in blood were measured by routine blood analysis to calculate Systemic Immune Inflammation Index (SII), which can reflect local immune response and systemic inflammation, and has been widely used in disease prediction and research (29–31). Enzyme-linked immunosorbent assays were used to analyze Thyroid-Stimulating Hormone (TSH), Free Triiodothyronine (FT3), Free Thyroxine (FT4), sex hormones (testosterone and estradiol), and inflammation markers.
Participants’ levels of depression were assessed using the Patient Health Questionnaire-9 (PHQ-9). This questionnaire consists of nine items, with scores ranging from 0 (not at all) to 3 (nearly every day), with higher scores indicating more severe depressive symptoms. The PHQ-9 demonstrates good sensitivity and specificity and is widely used for depression screening in primary care and research settings (32).
Taking into account the economic costs involved, we applied a stratified random sampling method to recruit participants for this study. Through this process, a cohort of 71 people was eventually established, of which 35 were in the tea drinking group and 36 were in the control group.
Structural MRI data was acquired on a 3-T UIH scanner (uMR 780) at Three Gorges Uni Renhe Hospital, and utilized the following parameters: repetition time (TR) = 7.2 ms; echo time (TE) = 3.1 ms; 176 slices; field of view (FOV) = 256 × 256 mm; matrix = 256 × 256.
Structural MRI data was preprocessing using FreeSurfer v7.4.0.1 Specifically, analysis was performed following these steps: (1) all images were inspected for data quality prior to processing; (2) skull stripping, bias field correction, and gray-white matter segmentation (33); (3) reconstruction of cortical surface models; (4) labeling of regions on the cortical surface, as well as subcortical brain structures; and (5) nonlinear registration of the cortical surface of an individual.
Then, we derived the information of cerebral cortex regions’ thickness and volume according to the default atlas, as well as the volume of subcortical regions like amygdala or putamen, to carry on the further statistical analysis.
Analysis was performed using SPSS Statistics 23.0. Categorical variables are represented using N and %, while quantitative data are represented using means and quartiles. Group differences were analyzed using chi-square tests and non-parametric tests. To verify the correlation between tea consumption and depression levels, a Spearman analysis was first conducted, including all potential factors influencing depression levels. Subsequently, ordered logistic regression analysis was employed.
To further explore the indirect effects of tea drinking on depression, path analysis was performed by structural equation model using Amos 26.0. The bootstrapping method (5,000 resamples) was employed to estimate the 95% confidence intervals (CI) of the mediators’ indirect effect. When the 95% CI did not contain zero, the indirect effect was considered statistically significant (34). The level of significance was set at 0.05, two-tailed.
Since the volunteers included in the study have consistent ethnicity, religious beliefs, and residence, they are not included in the statistical analysis. Subgroups with sample sizes that do not meet statistical requirements are merged with other groups for analysis.
The analyses of thickness and volume were performed using Python v3.11.7. For volume, we first got standardized volume by calculating the ratio of volume to estimated total intracranial volume (eTIV). Then, the general linear model was applied to test difference between two groups on thickness and volume of cerebral cortex or subcortical regions, with age and education were included as covariates. Following the application of the Benjamini-Hochberg method for controlling the False Discovery Rate (FDR), a threshold of p < 0.05 is utilized to determine statistical significance.
A total of 280 individuals participated in this study, with 151 individuals in the tea-drinking group. There were no significant differences between the two groups in basic sociodemographic information such as age (p = 0.175), only child status (p = 0.353), marital status (p = 0.375), education level (p = 0.508), annual income (p = 0.943), smoking status (p = 0.397), alcohol consumption (p = 0.318), living alone (p = 0.052), occupation (p = 0.255), and social support (p = 0.913). Similarly, there were no significant differences in biological information such as heart rate (p = 0.590), systolic pressure (SP) (p = 0.149), diastolic pressure (DP) (p = 0.167), TSH level (p = 0.351), FT4 level (p = 0.794), FT3 level (p = 0.934), and estradiol concentration (p = 0.246). However, significant differences were observed between the two groups in sleep duration (p = 0.031), insomnia severity (p = 0.008), depression severity (p = 0.003), body mass index (BMI) (p = 0.002), SII (p = 0.009), and testosterone concentration (p = 0.001) (see Tables 1, 2 for details).
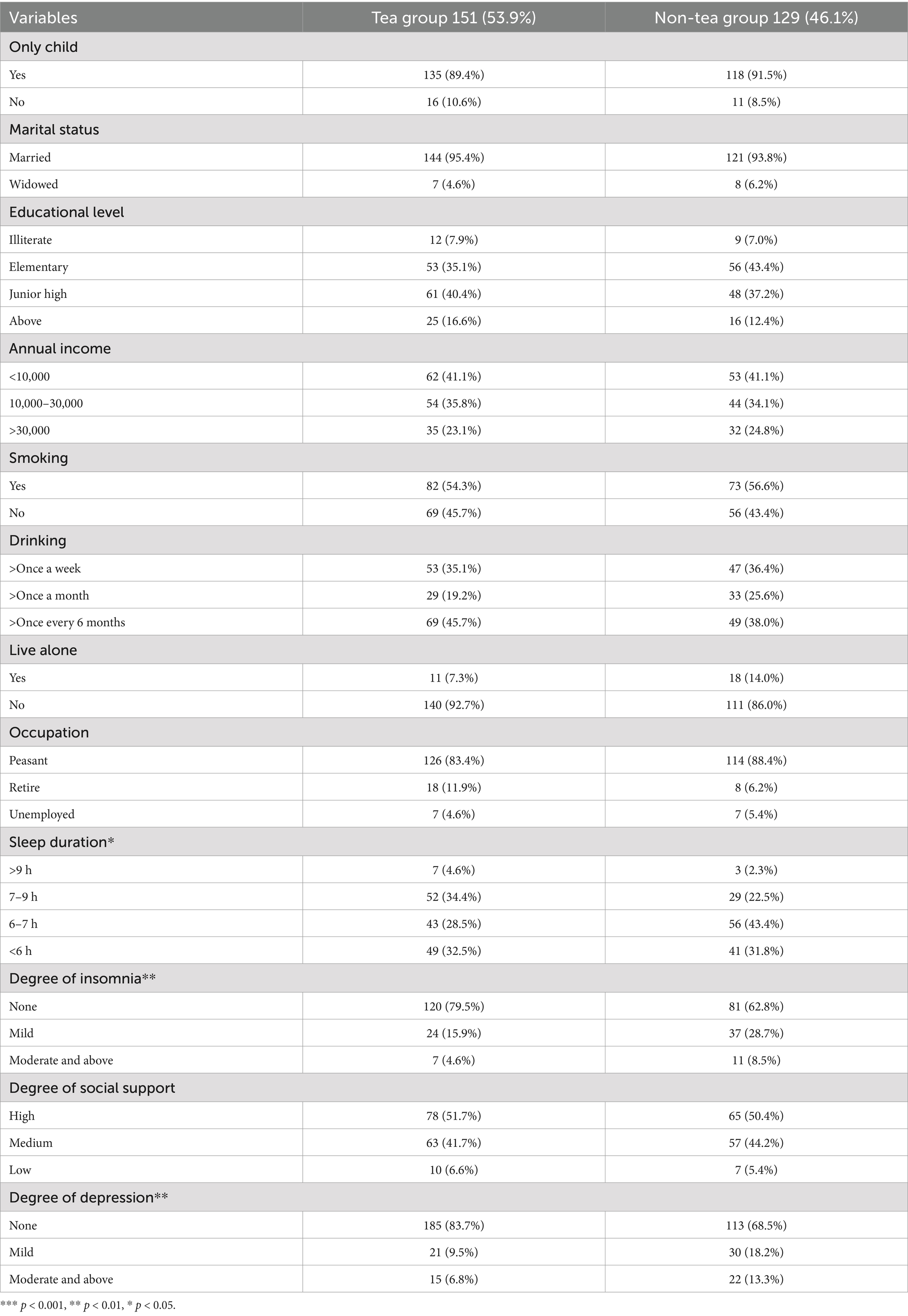
Table 1. The characteristics and differences between the two groups on different variables (Categorical variables).
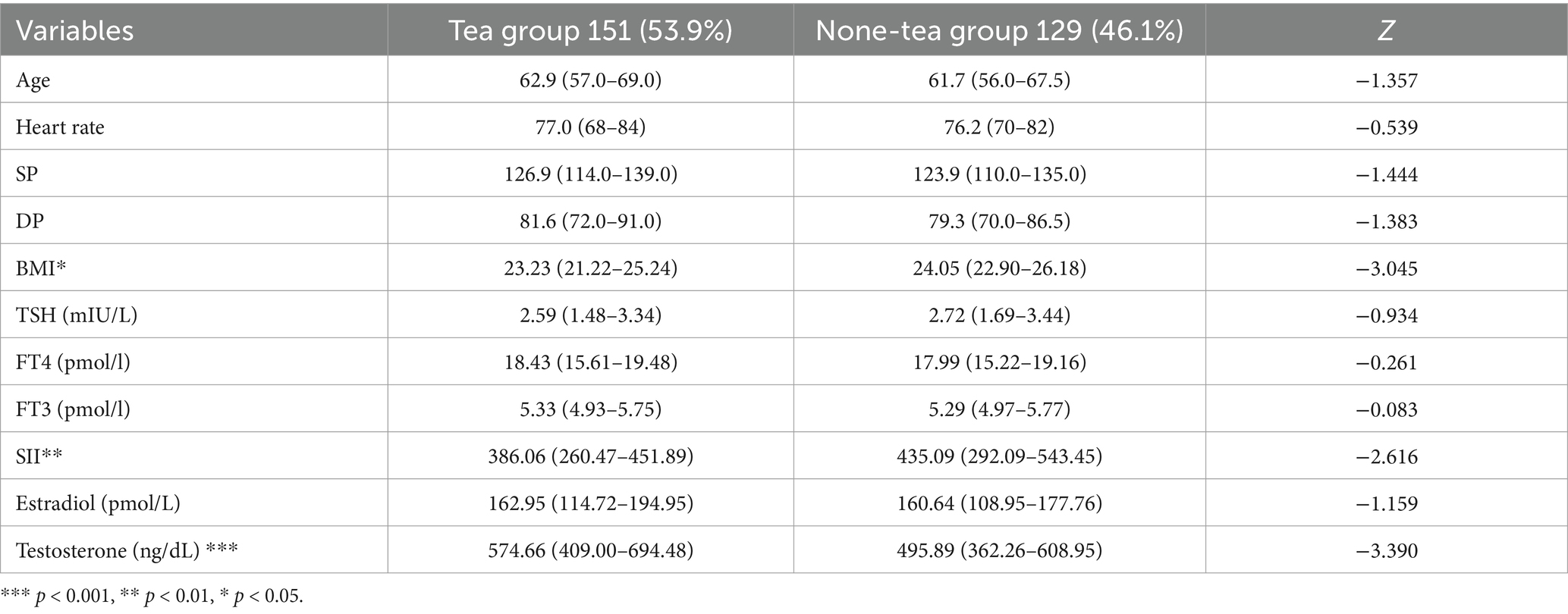
Table 2. The characteristics and differences between the two groups on different variables (Numerical variables).
First, Spearman correlation analysis was used to assess the relationship between certain biological and sociodemographic information and depression. Subsequently, factors potentially influencing depression severity were included, and ordered logistic regression was employed to explore the relationship between long-term tea drinking and depression severity. The results indicate significant correlations between systemic immune inflammation index (SII) (p < 0.01), heart rate (p < 0.01), sleep duration (p < 0.01), insomnia severity (p < 0.01), and social support (p < 0.05) with depression severity. Subsequent ordered logistic regression analysis revealed that the depression severity in the long-term tea-drinking group was significantly lower (p = 0.017) (The detailed content can be found in Figures 1, 2).
With tea drinking as the independent variable, the degree of depression as the dependent variable, SII and the degree of insomnia as the mediating variables, structural equation model was constructed (Figure 3). The fitting indexes of the structural equation model were as follows: χ2 / df = 3.348, GFI = 0.994, RMSEA = 0.092, CFI = 0.975, AGFI = 0.941, NFI = 0.967.
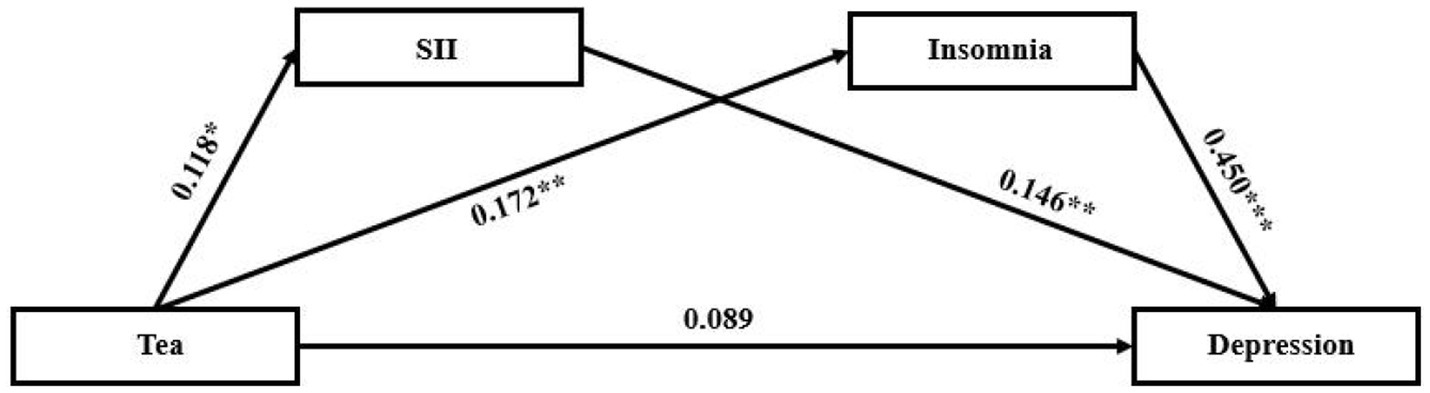
Figure 3. The structural equation model of the relationship between tea and depression. The value on each path indicates the standardized coefficient. SII, systemic immune inflammation. *** p < 0.001, ** p < 0.01, * p < 0.05.
The results showed that there are significant indirect effects of SII and insomnia on the relationship between tea drinking and the degree of depression (Table 3; Figure 3). The total indirect effect of tea drinking on the degree of depression was 0.103 (SE = 0.035, 95% CI [0.041, 0.180], p = 0.002), accounting for 51.6% of the total effect. Considering that the direct effect was not significant (0.089, SE = 0.057, 95% CI [−0.016, 0.189], p = 0.094), tea drinking could influence the degree of depression major by the mediating variables including SII and the degree of insomnia. The mediating effects of SII and the degree of insomnia were both significant, and were 0.019 (SE = 0.014, 95% CI [0.001, 0.059], p = 0.041), and 0.084 (SE = 0.032, 95% CI [0.032, 0.157], p = 0.003), accounted for 9.4 and 42.2% of the total effect, respectively. Figure 1 displayed the standardized path coefficients of the model.
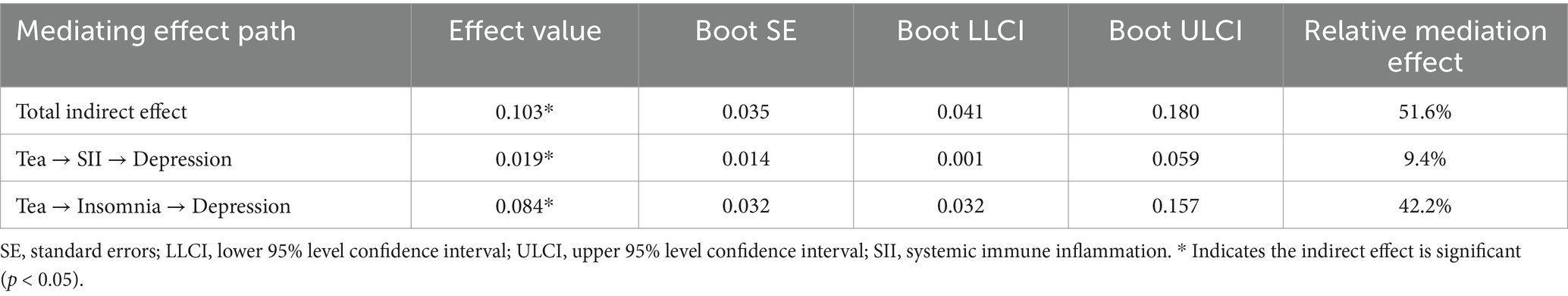
Table 3. Structural equation model results for the relationship between tea drinking and depression.
Compared to individuals who never drink tea, tea drinkers demonstrated a higher gray matter volume in the left medial occipito-temporal and parahippocampal gyrus (lh_G_oc_temp_med_Parahip, FDR q = 0.01378, Figure 4). No group differences were observed in the gray matter volumes of other brain regions and subcortical structures, or in cortical thickness.

Figure 4. Group differences of gray matter volume in lh_G_oc_temp_med_Parahip. (A) Anatomical position of lh_G_oc_temp_med_Parahip. (B) Standardized volume (volume/eTIV) of two groups of lh_G_oc_temp_med_Parahip. lh_G_oc_temp_med_Parahip, the left medial occipito-temporal and parahippocampal gyrus. FDR q < 0.05.
Green tea, as a globally popular beverage, has been widely recognized for its health benefits (35). However, the research conclusions regarding the correlation between green tea and the risk of depression remain controversial, with evidence both supporting and refuting its role in reducing depression risk (19, 36). The impact on endocrine function is also subject to debate, although abundant evidence confirms the effects of bioactive compounds in green tea on various hormones (37, 38), discrepancies in research conclusions exist due to differences in experimental methods and intervention duration (24, 25, 39, 40). In everyday life, green tea is highly favored and consumed over the long term by middle-aged and older adult Chinese men (41). Therefore, this study did not consider the frequency or quantity of tea consumption as observational indicators. Instead, it emphasized the impact of long-term tea drinking as a lifestyle habit on their endocrine function, level of depression, and brain structure.
The results indicate that the testosterone concentration in individuals who consume tea over the long term is significantly higher than that in the control group. Some studies have shown that the main active compound in green tea, EGCG, can acutely inhibit basal and kinase-stimulated testosterone production (39). However, an animal study administering EGCG to rats via the jugular vein found a significant increase in plasma testosterone levels several hours later (40). These studies suggest that the effect of green tea on testosterone concentration appears to be time-dependent. This study confirms the promoting effect of long-term tea drinking on testosterone, which may contribute to improving sexual function, enhancing mood, and alleviating depressive symptoms in older adult men (42). Many studies have affirmed the influence of green tea on estradiol, suggesting that drinking green tea helps increase estradiol levels in premenopausal women (43, 44), although contradictory evidence has also been presented (45). Perhaps because the overall estradiol levels in middle-aged and older adult men are lower (46), the effect of long-term green tea consumption on it is not significant. However, the majority of related research has focused on women, and due to the existence of gender differences, more exploration is needed on the effects of green tea on male estrogen.
The concentrations of TSH, FT3, and FT4 also showed no significant differences between the two groups of people. Many animal studies suggest that active substances in green tea (mainly catechins) have a significant impact on thyroid hormones by inhibiting TPO activity, thereby reducing T3 and T4 levels and increasing TSH levels (47). However, the effects on humans may not be the same, as rodents lack high-affinity thyroxine-binding globulin, making their thyroid more susceptible to influence. Additionally, different concentrations of catechins have varying effects on the thyroid of mice (48), with only higher concentrations potentially affecting the thyroid gland and its function (49). Therefore, some researchers believe that normal consumption of green tea in humans is unlikely to lead to thyroid dysfunction (50). This study also provides evidence supporting this notion, demonstrating that long-term consumption of green tea does not significantly affect thyroid hormones in healthy middle-aged and older adult men. However, it is important to emphasize that the conclusions of animal studies should not be ignored. For populations with metabolic issues or those who consume green tea at high concentrations for an extended period, attention should still be paid to the effects of green tea on thyroid function.
Furthermore, the results indicate that long-term tea consumption significantly increases sleep duration, improves sleep quality, and reduces BMI. Green tea is rich in caffeine, which has an activating effect, and also contains theanine, which has a sleep-promoting effect (51), leading to almost contradictory effects of green tea on sleep behavior. Interestingly, green tea often has adverse effects on the sleep of individuals with initial or short-term exposure (52, 53), while older adult individuals who consume tea over the long term exhibit better sleep behavior (21). This suggests that the association between green tea and sleep behavior may be influenced by the timing of intake, and the results of this study affirm the benefits of long-term tea consumption for sleep.
The depression levels in the tea-drinking group were also significantly lower, but this conclusion faces challenges from other studies (17, 19). A follow-up study provides evidence for our conclusion. Researchers found that despite initially no difference in depression levels between the two groups, the overall depression levels in the tea-drinking group showed a more significant improvement over time (54). Similar to the impact of tea consumption on sleep mentioned earlier, the protective effect of green tea against depression may also be time-dependent, with long-term tea consumption having a more affirmative role in reducing depression risk. Additionally, pathway models indicate that tea consumption can reduce depression levels by improving sleep quality and reducing SII concentrations. There is a significant bidirectional relationship between sleep and depression (55), and the theanine in green tea can enhance sleep quality through its anti-anxiety effects (56), effectively suppressing the occurrence of chronic sleep disorders and reducing the risk of depression. The anti-inflammatory effect of green tea has been well established in many previous studies (57, 58). Inflammation is involved in the occurrence and development of almost all systemic diseases (59–62), and it is a major cause of the onset and progression of depression (63).
Some recent studies have suggested that the active compounds in green tea can inhibit the loss of hippocampal neurons in mice (64), potentially providing neuroprotective effects for older adult individuals (65, 66). Therefore, we conducted an exploratory study to compare the differences in brain structure between two groups of people. we found that the participants who never drink tea had lower GM volume in the left medial occipito-temporal and parahippocampal gyrus. Reduced volume of gray matter in wide brain regions was often regarded as the important characteristic of aging (67). Therefore, higher GM volume in this study may revealed a delay of reduced gray matter volume. The left medial occipito-temporal and parahippocampal gyrus is a set of brain regions adjacent to the hippocampus, closely associated with the limbic system. Previous studies have confirmed its relationship with memory formation and retrieval, as well as higher-order visual processing (68, 69). This may be attribute to the acts neuroprotectively of EGCG in green tea (70, 71). Our study suggests that long-term tea consumption may potentially delay brain aging in older men.
This study explored the multifaceted effects of long-term green tea consumption on middle-aged and older adult individuals. It indicated that long-term tea drinking can help reduce the BMI of middle-aged and older adult men, increase testosterone levels, and also lower the risk of depression by reducing inflammation and improving sleep quality. Furthermore, long-term tea consumption may delay brain aging in middle-aged and older adult men. However, the study’s limitations cannot be ignored: it did not use relatively precise indicators such as green tea concentration, frequency, and quantity of consumption, making it difficult to provide more accurate guidance; while the study provided evidence for the long-term benefits of tea consumption, it could not confirm how long a history of tea consumption is needed to have a significant impact on the body; moreover, the study only focused on the effects of green tea on middle-aged and older adult individuals, and as more people begin to consume green tea, a broader range of age groups should be investigated. Considering that there are large differences in hormones between middle-aged and older men and women (72), we studied the two sexes separately. The study on women can be found in another article (73). Finally, food factors and some other beverages with potential effects on hormones and inflammation were not considered in this study, and future studies need to exclude or include all foods, beverages, and dietary supplements that may affect hormones and inflammation to make the results more accurate.
Long-term tea consumption helps reduce BMI and increase testosterone levels in middle-aged and older adult men, and it can also reduce their risk of depression by lowering inflammation and improving sleep quality. Additionally, long-term tea consumption may have the potential to delay brain aging in middle-aged and older adult men.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Second Xiangya Hospital, Central South University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
ZW: Conceptualization, Formal analysis, Investigation, Writing – original draft. QW: Investigation, Project administration, Resources, Writing – review & editing. XQ: Conceptualization, Formal analysis, Investigation, Writing – review & editing. GW: Writing – review & editing. HL: Investigation, Resources, Writing – review & editing. YJ: Investigation, Resources, Writing – review & editing. BW: Investigation, Resources, Writing – review & editing. LA: Investigation, Resources, Writing – review & editing. JW: Investigation, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Science and Technology Innovation 2030-Major program of “Brain Science and Brain-Like Research”, grant number 2021ZD0200701.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Malhi, GS, and Mann, JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
3. Kok, RM, and Reynolds, CF 3rd. Management of Depression in older adults: a review. JAMA. (2017) 317:2114–22. doi: 10.1001/jama.2017.5706
4. Zhou, F, He, S, Shuai, J, Deng, Z, Wang, Q, and Yan, Y. Social determinants of health and gender differences in depression among adults: a cohort study. Psychiatry Res. (2023) 329:115548. doi: 10.1016/j.psychres.2023.115548
5. Beghi, M, Butera, E, Cerri, CG, Cornaggia, CM, Febbo, F, Mollica, A, et al. Suicidal behaviour in older age: a systematic review of risk factors associated to suicide attempts and completed suicides. Neurosci Biobehav Rev. (2021) 127:193–211. doi: 10.1016/j.neubiorev.2021.04.011
6. Zhu, CE, Zhou, L, and Zhang, X. Effects of leisure activities on the cognitive ability of older adults: a latent variable growth model analysis. Front Psychol. (2022) 13:838878. doi: 10.3389/fpsyg.2022.838878
7. Gao, Y, Jia, Z, Zhao, L, and Han, S. The effect of activity participation in middle-aged and older people on the trajectory of depression in later life: National Cohort Study. JMIR Public Health Surveill. (2023) 9:e44682. doi: 10.2196/44682
8. Zhang, S, Xiang, K, Li, S, and Pan, HF. Physical activity and depression in older adults: the knowns and unknowns. Psychiatry Res. (2021) 297:113738. doi: 10.1016/j.psychres.2021.113738
9. Feng, Q, Fong, JH, Zhang, W, Liu, C, and Chen, H. Leisure activity engagement among the oldest old in China, 1998–2018. Am J Public Health. (2020) 110:1535–7. doi: 10.2105/AJPH.2020.305798
10. Tian, T, Lv, J, Jin, G, Yu, C, Guo, Y, Bian, Z, et al. Tea consumption and risk of stroke in Chinese adults: a prospective cohort study of 0.5 million men and women. Am J Clin Nutr. (2020) 111:197–206. doi: 10.1093/ajcn/nqz274
11. Chen, ZM, and Lin, Z. Tea and human health: biomedical functions of tea active components and current issues. J Zhejiang Univ Sci B. (2015) 16:87–102. doi: 10.1631/jzus.B1500001
12. Yang, X, Dai, H, Deng, R, Zhang, Z, Quan, Y, Giri, M, et al. Association between tea consumption and prevention of coronary artery disease: a systematic review and dose-response meta-analysis. Front Nutr. (2022) 9:1021405. doi: 10.3389/fnut.2022.1021405
13. Pang, J, Zhang, Z, Zheng, TZ, Bassig, BA, Mao, C, Liu, X, et al. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta-analysis. Int J Cardiol. (2016) 202:967–74. doi: 10.1016/j.ijcard.2014.12.176
14. Wang, ZY, Khan, WA, Bickers, DR, and Mukhtar, H. Protection against polycyclic aromatic hydrocarbon-induced skin tumor initiation in mice by green tea polyphenols. Carcinogenesis. (1989) 10:411–5. doi: 10.1093/carcin/10.2.411
15. Dinh, TC, Thi Phuong, TN, Minh, LB, Minh Thuc, VT, Bac, ND, van Tien, N, et al. The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders – an existing update. Diabetes Metab Syndr. (2019) 13:1667–73. doi: 10.1016/j.dsx.2019.03.021
16. Miki, T, Kochi, T, Kuwahara, K, Eguchi, M, Kurotani, K, Tsuruoka, H, et al. Dietary patterns derived by reduced rank regression (RRR) and depressive symptoms in Japanese employees: the Furukawa nutrition and health study. Psychiatry Res. (2015) 229:214–9. doi: 10.1016/j.psychres.2015.07.033
17. Li, FD, He, F, Ye, XJ, Shen, W, Wu, YP, Zhai, YJ, et al. Tea consumption is inversely associated with depressive symptoms in the elderly: a cross-sectional study in eastern China. J Affect Disord. (2016) 199:157–62. doi: 10.1016/j.jad.2016.04.005
18. Dong, X, Yang, C, Cao, S, Gan, Y, Sun, H, Gong, Y, et al. Tea consumption and the risk of depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. (2015) 49:334–45. doi: 10.1177/0004867414567759
19. Nanri, A, Eguchi, M, Kochi, T, Kabe, I, and Mizoue, T. Green tea consumption and depressive symptoms among Japanese workers: the Furukawa nutrition and health study. Nutrients. (2021) 14:167. doi: 10.3390/nu14010167
20. Kimura, Y, Suga, H, Kobayashi, S, and Sasaki, S Three-Generation Study of Women on Diets and Health Study Group. Intake of coffee associated with decreased depressive symptoms among elderly Japanese women: a multi-center cross-sectional study. J Epidemiol. (2020) 30:338–44. doi: 10.2188/jea.JE20190010
21. Wei, C, Zhang, J, Chen, N, Xu, Z, and Tang, H. Does frequent tea consumption provide any benefit to cognitive function in older adults? Evidence from a national survey from China in 2018. Front Public Health. (2023) 11:1269675. doi: 10.3389/fpubh.2023.1269675
22. Yao, Y, Chen, H, Chen, L, Ju, SY, Yang, H, Zeng, Y, et al. Type of tea consumption and depressive symptoms in Chinese older adults. BMC Geriatr. (2021) 21:331. doi: 10.1186/s12877-021-02203-z
23. Chen, X, Lu, W, Zheng, Y, Gu, K, Chen, Z, Zheng, W, et al. Exercise, tea consumption, and depression among breast cancer survivors. J Clin Oncol. (2010) 28:991–8. doi: 10.1200/JCO.2009.23.0565
24. Monteiro, R, Assunção, M, Andrade, JP, Neves, D, Calhau, C, and Azevedo, I. Chronic green tea consumption decreases body mass, induces aromatase expression, and changes proliferation and apoptosis in adult male rat adipose tissue. J Nutr. (2008) 138:2156–63. doi: 10.1093/jn/138.11.2156
25. Shen, CL, Wang, P, Guerrieri, J, Yeh, JK, and Wang, JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. (2008) 19:979–90. doi: 10.1007/s00198-007-0527-5
26. Chou, K-L. Assessing Chinese adolescents’ social support: the multidimensional scale of perceived social support. Personal Individ Differ. (2000) 28:299–307. doi: 10.1016/S0191-8869(99)00098-7
27. Thorndike, FP, Ritterband, LM, Saylor, DK, Magee, JC, Gonder-Frederick, LA, and Morin, CM. Validation of the insomnia severity index as a web-based measure. Behav Sleep Med. (2011) 9:216–23. doi: 10.1080/15402002.2011.606766
28. Bastien, CH, Vallières, A, and Morin, CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. (2001) 2:297–307. doi: 10.1016/s1389-9457(00)00065-4
29. Mazza, MG, Palladini, M, De Lorenzo, R, Magnaghi, C, Poletti, S, Furlan, R, et al. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav Immun. (2021) 94:138–47. doi: 10.1016/j.bbi.2021.02.021
30. Tian, T, Lu, J, Zhao, W, Wang, Z, Xu, H, Ding, Y, et al. Associations of systemic inflammation markers with identification of pulmonary nodule and incident lung cancer in Chinese population. Cancer Med. (2022) 11:2482–91. doi: 10.1002/cam4.4606
31. Zhang, F, Niu, M, Wang, L, Liu, Y, Shi, L, Cao, J, et al. Systemic-immune-inflammation index as a promising biomarker for predicting perioperative ischemic stroke in older patients who underwent non-cardiac surgery. Front Aging Neurosci. (2022) 14:865244. doi: 10.3389/fnagi.2022.865244
32. Negeri, ZF, Levis, B, Sun, Y, He, C, Krishnan, A, Wu, Y, et al. Accuracy of the patient health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ. (2021) 375:n2183. doi: 10.1136/bmj.n2183
33. Reuter, M, Rosas, HD, and Fischl, B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. (2010) 53:1181–96. doi: 10.1016/j.neuroimage.2010.07.020
34. Preacher, KJ, and Hayes, AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. (2008) 40:879–91. doi: 10.3758/brm.40.3.879
35. Singh, BN, Shankar, S, and Srivastava, RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. (2011) 82:1807–21. doi: 10.1016/j.bcp.2011.07.093
36. Kim, J, and Kim, J. Green tea, coffee, and caffeine consumption are inversely associated with self-report lifetime depression in the Korean population. Nutrients. (2018) 10:1201. doi: 10.3390/nu10091201
37. Shen, W, Pan, Y, Jin, B, Zhang, Z, You, T, Qu, Y, et al. Effects of tea consumption on anthropometric parameters, metabolic indexes and hormone levels of women with polycystic ovarian syndrome: a systematic review and Meta-analysis of randomized controlled trials. Front Endocrinol (Lausanne). (2021) 12:736867. doi: 10.3389/fendo.2021.736867
38. Alufer, L, Tsaban, G, Rinott, E, Kaplan, A, Meir, AY, Zelicha, H, et al. Long-term green-Mediterranean diet may favor fasting morning cortisol stress hormone; the DIRECT-PLUS clinical trial. Front Endocrinol (Lausanne). (2023) 14:1243910. doi: 10.3389/fendo.2023.1243910
39. Figueiroa, MS, César Vieira, JS, Leite, DS, Filho, RC, Ferreira, F, Gouveia, PS, et al. Green tea polyphenols inhibit testosterone production in rat Leydig cells. Asian J Androl. (2009) 11:362–70. doi: 10.1038/aja.2009.2
40. Yu, PL, Pu, HF, Chen, SY, Wang, SW, and Wang, PS. Effects of catechin, epicatechin and epigallocatechin gallate on testosterone production in rat leydig cells. J Cell Biochem. (2010) 110:333–42. doi: 10.1002/jcb.22541
41. Feng, L, Yan, Z, Sun, B, Cai, C, Jiang, H, Kua, EH, et al. Tea consumption and depressive symptoms in older people in rural China. J Am Geriatr Soc. (2013) 61:1943–7. doi: 10.1111/jgs.12496
42. Snyder, PJ, Bhasin, S, Cunningham, GR, Matsumoto, AM, Stephens-Shields, AJ, Cauley, JA, et al. Testosterone trials investigators. Effects of testosterone treatment in older men. N Engl J Med. (2016) 374:611–24. doi: 10.1056/NEJMoa1506119
43. Kotsopoulos, J, Eliassen, AH, Missmer, SA, Hankinson, SE, and Tworoger, SS. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer. (2009) 115:2765–74. doi: 10.1002/cncr.24328
44. Schliep, KC, Schisterman, EF, Mumford, SL, Pollack, AZ, Zhang, C, Ye, A, et al. Caffeinated beverage intake and reproductive hormones among premenopausal women in the BioCycle study. Am J Clin Nutr. (2012) 95:488–97. doi: 10.3945/ajcn.111.021287
45. Nagata, C, Kabuto, M, and Shimizu, H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer. (1998) 30:21–4. doi: 10.1080/01635589809514635
46. van den Beld, AW, de Jong, FH, Grobbee, DE, Pols, HA, and Lamberts, SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. (2000) 85:3276–82. doi: 10.1210/jcem.85.9.6825
47. Chandra, AK, and De, N. Goitrogenic/antithyroidal potential of green tea extract in relation to catechin in rats. Food Chem Toxicol. (2010) 48:2304–11. doi: 10.1016/j.fct.2010.05.064
48. Chandra, AK, and De, N. Catechin induced modulation in the activities of thyroid hormone synthesizing enzymes leading to hypothyroidism. Mol Cell Biochem. (2013) 374:37–48. doi: 10.1007/s11010-012-1503-8
49. Sakamoto, Y, Mikuriya, H, Tayama, K, Takahashi, H, Nagasawa, A, Yano, N, et al. Goitrogenic effects of green tea extract catechins by dietary administration in rats. Arch Toxicol. (2001) 75:591–6. doi: 10.1007/s00204-001-0286-6
50. Satoh, K, Sakamoto, Y, Ogata, A, Nagai, F, Mikuriya, H, Numazawa, M, et al. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol. (2002) 40:925–33. doi: 10.1016/s0278-6915(02)00066-2
51. Baba, Y, Takihara, T, and Okamura, N. Theanine maintains sleep quality in healthy young women by suppressing the increase in caffeine-induced wakefulness after sleep onset. Food Funct. (2023) 14:7109–16. doi: 10.1039/d3fo01247f
52. Watson, EJ, Banks, S, Coates, AM, and Kohler, MJ. The relationship between caffeine, sleep, and behavior in children. J Clin Sleep Med. (2017) 13:533–43. doi: 10.5664/jcsm.6536
53. Thakre, TP, Deoras, K, Griffin, C, Vemana, A, Podmore, P, and Krishna, J. Caffeine awareness in children: insights from a pilot study. J Clin Sleep Med. (2015) 11:741–6. doi: 10.5664/jcsm.4848.25845895
54. Ng, TP, Gao, Q, Gwee, X, and Chua, DQL. Tea consumption and depression from follow up in the Singapore longitudinal ageing study. J Nutr Health Aging. (2021) 25:295–301. doi: 10.1007/s12603-020-1526-x
55. de Feijter, M, Kocevska, D, Ikram, MA, and Luik, AI. The bidirectional association of 24-h activity rhythms and sleep with depressive symptoms in middle-aged and elderly persons. Psychol Med. (2023) 53:1418–25. doi: 10.1017/S003329172100297X
56. Rao, TP, Ozeki, M, and Juneja, LR. In search of a safe natural sleep aid. J Am Coll Nutr. (2015) 34:436–47. doi: 10.1080/07315724.2014.926153
57. Luvián-Morales, J, Varela-Castillo, FO, Flores-Cisneros, L, Cetina-Pérez, L, and Castro-Eguiluz, D. Functional foods modulating inflammation and metabolism in chronic diseases: a systematic review. Crit Rev Food Sci Nutr. (2022) 62:4371–92. doi: 10.1080/10408398.2021.1875189
58. Ntamo, Y, Jack, B, Ziqubu, K, Mazibuko-Mbeje, SE, Nkambule, BB, Nyambuya, TM, et al. Epigallocatechin gallate as a nutraceutical to potentially target the metabolic syndrome: novel insights into therapeutic effects beyond its antioxidant and anti-inflammatory properties. Crit Rev Food Sci Nutr. (2024) 64:87–109. doi: 10.1080/10408398.2022.2104805
59. Arnold, N, and Koenig, W. Infection, inflammation, and cardiovascular risk: are we underestimating an old disease? Eur Heart J. (2024) 45:1521–3. doi: 10.1093/eurheartj/ehae104
60. Li, C, Ke, B, Chen, J, Xiao, Y, Wang, S, Jiang, R, et al. Systemic inflammation and risk of Parkinson’s disease: a prospective cohort study and genetic analysis. Brain Behav Immun. (2024) 117:447–55. doi: 10.1016/j.bbi.2024.02.013
61. Ashton, JJ, Satsangi, J, and Uhlig, HH. Intestinal inflammation and Extraintestinal disease: understanding dynamic risk. Gastroenterology. (2024) 167:205–8. doi: 10.1053/j.gastro.2024.03.040
62. Yang, W, and Liu, J. Regarding the role of systemic immune-inflammation index on the risk of chronic kidney disease. Int J Surg. (2024) 110:2467–8. doi: 10.1097/JS9.0000000000001097
63. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
64. Stazi, M, Lehmann, S, Sakib, MS, Pena-Centeno, T, Büschgens, L, Fischer, A, et al. Long-term caffeine treatment of Alzheimer mouse models ameliorates behavioural deficits and neuron loss and promotes cellular and molecular markers of neurogenesis. Cell Mol Life Sci. (2021) 79:55. doi: 10.1007/s00018-021-04062-8
65. Xu, H, Fiocco, AJ, Liu, X, Wang, T, Li, G, Xiao, S, et al. Association between tea consumption and cognitive function in cognitively healthy older adults and older adults with mild cognitive impairment. Gen Psychiatr. (2021) 34:e100512. doi: 10.1136/gpsych-2021-100512
66. Kaplan, A, Zelicha, H, Yaskolka Meir, A, Rinott, E, Tsaban, G, Levakov, G, et al. The effect of a high-polyphenol Mediterranean diet (green-MED) combined with physical activity on age-related brain atrophy: the dietary intervention randomized controlled trial polyphenols unprocessed study (DIRECT PLUS). Am J Clin Nutr. (2022) 115:1270–81. doi: 10.1093/ajcn/nqac001
67. Fjell, AM, and Walhovd, KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. (2010) 21:187–221. doi: 10.1515/revneuro.2010.21.3.187
68. Epstein, R, and Kanwisher, N. A cortical representation of the local visual environment. Nature. (1998) 392:598–601. doi: 10.1038/33402
69. Grill-Spector, K, and Malach, R. The human visual cortex. Annu Rev Neurosci. (2004) 27:649–77. doi: 10.1146/annurev.neuro.27.070203.144220
70. Ortiz-López, L, Márquez-Valadez, B, Gómez-Sánchez, A, Silva-Lucero, MD, Torres-Pérez, M, Téllez-Ballesteros, RI, et al. Green tea compound epigallo-catechin-3-gallate (EGCG) increases neuronal survival in adult hippocampal neurogenesis in vivo and in vitro. Neuroscience. (2016) 322:208–20. doi: 10.1016/j.neuroscience.2016.02.040
71. Salameh, A, Einenkel, A, Kühne, L, Grassl, M, von Salisch, S, Kiefer, P, et al. Hippocampal neuroprotection by minocycline and Epigallo-Catechin-3-Gallate against cardiopulmonary bypass-associated injury. Brain Pathol. (2015) 25:733–42. doi: 10.1111/bpa.12242
72. Hunter, SK, and Senefeld, JW. Sex differences in human performance. J Physiol. (2024) 602:4129–56. doi: 10.1113/JP284198
Keywords: middle-aged and older adult, depression, mental health, green tea, inflammation, hormone
Citation: Wan Z, Wan Q, Qin X, Wang G, Lin H, Jin Y, Wan B, Ai L and Wei J (2025) Long-term consumption of green tea protects the mental health of middle-aged and older adult men by improving inflammation levels. Front. Public Health. 13:1531953. doi: 10.3389/fpubh.2025.1531953
Received: 21 November 2024; Accepted: 07 February 2025;
Published: 25 February 2025.
Edited by:
Emilia Vassilopoulou, International Hellenic University, GreeceReviewed by:
Majid Hajifaraji, National Nutrition and Food Technology Research Institute, IranCopyright © 2025 Wan, Wan, Qin, Wang, Lin, Jin, Wan, Ai and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaohua Wang, d2doNjQwMkB3aHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.