- 1College of Physical Education and Health, Guangxi Normal University, Guilin, China
- 2Department of Physical Education, Hebei University of Environmental Engineering, Qinhuangdao, China
- 3Outdoor Sports Academy, Guilin Tourism University, Guilin, China
Objective: This study aims to assess the relationship between modifiable dementia risk factors and both dementia and cognitive decline.
Methods: Data were obtained from the Health and Retirement Study (HRS) [2008–2020], the China Health and Retirement Longitudinal Study (CHARLS) [2011–2020], and the English Longitudinal Study of Ageing (ELSA) [2010–2020]. After adjusting for confounding factors, multivariable logistic regression was utilized to analyze the relationship between modifiable dementia risk factors and dementia, while multivariable linear regression was employed to examine the relationship between these risk factors and cognitive decline. Additionally, the Cox proportional hazards model was used to assess the relationship between the number of risk factor events, clusters, and dementia risk.
Results: A total of 30,113 participants from HRS, CHARLS, and ELSA were included (44.6% male, mean age 66.04 years), with an average follow-up period of 7.29 years. A low education level was significantly associated with an increased risk of dementia and accelerated cognitive decline (Overall, OR = 2.93, 95% CI: 2.70–3.18; Overall, β = −0.25, 95% CI: −0.60 to-0.55). The presence of multiple dementia risk factors correlated with a higher dementia risk; Specifically, compared with more than 5 risk factor events, both having no dementia risk factors and having only one dementia risk factor were associated with a significantly lower risk of dementia (Overall, HR = 0.15, 95% CI: 0.11–0.22, HR = 0.22, 95% CI: 0.18–0.25). Compared to the group with no coexistence of risk factors, the clusters of excessive alcohol, diabetes, vision loss, and hearing loss (HR = 4.11; 95% CI = 3.42–4.95; p < 0.001); excessive alcohol, vision loss, smoking, and hearing loss (HR = 5.18; 95% CI = 4.30–6.23; p < 0.001); and excessive alcohol, obesity, diabetes, and smoking (HR = 5.96; 95% CI = 5.11–6.95; p < 0.001) were most strongly associated with dementia risk.
Conclusion: Among the 11 risk factors, educational attainment has the greatest impact on dementia risk and cognitive decline. A dose–response relationship exists between the number of modifiable risk factor events and dementia risk. The coexistence of multiple risk factors is associated with dementia risk, and these associations vary by risk factor cluster.
1 Introduction
Dementia represents a syndrome marked by swift cognitive deterioration, along with a decline in the capacity for daily living. From a clinical perspective, dementia can be categorized into early and late stages; during the initial phase, individuals might display only subtle cognitive decline, exerting negligible effects on daily activities (1). As the condition advances, individuals may undergo pronounced cognitive impairments and a loss of daily functioning, which greatly impacts both the patients and their caregivers, imposing a significant socioeconomic burden (2). With the rise in life expectancy and an expanding older adult demographic, the number of individuals affected by dementia has exceeded 57 million, significantly impacting social and economic frameworks, thereby establishing it as a prominent global health challenge (3). The principal manifestations of dementia include cognitive decline and functional impairment. The efficacy of pharmacological interventions for dementia remains restricted, necessitating considerable medical and caregiving resources. Therefore, the prevention and postponement of dementia’s onset have emerged as crucial focal points in contemporary research (4).
The Lancet Dementia Commission has recognized 14 modifiable risk factors for dementia through systematic reviews and meta-analyses of high-caliber studies, potentially linked to more than 45% of dementia cases (5). This discovery opens up promising avenues for interventions designed to prevent or delay dementia via targeted strategies (6). Certain modifiable risk factors may not exhibit a strong correlation with dementia yet show a significant relationship with cognitive decline, underscoring their potential role in dementia prevention (7, 8). A prospective cohort study utilizing the UK Biobank suggested that enhancing educational attainment during middle to late life could markedly diminish the risk of developing dementia in later years (9). Research conducted in South Korea revealed that women experiencing depression had a substantially elevated risk of dementia compared to their non-depressed counterparts (10).
Most prior research has primarily focused on the relationship between single modifiable risk factors and either dementia or cognitive abilities (11, 12). However, risk factors often coexist and mutually influence one another across different life stages, such as depression and social isolation. Social isolation may elevate the risk of depression, while early depression can exacerbate the likelihood of social isolation, illustrating a bidirectional relationship between these two factors (13–15). Limited research has explored the interplay between multiple coexisting modifiable risk factors and their effects on dementia and cognitive abilities across the lifespan. Consequently, this study aims to explore the association between modifiable dementia risk factors and both dementia and cognitive decline in individuals over the age of 50, while also examining the relationship between the number of these risk factor events, the coexistence of multiple risk factor events, and dementia risk.
2 Methods
2.1 Study design and population
HRS, CHARLS, and ELSA represent national prospective cohort studies that encompass older adults in the United States, China, and England, respectively. To guarantee comparability in dementia and cognitive function across the three cohorts while maintaining a consistent time frame, this study employed the 9th wave of the HRS survey (2008), the 1st wave of the CHARLS survey (2011), and the 4th wave of the ELSA survey (2010) as baseline data. The 15th wave of the HRS survey (2020), the 5th wave of the CHARLS survey (2020), and the 9th wave of the ELSA survey (2020) functioned as the final follow-up assessments. Ethical approval for HRS, CHARLS, and ELSA was secured from the institutional review boards of the University of Michigan, Peking University, and the London Multicentre Research Ethics Committee, respectively. All participants provided their written informed consent.
This study identified 11 out of the 14 modifiable risk factors (low education level, hearing loss, hypertension, smoking, obesity, depression, physical activity, diabetes, alcohol consumption, social isolation, and vision loss) as independent variables by analyzing the matching levels of modifiable dementia risk factors across the three cohorts. The reasons for excluding the three risk factors from this study include: data on traumatic brain injury (TBI) was unavailable in ELSA; air pollution data could not be acquired for all three countries; and high LDL cholesterol data exhibited excessive missing values from the baseline sampling in CHARLS.
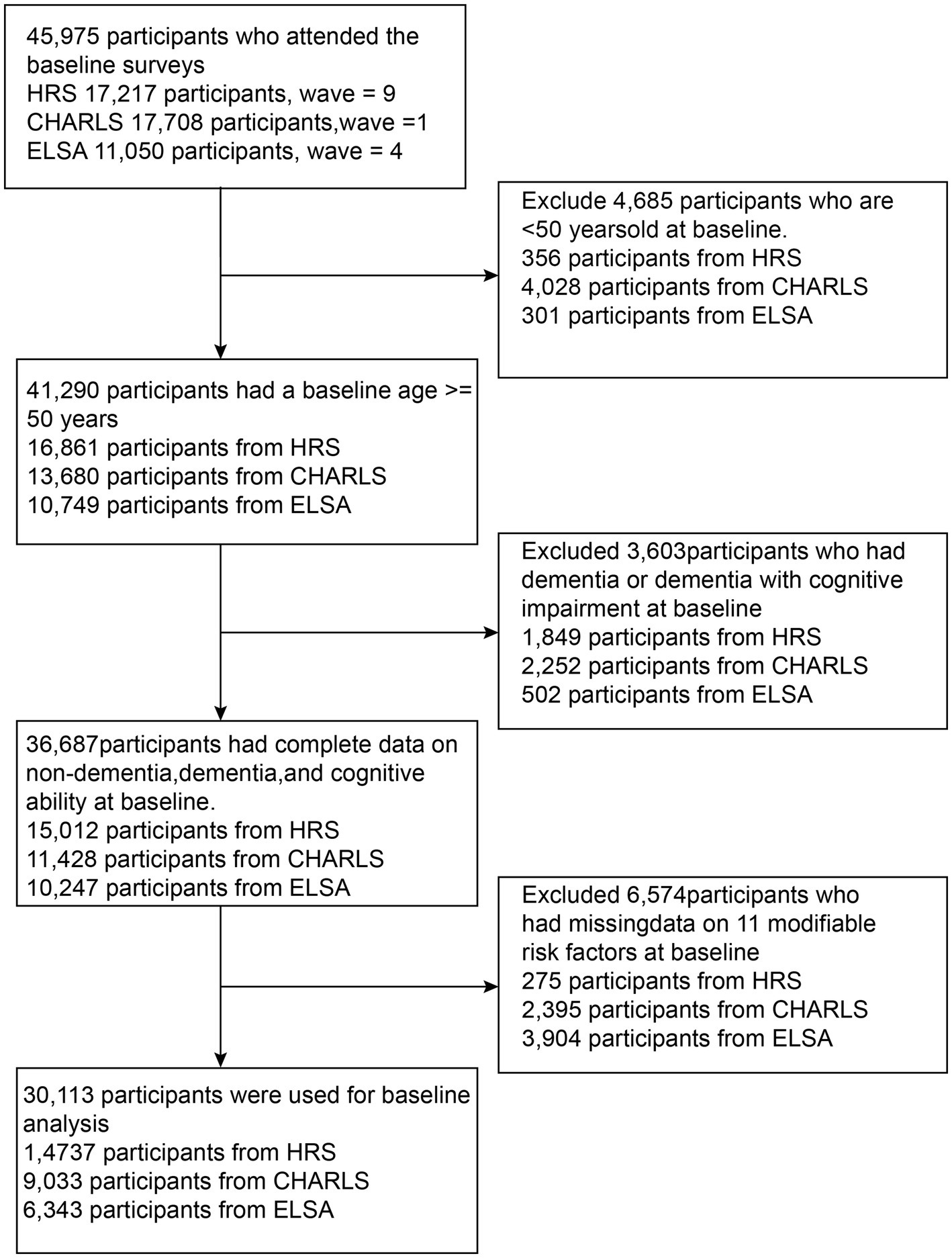
Figure 1 shows the selection process of the study population. The HRS sample included 17,217 participants recruited at baseline, with 2,480 excluded (356 under 50 years, 1,849 with dementia or unable to provide cognitive and dementia status, and 275 without baseline modifiable dementia risk factors). The CHARLS sample included 17,708 participants recruited at baseline, with 8,675 excluded (4,028 under 50 years, 2,252 with dementia, and 2,395 without baseline modifiable dementia risk factors). The ELSA sample consisted of 11,050 participants, with 4,646 excluded (301 under 50 years, 502 with dementia, and 3,904 without baseline modifiable dementia risk factors). Ultimately, a total of 30,113 participants (14,737 from HRS, 9,033 from CHARLS, and 6,343 from ELSA) were included in the study.
2.2 Modifiable dementia risk factors
The risk factors delineated in this study are as follows: Education level is categorized according to the International Standard Classification of Education (ISCE 2011), with individuals completing junior high school or below classified as having a low educational attainment (16, 17). Hearing loss is characterized by either the use of hearing aids or self-reported poor auditory ability (18, 19). In alignment with prior studies, hypertension is defined as having a systolic/diastolic blood pressure of ≥140/90 mmHg or self-reported hypertension (20). Smoking is determined by self-reported current smoking status. Obesity is defined as a body mass index (BMI) of ≥30 kg/m2.
Depression is evaluated using various scales: the CESD-10 questionnaire for CHARLS and the CESD-8 questionnaire for HRS and ELSA, with depression operationally defined as a CESD-10 score of ≥10 in CHARLS and a CESD-8 score of ≥3 in HRS and ELSA (21, 22). Physical inactivity is defined as engaging in vigorous or moderate activities for fewer than three days per week, with each session lasting at least 10 min (23). Diabetes is characterized by blood tests indicating a fasting blood glucose level of ≥126 mg/dL or HbA1c of ≥6.5%, or by self-reported physician-diagnosed diabetes (24). Excessive alcohol consumption is defined as self-reported drinking frequency exceeding half the week, encompassing consumption 4–6 days per week, daily, twice a day, or more than twice daily (25).
To evaluate social isolation, participants were assigned a social isolation score based on the following criteria: (i) unmarried, (ii) living alone, (iii) contact with children less than once a week, (iv) contact with parents, relatives, or friends less than once a week, and (v) no participation in any groups, clubs, or organizations in the past month (HRS, CHARLS) or past year (ELSA). The scoring ranges from 0 to 5, with higher scores indicating increased levels of social isolation. Based on prior studies, a score of ≥2 is designated as indicating social isolation (26, 27). To evaluate vision loss, in CHARLS, it is defined as a self-reported poor score in nearsightedness or farsightedness, whereas in HRS and ELSA, it is characterized as self-reported vision described as “blind” or “poor” (28–30). We defined the number of modifiable dementia risk factor events as a continuous variable ranging from 0 to 11 and as a categorical variable with the following categories: 0, 1, 2, 3, 4, 5, and > 5. The definition of multiple risk factor coexistence is the presence of at least 2 out of 11 conditions. Participants with 0 or 1 condition are defined as having no coexistence and serve as the reference group in the analysis (31).
2.3 Cognitive decline and dementia
In all three cohorts, cognitive function is predominantly assessed using tests that evaluate executive function, orientation, and memory. Elevated test scores signify enhanced cognitive abilities. The rate of cognitive decline is assessed by calculating z-scores. The z-score for each cognitive domain is computed by subtracting the baseline mean score and dividing by the baseline standard deviation (SD). The overall cognitive z-score is obtained by averaging the z-scores of the three cognitive test domains, followed by standardization through subtracting the baseline mean and dividing by the baseline SD. A global cognitive z-score of-1 indicates that the score is 1 SD lower than the mean cognitive score at baseline (32, 33). This study focuses exclusively on the rate of overall cognitive decline among participants.
In HRS, cognitive function scores were utilized to evaluate dementia, encompassing both memory and executive function domains, with a score range from 0 to 27. A score of ≤6 is deemed indicative of dementia (34). In ELSA and CHARLS, dementia is defined as the coexistence of cognitive impairment (involving two or more impaired cognitive function domains) and functional impairment (35–38). Cognitive impairment is characterized by scores in two or more cognitive function domains that are equal to or fall below 1.5 standard deviations beneath the mean, standardized for a population aged 50–80 with comparable education levels, akin to the criteria for cognitive impairment without dementia (CIND) (35). Functional impairment is described as challenges in performing one or more activities of daily living, which include moving around the room, dressing, bathing, eating, getting in and out of bed, and using the toilet (37).
2.4 Covariates
The covariates considered in this study encompass age, gender, history of stroke, and heart disease. Age is determined by subtracting the birth year from the survey year, whereas stroke and heart disease are self-reported by the participants.
2.5 Statistical analysis
For descriptive statistics, continuous variables are presented as mean (SD), while categorical variables are represented as counts (percentages). Multiple imputation was utilized to address missing data for physical activity variables in the CHARLS cohort. Multivariable logistic regression was employed to assess the association between dementia (dependent variable) and modifiable dementia risk factors (independent variables). The Cox proportional hazards model was used to assess the relationship between the coexistence of modifiable dementia risk factors and dementia risk. Additionally, multivariable linear regression was applied to determine the relationship between modifiable risk factors and cognitive decline. We utilized Population Attributable Fractions (PAF) to quantify the contribution of controlling individual risk factors to reducing the burden of dementia.
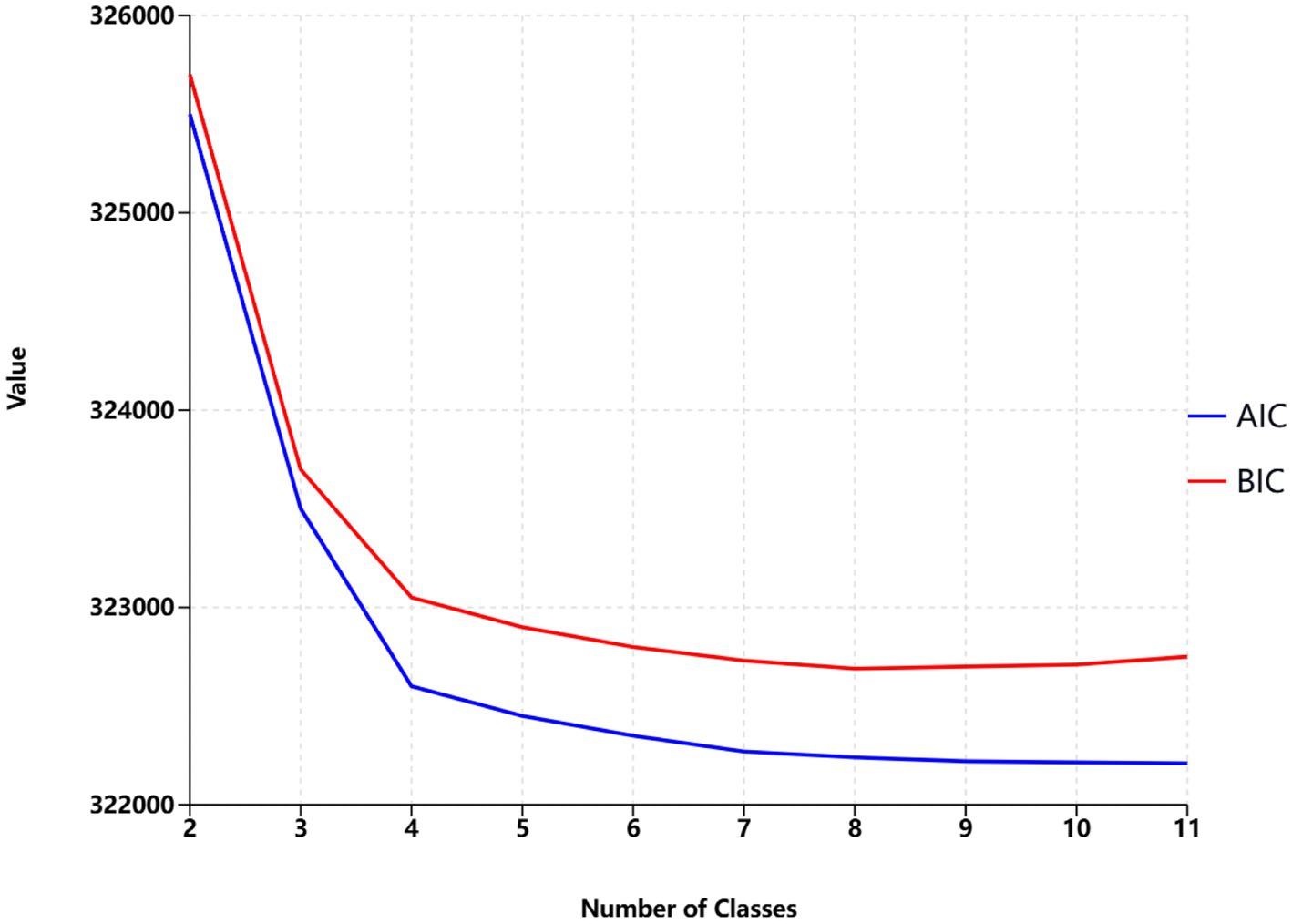
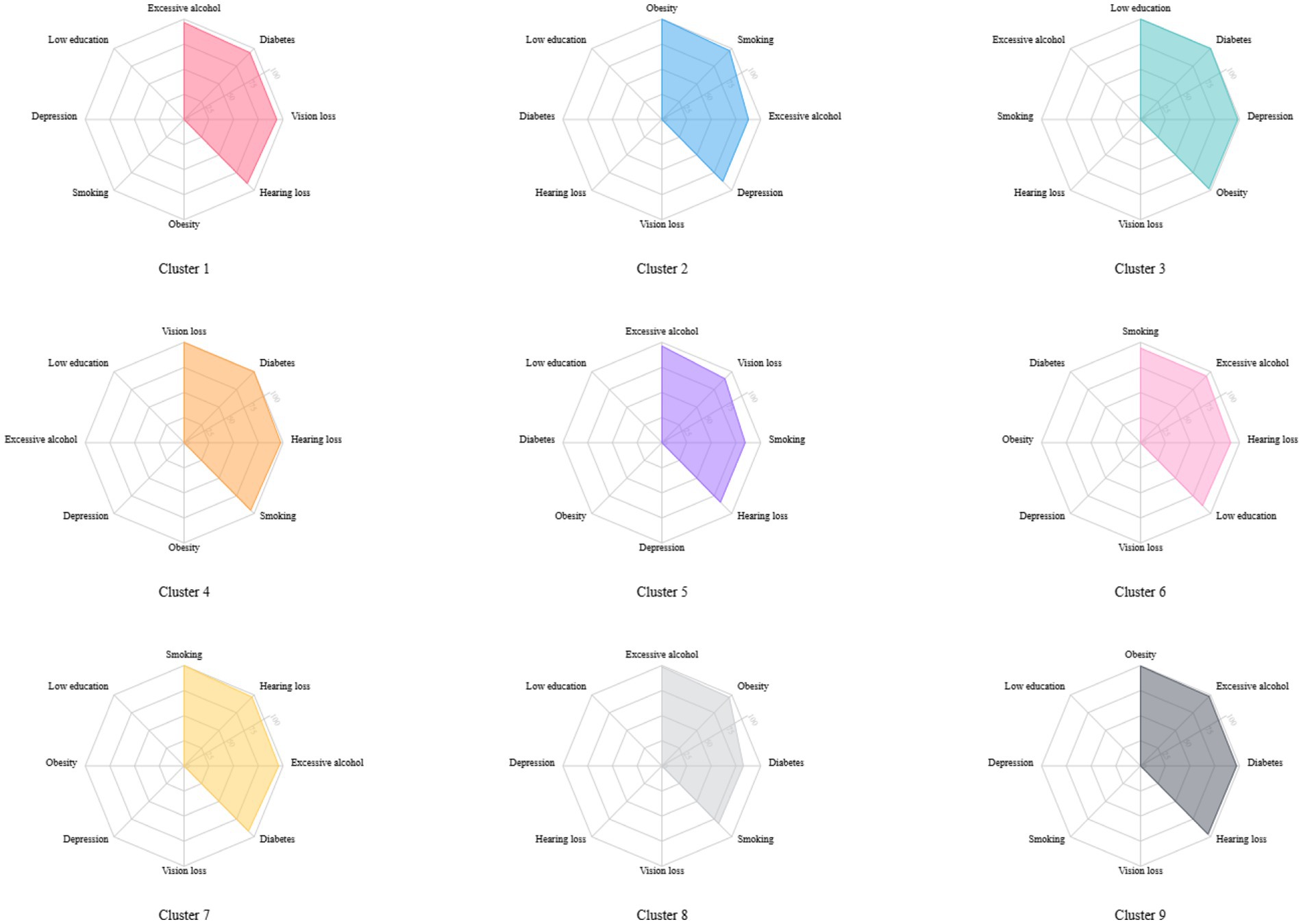
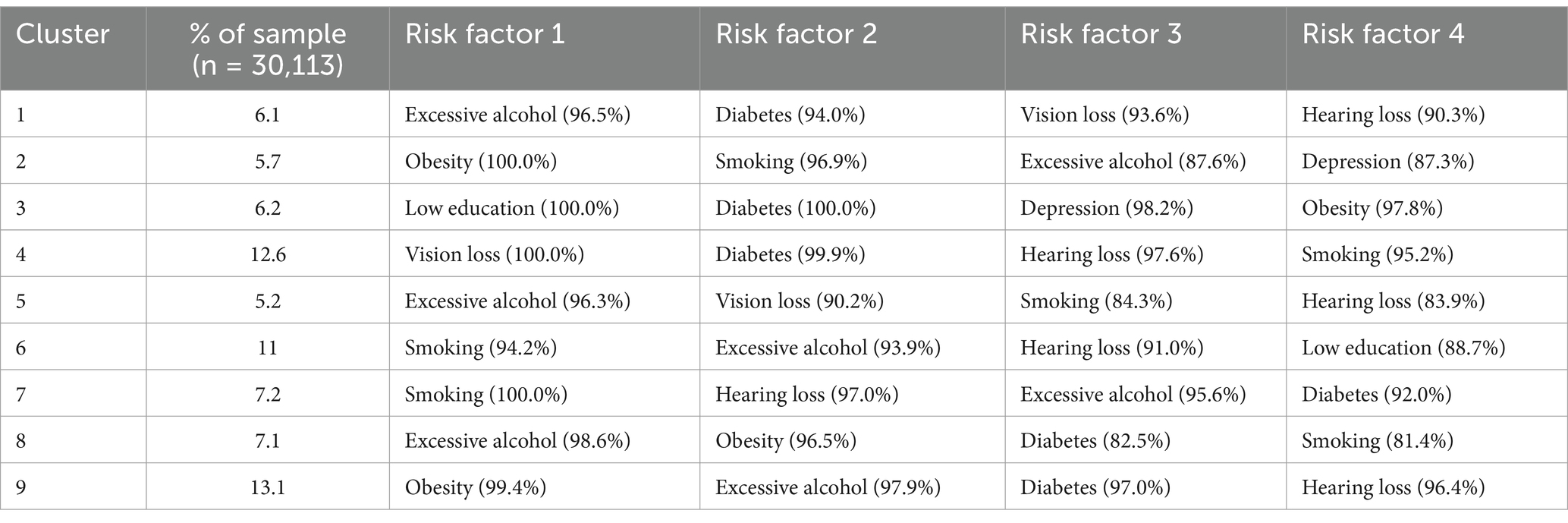
Latent class analysis was used to identify clusters of risk factors, assigning each participant with multiple concurrent risk factors to a non-overlapping cluster while allowing health conditions to contribute to multiple clusters with different probabilities. A random training sample of participants with multiple risk factors was used to determine the optimal number of clusters, followed by an estimation of the association between dementia risk factor clusters and dementia risk. Statistical metrics were generated for multiple latent class analysis models of clustering solutions, with the optimal number of clusters determined by the Bayesian Information Criterion adjusted for sample size and by restricting the minimum cluster size to more than 5% of the sample. The optimal number of clusters was determined to be 4 (see Figure 2), A total of nine clusters were included in this study (see Figure 3). To validate the reasonableness of selecting the 4-cluster model as the best-fitting solution, we calculated the posterior probability distribution based on BIC values using the formula: The posterior probabilities for k = 1, k = 2, k = 3, k = 4, k = 5, k = 6 were calculated as 0, 0.016, 0.313, 0.329, and 0.342, respectively. The results show that the 4-cluster model exhibits a relatively high posterior probability (0.313). While the posterior probabilities for the 5-and 6-cluster models were slightly higher, the differences were not significant. Considering model simplicity and interpretability, the selection of the 4-cluster model as the optimal solution is reasonable. Each cluster was characterized by the four most influential risk factors specific to that cluster (see Table 1). In addition, we conducted PAF analyses for each cluster to quantify the potential benefits of interventions.
All statistical analyses were two-sided, with p < 0.05 considered statistically significant. Analyses were conducted using Stata version 17 (Stata Corp, College Station, Texas) and R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline characteristics of the study participants
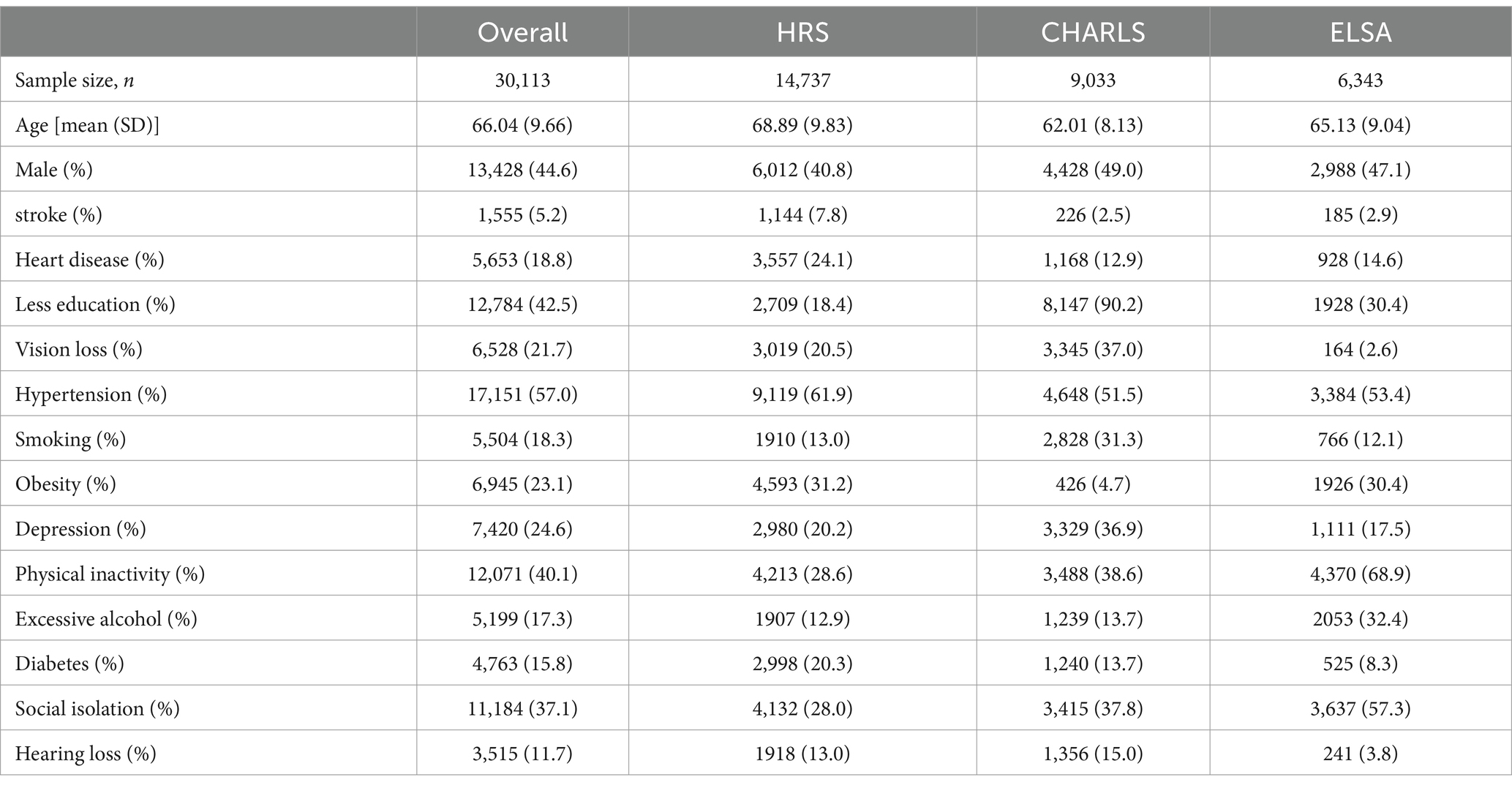
Table 2 presents the baseline characteristics of participants from HRS, CHARLS, ELSA, and the overall cohort. Overall, participants included in the study had a mean age of 66.04 years, with 44.6% being male. The prevalence of low education (42.5%), physical inactivity (40.1%), hypertension (57.0%), and social isolation (37.1%) was notably high. In HRS, there were 14,737 participants (mean age 68.89 years, 40.8% male); in CHARLS, there were 9,033 participants (mean age 62.01 years, 49.0% male); and in ELSA, there were 6,343 participants (mean age 65.13 years, 47.1% male). Among the three prospective cohorts, HRS participants had the highest prevalence of hypertension (61.9%), while CHARLS participants reported the highest rates of low education (90.2%), depression (36.9%), and vision loss (37.0%). The obesity rate was lowest in CHARLS (4.7%), whereas ELSA participants exhibited high rates of physical inactivity (68.9%), excessive alcohol consumption (32.4%), and social isolation (57.3%). Overall, among 27,560 follow-up participants, 3,400 cases of dementia were reported over an average follow-up period of 7.29 years. During an average follow-up of 6.84 years in HRS, 1,293 cases of dementia occurred among 13,213 participants. In CHARLS, during an average follow-up of 7.38 years, 1,438 cases of dementia were reported among 8,427 participants. In ELSA, during an average follow-up of 8.17 years, 669 cases of dementia occurred among 5,920 participants.
3.2 Association between modifiable dementia risk factors and dementia
Multivariable logistic regression analysis was conducted to compare the direction and magnitude of associations between modifiable dementia risk factors and dementia among the overall cohort, as well as participants from HRS, CHARLS, and ELSA. Low education level, depression, and hearing loss were significantly associated with an increased risk of dementia. In the overall cohort, hypertension was found to promote the risk of dementia, although the statistical significance was weak (OR = 1.07; 95% CI = 0.99–1.16; p = 0.084), with similar results in HRS and ELSA. In contrast, CHARLS exhibited a stronger association (OR = 1.14; 95% CI = 1.00–1.28; p = 0.043). Smoking also increased the risk of dementia in the overall cohort, but the statistical significance was weak (OR = 1.06; 95% CI = 0.96–1.17; p = 0.252). However, stronger associations were observed in HRS, CHARLS, and ELSA (OR = 1.28; 95% CI = 1.05–1.54; p = 0.013; OR = 1.25; 95% CI = 1.05–1.47; p = 0.01; OR = 1.81; 95% CI = 1.44–2.26; p < 0.001). Notably, a lack of physical activity was linked to an increased risk of dementia across the overall cohort, although the statistical significance was weak (OR = 1.06; 95% CI = 0.98–1.15; p = 0.136). The results for HRS and CHARLS were consistent; however, a stronger association was observed in ELSA (OR = 1.83; 95% CI = 1.47–2.29; p < 0.001). Vision loss was also associated with an increased risk of dementia in the overall cohort, although the statistical significance was weak (OR = 1.07; 95% CI = 0.98–1.17; p = 0.141). The results were consistent with HRS; however, CHARLS and ELSA showed stronger associations (OR = 1.26; 95% CI = 1.11–1.43; p < 0.001; OR = 1.59; 95% CI = 1.04–2.38; p = 0.028). Additionally, obesity and diabetes were significantly associated with an increased risk of dementia in the overall cohort (see Table 3). In addition, we performed PAF analyses on the aggregated cohort from three countries to assess the potential benefits of controlling various risk factors. Among these, low educational attainment (PAF = 45.1%), depression (PAF = 17.8%), social isolation (PAF = 6.3%), and hearing loss (PAF = 5.9%) were identified as the four risk factors with the highest potential contributions to the burden of dementia. Association between modifiable dementia risk factors and cognitive decline.
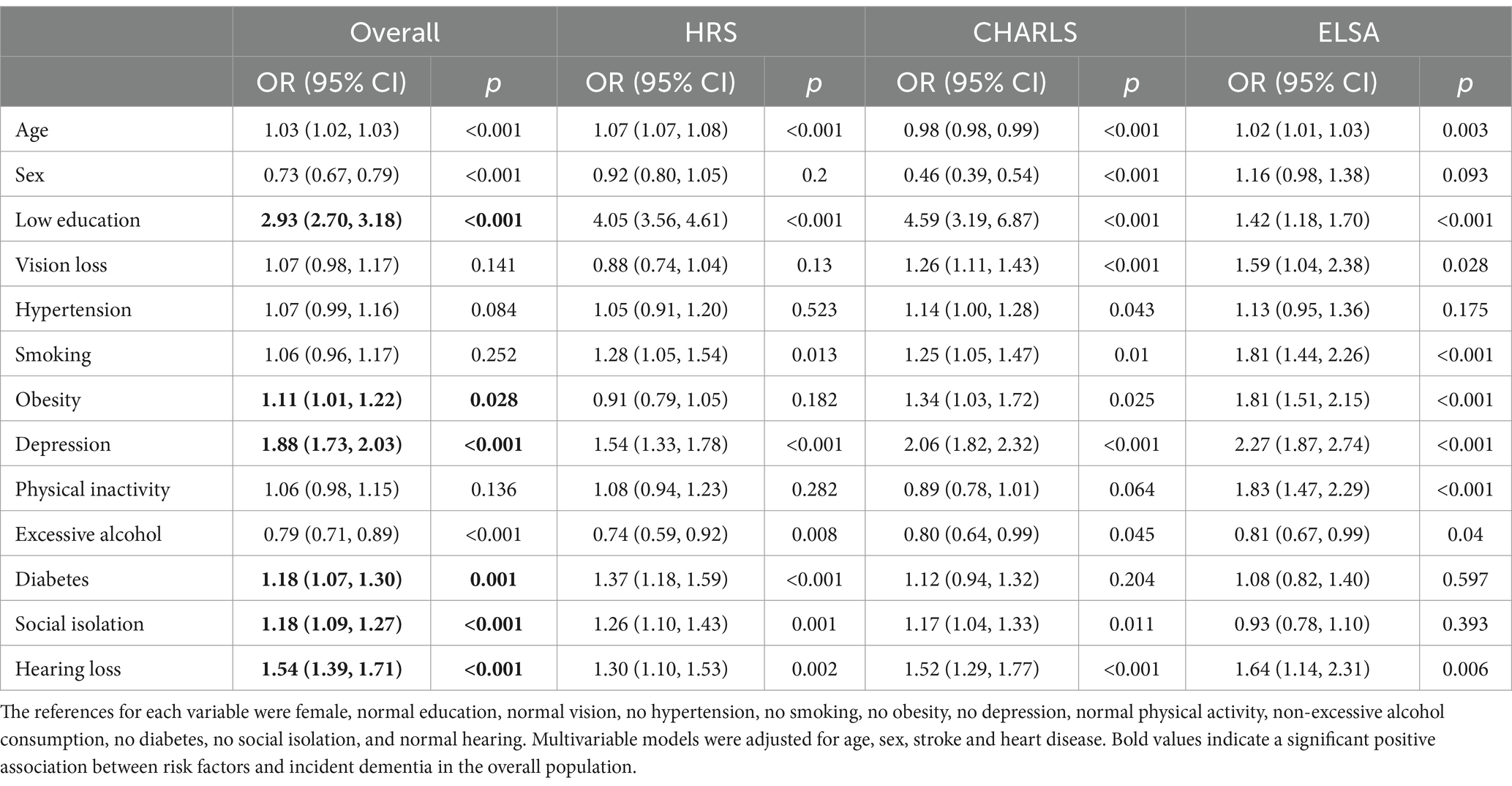
Table 3. Association between modifiable risk factors and dementia: Multinomial logistic regression analysis.
In the overall cohort, as well as in HRS, CHARLS, and ELSA, low education level, smoking, depression, diabetes, vision loss, and hearing loss were associated with accelerated cognitive decline. In HRS and ELSA, a lack of physical activity was related to accelerated cognitive decline, whereas in the overall cohort and CHARLS, a lack of physical activity was associated with a slowdown in cognitive decline. In the overall cohort, obesity was found to promote a slowdown in cognitive decline, although the statistical significance was weak (β = 0.01; 95% CI = 0.00–0.05; p = 0.1). Hypertension was significantly associated with accelerated cognitive decline, and excessive alcohol consumption was linked to a slowdown in cognitive decline. Additionally, social isolation was also associated with accelerated cognitive decline (see Table 4).
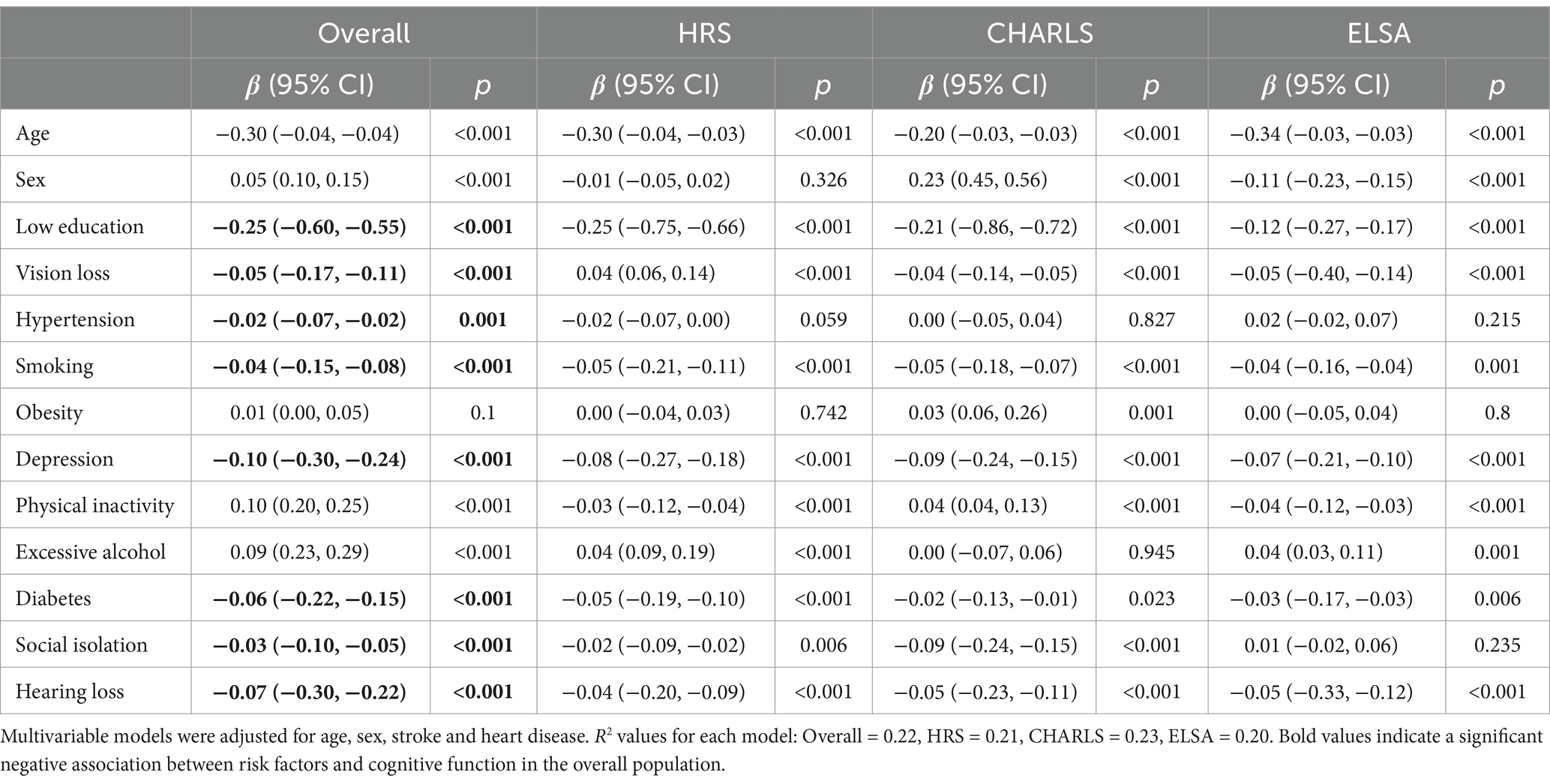
Table 4. Relationship between modifiable risk factors and cognitive decline: Linear regression with multiple variables analysis.
3.3 Correlation between the number of modifiable dementia risk factors and dementia risk
Multiple potentially modifiable risk factors for dementia frequently coexist in the older adult population. On average, participants in the entire cohort had 3.35 risk factors, while HRS participants had 2.61 risk factors, CHARLS participants had 4.67 risk factors, and ELSA participants had 3.14 risk factors. When the number of modifiable risk factors for dementia was considered a continuous variable, for each additional risk factor in the entire cohort, the risk of dementia increased by 39% (HR = 1.32; 95% CI = 1.30–1.35; p < 0.001). In the HRS, each additional risk factor was associated with a 39% increased risk of dementia (HR = 1.39; 95% CI = 1.34–1.43; p < 0.001). In CALLS, each additional risk factor was associated with a 25% increased risk of dementia (HR = 1.25; 95% CI = 1.21–1.29; p < 0.001). In ELSA, each additional risk factor corresponded to a 51% increased risk of dementia (HR = 1.51; 95% CI = 1.44–1.59; p < 0.001). When the number of modifiable risk factors for dementia was considered a categorical variable, there was a dose–response relationship with fewer risk factors being associated with a lower risk of dementia compared with more than five risk factors, both overall and in the three cohorts separately (see Table 5).
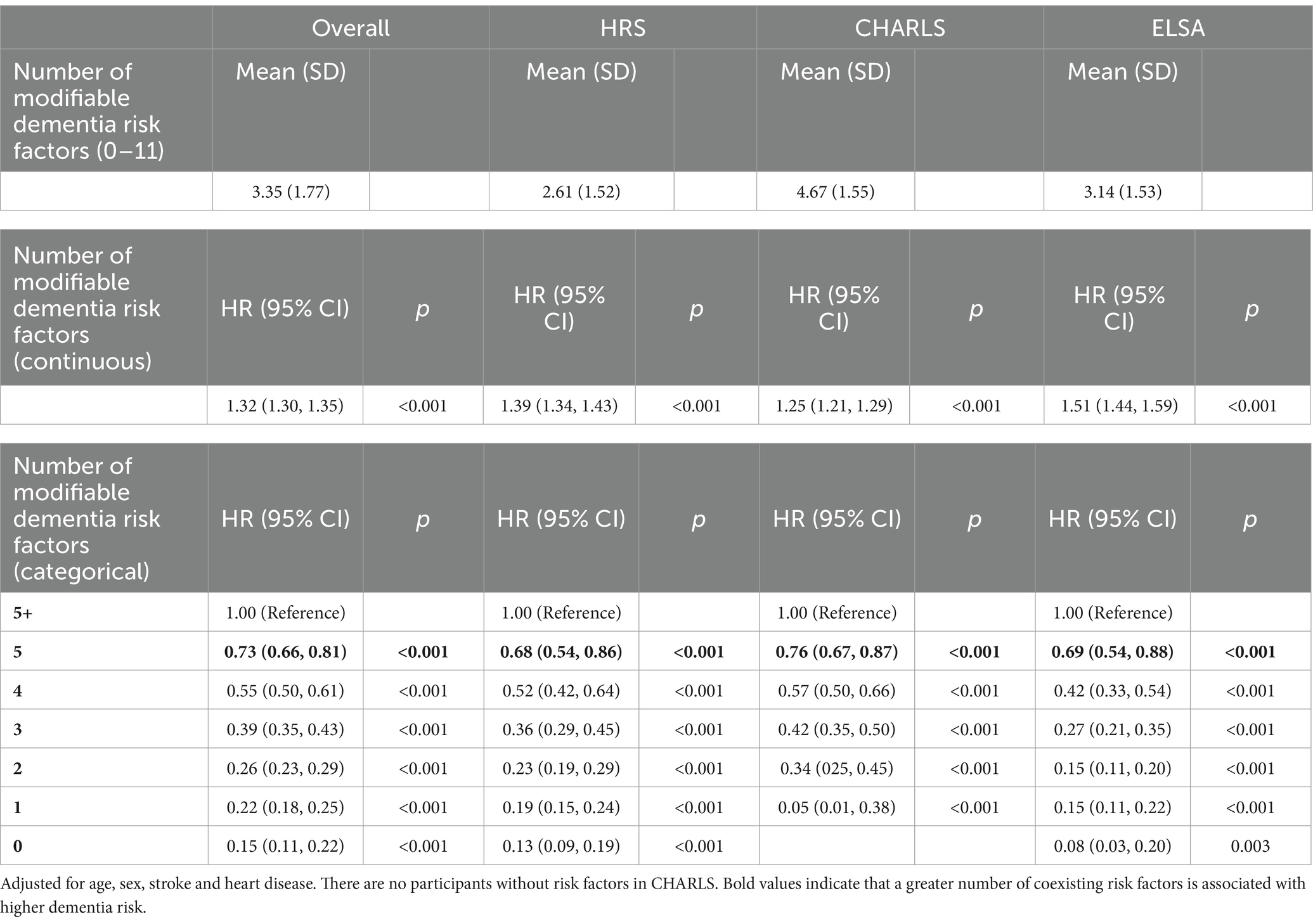
Table 5. Relationship between number of modifiable risk factors and dementia: Cox multivariate analysis.
3.4 Association of clusters of modifiable dementia risk factors with dementia risk
In all clusters, the four risk factors represent the optimal number of clusters, with the cluster formed by low education, diabetes, depression, and obesity showing no association with dementia risk (HR = 0.97; 95% CI = 0.76–1.24; p = 0.81). All other clusters were associated with an increased risk of dementia. Compared to the group with no coexistence of risk factors, the clusters of excessive alcohol, diabetes, vision loss, and hearing loss (HR = 4.11; 95% CI = 3.42–4.95; p < 0.001); excessive alcohol, vision loss, smoking, and hearing loss (HR = 5.18; 95% CI = 4.30–6.23; p < 0.001); and excessive alcohol, obesity, diabetes, and smoking (HR = 5.96; 95% CI = 5.11–6.95; p < 0.001) were most strongly associated with dementia risk (see Table 6). In addition, we conducted PAF analyses for each cluster within the overall cohort to assess the potential benefits of controlling risk factors in each cluster. The clusters with the highest potential contributions to the dementia disease burden were excessive alcohol, obesity, diabetes, and smoking (PAF = 58.6%), excessive alcohol, vision loss, smoking, and hearing loss (PAF = 44.6%), and excessive alcohol, diabetes, vision loss, and hearing loss (PAF = 43.1%).
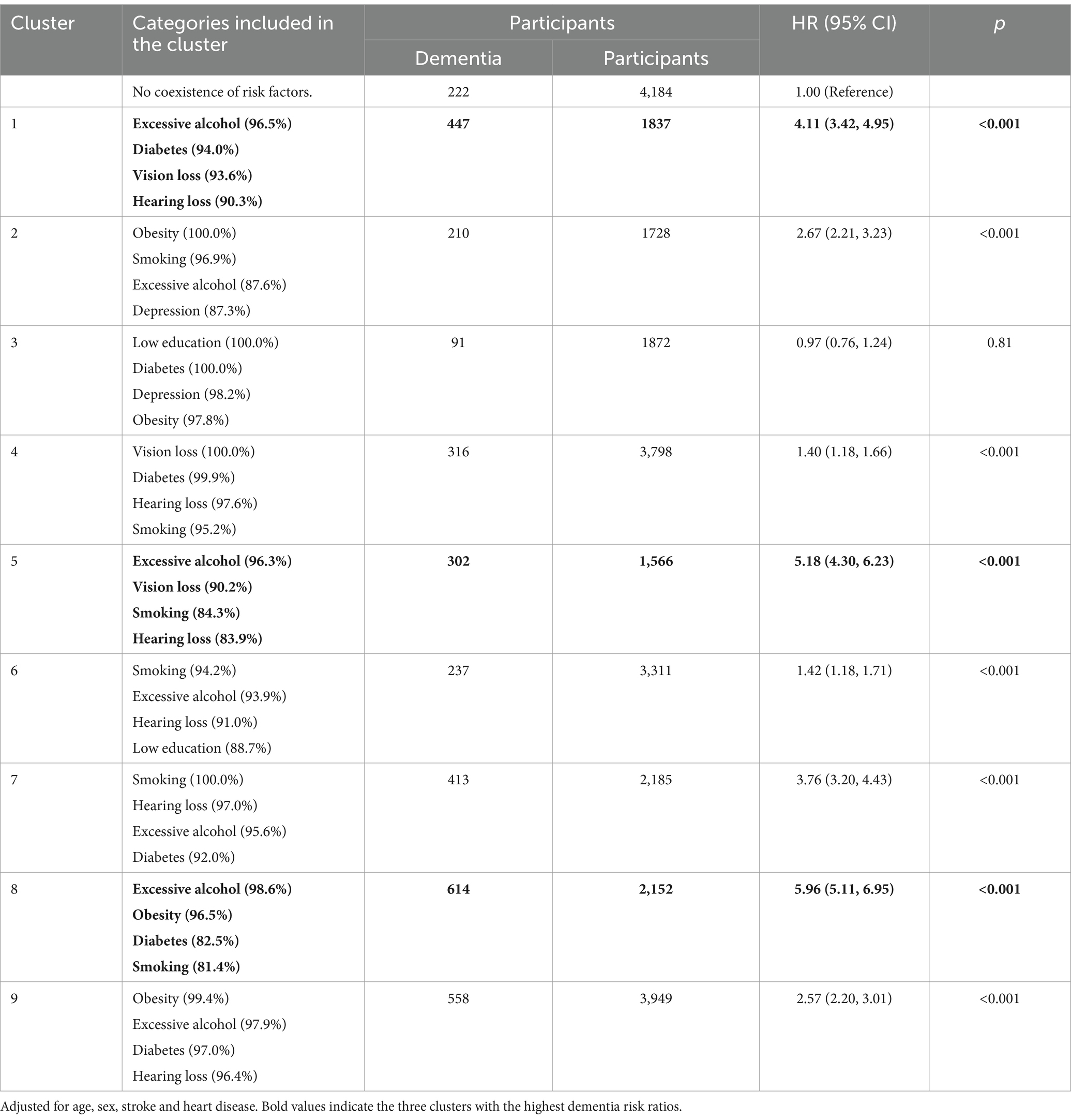
Table 6. The relationship between risk factor clusters and dementia risk: Cox proportional-hazards model.
4 Discussion
This study indicates that low educational attainment is the most significant factor contributing to an increased risk of dementia and accelerated cognitive decline, followed closely by depression and hearing loss. The risk of dementia shows a clear dose–response relationship with the number of modifiable risk factors, and the association between dementia risk and different clusters of risk factors varies considerably. This point is also validated by the PAF calculated for both individual risk factors and clustered risk factors.
Prior research has established a significant correlation between education level and dementia risk, with one study illustrating that higher education levels are associated with a 27% increase in survival time for individuals without dementia when compared to those with lower educational attainment (16). This may be associated with higher education levels facilitating greater neuronal connectivity and synaptic stimulation in the brain, thereby enhancing neural plasticity and increasing the network density of the cerebral cortex. Prolonged cognitive activities, such as learning and education, can increase the number of synaptic connections. The enhancement of synaptic density is closely linked to elevated levels of brain-derived neurotrophic factor (BDNF), a critical molecule that supports neuronal survival, synaptogenesis, and plasticity (39, 40). A study conducted in China revealed that the incidence and prevalence of dementia markedly increased among populations with lower education levels (41). Furthermore, a study involving Asian Americans reported that individuals with higher education levels are linked to a reduced risk of dementia (42). In our study, a greater proportion of participants in the CHARLS cohort exhibited lower education levels and a correspondingly higher incidence of dementia. This relationship is linked to the rising life expectancy of older adults in China, coupled with the lower educational attainment experienced by participants earlier in their life course (43). In developing nations, education represents one of the most critical modifiable risk factors, making it imperative to address educational attainment (44, 45).
Hearing loss has been identified as being associated with dementia and cognitive decline across all participant cohorts. Prior meta-analyses have documented a significant association between hearing loss and subsequent dementia (46–48). The Health ABC study revealed that older adults experiencing hearing loss faced a 55% increased risk of developing dementia (49). The Baltimore Longitudinal Study of Aging identified a significant correlation between the severity of baseline hearing loss and the risk of dementia (50). Hearing loss can lead to loneliness, depression, and social isolation, which interact with brain pathology. Hearing impairment may result in irreversible damage, subsequently disrupting cortical activity in the brain and potentially triggering the onset of dementia (51).
Depression is associated with dementia and cognitive decline in middle-aged and older adults, aligning with previous research that indicates a relationship between depression and both dementia and cognitive function. A cohort study in Denmark found that depression in middle or late life is significantly associated with increased dementia risk Elser (52). A cohort study in the UK reported a 51% increase in dementia risk among individuals aged 50–70 with baseline depression (53). Firstly, both depression and dementia are classified as mental health disorders and may share common pathological features. Secondly, depression can increase cortisol secretion, leading to hippocampal atrophy or triggering inflammatory responses (54). Therefore, treatment for depression may help slow down or prevent the onset of dementia (55).
In summary, education level, depression, and hearing loss are significant risk factors influencing dementia and cognitive ability. Therefore, it is essential to enhance educational opportunities early in life, promote cognitive stimulation throughout the life course to maintain a high level of cognitive reserve, and focus on mental health and living conditions to delay or prevent the onset of dementia.
Hypertension is likely to cause cortical white matter lesions and microvascular damage in the brain, which can subsequently lead to cognitive decline and the onset of dementia (56). Hypertension appeared to elevate the risk of dementia among participants; however, the statistical significance was weak in the overall cohort as well as in the HRS and ELSA studies. In high-income countries such as the United States and the United Kingdom, elevated levels of socioeconomic development may contribute to increased blood pressure in middle and late life, which could partially explain the weak associations observed in these cohorts (5).
Smoking is closely associated with the biological mechanisms of dementia, primarily through inducing oxidative stress and chronic inflammatory responses, which lead to neuronal damage and pathological changes in the brain, thereby increasing the risk of dementia. Additionally, smoking damages cerebrovascular health, resulting in insufficient blood supply to the brain and ischemic injuries. Over time, this can cause brain atrophy, further exacerbating cognitive decline. These changes are strongly linked to dementia types such as Alzheimer’s disease (57). Prior studies have demonstrated a significant correlation between midlife or current smoking and an elevated risk of dementia (58–60). In our study, smoking was linked to an increased risk of dementia and cognitive decline among participants in the overall cohort, HRS, CHARLS, and ELSA; however, the statistical significance was less pronounced in the overall cohort. This discrepancy may stem from the diversity of socioeconomic backgrounds, ethnicities, and lifestyles among participants, which could mask the true impact of smoking on dementia risk and result in attenuated statistical correlations. Furthermore, the method of assessing smoking—such as self-reporting—may introduce bias, potentially leading to an underestimation of the true impact of smoking behavior on dementia risk.
In this study, obesity was found to correlate with an elevated risk of dementia, supporting previous research that connects midlife and late-life obesity with a heightened risk of dementia (12). Obesity is associated with increased cortisol levels, inflammation, and negative health outcomes, which may contribute to the development of dementia (54). Nonetheless, some studies suggest that obesity in late life may be linked to a decreased risk of dementia (61). Our findings revealed that obesity was connected to a deceleration in cognitive decline across the overall cohort, including HRS and ELSA; in CHARLS, obesity similarly correlated with a slowdown in cognitive decline. This phenomenon may stem from the association between obesity and cognitive reserve, along with elevated levels of neurotrophic factors (62). Furthermore, the prevalence of obesity among older adults in China remains relatively low, at just 4.7%. In conclusion, while obesity is a modifiable risk factor for dementia, its impacts on dementia risk and cognitive abilities necessitate further exploration within specific national and social contexts.
Prior high-quality studies involving long-term follow-up research have demonstrated that physical activity can mitigate the risk of developing dementia. High levels of physical activity contribute to cognitive function, potentially by altering cerebral blood flow and increasing brain-derived neurotrophic factor (BDNF), ultimately enhancing brain plasticity and reducing neuroinflammation (63). A meta-analysis indicated that greater levels of physical activity correlate with lower incidence rates of dementia, implying that the beneficial impact of physical activity on dementia may necessitate both increased intensity and extended duration to manifest significant effects (64, 65). In this study, we observed that a deficiency in physical activity may elevate the risk of dementia; however, the statistical significance was weak within the overall cohort and HRS, while it was more pronounced in ELSA. Conversely, in CHARLS, a deficiency in physical activity correlated with a reduced risk of dementia, although this association was similarly weak. This discrepancy may stem from the diverse socioeconomic and cultural backgrounds of participants across the three cohorts, which could affect physical activity levels and health outcomes (66). Furthermore, the relatively brief follow-up duration in this study may also account for the observed weak statistical significance.
Excessive alcohol consumption induces oxidative stress and neuroinflammation, leading to neuronal damage and thereby increasing the risk of dementia. Furthermore, chronic heavy drinking can cause brain atrophy and disturbances in the neurotransmitter system, further exacerbating cognitive decline and increasing the likelihood of developing dementia (67). Previous cohort studies have indicated that heavy drinking correlates with an 8% increase in the risk of dementia (68). Conversely, our study revealed a negative correlation between excessive alcohol consumption and both dementia risk and cognitive decline. This finding contrasts with previous research. One potential explanation for this discrepancy is our definition of excessive drinking, which encompasses consumption more than half the week, including drinking 4–6 days per week, daily, or more than twice a day; all these data were self-reported and may introduce selection bias. Moreover, it is conceivable that participants consumed alcohol prior to the survey but ceased by the time of data collection, or they may not have been drinking during the survey period but resumed during follow-up. This could elucidate the observed negative correlation between excessive drinking and dementia risk and cognitive decline, necessitating further investigation.
This study additionally revealed that diabetes and social isolation are linked to an elevated overall risk of dementia and accelerated cognitive decline. Prior research has demonstrated a significant correlation between the age of onset of type 2 diabetes and the risk of developing dementia (69). Diabetes can lead to metabolic changes in the brain, resulting in increased β-amyloid toxicity, tau protein hyperphosphorylation, and neuroinflammation, all of which contribute to an increased risk of dementia (69). Furthermore, the World Health Organization recognizes diabetes as one of the detrimental factors affecting dementia across the life course (70). Previous meta-analyses indicate that regular social contact can mitigate the risk of dementia in older adults, while those with higher levels of social isolation encounter a greater risk (71, 72). Regular and extensive social interactions can foster cognitive reserve, alleviate loneliness, and encourage healthy behaviors, thereby decelerating cognitive decline and decreasing the incidence of dementia (73).
Vision loss may lead to reduced sensory input, increased cognitive load, and consequently affect brain structure and function (74). In this study, vision loss was significantly linked to accelerated cognitive decline. Prior research has similarly indicated significant correlations between vision loss, dementia, and cognitive abilities (75, 76). In our study, vision loss was found to elevate the risk of dementia among participants; however, the statistical significance was weak. This may be attributed to survival bias or limitations in the definitions and baseline assessments of modifiable risk factors, which might not adequately capture changes over the life course.
In conclusion, our findings suggest that, with the exception of excessive drinking, the impacts of the other ten risk factors on dementia and cognitive decline among all participants remain consistent.
Beyond evaluating individual modifiable risk factors, this study also explored the relationship between the quantity of modifiable risk factors and dementia risk among all participants. Older adults in China displayed the highest prevalence of coexisting risk factors, potentially linked to the limited medical and caregiving resources available to the aging population, as well as the suboptimal living conditions encountered earlier in their life course (77). Our findings demonstrate that the risk of dementia significantly escalates with the number of risk factor events, a result that aligns with observations from a comparable meta-analysis (78). Various risk factors may interact through biological pathways, intensifying neurodegenerative changes. For instance, biological processes like oxidative stress and neuronal damage may be exacerbated by the presence of coexisting risk factors, ultimately contributing to an elevated risk of dementia (79, 80).
A dose–response relationship was observed between modifiable dementia risk factors and dementia risk. Various clusters of risk factors have different associations with dementia risk; the clusters of excessive alcohol, diabetes, vision loss, and hearing loss; excessive alcohol, vision loss, smoking, and hearing loss; and excessive alcohol, obesity, diabetes, and smoking were associated with three times or more the risk of dementia. The clustering of these risk factors may reflect shared pathophysiological mechanisms, including the synergistic effects of vascular damage, chronic inflammation, and metabolic disorders. Specifically, the clusters of excessive alcohol, diabetes, vision loss, and hearing loss, as well as excessive alcohol, vision loss, smoking, and hearing loss, may collectively induce neuroinflammation and oxidative stress responses. These processes can accelerate neurodegenerative changes and increase the risk of dementia. Additionally, these clusters contribute to elevated stress and cognitive load, rendering individuals more vulnerable to dementia (57, 67, 81, 82). For the cluster comprising excessive alcohol, obesity, diabetes, and smoking, these risk factors are likely to jointly trigger systemic inflammation and oxidative stress, further impairing brain health. These processes not only accelerate neurodegenerative changes, increasing dementia risk, but also cause vascular damage, thereby disrupting cerebral blood flow and elevating the risk of vascular dementia (54, 57, 67, 69). Notably, excessive alcohol consumption appears consistently in all high-risk combinations, suggesting that alcohol may play a pivotal role in the interaction among multiple risk factors.
Our findings are consistent with a large cohort study in the UK, which found that multiple diseases are associated with an increased risk of dementia and that the coexistence of diseases poses a greater hazard for dementia (31). Our findings on the coexistence of multiple risk factors suggest that greater attention should be paid to the presence of multiple risk factors rather than focusing solely on individual ones. The different combinations of risk factors indicate the need for developing tailored prevention strategies targeting specific risk factor combinations. In this study, the cluster of low education, diabetes, depression, and obesity was not associated with dementia risk, possibly due to insufficient statistical power resulting from a limited sample size of dementia cases. However, when these factors coexist with other risk factors, they exhibit significant synergistic effects, reflecting a complex network of interactions among the risk factors.
The strengths of this study lie in its large sample size and the incorporation of three extensive, nationally representative cohorts, which facilitate long-term follow-up and comprehensive comparative analysis. Additionally, we integrated modifiable risk factors into the analysis of event frequency and coexistence associated with dementia risk. Nevertheless, our study has certain limitations. Although we adjusted for confounding variables, residual confounders—such as the APOE ε4 genotype and socioeconomic status—may persist. This study did not analyze the impact of changes in risk factors during follow-up on dementia and cognitive decline. Moreover, the majority of the modifiable dementia risk factors and covariates utilized in this study were self-reported, potentially introducing bias. Future research should strive to diversify and ensure more accurate measurement of risk factors to corroborate our findings.
5 Conclusion
In conclusion, modifiable risk factors for dementia are closely associated with an increased risk of dementia onset and accelerated cognitive decline. Multiple risk factors frequently coexist and interact, and dementia risk significantly escalates as the number of modifiable risk factors increases over a lifetime. Our findings emphasize the strengthening effect of coexisting modifiable risk factors on dementia onset, suggesting that more attention should be given to the coexistence of multiple risk factors. As the global number of dementia cases continues to rise, there is an urgent need for more effective strategies to address modifiable risk factors for dementia to prevent and mitigate its onset. Future research should focus on conducting prospective cohort studies targeting young and middle-aged populations to evaluate the combined effects of multifactorial interventions. These interventions may include promoting physical exercise, controlling alcohol consumption, smoking cessation, adopting a healthy lifestyle, and improving education levels. Furthermore, future studies should explore the relationships between risk factors and the mechanisms underlying dementia development. In clinical practice, it is recommended to incorporate multidimensional risk assessment into routine health check-ups and develop integrated intervention models through interdisciplinary collaboration. Early control or simultaneous intervention of multiple risk factors may prove to be a promising approach to reducing the incidence of dementia.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Health and Retirement Study (HRS), China Health and Retirement Longitudinal Study, English Longitudinal Study of Ageing. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MW: Formal analysis, Writing – original draft. CF: Writing – original draft, Formal analysis. YaH: Formal analysis, Writing – review & editing. YW: Formal analysis, Writing – review & editing. HC: Writing – original draft. WZ: Data curation, Writing – review & editing. XY: Methodology, Writing – review & editing. ZW: Methodology, Writing – review & editing. HW: Methodology, Writing – review & editing. YiH: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Social Science Foundation of China (Grant No. 21XTY018).
Acknowledgments
Thanks to all HRS, CHARLS and ELSA all participants and researchers for their support and contribution to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World alzheimer report 2024: global changes in attitudes to dementia. (2024). Available at: https://www.alzint.org/resource/world-alzheimer-report-2024/ (Accessed November 4, 2024).
2. 2024 Alzheimer's Association. Alzheimer’s disease facts and figures. Alzheimers Dement. (2024) 20:3708–821. doi: 10.1002/alz.13809
3. GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. (2022) 7:e105–25. doi: 10.1016/S2468-2667(21)00249-8
4. Abdul Manap, AS, Almadodi, R, Sultana, S, Sebastian, MG, Kavani, KS, Lyenouq, VE, et al. Alzheimer’s disease: a review on the current trends of the effective diagnosis and therapeutics. Front Aging Neurosci. (2024) 16:1429211. doi: 10.3389/fnagi.2024.1429211
5. Livingston, G, Huntley, J, Liu, KY, Costafreda, SG, Selbæk, G, Alladi, S, et al. Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet Lond Engl. (2024) 404:572–628. doi: 10.1016/S0140-6736(24)01296-0
6. Selbæk-Tungevåg, S, Selbæk, G, Strand, BH, Myrstad, C, Livingston, G, Lydersen, S, et al. Insomnia and risk of dementia in a large population-based study with 11-year follow-up: the HUNT study. J Sleep Res. (2023) 32:e13820. doi: 10.1111/jsr.13820
7. Lachner, C, Craver, EC, Babulal, GM, Lucas, JA, Ferman, TJ, White, RO, et al. Disparate dementia risk factors are associated with cognitive impairment and rates of decline in african americans. Ann Neurol. (2024) 95:518–29. doi: 10.1002/ana.26847
8. Xiong, LY, Wood Alexander, M, Wong, YY, Wu, C-Y, Ruthirakuhan, M, Edwards, JD, et al. Latent profiles of modifiable dementia risk factors in later midlife: relationships with incident dementia, cognition, and neuroimaging outcomes. Mol Psychiatry. (2024). doi: 10.1038/s41380-024-02685-4
9. Takeuchi, H, and Kawashima, R. Effects of adult education on cognitive function and risk of dementia in older adults: a longitudinal analysis. Front Aging Neurosci. (2023) 15:1212623. doi: 10.3389/fnagi.2023.1212623
10. Yoo, JE, Yoon, DH, Jin, EH, Han, K, Choi, S-Y, Choi, SH, et al. Association between depression and young-onset dementia in middle-aged women. Alzheimers Res Ther. (2024) 16:137. doi: 10.1186/s13195-024-01475-y
11. Chun, MY, Chae, W, Seo, SW, Jang, H, Yun, J, Na, DL, et al. Effects of risk factors on the development and mortality of early-and late-onset dementia: an 11-year longitudinal nationwide population-based cohort study in South Korea. Alzheimers Res Ther. (2024) 16:92. doi: 10.1186/s13195-024-01436-5
12. Hwang, PH, Ang, TFA, De Anda-Duran, I, Liu, X, Liu, Y, Gurnani, A, et al. Examination of potentially modifiable dementia risk factors across the adult life course: the Framingham heart study. Alzheimers Dement. (2023) 19:2975–83. doi: 10.1002/alz.12940
13. Domènech-Abella, J, Mundó, J, Haro, JM, and Rubio-Valera, M. Anxiety, depression, loneliness and social network in the elderly: longitudinal associations from the irish longitudinal study on ageing (TILDA). J Affect Disord. (2019) 246:82–8. doi: 10.1016/j.jad.2018.12.043
14. Luo, M. Social isolation, loneliness, and depressive symptoms: a twelve-year population study of temporal dynamics. J Gerontol Ser B. (2023) 78:280–90. doi: 10.1093/geronb/gbac174
15. Zhu, S, Kong, X, Han, F, Tian, H, Sun, S, Sun, Y, et al. Association between social isolation and depression: evidence from longitudinal and mendelian randomization analyses. J Affect Disord. (2024) 350:182–7. doi: 10.1016/j.jad.2024.01.106
16. Hyun, J, Hall, CB, Katz, MJ, Derby, CA, Lipnicki, DM, Crawford, JD, et al. Education, occupational complexity, and incident dementia: a COSMIC collaborative cohort study. J Alzheimers Dis JAD. (2022) 85:179–96. doi: 10.3233/JAD-210627
17. Makkar, SR, Lipnicki, DM, Crawford, JD, Kochan, NA, Castro-Costa, E, Lima-Costa, MF, et al. Education and the moderating roles of age, sex, ethnicity and apolipoprotein epsilon 4 on the risk of cognitive impairment. Arch Gerontol Geriatr. (2020) 91:104112. doi: 10.1016/j.archger.2020.104112
18. Ask, H, Krog, NH, and Tambs, K. Impact of hearing impairment on spousal mental health: the Nord-trøndelag health study. Eur J Pub Health. (2010) 20:271–5. doi: 10.1093/eurpub/ckp176
19. Du, Y, Luo, Y, Ren, Z, Gram, LZ, Zheng, X, and Liu, J. What impact does hearing impairment have on cognitive health in older married couples in China? Soc Sci Med. (2024) 352:116999. doi: 10.1016/j.socscimed.2024.116999
20. Ding, L, Zhu, X, Xiong, Z, Yang, F, and Zhang, X. The association of age at diagnosis of hypertension with cognitive decline: the China health and retirement longitudinal study (CHARLS). J Gen Intern Med. (2023) 38:1431–8. doi: 10.1007/s11606-022-07951-1
21. Wang, Y, Liu, M, Yang, F, Chen, H, Wang, Y, and Liu, J. The associations of socioeconomic status, social activities, and loneliness with depressive symptoms in adults aged 50 years and older across 24 countries: findings from five prospective cohort studies. Lancet Healthy Longev. (2024) 5:100618. doi: 10.1016/j.lanhl.2024.07.001
22. Yan, R, Liu, X, Xue, R, Duan, X, Li, L, He, X, et al. Association between internet exclusion and depressive symptoms among older adults: panel data analysis of five longitudinal cohort studies. EClinicalMedicine. (2024) 75:102767. doi: 10.1016/j.eclinm.2024.102767
23. Moreno-Agostino, D, Daskalopoulou, C, Wu, Y-T, Koukounari, A, Haro, JM, Tyrovolas, S, et al. The impact of physical activity on healthy ageing trajectories: evidence from eight cohort studies. Int J Behav Nutr Phys Act. (2020) 17:92. doi: 10.1186/s12966-020-00995-8
24. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44:S15–33. doi: 10.2337/dc21-S002
25. Ma, Y, Hua, R, Yang, Z, Zhong, B, Yan, L, and Xie, W. Different hypertension thresholds and cognitive decline: a pooled analysis of three ageing cohorts. BMC Med. (2021) 19:287. doi: 10.1186/s12916-021-02165-4
26. Li, L. Internet use and frailty in middle-aged and older adults: findings from developed and developing countries. Glob Health. (2024) 20:53. doi: 10.1186/s12992-024-01056-6
27. Steptoe, A, Shankar, A, Demakakos, P, and Wardle, J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci. (2013) 110:5797–801. doi: 10.1073/pnas.1219686110
28. de la Fuente, J, Hjelmborg, J, Wod, M, de la Torre-Luque, A, Caballero, FF, Christensen, K, et al. Longitudinal associations of sensory and cognitive functioning: a structural equation modeling approach. J Gerontol B Psychol Sci Soc Sci. (2019) 74:1308–16. doi: 10.1093/geronb/gby147
29. Ge, S, Pan, W, Wu, B, Plassman, BL, Dong, X, and McConnell, ES. Sensory impairment and cognitive decline among older adults: an analysis of mediation and moderation effects of loneliness. Front Neurosci. (2022) 16:1092297. doi: 10.3389/fnins.2022.1092297
30. Zhao, X, Zhou, Y, Wei, K, Bai, X, Zhang, J, Zhou, M, et al. Associations of sensory impairment and cognitive function in middle-aged and older Chinese population: the China health and retirement longitudinal study. J Glob Health. (2021) 11:08008. doi: 10.7189/jogh.11.08008
31. Calvin, CM, Conroy, MC, Moore, SF, Kuźma, E, and Littlejohns, TJ. Association of Multimorbidity, disease clusters, and modification by genetic factors with risk of dementia. JAMA Netw Open. (2022) 5:e2232124. doi: 10.1001/jamanetworkopen.2022.32124
32. Jin, Y, Liang, J, Hong, C, Liang, R, and Luo, Y. Cardiometabolic multimorbidity, lifestyle behaviours, and cognitive function: a multicohort study. Lancet Healthy Longev. (2023) 4:e265–73. doi: 10.1016/S2666-7568(23)00054-5
33. Wang, K, Fang, Y, Zheng, R, Zhao, X, Wang, S, Lu, J, et al. Associations of socioeconomic status and healthy lifestyle with incident dementia and cognitive decline: two prospective cohort studies. eClinicalMedicine. (2024) 76:102831. doi: 10.1016/j.eclinm.2024.102831
34. Crimmins, EM, Kim, JK, Langa, KM, and Weir, DR. Assessment of cognition using surveys and neuropsychological assessment: the health and retirement study and the aging, demographics, and memory study. J Gerontol Ser B. (2011) 66B:i162–71. doi: 10.1093/geronb/gbr048
35. Ahmadi-Abhari, S, Guzman-Castillo, M, Bandosz, P, Shipley, MJ, Muniz-Terrera, G, Singh-Manoux, A, et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ. (2017) 358:j2856. doi: 10.1136/bmj.j2856
36. Chen, S, Chen, X, Hou, X, Fang, H, Liu, GG, and Yan, LL. Temporal trends and disparities of population attributable fractions of modifiable risk factors for dementia in China: a time-series study of the China health and retirement longitudinal study (2011-2018). Lancet Reg Health West Pac. (2024) 47:101106. doi: 10.1016/j.lanwpc.2024.101106
37. Li, C, Zhu, Y, Ma, Y, Hua, R, Zhong, B, and Xie, W. Association of cumulative blood pressure with cognitive decline, dementia, and mortality. J Am Coll Cardiol. (2022) 79:1321–35. doi: 10.1016/j.jacc.2022.01.045
38. Lu, X, Yao, Y, and Jin, Y. Digital exclusion and functional dependence in older people: findings from five longitudinal cohort studies. EClinicalMedicine. (2022) 54:101708. doi: 10.1016/j.eclinm.2022.101708
39. Miranda, M, Morici, JF, Zanoni, MB, and Bekinschtein, P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. (2019) 13:363. doi: 10.3389/fncel.2019.00363
40. Rauti, R, Cellot, G, D’Andrea, P, Colliva, A, Scaini, D, Tongiorgi, E, et al. BDNF impact on synaptic dynamics: extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol Brain. (2020) 13:43. doi: 10.1186/s13041-020-00582-9
41. Ding, D, Zhao, Q, Wu, W, Xiao, Z, Liang, X, Luo, J, et al. Prevalence and incidence of dementia in an older chinese population over two decades: the role of education. Alzheimers Dement. (2020) 16:1650–62. doi: 10.1002/alz.12159
42. Hayes-Larson, E, Ikesu, R, Fong, J, Mobley, TM, Gee, GC, Brookmeyer, R, et al. Association of education with dementia incidence stratified by ethnicity and nativity in a cohort of older asian american individuals. JAMA Netw Open. (2023) 6:e231661. doi: 10.1001/jamanetworkopen.2023.1661
43. Mukadam, N, Sommerlad, A, Huntley, J, and Livingston, G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Health. (2019) 7:e596–603. doi: 10.1016/S2214-109X(19)30074-9
44. Avila, JF, Rentería, MA, Jones, RN, Vonk, JMJ, Turney, I, Sol, K, et al. Education differentially contributes to cognitive reserve across racial/ethnic groups. Alzheimers Dement J Alzheimers Assoc. (2021) 17:70–80. doi: 10.1002/alz.12176
45. Suemoto, CK, Bertola, L, Grinberg, LT, Leite, REP, Rodriguez, RD, Santana, PH, et al. Education, but not occupation, is associated with cognitive impairment: the role of cognitive reserve in a sample from a low-to-middle-income country. Alzheimers Dement. (2022) 18:2079–87. doi: 10.1002/alz.12542
46. Liang, Z, Li, A, Xu, Y, Qian, X, and Gao, X. Hearing loss and dementia: a meta-analysis of prospective cohort studies. Front Aging Neurosci. (2021) 13:695117. doi: 10.3389/fnagi.2021.695117
47. Loughrey, DG, Kelly, ME, Kelley, GA, Brennan, S, and Lawlor, BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Neck Surg. (2018) 144:115–26. doi: 10.1001/jamaoto.2017.2513
48. Yu, R-C, Proctor, D, Soni, J, Pikett, L, Livingston, G, Lewis, G, et al. Adult-onset hearing loss and incident cognitive impairment and dementia – a systematic review and meta-analysis of cohort studies. Ageing Res Rev. (2024) 98:102346. doi: 10.1016/j.arr.2024.102346
49. Deal, JA, Betz, J, Yaffe, K, Harris, T, Purchase-Helzner, E, Satterfield, S, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the health ABC study. J Gerontol A Biol Sci Med Sci. (2016) 72:glw069–709. doi: 10.1093/gerona/glw069
50. Lin, FR, Metter, EJ, O’Brien, RJ, Resnick, SM, Zonderman, AB, and Ferrucci, L. Hearing loss and incident dementia. Arch Neurol. (2011) 68:214–20. doi: 10.1001/archneurol.2010.362
51. Griffiths, TD, Lad, M, Kumar, S, Holmes, E, McMurray, B, Maguire, EA, et al. How can hearing loss cause dementia? Neuron. (2020) 108:401–12. doi: 10.1016/j.neuron.2020.08.003
52. Elser, H, Horváth-Puhó, E, Gradus, JL, Smith, ML, Lash, TL, Glymour, MM, et al. Association of early-, middle-, and late-life depression with incident dementia in a danish cohort. JAMA Neurol. (2023) 80:949–58. doi: 10.1001/jamaneurol.2023.2309
53. Yang, L, Deng, Y-T, Leng, Y, Ou, Y-N, Li, Y-Z, Chen, S-D, et al. Depression, depression treatments, and risk of incident dementia: a prospective cohort study of 354,313 participants. Biol Psychiatry. (2023) 93:802–9. doi: 10.1016/j.biopsych.2022.08.026
54. Ouanes, S, and Popp, J. High cortisol and the risk of dementia and alzheimer’s disease: a review of the literature. Front Aging Neurosci. (2019) 11:43. doi: 10.3389/fnagi.2019.00043
55. Tetsuka, S. Depression and dementia in older adults: a neuropsychological review. Aging Dis. (2021) 12:1920–34. doi: 10.14336/AD.2021.0526
56. O’Rourke, MF, and Safar, ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertens Dallas Tex. (2005) 46:200–4. doi: 10.1161/01.HYP.0000168052.00426.65
57. Sivandzade, F, Alqahtani, F, Sifat, A, and Cucullo, L. The cerebrovascular and neurological impact of chronic smoking on post-traumatic brain injury outcome and recovery: an in vivo study. J Neuroinflammation. (2020) 17:133. doi: 10.1186/s12974-020-01818-0
58. Juul Rasmussen, I, Rasmussen, KL, Nordestgaard, BG, Tybjærg-Hansen, A, and Frikke-Schmidt, R. Impact of cardiovascular risk factors and genetics on 10-year absolute risk of dementia: risk charts for targeted prevention. Eur Heart J. (2020) 41:4024–33. doi: 10.1093/eurheartj/ehaa695
59. Raggi, M, Dugravot, A, Valeri, L, Machado-Fragua, MD, Dumurgier, J, Kivimaki, M, et al. Contribution of smoking towards the association between socioeconomic position and dementia: 32-year follow-up of the Whitehall II prospective cohort study. Lancet Reg Health Eur. (2022) 23:100516. doi: 10.1016/j.lanepe.2022.100516
60. Zhong, G, Wang, Y, Zhang, Y, Guo, JJ, and Zhao, Y. Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One. (2015) 10:e0118333. doi: 10.1371/journal.pone.0118333
61. Atti, AR, Palmer, K, Volpato, S, Winblad, B, De Ronchi, D, and Fratiglioni, L. Late-life body mass index and dementia incidence: nine-year follow-up data from the kungsholmen project. J Am Geriatr Soc. (2008) 56:111–6. doi: 10.1111/j.1532-5415.2007.01458.x
62. Ihle, A, Mons, U, Perna, L, Oris, M, Fagot, D, Gabriel, R, et al. The relation of obesity to performance in verbal abilities, processing speed, and cognitive flexibility in old age: the role of cognitive reserve. Dement Geriatr Cogn Disord. (2016) 42:117–26. doi: 10.1159/000448916
63. Wang, M, Zhang, H, Liang, J, Huang, J, and Chen, N. Exercise suppresses neuroinflammation for alleviating alzheimer’s disease. J Neuroinflammation. (2023) 20:76. doi: 10.1186/s12974-023-02753-6
64. Iso-Markku, P, Kujala, UM, Knittle, K, Polet, J, Vuoksimaa, E, and Waller, K. Physical activity as a protective factor for dementia and alzheimer’s disease: systematic review, meta-analysis and quality assessment of cohort and case–control studies. Br J Sports Med. (2022) 56:701–9. doi: 10.1136/bjsports-2021-104981
65. James, S-N, Chiou, Y-J, Fatih, N, Needham, LP, Schott, JM, and Richards, M. Timing of physical activity across adulthood on later-life cognition: 30 years follow-up in the 1946 british birth cohort. J Neurol Neurosurg Psychiatry. (2023) 94:349–56. doi: 10.1136/jnnp-2022-329955
66. Minicuci, N, Naidoo, N, Corso, B, Rocco, I, Chatterji, S, and Kowal, P. Data resource profile: cross-national and cross-study sociodemographic and health-related harmonized domains from SAGE plus CHARLS, ELSA, HRS, LASI and SHARE (SAGE+ wave 2). Int J Epidemiol. (2019) 48:14–14j. doi: 10.1093/ije/dyy227
67. Topiwala, A, and Ebmeier, KP. Effects of drinking on late-life brain and cognition. BMJ Ment Health. (2018) 21:12–5. doi: 10.1136/eb-2017-102820
68. Jeon, KH, Han, K, Jeong, S-M, Park, J, Yoo, JE, Yoo, J, et al. Changes in alcohol consumption and risk of dementia in a nationwide cohort in South Korea. JAMA Netw Open. (2023) 6:e2254771. doi: 10.1001/jamanetworkopen.2022.54771
69. Amidei, CB, Fayosse, A, Dumurgier, J, Machado-Fragua, MD, Tabak, AG, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA. (2021) 325:1640–9. doi: 10.1001/jama.2021.4001
70. WHO. Risk reduction of cognitive decline and dementia: WHO guidelines. Geneva: World Health Organization (2019).
71. Penninkilampi, R, Casey, A-N, Singh, MF, and Brodaty, H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. (2018) 66:1619–33. doi: 10.3233/JAD-180439
72. Shen, C, Rolls, ET, Cheng, W, Kang, J, Dong, G, Xie, C, et al. Associations of social isolation and loneliness with later dementia. Neurology. (2022) 99:e164–75. doi: 10.1212/WNL.0000000000200583
73. Sommerlad, A, Kivimäki, M, Larson, EB, Röhr, S, Shirai, K, Singh-Manoux, A, et al. Social participation and risk of developing dementia. Nat Aging. (2023) 3:532–45. doi: 10.1038/s43587-023-00387-0
74. Ehrlich, JR, Goldstein, J, Swenor, BK, Whitson, H, Langa, KM, and Veliz, P. Addition of vision impairment to a life-course model of potentially modifiable dementia risk factors in the US. JAMA Neurol. (2022) 79:623–6. doi: 10.1001/jamaneurol.2022.0723
75. Chen, SP, Bhattacharya, J, and Pershing, S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. (2017) 135:963–70. doi: 10.1001/jamaophthalmol.2017.2838
76. Kuźma, E, Littlejohns, TJ, Khawaja, AP, Llewellyn, DJ, Ukoumunne, OC, and Thiem, U. Visual impairment, eye diseases, and dementia risk: a systematic review and meta-analysis. J Alzheimers Dis. (2021) 83:1073–87. doi: 10.3233/JAD-210250
77. Chen, X, Giles, J, Yao, Y, Yip, W, Meng, Q, Berkman, L, et al. The path to healthy ageing in China: a Peking university-lancet commission. Lancet Lond Engl. (2022) 400:1967–2006. doi: 10.1016/S0140-6736(22)01546-X
78. Peters, R, Booth, A, Rockwood, K, Peters, J, D’Este, C, and Anstey, KJ. Combining modifiable risk factors and risk of dementia: a systematic review and meta-analysis. BMJ Open. (2019) 9:e022846. doi: 10.1136/bmjopen-2018-022846
79. Singh, A, Kukreti, R, Saso, L, and Kukreti, S. Oxidative stress: a key modulator in neurodegenerative diseases. Mol Basel Switz. (2019) 24:1583. doi: 10.3390/molecules24081583
80. Zhang, W, Xiao, D, Mao, Q, and Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. (2023) 8:267. doi: 10.1038/s41392-023-01486-5
81. Byeon, G, Oh, GH, Jhoo, JH, Jang, J-W, Bae, JB, Han, JW, et al. Dual sensory impairment and cognitive impairment in the korean longitudinal elderly cohort. Neurology. (2021) 96:e2284–95. doi: 10.1212/WNL.0000000000011845
82. van Gennip, ACE, Stehouwer, CDA, van Boxtel, MPJ, Verhey, FRJ, Koster, A, Kroon, AA, et al. Association of type 2 diabetes, according to the number of risk factors within target range, with structural brain abnormalities, cognitive performance, and risk of dementia. Diabetes Care. (2021) 44:2493–502. doi: 10.2337/dc21-0149
Keywords: modifiable risk factors, coexistence, dementia, cognitive decline, cohort study
Citation: Wang M, Fan C, Han Y, Wang Y, Cai H, Zhong W, Yang X, Wang Z, Wang H and Han Y (2025) Associations of modifiable dementia risk factors with dementia and cognitive decline: evidence from three prospective cohorts. Front. Public Health. 13:1529969. doi: 10.3389/fpubh.2025.1529969
Edited by:
Vahid Rashedi, University of Social Welfare and Rehabilitation Sciences, IranReviewed by:
Vishal Singh, Rutgers University, United StatesKangni Liang, Zhejiang Chinese Medical University, China
Copyright © 2025 Wang, Fan, Han, Wang, Cai, Zhong, Yang, Wang, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Han, eWltaW5naGFuQG1haWxib3guZ3hudS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Mengzhao Wang
Mengzhao Wang Changming Fan2†
Changming Fan2† Yifei Wang
Yifei Wang Hejia Cai
Hejia Cai Xin Yang
Xin Yang Hongli Wang
Hongli Wang