
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Public Health , 05 March 2025
Sec. Aging and Public Health
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1529519
Background: Sleep problems are prevalent among the older adult population, with a significant impact on their health and overall well-being. Several randomized controlled trials (RCTs) have yielded controversial results regarding the efficacy of exercise interventions in the improvement of sleep among older adult people. This systematic review and meta-analysis aim to assess the influence of exercise interventions on sleep quality within this demographic.
Methods: A search was conducted across four databases, namely Web of Science, PubMed, Embase, and SportDiscus, in order to identify randomized controlled trials investigating exercise interventions and sleep in the older adult. The quality of the studies included was evaluated by two researchers according to the PEDro scale. Meta-analysis and sensitivity analysis were performed utilizing RevMan 5.4 and Stata 17 software.
Result: A total of fifty studies encompassing 3,937 participants were included in the analysis. Regarding patient-reported sleep parameters, exercise interventions exhibited enhancements in sleep quality (WMD = −2.18, 95%CI: −2.83 to −1.53, p < 0.01) and reductions in insomnia severity (SMD = −0.52, 95%CI: −0.79 to −0.25, p < 0.01), albeit without significant improvements in daytime sleepiness (SMD = −0.66, 95%CI: −1.41 to 0.09, p = 0.09). In terms of clinician-reported sleep parameters, exercise interventions resulted in increased total sleep time (WMD = 8.98, 95%CI: 1.19 to 16.78, p < 0.05) and sleep efficiency (WMD = 3.66, 95%CI: 2.46 to 4.85, p < 0.01), and reduced wake time after sleep onset (WMD = −11.85, 95%CI: −15.58 to −8.11, p < 0.01), but did not decrease sleep onset latency (WMD = −3.05, 95%CI: −6.23 to 0.13, p = 0.06) or the number of awakenings during sleep (WMD = −0.73, 95%CI: −1.98 to 0.52, p = 0.25).
Conclusion: Exercise interventions have demonstrated positive effects on enhancing sleep quality among the older adult population. This study lends support to the utilization of exercise interventions as a safe, feasible, and effective non-pharmacological treatment approach for enhancing sleep among older individuals.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024530227, Identifier CRD42024530227.
Sleep, as a crucial restorative behavior, has a profound influence on individual’s health and well-being (1). As a sensitive indicator of individual health, sleep is closely linked to both physical and mental health, as well as several key psychological and behavioral structures. Sleep of high quality is essential for health, learning, memory, and energy, playing critical roles in physiological restoration, cognitive enhancement, and immune function regulation (2, 3). However, sleep problems have become a global public health concern, affecting individuals worldwide. According to data from the Centers for Disease Control and Prevention in the United States, approximately one-third of adults experience sleep disorders, with higher prevalence rates observed among the older adult demographic compared to their younger counterparts (4). As individuals age, sleep patterns gradually change, characterized by diminished sleep duration and efficiency, heighted sleep fragmentation, decreased duration of rapid eye movement (REM) and slow-wave sleep (5). Additionally, older adult people frequently suffer from chronic systemic diseases such as hypertension, diabetes, and rheumatism, the symptomatic manifestations of which can compromise sleep quality (6). All these factors contribute to poorer sleep condition among older adult population compared to younger individuals. Epidemiological studies indicate that more than half of older adult individuals report sleep-related complaints, manifesting as difficulties initiating sleep, poor sleep continuity, premature awakening, and diurnal somnolence (7, 8). Prolonged sleep disturbances can exacerbate physiological and pathological aging among the older adult, raising the likelihood of diseases such as Alzheimer’s, stroke, and atherosclerosis (9–11), in addition to anxiety, depression, and other psychological disorders (12). How to effectively prevent sleep problems in older adult people and improve their sleep have garnered widespread clinical attention.
In clinical practice, strategies aimed at optimizing sleep quality among the older adult encompass both pharmacological and non-pharmacological treatments. Commonly used medications for pharmacological treatment include sedative-hypnotic drugs, atypical antipsychotics, antidepressants, and melatonin, along with melatonin receptor agonists (13–16). In spite of the short-term improvement in sleep quality among the older adult population, prolonged pharmacological intervention is discouraged owing to the attendant risks of tolerance and dependence (17). Furthermore, the majority of pharmacological treatments are associated with the risk of cognitive and behavioral changes, including memory loss, dizziness, or loss of balance leading to falls (18). Non-pharmacological interventions refer to methods of improving sleep that do not involve medication. Compared to pharmacological interventions, non-pharmacological therapies may have longer-lasting effects and lower risk of adverse events, rendering them preferable for older adult individuals experiencing sleep disturbances (19, 20). The most common is psychotherapy, with cognitive-behavioral therapy and mindfulness-based stress reduction therapy included, both of which have been proven to have positive impact on sleep quality among the older adult demographic (21, 22). Nevertheless, psychotherapy requires a longer duration for sleep improvement, coupled with the necessity for administration by trained therapists, rendering it costly and less accessible to a large number of patients (23). Therefore, it is indispensable to explore simpler and cost-effective non-pharmacological treatment options.
Exercise therapy is a non-pharmacological treatment option, in the form of exercise prescriptions for patients or sub-healthy populations, offering advantages such as minimal side effects, wide accessibility, and lower investment costs (24). Some studies have investigated the relationship between exercise and sleep in the older adult population. Epidemiological research indicates that regular exercise is connected with better self-reported sleep quality among older adult people (25), while less physical activity may lead to insomnia in later life (26). Additionally, several RCTs have examined the effects of exercise therapy on sleep among older adult individuals, but inconsistent results exist possibly due to differences in measurement tools, sample characteristics, and other factors. Noteworthy are the contradictory conclusions drawn in the two recent studies. One examined the effects of a 12-week exercise program on sleep quality in older adult people, showing improvements in subjective sleep quality and clinician-reported sleep parameters compared to a placebo control group (27). Another found that a 12-week exercise intervention did not improve sleep quality significantly in older adult community residents compared to routine care controls (28). These conflicting findings pose challenges for clinical practice.
In recent years, several systematic or narrative reviews have included discussions on the relationship between exercise and sleep, whose conclusions suggest that exercise therapy has a positive impact on self-reported sleep quality in adults to certain degree, particularly on sleep latency and efficiency (29, 30). Nonetheless, most of these studies focused on relatively younger adults, therefore these findings may not apply to older populations. Due to prevalent chronic diseases among older adult population as well as their declining physical capabilities, exercise regimens designed for general adults may not be suitable for the older adult. Recently, a systematic review evaluated the effects of physical exercise on sleep in older adult people (31). Although it demonstrated positive effects of exercise programs on various aspects of sleep in this population, only six of the included studies were RCTs. Given the limited trials, small sample sizes, and the interventions used predominantly involving mind–body exercises such as yoga, Tai Chi, and Baduanjin, the overall impact of exercise intervention on sleep remains unclear. Furthermore, new evidence from additional RCTs has emerged since these studies were published. Therefore, there is a need to update and synthesize the existing evidence to further confirm the relationship between exercise therapy and sleep in older adult population. The primary objective of the present study is to conduct a systematic review and meta-analysis to evaluate the effectiveness of exercise therapy in the improvement of sleep quality among older adult individuals and to evaluate whether and how exercise interventions may improve patient-reported or clinician-reported sleep outcomes among this demographic cohort.
To ensure methodological rigor and scientific integrity, this meta-analysis strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement in both the conduct of the analysis and the writing of the report (32). The protocol for this review has been registered on the PROSPERO platform1 under registration number CRD42024530227.
This study established eligibility criteria based on the evidence-based medicine PICOS framework (33).
Participant: We included studies focusing on older adult people with a mean age of 60 years or above, regardless of their diagnostic status regarding sleep disorders. In other words, no specific baseline sleep condition threshold was imposed, whereas participants with acute illnesses were excluded. Intervention: The intervention was a pre-determined regular exercise program, including exercises of any mode, intensity, duration, and frequency. Studies combining exercise therapy with non-exercise interventions were excluded due to the challenge in isolating the effect of exercise on sleep in older adult people. Comparison: We included studies comparing exercise interventions with no additional exercise or physical activity in the control group. Control conditions may include no intervention, placebo, usual care, waitlist, health education, etc. Outcome: Included studies reported sleep-related outcomes pre-and post-intervention, providing sufficient statistical data (sample size, mean, standard deviation, or standard error). The measurement tools employed in the studies were not restricted, ranging from standardized scales such as the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), Athens Insomnia Scale (AIS), Insomnia Severity Index (ISI), as well as objective measurement instruments like polysomnography (PSG), actigraphy, etc. Study design: To obtain high-level evidence, only randomized controlled trials (RCTs) were included, thereby reducing heterogeneity across studies and enhancing the validity of combined results. These studies were sourced from peer-reviewed English journals. Additionally, studies manifesting significant baseline disparities between the exercise intervention group and the control group were excluded.
We conducted searches in four electronic databases—Web of Science, PubMed, Embase, and SPORTDiscus—to identify studies on the impact of exercise interventions on sleep in older adult people. The search period spanned from the inception of each database to October 24, 2024. The initial search employed four key terms: aged or older adult, exercise or physical activity, sleep or sleep quality, and randomized controlled trials. Following the retrieval strategies of previous relevant reviews, search keywords were designed for each major term. Additionally, based on guidance from experienced librarians, we combined subject headings and free terms corresponding to the search keywords from the database thesauri to maximize the retrieval of relevant records.
The study selection process involved three steps: First, EndNote X9 literature management software was adopted in order to merge search results from each database and remove duplicate records of the same report. Second, titles and abstracts of the remaining reports were reviewed to eliminate off-topic reports. Finally, the full text of potentially relevant reports was obtained and examined to assess their eligibility for inclusion. Two members of the systematic review team (DG and XL) participated in the screening of titles and abstracts, followed by independent full-text reviews of potentially relevant reports. In cases where disagreements could not be resolved through discussion, arbitration was provided by the team leader (GS). In cases of multiple study reports based on identical sample, only the most comprehensive report or the study with the largest sample size was included.
To ensure data accuracy and minimize potential bias, data extraction was performed independently by two experienced members (DG and XL) in a double-blind manner. A pre-designed electronic spreadsheet facilitated meticulous entry of extracted data, including: (1) literature information (author names, publication year, country/region); (2) participant details (source, sample size, age); (3) intervention details (type, duration per session, frequency, duration); (4) control conditions; and (5) sleep-related index measurement tools and outcomes (mean, standard deviation). In cases where studies lacked sufficient information, authors were contacted via email for clarification. Any disagreement during data extraction that could not be resolved through discussion was arbitrated by GS.
This study utilized the Physiotherapy Evidence Database (PEDro) scale to assess the included literature (34). The PEDro scale is specifically designed to evaluate the methodological quality of randomized controlled trials in physiotherapy, with reliability and validity (35). It consists of 11 criteria, such as participant eligibility criteria, random allocation, concealment of allocation, baseline comparability, blinding (participants, therapists, and assessors), attrition rate (<15%), intention-to-treat analysis, between-group comparisons, and point and variability measures. The first criterion is not scored, and the remaining criteria are each scored as 1 point, contributing to a total score of 10. One point is achieved for meeting each criterion and 0 point for failing to do it. However, a review has highlighted that blinding participants and therapists may often be infeasible in many exercise intervention trials. Consistent with Liang et al., we categorized the quality of the included studies into three levels: high quality (score ≥ 6), adequate quality (score 4–5), and low quality (score ≤ 3) (36). Two team members (DG and XL) scored the included literature independently based on the assessment criteria. Any disagreements in quality assessment that could not be resolved through discussion were arbitrated by GS.
To explore the impact of exercise interventions on sleep quality among elder individuals, this meta-analysis adopted Review Manager 5.4 software to statistically combine results from multiple independent studies. We utilized post-intervention outcome indicator data (sample size, mean, standard deviation) from intervention and control groups. In studies where standard errors were provided, we calculated standard deviations by multiplying the standard error by the square root of the sample size. In cases where measurement units were consistent across the studies included, we used the weighted mean difference (WMD) as the effect measure. For studies with different measurement units or methods, the standardized mean difference (SMD) served as the effect measure. When aggregating the effect sizes from individual studies, we employed the inverse variance method to determine the weight assigned to each study. The threshold for statistical significance of combined effect sizes was set at p < 0.05. The heterogeneity of included studies was assessed using Q-test and I2 statistic values. The I2 value represents the degree of heterogeneity, where 25, 50, and 75% correspond to low, moderate, and high heterogeneity, respectively (37). If I2 ≥ 50%, a random-effects model was utilized to calculate combined effect sizes and 95% confidence intervals (CIs); a fixed-effects model was adopted otherwise. To explore potential sources of heterogeneity, subgroup analysis was conducted based on participant sources, control types, and study quality. Sensitivity analysis was conducted using the one-by-one removal method to evaluate the influence of each study on the overall results. When the number of included studies was ≥10, a funnel plot was generated using Review Manager 5.4 to visually assess publication bias. Additionally, Egger’s regression test was performed using Stata 17.0 to detect significant publication bias (p < 0.05). Given the aim of investigating the impact of exercise interventions on sleep in older individuals, in cases where two or more subgroups were included in a study, they were merged into a single exercise intervention group for comparison with the control group. The formula for the combination of subgroup data is as follows: , , where M represents the mean, N represents the sample size, and SD represents the standard deviation (38).
This study searched four databases, yielding a total of 7,733 records. After removing duplicates utilizing EndNote X9 literature management software, 6,064 records remained. Initial screening based on titles and abstracts led to the exclusion of 5,972 irrelevant records. Subsequently, full-text screening of the remaining 92 records resulted in the exclusion of 42 articles for various reasons: data duplication (n = 1), unavailable data (n = 28), non-matching control conditions (n = 2), non-English language (n = 1), non-RCTs (n = 1), outcome indicators mismatch (n = 4), intervention content mismatch (n = 4), and ineligible subject (n = 1). Ultimately, 50 studies (27, 28, 39–86) were included in the meta-analysis. The literature screening process is illustrated in Figure 1.
The basic characteristics of the included studies are summarized in Table 1. This study encompassed 50 trials conducted in various countries and regions including the United States, United Kingdom, Germany, Mainland China, Taiwan, Hong Kong, Tunisia, Brazil, Spain, Thailand, Turkey, Saudi Arabia, South Korea, India, Vietnam, and Iran. These studies collectively recruited 3,937 participants from community settings, nursing homes, public health centers, outpatient clinics, and hospitals. The participant populations encompassed a diverse range, including general older adult people, individuals with muscular dystrophy, metabolic syndrome, knee osteoarthritis, stroke, chronic insomnia, mild cognitive impairment, depression, sleep disorders, postmenopausal women, heart failure patients, colon cancer patients, lung cancer patients, individuals with memory complaints, Alzheimer’s disease patients.
Among the 50 studies included, the exercise interventions varied widely in their approaches, including aerobic exercise, resistance training, interval training, water-based exercises, Tai Chi, Baduanjin, walking, Qigong, yoga, cycling, and Pilates. The duration of the entire intervention period ranged from 4 weeks to 12 months, with a maximum duration of 12 weeks in most studies. The duration of each exercise session also varied, ranging from 30 to 120 min, with the most common duration being 60 min per session. The frequency of intervention per week showed significant differences as well, ranging from once a week to seven times a week, with most interventions occurring three times per week.
In the 39 studies, exercise interventions were compared with no specific treatment, and control conditions included standard care, no intervention, waiting list controls, singing, and prayer. The remaining 11 studies compared exercise interventions with active treatments, such as health education and sleep hygiene education. 34 studies exclusively used subjective measurement scales to assess post-intervention patient-reported sleep outcome. The measurement tools included PSQI, ESS, ISI, AIS, and Oviedo Sleep Questionnaire (OSQ). Five studies solely employed PSG or actigraphy in the measurement of clinician-reported sleep parameters in participants. Additionally, eleven studies combined qualitative and quantitative tools to evaluate participants’ sleep. Overall, the PSQI was the most commonly utilized assessment tool, as it was employed in 41 out of the 50 studies.
Table 2 describes the methodological quality assessment of the studies included. Each study met at least four criteria and achieved a moderate quality level or higher. The number of studies with high and moderate quality ratings were 33 and 17, respectively, yielding an average score of 6.06, indicating an overall high level of methodological quality, characterized by transparent participant recruitment criteria, random participant allocation and comprehensive reporting of point measures, variability measures, and between-group statistical results of the key outcomes. Additionally, the groups were comparable at baseline for the most important prognostic indicators. More than two-thirds of the studies included maintained a high retention rate during the intervention period. However, due to the limitations of exercise interventions, blinding of participants and therapists was not implemented in most studies.
Of the included studies, 41 reported the post-intervention PSQI total score of the participants. The PSQI is primarily used in clinical and basic research to assess subjective sleep quality, with a total score ranging from 0 to 21, where higher scores indicate poorer sleep quality. Figure 2 presents a forest plot of the difference in PSQI total scores between the experimental and control groups. Due to an I2 = 88%, a random-effects model was used for calculation, indicating a significantly beneficial effect of exercises on sleep quality as reflected by the PSQI total score (WMD = −2.18, 95%CI: −2.83 to −1.53, p < 0.01). Additionally, seven studies reported the post-intervention severity of insomnia among participants, using assessment tools such as ISI, AIS, and OSQ, which are tools utilized for self-reported insomnia severity measurement, where higher scores indicate more severe insomnia. Figure 3 shows a forest plot of the difference in insomnia severity between the experimental and control groups. Using a random-effects model, the results indicated a notable reduction in insomnia severity in the experimental group compared to the control group (SMD = −0.52, 95%CI: −0.79 to −0.25, p < 0.01). Besides, five studies reported the daytime sleepiness of participants post-intervention. Figure 4 presents a forest plot of the difference in daytime sleepiness, showing no significant difference between the experimental and control groups (SMD = −0.66, 95%CI: −1.41 to 0.09, p = 0.09).
Regarding clinician-reported sleep parameters, participants’ total sleep time (TST), sleep latency (SL), sleep efficiency (SE), wake after sleep onset (WASO), and the number of awakenings before and after the intervention were measured in the studies utilizing PSG or actigraphy. Figure 5 depicts the combined effect sizes of these clinician-reported sleep parameters. Fifteen studies reported TST of pre-intervention and post-intervention, and the combined results showed a significant difference between the experimental and control groups (WMD = 8.98, 95%CI: 1.19 to 16.78, p < 0.05). Thirteen studies compared changes in pre-intervention and post-intervention SE between the experimental and control groups, with combined results indicating a positive effect of exercise interventions on SE (WMD = 3.66, 95%CI: 2.46 to 4.85, p < 0.01). Combining results from nine studies reporting WASO outcomes revealed that the experimental group had significantly less WASO compared to the control group (WMD = −11.85, 95%CI: −15.58 to −8.11, p < 0.01). However, no significant differences were found between the experimental and control groups in terms of SL (WMD = −3.05, 95%CI: −6.23 to 0.13, p = 0.06) and the number of awakenings (WMD = −0.73, 95%CI: −1.98 to 0.52, p = 0.25).
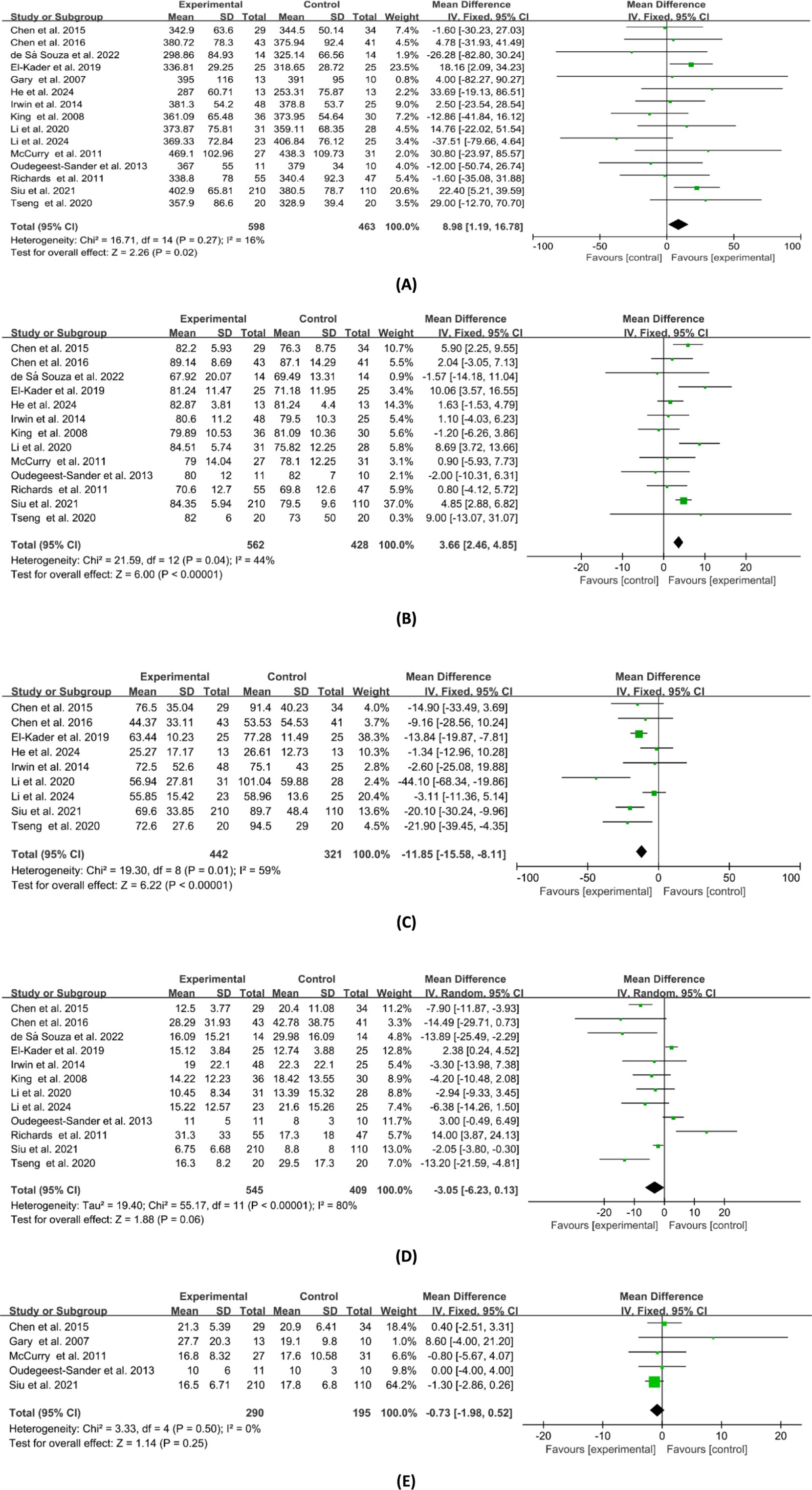
Figure 5. Forest plot of the effects of exercises on clinician-reported sleep parameters. (A) TST; (B) SE; (C) WASO; (D) SL; (E) no. of awakenings.
The sensitivity analysis conducted on the combined results of patient-reported sleep parameters demonstrated that the exclusion of any single study did not lead to significant changes in the combined effect size and 95% confidence intervals (CIs). This indicates a high level of stability in the analysis results for patient-reported sleep parameters. Regarding the sensitivity analysis of combined results for clinician-reported sleep parameters, the analyses for SE, WASO, and the number of awakenings were found to be stable. However, variability was noted in TST and SL. Specifically, after the exclusion of the study by Siu et al., the combined result for TST changed substantially from (WMD = 8.98, 95%CI: 1.19 to 16.78, p < 0.05) to (WMD = 5.51, 95%CI: −3.24 to 14.25, p = 0.22). Similarly, after excluding the study by Richards et al., the combined result for SL shifted from (WMD = −3.05, 95%CI: −6.23 to 0.13, p = 0.06) to (WMD = −3.94, 95%CI: −7.02 to −0.86, p < 0.05). Therefore, caution should be exercised in the interpretation of the TST and SL results.
This study conducted subgroup analysis on the meta-analysis results for PSQI total score, TST, SE, and WASO. Table 3 depicts the subgroup meta-analysis results based on participant source, control type, and study quality. No significant differences were observed among the indicators based on participant source and study quality. As for control type, significant intergroup differences were found in SE (p < 0.05). Besides, only when the controlled conditions were non-active treatment, the combined results of TST and SE were of statistical significance.
Since the meta-analyses for PSQI total score, TST, SL, and SE included more than 10 studies each, we investigated the publication bias in them. Figure 6 shows that most studies are positioned above the center line, and the distribution is roughly symmetrical on both sides. Additionally, Egger’s test p-values for these analyses were 0.978, 0.160, 0.241, and 0.410, respectively, all of which are greater than 0.05. In summary, these results suggest no notable publication bias in the studies included in each of these meta-analyses.
Sleep is a complex physiological phenomenon, whose quality should be assessed by integrating subjective sleep perceptions and clinician-reported sleep parameters (87). This study conducted a systematic review and meta-analysis of RCTs with the highest-level evidence to summarize the effectiveness of exercise interventions in the improvement of sleep quality among older adult population. The results of the study indicate a positive impact of exercise interventions on sleep among older adult people. This positive impact is specifically manifested in three aspects: First, a significant decrease in overall PSQI scores reflects improved subjective sleep quality. Second, after exercise interventions, self-reported severity of insomnia is effectively alleviated. Finally, in terms of clinician-reported sleep outcomes, improvements in TST, SE, and WASO with exercise interventions show clinical relevance compared to active treatments, routine care, or waitlist controls. To our knowledge, there is currently no meta-analysis comprehensively assessing the impact of physical exercise on clinician-reported sleep outcomes in older adult population. Therefore, our study has provided more comprehensive and robust evidence in the summary and confirmation of the true significance of exercise on sleep among elder individuals.
The PSQI is a classic subjective sleep quality assessment tool widely used in epidemiological studies of sleep disorders. In our Meta-analysis, the PSQI total score showed a change of −2.18 points, which was of statistical significance (88). Regarding the minimal clinically important difference (MCID) for the PSQI, studies suggest a range between 1.14 to 1.80. The score change we observed aligns closely with the two recent meta-analyses. In the study by Solis-Navarro et al., older adult people in the exercise intervention group showed a decrease of 2.49 in PSQI scores compared to the control group, with merely 8 studies included in their analysis (89). Wu et al.’s meta-analysis demonstrated a significant decrease of 2.34 in PSQI scores among older adult people participating in traditional Chinese exercises or general aerobic exercises compared to control conditions (90). Compared to these studies, our research included more high-quality RCTs and encompassed a broader range of intervention measures. Our study, therefore, further confirms that exercise interventions obtain clinically meaningful benefits for subjective sleep quality in older adult people. Currently, there are several explanations for the mechanisms underlying the relationship between exercise and sleep quality. One viewpoint suggests that elevated levels of pro-inflammatory cytokines can worsen insomnia, while exercise can help restore a stable sleep–wake cycle by improving TNF-α, IL-1β, IL-6, and other pro-inflammatory cytokines (91, 92). Other proposed mechanisms include increasing exposure to sunlight, enhancing metabolic capacity, alleviating stress and anxiety, and regulating temperature changes (75, 93–95). It’s important to note that our analysis revealed a high degree of heterogeneity in PSQI scores among the studies (I2 = 88%). However, subgroup meta-analysis based on participant source, control type, and methodological quality showed no significant inter-group differences. Given the varied nature of exercise interventions included in our study, we speculate that this may be an important factor contributing to the observed heterogeneity.
Moreover, this study delved into the impact of exercise intervention on the severity of insomnia and daytime sleepiness. According to Cohen’s recommended effect size criteria (96), exercise intervention demonstrated a moderate effect size (d = −0.52) on reducing insomnia severity in the older adult, consistent with previous research focusing on adults clinically diagnosed with insomnia (97). Therefore, it appears that the influence of exercise on insomnia severity is not affected by age. Our study results suggest that exercise does not have a significant positive impact on daytime sleepiness in older adult people. Some studies suggest that the effect of exercise on daytime sleepiness depends on the timing of exercise, with benefits observed when exercise is performed in the morning (98). After waking up in the morning, the cerebral cortex is in an inhibited state, and moderate exercise can increase cortical excitability, thereby reducing daytime sleepiness (99). However, our meta-analysis on daytime sleepiness was based on merely five studies, none of which reported the timing of exercise. Therefore, it is challenging to determine whether our results are related to the timing of interventions included in the studies. Future research should include more high-quality trials to confirm the impact of exercise on daytime sleepiness in older adult people and investigate on whether the timing of exercise plays a regulatory role.
Older adult individuals may exhibit biases in self-assessment of their sleep conditions, emphasizing the need for objective measurements to validate these assessments (87). To comprehensively evaluate the effectiveness of exercise on sleep in older adult people, we analyzed clinician-reported parameters measured via PSG or actigraphy, including TST, SE, SL, WASO, and the frequency of awakenings. The results demonstrate that exercise has beneficial effects on many clinician-reported sleep indicators in older adult people. Consistent with previous research (89), we found that exercise positively impacts SE among this demographic cohort. However, there is rather limited understanding of exercise’s effect on SE. A recent survey revealed that pain, nocturia, and sleep medication use are closely associated with SE in older adult people (100), with those using sleep medications more likely to have lower SE (13). Previous evidence suggests that regular physical exercises can alleviate chronic pain in older adult population, as well as reduce nocturia, and decrease sleep medication use (101–103). The beneficial effects of exercise on these influencing factors may represent potential mechanisms explaining the improvement in SE in older adult people. Additionally, it is found that exercise positively influences TST and WASO in older adult people, which has not been reported previously. This finding is of great significance in that these factors are key diagnostic indicators of sleep disorders and are related to increased mortality in older adult people (104). In this study, we observed that exercise did not reduce SL or the number of awakenings. Frequency of awakenings refer to the number of awakenings lasting 1 min or above during the night of sleep, excluding the last awakening before waking up (105). Some studies suggest that baseline physical activity levels are an important moderating variable affecting the relationship between exercise and the number of awakenings in older adult people, with greater improvements observed in older sedentary individuals (106). However, most of the 5 studies included in our analysis did not report participants’ baseline physical activity levels, making it difficult to determine whether our results are related to this variable. It is important to note that sensitivity analysis revealed unstable results for TST and SL, indicating significant potential bias factors related to exercise intervention efficacy that require further investigation for validation.
Furthermore, in our subgroup analysis based on control types, insightful revelations have been discovered. The combined effect sizes of TST and SE proved statistically significant in the non-active treatment subgroup, but not in the active treatment subgroup. The active treatment subgroup included control conditions such as sleep hygiene education or health promotion courses, indicating they may have some positive impact on sleep in older adult people. Limited evidence suggests that sleep hygiene education interventions lead to significant improvements in sleep complaints or insomnia in patients, achieving small to moderate effect sizes (107). Future research could explore the impact of combined exercise interventions with sleep hygiene education on sleep among the older adult.
This review is beset by several limitations. Firstly, some studies exhibited high heterogeneity among them, therefore our results should be interpreted with caution. Subgroup analyses based on participant sources, control types, and methodological quality inadequately explicate the origins of this heterogeneity. Future investigations need additional moderating variables for grouping and analysis, such as gender, physical activity levels, types of exercise, exercise intensity, and exercise duration. Secondly, most studies did not implement blinding of participants and therapists, which could potentially exaggerate the current findings. Lastly, we solely included studies published in English in peer-reviewed journals. Future endeavors could incorporate broader literature searches and the inclusion of relevant studies from a more extensive range of sources.
Our research findings indicate that exercise has a beneficial effect on enhancing sleep in older adult people, offering a safe and effective approach. Following exercise intervention, older adult individuals reported significant improvements in subjective sleep quality and reductions in insomnia severity, along with positive impacts on objective measures such as TST, SE, and WASO. It not only provides evidence-based support for the formulation of exercise prescriptions and health management policies, but also offers references for other researchers engaged in this field.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
DG: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. XL: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. GS: Conceptualization, Supervision, Validation, Writing – review & editing, Project administration.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the project of Sichuan Federation of Social Science Association (no. SCJJ23ND360).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1529519/full#supplementary-material
1. Kudrnáčová, M, and Kudrnáč, A. Better sleep, better life? Testing the role of sleep on quality of life. PLoS One. (2003) 18:e0282085. doi: 10.1371/journal.pone.0282085
2. Murre, JMJ, Kristo, G, and Janssen, SMJ. The effect of self-reported habitual sleep quality and sleep length on autobiographical memory. Memory. (2014) 22:633–45. doi: 10.1080/09658211.2013.811253
3. Gamaldo, CE, Shaikh, AK, and McArthur, JC. The sleep-immunity relationship. Neurol Clin. (2012) 30:1313–43. doi: 10.1016/j.ncl.2012.08.007
4. National Institutes of Health. National Institutes of Health state of the science conference statement on manifestations and management of chronic insomnia in adults. Sleep. (2005) 28:1049–57. doi: 10.1093/sleep/28.9.1049
5. Mander, BA, Winer, JR, and Walker, MP. Sleep and Human Aging. Neuron. (2017) 94:19–36. doi: 10.1016/j.neuron.2017.02.004
6. Garcia, AD. The effect of chronic disorders on sleep in the elderly. Clin Geriatr Med. (2008) 24:27–38. doi: 10.1016/j.cger.2007.08.008
7. Wang, P, Song, L, Wang, KL, Han, XL, Cong, L, Wang, YX, et al. Prevalence and associated factors of poor sleep quality among Chinese older adults living in a rural area: a population-based study. Aging Clin Exp Res. (2020) 32:125–31. doi: 10.1007/s40520-019-01171-0
8. Ganguli, M, Reynolds, CF, and Gilby, JE. Prevalence and persistence of sleep complaints in a rural older community sample: the MoVIES project. J Am Geriatr Soc. (1996) 44:778–84. doi: 10.1111/j.1532-5415.1996.tb03733.x
9. Irwin, MR, and Vitiello, MV. Implications of sleep disturbance and inflammation for Alzheimer's disease dementia. Lancet Neurol. (2019) 18:296–306. doi: 10.1016/S1474-4422(18)30450-2
10. Ji, AL, Lou, HQ, Lou, PA, Xu, CR, Zhang, P, Qiao, C, et al. Interactive effect of sleep duration and sleep quality on risk of stroke: An 8-year follow-up study in China. Sci Rep. (2020) 10:8690. doi: 10.1038/s41598-020-65611-y
11. Domínguez, F, Fuster, V, Fernández-Alvira, JM, Fernández-Friera, L, López-Melgar, B, Blanco-Rojo, R, et al. Association of Sleep Duration and Quality with Subclinical Atherosclerosis. J Am Coll Cardiol. (2019) 73:134–44. doi: 10.1016/j.jacc.2018.10.060
12. Rio João, KAD, de Jesus, SN, Carmo, C, and Pinto, P. Sleep quality components and mental health: study with a non-clinical population. Psychiatry Res. (2018) 269:244–50. doi: 10.1016/j.psychres.2018.08.020
13. Béland, SG, Préville, M, Dubois, MF, Lorrain, D, Grenier, S, Voyer, P, et al. Benzodiazepine use and quality of sleep in the community-dwelling elderly population. Aging Ment Health. (2010) 14:843–50. doi: 10.1080/13607861003781833
14. Thompson, W, Quay, TAW, Rojas-Fernandez, C, Farrell, B, and Bjerre, LM. Atypical antipsychotics for insomnia: a systematic review. Sleep Med. (2016) 22:13–7. doi: 10.1016/j.sleep.2016.04.003
15. Everitt, H, Baldwin, DS, Stuart, B, Lipinska, G, Mayers, A, Lmalizia, A, et al. Antidepressants for insomnia in adults. Cochrane Database of Syst Rev. (2018) 2018:CD010753. doi: 10.1002/14651858.CD010753.pub2
16. Pandi-Perumal, SR, Zisapel, N, Srinivasan, V, and Cardinali, DP. Melatonin and sleep in aging population. Exp Gerontol. (2005) 40:911–25. doi: 10.1016/j.exger.2005.08.009
17. Wilt, TJ, MacDonald, R, Brasure, M, Olson, CM, Carlyle, M, Fuchs, E, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. (2016) 165:103–12. doi: 10.7326/M15-1781
18. Glass, J, Lanctôt, KL, Herrmann, N, Herrmann, BA, and Busto, UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ-Brit Med J. (2005) 331:1169–73. doi: 10.1136/bmj.38623.768588.47
19. Petit, L, Azad, N, Byszewski, A, Sarazan, FFA, and Power, B. Non-pharmacological management of primary and secondary insomnia among older people: review of assessment tools and treatments. Age Ageing. (2003) 32:19–25. doi: 10.1093/ageing/32.1.19
20. Schutte-Rodin, S, Broch, L, Buysse, D, Dorsey, C, and Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. (2008) 4:487–504. doi: 10.5664/jcsm.27286
21. Lee, S, and Yu, S. Effectiveness of information and communication technology (ICT) interventions in Elderly’s sleep disturbances: a systematic review and Meta-analysis. Sensors. (2021) 21:6003. doi: 10.3390/s21186003
22. Samara, MT, Huhn, M, Chiocchia, V, Schneider-Thoma, J, Wiegand, M, Salanti, G, et al. Efficacy, acceptability and tolerability of all available treatments for insomnia in the elderly: a systematic review and network meta-analysis. Acta Psychiat Scand. (2020) 142:6–17. doi: 10.1111/acps.13201
23. MacLeod, S, Musich, S, Kraemer, S, and Wicker, E. Practical non-pharmacological intervention approaches for sleep problems among older adults. Geriatr Nurs. (2018) 39:506–12. doi: 10.1016/j.gerinurse.2018.02.002
24. Pedersen, BK, and Saltin, B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Spor. (2015) 25:1–72. doi: 10.1111/sms.12581
25. Makizako, H, Kiyama, R, Nishimoto, D, Nishio, I, Masumitsu, T, Ikeda, Y, et al. Association between regular exercise and self-rated health and sleep quality among adults in Japan during the COVID-19 pandemic. Int J Env Res Pub He. (2021) 18:10515. doi: 10.3390/ijerph181910515
26. Morgan, K. Daytime activity and risk factors for late-life insomnia. J Sleep Res. (2003) 12:231–8. doi: 10.1046/j.1365-2869.2003.00355.x
27. de Sá Souza, HD, de Melo, CM, Piovezan, RD, Miranda, REEPC, Carneiro, MA, Silva, BM, et al. Resistance training improves sleep and anti-inflammatory parameters in Sarcopenic older adults: a randomized controlled trial. Int J Env Res Pub He. (2022) 19:16322. doi: 10.3390/ijerph192316322
28. Marupuru, S, Bell, ML, Grandner, MA, and Taylor-Piliae, RE. The effect of physical activity on sleep quality among older stroke survivors: secondary analysis from a randomized controlled trial. Int J Env Res Pub He. (2022) 19:13320. doi: 10.3390/ijerph192013320
29. Lowe, H, Haddock, G, Mulligan, LD, Gregg, L, and Kyle, SD. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin Psychol Rev. (2018) 68:1–12. doi: 10.1016/j.cpr.2018.11.002
30. Sejbuk, M, Mironczuk-Chodakowska, I, and Witkowska, AM. Sleep quality: a narrative review on nutrition, stimulants, and physical activity as important factors. Nutrients. (2022) 14:1912. doi: 10.3390/nu14091912
31. Vanderlinden, J, Boen, F, and van Uffelen, JGZ. Effects of physical activity programs on sleep outcomes in older adults: a systematic review. Int J Behav Nutr Phys Act. (2020) 17:11. doi: 10.1186/s12966-020-0913-3
32. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ-Brit Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Liberati, A, Altman, DG, Tetzlaff, J, Mulrow, C, Gotzsche, PC, Ioannidis, JPA, et al. The PRISMA statement for reporting systematic reviews and Meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. (2009) 151:W-65–94. doi: 10.7326/0003-4819-151-4-200908180-00136
34. Cashin, AG, and McAuley, JH. Clinimetrics: physiotherapy evidence database (PEDro) scale. J Physiother. (2020) 66:59. doi: 10.1016/j.jphys.2019.08.005
35. Maher, CG, Sherrington, C, Herbert, RD, Moseley, AM, and Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. (2003) 83:713–21. doi: 10.1093/ptj/83.8.713
36. Liang, X, Li, R, Wong, SHS, Sum, RKW, Wang, P, Yang, BR, et al. The effects of exercise interventions on executive functions in children and adolescents with autism Spectrum disorder: a systematic review and Meta-analysis. Sports Med. (2022) 52:75–88. doi: 10.1007/s40279-021-01545-3
37. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ-Brit Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
38. Shu, Y, He, Q, Xie, Y, Zhang, WR, Zhai, S, and Wu, T. Cognitive gains of aerobic exercise in patients with ischemic cerebrovascular disorder: a systematic review and Meta-analysis. Front Cell Dev Biol. (2020) 8:582380. doi: 10.3389/fcell.2020.582380
39. Wang, C, Jiang, T, Li, H, Cao, G, and Zhang, G. The effects of tai chi exercise on sleep quality among the elderly: a study based on polysomnographic monitoring. Front Neurol. (2024) 15:1304463. doi: 10.3389/fneur.2024.1304463
40. Song, D, Yu, D, Liu, T, and Wang, J. Effect of an aerobic dancing program on sleep quality for older adults with mild cognitive impairment and poor sleep: a randomized controlled trial. J Am Med Dir Assoc. (2024) 25:494–9. doi: 10.1016/j.jamda.2023.09.020
41. Sánchez-Alcalá, M, Aibar-Almazán, A, Hita-Contreras, F, Castellote-Caballero, Y, Carcelén-Fraile, MD, Infante-Guedes, A, et al. Effects of dance-based aerobic training on mental health and quality of life in older adults with mild cognitive impairment. J Pers Med. (2024) 14:844. doi: 10.3390/jpm14080844
42. Li, LY, Xie, X, Jiang, HX, and Yu, J. Improving memory through better sleep in community-dwelling older adults: a tai chi intervention study. J Gerontol B-Psychol. (2024) 79:39269015. doi: 10.1093/geronb/gbae156
43. He, JL, Chan, SHW, Lin, JX, and Tsang, HWH. Integration of tai chi and repetitive transcranial magnetic stimulation for sleep disturbances in older adults: a pilot randomized controlled trial. Sleep Med. (2024) 122:35–44. doi: 10.1016/j.sleep.2024.07.029
44. Teruel-Hernández, E. Improving sleep quality, daytime sleepiness, and cognitive function in patients with dementia by therapeutic exercise and NESA Neuromodulation: a multicenter clinical trial. Int J Environ Res Pub He. (2023) 20:7027. doi: 10.3390/ijerph20217027
45. Tung, HT, Chen, KM, Chou, CP, Belcastro, F, Hsu, HF, and Kuo, CF. Acupunch exercise improved muscle mass, hand grip strength, and sleep quality of institutional older adults with probable sarcopenia. J Appl Ecol. (2023) 42:888–97. doi: 10.1177/07334648221141413
46. Baklouti, S, Fekih-Romdhane, F, Guelmami, N, Bonsaksen, T, Baklouti, H, Aloui, A, et al. The effect of web-based hatha yoga on psychological distress and sleep quality in older adults: a randomized controlled trial. Complement Ther Clin. (2023) 50:101715. doi: 10.1016/j.ctcp.2022.101715
47. Zhou, Y, Wu, WL, Zou, YQ, Huang, WT, Lin, SS, Ye, JS, et al. Benefits of different combinations of aerobic and resistance exercise for improving plasma glucose and lipid metabolism and sleep quality among elderly patients with metabolic syndrome: a randomized controlled trial. Endocr J. (2022) 69:819–30. doi: 10.1507/endocrj.EJ21-0589
48. Song, JL, Wei, LJ, Cheng, K, Lin, Q, Xia, P, Wang, XW, et al. The effect of modified tai chi exercises on the physical function and quality of life in elderly women with knee osteoarthritis. Front Aging Neurosci. (2022) 14:860762. doi: 10.3389/fnagi.2022.860762
49. Siu, PM, Yu, AP, Tam, BT, Chin, EC, Yu, DS, Chung, KF, et al. Effects of tai chi or exercise on sleep in older adults with insomnia: a randomized clinical trial. JAMA Netw Open. (2021) 4:e2037199. doi: 10.1001/jamanetworkopen.2020.37199
50. Li, ZH, Li, JX, Yu, GL, Yu, F, Li, K, and Szanton, S. The effect of resistance training on sleep in Chinese older adults: a randomized controlled trial. Geriatr Nurs. (2020) 42:289–94. doi: 10.1016/j.gerinurse.2020.09.002
51. Shree Ganesh, HR, Subramanya, P, Raghavendra, RM, and Udupa, V. Role of yoga therapy in improving digestive health and quality of sleep in an elderly population: a randomized controlled trial. J Bodyw Mov Ther. (2021) 27:692–7. doi: 10.1016/j.jbmt.2021.04.012
52. de Lima, BE, Passos, GS, Youngstedt, SD, Bandeira Santos Júnior, LC, and Santana, MG. Effects of Xbox Kinect exercise training on sleep quality, anxiety and functional capacity in older adults. J Bodyw Mov Ther. (2021) 28:271–5. doi: 10.1016/j.jbmt.2021.07.029
53. Jiménez-García, JD, Hita-Contrera, F, de la Torre-Cruz, MJ, Aibar-Almazán, A, Achalandabaso-Ochoa, A, Fábrega-Cuadros, R, et al. Effects of HIIT and MIIT suspension training programs on sleep quality and fatigue in older adults: randomized controlled clinical trial. Int J Environ Res Pub He. (2021) 18:1211. doi: 10.3390/ijerph18031211
54. Wang, L, Wu, B, Tao, H, Chai, N, Zhao, X, Zhen, X, et al. Effects and mediating mechanisms of a structured limbs-exercise program on general cognitive function in older adults with mild cognitive impair-ment: a randomized controlled trial. Int J Nurs Stud. (2020) 110:103706. doi: 10.1016/j.ijnurstu.2020.103706
55. Tseng, TH, Chen, HC, Wang, LY, and Chien, MY. Effects of exercise training on sleep quality and heart rate variability in middle-aged and older adults with poor sleep quality: a randomized controlled trial. J Clin Sleep Med. (2020) 16:1483–92. doi: 10.5664/jcsm.8560
56. Phansuea, P, Tangwongchai, S, Rattananupong, T, Lohsoonthorn, V, and Lertmaharit, S. Effectiveness of a qigong program on sleep quality among community-dwelling older adults with mild to moderate depression: a randomized controlled trial. J Health Res. (2020) 34:305–15. doi: 10.1108/JHR-04-2019-0091
57. Fan, BF, Song, WD, Zhang, JH, Er, YL, Xie, B, Zhang, HM, et al. The efficacy of mind-body (Baduanjin) exercise on self-reported sleep quality and quality of life in elderly subjects with sleep disturbances: a randomized controlled trial. Sleep Breath. (2020) 24:695–701. doi: 10.1007/s11325-019-01999-w
58. Song, D, and Yu, DSF. Effects of a moderate-intensity aerobic exercise Programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled Tria. Int J Nurs Stud. (2019) 93:97–105. doi: 10.1016/j.ijnurstu.2019.02.019
59. Gümüş Şekerci, Y, and Kir Biçer, E. The effect of walking exercise on quality of life and sleep in elderly individuals: randomized controlled study. Turk Geriatri Dergisi. (2019) 22:443–53. doi: 10.31086/tjgeri.2020.123
60. Bademli, K, Lok, N, Canbaz, M, and Lok, S. Effects of physical activity program on cognitive function and sleep quality in elderly with mild cognitive impairment: a randomized controlled trial. Perspect Psychiatr C. (2019) 55:401–8. doi: 10.1111/ppc.12324
61. Aibar-Almazan, A, Hita-Contreras, F, Cruz-Díaz, D, de la Torre-Cruz, M, Jiménez-García, JD, and Martínez-Amat, A. Effects of Pilates training on sleep quality, anxiety, depression and fatigue in postmenopausal women: a randomized controlled trial. Maturitas. (2019) 124:62–7. doi: 10.1016/j.maturitas.2019.03.019
62. El-Kader, SM, and Al-Jiffri, OH. Aerobic exercise modulates cytokine profile and sleep quality in elderly. Afri Health Sci. (2019) 19:2198–207. doi: 10.4314/ahs.v19i2.45
63. Pourhabib, A, Fotokian, Z, Abrotan, S, and Nasiri, M. Effects of aerobic and resistance exercise program on sleep quality in the elderlies with heart failure. J Babol Univ Med Sci. (2018) 20:63–7. doi: 10.18502/ssu.v27i7.1935
64. Curi, VS, Vilaça, J, Haas, AN, and Fernandes, HM. Effects of 16-weeks of Pilates on health perception and sleep quality among elderly women. Arch Gerontol Geriat. (2018) 74:118–22. doi: 10.1016/j.archger.2017.10.012
65. Choi, MJ, and Sohng, KY. The effects of floor-seated exercise program on physical fitness, depression, and sleep in older adults: a cluster randomized controlled trial. Int J Gerontol. (2017) 12:116–21. doi: 10.1016/j.ijge.2017.06.003
66. Laredo-Aguilera, JA, Carmona-Torres, JM, García-Pinillos, F, and Latorre-Román, PÁ. Effects of a 10-week functional training programme on pain, mood state, depression, and sleep in healthy older adults. Psychogeriatrics. (2018) 18:292–8. doi: 10.1111/psyg.12323
67. Cramer, H, Pokhrel, B, Fester, C, Meier, B, Gass, F, Lauche, R, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psycho-Oncology. (2016) 25:412–20. doi: 10.1002/pon.3927
68. Chen, LJ, Fox, KR, Ku, PW, and Chang, YW. Effects of aquatic exercise on sleep in older adults with mild sleep impairment: a randomized controlled trial. Int J Behav Med. (2015) 25:412–20. doi: 10.1007/s12529-015-9492-0
69. Chen, HM, Tsai, CM, Wu, YC, Lin, KC, and Lin, CC. Effect of walking on circadian rhythms and sleep quality of patients with lung cancer: a randomised controlled trial. Brit J Cancer. (2016) 115:1304–12. doi: 10.1038/bjc.2016.356
70. Sharif, F, Seddigh, M, Jahanbin, I, and Keshavarzi, S. The effect of aerobic exercise on quantity and quality of sleep among elderly people referring to health centers of Lar city, southern of Iran; a randomized controlled clinical trial. Curr Aging Sci. (2015) 8:248–55. doi: 10.2174/1874609808666150727113127
71. Taylor-Piliae, RE, Hoke, TM, Hepworth, JT, Latt, LD, Najafi, B, and Coull, BM. Effect of tai chi on physical function, fall rates and quality of life among older stroke survivors. Arch Phys Med Rehab. (2014) 95:816–24. doi: 10.1016/j.apmr.2014.01.001
72. Irwin, MR, Olmstead, R, Carrillo, C, Sadeghi, N, Breen, EC, Witarama, T, et al. Cognitive behavioral therapy vs. tai chi for late life insomnia and inflammatory risk: a randomized controlled comparative efficacy trial. Sleep. (2014) 37:1543–52. doi: 10.5665/sleep.4008
73. Cheung, C, Wyman, JF, Resnick, B, and Savik, K. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC Complem Altern M. (2014) 14:160. doi: 10.1186/1472-6882-14-160
74. Hariprasad, VR, Sivakumar, PT, Koparde, V, Varambally, S, Thirthalli, J, Varghese, M, et al. Effects of yoga intervention on sleep and quality-of-life in elderly: a randomized controlled trial. Indian J Psychiat. (2013) 55:364–S368. doi: 10.4103/0019-5545.116310
75. Oudegeest-Sander, MH, Eijsvogels, THM, Verheggen, RJHM, Poelkens, F, Hopman, MTE, Jones, H, et al. Impact of physical fitness and daily energy expenditure on sleep efficiency in young and older humans. Gerontology. (2013) 59:8–16. doi: 10.1159/000342213
76. Nguyen, MH, and Kruse, A. A randomized controlled trial of tai chi for balance, sleep quality and cognitive performance in elderly Vietnamese. Clin Interv Aging. (2012) 7:185–90. doi: 10.2147/CIA.S32600
77. Chen, MC, Liu, HE, Huang, HY, and Chiou, AF. The effect of a simple traditional exercise programme (Baduanjin exercise) on sleep quality of older adults: a randomized controlled trial. Int J Nurs Stud. (2012) 49:265–73. doi: 10.1016/j.ijnurstu.2011.09.009
78. Richards, KC, Lambert, C, Beck, CK, Bliwise, DL, Evans, WJ, Kalra, GK, et al. Strength training, walking, and social activity improve sleep in nursing home and assisted living residents: randomized controlled trial. J Am Geriatr Soc. (2011) 59:214–23. doi: 10.1111/j.1532-5415.2010.03246.x
79. McCurry, SM, Lambert, C, Beck, CK, Bliwise, DL, Evans, WJ, Kalra, GK, et al. Increasing walking and bright light exposure to improve sleep in community-dwelling persons with Alzheimer's disease: results of a randomized. Controlled Trial J AM Geriatr Soc. (2011) 59:1393–402. doi: 10.1111/j.1532-5415.2011.03519.x
80. Hosseini, H, Esfirizi, MF, Marandi, SM, and Rezaei, A. The effect of Ti chi exercise on the sleep quality of the elderly residents in Isfahan, Sadeghieh elderly home. Iran J Nurs Midwife. (2011) 16:55–60.
81. Chen, KM, Chen, MH, Chao, HC, Hung, HM, Lin, HS, and Li, CH. Sleep quality, depression state, and health status of older adults after silver yoga exercises: cluster randomized trial. Int J Nurs Stud. (2009) 46:154–63. doi: 10.1016/j.ijnurstu.2008.09.005
82. King, AC, Pruitt, LA, Woo, S, Castro, CM, Ahn, DK, Vitiello, MV, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A-Biol. (2008) 63:997–1004. doi: 10.1093/gerona/63.9.997
83. Irwin, MR, Olmstead, R, and Motivala, SJ. Improving sleep quality in older adults with moderate sleep complaints: a randomized controlled trial of tai chi chih. Sleep. (2008) 31:1001–8. doi: 10.1016/j.seizure.2008.03.007
84. Gary, R, and Lee, SY. Physical function and quality of life in older women with diastolic heart failure: effects of a progressive walking program on sleep patterns. Prog Cardiovasc Nurs. (2007) 22:72–80. doi: 10.1111/j.0889-7204.2007.05375.x
85. Frye, B, Scheinthal, S, Kemarskaya, T, and Pruchno, R. Tai chi and low impact exercise: effects on the physical functioning and psychological well-being of older people. J Appl Gerontol. (2007) 26:433–53. doi: 10.1177/0733464807306915
86. Singh, NA, Clements, KM, and Fiatarone, MA. A randomized controlled trial of the effect of exercise on sleep. Sleep. (1997) 20:95–101. doi: 10.1093/sleep/20.2.95
87. Landry, GJ, Best, JR, and Liu-Ambrose, T. Measuring sleep quality in older adults: a comparison using subjective and objective methods. Front Aging Neurosci. (2015) 7:166. doi: 10.3389/fnagi.2015.00166
88. Lu, TY, Li, Y, Pan, JY, and Wu, DR. Study on minimal important difference of the Pittsburgh sleep quality index based on clinical trial of traditional Chinese medicine. J Guangzhou Univ Tradit Chin Med. (2013) 30:574–8. doi: 10.13359/j.cnki.gzxbtcm.2013.04.020
89. Solis-Navarro, L, Masot, O, Torres-Castro, R, Otto-Yanez, M, Fernandez-Jane, C, Sola-Madurell, M, et al. Effects on sleep quality of physical exercise programs in older adults: a systematic review and Meta-analysis. Clocks Sleep. (2023) 5:152–66. doi: 10.3390/clockssleep5020014
90. Wu, YHT, He, WB, Gao, YY, and Han, XM. Effects of traditional Chinese exercises and general aerobic exercises on older adults with sleep disorders: a systematic review and meta-analysis. J Integr Med. (2021) 19:493–502. doi: 10.1016/j.joim.2021.09.007
91. Santos, RVT, Tufik, S, and De Mello, MT. Exercise, sleep and cytokines: Is there a relation? Sleep Med Rev. (2007) 11:231–9. doi: 10.1016/j.smrv.2007.03.003
92. Ghilotti, F, Bellocco, R, Lagerros, YT, Thorson, A, Theorell-Haglöw, J, Åkerstedt, T, et al. Relationship between sleep characteristics and markers of inflammation in Swedish women from the general population. J Sleep Res. (2020) 30:e13093. doi: 10.1111/jsr.13093
93. O'Connor, PJ, and Youngstedt, SD. Sleep quality in older adults: effects of exercise training and influence of sunlight exposure. JAMA-J AM Med Assoc. (1997) 277:1034–5. doi: 10.1001/jama.1997.03540370024017
94. Passos, GS, Poyares, D, Santana, MG, Garbuio, SA, Tufik, S, and Mello, MT. Effect of acute physical exercise on patients with chronic primary insomnia. J Clin Sleep Med. (2010) 6:270–5. doi: 10.5664/jcsm.27825
95. Murphy, PJ, and Campbell, SS. Nighttime drop in body temperature: a physiological trigger for sleep onset? Sleep. (1997) 20:505–11. doi: 10.1093/sleep/20.7.505
96. Cohen, J. Statistical power analysis for the behavioral sciences. 2nd ed. Hoboken, NJ: Taylor and Francis (2013).
97. D’Aurea, CV, Frange, C, Poyares, D, Souza, AA, and Lenza, M. Physical exercise as a therapeutic approach for adults with insomnia: systematic review and meta-analysis. Einstein (São Paulo). (2022) 20:eAO8058. doi: 10.31744/einstein_journal/2022AO8058
98. Leproult, R, VanReeth, O, Byrne, MM, Sturis, J, and VanCauter, E. Sleepiness, performance, and neuroendocrine function during sleep deprivation: effects of exposure to bright light or exercise. J Biol Rhythm. (1997) 12:245–58. doi: 10.1177/074873049701200306
99. Neva, JL, Brown, KE, Mang, CS, Francisco, BA, and Boyd, LA. An acute bout of exercise modulates both intracortical and interhemispheric excitability. Eur J Neurosci. (2017) 45:1343–55. doi: 10.1111/ejn.13569
100. Desjardins, S, Lapierre, S, Hudon, C, and Desgagné, A. Factors involved in sleep efficiency: a population-based study of community-dwelling elderly persons. Sleep. (2019) 42:zsz038. doi: 10.1093/sleep/zsz038
101. Tse, MMY, Wan, VTC, and Ho, SSK. Physical exercise: does it help in relieving pain and increasing mobility among older adults with chronic pain? J Clin Nurs. (2011) 20:635–44. doi: 10.1111/j.1365-2702.2010.03548.x
102. Sugaya, K, Nishijima, S, Owan, T, Oda, M, Miyazato, M, and Ogawa, Y. Effects of walking exercise on nocturia in the elderly. Biomed Res. (2007) 28:101–5. doi: 10.2220/biomedres.28.101
103. Fank, F, Pereira, FDS, dos Santos, L, de Mello, MT, and Mazo, GZ. Effects of Exercise on Sleep in Older Adults: An Overview of Systematic Reviews and Meta-Analyses. J Aging Phys Activ. (2022) 30:1101–17. doi: 10.1123/japa.2021-0444
104. Dew, MA, Hoch, CC, Buysse, DJ, Monk, TH, Begley, AE, Houck, PR, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. (2003) 65:63–73. doi: 10.1097/01.PSY.0000039756.23250.7C
105. Buysse, DJ, Ancoli-Israel, S, Edinger, JD, Lich-stein, KL, and Morin, CM. Recommendations for a standard research assessment of insomnia. Sleep. (2006) 29:1155–73. doi: 10.1093/sleep/29.9.1155
106. Buman, MP, Hekler, EB, Bliwise, DL, and King, AC. Moderators and Mediators of Exercise-Induced Objective Sleep Improvements in Mid life and Older Adults With Sleep Complaints. Health Psychol. (2011) 30:579–87. doi: 10.1037/a0024293
Keywords: meta-analysis, exercise, physical activity, sleep, older people
Citation: Geng D, Li X and Sun G (2025) The effectiveness of exercise interventions in the improvement of sleep in older adult people: a meta-analysis. Front. Public Health. 13:1529519. doi: 10.3389/fpubh.2025.1529519
Received: 17 November 2024; Accepted: 14 February 2025;
Published: 05 March 2025.
Edited by:
Mika Venojärvi, University of Eastern Finland, FinlandReviewed by:
Nasr Chalghaf, University of Gafsa, TunisiaCopyright © 2025 Geng, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guotao Sun, Z3VvdGFvc3VuNTIwQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.