
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 25 March 2025
Sec. Infectious Diseases: Epidemiology and Prevention
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1526990
This article is part of the Research TopicEmerging Zoonotic Diseases: Understanding and Mitigating Risks at Animal-Human InterfacesView all 7 articles
Background: Psittacosis is a global and underappreciated zoonosis, with increasing reported cases in many countries. There have been several outbreaks and even deaths of psittacosis reported in China. Understanding its epidemiological characteristics and dimensions is crucial for formulating precise prevention and control strategies. This study aimed to analyze the epidemiological characteristics of human psittacosis in Guangzhou, China.
Methods: The demographic characteristics, clinical manifestations, temporal patterns, geographic distribution and potential exposures of psittacosis in Guangzhou were analyzed based on the surveillance data and epidemiological investigation conducted between January 2021 and June 2024. Seasonal and trend decomposition using LOESS was applied to decompose the number of psittacosis cases into trend, seasonal and remainder component.
Results: A total of 148 cases were reported, with a significant increase in the number of psittacosis cases over the study period. Most of cases were sporadic and detected by metagenomic next-generation sequencing (mNGS). Psittacosis was predominant males aged 40–79 years. Fever and pneumonia were the most commonly observed clinical manifestations. A seasonal trend was observed in the number of psittacosis cases with a high prevalence of cases in December and March. A total of 108 local cases (87%) occurred in rural regions. Among local cases, 67.7% reported a history of contact with birds or poultry, and 17.7% had been exposed to a related environment. The suspected source of infection differed between urban and rural areas, with parrots being the primary source in urban areas and poultry in rural areas.
Conclusion: Increasing clinicians’ awareness, enhancing epidemiological surveillance, paying close attention to the epidemic in rural areas, and implementing measures against avian influenza, will be conducive to preventing and controlling psittacosis.
Psittacosis, commonly referred to as parrot fever, is a zoonotic infectious disease caused by Chlamydia psittaci (C. psittaci). More than 450 bird species, including poultry, are susceptible to C. psittaci infection (1) and the prevalence of C. psittaci infections in birds is estimated to be approximately 20% (2). The pathogen can be excreted in the urine, feces and other excretions of infected birds such as chickens, ducks, pigeons and parrots, and can remain infectious in the environment for months. Humans can be infected by inhaling aerosols containing C. psittaci, with manifestations from mild symptoms to severe pneumonia (3). Psittacosis has been proven to be an important cause of community-acquired pneumonia (CAP), but patients are often misdiagnosed and under inappropriate treatment due to nonspecific symptoms (4). The public health risk and significance of psittacosis remain largely underappreciated.
In recent years, the development of metagenomic next-generation sequencing (mNGS) and commercial polymerase chain reaction (PCR) detection reagents has significantly increased the detection rate of C. psittaci (5, 6). Several outbreaks and even deaths of psittacosis reported in China, including in Zhejiang province (7), Shandong province (8) and other places, which raised public concern. However, routine surveillance of psittacosis has not been implemented in China, as it is not a notifiable infectious disease (9). Since 2021, Guangzhou, for the first time, has started to require medical institutions at all levels to implement the all-reporting mechanism for psittacosis cases to strengthen disease surveillance. Epidemiological investigations were conducted to identify suspected infection source and clusters of cases. In this study, we aim to elucidate the epidemiological characteristics of human psittacosis in Guangzhou and to provide scientific evidence for future policymaking.
Guangzhou, the capital of Guangdong province, is located in the south of China, with a resident population of 18 million. The city covers an area of 7434.4 km2 and comprises six urban administrative regions (Yuexiu, Liwan, Tianhe, Haizhu, Baiyun, and Huangpu) and five rural administrative regions (Panyu, Huadu, Nansha, Zengcheng, and Conghua).
This study was conducted by collecting data of psittacosis in Guangzhou between January 1, 2021 and June 30, 2024. Psittacosis cases data were extracted from the China Information System for Disease Control and Prevention (CISDCP). CISDCP is an Internet-based real-time system for infectious diseases in China established in 2004. Medical institutions in Guangzhou were required to report psittacosis cases to CISDCP upon obtaining a positive result via any of the following methods: detection of (1) C. psittaci by culture, or (2) C. psittaci gene fragments by mNGS in specimens, like broncho-alveolar lavage fluid (BALF), blood, or sputum, meeting the criteria for a positive mNGS result, or (3) the C. psittaci genome by PCR. Guangzhou Centre for Disease Control and Prevention (CDC) and the local CDC conducted epidemiological investigations on all psittacosis cases or their relatives during the study period. The information of psittacosis included demographic characteristics (e.g., age, sex, occupation, address), exposure history, symptoms, date of illness onset, date of medical institutions diagnosed and reported, types of specimens, and the mNGS examination results (if available).
This study described the demographic characteristics, clinical manifestations, temporal patterns and geographic distribution of psittacosis cases. Categorical variables were presented as percentages. Continuous variables were presented as means ± standard deviation (SD) for normal distribution, or as medians with inter quartile range (IQR) for non-normal distribution. Seasonal and trend decomposition using LOESS (STL) was performed to decompose the number of psittacosis cases into trend component, seasonal component and remainder component. The chi-square test was used to assess whether the suspected infection source was different in urban and rural areas. All statistical analyses were performed using Microsoft Excel 2016 and R 4.1.1.
Between 1 January 2021 and 30 June 2024, 148 psittacosis cases were reported in Guangzhou, including one associated death (case fatality ratio: 0.7%). Among the 148 cases, two involved cohabiting couples, and two others involved a mother and son. Except for these two clusters, the majority of the cases (144, 97.3%) were sporadic.
Psittacosis cases were predominantly males (91, 61.5%) with a sex ratio of 1.6:1. The mean age was 59 years [standard deviation (SD) = 13, range from 21 to 93]. Middle-aged and older adult aged 40–79 years represented 89.2% of cases. Household and unemployed persons, farmers, and retirees accounted for about 70% of the psittacosis cases (Table 1).
Almost all psittacosis cases (147, 99.3%) were diagnosed by mNGS, with only one case was identified by PCR through active case search. Cases were detected by mNGS for 133 BALF samples, 6 blood samples, and 4 sputum samples. Four of the cases underwent both BALF and blood mNGS testing, and the results were positive.
We collected the clinical manifestations data of 140 psittacosis cases through epidemiological investigation and medical records. The most common symptom was fever, which observed in almost all cases (136, 97.1%). Of these, 107 cases reported their highest body temperature as illness onset, ranging from 38°C to 42°C. Other common symptoms were cough (70.0%), expectoration (45.7%), fatigue (40.7%), chest tightness and shortness of breath (37.1%), chills (30.7%), and headache (29.7%). Other reported symptoms included myalgia, dyspnea, dizziness, sore throat, and gastrointestinal problems (nausea and vomiting, abdominal pain and diarrhea). All cases underwent X-ray or CT scan. According to examination, all patients showed unilateral pneumonia or bilateral pneumonia. The median duration from symptom onset to diagnosis was 11 days (IQR, 7 to 16). All clinical manifestations are shown in Table 2.
Figure 1A showed the change in the number of psittacosis cases in the time series between January 2021 and June 2024. The number of psittacosis cases in Guangzhou jumped from 6 in 2021 to 21 in 2022 and 63 in 2023. In the first half of 2024, 58 psittacosis cases have been reported. In Figure 1B, a significant rising secular trend was observed over the period from January 2021 to June 2024 with an accelerating growth rate. Figure 1C showed the seasonal trends of psittacosis throughout the year. The number of cases in December and from February to March were above the annual average, with λ values of 2.13, 1.00 and 2.48, respectively. The highest number occurred in March. Figure 1D presented the remainder component of the time series. The auto-correlation function (ACF) and partial auto-correlation function (PACF) of the remainder were performed to analyze the decomposition effect. The ACF and PACF plots showed no significant auto-correlation of the residuals. The Ljung-Box test was also applied to verify the remainder was a white noise (p>0.05), indicating that the decomposition effect was satisfactory.
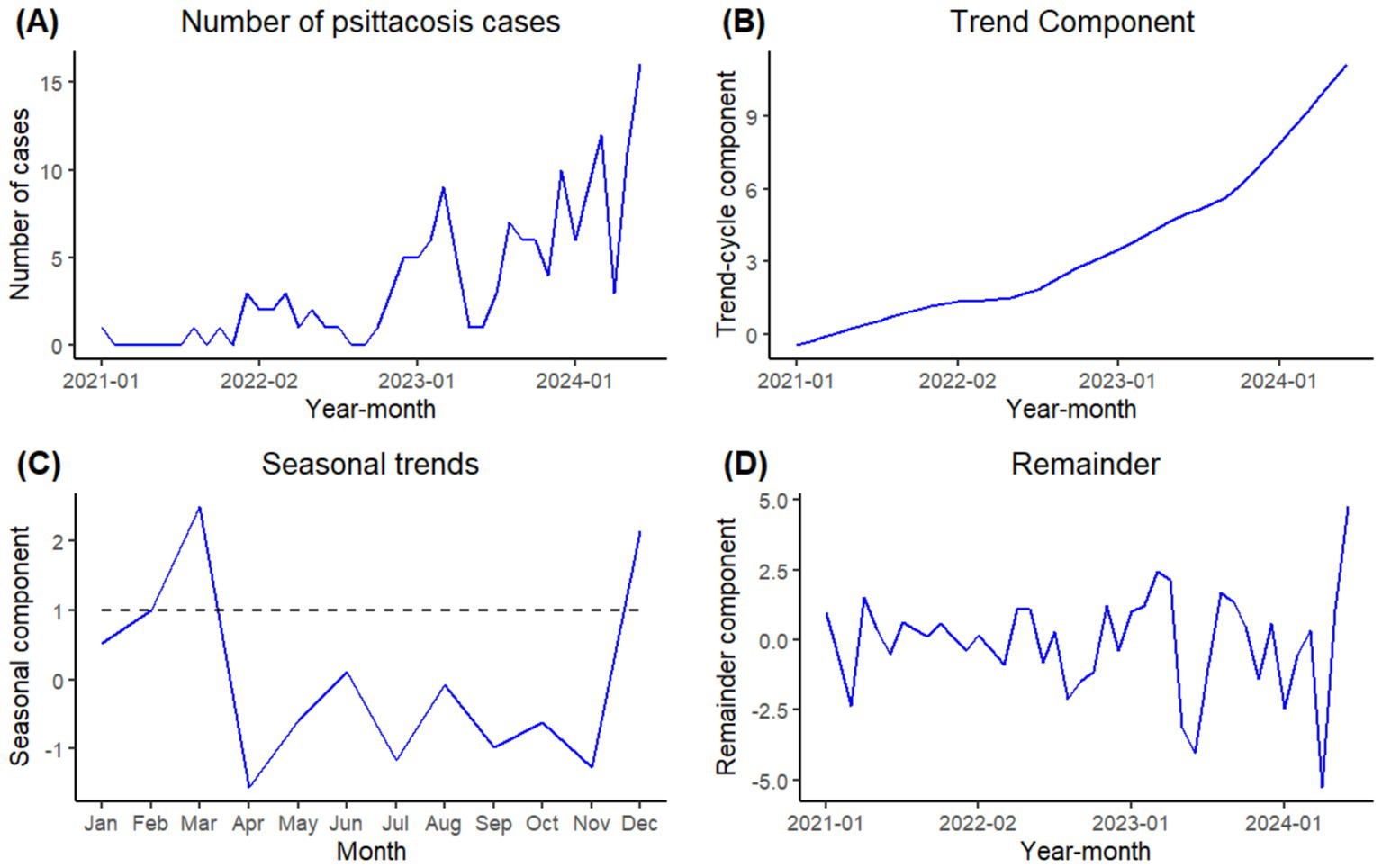
Figure 1. Time series of psittacosis cases number, secular trend, seasonal trend and remainder by STL decomposition. (A: Number of psittacosis cases; B: secular trend component; C: seasonal component; D: remainder component).
For 148 cases, 124 were locally transmitted cases and 24 were imported from surrounding cities. During the study period, locally transmitted cases of psittacosis occurred in 10 districts in Guangzhou, with the exception of the Tianhe district. Five rural administrative regions, where had lower population density, reported significantly more psittacosis cases than the six urban administrative regions. Eighty-seven percent of cases occurred in rural administrative regions, with the highest number (44 cases) in Zengcheng, followed by 28 cases in Panyu and 21 cases in Nansha (Figure 2).

Figure 2. Geographical distribution of psittacosis cases by district in Guangzhou during January 2021 to June 2024.
Among the locally transmitted cases, 84 cases (67.7%) reported a history of contact with birds or poultry before illness onset, and 22 cases (17.7%) did not contact birds or poultry directly but had been exposed to a related environment, such as live poultry markets, henneries, aviaries, or bird habitats. Only 16 cases (12.9%) denied having exposed to poultry or birds. The sources for the remaining 2 cases (1.6%) were unknown/unreported. Among the 84 cases with a suspected infection source, chicken (n = 36, 42.9%) was the most common, followed by parrots (n = 23, 27.4%) and ducks (n = 22, 26.2%). Most had contact though activities such as raising birds and poultry, slaughtering poultry, or handling infected birds. Only a small number of patients (n = 12, 14.3%) had one-time exposure to birds, such as buying live poultry.
The suspected infection source differed between urban and rural areas (p < 0.05). In urban areas, most cases (n = 8, 80%) reported contact with parrots with none reporting exposure to pigeons. In contrast, cases in rural areas reported more contact with poultry (n = 53, 71.6%) than parrots (n = 15, 20.3%) (Table 3).
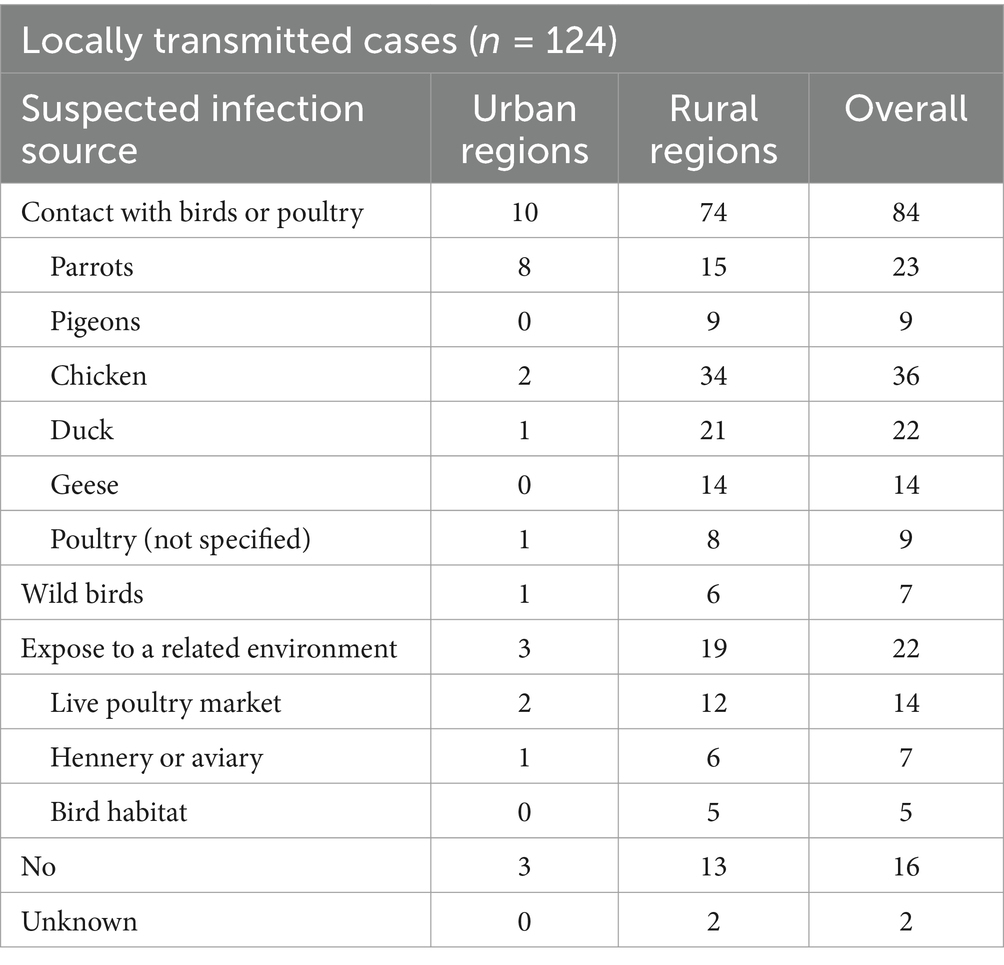
Table 3. Suspected infection source of psittacosis cases in Guangzhou during January 2021 to June 2024.
Human psittacosis is considered an emerging public health risk. As it is not a nationally notifiable disease in China, previous studies on psittacosis were case studies (10, 11), case series analysis (12), and multi-center observational studies (13–15), which rarely described the underlying situation of the disease in China. This study is the largest cross-sectional, observational study of human psittacosis in China, which analyzed the epidemiological characteristics of psittacosis in Guangzhou from 1 January 2021 to 30 June 2024. The finding is of importance to provide information on the public health risk of human psittacosis and to formulate prevention and control strategies.
This study showed that the number of psittacosis cases in Guangzhou tripled annually since 2021, with 99.3% of cases detected by mNGS. The recent development of mNGS technology has led many hospitals to send specimens (usually BALF) from patients with unexplained severe pneumonia to third-party institutions for testing, which has directly contributed to the significant increase in the detection rate of psittacosis. There was no significant upward trend in psittacosis cases over the past 10 years in the United States and Australia (16, 17) and the annual number and notification rate in Japan declined (18). However, a similar increase in case numbers was observed in several European countries (19, 20). Austria, Denmark, Germany, Sweden, and Netherlands reported an unusual and unexpected increase in psittacosis cases from 2023 to early 2024. In Sweden, the general increase since 2017 could be attributed to the increased use of more sensitive PCR panels, whereas testing procedures of the other four European countries have not changed in recent years (20). The difference in trends between China and other countries requires further investigation. It is important to determine whether it is a true secular trend in cases or if it reflects differences in the sensitivity of surveillance systems or diagnostic techniques, as a large number of mNGS-identified cases have been reported primarily in China. The actual number of psittacosis cases in Guangzhou is likely underestimated, as most cases are reported in CISDCP after the diagnosis of pneumonia in hospitals. It is possible that cases of psittacosis remain undetected due to atypical or mild manifestations, or because they improve after anti-infective treatment without being detected, or was diagnosed as CAP. Given these factors, it is foreseeable that the number of psittacosis cases will continue to rise in the future.
Through the epidemiological investigation of psittacosis cases reported in Guangzhou, we found only two clusters. Within the two clusters, the cases both had contact with sick parrots and their illness onset occur close in time, suggesting co-exposure to infected parrots as the likely cause. Other cases did not show any family, workplace or temporal clustering, without evidence of person-to-person transmission. In addition, over 85% of locally transmitted cases reported direct contact with birds or poultry, or exposure to related environments before illness onset. This study revealed that human psittacosis is primarily an opportunistic disease caused by contacting with birds or poultry, although C. psittaci has the potential to evolve human-to-human transmission via various routes (8).
The epidemic process of psittacosis can be viewed as a chain with three major links. The first link is the source of infection. C. psittaci is a globally distributed zoonotic bacterium, and bird infections are common. While parrots have traditionally been considered the primary host of psittacosis (3), but there are also reports that poultry, turkey, pigeons, and even some mammals, such as horses (21) and sheep (22), can be infected with C. psittaci and be a source of infection. In Guangzhou, most psittacosis cases can be traced to poultry (especially chickens) and parrots, with a lesser number linked to wild birds and pigeons. This finding aligns with the study by Wang et al., which identified poultry as the most common source of infection in humans (51.91%), followed by wild birds (5.53%), pigeons (2.98%) and parrots (2.56%) (23). However, this result contrasts with findings from Denmark, where 80% of cases were linked to wild birds (20). This discrepancy suggested the source of infection may vary by regions, as the distribution of bird species and their populations differ significantly across areas. The source of infection was significantly different between urban and rural areas in Guangzhou. In urban areas, parrots were the most suspected source of infection, while in rural areas, poultry was more likely to be the source. The difference in the types of birds people are exposed to in urban versus rural areas may help explain this variation (24). Since much of Guangzhou’s urban area restricts live poultry operations (25), the exposure of rural people to live poultry is much more than that of urban areas.
The second link is the route of transmission. The most common route of transmission in humans is through inhalation of contaminated aerosols from feathers (e.g., flapping wings), excreta (e.g., drying), or the environment (e.g., sand and cages), as well as handling of feathers, tissues of infected birds (e.g., dissecting or eviscerating). In Guangzhou, most cases involved poultry farmers or pet parrot owners, who were likely infected through prolonged contact with birds or through close operations such as slaughtering and cleaning up feces. One case was also reported where the person was bitten by a sick parrot. Psittacosis cases who did not contact with birds or were just exposed to the related environment for a short time have also been found, but less frequently. This aligns with findings from Denmark and Netherlands, where several cases every year report no direct contact with birds (20). It suggests that the transmission of C. psittaci from birds (including poultry) to humans is not easy and that direct contact with infected birds or objects contaminated by their excreta remains the primary route of transmission for human psittacosis.
The third link is high-risk groups. Human psittacosis most commonly occurs in persons with a history of contact with birds or poultry, either in occupational settings or through exposure to companion bird. In Guangzhou, psittacosis cases reported were predominantly observed in males aged 40 to 79 years, and among households and unemployed persons, farmers, and retirees. This finding is consistent with the age and gender distribution observed in Japan (18), Belgium (19) and Fujian and Zhejiang, China (26, 27). We hypothesized that there were two reasons for this: first, many families in rural regions had small poultry-raising areas and middle-aged and older adults in China were more likely to be in close contact with poultry. The second was that middle-aged and older adults were more susceptible to infection because they had weaker immunity or more underlying diseases than younger people.
Human psittacosis occurred all year, with a peak season from December to March of the following year, which was consistent with other respiratory diseases. This period also coincides with the Chinese Lunar New Year, when the demand for live poultry increases, leading to more frequent transportation, trade, and consumption of live poultry. Psittacosis cases typically present with sudden onset, with fever being the most common symptom. The body temperature of fever cases was between 38 and 42°C. Another common feature of psittacosis cases was that chest radiography images show unilateral pneumonia or bilateral pneumonia. Even in the mild case found by active case search, CT scan revealed infection in the right lower lobe. A similar situation was observed in Germany, where almost all cases had pneumonia (18/19) (20). In addition to fever, some patients displayed non-specific symptoms of respiratory infection, including cough, expectoration, fatigue and chest tightness and shortness of breath. Unlike typical bacterial pneumonia, which primarily affects the lungs, systematic symptoms (like chills, headache, or muscle aches) and gastrointestinal presentations (such as nausea and vomiting, abdominal pain, or diarrhea) were also reported in this study. Clinicians should consider psittacosis when encountering patients with a history of avian or bird exposure during the peak season, especially those presenting with high fever, ineffective conventional treatments, and pneumonia seen on chest CT scans.
Geographically, the regions with the largest number of cases were Zengcheng, Panyu, and Nansha districts. Most psittacosis cases in Guangzhou occurred in rural administrative regions, where live poultry trade is permitted. This distribution is similar to that observed in Netherlands, where cases primarily occurred in poultry-dense areas, particularly in the main poultry production zones (28). It’s worth noting that this pattern was similar to the geographical distribution of human avian influenza in China in recent years (29). To prevent and control human avian influenza, almost all urban administrative regions in Guangzhou are designated as restricted zones for live poultry operations, prohibiting the trade of live poultry (25). However, Guangzhou is a big poultry-consuming city and live poultry stocks are still prevalent. Many families in rural areas operated small-scale poultry farms or keep backyard poultry (30). These families depend on poultry for income and food, even when poultry show symptoms of illness, they may still be slaughtered for sale or consumption. We analyzed the measures taken by the Guangzhou government to control avian influenza, which also helped to control the spread of psittacosis in urban areas to some extent because the two pathogens share similar routes and modes of transmission.
This study revealed the epidemiological characteristics of psittacosis in Guangzhou, China. More localized intervention policy for disease prevention and control are required. First, it is important to train medical personnel to improve the awareness and diagnostic ability of psittacosis and make full use of mNGS to screen unexplained pneumonia cases with high fever and a clear exposure history of poultry or birds. Second, both health and agriculture departments should enhance surveillance of psittacosis in humans and birds, respectively. C. psittaci is suggested to be included in veterinary legal quarantine and the list of notifiable infectious diseases in China. Third, the government is suggested to monitor closely the epidemic situation in rural regions and poultry-dense areas, and sustain preventive and control measures related to avian influenza (e.g., restricted zones for live poultry operations). Fourth, it should attach great importance to the publicity and health education on psittacosis among poultry farm workers, bird owners, and individuals who frequent contact with avians.
There are several limitations in this study. First, there were non-response bias and recall bias in tracing the exposure history. Second, current surveillance has found that psittacosis was mostly hospitalized and severe, but may not find some asymptomatic infection and mild cases. As a result, the true number of individuals infected with C. psittaci may be underestimated, and the severity of the disease could be overestimated.
The number of psittacosis increased rapidly in Guangzhou from 2021 to 2024, largely due to the widespread use of mNGS in unexplained pneumonia cases. The transmission route was mainly avian-to-human transmission and the peak season was from December to the following March. Most cases showed fever, pneumonia, and non-specific respiratory symptoms, with a history of contact with birds or exposure to a related environment. The majority of cases occurred in middle-aged and older adults, primarily in rural regions. In the future, increasing clinicians’ awareness about psittacosis, enhancing surveillance in humans and birds, paying close attention to the epidemic situation in rural areas, and continuing to take prevention and control measures against avian influenza, will be conducive to the prevention and control of the spread of psittacosis in humans.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethic Committee of Guangzhou center for disease control and prevention (approval number GZCDC-ECHR-2022P0015). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the patient or patient legal guardian/next of kin.
YW: Formal analysis, Funding acquisition, Writing – original draft, Writing – review & editing, Visualization. WZ: Data curation, Writing – review & editing, Funding acquisition. YL: Investigation, Writing – original draft. XL: Investigation, Writing – review & editing. JX: Project administration, Writing – review & editing. RZ: Conceptualization, Project administration, Writing – review & editing, Funding acquisition. PQ: Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the fund for Health Science and Technology Project of Guangzhou (20231A011072, 20241A011065), Guangzhou Municipal Science and Technology Project (2025A03J3695) and the Key Project of Medicine Discipline of Guangzhou (2025-2027-11).
We acknowledged the hard work of local CDC in the epidemiological investigation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Stokes, HS, Berg, ML, and Bennett, ATD. A review of chlamydial infections in wild birds. Pathogens (Basel, Switzerland). (2021) 10. doi: 10.3390/pathogens10080948
2. Sukon, P, Nam, NH, Kittipreeya, P, Sara-In, A, Wawilai, P, Inchuai, R, et al. Global prevalence of chlamydial infections in birds: a systematic review and meta-analysis. Prev Vet Med. (2021) 192:105370. doi: 10.1016/j.prevetmed.2021.105370
3. Dembek, ZF, Mothershead, JL, Owens, AN, Chekol, T, and Wu, A. Psittacosis: An underappreciated and often undiagnosed disease. Pathogens (Basel, Switzerland). (2023) 12. doi: 10.3390/pathogens12091165
4. Hogerwerf, L, de Gier, B, Baan, B, and Van Der Hoek, W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. (2017) 145:3096–105. doi: 10.1017/S0950268817002060
5. Tang, J, Tan, W, Luo, L, Xu, H, and Li, N. Application of metagenomic next-generation sequencing in the diagnosis of pneumonia caused by Chlamydia psittaci. Microbiol Spectrum. (2022) 10:e0238421. doi: 10.1128/spectrum.02384-21
6. Nieuwenhuizen, AA, Dijkstra, F, Notermans, DW, and van der Hoek, W. Laboratory methods for case finding in human psittacosis outbreaks: a systematic review. BMC Infect Dis. (2018) 18:442. doi: 10.1186/s12879-018-3317-0
7. Yao, W, Chen, X, Wu, Z, Wang, L, Shi, G, Yang, Z, et al. A cluster of psittacosis cases in Lishui, Zhejiang Province, China, in 2021. Front Cell Infect Microbiol. (2022) 12:1044984. doi: 10.3389/fcimb.2022.1044984
8. Zhang, Z, Zhou, H, Cao, H, Ji, J, Zhang, R, Li, W, et al. Human-to-human transmission of Chlamydia psittaci in China, 2020: an epidemiological and aetiological investigation. Lancet Microbe. (2022) 3:e512–20. doi: 10.1016/S2666-5247(22)00064-7
9. Liu, S, Cui, Z, Carr, MJ, Meng, L, Shi, W, and Zhang, Z. Chlamydia psittaci should be a notifiable infectious disease everywhere. Lancet Microbe. (2023) 4:e62–3. doi: 10.1016/S2666-5247(22)00306-8
10. Cao, J, Xie, X, Lei, Y, Li, S, Song, X, Lei, Y, et al. Epidemiological and clinical characteristics of a family cluster of psittacosis: a case report. IDCases. (2023) 33:e01845. doi: 10.1016/j.idcr.2023.e01845
11. Wu, Y, Xu, X, Liu, Y, Jiang, X, Wu, H, Yang, J, et al. Case report: clinical analysis of a cluster outbreak of chlamydia psittaci pneumonia. Front Cell Infect Microbiol. (2023) 13:1214297. doi: 10.3389/fcimb.2023.1214297
12. Teng, XQ, Gong, WC, Qi, TT, Li, GH, Qu, Q, Lu, Q, et al. Clinical analysis of metagenomic next-generation sequencing confirmed Chlamydia psittaci pneumonia: a case series and literature review. Infect Drug Resist. (2021) 14:1481–92. doi: 10.2147/IDR.S305790
13. Yang, M, Yang, DH, Yang, H, Ding, SZ, Liu, CH, Yin, HM, et al. Clinical characteristics of Chlamydia psittaci pneumonia infection in central South China. Infect Dis Ther. (2022) 11:1631–47. doi: 10.1007/s40121-022-00662-4
14. Huang, W, Wang, F, Cai, Q, Xu, H, Hong, D, Wu, H, et al. Epidemiological and clinical characteristics of psittacosis among cases with complicated or atypical pulmonary infection using metagenomic next-generation sequencing: a multi-center observational study in China. Ann Clin Microbiol Antimicrob. (2023) 22:80. doi: 10.1186/s12941-023-00631-w
15. Tang, X, Wang, N, Liu, G, Tan, H, Li, AM, Gao, YQ, et al. Psittacosis caused severe community-acquired pneumonia accompanied by acute hypoxic respiratory failure: a multicenter retrospective cohort study from China. BMC Infect Dis. (2023) 23:532. doi: 10.1186/s12879-023-08283-z
16. U.S. Centers for Disease Control and Prevention. National Notifiable Disease Surveillance System Annual Summary Data (2016–2022) of psittacosis in U.S. Available online at: https://wonder.cdc.gov/nndss-annual-summary.html (Accessed February 20, 2025)
17. Australia Government Department of Health and Aged Care. National notifiable diseases surveillance system annual notifications (1991–2018) of psittacosis/ornithosis in Australia. Available online at: https://www.health.gov.au/resources/apps-and-tools/national-notifiable-diseases-surveillance-system-nndss-data-visualisation-tool (Accessed February 20, 2025)
18. Kozuki, E, Arima, Y, Matsui, T, Sanada, Y, Ando, S, Sunagawa, T, et al. Human psittacosis in Japan: notification trends and differences in infection source and age distribution by gender, 2007 to 2016. Ann Epidemiol. (2020) 44:60–3. doi: 10.1016/j.annepidem.2020.03.001
19. Rybarczyk, J, Versteele, C, Lernout, T, and Vanrompay, D. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. (2020) 75:42–8. doi: 10.1080/17843286.2019.1590889
20. World Health Organization. Disease outbreak news; psittacosis–European region; (2024) [updated 5 March 2024]. Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON509 (Accessed February 20, 2025)
21. Chan, J, Doyle, B, Branley, J, Sheppeard, V, Gabor, M, Viney, K, et al. An outbreak of psittacosis at a veterinary school demonstrating a novel source of infection. One Health (Amsterdam, Netherlands). (2017) 3:29–33. doi: 10.1016/j.onehlt.2017.02.003
22. Osman, KM, Ali, HA, ElJakee, JA, and Galal, HM. Chlamydophila psittaci and Chlamydophila pecorum infections in goats and sheep in Egypt. Rev Sci Tech. (2011) 30:939–48. doi: 10.20506/rst.30.3.2088
23. Wang, J, Wang, B, Xiao, J, Chen, Y, and Wang, C. Chlamydia psittaci: a zoonotic pathogen causing avian chlamydiosis and psittacosis. Virulence. (2024) 15:2428411. doi: 10.1080/21505594.2024.2428411
24. Peng, Z, Wu, P, Ge, L, Fielding, R, Cheng, X, Su, W, et al. Rural villagers and urban residents exposure to poultry in China. PloS One. (2014) 9:e95430. doi: 10.1371/journal.pone.0095430
25. Chen, Y, Cheng, J, Xu, Z, Hu, W, and Lu, J. Live poultry market closure and avian influenza a (H7N9) infection in cities of China, 2013-2017: an ecological study. BMC Infect Dis. (2020) 20:369. doi: 10.1186/s12879-020-05091-7
26. Liu, K, Wu, L, Chen, G, Zeng, D, Zhong, Q, Luo, L, et al. Clinical characteristics of Chlamydia psittaci infection diagnosed by metagenomic next-generation sequencing: a retrospective multi-center study in Fujian, China. Infect Drug Resist. (2024) 17:697–708. doi: 10.2147/IDR.S443953
27. Su, S, Su, X, Zhou, L, Lin, P, Chen, J, Chen, C, et al. Severe Chlamydia psittaci pneumonia: clinical characteristics and risk factors. Ann Palliat Med. (2021) 10:8051–60. doi: 10.21037/apm-21-1502
28. Hogerwerf, L, Holstege, MMC, Benincà, E, Dijkstra, F, and van der Hoek, W. Temporal and spatial analysis of psittacosis in association with poultry farming in the Netherlands, 2000-2015. BMC Infect Dis. (2017) 17:519. doi: 10.1186/s12879-017-2608-1
29. Zhou, L, Tan, Y, Kang, M, Liu, F, Ren, R, Wang, Y, et al. Preliminary epidemiology of human infections with highly pathogenic avian influenza a(H7N9) virus, China, 2017. Emerg Infect Dis. (2017) 23:1355–9. doi: 10.3201/eid2308.170640
Keywords: psittacosis, metagenomic next-generation sequencing (mNGS), rural areas, Chlamydia psittaci (C. psittaci), avian-to-human transmission
Citation: Wen Y, Zhang W, Li Y, Liao X, Xu J, Zhen R and Qin P (2025) Epidemiological characteristics of human psittacosis in Guangzhou, China, January 2021 to June 2024. Front. Public Health. 13:1526990. doi: 10.3389/fpubh.2025.1526990
Received: 12 November 2024; Accepted: 10 March 2025;
Published: 25 March 2025.
Edited by:
Christopher Hamilton-West, University of Chile, ChileReviewed by:
Hong-Juan Peng, Southern Medical University, ChinaCopyright © 2025 Wen, Zhang, Li, Liao, Xu, Zhen and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruonan Zhen, a2FubnkwNzIwQG91dGxvb2suY29t; Pengzhe Qin, cGV0Z3l5QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.