- 1National Center for Tuberculosis Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
- 2Medical College of Xiangyang Polytechnic, Xiangyang, Hubei, China
- 3Department of Tuberculosis Control and Prevention, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, Zhejiang, China
- 4National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, Chinese Center for Disease Control and Prevention, Beijing, China
Background: Understanding health system delay (HSD) in pulmonary tuberculosis (PTB) diagnosis aids in tailoring interventions for case detection and curbing transmission. However, recent nationwide studies on HSD in PTB diagnosis have been scarce. This study assesses HSD and its risk factors in China, taking into account the impact of the COVID-19 epidemic.
Methods: Patients diagnosed with PTB between 2019 and 2022 were selected using a multistage stratified clustering method. A semi-structured questionnaire was employed to assess HSD, which was defined as the interval between the patient’s initial visit to a health facility and the definitive PTB diagnosis. The HSD was then compared between 2019 (before the epidemic) and 2020–2022 (during the epidemic). Factors associated with long health system delay (LHSD, defined as HSD > 14 days) were examined using both univariate and multivariate analyses with chi-square tests and binary logistic regression, respectively.
Results: In total, 958 patients with PTB were analyzed: 478 before and 480 during the epidemic. The HSD was 14 (interquartile range, 7–30) days for all patients, and the HSD before and during the epidemic also shared this value. A total of 199 patients (20.8%) had LHSD. LHSD was more prevalent in patients presenting solely with cough and expectoration (Odds ratio [OR]: 1.482, 95% confidence interval [CI]: 1.015–2.162) and those visiting ≥2 health facilities before definitive diagnosis (2 health facilities: OR = 2.469, 95%CI: 1.239–4.920; ≥3 health facilities: OR = 8.306, 95%CI: 4.032–17.111). Additionally, patients with negative bacteriological results were independently associated with higher LHSD risk (OR = 1.485, 95%CI: 1.060–2.080).
Conclusion: In China, HSD in PTB diagnosis remains relatively low and is primarily mediated by factors associated with health providers. No significant impact on HSD from the COVID-19 epidemic has been found. Implementing targeted training programs to enhance health providers’ awareness of chronic respiratory symptoms and maintain vigilance for PTB; strengthening presumptive PTB identification capabilities at grassroots health facilities, and promoting the use of Mycobacterium tuberculosis (MTB) bacteriological technologies are recommended to shorten the HSD.
1 Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a major global public health challenge as one of the top infectious disease killers worldwide (1). According to the latest World Health Organization (WHO) estimates, TB was responsible for approximately 10.8 million new cases and 1.25 million deaths globally in 2023 (1). In response to this persistent epidemic, the WHO launched the “End TB Strategy” with ambitious targets for 2035, including a 90% reduction in TB incidence and 95% reduction in TB deaths compared to 2015 levels (2). Early case detection has been identified as a fundamental component of this strategy, as prompt TB diagnosis and subsequent correct treatment can alleviate patients’ suffering and lead to positive prognosis (3). More importantly, it can reduce the duration of MTB transmission in the community. Therefore, minimizing diagnostic delays is crucial for achieving effective TB control and meeting global elimination targets.
According to the WHO (4), diagnostic delay can be divided into two segments, the former is the interval from the onset of symptoms to the first visit to a health provider, which is called patient delay, and the interval between the initial health facility visit and definitive TB diagnosis, also known as health system delay (HSD). Extensive research conducted in this field has revealed considerable spatiotemporal heterogeneity in the degree and determinants of HSD (5). The median HSD across different countries spans from 2 to 128.5 days in low- and middle- income countries in a systematic review study (6). Chakma et al. (7) reported that the HSD among TB patients in Portugal exhibited a slight increasing trend over time from 2007 to 2018. Regarding the factors influencing HSD, previous studies have demonstrated these factors originate from individuals’ and health providers’ aspects (8, 9). However, significant variations exist across studies regarding the relative importance of these factors (9). This heterogeneity underscores the need for updated, context-specific analyses of HSD patterns and determinants to inform targeted interventions for optimizing TB diagnostic pathways.
China ranks third among 30 countries with the highest burden of new TB cases (1), with pulmonary tuberculosis (PTB) accounting for the majority of the cases (10), making PTB a top priority in infectious disease prevention and control efforts (11). In recent years, several studies have focused on HSD and related factors. Li et al. (12) reported that the average HSD of 50,606 patients with PTB diagnosed from 2016 to 2022 in Gansu province was 6.88 days, with 91.12% of patients being diagnosed within 14 days. A study in Shanghai on patients with PTB diagnosed during 2018–2020 found that, 22.12% of migrants and 16.52% of locals patients experienced HSD exceeding 14 days; and migrant patients and initial care seeking at general hospitals were associated with a higher probability of HSD > 14 days (13). Hao et al. (14) conducted a survey in 2020 and found that the median HSD for patients with PTB from western China was 12 days. Furthermore, the use of molecular biological methods significantly shortened the HSD. However, most of these studies were regional or based on tuberculosis surveillance data, making it difficult to quantify the overall national situation, and surveillance data often lack variables such as number of health facilities patient visited before definitive diagnosis may be related to HSD (15).
Besides, during the COVID-19 epidemic (hereafter referred to as the epidemic), TB services experienced varying degrees of disruption both domestically and internationally, significantly affecting PTB diagnosis and management (16, 17). In response, Chinese health authorities implemented strategic directives to maintain essential TB services while addressing COVID-19 priorities, ensuring the continuity of basic TB diagnosis and treatment (18). The epidemic intensity exhibited substantial fluctuations between January 2020 and November 2022, prompting corresponding adjustments in non-pharmacological interventions (NPIs) (19). Understanding HSD in PTB diagnosis within this complex context is essential for developing resilient TB diagnostic services in the future. However, existing research in China has primarily focused on localized regions and the initial epidemic phases (20, 21), leaving the nationwide impact on HSD largely unexplored. To address this knowledge gap, our study employs comprehensive patient surveys, incorporating COVID-19-related factors, to evaluate the current status and identify determinants of HSD in PTB diagnosis across China. The findings will provide critical evidence to inform policy development and strengthen TB control strategies in the post-epidemic era.
2 Methods
2.1 Study design and settings
This is a retrospective survey to investigate the HSD in PTB diagnosis. The study sites were chosen by multistage random sampling method. Taking into account the evolving COVID-19 control policies in China (22), we divided the epidemic period into four distinct stages: stage I, January to April 2020; stage II, May 2020 to July 2021; stage III, August 2021 to March 2022; and stage IV, April 2022 to November 2022. Meanwhile, we classified all counties into three levels based on the stringency of NPIs implemented for epidemic during this period, namely strict, moderate, and general. The classification of NPI stringency was determined by experts from the China CDC. Here are the methodologies employed for selecting the study sites during stage I.
Initially, we picked a month from January to April 2020 to serve as the starting point for selecting participants during the epidemic, with this month meet the following conditions: (1) at least two provinces reported more than one hundred COVID-19 cases. Each of these provinces had at least one county implementing strict NPIs, one implementing moderate NPIs, and one implementing general NPIs, all lasting for more than 15 days during that month. (2) Each of the selected counties reported no fewer than 120 PTB cases in 2019. If more than 1 month meet these conditions, using the random number method to select one. Next, selected two provinces after determining the month, if more than two provinces meet the requirements, randomly select two of them. Then, in the selected provinces, randomly chose one county each that implemented strict, moderate, and general NPIs during the selected month. Finally, for stage I, six counties located in Hubei and Henan provinces were selected as study sites, and the selection of patients began during the epidemic, specifically in March 2020.
For the other three stages, the methodology for study sites selection was similar to that of stage I, ensuring that no province was chosen more than once. Eventually, 24 counties across 8 provinces were selected (Figure 1). The starting time patients to be selected during the epidemic began in stage II-IV were January 2021, November 2021, and May 2022, respectively.
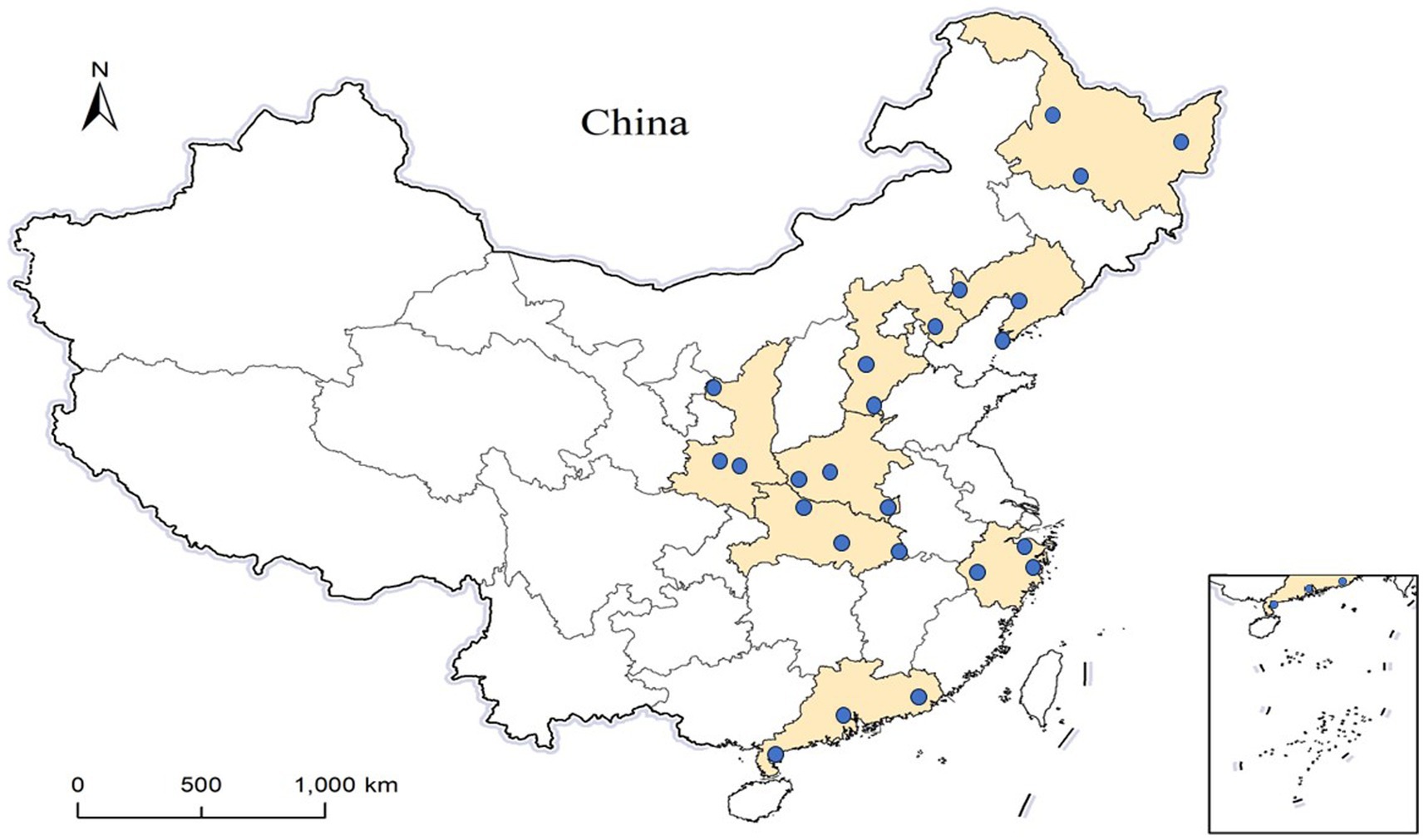
Figure 1. The location of the 24 study sites. The colored areas are 8 provinces and the blue dots represent the geographical locations of the selected counties.
2.2 Sample size
The sample size was estimated for this study using the formula, n = z2p(1-p)/δ2. Taking into account a 95% confidence level, a proportion of LHSD within PTB patients (p = 0.36) derived from a meta-analysis conducted in China (23), a 5% allowable error, and a design effect of 2.5 due to the cluster sampling method (24), the calculation was performed. Thus, at least 885 PTB participants need to be included in the study. After dividing by 24, approximately 37 patients were needed in each county. Therefore, we decided to select 40 patients in each county, with 20 before and 20 during the epidemic.
2.3 Participants
Using a multistage clustering sampling method to select participants in the TB-designated hospitals of the selected counties, patients diagnosed with PTB and notified to the Chinese Tuberculosis Information Management System (TBIMS) were selected. Notably, in China, it is a legal requirement for all confirmed PTB cases to be registered within TBIMS (25).
For the six counties selected in stage I, starting from the first patient registered in March 2020, a consecutive series of 20 patients were contacted. In case of death, loss to follow-up, or refusal to participate, these patients were replaced sequentially based on their registration numbers. Using the same method, 20 patients from each of 6 counties in 2019 were selected, consistent with the epidemic period starting March 2019. For the remaining 18 counties in stages II to IV, the selection of patients began during the epidemic at determined month, and patients before the epidemic were selected starting from the corresponding month in 2019. The patient selection methodology in these counties remained consistent with that used for the 6 counties in stage I.
2.4 Data collection
Data were collected using a pre-tested, interviewer-administered semi-structured questionnaire between September and December 2023. The survey covered the participants’ sociodemographic characteristics (including sex, age, education level, occupation, marital status, residence), TB treatment history, PTB symptoms, pathway, and duration (days) from their first health facility visit to definitive diagnosis. The case-finding method, health facility types, and number of health facilities visited before diagnosis were determined based on the patients’ pathways. Specifically, the patient’s diagnosis after self-presentation to a health facility due to PTB symptoms was classified as passive case-finding, whereas other scenarios were classified as active case-finding. Health facilities were classified into four types: grassroots health facilities (including village clinics, private clinics, and township health centers), general hospitals, other non-TB-designated hospitals, and TB-designated hospitals. Repeated visits to the same health facility were counted as a single facility. Additionally, data on TB bacteriological results were collected through a review of medical records. For patients with communication barriers, under 15 years of old, or with mental disorders, a caregiver or guardian was asked to respond on their behalf.
2.5 Relevant definitions
Based on previous study (4), HSD was defined as the time interval from the patient’s initial visit to a health facility before the definitive diagnosis of PTB. Long health system delay (LHSD) occurs when this interval exceeds 14 days (26).
PTB symptoms: at least one of the following symptoms persisting or recurring prior to PTB diagnosis: cough and expectoration, hemoptysis, fever, chest pain, night sweats, fatigue, appetite loss, or weight loss.
Bacteriological results: results of the MTB test when patients were diagnosed by sputum smear microscopy, culture, or PCR; a “positive” result indicates the detection of MTB, while a “negative” result indicates that MTB was not detected.
2.6 Ethical approval
Ethical approval was obtained from the Ethics Committee of the Chinese Center for Disease Control and Prevention. All the study participants provided informed consent. The data were anonymized before further analyses.
2.7 Statistical analysis
Survey data were subjected to a double-entry process using Epidata3.1 (The Epidata Association Odense, Denmark) to ensure precision before being exported to a Microsoft Excel (version 2016; Microsoft Corporation, WA, United States) spreadsheet. Statistical analyses were performed using SPSS22.0™ (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Diagrams were created using R software (version 4.4.1, Auckland, New Zealand) and Sankey MATIC (an online Sankey diagram tool).
The statistical analysis was conducted using appropriate methods based on variable types and distribution characteristics. Categorical variables were presented as frequencies and percentages, with between-group comparisons performed using chi-square tests. For continuous variables, we first assessed data distribution using the one-sample Kolmogorov–Smirnov test (K–S test). Normally distributed data were expressed as mean ± standard deviation (SD), while non-normally distributed variables were summarized using median and interquartile range (IQR). To compare HSD distributions between before and during the epidemic, we employed the two-sample K–S test. Group comparisons of HSD duration were conducted using either the Student’s t-test (for normally distributed data) or the Mann–Whitney U test (for non-normal distributions), as appropriate. Univariate analysis was performed using chi-square tests to identify potential predictors of LHSD. To minimize the exclusion of potentially significant variables, we adopted a liberal p-value threshold of ≤0.2 for inclusion in subsequent multivariate analysis. Significant variables from the univariate analysis were then included in a binary logistic regression model to identify independent determinants of LHSD. The final multivariate model results were expressed as adjusted odds ratios (OR) with corresponding 95% confidence intervals (CI). A two-tailed p-value<0.05 was considered statistically significant.
3 Results
3.1 Characteristics of participants
A total of 1,052 PTB cases were initially contacted for participation. After excluding 92 cases due to death (n = 5), loss to follow-up (n = 46), and refusal to participate (n = 41), 960 patients completed the survey. Two additional cases were excluded due to missing medical records, resulting in 958 cases included in the final analysis. The cohort comprised 478 patients diagnosed before the epidemic and 480 patients diagnosed during the epidemic period (Figure 2). Comparative analysis revealed no significant differences between analyzed and excluded patients in terms of: mean age (48.4 ± 17.5 years vs. 48.8 ± 16.7 years; p = 0.846), gender distribution (68.6% vs. 73.4% male; p = 0.334).
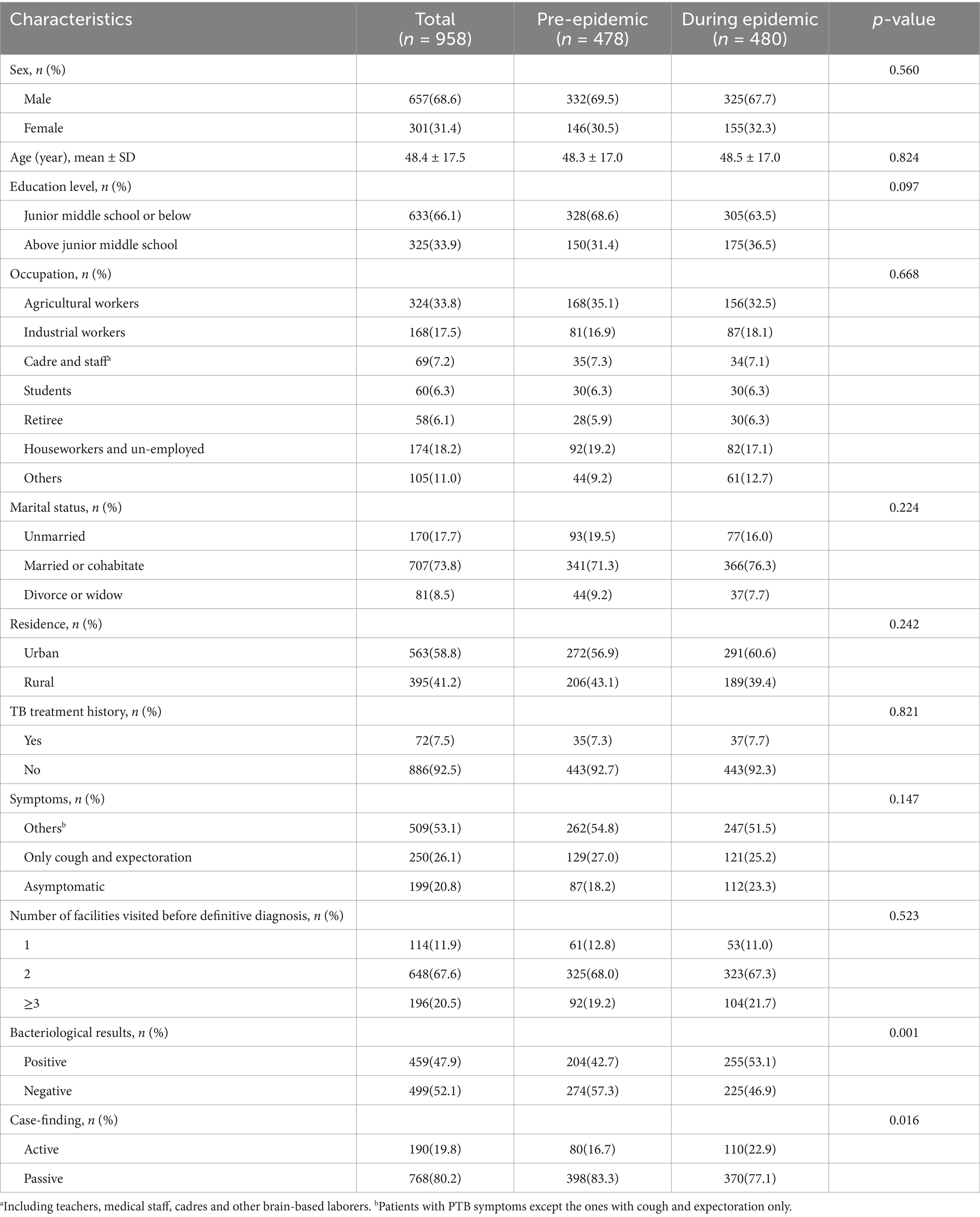
The majority of participants were male 657 (68.6%). Slightly more than half of the patients were aged between 35 and 64. Nearly two-thirds of the participants had completed junior middle school or below. One-third of participants were engaged in agricultural labor. Nearly 74% of patients were married or cohabiting. Of all the participants, 563 (58.8%) lived in urban areas. A total of 72 (7.5%) had a history of TB treatment. Up to 759 (79.2%) patients presented with PTB symptoms, and 250 (26.1%) only had cough and expectoration. Among them, 648 patients (67.6%) visited two health facilities before being confirmed. There were no statistical differences in the distribution of the above characteristics between patients diagnosed before and during the epidemic. Of the 958 patients, 459 (47.9%) were confirmed as bacteriologically positive. Among the 958 patients included in the analysis, 459 (47.9%) were bacteriologically confirmed through positive test results. Active case-finding accounted for 190 (19.8%) of all diagnoses. Comparative analysis revealed significant differences between patients diagnosed before and during the epidemic: Higher proportion of bacteriologically positive cases during the epidemic (p < 0.05) and increased rate of diagnosis through active case-finding during the epidemic (p<0.05). More details were displayed in Table 1.
3.2 Patient pathway
All 958 patients received definitive diagnoses at TB-designated hospitals. A total of 114, 648, 184, and 12 patients (11.9, 67.6, 19.2, and 1.3%, respectively) visited 1, 2, 3, and 4 health facilities, respectively, until the definitive diagnosis. Of all patients, 226 (23.6%), 460 (48.0%), 154 (16.1%) initially visited grassroots health facilities, general hospitals, and other non-TB-designated hospitals, respectively; while 118 (12.3%) patients selected TB-designated hospitals as their first-contact health facilities (Figure 3).
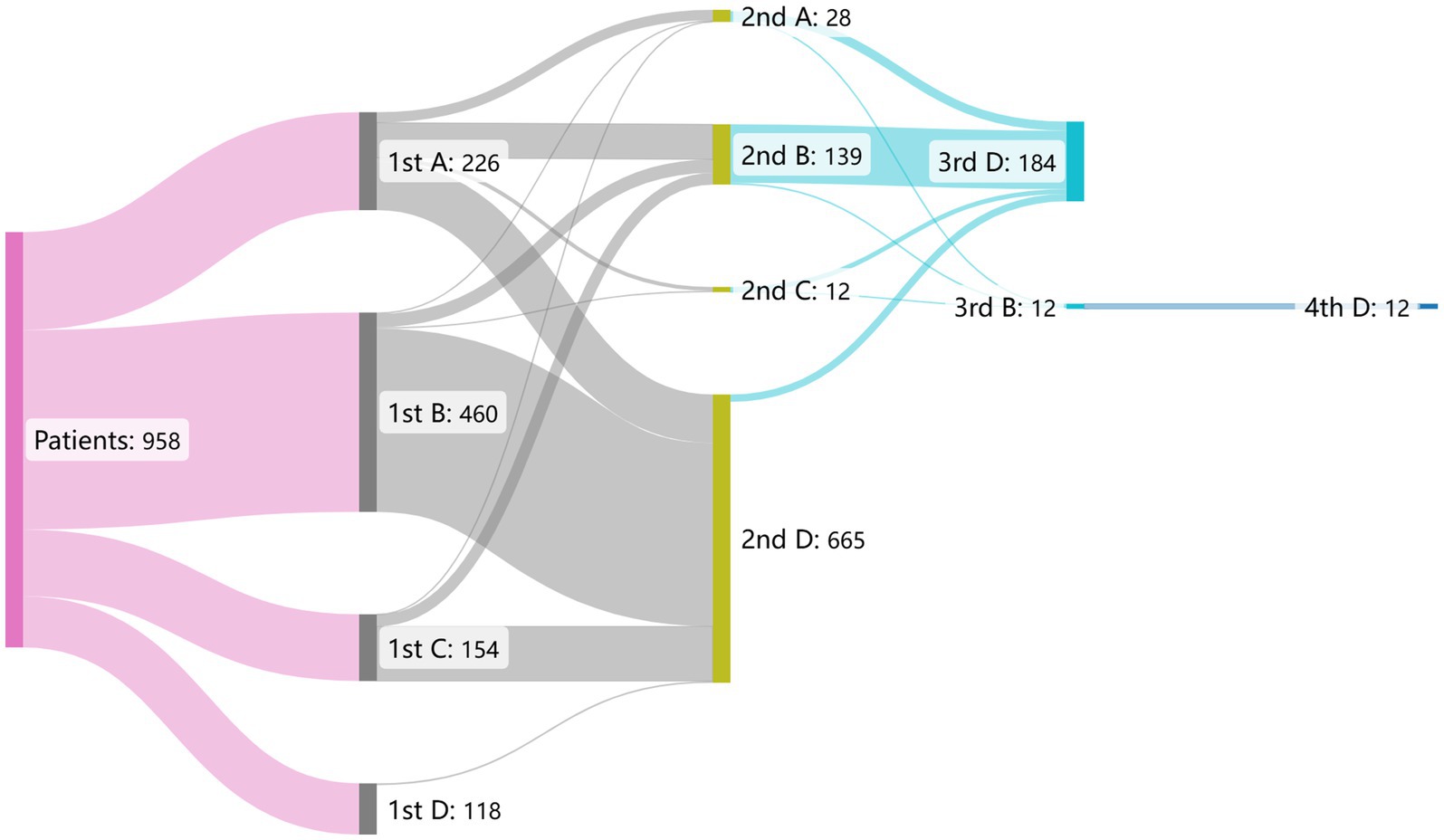
Figure 3. The flow gram of patient pathway before pulmonary tuberculosis diagnosis in 958 patients. The alphabet A–D in the figure represents distinct health facilities. A: grassroots health facilities, B: general hospitals, C: other non-TB-designated hospitals, D: TB-designated hospitals.
Among the 226 patients who chose grassroots health facilities for their first visit, 112 (49.6%) chose TB-designated hospitals as their secondary health provider. This number (proportion) was 423 (92.0%) and 126 (79.7%) for those who initially visited general hospitals and other non-TB-designated hospitals, respectively. Among the 118 patients who first chose TB-designated hospitals, 114 (96.6%) were diagnosed there.
3.3 Health system delay
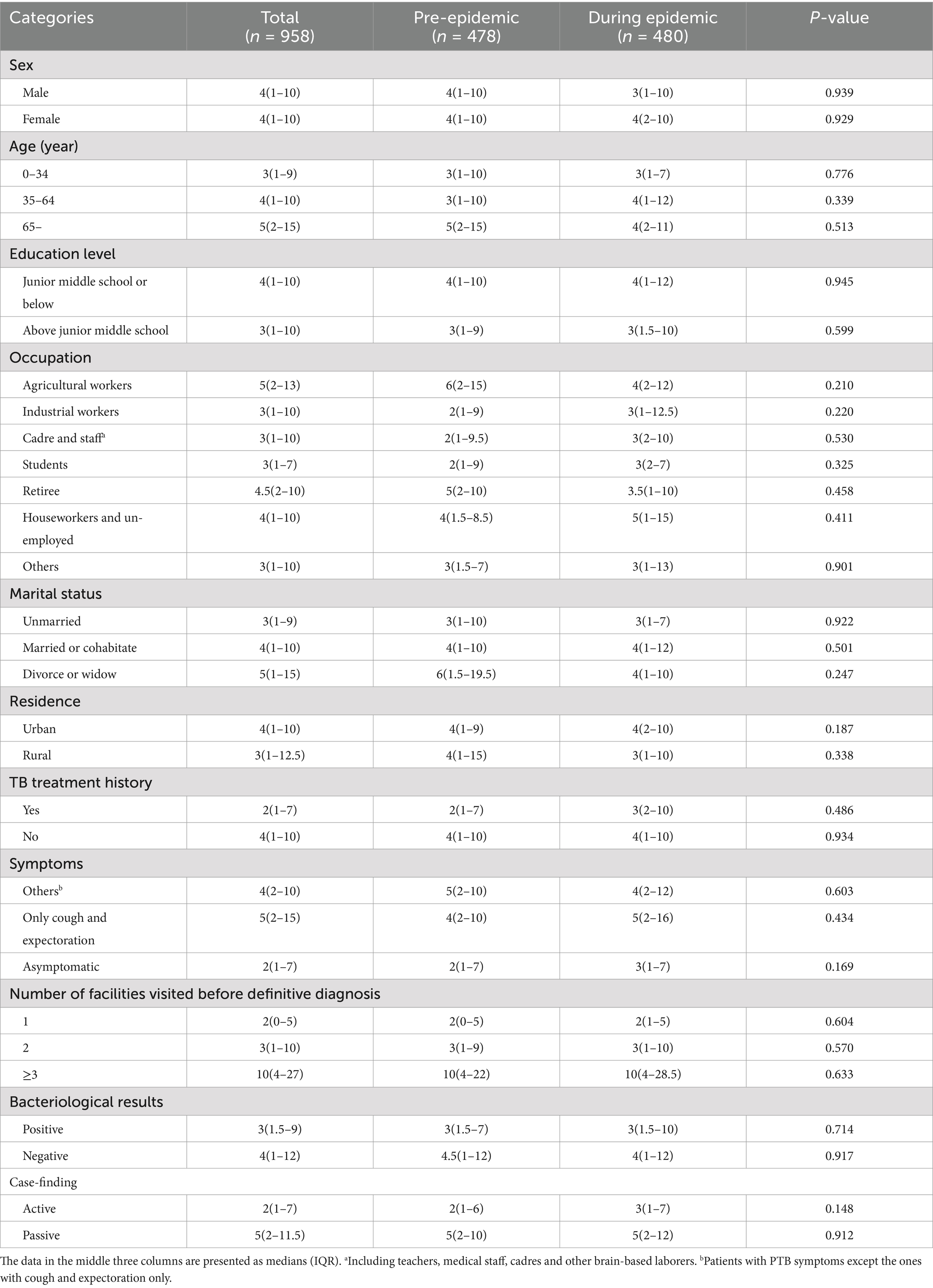
Among the 958 patients with PTB, the distribution of HSD was positively skewed, ranging from 0 to 64 days (Figure 4). The K–S test showed no statistical difference in the distribution of HSD before and during the epidemic (D = 0.405, p = 0.997). The HSD for all participants was 4 (1–10) days, whereas patients diagnosed before and during the epidemic shared the same HSD (4[1–10] days) with no statistical significance (Mann–Whitney U test, p = 0.793). All the medians of HSD in subcategories varied from 1 to 5 days except for patients visiting ≥3 health facilities before PTB confirmation, who had a much longer HSD (10[4–27] days). Patients in all subcategories, whether diagnosed during or before the epidemic, showed no significant differences in HSD (Table 2).
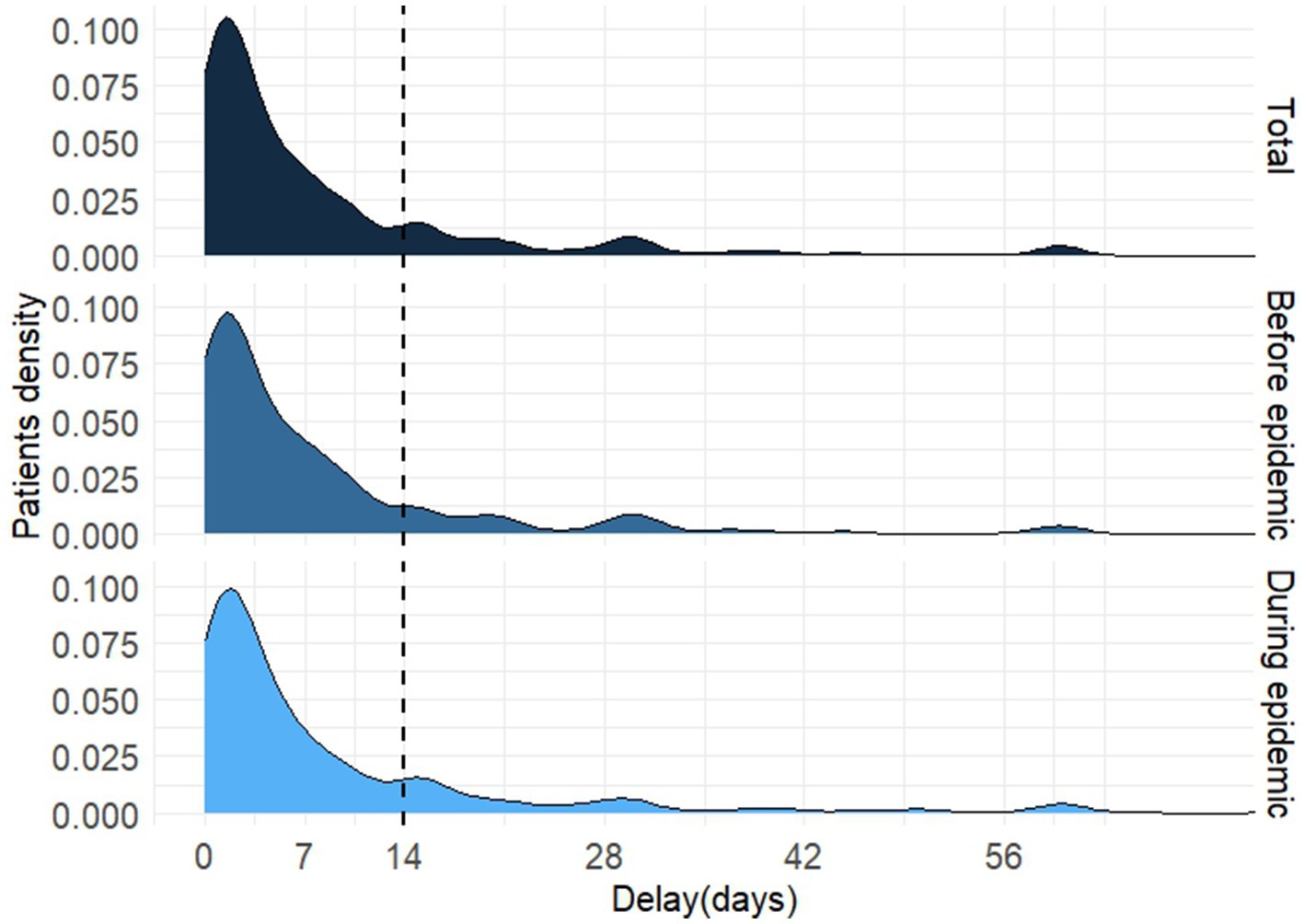
Figure 4. The distribution of health system delay of diagnosis in patients with pulmonary tuberculosis.
Among all the participants, 13 patients diagnosed during the epidemic recalled that, due to fever symptoms, they were requested to undergo chest CT scans to rule out COVID-19 with prompt detection of PTB. However, another feverish patient tested negative for SARS-CoV-2 nucleic acid test and was considered with cold by doctor, due to the worsening of the illness, a chest CT scan confirmed the diagnosis of PTB 20 days later.
3.4 Long health system delay and related factors
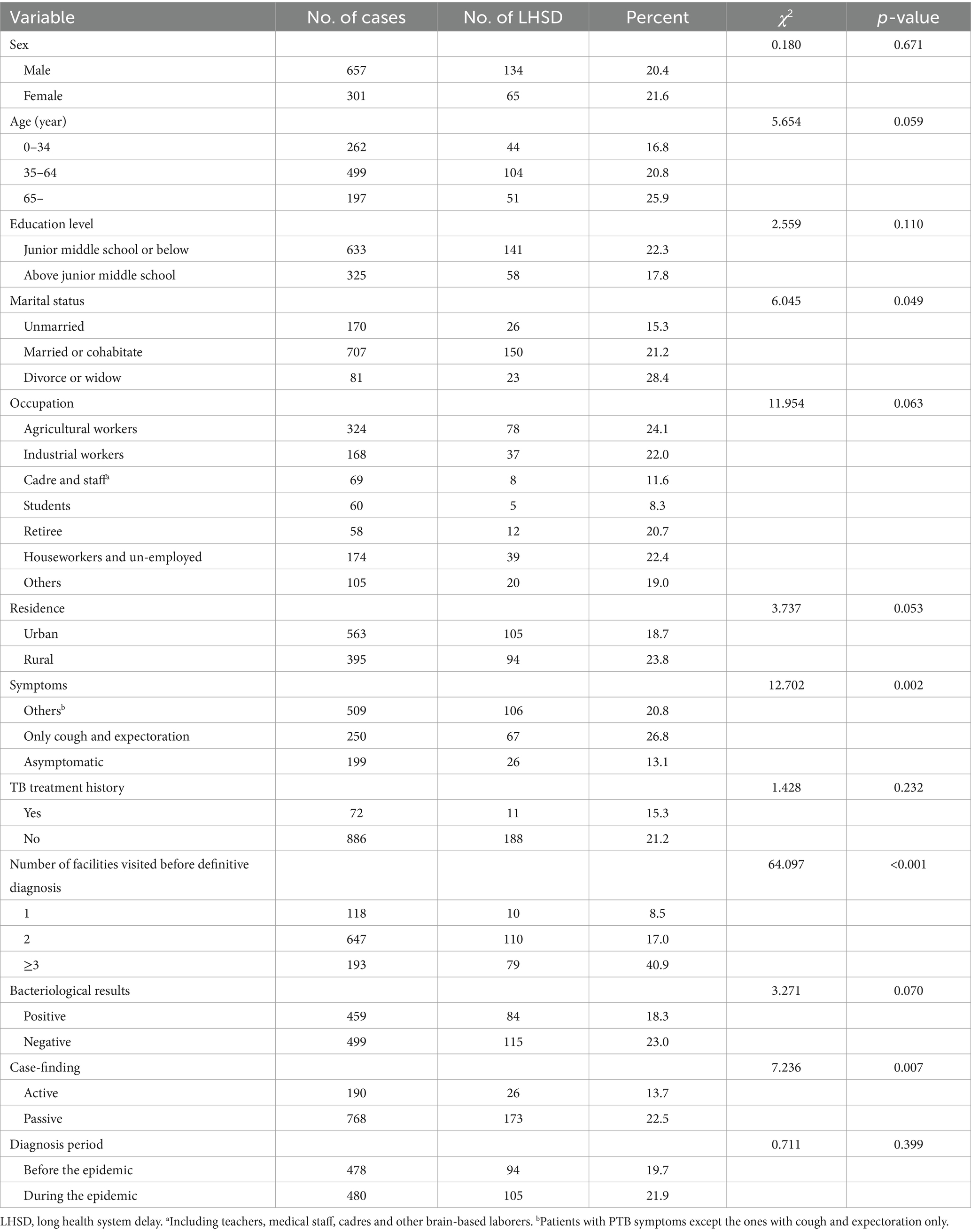
A total of 199 (20.8%) participants had experienced LHSD. Univariate analysis revealed no significant association between the LHSD and sex, education level, TB treatment history, or diagnosis period. Similarly, age, occupation, residence, and bacteriological results were not found to be associated with LHSD, with p-values slightly exceeding the threshold of 0.05. Marital status, symptoms, number of health facilities visited before definitive diagnosis, and case-finding method were related to LHSD (p < 0.05) (Table 3). However, after adjusting possible confounders, multivariate analysis confirmed that LHSD was more common in patients with cough and expectoration solely (OR: 1.482, 95%CI: 1.015–2.162) and those visiting ≥2 health facilities before diagnosis (2 health facilities: OR = 2.469, 95%CI: 1.239–4.920; ≥3 health facilities: OR = 8.306, 95%CI:4.032–17.111). Additionally, negative bacteriological results were independently associated with a higher risk of LHSD (OR = 1.485, 95%CI: 1.060–2.080) (Table 4). Subgroup analysis revealed no statistically significant interaction effects between clinical symptoms, the number of health facilities visited prior to diagnosis, and bacteriological results in their association with LHSD (all interaction p-values >0.05).
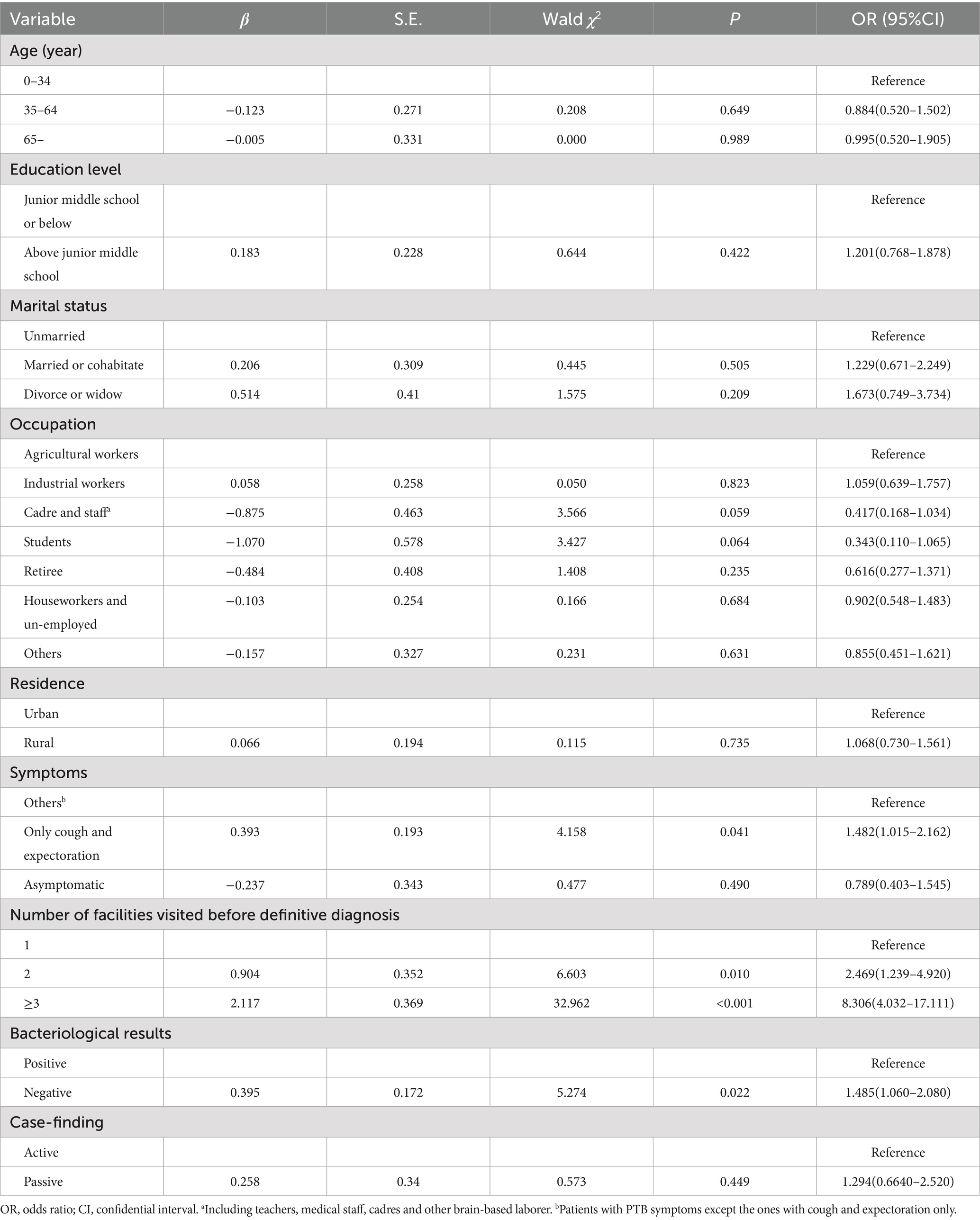
Table 4. Multivariate analysis for health system delay in pulmonary tuberculosis diagnosis (N = 958).
4 Discussion
This study represents an updated nationwide multi-center survey on the HSD of PTB diagnosis, the results indicate that the HSD of PTB diagnosis is at a relatively low level in China, and no impact of the COVID-19 epidemic on HSD was observed. It is still noteworthy that patients presenting non-specific cough and expectoration, visiting multiple facilities before definitive diagnosis, and with negative bacteriological results were identified as risk factors for LHSD.
In our study, the HSD was 4 (1–10) days. This was consistent with a systematic review published in 2021, the HSD in five upper-middle-income countries with a high TB burden was 4 (95% confidence interval: 2–4) days (27). However, a multi-center study based on 20 hospitals in China found that the HSD was 20 (7–72) days (28), which is much longer than our survey result. The most likely reason for this large gap is that the participants in the former survey were diagnosed by TB-designated hospitals at the prefecture or provincial level, whereas the majority of patients with PTB in China are diagnosed and cured by TB-designated hospitals at the county level. Patients with complex conditions that are difficult to diagnose are referred or self-directed to higher-level TB hospitals (29), which lasts more time until diagnosis. Our survey results were also lower than those of a survey conducted in two districts of Beijing in 2021, which showed the HSD was 8 (0–18) days (30). The likely cause is Beijing’s abundant health resources attracting a high proportion of out-of-town TB patients (31), and they may have consulted locally health facilities but faced prolonged un-diagnosis periods, leading to longer HSD. The relatively low HSD in China can be attributed to three reasons. First, China’s TB prevention and control system with clear responsibilities and efficient coordination. Detailed regulations have been established for the reporting and referral of suspected PTB discovered in non-TB-designated hospitals, and corresponding tracking measures are in place for those not properly referred, shortening the time from the patient’s initial visit to definitive diagnosis (29). Second, improved self-health awareness and economic status promote the suspects promptly visit TB-designated hospitals for further diagnosis and treatment after detecting abnormalities during the initial visit (32). Third, according to “13th Five-Year Plan” for TB Control (31), at least 70% of county-level TB-designated hospitals were required to possess molecular biological diagnostic capabilities. This technique (e.g., GeneXpert), characterized by high sensitivity and rapid detection speed (33), has facilitated early diagnosis for PTB patients.
It is worth mentioning that, 13 patients with fever symptoms underwent COVID-19 quarantine measures without experiencing LHSD. There may be a detection symptom bias here, which potentially overstated the role of COVID-19 quarantine measures in shortening the HSD, as even without the COVID-19 quarantine measures, these PTB patients could still had been diagnosed earlier. However, the role of these stringent quarantine measures in promptly identifying some patients with fever symptoms is undeniable. Nevertheless, there is also a risk that patients with fever might be overlooked after testing negative for SARS-CoV-2 nucleic acid tests, leading to a prolongation of their diagnostic time. This highlights the importance of concurrent screening for PTB during future epidemic of similar infectious diseases (34). Over all, we observed no statistical difference in HSD for patients with PTB diagnosed before and during the COVID-19 epidemic, which according to a survey in Ningxia (20). The most plausible explanation is that NPIs and patients fearing of SARS-CoV-2 infection can lead to delays in seeking medical (35, 36), but once patients were able to access the health system, these impact on diagnostic delays is relatively minor.
The current study also showed that sex, age and other socio-demographic factors were not associated with the LHSD, which is consistent with the findings of a cross-sectional survey conducted in the Gurage and Siltie regions of southern Ethiopia (37). Similarly, interview-based studies conducted in Tanzania and Italy also revealed that there was no correlation between the patients’ demographic and sociological characteristics and HSD (38, 39). This consistent evidence across diverse settings suggests that HSD in PTB is primarily influenced by health provider’ factors rather than patient characteristics. Specifically, the timeliness of PTB diagnosis appears to depend largely on the diagnostic capacity of the health facilities patients visited.
We found that compared to patients with PTB presenting solely with cough and expectoration, those exhibiting other PTB symptoms were diagnosed more promptly, consistent with previous studies (40, 41). If patients have more specific symptoms of PTB, such as chest pain and hemoptysis, it would not only attract patients’ attention and shorten the time to seek healthcare but may necessitate health providers to investigate PTB early. Cough plays a critical role in the MTB transmission (42), warranting careful attention. However, the symptoms of sole cough and expectoration caused by PTB are often clinically similar to those of other respiratory diseases, posing challenges for differentiation (43). This study found that more than a quarter of these PTB patients experienced LHSD. When combined with the patient delay, it can be inferred that many patients experienced cough and expectoration for more than 2 weeks. Therefore, targeted training programs are imperative to enhance the awareness of health providers regarding patients with chronic respiratory symptoms, ensuring that PTB-related examinations are conducted when necessary to facilitate early detection of PTB cases.
Our analysis revealed a strong association between more health facility visits and LHSD, with patients visiting two or more facilities demonstrating significantly higher HSD incidence. This finding aligns with the reports from Ghana (44), Iran (45) and Afghanistan (46), where multiple visits to health care providers strongly predict increased diagnostic delay. Notably, similar to a domestic study, we observed a dose–response relationship, wherein the likelihood of LHSD increased proportionally with the number of facilities visited (15). The existing policy stipulates that tuberculosis patients should be treated centrally at TB-designated hospitals. Suspected cases from other health facilities must be referred to TB-designated hospitals for confirmation, registration, and treatment (47). Compared with other health facilities, TB-designated hospitals are more professional in PTB diagnosis, and require a shorter time for diagnosis (48). Our findings support this, showing that over 95% of patients initially visiting TB-designated hospitals received definitive diagnoses there. Thus, initial visits to TB-designated hospitals or prompt referral from other facilities could significantly reduce HSD. However, patient pathway analysis revealed that only 12.3% of patients chose a TB-designated hospital for their first visit, this value is close to a systematic review that encompassed 9,891 PTB patients in China (13%) (23). The ability of non-TB-designated facilities to identify and refer suspected PTB cases is critical. Yet, among patients initially visiting other facilities, particularly grassroots-level ones, only 50% selected TB-designated hospitals as their secondary facility. This indicates that primary healthcare providers failed to identify half of these patients, leading to further visits to non-TB-designated hospitals, additional referrals, and prolonged HSD. These findings highlight significant deficiencies in presumptive PTB recognition at grassroots facilities (49, 50), and necessitates the targeted training for grassroots doctors to enhance their ability to identify PTB.
In our study, patients with negative bacteriological results demonstrated a significantly higher likelihood of experiencing LHSD. This finding is similar to a study conducted in the Taiwan area (41). The association between negative bacteriological results and LHSD can be attributed to several factors. Primarily, the bacteriological detection of MTB serves as a critical component for the timely and accurate diagnosis of PTB (51). Furthermore, current clinical guidelines recommend a 14-day course of non-specific broad-spectrum antibiotic therapy for suspected PTB cases with negative bacteriological results prior to definitive diagnosis (29). This diagnostic approach, while clinically justified, potentially extends the time to diagnosis and may contribute to LHSD. The findings underscore the critical need to implement bacteriological diagnostic technologies for PTB to effectively mitigate HSD.
5 Strength and limitation
This nationwide survey provides valuable insights into HSD in the diagnosis of PTB patients in China. However, several limitations should be acknowledged. First, despite our implementation of consecutive enrollment based on registration numbers, a small proportion of patients were excluded due to loss to follow-up, mortality, refusal to participate, or incomplete data. Although comparative analysis revealed no significant differences in age distribution or gender composition between analyzed and non-analyzed participants, the potential for selection bias cannot be entirely ruled out. Second, our study was limited to patients notified at county-level TB-designated hospitals, excluding those notified at municipal or higher-level TB-designated hospitals. This exclusion criterion may introduce a degree of selection bias, potentially limiting the generalizability of our findings to the broader PTB patient population. Third, while we employed medical record verification to supplement and validate patient recall during the questionnaire administration, the retrospective nature of our study design inevitably introduces some degree of recall bias regarding illness progression and patient pathways. These limitations notwithstanding, our study provides crucial epidemiological data that contribute to the understanding of HSD in PTB management within the Chinese health system.
6 Conclusion
Health system delay in PTB diagnosis remains at a relatively low level in China. No significant impact on the HSD from the COVID-19 epidemic has been noted, although a small number of febrile patients with PTB were diagnosed in timely manner through COVID-19 quarantine protocols. The study identified patients with only cough and expectoration, contacted ≥2 health facilities, and negative bacteriological results increased the risk of LHSD. Based on these findings, we recommend the following interventions to further reduce HSD: Implementing targeted training programs to enhance health providers’ awareness of chronic respiratory symptoms and maintain vigilance for PTB; strengthening the presumptive PTB patients recognition capabilities of grassroots health facilities, and promoting the use of MTB bacteriological technologies. These evidence-based strategies could significantly improve early PTB detection and reduce HSD, ultimately contributing to better TB control outcomes in China.
Data availability statement
All data relevant to the study are included in the article and can be obtained from the corresponding authors upon reasonable request.
Ethics statement
The studies involving humans were approved by Ethics Committee Review Board of the Chinese Center for disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MF: Formal analysis, Investigation, Writing – original draft. YSL: Investigation, Visualization, Writing – original draft. KL: Methodology, Visualization, Writing – original draft. XL: Project administration, Resources, Writing – review & editing. YHL: Project administration, Resources, Writing – review & editing. TL: Writing – review & editing. CZ: Writing – review & editing. HZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. JC: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Research Project of Chinese Center for Disease Control and Prevention (Grant no. JY22-3-13) and The National Thirteenth Five-year Mega-Scientific Projects of China (Grant no. 2017ZX10201302).
Acknowledgments
We acknowledge all participants in the study, and thanks to the health staff in CDCs and TB-designated hospitals organizing and participating in patients survey. We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Global tuberculosis report 2024. Geneva: World Health Organization (2024).
2. Uplekar, M, Weil, D, Lonnroth, K, Jaramillo, E, Lienhardt, C, Dias, HM, et al. WHO’s new end TB strategy. Lancet. (2015) 385:1799–801. doi: 10.1016/S0140-6736(15)60570-0
3. Zhu, XH, Tao, NN, Zhang, QY, Song, WM, An, QQ, Liu, SQ, et al. Association between diagnostic delay and prognosis of pulmonary tuberculosis in Shandong, China: a retrospective study. BMC Pulm Med. (2022) 22:309. doi: 10.1186/s12890-022-02101-z
4. World Health Organization. Regional Office for the Eastern Mediterranean. (2006). Diagnostic and treatment delay in tuberculosis. Available at: https://iris.who.int/handle/10665/116501 (accessed September 15, 2024).
5. Jiang, Y, Zhang, P, Deng, G, and Chen, J. Research progress of delay in diagnosis of tuberculosis and its influencing factors. J Tuber Lung Dis. (2021) 2:289–93. doi: 10.3969/j.issn.2096-8493.20210073
6. Getnet, F, Demissie, M, Assefa, N, Mengistie, B, and Worku, A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med. (2017) 17:202. doi: 10.1186/s12890-017-0551-y
7. Chakma, B, Gomes, D, Filipe, PA, Soares, P, de Sousa, B, and Nunes, C. A temporal analysis on patient and health service delays in pulmonary tuberculosis in Portugal: inter and intra-regional differences and in (equalities) between gender and age. BMC Public Health. (2022) 22:1830. doi: 10.1186/s12889-022-14216-3
8. Seid, A, and Metaferia, Y. Factors associated with treatment delay among newly diagnosed tuberculosis patients in Dessie city and surroundings, northern Central Ethiopia: a cross-sectional study. BMC Public Health. (2018) 18:931. doi: 10.1186/s12889-018-5823-9
9. Cai, J, Wang, X, Ma, A, Wang, Q, Han, X, and Li, Y. Factors associated with patient and provider delays for tuberculosis diagnosis and treatment in Asia: a systematic review and meta-analysis. PLoS One. (2015) 10:e0120088. doi: 10.1371/journal.pone.0120088
10. Li, T, Yan, X, Du, X, Huang, F, Wang, N, Ni, N, et al. Extrapulmonary tuberculosis in China: a national survey. Int J Infect Dis. (2023) 128:69–77. doi: 10.1016/j.ijid.2022.12.005
11. Zhu, B, Fu, Y, Liu, J, and Mao, Y. Spatial distribution of 12 class B notifiable infectious diseases in China: a retrospective study. PLoS One. (2018) 13:e0195568. doi: 10.1371/journal.pone.0195568
12. Li, J, Zhao, J, Bai, Y, and Zhang, L. Medical facility visit and diagnosis delays of tuberculosis patients in Gansu based on surveillance during 2016–2022. Dis Surveill. (2024) 39:412–9. doi: 10.3784/jbjc.202311180625
13. Jia, Y, Jiang, W, Xiao, X, Lou, Z, Tang, S, Chen, J, et al. Patient delay, diagnosis delay, and treatment outcomes among migrant patients with tuberculosis in Shanghai, China, 2018-2020: a mixed-methods study. BMJ Open. (2024) 14:e082430. doi: 10.1136/bmjopen-2023-082430
14. Hao, D, Li, T, and Xu, C. Influencing factors associated with health-care seeking delay and diagnosis delay of pulmonary tuberculosis patients in western China, 2020. Dis Surveill. (2023) 38:1294–300. doi: 10.3784/jbjc.202307200354
15. Lu, X, Liu, X, Li, T, Ye, J, Zhang, M, Yu, Z, et al. Factors associated with health care-seeking delay and treatment delay in pulmonary tuberculosis patients in Shishou and Badong of Hubei. Dis Surveill. (2021) 36:1010–6. doi: 10.3784/jbjc.202104270229
16. World Health Organization. Global tuberculosis report 2020. Geneva: World Health Organization (2020).
17. Fei, H, Yinyin, X, Hui, C, Ni, W, Xin, D, Wei, C, et al. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Reg Health West Pac. (2020) 3:100032. doi: 10.1016/j.lanwpc.2020.100032
18. General Office of the National Health Commission. Notice of on strengthening medical service management during the epidemic period to meet the basic medical needs of the masses. (2020). Available online at: https://www.gov.cn/zhengce/zhengceku/2020-02/17/content_5480176.htm (Accessed October 12, 2024).
19. Ba, Z, Li, Y, Ma, J, Qin, Y, Tian, J, Meng, Y, et al. Reflections on the dynamic zero-COVID policy in China. Prev Med Rep. (2023) 36:102466. doi: 10.1016/j.pmedr.2023.102466
20. Wang, X, He, W, Lei, J, Liu, G, Huang, F, and Zhao, Y. Impact of COVID-19 pandemic on pre-treatment delays, detection, and clinical characteristics of tuberculosis patients in Ningxia Hui autonomous region, China. Front Public Health. (2021) 9:9. doi: 10.3389/fpubh.2021.644536
21. Zhang, G, Yu, Y, Zhang, W, Shang, J, Chen, S, Pang, X, et al. Influence of COVID-19 for delaying the diagnosis and treatment of pulmonary tuberculosis–Tianjin, China. Front Public Health. (2022) 10:10. doi: 10.3389/fpubh.2022.937844
22. Press Conference of the Joint Prevention and Control Mechanism of the State Council. Where is China's COVID-19 prevention and control at? The latest research and judgment of Liang Wannian. (2022). Available at: https://baijiahao.baidu.com/s?id=1732282042170283211&wfr=spider&for=pc (accessed October 20, 2024)
23. Wang, Y, Fang, S, Lu, X, and Wen, L. Three types of delay among tuberculosis patients in China and their associations with economic factors: a meta-analysis. Chin J Evid Based Med. (2022) 22:791–8. doi: 10.7507/1672-2531.202112089
24. Neuhaus, JM, and Segal, MR. Design effects for binary regression models fitted to dependent data. Stat Med. (1993) 12:1259–68. doi: 10.1002/sim.4780121307
25. Huang, F, Cheng, S, Du, X, Chen, W, Scano, F, Falzon, D, et al. Electronic recording and reporting system for tuberculosis in China: experience and opportunities. J Am Med Inform Assoc. (2014) 21:938–41. doi: 10.1136/amiajnl-2013-002001
26. Liu, K, Ge, R, Luo, D, Zheng, Y, Shen, Z, Chen, B, et al. Delay analysis of pulmonary tuberculosis in the eastern coastal county of China from 2010 to 2021: evidence from two surveillance systems. Front Public Health. (2023) 11:1233637. doi: 10.3389/fpubh.2023.1233637
27. Teo, A, Singh, SR, Prem, K, Hsu, LY, and Yi, S. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res. (2021) 22:251. doi: 10.1186/s12931-021-01841-6
28. Zhang, L, Weng, TP, Wang, HY, Sun, F, Liu, YY, Lin, K, et al. Patient pathway analysis of tuberculosis diagnostic delay: a multicentre retrospective cohort study in China. Clin Microbiol Infect. (2021) 27:1000–6. doi: 10.1016/j.cmi.2020.12.031
29. National health commission of the people' republic of China. Technical specification for tuberculosis prevention and control in China (2020 edition). (2020). Available at: https://wst.hainan.gov.cn/sjkzx/info/1077/4576.htm (accessed September 15, 2024)
30. Zhang, L, Ma, X, Gao, H, Bao, C, Wu, Y, Wu, S, et al. Analysis of care-seeking and diagnosis delay among pulmonary tuberculosis patients in Beijing, China. Front Public Health. (2024) 12:1369541. doi: 10.3389/fpubh.2024.1369541
31. Yang, X, Chen, S, Yi, J, Zhao, Y, Chen, H, Dai, X, et al. Analysis of tuberculosis epidemiological characteristics and drug resistance among the floating population in Beijing in 2019. Chin J Epidemiol. (2023) 44:1746–52. doi: 10.3760/cma.j.cn112338-20220522-00446
32. Li, S, Ju, H, Li, G, and Yang, G. Analysis of factors on the failures in referring TB suspects from third grade class a hospital in Tianjin. Chin J Antituber. (2009) 31:446–8.
33. Lee, JH, Garg, T, Lee, J, McGrath, S, Rosman, L, Schumacher, SG, et al. Impact of molecular diagnostic tests on diagnostic and treatment delays in tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. (2022) 22:940. doi: 10.1186/s12879-022-07855-9
34. Scott, AJ, Limbada, M, Perumal, T, Jaumdally, S, Kotze, A, van der Merwe, C, et al. Integrating molecular and radiological screening tools during community-based active case-finding for tuberculosis and COVID-19 in southern Africa. Int J Infect Dis. (2024) 145:107081. doi: 10.1016/j.ijid.2024.107081
35. Xia, Y, Huang, F, Chen, H, Wang, N, Du, X, Chen, W, et al. The impact of COVID-19 on tuberculosis Patients' behavior of seeking medical care – China, 2020. China CDC Wkly. (2021) 3:553–6. doi: 10.46234/ccdcw2021.143
36. Feyisa, JW, Kitila, KM, Lemu, JC, Hunde, MD, and Hunde, AD. Healthcare-seeking delay during COVID-19 pandemic among tuberculosis patients in Ilubabor zone health facilities, south-West Ethiopia. SAGE Open Med. (2022) 10:382974011. doi: 10.1177/20503121221142469
37. Wako, WG, Wasie, A, Wayessa, Z, and Fikrie, A. Determinants of health system diagnostic delay of pulmonary tuberculosis in Gurage and Siltie zones, South Ethiopia: a cross-sectional study. BMJ Open. (2021) 11:e047986. doi: 10.1136/bmjopen-2020-047986
38. Said, K, Hella, J, Mhalu, G, Chiryankubi, M, Masika, E, Maroa, T, et al. Diagnostic delay and associated factors among patients with pulmonary tuberculosis in Dar Es Salaam, Tanzania. Infect Dis Poverty. (2017) 6:64. doi: 10.1186/s40249-017-0276-4
39. Peri, AM, Bernasconi, DP, Galizzi, N, Matteelli, A, Codecasa, L, Giorgio, V, et al. Determinants of patient and health care services delays for tuberculosis diagnosis in Italy: a cross-sectional observational study. BMC Infect Dis. (2018) 18:690. doi: 10.1186/s12879-018-3609-4
40. Xu, CH, Zhang, XM, Liu, Y, Hu, DM, Xia, YY, Wang, L, et al. Factors associated with diagnostic delay of pulmonary tuberculosis in China. Biomed Environ Sci. (2022) 35:73–8. doi: 10.3967/bes2022.010
41. Chiang, CY, Chang, CT, Chang, RE, Li, CT, and Huang, RM. Patient and health system delays in the diagnosis and treatment of tuberculosis in southern Taiwan. Int J Tuberc Lung Dis. (2005) 9:1006–12.
42. Turner, RD. Cough in pulmonary tuberculosis: existing knowledge and general insights. Pulm Pharmacol Ther. (2019) 55:89–94. doi: 10.1016/j.pupt.2019.01.008
43. Isangula, KG, and Haule, RJ. Leveraging AI and machine learning to develop and evaluate a contextualized user-friendly cough audio classifier for detecting respiratory diseases: protocol for a diagnostic study in rural Tanzania. JMIR Res Protoc. (2024) 13:e54388. doi: 10.2196/54388
44. Osei, E, Akweongo, P, and Binka, F. Factors associated with DELAY in diagnosis among tuberculosis patients in Hohoe municipality, Ghana. BMC Public Health. (2015) 15:721. doi: 10.1186/s12889-015-1922-z
45. Ebrahimi, KM, Yekrang, SH, Kelkar, V, Harrison, SH, Goins, GD, Asghari, JM, et al. The identification of risk factors associated with patient and healthcare system delays in the treatment of tuberculosis in Tabriz, Iran. BMC Public Health. (2018) 18:174. doi: 10.1186/s12889-018-5066-9
46. Sabawoon, W, Sato, H, and Kobayashi, Y. Delay in the treatment of pulmonary tuberculosis: a report from Afghanistan. Environ Health Prev Med. (2012) 17:53–61. doi: 10.1007/s12199-011-0219-9
47. Ministry of Health of the People's Republic of China. Regulations on tuberculosis prevention and control. (2013). Available at: https://www.gov.cn/zhengce/2013-02/20/content_5713798.htm (accessed October 20, 2024)
48. Bian, X, Meng, Q, Sun, Q, Sun, X, Yan, Y, Zhang, J, et al. The study of multi-drug resistant tuberculosis patients in the first visit Progress. Med Philos. (2010) 31:32–4.
49. Davies, PD, and Pai, M. The diagnosis and misdiagnosis of tuberculosis. Int J Tuberc Lung Dis. (2008) 12:1226–34.
50. Chimbatata, N, Zhou, C, Chimbatata, CM, Mhango, L, Diwan, VK, and Xu, B. Barriers to prompt TB diagnosis-a comparative study between northern Malawi and eastern rural China. Trans R Soc Trop Med Hyg. (2017) 111:504–11. doi: 10.1093/trstmh/try002
Keywords: tuberculosis, lung, delay, diagnosis, risk factors
Citation: Fan M, Liu Y, Liu K, Liu X, Li Y, Li T, Zhang C, Zhang H and Cheng J (2025) Health system delay and risk factors in pulmonary tuberculosis diagnosis before and during the COVID-19 epidemic: a multi-center survey in China. Front. Public Health. 13:1526774. doi: 10.3389/fpubh.2025.1526774
Edited by:
Chongguang Yang, Sun Yat-sen University, ChinaReviewed by:
Cheng Chen, Jiangsu Provincial Center for Disease Control and Prevention, ChinaMesfin Abebe, Dilla University, Ethiopia
Copyright © 2025 Fan, Liu, Liu, Liu, Li, Li, Zhang, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Zhang, emhhbmdodWlAY2hpbmFjZGMuY24=; Jun Cheng, Y2hlbmdqdW5AY2hpbmFjZGMuY24=
 Mingkuan Fan
Mingkuan Fan Yushu Liu1
Yushu Liu1 Kui Liu
Kui Liu Tao Li
Tao Li Canyou Zhang
Canyou Zhang