- 1Physical Examination Center, Hebei General Hospital, Shijiazhuang, Hebei, China
- 2Department of Obstetrics and Gynecology, Hebei General Hospital, Shijiazhuang, Hebei, China
- 3Department of Obstetrics and Gynecology, Affiliated Hospital of Chengde Medical University, Chengde, Hebei, China
- 4Department of Obstetrics and Gynecology, Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Background: Endometriosis affects up to 15% of women of reproductive age and can lead to various symptoms. More than 200 million people worldwide are at risk of higher than safe levels of arsenic exposure through drinking water. Studies investigating the relationship between arsenic and endometriosis are very limited and have yielded inconsistent results. This study aimed to explore the relationship between total urinary arsenic, arsenic species (Urinary arsenous acid, Urinary Arsenic acid, Urinary Arsenobetaine, Urinary Arsenocholine, Urinary Dimethylarsinic acid, Urinary Monomethylarsonic acid) and endometriosis.
Methods: We utilized a nationally representative dataset from the National Health and Nutrition Examination Survey (NHANES) from 2003 to 2006. A total of 650 participants were included. We examined the association between total urinary arsenic and different arsenic species with endometriosis using weighted multivariate logistic regression models.
Results: Urinary arsenous acid and urinary monomethylarsonic acid (MMA) were positively correlated with endometriosis (p < 0.05). After adjusting for potential confounding factors, the positive correlation of urinary MMA remained significant (OR: 1.317, 95%CI: 1.074–1.615). Subgroup analyses and interaction tests indicated that this association was not dependent.
Conclusion: Our research underscores a significant positive association observed between factors urinary MMA and endometriosis. Future research is needed to elucidate the specific mechanisms behind this association.
1 Introduction
Endometriosis is characterized by the proliferation of endometrial-like tissue, including glandular cells and stroma, beyond the uterine cavity. The clinical presentation typically encompasses cyclical hemorrhage, dysmenorrhea, and dyspareunia (1). Epidemiological data suggests a prevalence rate of 10–15% in the female population of childbearing age (2). At present, the pathogenesis of endometriosis remains elusive. Proposed etiological hypotheses comprise retrograde menstruation, vascular or lymphatic dissemination, aberrant coelomic metaplasia, immune system dysregulation, and factors of cellular determinism, along with hereditary and environmental influences (2).
Arsenic is one of the most extensively studied elements in the field of metal toxicity, second only to lead (Pb) (3). Arsenic, a metalloid, is ubiquitously present in water, soil, and air, originating from both natural processes and human activities, existing in both inorganic and organic forms (4). Arsenic exposure through drinking water represents the primary mechanism of human contact, with over 200 million individuals worldwide facing the threat of encountering arsenic levels surpassing safety thresholds (5). Arsenic, a well-known metalloid, can cause endocrine disruption and reproductive disorders in women (6). Existing studies have linked arsenic exposure to ovarian dysfunction and infertility (7, 8). Exposure to high doses of arsenic during pregnancy increases the risk of miscarriage, fetal defects, and toxicity to the fetus (9, 10).
However, the relationship between arsenic exposure and endometriosis remains under-researched and is marked by contradictory findings (11, 12). Previous studies have not investigated the correlation between arsenic forms and endometriosis. Consequently, our research is designed to investigate the link between urinary total arsenic and different arsenic (urinary arsenous acid, urinary arsenic acid, urinary arsenobetaine, urinary arsenocholine, urinary dimethylarsinic acid, urinary monomethylarsonic acid) forms with endometriosis.
2 Methods
2.1 Data source
In this cross-sectional investigation, we employed data from the National Health and Nutrition Examination Survey (NHANES), which utilizes a sophisticated, multi-stage, stratified probability sampling method, ensuring samples that are representative of the national populace. The National Center for Health Statistics Institutional Review Board approved the data collection methods employed in the NHANES study, securing informed consent from all contributors. NHANES data are publicly available, enhancing transparency and facilitating scientific research across various fields. People can access extensive NHANES datasets, comprehensive operational manuals, consent forms, and periodical guides on the NHANES portal.1
2.2 Study participants
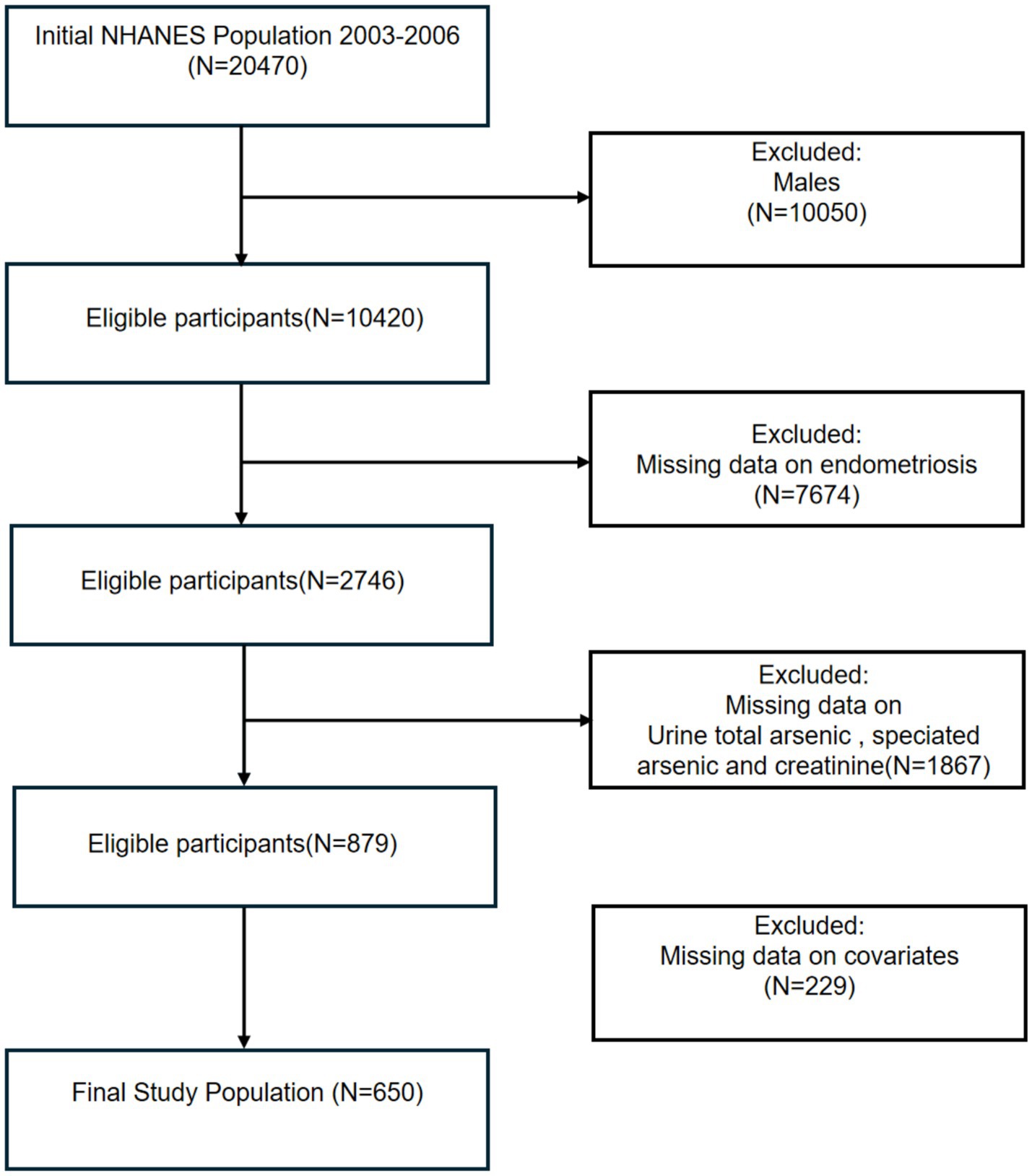
This study analyzed data from two NHANES cycles (2003–2004 and 2005–2006), and all groups were initially included; the male group, those with missing information on endometriosis, those with urinary arsenic levels, and relevant covariates were excluded in turn, and 650 participants were included in the final analysis (Figure 1).
2.3 Urinary arsenic and creatinine measurement
This analysis used NHANES datasets for arsenic from 2003–2004 (L06UAS_C) and 2005–2006 (UAS_D), utilizing High-Performance Liquid Chromatography (HPLC) for species separation, followed by ICP-DRC-MS for detection of total urinary arsenic and species concentrations. The following arsenic species and their respective lower limit of detection (LLOD) were analyzed: Urinary total arsenic 0.74 μg/L, urinary arsenous acid 1.2 μg/L, urinary arsenic acid 1.0 μg/L, urinary arsenobetaine 0.4 μg/L, urinary arsenocholine 0.6 μg/L, urinary dimethylarsinic acid (DMA) 1.7 μg/L, and urinary monomethylarsonic acid (MMA) 0.9 μg/L. Concentrations below detection limits were substituted with LLOD/ √2 values. To correct for urine arsenic in urine cadmium concentration, we used urinary creatinine concentration (mg/dl) data from the same dataset.
2.4 Endometriosis status
The diagnosis of endometriosis was determined through a reproductive health questionnaire for women aged 20–54 years, asking, “Has a doctor or other health professional ever told you that you had endometriosis?”
2.5 Covariates assessment
Drawing from existing research and clinical insights, our analysis included the following covariates to minimize potential confounding effects. We gathered data on demographic aspects such as age, ethnicity (Non-Hispanic Black, Non-Hispanic White, Other), educational level (high school and below, above high school), marital status (married/living with a partner, widowed/divorced/separated, never married), and the poverty income ratio (PIR). The Body Mass Index (BMI), determined by the individual’s mass in kilograms divided by the square of their height in meters, was classified into either normal or underweight (BMI < 25 kg/m2) and overweight or obese (BMI ≥ 25 kg/m2). We queried participants about their smoking status with the question, “Have you smoked at least 100 cigarettes in your lifetime?” and measured alcohol consumption by asking, “In the past 12 months, have you consumed 4 to 5 or more drinks per day?” Histories of diabetes and hypertension were acquired from self-reports in our health survey questionnaire. Additional data on age at menarche, fertility status were gleaned from the reproductive health questionnaire.
2.6 Statistical analysis
To delineate the variations in baseline characteristics between individuals with and without endometriosis, categorical variables were represented as weighted proportions, and continuous variables as weighted means with standard deviation or weighted medians with interquartile ranges. Prior to conducting logistic regression analyses, we evaluated the correlations between different arsenic species using Spearman’s correlation coefficients. To avoid potential multicollinearity issues, we examined the variance inflation factors for all arsenic species. The variance inflation factors values were all below 5, indicating no significant multicollinearity among the arsenic species. Based on these analyses, we conducted separate logistic regression models for each arsenic species to avoid the potential confounding effects of inter-species correlations. Weighted logistic regression analyses, both univariate and multivariate, were applied to gauge the relationship between six arsenic species and endometriosis. Model 1 did not control for any covariates, Model 2 was adjusted for age and ethnicity, and Model 3 further controlled for marital status, educational attainment, smoking status, BMI, PIR, menarche onset, alcohol use, hypertension, diabetes, fertility status and creatinine levels in urine. The adoption of restricted cubic splines (RCS) served to address the nonlinear relationship between MMA and endometriosis. Finally, statistical p for interaction among covariates and endometriosis prevalence rate was assessed, and subgroup analyses were performed to enhance understanding of these findings. In addition, age-stratified analysis using 40 years as the cutoff point was conducted, taking into account both the natural course of endometriosis and the potential cumulative effects of arsenic exposure. Besides, the multiple imputation (MI) approaches were applied by us to fill in the missing values that exist in the data through the “MICE” package of the R software. R and RStudio (version 4.3.0) facilitated all statistical computations, with p-values below 0.05 denoting statistical significance.
3 Results
3.1 Characteristics of the participants
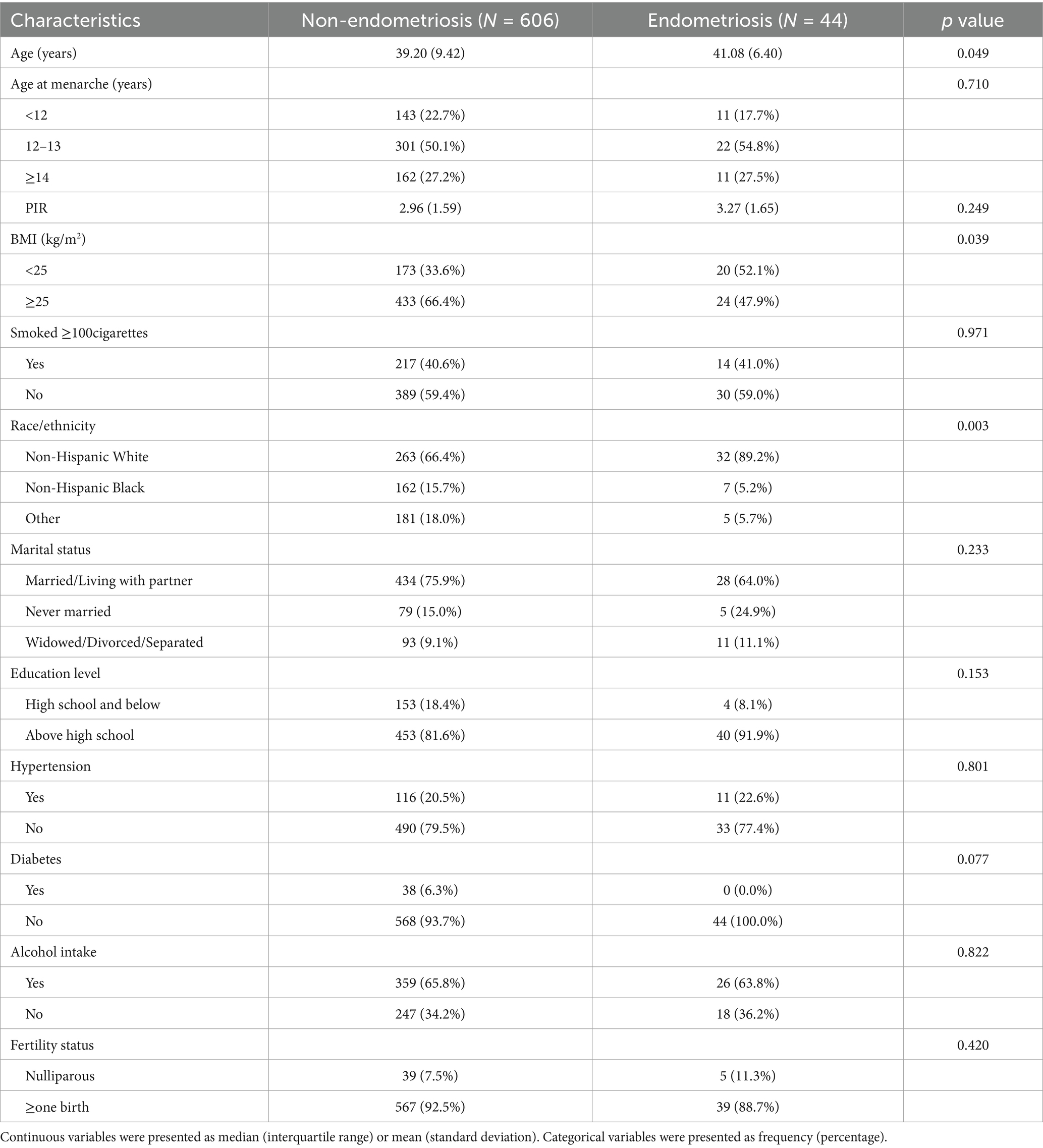
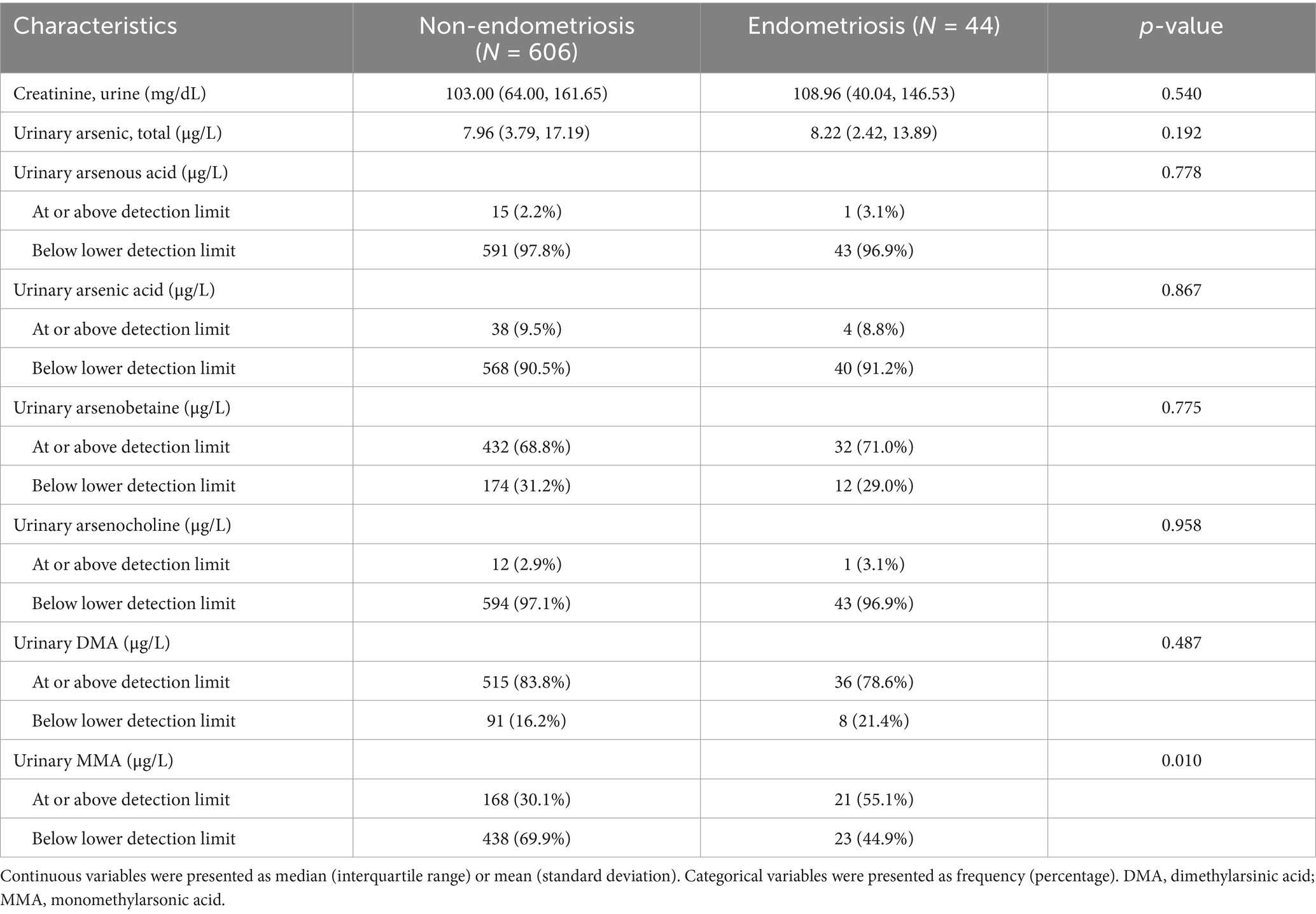
The baseline characteristics and weighted estimates of the 650 participants were detailed in Table 1. Out of the total participants, 44 were afflicted with endometriosis, while 606 remained unaffected. Substantial variations (p < 0.05) were evident between the endometriosis and non-endometriosis cohorts concerning age, BMI, ethnicity. There were no significant differences (p > 0.05) in menarche age, PIR, marital status, smoking status, education level. The distribution of laboratory measurement results among participants was displayed in Table 2, with MMA being the only indicator significantly associated with endometriosis. Individuals with endometriosis were more likely to have urinary MMA concentrations at or above the detection limit.
3.2 Weighted logistic regression and subgroup analyses
Weighted univariate and multivariate logistic regression analyses were utilized to examine the correlation between six types of arsenic and endometriosis. As depicted in Table 3, Model 1 did not adjust for covariates, Model 2 adjusted for age and race, and Model 3 was subjected to further adjustments incorporating marital status, education level, smoking status, BMI, PIR, age at menarche, alcohol use, hypertension, diabetes, fertility status and urine creatinine levels. Associations were reported using odds ratios (OR) with 95% confidence intervals (CI). Higher concentrations of arsenous acid and MMA in urine were positively correlated with endometriosis (p < 0.05). Although the positive correlation of arsenous acid weakened after adjusting for potential confounding factors, MMA remained significantly correlated with endometriosis (MMA: OR: 1.317, 95%CI: 1.074–1.615).
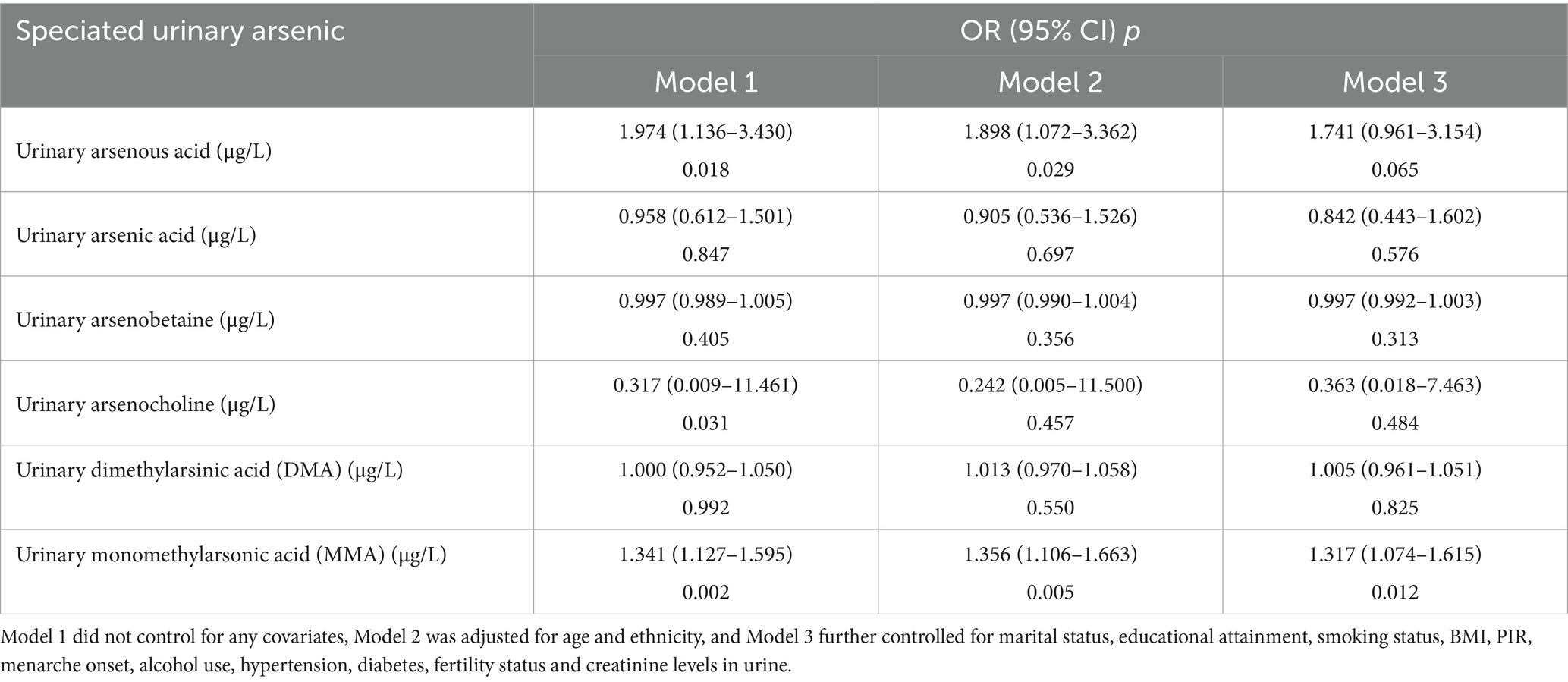
Table 3. Unadjusted and adjusted odds ratios (OR) and 95% confidence intervals (CI) of the association between arsenic and endometriosis NHANES 2003–2006.
After adjusting for covariates in Model 3, although RCS did not reveal a nonlinear relationship between MMA and endometriosis, the prevalence rate of endometriosis seemed to increase with higher exposure levels (Figure 2). Furthermore, we found that the correlation between MMA and endometriosis was not significant in individuals under 40 years old but was significant in those over 40 (Figure 3), possibly related to long-term arsenic exposure. The sensitivity analysis results after multiple interpolation indicate that they were still robust (Supplementary Table S1).
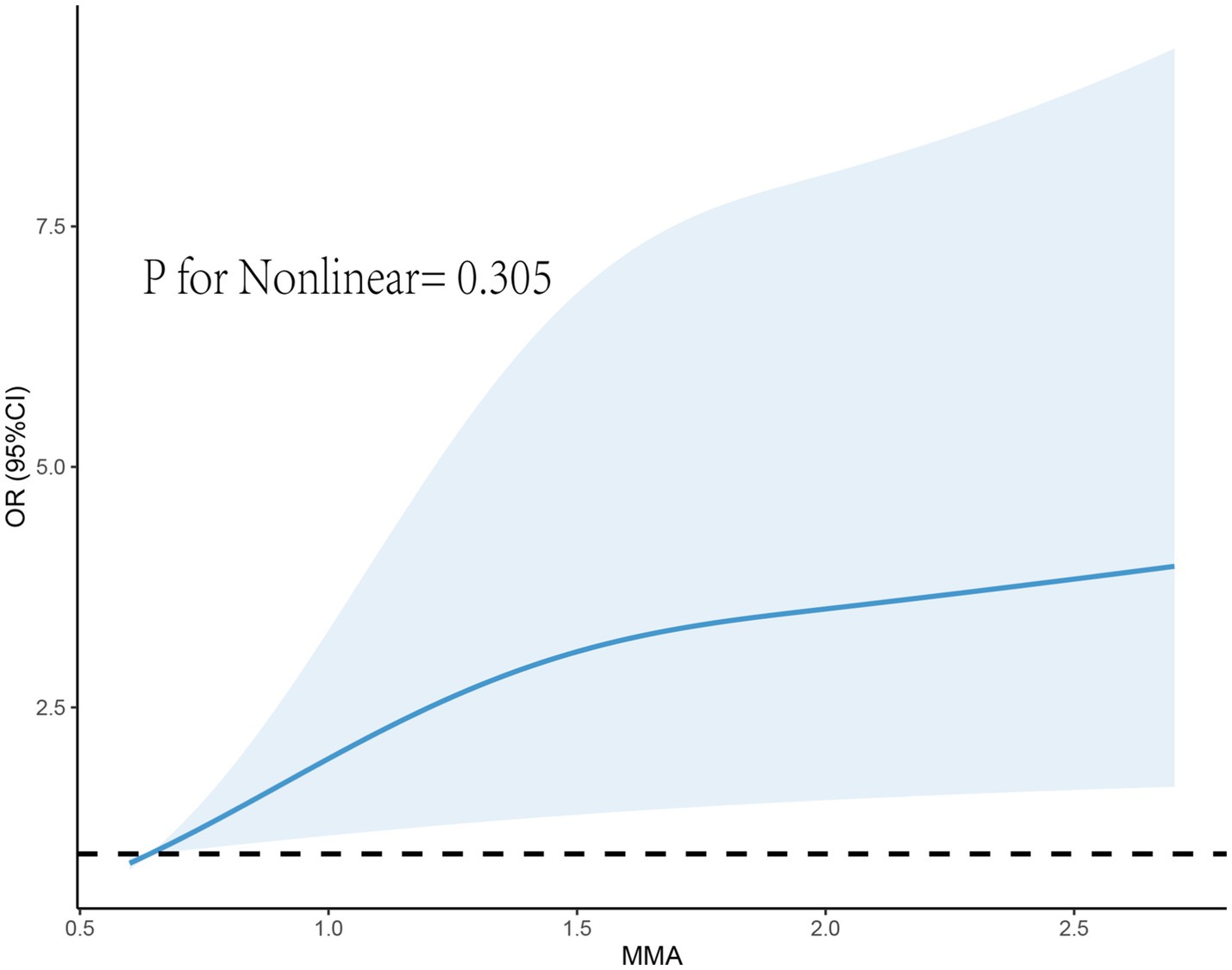
Figure 2. Restricted cubic spline plot of the association between monomethylarsonic acid (MMA) and endometriosis.
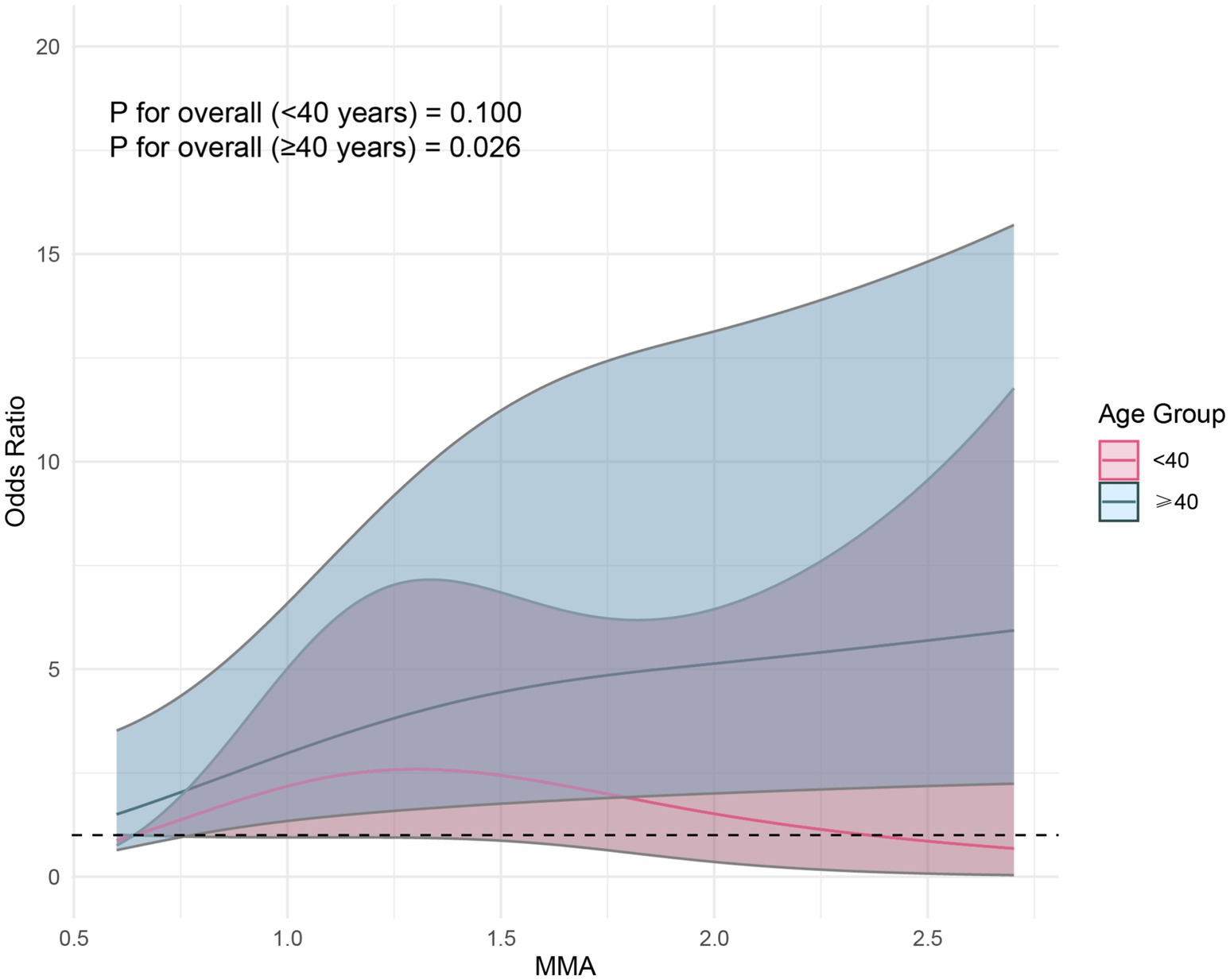
Figure 3. Restricted cubic spline plot of the association between monomethylarsonic acid (MMA) and endometriosis stratified by age.
As illustrated in Figure 4, when observing the association between MMA and endometriosis, grouped by age, marital status, education level, BMI, age at menarche, smoking status, and race, no interaction of the related covariates was observed in this association (p for interaction > 0.05), indicating that the correlation between MMA and endometriosis does not vary due to age, BMI, age at menarche, smoking status, and hypertension.
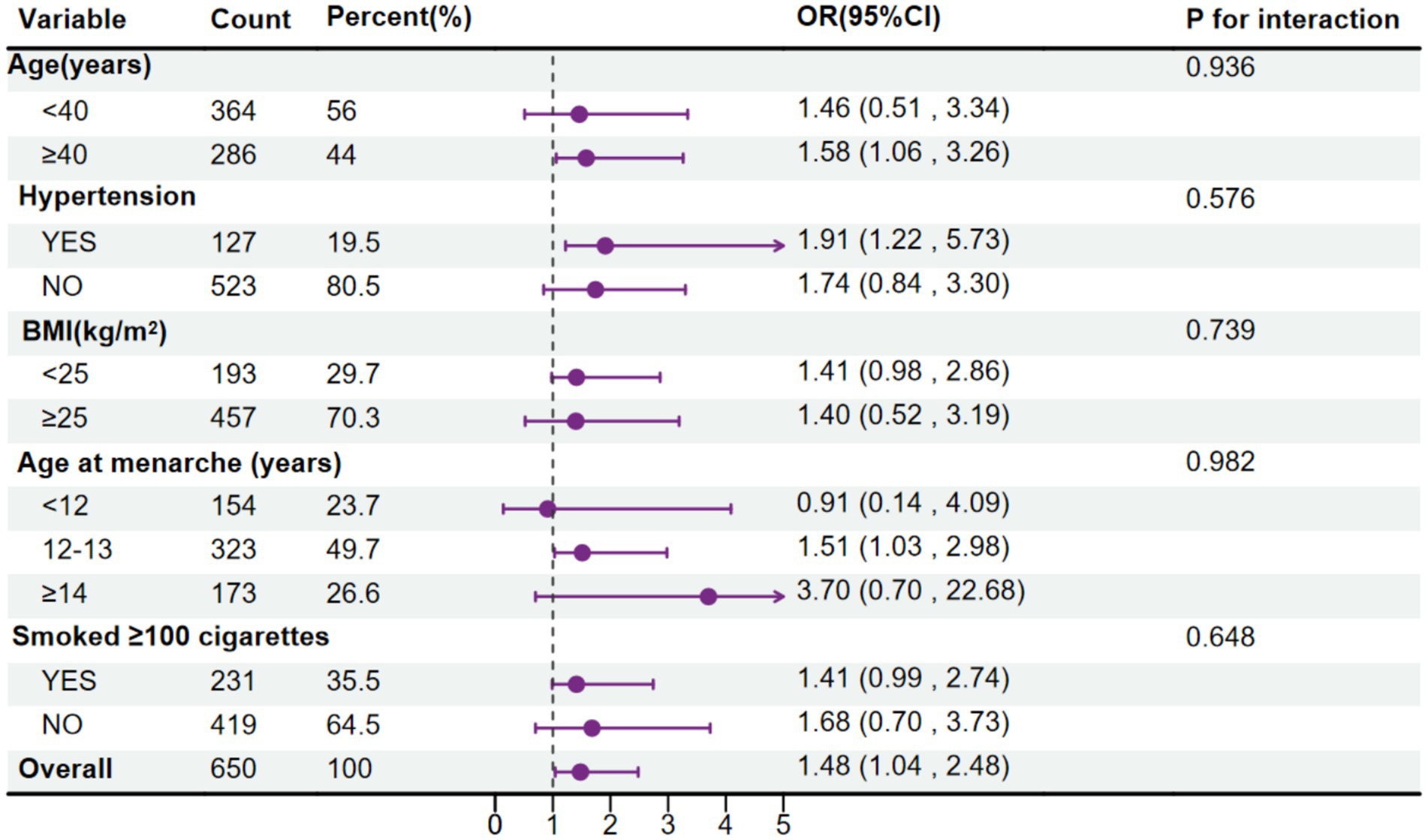
Figure 4. Forest plot for the association between urinary monomethylarsonic acid (MMA) and endometriosis, stratified by age, body mass index (BMI), age at menarche, hypertension and smoking status.
4 Discussion
In our nationally representative study of American women, we found a correlation between urinary MMA and self-reported physician-diagnosed endometriosis, after adjusting for potential confounders. As far as we are aware, this serves as the inaugural large-scale, nationally representative inquiry into the relationship between total urinary arsenic, arsenic species, and endometriosis, unveiling a positive correlation between urinary MMA and endometriosis. Studies on arsenic and endometriosis are limited and inconsistent; a previous cohort study involving 473 women did not observe a correlation between urinary arsenic and endometriosis (12). In China, a recent study on the association between exposure to various toxic metals and endometriosis found that the concentration of arsenic in blood or follicular fluid contributed the most, reporting a positive correlation with endometriosis (11).
As an estrogen-dependent chronic inflammatory disease, endometriosis has the highest prevalence rate between the ages of 25–35 (13). Estrogen plays a crucial role in the disease’s pathogenic mechanism; increased local production of estrogen fosters the survival, implantation, and proliferation of ectopic endometrial tissue (14). The relationship between arsenic and estrogen is still controversial. A study based on adolescents showed a positive correlation between total arsenic, its metabolites, and estradiol (14), while two experimental studies indicated a significant decrease in estradiol levels after arsenic exposure (15, 16). Arsenic interacts with the ligand-binding domain of estrogen receptor-alpha (ERα) with high affinity, exhibiting potent estrogenic activity in vitro and in vivo (17). Moreover, arsenic, characterized by its properties as a metalloid with both metallic and nonmetallic attributes, predominantly manifests its toxic effects through oxidative stress, widely acknowledged as the principal mechanism of arsenic toxicity (18, 19). Reactive oxygen species (ROS) production can alter redox balance, ultimately leading to reproductive abnormalities such as miscarriage, endometriosis, and polycystic ovary syndrome (20, 21). Studies have found that antioxidant defense mechanisms in the uterus of mice, Including enzymes like peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT), are reduced by 2.5, 2.6, and 2.9 times, respectively, in arsenic-exposed groups (16). MMA is a major component of arsenic metabolites, with up to 11% of total urinary arsenic excreted as MMA (22). It is one of the most toxic forms of arsenic in the human body (22, 23), possibly due to its higher cellular uptake rate (24) and high affinity for thiol (-SH) groups (22). Although the toxicological characteristics of arsenic metabolites vary in the body, the toxicity phenotype of MMA is mostly associated with ROS production (3). Arsenic exposure also leads to an increase in pro-inflammatory cytokines such as IL-6, IL-10, and TNF-α, which are closely related to inflammatory responses (25). The inflammatory pathway is also involved in the mechanism of endometriosis formation (26). The complexity of arsenic toxicity is manifested through its multifaceted nature (27). Nevertheless, owing to the scarcity of specific research examining arsenic exposure in relation to endometriosis, further experiments are warranted to elucidate the involved mechanisms.
This study has the following limitations: Firstly, as it is a cross-sectional study design, potential reverse causation cannot be ruled out. The study measures current arsenic levels but cannot account for the time elapsed between endometriosis diagnosis and arsenic measurement. Secondly, although urinary arsenic concentration is commonly used as a typical biomarker for arsenic exposure, the rapid metabolism in humans means that data from a single urine sample may limit our ability to accurately assess the average level of arsenic exposure. Additionally, the distribution of arsenic forms differs between blood and urine, with blood arsenic potentially providing a more accurate reflection of exposure levels in target tissues and organs (28). Furthermore, our analytical approach, which focused on single urinary arsenic measurements, represents a methodological limitation. More sophisticated mixed-effects analyses, such as weighted quantile sum regression or Bayesian kernel machine regression, could provide deeper insights into the complex relationships between multiple arsenic species and endometriosis risk. Lastly, most of the data used were collected through interviews or self-reported questionnaires, which could be subject to recall and reporting biases.
5 Conclusion
In conclusion, this investigation establishes the initial evidence of a direct link between urinary MMA and the prevalence of endometriosis. Considering the significant prevalence of endometriosis and the pervasive exposure to arsenic in the general populace, it is vital to pursue further experimental studies to validate our findings and elucidate the specific mechanisms involved.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: all the data analyzed in our study can be obtained from NHANES database.
Author contributions
LS: Writing – original draft. YR: Writing – review & editing. RX: Writing – review & editing. SZ: Writing – review & editing. WL: Writing – review & editing. CM: Writing – review & editing. XZ: Writing – review & editing. ZD: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We acknowledge NHANES database for providing their platforms and contributors for uploading their meaningful datasets and we thank all participants included in our present study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1525986/full#supplementary-material
Abbreviations
NHANES, National Health and Nutrition Examination Survey; MMA, Monomethylarsonic acid; HPLC, High-Performance Liquid Chromatography; LLOD, Lower limit of detection; DMA, Dimethylarsinic acid; PIR, Poverty income ratio; BMI, Body Mass Index; RCS, Restricted cubic splines; OR, Odds ratios; ERα, Estrogen receptor-alpha; ROS, Reactive oxygen species; GPx, Glutathione peroxidase; SOD, Superoxide dismutase.
Footnotes
References
1. Eskenazi, B, and Warner, ML. Epidemiology of endometriosis. Obstet Gynecol Clin N Am. (1997) 24:235–58. doi: 10.1016/s0889-8545(05)70302-8
2. Smolarz, B, Szyłło, K, and Romanowicz, H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (review of literature). Int J Mol Sci. (2021) 22:10554. doi: 10.3390/ijms221910554
3. Flora, SJS. Arsenic-induced oxidative stress and its reversibility. Free Radic Biol Med. (2011) 51:257–81. doi: 10.1016/j.freeradbiomed.2011.04.008
4. Pan, W, Ye, X, Zhu, Z, Li, C, Zhou, J, and Liu, J. A case-control study of arsenic exposure with the risk of primary ovarian insufficiency in women. Environ Sci Pollut Res. (2020) 27:25220–9. doi: 10.1007/s11356-020-08806-0
5. Naujokas, MF, Anderson, B, Ahsan, H, Aposhian, HV, Graziano, JH, Thompson, C, et al. The broad scope of health effects from chronic arsenic exposure: update on a worldwide public health problem. Environ Health Perspect. (2013) 121:295–302. doi: 10.1289/ehp.1205875
6. De Coster, S, and van Larebeke, N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health. (2012) 2012:713696. doi: 10.1155/2012/713696
7. Yunus, FM, Rahman, MJ, Alam, MZ, Hore, SK, and Rahman, M. Relationship between arsenic skin lesions and the age of natural menopause. BMC Public Health. (2014) 14:419. doi: 10.1186/1471-2458-14-419
8. Lei, HL, Wei, HJ, Ho, HY, Liao, KW, and Chien, LC. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: a cross-sectional study from Taiwan. BMC Public Health. (2015) 15:1220. doi: 10.1186/s12889-015-2564-x
9. Ommati, MM, Shi, X, Li, H, Zamiri, MJ, Farshad, O, Jamshidzadeh, A, et al. The mechanisms of arsenic-induced ovotoxicity, ultrastructural alterations, and autophagic related paths: an enduring developmental study in folliculogenesis of mice. Ecotoxicol Environ Saf. (2020) 204:110973. doi: 10.1016/j.ecoenv.2020.110973
10. Broberg, K, Ahmed, S, Engström, K, Hossain, MB, Jurkovic Mlakar, S, Bottai, M, et al. Arsenic exposure in early pregnancy alters genome-wide DNA methylation in cord blood, particularly in boys. J Dev Orig Health Dis. (2014) 5:288–98. doi: 10.1017/S2040174414000221
11. Shen, L, Liang, C, Li, D, Zhang, Z, Wang, X, Jiang, T, et al. The association between exposure to multiple toxic metals and the risk of endometriosis: evidence from the results of blood and follicular fluid. Sci Total Environ. (2023) 855:158882. doi: 10.1016/j.scitotenv.2022.158882
12. Pollack, AZ, Louis, GMB, Chen, Z, Peterson, CM, Sundaram, R, Croughan, MS, et al. Trace elements and endometriosis: the ENDO study. Reprod Toxicol Elmsford N. (2013) 42:41–8. doi: 10.1016/j.reprotox.2013.05.009
13. Nahir, B, Eldar-Geva, T, Alberton, J, and Beller, U. Symptomatic diaphragmatic endometriosis ten years after total abdominal hysterectomy. Obstet Gynecol. (2004) 104:1149–51. doi: 10.1097/01.AOG.0000125000.20582.64
14. Zhang, Y, Xing, H, Hu, Z, Xu, W, Tang, Y, Zhang, J, et al. Independent and combined associations of urinary arsenic exposure and serum sex steroid hormones among 6-19-year old children and adolescents in NHANES 2013-2016. Sci Total Environ. (2023) 863:160883. doi: 10.1016/j.scitotenv.2022.160883
15. Chen, Y, Sun, Y, Zhao, A, Cai, X, Yu, A, Xu, Q, et al. Arsenic exposure diminishes ovarian follicular reserve and induces abnormal steroidogenesis by DNA methylation. Ecotoxicol Environ Saf. (2022) 241:113816. doi: 10.1016/j.ecoenv.2022.113816
16. Dash, M, Dey, A, and Chattopadhyay, S. Mitigation of arsenic driven utero-ovarian malfunction and changes of apoptotic gene expression by dietary NAC. Ecotoxicol Environ Saf. (2020) 199:110675. doi: 10.1016/j.ecoenv.2020.110675
17. Parodi, DA, Greenfield, M, Evans, C, Chichura, A, Alpaugh, A, Williams, J, et al. Alteration of mammary gland development and gene expression by in utero exposure to arsenic. Reprod Toxicol Elmsford N. (2015) 54:66–75. doi: 10.1016/j.reprotox.2014.12.011
18. Ercal, N, Gurer-Orhan, H, and Aykin-Burns, N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. (2001) 1:529–39. doi: 10.2174/1568026013394831
19. Yu, H, Kuang, M, Wang, Y, Rodeni, S, Wei, Q, Wang, W, et al. Sodium Arsenite injection induces ovarian oxidative stress and affects steroidogenesis in rats. Biol Trace Elem Res. (2019) 189:186–93. doi: 10.1007/s12011-018-1467-y
20. Webster, RP, Roberts, VHJ, and Myatt, L. Protein nitration in placenta – functional significance. Placenta. (2008) 29:985–94. doi: 10.1016/j.placenta.2008.09.003
21. Liang, C, Zhang, Z, Cao, Y, Wang, J, Shen, L, Jiang, T, et al. Exposure to multiple toxic metals and polycystic ovary syndrome risk: endocrine disrupting effect from as, Pb and Ba. Sci Total Environ. (2022) 849:157780. doi: 10.1016/j.scitotenv.2022.157780
22. Petrick, JS, Jagadish, B, Mash, EA, and Aposhian, HV. Monomethylarsonous acid (MMA(III)) and arsenite: LD(50) in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem Res Toxicol. (2001) 14:651–6. doi: 10.1021/tx000264z
23. Xu, H, Medina, S, Lauer, FT, Douillet, C, Liu, KJ, Stýblo, M, et al. Genotoxicity induced by monomethylarsonous acid (MMA+3) in mouse thymic developing T cells. Toxicol Lett. (2017) 279:60–6. doi: 10.1016/j.toxlet.2017.07.897
24. Dopp, E, Kligerman, AD, and Diaz-Bone, RA. Organoarsenicals. Uptake, metabolism, and toxicity. Met Ions Life Sci. (2010) 7:231–65. doi: 10.1039/bk9781847551771-00231
25. Tutkun, L, Gunduzoz, M, Turksoy, VA, Deniz, S, Oztan, O, Cetintepe, SP, et al. Arsenic-induced inflammation in workers. Mol Biol Rep. (2019) 46:2371–8. doi: 10.1007/s11033-019-04694-x
26. Giacomini, E, Minetto, S, Li Piani, L, Pagliardini, L, Somigliana, E, and Viganò, P. Genetics and inflammation in endometriosis: improving knowledge for development of new pharmacological strategies. Int J Mol Sci. (2021) 22:9033. doi: 10.3390/ijms22169033
27. Dangleben, NL, Skibola, CF, and Smith, MT. Arsenic immunotoxicity: a review. Environ Health Glob Access Sci Source. (2013) 12:73. doi: 10.1186/1476-069X-12-73
Keywords: endometriosis, arsenic, NHANES, gynecology, chronic inflammatory disease
Citation: Su L, Ren Y, Xu R, Zhao S, Liu W, Meng C, Zhou X and Du Z (2025) Association between urinary arsenic and the prevalence of endometriosis in women in the United States. Front. Public Health. 13:1525986. doi: 10.3389/fpubh.2025.1525986
Edited by:
Shuna Jin, Hubei University of Chinese Medicine, ChinaReviewed by:
Rima Kregzdyte, Lithuanian University of Health Sciences, LithuaniaYue Cao, Southern Medical University, China
Copyright © 2025 Su, Ren, Xu, Zhao, Liu, Meng, Zhou and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zeqing Du, Mjg4MDQ0MjFAaGVibXUuZWR1LmNu
 Luyang Su
Luyang Su Yanan Ren
Yanan Ren Ren Xu2
Ren Xu2 Xuan Zhou
Xuan Zhou