- 1Departement of Pharmacy, Asrat Woldeyes Health Science Campus, Debre Berhan University, Debre Berhan, Ethiopia
- 2School of Medicine, Asrat Woldeyes Health Science Campus, Debre Berhan University, Debre Berhan, Ethiopia
- 3School of Pharmacy, College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
Background: The global older adult population is expected to increase from 524 million in 2010 to 1.5 billion by 2050, mainly in developing countries. Age-related diseases, comorbidities, and polypharmacy make appropriate prescribing crucial. This study aimed to assess the prevalence of polypharmacy, drug–drug interaction, and potentially inappropriate medication use and its factors in an Ethiopian hospital.
Methods: A facility-based cross-sectional study on 236 patients aged 65 and above at Dessie Comprehensive Specialized Hospital (Jan 2022–Apr 2023) used the 2023 Beers Criteria and START/STOP V.3 to identify potentially inappropriate medications. Polypharmacy and potential drug–drug interactions were assessed using Micromedex®, with descriptive statistics and binary logistic regression performed in SPSS version 26.
Result: Of the 236 patients in this study, 94 (39.8, 95% CI: 35.7–44.5%) were prescribed at least one potentially inappropriate medication per the STOPP/START criteria, with 81 (34.3%) identified by STOPP and 13 (5.5%) by START. According to the Beers Criteria, 108 patients (45.7, 95% CI: 40.1–51.0%) received at least one potentially inappropriate medication. Polypharmacy was observed in 80 patients (33.9, 95% CI: 29.1–38.5%), and potential drug–drug interactions were identified in 111 patients (47.0%). Being female (AOR: 2.93), age ≥75 (AOR: 1.52), and polypharmacy (AOR: 3.20) were linked to potentially inappropriate medication use per Beers Criteria. Age 70–74 (AOR: 2.30) and polypharmacy (AOR: 3.10) were also associated per STOPP/START criteria.
Conclusion: Polypharmacy, drug–drug interactions, and potentially inappropriate medications are common among older Ethiopian patients, with age, sex, and polypharmacy as contributing factors. Future studies are needed to assess the health and economic impacts of potentially inappropriate medications use.
Background
The global population of individuals aged 65 and above is projected to increase significantly, from 524 million in 2010 to an estimated 1.5 billion by 2050, with the majority of this growth occurring in developing countries (1). Drug therapy for the older adult requires special consideration due to age-related changes in pharmacokinetics and drug sensitivity (2). Older adults are at higher risk for age-related comorbidities, often necessitating multiple medications, which can lead to inappropriate and potentially harmful prescribing practices. Inappropriate prescriptions remain a significant health concern in the older adult population (3).
Older adults face a high risk of inappropriate medication use due to their complex medical conditions and the use of multiple medications (4). Certain drugs pose an increased risk of adverse drug events in this population and are thus classified as potentially inappropriate medications (PIMs) for older adults (5). Medication appropriateness is crucial in geriatrics due to their heightened vulnerability to medication issues. Screening and intervention strategies using accessible tools can be integrated into clinical workflows and, when possible, electronic medical records to improve medication safety (4). Among nursing home residents, approximately 50% are exposed to PIMs, with data suggesting an increasing prevalence over time (6).
Inappropriate prescribing is significantly associated with the number of medications prescribed to older adult patients (7). The prevalence of PIM varies across countries; for instance, studies report PIM exposure rates of 52.5% in Saudi Arabia (5), 11% in the USA (8), 66% in India (9), and 88% in Germany (10) among older adult patients. A retrospective study in the USA on cardiovascular patients observed a high prevalence of PIM use in the geriatric population (11). Similarly, a retrospective study in northwest Ethiopia involving 1,252 patients found that 347 (27.7%) received at least one PIM, with immediate-release nifedipine (53.9%) being the most inappropriately prescribed, followed by diclofenac (22.2%), ibuprofen (7.8%), and indomethacin (5.2%) (12). Additionally, an observational study in India indicated a high prevalence of potentially inappropriate prescribing and adverse drug reactions among hospitalized older adults (13).
A significant association was found between PIMs and adverse outcomes, including increased hospitalizations, emphasizing the need for interventional strategies to prevent PIM use, especially in patients with multiple chronic conditions (14). The use of PIMs among the geriatric population is linked to an increased risk of unplanned hospitalizations, especially among patients undergoing polypharmacy, warranting caution in PIM prescriptions for older adults (15). PIMs pose a substantial risk of adverse drug events in older adult patients (5), and their use is a significant concern due to the higher demand for emergency care resources annually (16). Reducing PIM utilization can decrease adverse drug reactions, lower treatment costs, enhance medication adherence, and reduce hospitalization risk in older patients (7, 17).
In Ethiopia, the absence of specific guidelines for geriatric medication management underscores the importance of this study. Findings may inform the development of guidelines to improve medication appropriateness and reduce PIMs among older adults. Additionally, this study can aid institutions in updating formularies and incorporating safer alternatives, ultimately optimizing drug therapy for older adult patients.
Methods
Study design and study population
A facility-based cross-sectional study was conducted among patients aged 65 and above who attended the medical referral clinic at Dessie Comprehensive Specialized Hospital from January 1, 2022, to April 30, 2023. In these clinics, patients typically have a maximum follow-up period of 4 months; hence, data collection was conducted over 4 months. Patients with incomplete medical or medication records such as missing dosage or frequency information and those on follow-up without any prescribed medications were excluded from the study.
Sample size determination
The sample size was calculated using a single proportion formula, based on a previous study that reported a 28.6% prevalence of PIMs (12), with a 5% margin of error and a 95% confidence level. During the study period, 675 older adult patients visited Dessie Comprehensive Specialized Hospital. After applying a finite population correction, a final sample of 236 patients was determined. Participants were selected through simple random sampling.
By adjusting this number = 214 by adding 10% 236.
Data collection, procedure, and instrument
To enhance patient safety, the STOPP/START criteria and AGS Beers Criteria provide updated guidelines for PIMs in older adults. These guidelines highlight medications that may increase the risk of adverse drug reactions and offer safer alternatives or recommendations for cautious use. The STOPP/START version 3 criteria serve as tools to improve medication management in this population. The STOPP criteria identify potentially inappropriate medications to be avoided, while the START criteria emphasize essential medications that may be under-prescribed. This version focuses on reducing medication-related risks by optimizing treatment and minimizing unnecessary polypharmacy. A data abstraction tool was developed utilizing the 2023 AGS Beers Criteria (18), the STOPP/START version 3 (19), and previously published articles (20, 21). This tool was employed to extract essential information, including patient demographics, medical conditions, medication-related details, and relevant investigative data, using a pre-designed data abstraction format.
Variables of the study
The analysis considered several independent variables, including demographic factors (age in years and gender), the number of comorbidities, specific comorbid conditions, and the total number of prescribed medications. Chronic disease conditions were classified: as cardiovascular, endocrine, respiratory, neurological, hematologic, and ophthalmic disorders, which were treated as additional independent variables. The primary outcome of interest was the incidence of PIMs, while secondary outcomes included instances of polypharmacy and drug–drug interactions. While no universally accepted definition of polypharmacy exists, this study defined polypharmacy as the concurrent prescription of five or more medications (22). Potential drug–drug interactions (pDDIs) were evaluated using the online computerized verification system available through Micromedex®. This tool classifies pDDIs by severity: mild or minor drug interactions are deemed to have minimal clinical significance and are unlikely to produce relevant clinical effects. In contrast, moderate interactions may have clinical implications and should be approached with caution, necessitating close monitoring, as they could exacerbate the patient’s condition or require therapy adjustments. Severe drug interactions are clinically significant, potentially life-threatening, and may necessitate medical intervention to mitigate or prevent serious adverse effects (23).
Data entry and analysis
Data were coded, cleaned, entered, and analyzed using SPSS version 26. Descriptive statistics, including mean, median, and percentages, were calculated, and results were presented in tables and charts. Binary logistic regression analysis was performed, with variables exhibiting a p-value of <0.05 considered statistically significant in the multivariate analysis. The odds ratio (OR) and 95% confidence interval were calculated for each variable to assess the strength of the associations.
Results
Socio-demographic and clinical characteristics of patients
In this study, the mean age of participants was 70.5 ± 5.9 years, with males representing 119 (50.4%) of the cohort. A substantial proportion, 169 (71.6%), of participants had comorbid conditions. A total of 743 medications were prescribed, resulting in an average of 3.2 ± 1.7 medications per patient. Notably, 66 (28%) of patients were prescribed only one medication, whereas 80 (33.9%) received five or more medications (Table 1).
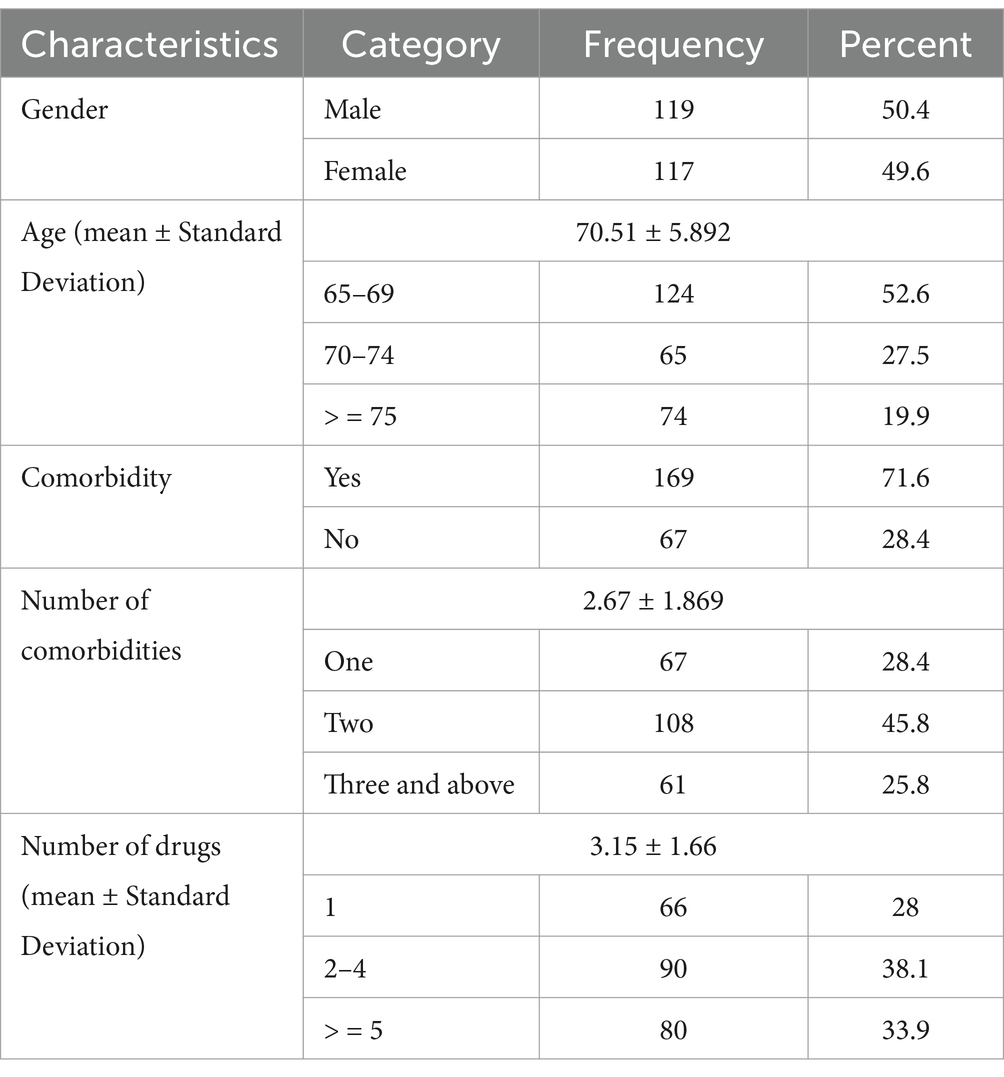
Table 1. Demographic characteristics of study participants at Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Disease characteristics
Diabetes mellitus was the most prevalent condition, with a prevalence of 99 (42%), followed by hypertension at 70 (29.7%). Chronic kidney disease and chronic obstructive pulmonary disease were the least prevalent, affecting 5 (2.1%) and 4 (1.7%) of participants, respectively (Table 2).
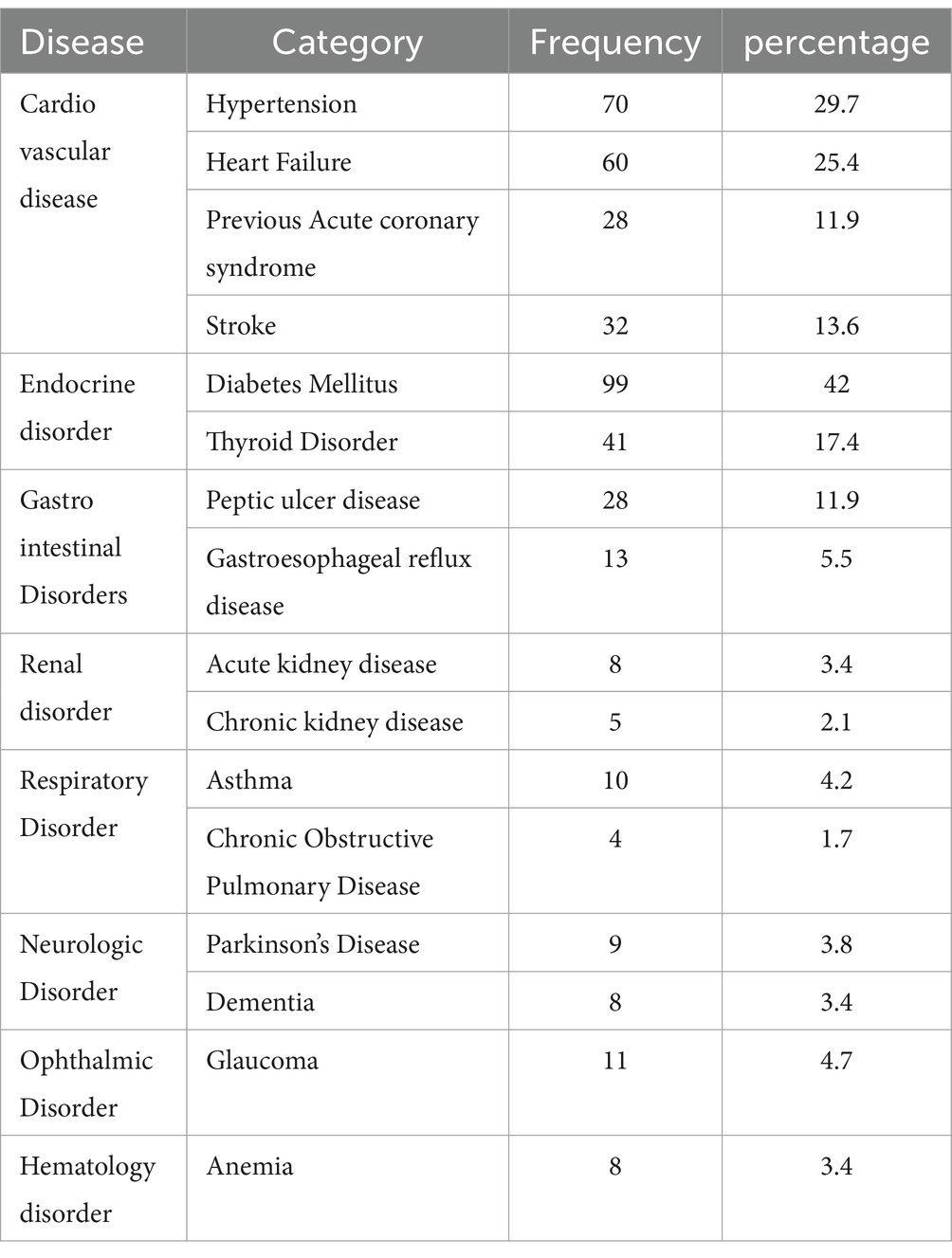
Table 2. Disease characteristics of the study participants at Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Potentially inappropriate medications based on STOPP/START criteria
According to the STOPP/START criteria, 94 participants (39.8, 95% CI: 35.7–44.5%) were prescribed at least one PIM, with 81 (34.3%) identified by STOPP criteria and 13 (5.5%) by START criteria. Among the STOPP-listed medications, long-acting sulfonylureas (Glibenclamide) were the most frequently prescribed, affecting 61 patients. From the START criteria, the most common omission was the failure to initiate cardio-selective beta-blockers in patients with potential added benefits, affecting 4 (1.7%) patients (Table 3).
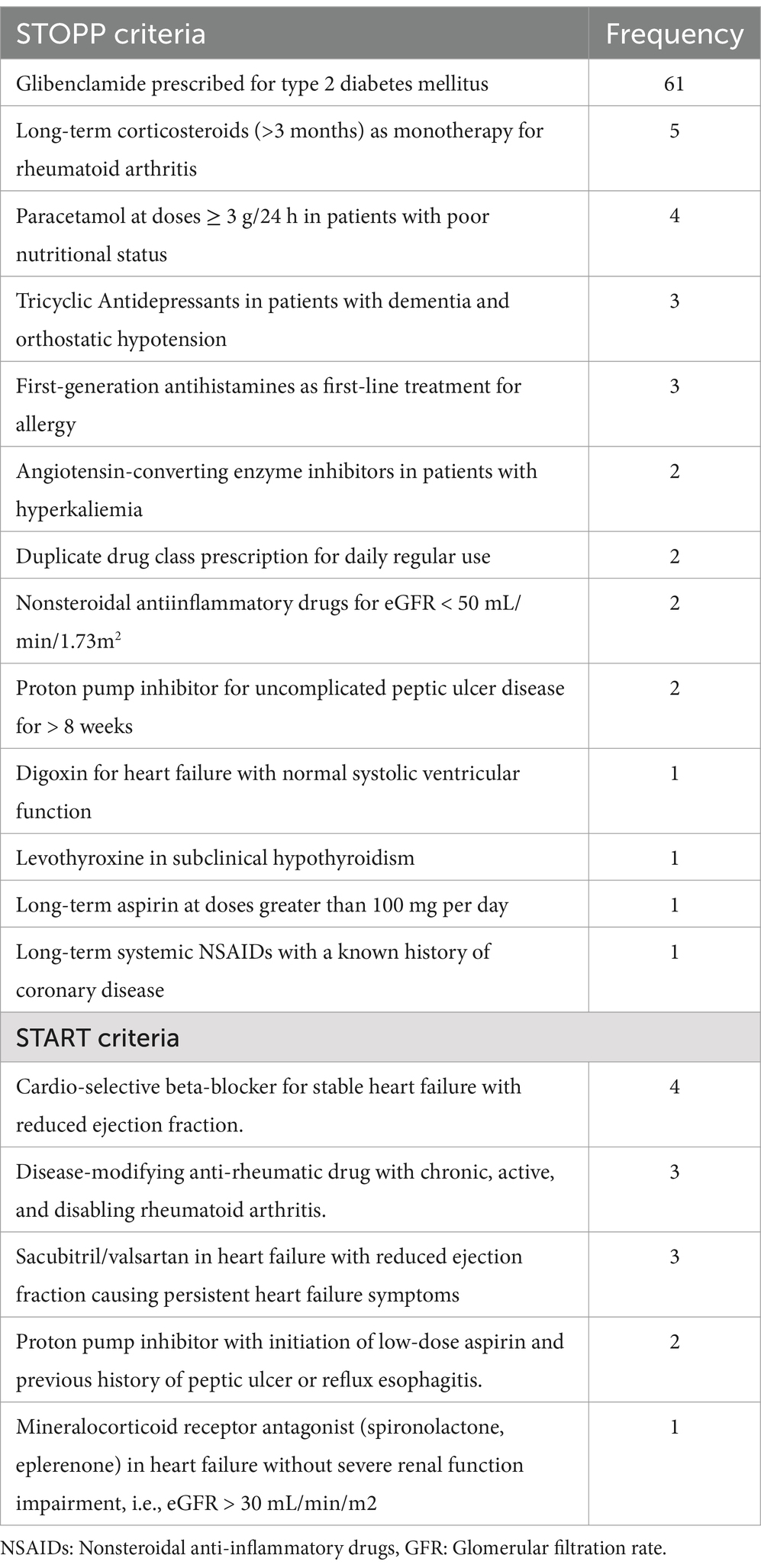
Table 3. Potentially inappropriate medications based on STOPP/START criteria at Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Potentially inappropriate medications based on AGS Beers Criteria
A total of 108 older patients (45.7%; 95% CI: 40.1–51.0%) were prescribed at least one PIM according to the Beers Criteria. The medication class deemed potentially inappropriate had the highest occurrence, affecting 81 patients, while medications requiring dosage adjustment based on renal function but not adjusted had the lowest prevalence, involving 5 patients (Table 4).
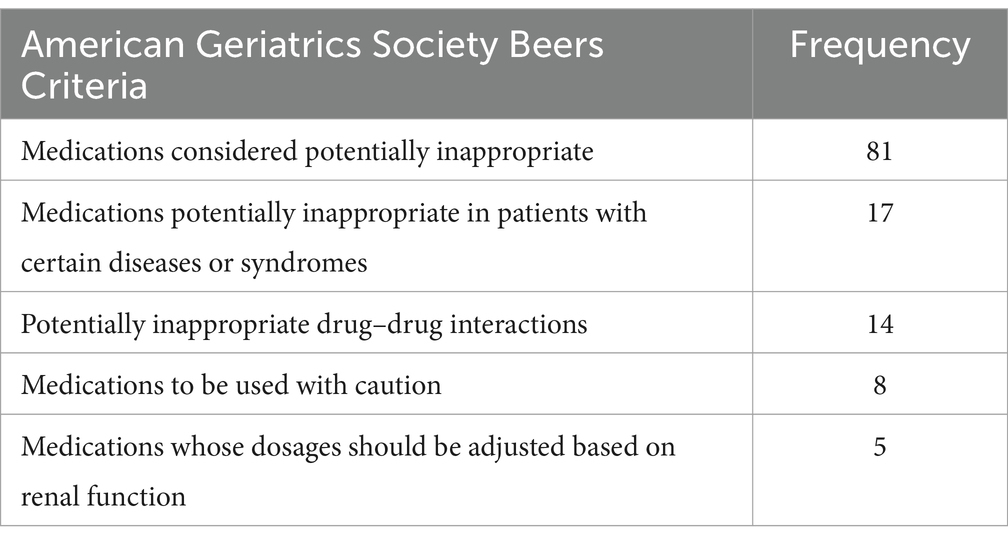
Table 4. Potentially inappropriate medications based on Beers Criteria Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Polypharmacy and drug–drug interaction
In this study, 33.9% of patients (95% CI: 29.1–38.5%) experienced polypharmacy, defined as the use of five or more medications. All polypharmacy patients had at least two comorbidities. Potential drug–drug interactions were found in 47.0% of patients (111), with an average of 2.36 ± 2.10 interactions per patient, totaling 551 interactions. The majority were moderate (47.9%, 264), followed by major (35.2%, 194) and minor (29.8%, 93) interactions (Table 5).
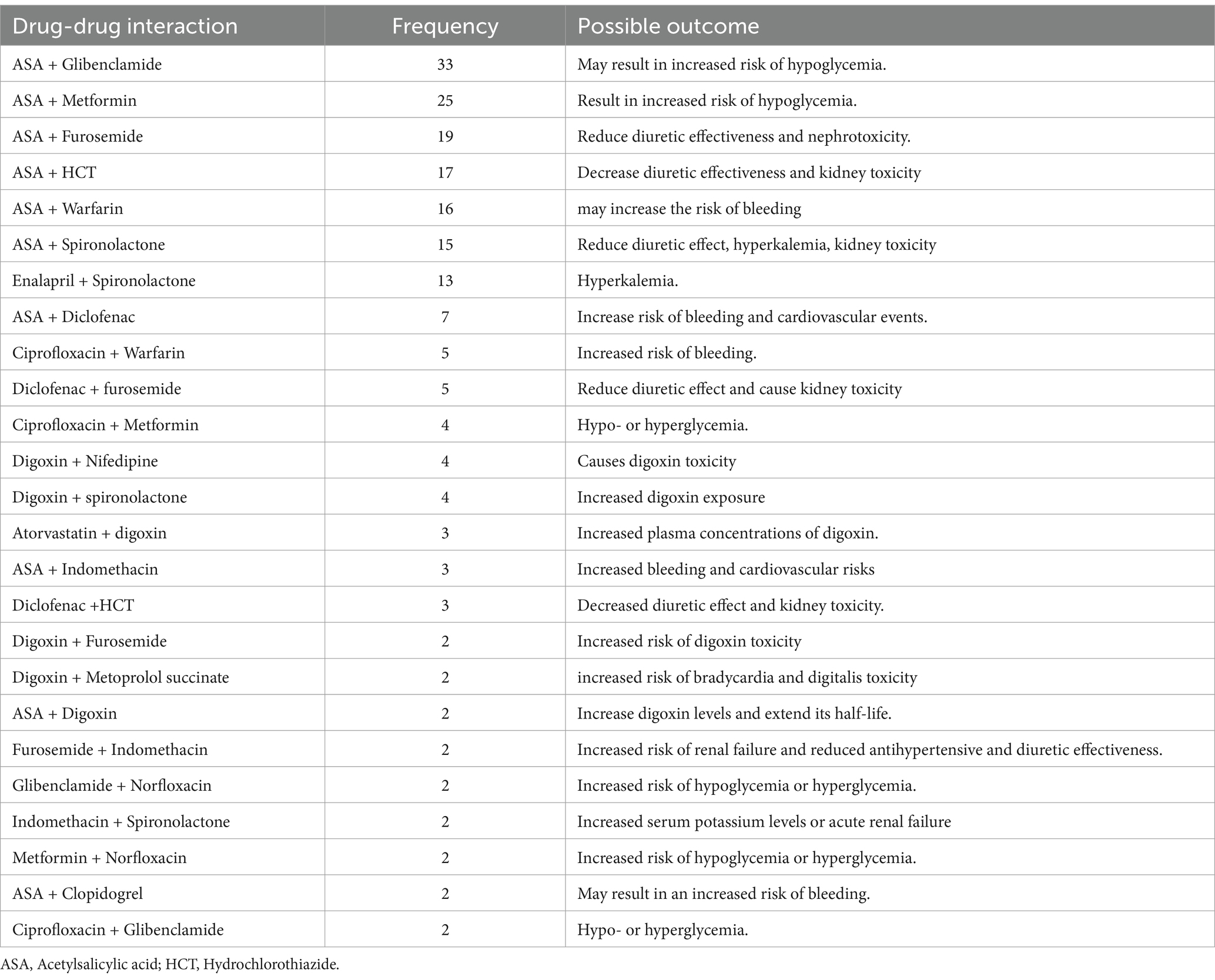
Table 5. Major drug–drug interaction at Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Factors associated with Beer’s PIM criteria
Sex, age, presence of comorbidity, and number of drugs were analyzed independently by bivariate and multivariate logistic regression for Beers and STOPP/ START PIMs. Female patients≥ 75 years old are 2.9 3 and 1.52 times more likely to have Beer’s PIMs. Polypharmacy prescription (≥5 drugs) increases the likelihood of Beer’s PIMs by 3.20 times as compared to the single drug. For STOPP/START criteria. Age of 70–74 patients 2.3 times and polypharmacy (≥5) drugs 3.20 times chance of having STOPP/ START PIMs list as compared to the age of 65–69 and only on one drug (Table 6).
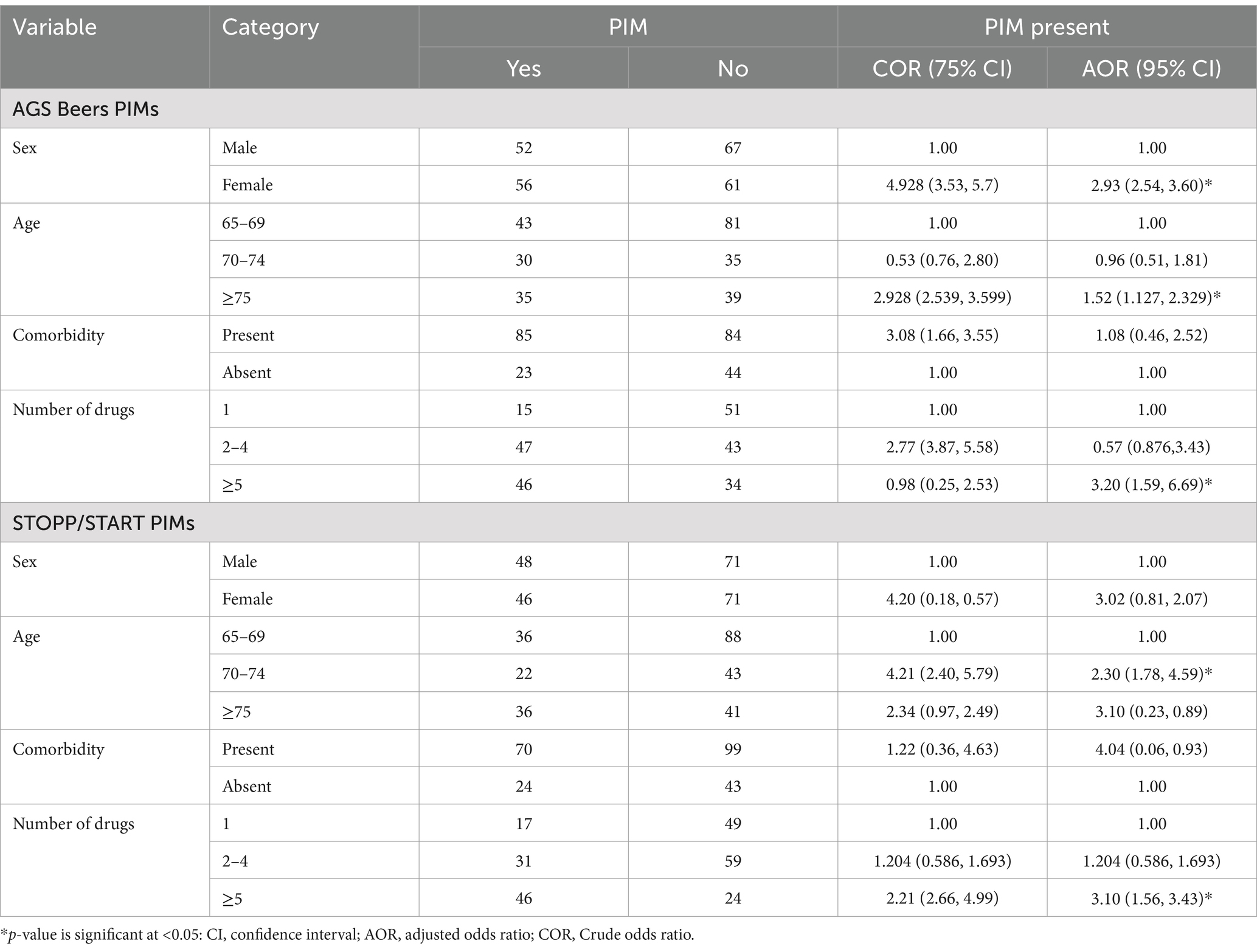
Table 6. Association of contributing factors and PIMs in Dessie Comprehensive Specialized Hospital, Ethiopia (N = 236).
Discussion
This study revealed a prevalence of 39.8% for STOPP/START criteria and 45.7% for Beers criteria PIMs, which aligns with findings from various countries worldwide, ranging from 44 to 52% (24–27). The slightly lower prevalence compared to other studies (9, 28–32) may be due to differences in study settings, study designs, and the criteria used to identify PIMs. Additional possible reasons for the discrepancy may include variations in the availability of listed medications in the study setting, the multi-centered nature of some studies, and a higher proportion of older adults with comorbid conditions in other research.
This figure was higher than in previous studies (15, 33, 34). The higher prevalence could be attributed to starting medications before confirming a specific diagnosis with objective findings (empiric treatment), the inclusion of multi-morbid patients, available medications, and varying prescribing patterns. For example, Inamdar & Kulkarni enrolled only DM patients, whereas Lim et al. included older adults regardless of disease condition. Meanwhile, this figure is considerably higher than findings from other Ethiopian hospital studies: 28.6% in northern Ethiopia (35) and 27.7% in northwest Ethiopia (12). The discrepancy may be due to differences in prescribing patterns, study settings, and study populations; for instance, the northern Ethiopia study included admitted patients across different wards. Additionally, over half of the patients on antibiotics had an infectious disease, resulting in a higher percentage of antibiotics prescribed, whereas the Beers list predominantly includes non-antibiotic drugs. The long-acting sulfonylurea glibenclamide accounted for the largest share of PIMs in both the Beers and STOPP/START criteria. Long-acting sulfonylureas, including chlorpropamide, glimepiride, and glibenclamide, can cause prolonged hypoglycemia and should therefore be avoided in older adults. Geriatric guidelines and scientific organizations recommend short-acting sulfonylureas, such as glipizide, for older adult patients to prevent prolonged hypoglycemia (18, 19). Although glibenclamide is classified as a PIM, it is included as a first-line treatment option for type 2 diabetes as an alternative to metformin in Ethiopia’s standard treatment guidelines (27). This recommendation is based on the availability and low cost of glibenclamide, making it accessible and affordable for patients.
Polypharmacy, being female, and age ≥75 were significantly associated with Beers PIMs, while age between 70 and 74 and polypharmacy were associated with STOPP/START PIMs. Consistent with other studies (11, 35–37), polypharmacy is an important predictor of PIMs. Age is another contributing factor, as supported by previous studies (37). Unlike other studies (11, 34, 35), comorbidity showed no association with the occurrence of PIMs in this study. However, earlier studies have reported an increased prevalence of PIMs in older patients with comorbidities such as diabetes, ischemic heart disease, heart failure, chronic kidney disease, cancer, osteoarthritis, osteoporosis, and anxiety (28).
This study highlights that the prevalence of polypharmacy (defined as 5 or more drugs) was 33.9%, aligning with findings from systematic reviews and meta-analyses (38–40, and). This prevalence is higher than that reported in Iran (23.1%) (41) but lower than in Germany, where polypharmacy of 5–9 drugs was reported at 58.3% and ≥10 drugs at 28.5% (40). In this study, the prevalence of potential drug–drug interactions was 47.0%. This finding differs from reports in a systematic review and meta-analysis, which showed a prevalence of 57.8% (42), in Pakistan at 70.17% (43), a multicenter study across Bern, Brussels, Cork, and Utrecht at 54% (44), and studies using Lexicomp®, Micromedex®, and DDInter checker software, which reported prevalences of 32.22, 32.93, and 22.62%, respectively (45). Scientific reasons for these discrepancies may include differences in study design (e.g., pooled prevalence in systematic reviews and meta-analyses), multicenter approaches, variations in study populations (such as admitted patients), and the use of different drug interaction checker software.
Older patients benefit from special considerations before and after drug prescriptions. To achieve this, physicians should be assisted by pharmacists and, ideally, by patients themselves (23). All health professionals should be aware of the basic changes in drug pharmacokinetics and pharmacodynamics that occur with aging. Specifically, those prescribing medications should always consider these changes to prevent compromising the health of older adult patients through inappropriate prescriptions. Pharmacists are key professionals in avoiding the use of PIMs, as they can identify possible contraindications in older adult patients for all drugs they dispense (46). In Ethiopia, current hospital reform implementation guidelines include clinical pharmacy services in inpatient, outpatient, and emergency departments. These services should be well-organized, recorded, documented, and reported (47).
An important aspect of this study is that it provides insight into the magnitude of polypharmacy, potential drug–drug interactions, and PIMs in ambulatory, chronically ill older patients in a developing setting. It encourages physicians to pay closer attention when prescribing medications, particularly for patients with cardiovascular diseases and endocrine disorders. This study has several strengths, including its prospective design, use of the latest criteria for identifying potentially inappropriate medications (PIMs), and the involvement of physicians in data collection discussions. It also emphasizes the potential for clinical pharmacists to enhance patient care by identifying, preventing, and addressing medication-related issues. However, there are limitations. First, over-the-counter medications were excluded, as only prescribed medications from patient charts were considered, potentially limiting the study’s generalizability. Second, the assessment of patients’ general health and comorbidities relied solely on available documents, which may have led to inaccurate estimates of PIMs. Finally, the study could not assess the outcomes of PIMs due to incomplete data records.
Conclusion
This study revealed that polypharmacy, drug–drug interactions, and the prescribing of potentially inappropriate medications are common among older, chronically ill patients in Ethiopia. Polypharmacy, sex, and age were identified as contributing factors that increase the likelihood of PIM use in this population. Given the rapid growth of the older population, future studies with robust study designs are needed to further explore the adverse health outcomes and the economic burden associated with the use of PIMs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Wollo University College of Medicine and Health Sciences (CMHS/671/2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
BLE: Conceptualization, Data curation, Formal analysis, Investigation, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. MTK: Investigation, Methodology, Software, Supervision, Writing – review & editing. YWE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft. ASA: Conceptualization, Data curation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. WHO. Global health and aging. National Institute on Aging, National Institute of health U.S. Department of Health and Human Services. World Health Organization.(2011). 4 p.
2. Turnheim, K. Drug therapy in the elderly. Exp Gerontol. (2004) 39:1731–8. doi: 10.1016/j.exger.2004.05.011
3. Fialová, D, and Onder, G. Medication errors in elderly people: contributing factors and future perspectives. Br J Clin Pharmacol. (2009) 67:641–5. doi: 10.1111/j.13652125.2009.03419.x
4. Zullo, AR, Gray, SL, Holmes, HM, and Marcum, ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. (2018) 34:39–54. doi: 10.1016/j.cger.2017.09.003
5. Holt, S, Schmiedl, S, and Thürmann, PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int. (2010) 107:543–51. doi: 10.3238/arztebl.2010.0543
6. Morin, L, Laroche, ML, Texier, G, and Johnell, K. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc. (2016) 17:862.e1–9. doi: 10.1016/j.jamda.2016.06.011
7. Pradhan, S, Panda, A, Mohanty, M, Behera, J, Ramani, R, and Pradhan, P. A study of the prevalence of potentially inappropriate medication in elderly in a tertiary care teaching hospital in the state of Odisha. Int J Public Health. (2015) 5:344–8. doi: 10.4103/2230-8598.165108
8. Lichtman, SM, and Boparai, M. Potentially inappropriate medication use in elderly cancer patients. Crit Rev Oncol Hematol. (2008) 68:S40. doi: 10.1016/S1040-8428(08)70090-8
9. Narvekar, RS, Bhandare, NN, Gouveia, JJ, and Bhandare, PN. Utilization pattern of potentially inappropriate medications in geriatric patients in a tertiary care hospital: a retrospective observational study. J Clin Diagn Res. (2017) 11:FC04–8. doi: 10.7860/JCDR/2017/21080.9731
10. Wickop, B, Härterich, S, Sommer, C, Daubmann, A, Baehr, M, and Langebrake, C. Potentially inappropriate medication use in multimorbid elderly inpatients: differences between the FORTA, PRISCUS and STOPP ratings. Drugs Real World Outcomes. (2016) 3:317–25. doi: 10.1007/s40801-016-0085-2
11. Sheikh-Taha, M, and Dimassi, H. Potentially inappropriate home medications among older patients with cardiovascular disease admitted to a cardiology service in USA. BMC Cardiovasc Disord. (2017) 17:189. doi: 10.1186/s12872-017-0623-1
12. Mekonnen, AB, and Bhagavathula, AS. Inappropriate medication use in the elderly population attending Gondar University hospital. Int J Pharm Pharm Sci. (2014) 6:540–3.
13. Reich, O, Rosemann, T, Rapold, R, Blozik, E, and Senn, O. Potentially inappropriate medication use in older patients in Swiss managed care plans: prevalence, determinants and association with hospitalization. PLoS One. (2014) 9:e105425. doi: 10.1371/journal.pone.0105425
14. Rawat, RS. Evaluation of Potentially Inappropriate Medication Use and Risk of Adverse Drug Reactions in Hospitalized Older Adults: An Observational Study in a Tertiary Care Hospital. Indian J Pharm Prac. (2018) 11:79–85. doi: 10.5530/ijopp.11.2.17
15. Sarwar, MR, Dar, A, Mahar, SY, Riaz, T, Danish, U, and Iftikhar, S. Assessment of prescribing potentially inappropriate medications listed in beers criteria and its association with the unplanned hospitalization: a cross-sectional study in Lahore, Pakistan. Clin Interv Aging. (2018) 13:1485–95. doi: 10.2147/CIA.S173942
16. Chang, CB, Lai, HY, Hwang, SJ, Yang, SY, Wu, RS, Liu, HC, et al. Prescription of potentially inappropriate medication to older patients presenting to the emergency department: a nationally representative population study. Sci Rep. (2018) 8:11727. doi: 10.1038/s41598-018-30184-4
17. Henschel, F, Redaelli, M, Siegel, M, and Stock, S. Correlation of incident potentially inappropriate medication prescriptions and hospitalization: an analysis based on the PRISCUS list. Drugs Real World Outcomes. (2015) 2:249–59. doi: 10.1007/s40801-015-0035-4
18. American Geriatrics Society. Updated Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. (2023) 71:2052–81. doi: 10.1111/jgs.18372
19. O'Mahony, D, Cherubini, A, Guiteras, AR, Denkinger, M, Beuscart, JB, onder, G, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 3 [published correction appears in Eur Geriatr med. 2023;14(4):633. Doi: 10.1007/s41999-023-00812-y]. Eur Geriatr Med. (2023) 14:625–32. doi: 10.1007/s41999-023-00777-y
20. Tadesse, TA, Belayneh, A, Aynalem, MW, Yifru, YM, Amare, F, and Beyene, DA. Potentially inappropriate prescribing in elderly patients with epilepsy at two referral hospitals in Ethiopia. Front Med. (2024) 11:1403546. doi: 10.3389/fmed.2024.1403546
21. Schietzel, S, Zechmann, S, Rachamin, Y, Neuner-Jehle, S, Senn, O, and Grischott, T. Potentially inappropriate medication use in primary Care in Switzerland. JAMA Netw Open. (2024) 7:e2417988. doi: 10.1001/jamanetworkopen.2024.17988
22. Kaur, G. Polypharmacy: the past, present and the future. J Adv Pharm Technol Res. (2013) 4:224–5. doi: 10.4103/2231-4040.121418
23. Carpenter, M, Berry, H, and Pelletier, AL. Clinically relevant drug-drug interactions in primary care. Am Fam Physician. (2019) 99:558–64.
24. Bazargan, M, Smith, JL, and King, EO. Potentially inappropriate medication use among hypertensive older African-American adults. BMC Geriatr. (2018) 18:238. doi: 10.1186/s12877-018-0926-9
25. Lopes, LM, Figueiredo, TP, Costa, SC, and Reis, AM. Use of potentially inappropriate medications by the elderly at home. Utilização de medicamentos potencialmente inapropriados por idosos em domicílio. Ciênc Saúde Colet. (2016) 21:3429–38. doi: 10.1590/1413-812320152111.14302015
26. Al Odhayani, A, Tourkmani, A, Alshehri, M, Alqahtani, H, and Mishriky, A. Potentially inappropriate medications prescribed for elderly patients through family physicians. Saudi J Biol Sci. (2017) 24:200–7. doi: 10.1016/j.sjbs.2016.05.006
28. Alhawassi, TM, Alatawi, W, and Alwhaibi, M. Prevalence of potentially inappropriate medications use among older adults and risk factors using the 2015 American Geriatrics Society beers criteria. BMC Geriatr. (2019) 19:154. doi: 10.1186/s12877-019-1168-1
29. Al-Azayzih, A, Alamoori, R, and Altawalbeh, SM. Potentially inappropriate medications prescribing according to beers criteria among elderly outpatients in Jordan: a cross sectional study. Pharm Pract (Granada). (2019) 17:1439. doi: 10.18549/PharmPract.2019.2.1439
30. Simões, PA, Santiago, LM, Maurício, K, and Simões, JA. Prevalence of potentially inappropriate medication in the older adult population within primary care in Portugal: a Nationwide cross-sectional study. Patient Prefer Adherence. (2019) 13:1569–76. doi: 10.2147/PPA.S219346
31. Alturki, A, Alaama, T, Alomran, Y, Al-Jedai, A, Almudaiheem, H, and Watfa, G. Potentially inappropriate medications in older patients based on beers criteria: a cross-sectional study of a family medicine practice in Saudi Arabia. BJGP Open. (2020) 4:bjgpopen20X101009. doi: 10.3399/bjgpopen20X101009
32. Osei, EK, Berry-Cabán, CS, Haley, CL, and Rhodes-Pope, H. Prevalence of beers criteria medications among elderly patients in a military hospital. Gerontol Geriatr Med. (2016) 2:2333721416637790. doi: 10.1177/2333721416637790
33. Zaman Huri, H, and Chai, LL. Drug-related problems in type 2 diabetes mellitus patients with dyslipidemia. BMC Public Health. (2013) 13:1192. doi: 10.1186/1471-2458-13-1192
34. Lim, YJ, Kim, HY, Choi, J, Lee, JS, Ahn, AL, Oh, EJ, et al. Potentially inappropriate medications by beers criteria in older outpatients: prevalence and risk factors. Korean J Fam Med. (2016) 37:329–33. doi: 10.4082/kjfm.2016.37.6.329
35. Teka, F, Teklay, G, Ayalew, E, and Kassa, TT. Prevalence of potentially inappropriate medications in Ayder referral hospital, Tigray region, northern Ethiopia: prospective study. J Drug Delivery Therap. (2016) 6:16–21. doi: 10.22270/jddt.v6i6.1238
36. Samara, E, Nazzal, Z, and Naghnaghia, SALRamahi R. Potentially inappropriate medication uses and associated factors among elderly primary health care clinics attendees: a call to action. PLoS One. (2023) 18:e0290625. doi: 10.1371/journal.pone.0290625
37. Zhu, X, Zhang, F, Zhao, Y, Zhang, W, Zhang, Y, and Wang, J. Evaluation of potentially inappropriate medications for the elderly according to beers, STOPP, START, and Chinese criteria. Front Pharmacol. (2024) 14:1265463. doi: 10.3389/fphar.2023.1265463
38. Delara, M, Murray, L, Jafari, B, Bahji, A, Goodarzi, Z, Kirkham, J, et al. Prevalence and factors associated with polypharmacy: a systematic review and meta-analysis. BMC Geriatr. (2022) 22:601. doi: 10.1186/s12877-022-03279-x
39. Kitaw, TA, and Haile, RN. Prevalence of polypharmacy among older adults in Ethiopia: a systematic review and meta-analysis. Sci Rep. (2023) 13:17641. doi: 10.1038/s41598-023-45095-2
40. Bennie, M, Santa-Ana-Tellez, Y, Galistiani, GF, Trehony, J, Despres, J, Jouaville, LS, et al. The prevalence of polypharmacy in older Europeans: a multi-national database study of general practitioner prescribing. Br J Clin Pharmacol. (2024) 90:2124–36. doi: 10.1111/bcp.16113
41. Hosseini, SR, Zabihi, A, Jafarian Amiri, SR, and Bijani, A. Polypharmacy among the elderly. J Midlife Health. (2018) 9:97–103. doi: 10.4103/jmh.JMH_87_17
42. Hughes, JE, Waldron, C, Bennett, KE, and Cahir, C. Prevalence of drug-drug interactions in older community-dwelling individuals: a systematic review and Meta-analysis. Drugs Aging. (2023) 40:117–34. doi: 10.1007/s40266-022-01001-5
43. Shakeel, F, Aamir, M, Khan, AF, Khan, TN, and Khan, S. Epidemiology of potential drug-drug interactions in elderly population admitted to critical care units of Peshawar, Pakistan. BMC Pharmacol Toxicol. (2018) 19:85. doi: 10.1186/s40360-018-0276-4
44. Zerah, L, Henrard, S, Wilting, I, O’Mahony, D, Rodondi, N, Dalleur, O, et al. Prevalence of drug-drug interactions in older people before and after hospital admission: analysis from the OPERAM trial. BMC Geriatr. (2021) 21:571. doi: 10.1186/s12877-021-02532-z
45. Liu, Y, Wang, J, Gong, H, Li, C, Wu, J, Xia, T, et al. Prevalence and associated factors of drug-drug interactions in elderly outpatients in a tertiary care hospital: a cross-sectional study based on three databases. AnnTransl Med. (2023) 11:17. doi: 10.21037/atm-2
46. Bressler, R, and Bahl, JJ. Principles of drug therapy for the elderly patient. Mayo Clin Proc. (2003) 78:1564–77. doi: 10.4065/78.12.1564
Keywords: polypharmacy, drug–drug interaction, potential inappropriate medication, Beer’s Criteria, START/STOP criteria, comorbidity
Citation: Endalifer BL, Kassa MT, Ejigu YW and Ambaye AS (2025) Polypharmacy, drug–drug interactions, and potentially inappropriate medications among older adults: a cross-sectional study in Northeast Ethiopia. Front. Public Health. 13:1525079. doi: 10.3389/fpubh.2025.1525079
Edited by:
Ian Scott, Metro South Health, AustraliaReviewed by:
Ildefonso Rodriguez-Leyva, Autonomous University of San Luis Potosi, MexicoReginald Obiako, Ahmadu Bello University, Nigeria
Copyright © 2025 Endalifer, Kassa, Ejigu and Ambaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bedilu Linger Endalifer, bGluZ2VyZW5kYWxpZmVyQGdtYWlsLmNvbQ==
†ORCID: Bedilu Linger Endalifer, orcid.org/0000-0003-1639-6591
Mekuanint Terefe Kassa, orcid.org/0009-0002-3674-8363
Abyou Seyfu Ambaye, orcid.org/0000-0002-1598-8395
 Bedilu Linger Endalifer
Bedilu Linger Endalifer Mekuanint Terefe Kassa2†
Mekuanint Terefe Kassa2† Yenesew Wudu Ejigu
Yenesew Wudu Ejigu Abyou Seyfu Ambaye
Abyou Seyfu Ambaye