- 1Department of Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Sichuan University, Chengdu, China
- 3West China School of Public Health and West China Fourth Hospital, Sichuan University, Chengdu, China
- 4National Office for Maternal and Child Health Surveillance, West China Second University Hospital, Sichuan University, Chengdu, China
- 5Department of Pathology, West China Second University Hospital, Sichuan University, Chengdu, China
- 6Chengdu Shuangliu District Maternity and Child Healthcare Hospital, Chengdu, China
Introduction: Cervical cancer, driven by persistent high-risk human papillomavirus (hrHPV) infection, remains a global health challenge, especially in low- and middle-income areas such as western China. Despite the critical role of HPV testing in early detection, coverage in China remains low due to cultural, psychological, and other barriers. Self-collected urine and vaginal samples offer alternative methods for sample collection. This study aimed to evaluate the feasibility and acceptability of detecting hrHPV and cervical intraepithelial neoplasia grade 2 or worse (CIN2+) via urine and vaginal self-sampling compared with clinician sampling in urban areas of western China.
Methods: A cross-sectional survey was conducted from November 2022 to March 2023 in urban areas of western China. The participants provided self-collected urine and vaginal samples for hrHPV testing and completed questionnaires on acceptability of self-sampling. The HPV positivity, agreement, and kappa value were calculated to assess concordance between self- and clinician sampling. The sensitivity, specificity, agreement, predictive values, and likelihood ratios were used to evaluate the clinical performance of both methods for detecting CIN2+.
Results: A total of 2,228 female subjects aged 21–71 years were recruited, and self-collected urine samples, vaginal samples, and clinician-collected cervical samples were obtained. The sensitivity of clinician sampling, urine self-sampling and vaginal self-sampling were 80.00% (95% CI: 44.22–96.46), 70.00% (95% CI: 35.37–91.91) and 90.00% (95% CI: 54.12–99.48) for CIN2+; the specificity for <CIN2 were 98.33% (95% CI: 97.68–98.81), 98.23% (95% CI: 97.56–98.72) and 98.50% (95% CI: 97.87–98.95%); and the agreements for CIN2+ were 98.25% (95% CI: 97.59–98.74), 98.83 (95% CI: 98.26–99.22) and 98.82 (95% CI: 98.25–99.21). All methods yielded high negative predictive values, high positive likelihood ratios, and low negative likelihood ratios. Additionally, participants reported high acceptability of self-sampling, citing less discomfort and embarrassment than clinician sampling.
Conclusion: Self-collected urine and vaginal samples for the detection of hrHPV and CIN2+ demonstrate high diagnostic accuracy and acceptability, making them viable alternatives to clinician-collected samples. Self-sampling methods may improve screening accessibility and compliance, especially in resource-limited settings, thereby supporting the prevention and early detection of CIN2+.
1 Introduction
Cervical cancer, which is strongly associated with persistent high-risk human papillomavirus (hrHPV) infection, ranks as the fourth most common cancer in both incidence and mortality among women, with approximately 660,000 new cases and 350,000 deaths globally in 2022 (1). Notably, 80% of the decline in cases occurred in lower-middle-income countries (LMICs) (2). Unfortunately, China remains among the developing countries with a high burden of cervical cancer, making it the most prevalent gynecological cancer in terms of both incidence and mortality (3). Cervical cancer could be the first cancer eliminated worldwide through strategies such as the 90–70–90 targets proposed by the World Health Organization (WHO) in 2020. These targets aim for 90% of girls to be fully vaccinated with the HPV vaccine by age 15, 70% of women to be screened with a high-performance test by age 35 and again by age 45, and 90% of women diagnosed with precancer or invasive cancer to receive appropriate treatment (4).
In addition to vaccination, HPV testing is a crucial method for cervical cancer screening, offering greater effectiveness in reducing cervical cancer incidence compared to cervical cytology, due to its higher sensitivity and negative predictive value (5–8). As of 2021, 48 countries have recommended primary HPV-focused screening, either independently or in combination with other screening methods such as cytology (9). Low rates of HPV vaccination and cervical cancer screening coverage remain significant challenges in China, where the cumulative full vaccination rate among women aged 9–45 was only 6.21% (10), and 70.5% of women aged 20–64 years have never been screened (11). This situation is attributed to organizational barriers such as limited medical access and prolonged wait times; interpersonal challenges such as stigma and a lack of family support; and personal factors such as low risk perception, discomfort, and fear (12, 13).
HPV self-sampling techniques are recommended by the WHO to be incorporated into cervical cancer screening protocols, aiming to facilitate the achievement of the 2030 targets (4, 14). As of 2021, 17 countries have integrated self-sampling into national programs or guidelines, targeting underscreened populations or as a primary method. Several studies have shown the high feasibility and acceptability of the HPV-DNA test using self-collected urine/vaginal samples and clinician-collected cervical samples (13, 15–18). Advantages include being less invasive, offering greater accessibility for self-collection, and reducing the need for infrastructure such as examination beds and costly collection kits. Self-sampling methods have also been shown to be cost-effective (19–21), making them a promising way to increase women’s participation in cervical cancer prevention, especially in LMICs.
Despite evidence supporting the feasibility and acceptability of self-sampling methods, studies in China on these techniques remain limited (22, 23). Therefore, our study aimed to evaluate the feasibility and acceptability of detecting high-risk HPV (hrHPV) and cervical intraepithelial neoplasia grade 2 or worse (CIN2+) using urine and vaginal self-sampling compared with clinician sampling in urban areas of western China.
2 Methods
2.1 Study design and participants
A cross-sectional study was conducted from November 2022 to March 2023 in urban areas of western China. To be eligible for the study, women had to be between 21 and 71 years of age at the time of invitation, must have refrained from sexual intercourse, vaginal medication, vaginal contraceptives, and the use of vaginal cleansers for at least 48 h prior to the examination, and must have signed the informed consent. Patients were excluded from the study if they had a history of uterine or cervical surgery (such as hysterectomy), were pregnant or within 8 weeks postpartum, or were experiencing menstrual bleeding at the time of the examination.
Eligible patients were recruited at our partner hospital in Shuangliu District, Chengdu, where they registered, signed informed consent, underwent cervical and self-sampling, and completed a questionnaire. The physician-collected hrHPV-DNA test was performed at the partner hospital, while the self-sampling hrHPV-DNA test was conducted at Hangzhou central laboratory. Cytology and immunohistochemistry were handled by Guangzhou central laboratory and sent to our hospital’s pathology department for slide review. Colposcopy and sampling were performed at our partner hospital, with slides prepared and reviewed by our hospital’s pathology department.
2.2 Basic information collection, sample collection and acceptability assessment
After registration and informed consent, the participants completed a questionnaire covered basic participant information (such as age, education, marriage, family income), histories of cervical cancer prevention, previous female reproductive system diseases, menstrual cycles, and contraceptive use.
Before sample collection, video guidance based on the manufacturer’s instructions for self-sampling was given to all participants. The participants were instructed to collect at least 25 ccs of anterior first void in a sterile urine container. After urine collection, a self-sampling swab for vaginal sampling was obtained. Researchers remained outside the bathroom to offer further assistance and collect the samples. Finally, trained gynecologists conducted gynecological examinations and collected cervical samples, completing a corresponding examination questionnaire.
After sampling, the participants completed a questionnaire assessing the acceptability of the HPV screening protocol. This survey contained 20 questions evaluating their experiences, comfort, and safety during the procedure, as well as their method preferences and reasons for those choices. All paper questionnaires were transcribed into a Microsoft Access 2016 database by two data stewards for data cleaning and coding, with a third steward ensuring consistency of the double-entered data.
2.3 hrHPV-DNA detection, cytology, immunocytochemistry, colposcopy, and histopathology
Cervical samples were evaluated for hrHPV-DNA with HBRT-H14 (Hybribio Biotechnology Ltd. Corp., Guangdong, China) (24), whereas urine and vaginal self-samples were evaluated with CareHPV systems (QIAGEN, Hilden, Germany).
Liquid-based cytology testing (LCT) and P16/Ki-67 immunocytochemistry (ICC) were conducted on clinician-collected cervical samples if urine, vaginal, or cervical samples tested negative for HPV16/18 but positive for the other 12 types of hrHPV.
According to the screening results, women meeting any of the following criteria are required to undergo colposcopy within 1 month: (1) positive for HPV16/18; (2) positive for the other 12 types of hrHPV, along with a positive result for LCT [atypical squamous cells of undetermined significance (ASC-US) or higher] or P16/Ki-67 ICC. Tissue biopsies were performed by an experienced physician on women who tested positive during colposcopy. If abnormalities were detected, a direct biopsy was taken at the abnormal site. If a high-grade lesion was indicated by cytology but no abnormalities were found during colposcopy, endocervical curettage (ECC) and random biopsies (at positions 2, 4, 8, and 10 on the cervical squamocolumnar junction) were conducted.
All LCT, ICC, and histopathology samples were blinded and independently evaluated by two experienced cytopathologists or histopathologists. In cases of diagnostic discrepancies, the specimens were re-evaluated to reach a consensus. Cytological diagnoses were made according to the Bethesda System (3rd Ed.), and the histological results were based on the highest level of diagnosis.
2.4 Statistical analysis
Statistical analyses were conducted via SPSS version 23.0 (IBM Corp., Armonk, NY, United States) and VassarStats (online). The HPV positivity rate, agreement rate, and kappa value were calculated to assess the concordance between self-sampling and clinician sampling. The sensitivity, specificity, agreement rate, predictive values, and likelihood ratios were used to evaluate the clinical performance of self-sampling and clinician sampling for detecting CIN2+. HPV positivity rates between self-sampling and physician sampling were compared using McNemar’s test. Participants’ feelings about self-sampling were compared using Chi-Square test, and Bonferroni’s correction was used for multiple comparisons. The distributions of difficulty levels between self-sampling and physician sampling were compared using the Mann–Whitney U test. All tests were two-sided, and p values < 0.05 were considered statistically significant.
2.5 Ethics approval and consent to participate
This study was approved by the ethics committees of both the Peking Union Medical College Hospital (PUMCH) (approval code ZS-3293) and West China Second University Hospital (WCSUH). Written informed consent from all the study participants was obtained, and all materials complied with the Declaration of Helsinki and ethical standards.
3 Results
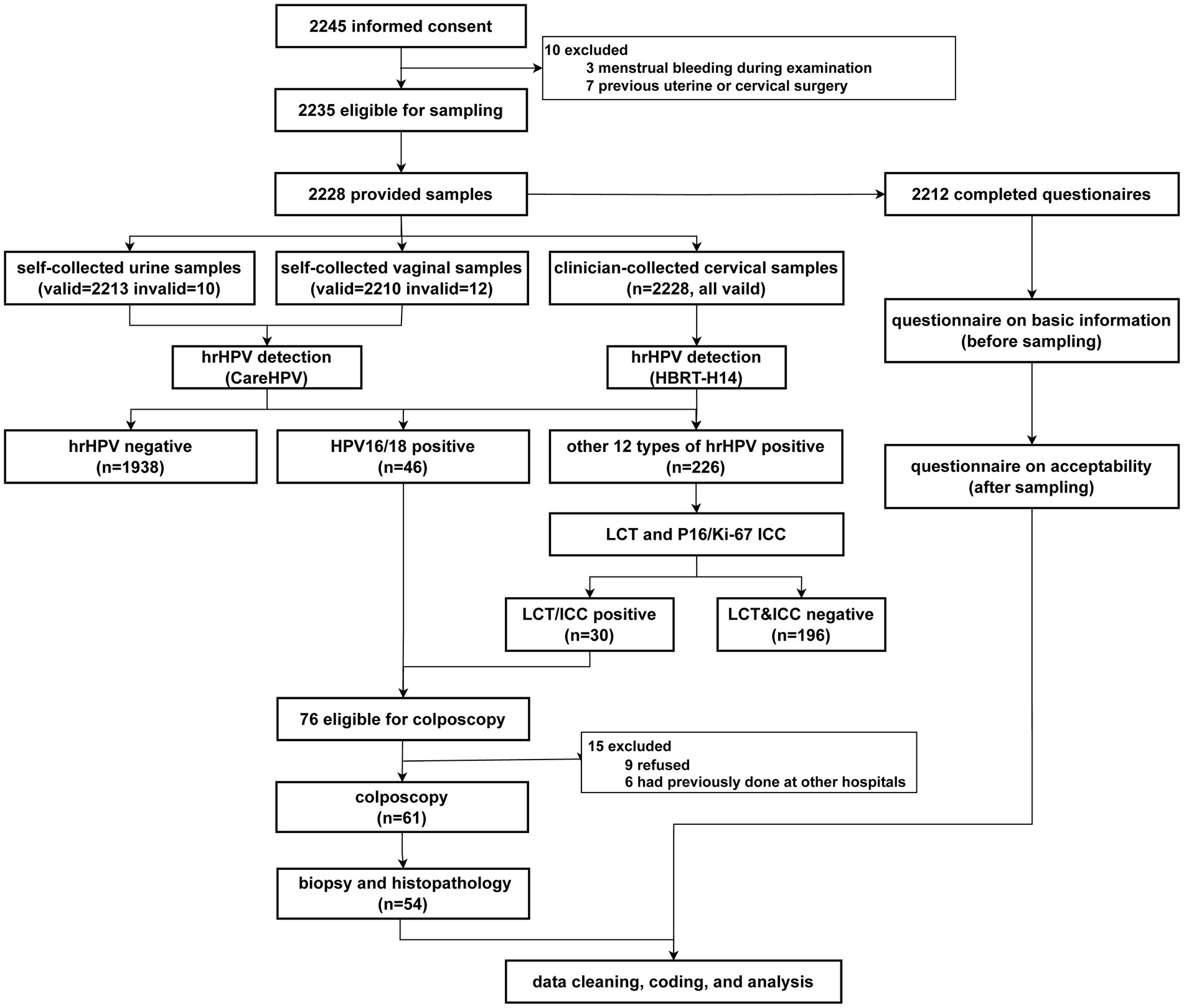
Figure 1 presents a flow chart of the study. Among the 2,235 eligible participants, 2,228 provided self-collected urine and vaginal samples, and 2,212 completed the questionnaire.
3.1 Basic characteristics
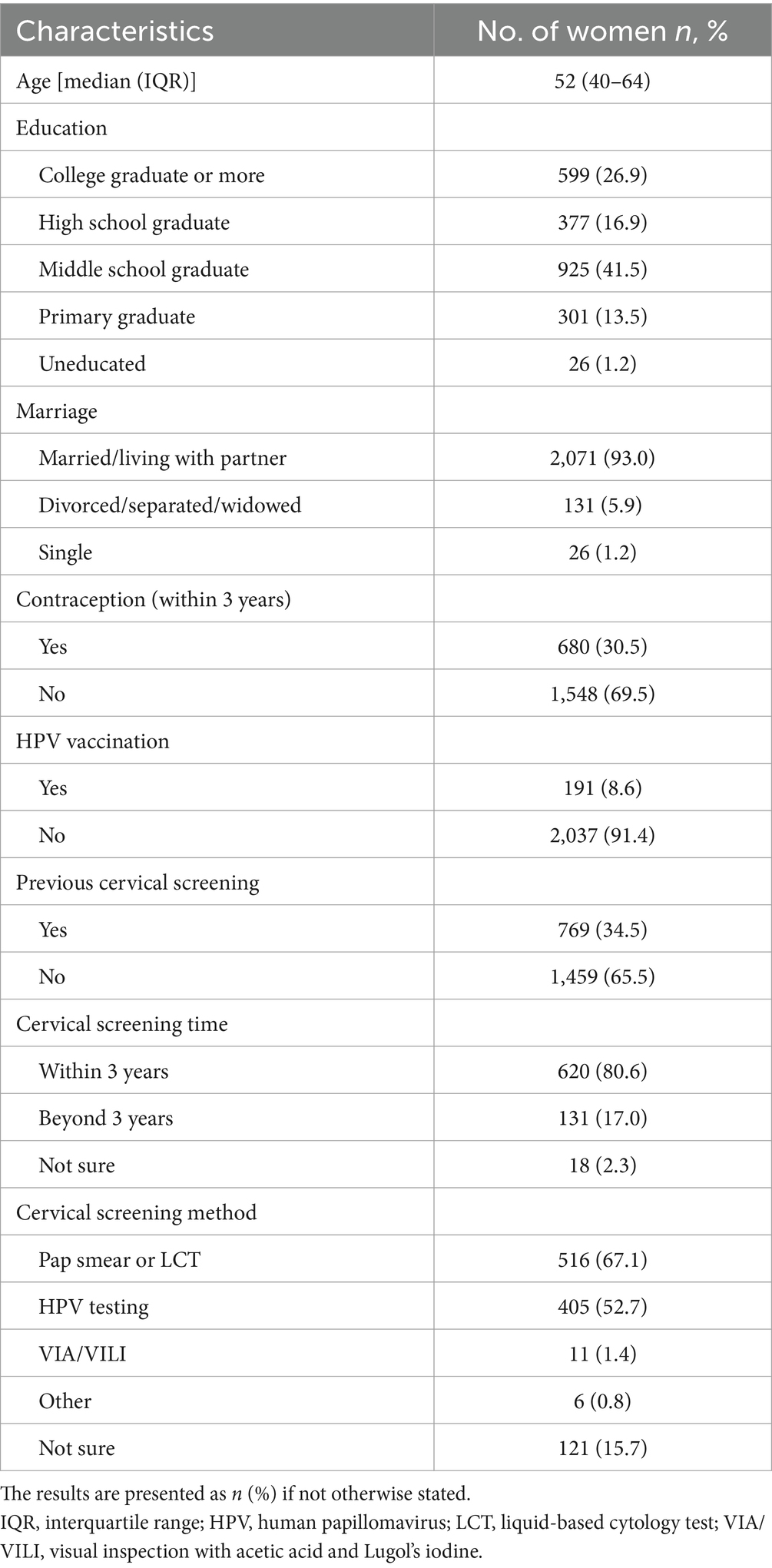
The median age of the 2,228 participants was 52 years (IQR: 40–64 years). Most participants were married (2,071/2,228, 93.0%), and approximately 98.8% (2,202/2,228) had completed primary or higher education. Additionally, 91.4% (2,037/2,228) had not received the HPV vaccine, and 65.5% (1,459/2,228) had never undergone cervical screening (Table 1).
3.2 Accordance of HPV testing between self-sampling and clinician sampling
The comparison among the tests is shown in Table 2 and Supplementary Table 1. The hrHPV positivity rate was similar between urine and clinician samples (9.9% vs. 9.9%, p > 0.05) and between vaginal and clinician samples (9.4% vs. 9.9%, p > 0.05).
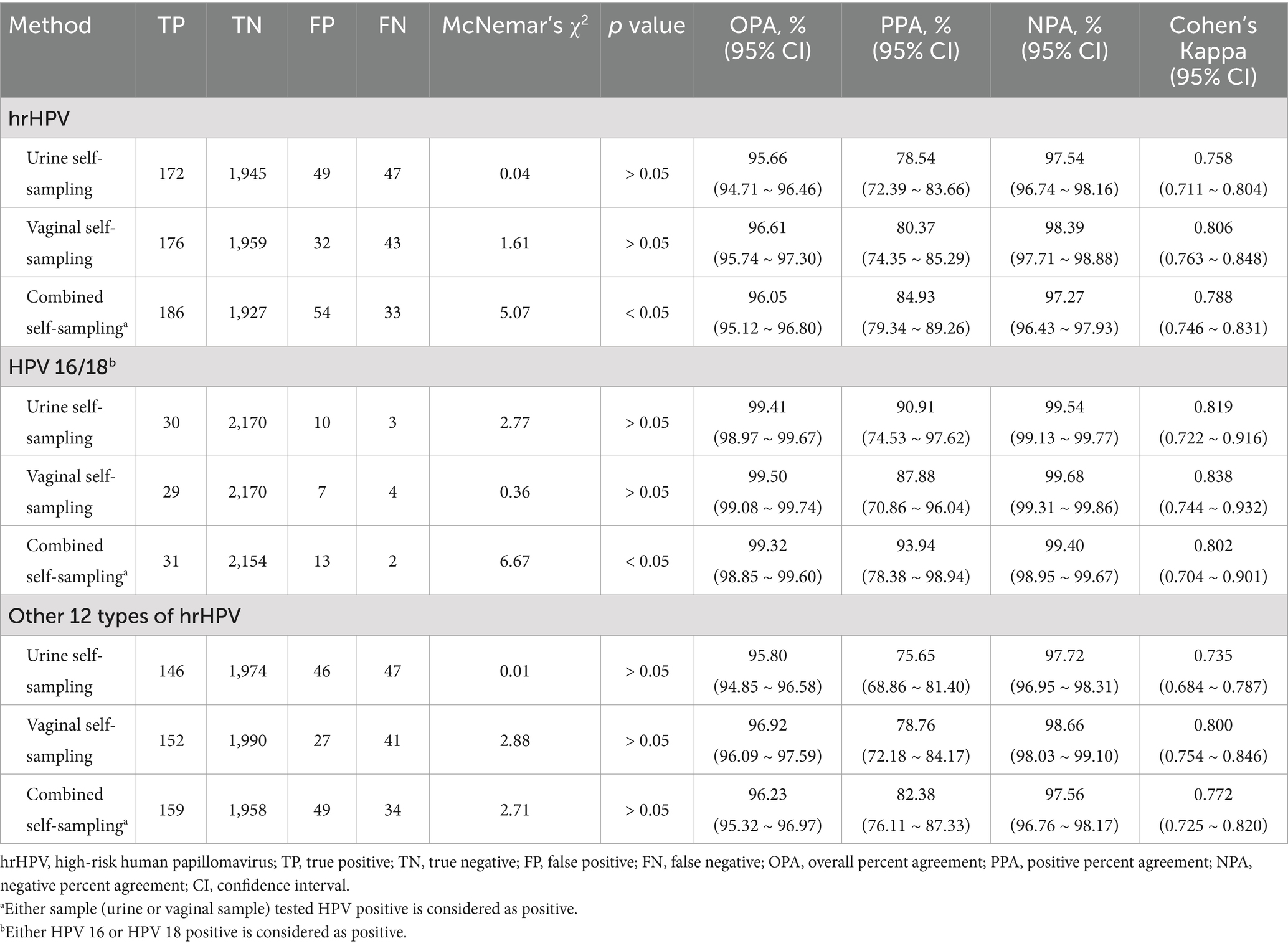
Table 2. Results of hrHPV testing—self-collected urine and vaginal samples compared with clinician-collected cervical samples.
Urine self-sampling had an overall percent agreement (OPA) of 95.66%, a positive percent agreement (PPA) of 78.54%, and a negative percent agreement (NPA) of 97.54%, with a Cohen’s kappa of 0.758. Vaginal self-sampling had an OPA of 96.61%, a PPA of 80.37%, and a NPA of 98.39%, with a Cohen’s kappa of 0.806. The combined self-sampling methods yielded an OPA of 96.05%, a PPA of 84.93%, and a NPA of 97.27%, with a Cohen’s kappa of 0.788.
3.3 Cytological and immunocytochemical findings of the population
Among those with cytological alterations (25/226, 10.8%), ASC-US was the most common (20/226, 8.6%). ICC was positive for both P16 and Ki-67 in 17 samples (7.5%). Overall, 30 samples (13.3%) were positive for either cytology (ASC-US or higher) or ICC [P16 (+), Ki-67 (+)] (Supplementary Table 2).
3.4 Histological findings of the population
Of the 76 participants eligible for colposcopy, 61 (80.2%) completed the procedure, with 54 cervical samples (71.1%) collected. Histological findings showed 24 women (44.4%) with less than cervical intraepithelial neoplasia grade 1 (< CIN1), 20 (37.0%) with CIN1, and 10 (18.5%) with grade 2 or worse (≥ CIN2) (Supplementary Table 3).
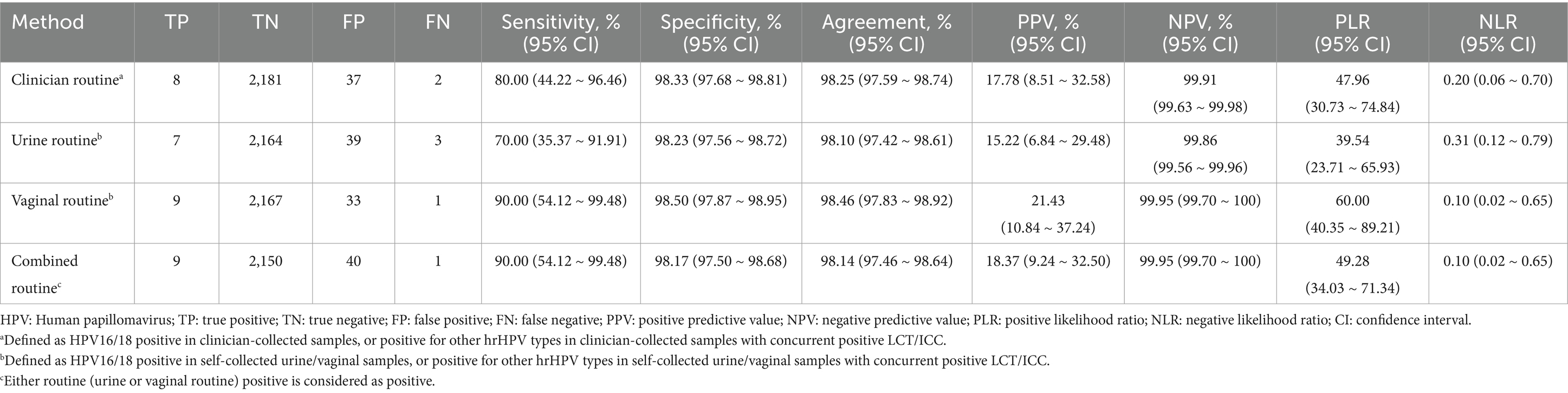
The accuracy rates for detecting CIN2+ lesions are shown in Table 3. Clinician-collected cervical samples had a sensitivity of 80.00% and a specificity of 98.33%, and an agreement of 98.25%. Urine self-sampling had a lower sensitivity of 70.00% and a specificity of 98.23%, and an agreement of 98.10%. Vaginal self-sampling had a sensitivity of 90.00% and a specificity of 98.50%, and an agreement of 98.46%. The combined approach of urine and vaginal self-sampling maintained a sensitivity of 90.00% and a specificity of 98.17%, with an agreement of 98.14%. All methods yielded high negative predictive values (NPVs), high positive likelihood ratios (PLRs), and low negative likelihood ratios (NLRs).
3.5 Awareness, feelings, and acceptability of self-sampling
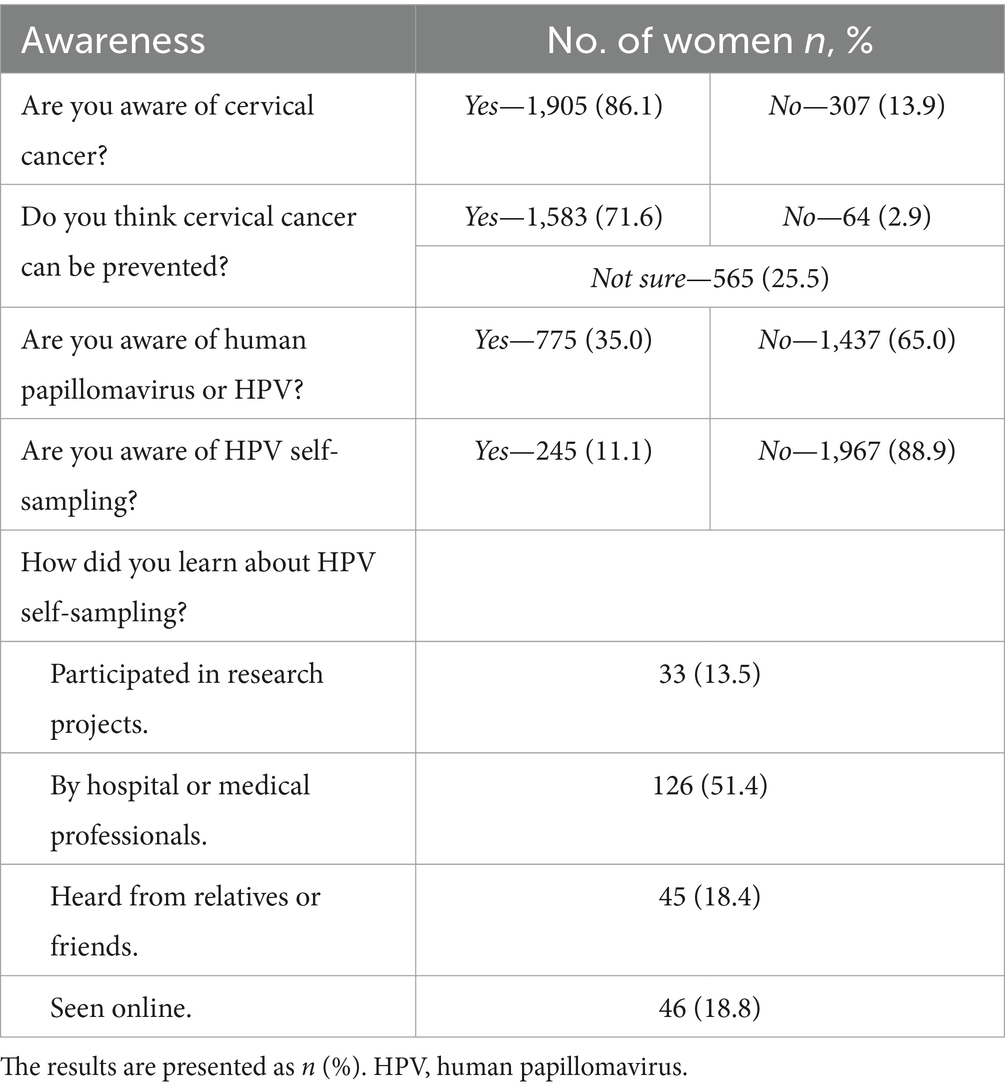
The participants’ awareness of cervical cancer, HPV, and self-sampling is presented in Table 4. Awareness of cervical cancer was high, with 86.1% (1,905/2,212) of participants aware of the disease, and 71.6% (1,583/2,212) believing it could be prevented. In contrast, only 35% (775/2,212) were aware of HPV, and 88.9% (245/2,212) had never heard of HPV self-sampling. Among those aware of HPV self-sampling, medical professionals were the primary source of information (51.4%, 126/245).
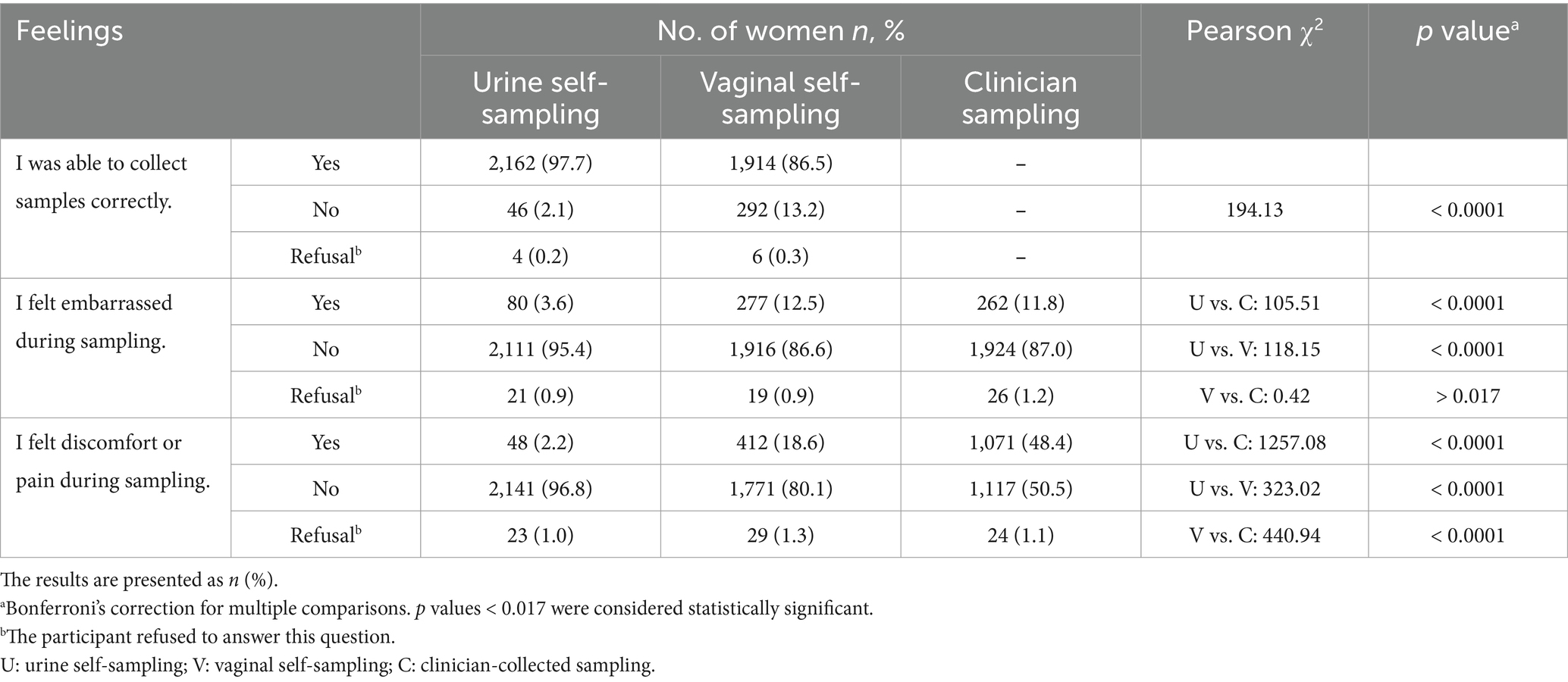
The participants’ feelings about self-sampling are presented in Table 5. Most were confident in performing self-sampling (urine vs. vaginal: 97.7% vs. 86.5%, p < 0.0001). Embarrassment was reported less frequently during urine self-sampling (3.6%, 80/2,212) than during vaginal (12.5%, 277/2,212, p < 0.0001) or clinician-collected sampling (11.8%, 262/2,212, p < 0.0001). Discomfort or pain was reported less frequently during urine self-sampling (2.2%, 48/2,212) than during vaginal (18.6%, 412/2,212, p < 0.0001) or clinician-collected sampling (48.4%, 1,071/2,212, p < 0.0001), and less frequently during vaginal self-sampling than during clinician-collected sampling (18.6% vs. 48.4%, p < 0.0001).
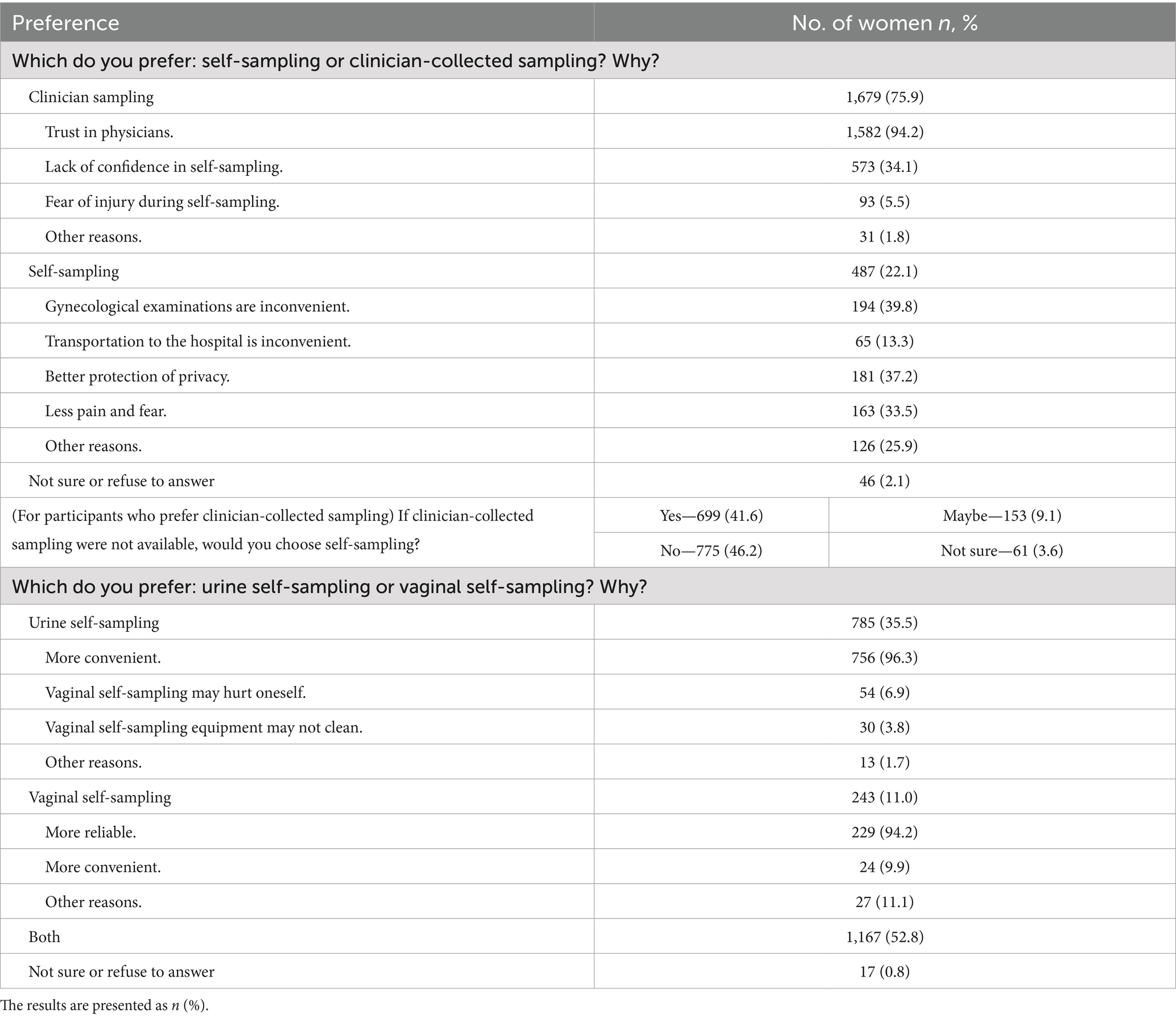
The participants’ preferences for self-sampling are presented in Table 6. Most (75.9%, n = 1,679) favored clinician sampling, mainly because of trust in physicians (94.2%, n = 1,582) and concerns about incorrect sampling (34.1%, n = 573). In contrast, 22.0% (n = 487) preferred self-sampling, citing the inconvenience of gynecological exams (39.8%, n = 194), better privacy (37.2%, n = 181), and less pain and fear (33.5%, n = 163). Among those preferring clinician sampling, 41.6% (n = 699) would choose self-sampling if clinician sampling was unavailable. When comparing urine to vaginal self-sampling, 35.5% (n = 785) preferred urine for its convenience (96.3%, n = 756), 11.0% (n = 243) preferred vaginal self-sampling for its reliability (94.2%, n = 229), 52.8% (n = 1,167) had no strong preference.
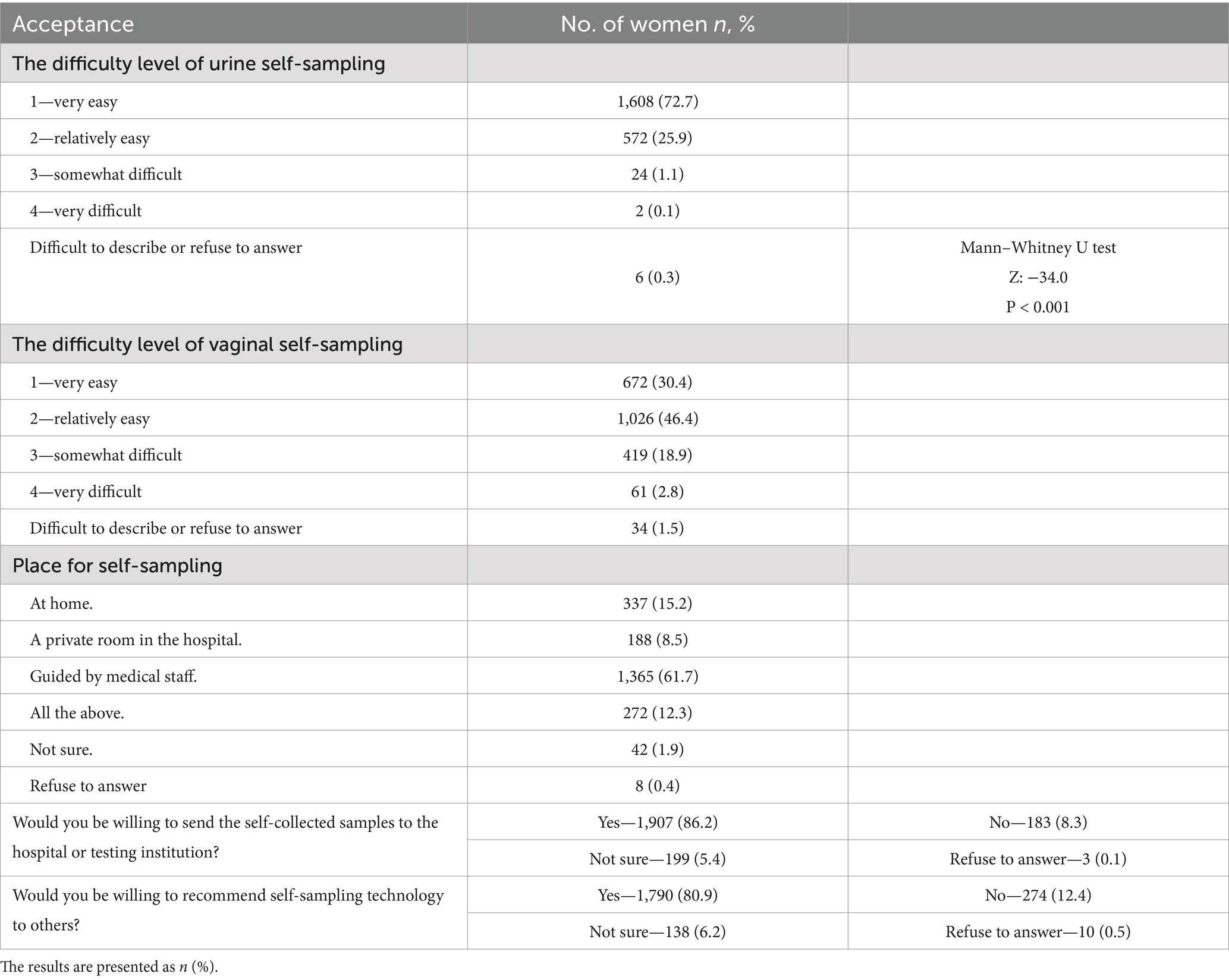
The participants’ acceptance of self-sampling is presented in Table 7. Urine self-sampling was perceived as easier than vaginal self-sampling (Z = −34.0, p < 0.001). Urine sampling was rated as “very easy” by 72.7% (n = 1,608) and “relatively easy” by 25.9% (n = 572), while for vaginal sampling, the rating were 30.4% (n = 672) and 46.4% (n = 1,026). Regarding preferred locations for self-sampling, 61.7% (n = 1,365) preferred guidance by medical staff in hospitals. Additionally, 86.2% (n = 1,907) were willing to send self-collected samples to a hospital or testing institution, and 80.9% (n = 1,790) were willing to recommend self-sampling to others.
4 Discussion
This study aimed to evaluate the feasibility and acceptability of detecting hrHPV and CIN2+ via urine and vaginal self-sampling compared with clinician sampling in urban areas of western China.
Our study evaluated the feasibility and acceptability of self-sampling in a population with low vaccination and screening rates. The HPV vaccination rate among the study population was 8.6% (191/2,228), which is higher than the national average in China (10) but lower than the global average of 15% (25). This may be because Shuangliu, the study location, was among the first regions in China to initiate cervical cancer vaccination programs. The overall cervical cancer screening rate was 34.5% (769/2,228), which was lower than the national average in China of 36.8% (95% CI: 35.1–38.4) (11).
Self-sampling could be a viable alternative in hrHPV screening, potentially improving accessibility and compliance. Arbyn’s 2022 meta-analysis (16) of 4 studies conducted in screening populations reported a pooled OPA between vaginal self-sampling and clinician sampling of 88.1% (95% CI: 81.8–93.3), a kappa statistic of 0.65 (95% CI: 0.51–0.79), a PPA of 74.8% (95% CI: 60.0–87.1), and a NPA of 92.0% (95% CI: 86.9–94.8). Bober’s 2021 meta-analysis (26) reported the diagnostic accuracy of first-void urine sampling versus clinician-collected sampling. For hrHPV detection, the sensitivity of urine self-sampling was 78% (95% CI: 70–84), and the specificity was 89% (95% CI: 81–94). For HPV16/18 detection, the sensitivity of urine self-sampling was 87% (95% CI: 74–94), and the specificity was 91% (95% CI: 83–96). Our study demonstrated strong concordance between urine and vaginal self-sampling and clinician-collected samples for hrHPV detection, with kappa values of 0.758 for urine, 0.806 for vaginal, and 0.788 for the combined methods. All three self-sampling methods (urine, vaginal and combined) resulted in high OPA, PPA, and NPA.
Our findings concerning the detection of CIN2+ are consistent with those of other studies investigating the accuracy of HPV testing via self-collected vaginal or urine samples. Previous studies, including clinical trials and meta-analyses, have shown that HPV testing on self-collected vaginal or urine samples has a similar accuracy to that of clinician-collected cervical samples (15, 27–32). For example, a 2018 meta-analysis by Arbyn et al. (27) revealed that compared with clinician sampling, PCR-based hrHPV assays had similar diagnostic accuracy for CIN2+ or CIN3+ patients via vaginal self-sampling (pooled sensitivity ratio for CIN2+: 99, 95% CI: 0.97–1.02). Additionally, two randomized trials (28, 32) confirmed comparable accuracy between self-collected and clinician-collected samples for detecting CIN2+ or CIN3+ lesions. The 2021 meta-analysis by Cho et al. (33) demonstrated that HPV testing via a PCR-based urine detection method showed similar clinical accuracy to clinician-collected samples in detecting CIN2 or more severe lesions.
In terms of participants’ awareness of cervical cancer, HPV and self-sampling, the majority were aware of cervical cancer and thought that it could be prevented. However, their knowledge of HPV is limited and may contribute to the low rates of HPV vaccination and cervical cancer screening in China. Most participants (88.9%, n = 1,967) were introduced to self-sampling techniques and underwent self-sampling for the first time throughout our project. Consequently, their experiences in this self-sampling initiative could directly influence their acceptance of the technique. Since self-sampling is not yet widely adopted for cervical cancer screening in China, our study offers a genuine reflection of initial impressions and acceptance levels among the screening population. Consistent with previous studies (34, 35), most participants were confident in their ability to collect samples correctly (97.7%, n = 2,162) and reported less embarrassment (3.6%, n = 80) and pain (2.2%, n = 48) during the process.
Nelson’s 2017 meta-analysis (36) evaluated patient acceptance and preferences for self-sampling versus clinician-collected sampling across 23 studies (N = 12,610), finding an average preference rate of 59% (95% CI: 0.48–0.69) for self-sampling. In our study, only 22.1% (n = 487) of the participants preferred self-sampling, whereas most (75.9%, n = 1,679) favored clinician sampling because of their trust in physicians (94.2%, n = 1,582), lack of confidence in self-sampling (34.1%, n = 573), and fear of self-inflicted injury (5.5%, n = 93). This may be due to low awareness of self-sampling and the fact that most participants were sampling themselves for the first time. However, if clinician assistance was unavailable, half (50.7%, n = 852) of those who preferred clinician sampling would opt for self-sampling. When asked about their preferred self-sampling method, most (50.7%, n = 852) reported that both urine and vaginal self-sampling were acceptable. Those favoring urine sampling cited convenience (96.3%, n = 756), whereas those preferring vaginal sampling considered it more reliable (94.2%, n = 229). Thus, in areas with limited medical resources, self-sampling could be a viable alternative to clinician-collected sampling. Urine self-sampling was considered easier (Mann–Whitney U test, Z: −34.0, p < 0.001), suggesting that it may achieve greater acceptance in resource-limited settings.
Nelson’s 2017 meta-analysis (36) reported that 97% (95% CI: 0.95–0.98) of patients reported that self-sampling was generally acceptable (7 studies, N = 1,470), and 87% (95% CI: 0.73–0.95) expressed a willingness to repeat it (9 studies, N = 2,660). In our study, 86.2% (n = 1,907) of the participants were willing to send self-collected samples to a hospital or testing institution, and 80.9% (n = 1,790) were willing to introduce self-sampling to others, indicating a promising outlook for promoting self-sampling. Di Gennaro’s meta-analysis (13) revealed similar preferences for home sampling (66, 95% CI: 57–74%) and clinical settings (67, 95% CI: 62–71%; p = 0.841), whereas Nishimura’s review (37) of 8 studies revealed a preference for home collection. In contrast, our study, which provided video and professional instructions, revealed that most participants (61.7%, n = 1,365) preferred self-sampling under medical guidance. This preference may stem from a lack of confidence in self-sampling skills and concerns about accessing treatment when needed. These findings suggest that combining media with professional guidance could effectively promote self-sampling adoption.
Our study presents certain innovations. It focuses on western China, where low- to middle-income populations are more common and where medical resources, screening rates, and compliance are lower than those in the more developed eastern regions. Given the limited research in this area, our study on the feasibility and acceptability of self-sampling provides valuable insights and potential strategies to improve screening rates and compliance in this region.
However, our study has several limitations. As this study focused on primary screening, the low number of CIN2+ cases may have affected the feasibility assessment. Expanding the screening scale is necessary to ensure a more representative evaluation of diagnostic accuracy. Self-sampling techniques, especially urine self-sampling, need further refinement to increase sensitivity and specificity. Additionally, multicenter, large-scale prospective studies are needed to provide higher-level evidence for the adoption of HPV self-sampling.
5 Conclusion
Self-collected urine and vaginal samples for the detection of hrHPV and CIN2+ demonstrate high diagnostic accuracy and acceptability, making them viable alternatives to clinician-collected samples. Self-sampling methods may improve screening accessibility and compliance, especially in resource-limited settings, thereby supporting the prevention and early detection of CIN2+.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Peking Union Medical College Hospital (PUMCH) (approval code ZS-3293) and West China Second University Hospital (WCSUH). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SX: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Software, Visualization. XZ: Conceptualization, Data curation, Methodology, Project administration, Writing – review & editing, Investigation. JL: Conceptualization, Writing – review & editing, Data curation, Methodology. LK: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. MX: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. LX: Investigation, Writing – review & editing. PF: Investigation, Project administration, Writing – review & editing, Supervision. MZ: Investigation, Writing – review & editing. MA: Investigation, Writing – review & editing. XY: Investigation, Writing – review & editing. DL: Investigation, Writing – review & editing. GL: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The author extends heartfelt appreciation to the dedicated staff at Chengdu Shuangliu District Maternal and Child Health Hospital and the Pathology Department of West China Second University Hospital for their invaluable support and assistance throughout the course of this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2025.1524796/full#supplementary-material
Abbreviations
ASC-US, atypical squamous cells of undetermined significance; CI, confidence interval; CIN2+, cervical intraepithelial neoplasia grade 2 or worse; HPV, human papillomavirus; hrHPV, high-risk human papillomavirus; ICC, immunocytochemistry; IQR, interquartile range; LCT, liquid-based cytology testing; LMICs, lower-middle-income countries; NLR, negative likelihood ratio; NPA, negative percent agreement; NPV, negative predictive value; OPA, overall percent agreement; PLR, positive likelihood ratio; PPA, positive percent agreement; PPV, positive predictive value; PUMCH, Peking Union Medical College Hospital; WCSUH, West China Second University Hospital; WHO, World Health Organization.
References
1. Bray, F, Laversanne, M, Sung, H, Ferlay, J, Siegel, RL, Soerjomataram, I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Canfell, K, Kim, JJ, Brisson, M, Keane, A, Simms, KT, Caruana, M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. (2020) 395:591–603. doi: 10.1016/S0140-6736(20)30157-4
3. Han, B, Zheng, R, Zeng, H, Wang, S, Sun, K, Chen, R, et al. Cancer incidence and mortality in China, 2022. J Natl Cancer Cent. (2024) 4:47–53. doi: 10.1016/j.jncc.2024.01.006
4. World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem; (2020). Available online at: https://www.who.int/publications/i/item/9789240014107 (Accessed July 15, 2024).
5. Bouvard, V, Wentzensen, N, Mackie, A, Berkhof, J, Brotherton, J, Giorgi-Rossi, P, et al. The IARC perspective on cervical Cancer screening. N Engl J Med. (2021) 385:1908–18. doi: 10.1056/NEJMsr2030640
6. Koliopoulos, G, Nyaga, VN, Santesso, N, Bryant, A, Martin-Hirsch, PP, Mustafa, RA, et al. Cytology versus HPV testing for cervical cancer screening in the general population. Cochrane Database Syst Rev. (2017) 2018:CD008587. doi: 10.1002/14651858.CD008587.pub2
7. Dillner, J, Rebolj, M, Birembaut, P, Petry, K-U, Szarewski, A, Munk, C, et al. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: Joint European Cohort Study. BMJ. (2008) 337:a1754. doi: 10.1136/bmj.a1754
8. Petry, K-U, Menton, S, Menton, M, van Loenen-Frosch, F, de Carvalho, GH, Holz, B, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer. (2003) 88:1570–7. doi: 10.1038/sj.bjc.6600918
9. Serrano, B, Ibáñez, R, Robles, C, Peremiquel-Trillas, P, de Sanjosé, S, and Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev Med. (2022) 154:106900. doi: 10.1016/j.ypmed.2021.106900
10. Chen, J, Zhang, Z, Pan, W, Song, Y, Zheng, L, Li, L, et al. Estimated human papillomavirus vaccine coverage among females 9-45 years of age – China, 2017-2022. China CDC Wkly. (2024) 6:413–7. doi: 10.46234/ccdcw2024.080
11. Zhang, M, Zhong, Y, Wang, L, Bao, H, Huang, Z, Zhao, Z, et al. Cervical Cancer screening coverage – China, 2018-2019. China CDC Wkly. (2022) 4:1077–82. doi: 10.46234/ccdcw2022.217
12. Hawkes, D, Keung, MHT, Huang, Y, McDermott, TL, Romano, J, Saville, M, et al. Self-collection for cervical screening programs: from research to reality. Cancers. (2020) 12:1053. doi: 10.3390/cancers12041053
13. Di Gennaro, G, Licata, F, Trovato, A, and Bianco, A. Does self-sampling for human papilloma virus testing have the potential to increase cervical cancer screening? An updated meta-analysis of observational studies and randomized clinical trials. Front Public Health. (2022) 10:1003461. doi: 10.3389/fpubh.2022.1003461
14. World Health Organization. WHO guideline on self-care interventions for health and well being, 2022 revision. (2022). Available online at: https://www.who.int/publications/i/item/9789240052192 (Accessed July 15, 2024).
15. Cho, H-W, Shim, SR, Lee, JK, and Hong, JH. Accuracy of human papillomavirus tests on self-collected urine versus clinician-collected samples for the detection of cervical precancer: a systematic review and meta-analysis. J Gynecol Oncol. (2022) 33:e4. doi: 10.3802/jgo.2022.33.e4
16. Arbyn, M, Castle, PE, Schiffman, M, Wentzensen, N, Heckman-Stoddard, B, and Sahasrabuddhe, VV. Meta-analysis of agreement/concordance statistics in studies comparing self- vs clinician-collected samples for HPV testing in cervical cancer screening. Int J Cancer. (2022) 151:308–12. doi: 10.1002/ijc.33967
17. Tatara, T, Wnuk, K, Miazga, W, Świtalski, J, Karauda, D, Mularczyk-Tomczewska, P, et al. The influence of vaginal HPV self-sampling on the efficacy of populational screening for cervical cancer-an umbrella review. Cancers. (2022) 14:5913. doi: 10.3390/cancers14235913
18. Sy, F, Greuel, M, Winkler, V, Bussmann, H, Bärnighausen, T, and Deckert, A. Accuracy of HPV testing on self-collected and clinician-collected samples for different screening strategies in African settings: a systematic review and meta-analysis. Gynecol Oncol. (2022) 166:358–68. doi: 10.1016/j.ygyno.2022.06.012
19. Meenan, RT, Troja, C, Buist, DSM, Tiro, JA, Lin, J, Anderson, ML, et al. Economic evaluation of mailed home-based human papillomavirus self-sampling kits for cervical cancer screening. JAMA Netw Open. (2023) 6:e234052. doi: 10.1001/jamanetworkopen.2023.4052
20. Zhao, X-L, Zhao, S, Xia, C-F, Hu, S-Y, Duan, X-Z, Liu, Z-H, et al. Cost-effectiveness of the screen-and-treat strategies using HPV test linked to thermal ablation for cervical cancer prevention in China: a modeling study. BMC Med. (2023) 21:149. doi: 10.1186/s12916-023-02840-8
21. Daponte, N, Valasoulis, G, Michail, G, Magaliou, I, Daponte, A-I, Garas, A, et al. HPV-based self-sampling in cervical cancer screening: an updated review of the current evidence in the literature. Cancers. (2023) 15:1669. doi: 10.3390/cancers15061669
22. Zhao, X-L, Xu, X-Q, Duan, X-Z, Rezhake, R, Hu, S-Y, Wang, Y, et al. Comparative performance evaluation of different HPV tests and triaging strategies using self-samples and feasibility assessment of thermal ablation in “colposcopy and treat” approach: a population-based study in rural China. Int J Cancer. (2020) 147:1275–85. doi: 10.1002/ijc.32881
23. Xu, H-F, Zhao, X-L, Zhao, S, Hu, S-Y, Zhang, X, Zhao, F-H, et al. Clinical performance of a newly developed domestic urine-based HPV test for cervical cancer screening in China. J Med Virol. (2023) 95:e28705. doi: 10.1002/jmv.28705
24. Bian, M-L, Cheng, J-Y, Ma, L, Cong, X, Liu, J, Chen, Y, et al. Evaluation of the detection of 14 high-risk human papillomaviruses with HPV 16 and HPV 18 genotyping for cervical cancer screening. Exp Ther Med. (2013) 6:1332–6. doi: 10.3892/etm.2013.1309
25. Bruni, L, Saura-Lázaro, A, Montoliu, A, Brotons, M, Alemany, L, Diallo, MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
26. Bober, P, Firment, P, and Sabo, J. Diagnostic test accuracy of first-void urine human papillomaviruses for presence cervical HPV in women: systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18:13314. doi: 10.3390/ijerph182413314
27. Arbyn, M, Smith, SB, Temin, S, Sultana, F, and Castle, PCollaboration on Self-Sampling and HPV Testing. Detecting cervical precancer and reaching under screened women by using HPV testing on self samples: updated meta-analyses. BMJ. (2018) 363:k4823. doi: 10.1136/bmj.k4823
28. Polman, NJ, Ebisch, RMF, Heideman, DAM, Melchers, WJG, Bekkers, RLM, Molijn, AC, et al. Performance of human papillomavirus testing on self-collected versus clinician-collected samples for the detection of cervical intraepithelial neoplasia of grade 2 or worse: a randomised, paired screen-positive, non-inferiority trial. Lancet Oncol. (2019) 20:229–38. doi: 10.1016/S1470-2045(18)30763-0
29. Pathak, N, Dodds, J, Zamora, J, and Khan, K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: systematic review and meta-analysis. BMJ. (2014) 349:g5264. doi: 10.1136/bmj.g5264
30. Enerly, E, Olofsson, C, and Nygård, M. Monitoring human papillomavirus prevalence in urine samples: a review. Clin Epidemiol. (2013) 5:67–79. doi: 10.2147/CLEP.S39799
31. Van Keer, S, Peeters, E, Vanden Broeck, D, De Sutter, P, Donders, G, Doyen, J, et al. Clinical and analytical evaluation of the real time high risk HPV assay in Colli-pee collected first-void urine using the VALHUDES protocol. Gynecol Oncol. (2021) 162:575–83. doi: 10.1016/j.ygyno.2021.06.010
32. Aarnio, R, Isacson, I, Sanner, K, Gustavsson, I, Gyllensten, U, and Olovsson, M. Comparison of vaginal self-sampling and cervical sampling by medical professionals for the detection of HPV and CIN2+: a randomized study. Int J Cancer. (2021) 148:3051–9. doi: 10.1002/ijc.33482
33. Cho, H-W, Hong, JH, Min, KJ, Ouh, Y-T, Seong, SJ, Moon, JH, et al. Performance and diagnostic accuracy of human papillomavirus testing on self-collected urine and vaginal samples in a referral population. Cancer Res Treat. (2021) 53:829–36. doi: 10.4143/crt.2020.1165
34. Hang, S, Haruyama, R, Uy, K, Fujita, N, Kimura, T, Koum, K, et al. Feasibility, accuracy and acceptability of self-sampled human papillomavirus testing using careHPV in Cambodia: a cross-sectional study. J Gynecol Oncol. (2024) 35:e6. doi: 10.3802/jgo.2024.35.e6
35. Madhivanan, P, Nishimura, H, Ravi, K, Pope, B, Coudray, M, Arun, A, et al. Acceptability and concordance of self-versus clinician-sampling for HPV testing among rural south Indian women. Asian Pac J Cancer Prev. (2021) 22:971–6. doi: 10.31557/APJCP.2021.22.3.971
36. Nelson, EJ, Maynard, BR, Loux, T, Fatla, J, Gordon, R, and Arnold, LD. The acceptability of self-sampled screening for HPV DNA: a systematic review and meta-analysis. Sex Transm Infect. (2017) 93:56–61. doi: 10.1136/sextrans-2016-052609
Keywords: vaginal self-sampling, urine self-sampling, human papillomavirus testing, cervical intraepithelial neoplasia, cervical cancer screening, acceptability
Citation: Xue S, Zeng X, Li J, Kang L, Xi M, Xu L, Fu P, Zhou M, Ao M, Yao X, Li D and Liao G (2025) Feasibility and acceptability of human papillomavirus self-sampling compared with clinician sampling in urban areas of western China: a cross-sectional survey. Front. Public Health. 13:1524796. doi: 10.3389/fpubh.2025.1524796
Edited by:
Marina Pekmezovic, Independent Researcher, Berlin, GermanyReviewed by:
Fausto Ciccacci, University of Rome Tor Vergata, ItalyNicholas Matovu, Queen’s University Belfast, United Kingdom
Copyright © 2025 Xue, Zeng, Li, Kang, Xi, Xu, Fu, Zhou, Ao, Yao, Li and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangdong Liao, NTEzNTY2MkBxcS5jb20=
 Shaolong Xue
Shaolong Xue Xi Zeng
Xi Zeng Jing Li
Jing Li Leni Kang4
Leni Kang4 Mingrong Xi
Mingrong Xi Mengyin Ao
Mengyin Ao Xiaoxi Yao
Xiaoxi Yao Dongmei Li
Dongmei Li