
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Public Health, 28 February 2025
Sec. Children and Health
Volume 13 - 2025 | https://doi.org/10.3389/fpubh.2025.1522959
This article is part of the Research TopicEarly Maternal and Child Health Management and the Impact of Living EnvironmentView all 12 articles
 Endalk Birrie Wondifraw1*
Endalk Birrie Wondifraw1* Fekadeselassie Belege Getaneh1
Fekadeselassie Belege Getaneh1 Muluken Amare1
Muluken Amare1 Setegn Mihret1
Setegn Mihret1 Gebeyaw Biset1
Gebeyaw Biset1 Birhanu Desu Tefera2
Birhanu Desu Tefera2 Mulusew Zeleke3
Mulusew Zeleke3 Fuad Ahmed4
Fuad Ahmed4 Ermias Sisay Chanie5
Ermias Sisay Chanie5Background: Poor adherence to antiretroviral therapy (ART) occurs when an individual with Human Immune deficiency Virus does not follow the prescribed treatment regimen correctly. This includes missing doses, not taking medication as scheduled, taking medication inconsistently or irregularly, and failing to adhere to specific instructions. The lack of adherence to antiretroviral therapy (ART) among children is a noteworthy issue that necessitates attention. The study aims to determine the level of non-adherence to antiretroviral therapy (ART) and its associated factors in children receiving ART in public hospitals in the South Wollo Zone.
Methods: A multi-center cross-sectional study was conducted among children receiving antiretroviral therapy at South Wollo Zone public hospitals. A single population proportion formula was used to determine the required sample size. A computer-generated simple random sampling method was employed to select the participants. The tools used to assess adherence for all participants were viral load monitoring, Self-reporting, Pill counts, and Pharmacy refill records. Data were collected through face-to-face interviews, and reviewing patients’ documents using a structured checklist. The data were entered into Epi Data version 4.1 and analyzed using STATA 17. Binary logistic regression was employed to evaluate the relationship between the factors and the outcome variable. Variables were considered significant if the p-value was less than 0.05.
Result: Of 291 participants, 286 were involved in the study, making the response rate 98.3%. The mean age of the participants was 7.8 years old (±3.64 SD), and half of the 146 children (51%) were male. The overall proportion of ART non-adherence was 24.1% (95% CI: 19.2–29.0%). Positive TB status (Adjusted odd ratio (AOR) = 4.10, 95% CI: 1.90–8.88), diagnostic status not disclosed (AOR = 2.69, 95% CI: 1.43–5.00), and poor caregiver knowledge (AOR = 2.18, 95% CI: 1.04–4.56) were significantly associated with poor adherence.
Conclusion: According to the current study, the level of non-adherence to antiretroviral therapy remains high compared to the targets set by the United Nations Joint Program on HIV/AIDS (UNAIDS) Project 95-95-95. TB co-infection, undisclosed diagnostic status, and poor caregiver knowledge were found to be significantly associated with non-adherence. Before and throughout ART, healthcare providers should provide intense and ongoing counseling to children and their caregivers.
In 2023, approximately 630,000 individuals died from HIV-related causes, and around 1.3 million people acquired HIV (1). By the end of the year, approximately 1.4 million children aged 0–14 were living with HIV, with 120,000 newly infected. Around 76,000 children succumbed to AIDS-related illnesses. Early testing and treatment are critical to decreasing HIV-related deaths and diseases in this vulnerable group (1). Without timely intervention, half of the children with HIV will die by age 2, and 80% will not survive past their fifth birthday (1–3).
According to an Ethiopian Public Health Institute report published in 2021, around 42,000 Ethiopian children were infected with HIV during the same year. The report also indicates that in 2013 the country’s ART coverage for children was only 12%. Additionally, it highlights that the HIV epidemic among Ethiopian youth has received minimal attention from government initiatives (4, 5).
ART is a combination of antiretroviral medications that can extend and enhance the quality of life of HIV patients by reducing viral loads and raising CD4 cell counts. ART is an HIV medication that should be taken constantly (6). Adherence to appropriate and prolonged treatment is critical to ART success (7).
Poor adherence (non-adherence) to ART occurs when an individual with HIV does not follow the prescribed treatment regimen correctly. This includes missing doses, not taking medication as scheduled, taking medication inconsistently or irregularly, failing to adhere to specific instructions such as taking the drug with food or at designated times, completely discontinuing medication, or not refilling prescriptions as required. Such poor adherence can result in less effective treatment, including treatment failure, higher viral loads, development of drug resistance, and disease progression. Contributing factors may include side effects, the complexity of the regimen, lack of support, or various personal and systemic issues (1, 8, 9).
The study shows that non-adherence levels in Ethiopia ranged from 4.5% at Tikur Anbessa Hospital (10) to 65.5% at Debre Birhan Hospital (11).
Some studies state factors that contribute to poor adherence among children. These factors include socio-economic, socio-demographic, and socio-cultural aspects, as well as problems with service delivery, which can have an impact on adherence to pediatric ART (12, 13). High pill burden, poor palatability, long-term toxicity, ART side effects, ART regimens, and medication dosing are additional factors that can affect adherence. Pediatric ART adherence has also been linked to children’s health, length of time on antiretroviral therapy (ART), knowledge of their HIV status, and psychological variables (10–18).
Routine assessment of medication adherence in clinical care should include increasing the frequency of viral load monitoring after starting or changing medications. Additionally, healthcare providers should ask the child/adolescent and caregiver about the number of missed doses over specific periods (e.g., 1, 3, or 7 days) and request details about the medication, such as its name, appearance, dosage, and frequency of intake. Engaging in conversations with the child and caregiver about potential adherence barriers and strategies to overcome them is essential. Pharmacy-based or clinic-based methods can be used to assess on-time medication refills. Both remote and in-person visits should be utilized to support families, observe ART preparation, handling, and administration, and conduct directly observed therapy (DOT) at home. Finally, announced and unannounced pill counts can be performed by asking individuals to bring medications to the clinic, conducting home visits, or referring them to community health nursing (19, 20).
Non-adherence to ART poses a major challenge to achieving optimal health outcomes for children living with HIV, leading to virologic failure, drug resistance, and increased morbidity and mortality. Understanding the extent of non-adherence is essential for improving pediatric HIV treatment. Ethiopia’s sociocultural, economic, and healthcare contexts significantly influence adherence, and identifying these factors can guide the development of targeted locally relevant interventions.
This study aligns with Ethiopia’s national HIV/AIDS strategic plan and global UNAIDS 95–95-95 goals, contributing to viral suppression and the effort to end the pediatric HIV epidemic. By quantifying non-adherence and its associated factors, the research provides critical data to inform policymakers and healthcare providers, enabling better resource allocation, caregiver training, and implementation of evidence-based strategies.
Non-adherence not only leads to treatment failure but also increases healthcare costs due to the need for second-line therapies and hospitalizations. Addressing adherence early can reduce this burden on the healthcare system. Limited data exist on pediatric ART adherence in Ethiopia, particularly in the study area. This research employs innovative methods, including adherence assessment through viral load measurement, which has not been extensively studied before. The findings will fill critical knowledge gaps, advance pediatric HIV care, and support Ethiopia’s fight against HIV/AIDS. This study aimed to determine the level of non-adherence to ART and its associated factors in children receiving ART in public hospitals in the South Wollo Zone.
An institution-based cross-sectional study was conducted from June 1 to June 30, 2023, among children receiving antiretroviral therapy at South Wollo Zone public hospitals. It was far from Addis Ababa, a distance of 401 km, and from Bahir Dar, a distance of 471 km. It has a total population of 2,518,862 and 499 health posts, 126 health centers, and 14 hospitals.
All HIV-infected children <15 years of age who started ART at South Wollo Zone Public Hospitals and their primary caregivers served as our source population for this study. Our study population includes all HIV-infected children <15 years of age who started ART at South Wollo Zone Public Hospitals from September 1, 2019, to June 30, 2023, and their primary caregivers.
The study included children under 15 who had been on ART for at least 1 month and their primary caregivers.
Participants with incomplete data (charts lacking significant explanatory and outcome variables) were excluded from this study.
A single population proportion formula was used to determine the required sample size with the following statistical assumptions: 22% proportion (p) of the level of non-adherence was taken from a study conducted in northwest Ethiopia (16); 5% margin of error; 10% incomplete or inconsistent data; and 95% confidence intervals (CI).
Where: n = the required sample size, Zα/2 = the standard normal variation, p = proportion (0.22) of the level of non-adherence, and d = margin of sampling error (0.05). By considering 10% of the incomplete or inconsistent data, the final sample size of our study was 291.
From the South Wollo zone hospitals, Dessie Comprehensive Specialized Hospital, Akasta (Hadar 11) General Hospital, Mekan Selam General Hospital, Negste Zewditw Primary Hospital, and Jamma Primary Hospital were selected through a simple random sampling method. In each hospital, the sample size was allocated proportionally. Using the ART registration logbook as the sampling frame, a simple random sampling technique was applied. From September 1, 2019, to June 30, 2023, children on ART were enrolled in the following hospitals: Dessie Comprehensive Specialized Hospital (550 children), Akasta Hadar 11 General Hospital (30 children), Mekan Selam General Hospital (16 children), Negste Zewditu Primary Hospital (14 children), and Jamma Primary Hospital (21 children) (Figure 1).
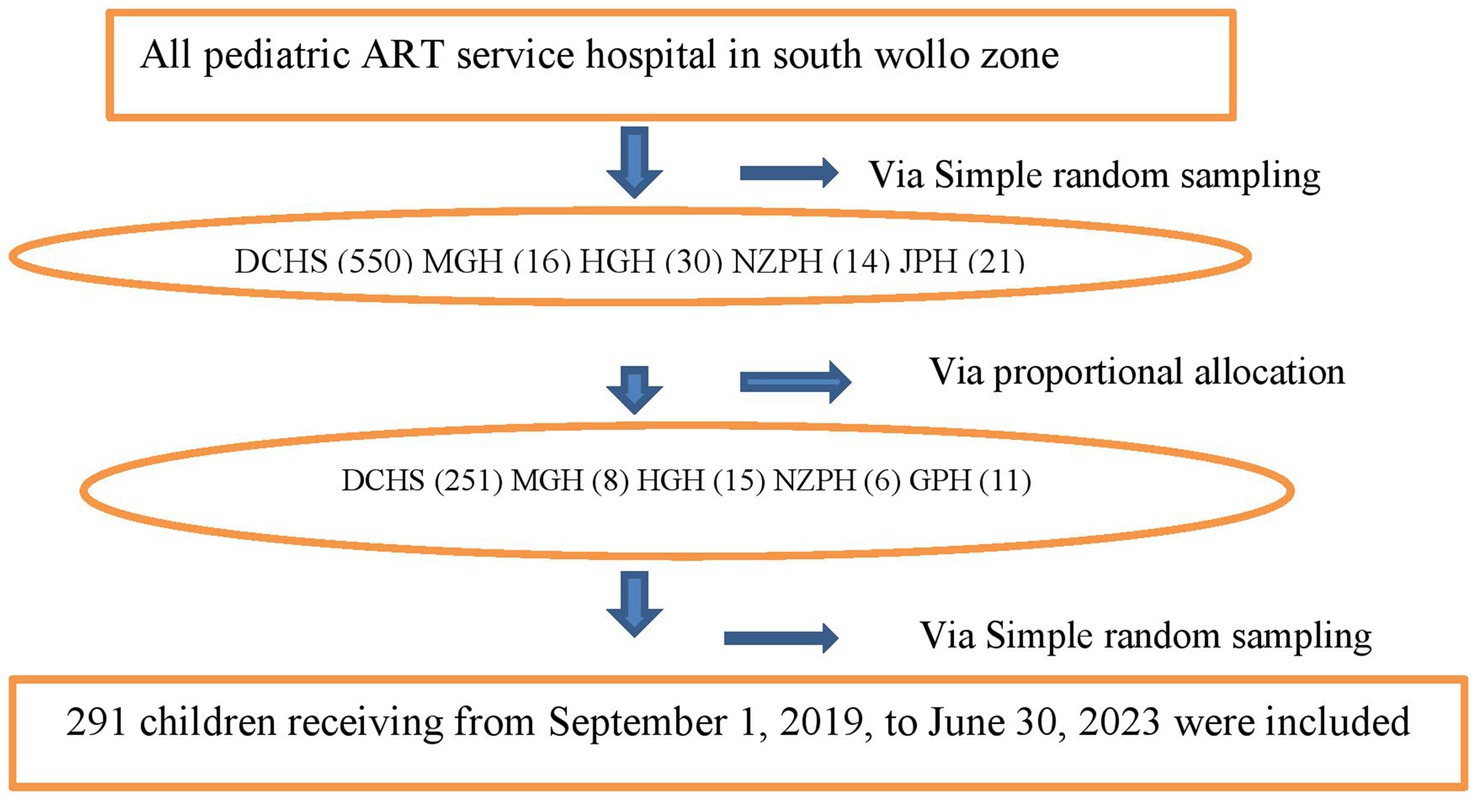
Figure 1. Schematic diagram of sampling procedure among children on antiretroviral therapy at South Woll zone public Hospitals. Dessie Comprehensive Specialized Hospital (DCHS), Hadar 11 General Hospital (HGH), Mekan Selam General Hospital (MGH), Negste Zewditw Primary Hospital (NZPH), and Jamma Primary Hospital (GPH).
The data collection checklist was developed using the Federal Ministry of Health’s HIV/ART follow-up and intake records. Data were collected through face-to-face interviews and reviewing patients’ documents using a structured checklist. The data extraction form includes information on socio-demographic variables, treatment and additional drugs, clinical and laboratory data, etc. Under the direction of one MSc nursing practitioner, three BSc nurses with ART service experience and training collected data for 4 weeks in July 2023. Pre-testing was performed on 13 newborns (5% of the sample population) at Dessie Compressive Specialized Hospital before the real data-gathering process.
Dependent variable: Adherence to first-line ART, which was classified as “non-adherent” or “adherent.”
Independent variables: age of the child, sex, residence, Caregiver knowledge, Caregiver marital status, Educational status, HIV disclosure Status, Weight for age, height for age, Hemoglobin (Hgb) level, CD4 counts or %, WHO clinical stages, Duration on ART, CPT (Co-trimoxazole Preventive Therapy), IPT (Isoniazid Preventive Therapy), Treatment failure, and TB status.
Level of ART adherence: the tools used to assess adherence for all participants were viral load monitoring, Self-reporting, Pill counts, and Pharmacy refill records.
To assess adherence using viral load, first measure the viral load before initiating ART to establish a baseline. This baseline helps in evaluating the treatment’s effectiveness over time. Regular viral load tests, typically conducted every 3–6 months, should be scheduled to track the amount of HIV in the blood. Consistently low viral loads suggest good adherence and effective viral suppression. An undetectable viral load usually indicates that the treatment is working well. However, if the viral load remains low but detectable, it may imply some level of adherence but might also require further evaluation or adjustment of the treatment regimen. Conversely, a high viral load often indicates poor adherence (non-adherence), possible treatment failure, or drug resistance, necessitating a review of adherence strategies and potentially a change in therapy (1, 19, 20).
A 1-Month Self-Recall Report (Visual Analogue Scale): Caregivers were asked to mark on a calibrated line scale from 1 to 10, reflecting how they administered medications to the child over the past month. The marks were converted into percentages to estimate their drug administration adherence for the entire month. Adherence was classified as follows: a mark below 8.5 (<85%) indicated poor adherence, 8.5–9.4 (85–94%) indicated fair adherence, and 9.5 or above (≥95%) indicated good adherence (21, 22).
Pill counts: counting the remaining pills in bottles during routine healthcare visits helps assess adherence (21). Adherence (%) = (Number of pills prescribed - Number of pills returned) × 100.
Pharmacy refill records: pharmacy refill records provide information on when individuals pick up their antiretroviral (ARV) drugs. Irregular pharmacy refill intervals may indicate non-adherence to antiretroviral therapy (ART). Calculation: Proportion of days covered (PDC) = (Number of days the patient had medication) ÷ (Number of days in the observation period) × 100.
Overall adherence measures combining these four adherence measures, an overall response rate of 95% or higher is considered good adherence, 85–94% is fair adherence, and below 85% is poor adherence (non-adherence) (19, 21–23).
Knowledge of caregiver on children ART adherent: caregivers who scored at or above the overall mean on the adherence knowledge assessment were categorized as having good knowledge, while those who scored below the mean were categorized as having poor knowledge (24, 25).
A CD4 count: CD4 below the threshold level was classified based on the age of the child (i.e infants CD4 < 1,500/mm3, 12–35 months <750/mm3, 36–59 months <350/mm3 and ≥ 5 years <200/mm3) (26).
Underweight or stunting was defined as weight for age Z-score < −2 SD for under-five children and BMI for age Z-score < −2 SD for older children (26).
Tuberculosis: Cases were detected using sputum or stomach aspirate microscopy, chest X-ray examination, and/or histology, following the Ethiopian Ministry of Health’s TB diagnosis guidelines (27).
ART treatment failure: when an antiretroviral regimen is unable to control HIV infection (21).
The World Health Organization (WHO) clinical staging system: categorizes HIV patients into four stages based on their immune deficiency and clinical symptoms (28).
The data were entered into Epi Data version 4.1, and STATA 17 was used for the analysis. The table and figures provide an exploration of the descriptive and summary statistics. The outcome level of ART adherence was divided into two categories: “non-adhered” and “adhered.” For each predictor variable, the bivariate logistic regression model was fitted. Furthermore, a multivariate logistic regression model was fitted for those variables with a p-value of less than 0.25 in a bivariate analysis. Adjusted odds ratios with 95% confidence intervals and p-values were employed to assess the strength of the relationship to determine statistically significant predictors. Variables with a p-value of less than 0.05 in multivariable analysis were regarded as significant predictors of poor levels of adherence. The multicollinearity assumption was assessed using a correlation matrix, confirming that all correlations were below 0.8. Hosmer-Lemeshow test data and the Omnibus assumption were also used to check the model fitness.
Approval was obtained from the Research and Ethical Review Committee of Wollo University College of Medicine and Health Sciences, with reference number CMHS/RCPGC/03/14. All hospital administrations and the ART clinic’s focal person provided permission letters. Written informed consent was obtained from caregivers willing to participate in the study. The names or identification numbers of the children were not permitted to be recorded in the data. Furthermore, the study was conducted by the ethical principles of the Declaration of Helsinki.
Of 291 participants, 286 were involved in the study and five incomplete ones were discarded. As a result, 286 children were included in the analysis, yielding a completion rate of 98.3%. The mean age of the participants was 7.8 years old (±3.64 SD), and half of the 146 (51%) children were male. More than half of the 176 (61.5%) children knew that they had HIV, and the vast majority (83.6%) lived in cities. Almost half of the 156 (54.5%) caregivers had a good understanding of the importance of ART (Table 1).
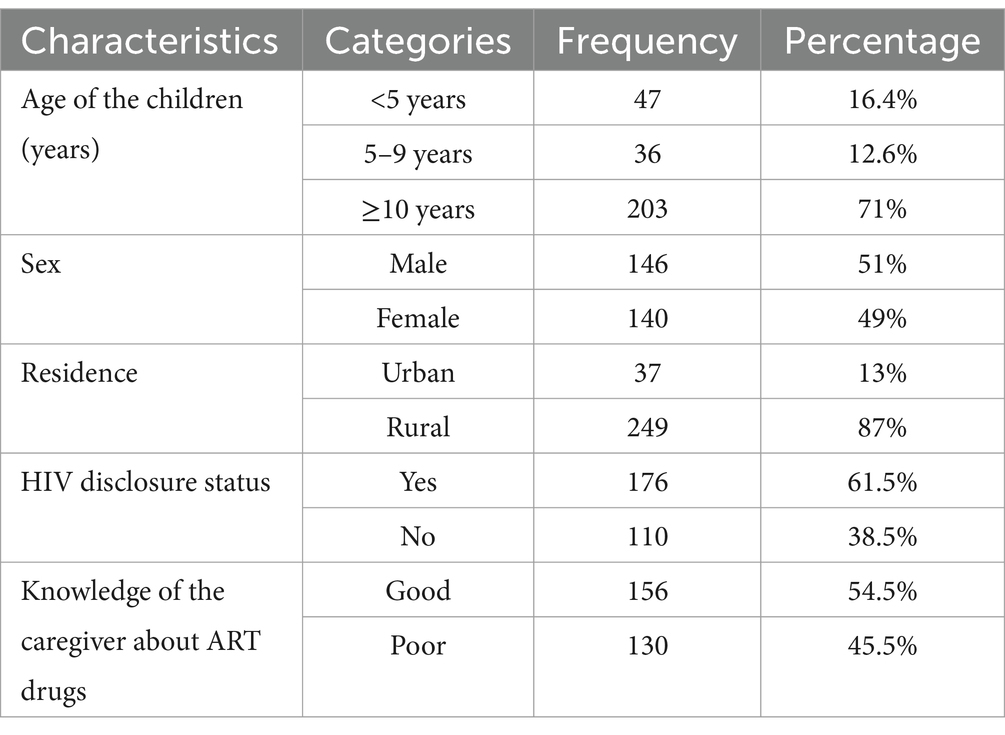
Table 1. Socio-demographic characteristics of children on antiretroviral therapy at South Wollo Zone Public hospitals, 2024.
Approximately half of the 175 children (55%) had a CD4 count or percentage above the threshold. Sixty-one percent (61%) of the participants were in WHO stages I and II, while 16.8% had TB. Regarding ART duration, 252 participants (88.1%) had been on ART for more than 34 months. Additionally, 111 participants (39%) had anemia (hemoglobin <10 mg/dL). During data collection, viral load tests were conducted for 217 children (75.8%) within the past 12 months. Of these, 59 children (20.7%) had a high viral load (≥1,000 copies/mL) (Table 2).

Table 2. Clinical and treatment-related characteristics of children on antiretroviral therapy at South Wollo Zone Public hospitals, 2024.
In this study, the overall proportion of ART non-adherence was 24.1% (95% CI: 19.2–29.0%) of the study participants.
Bivariate and multivariate logistic regressions were used to fit all variables that met the chi-square assumption. In the bivariable logistic regression analysis, age of the child, sex, residence, knowledge of the caregiver, HIV disclosure status, weight for age, height for age, HGB level, CD4 count or percentage, WHO stage, CPT user, IPT user, TB status, and duration of ART were each given a p-value of less than 0.25 and fitted into a multivariable logistic regression model. Positive TB status, diagnostic status not disclosure, and poor caregiver knowledge were found to be significantly associated with poor adherence at the multivariate level, with p-values of less than 0.05 (Table 3).
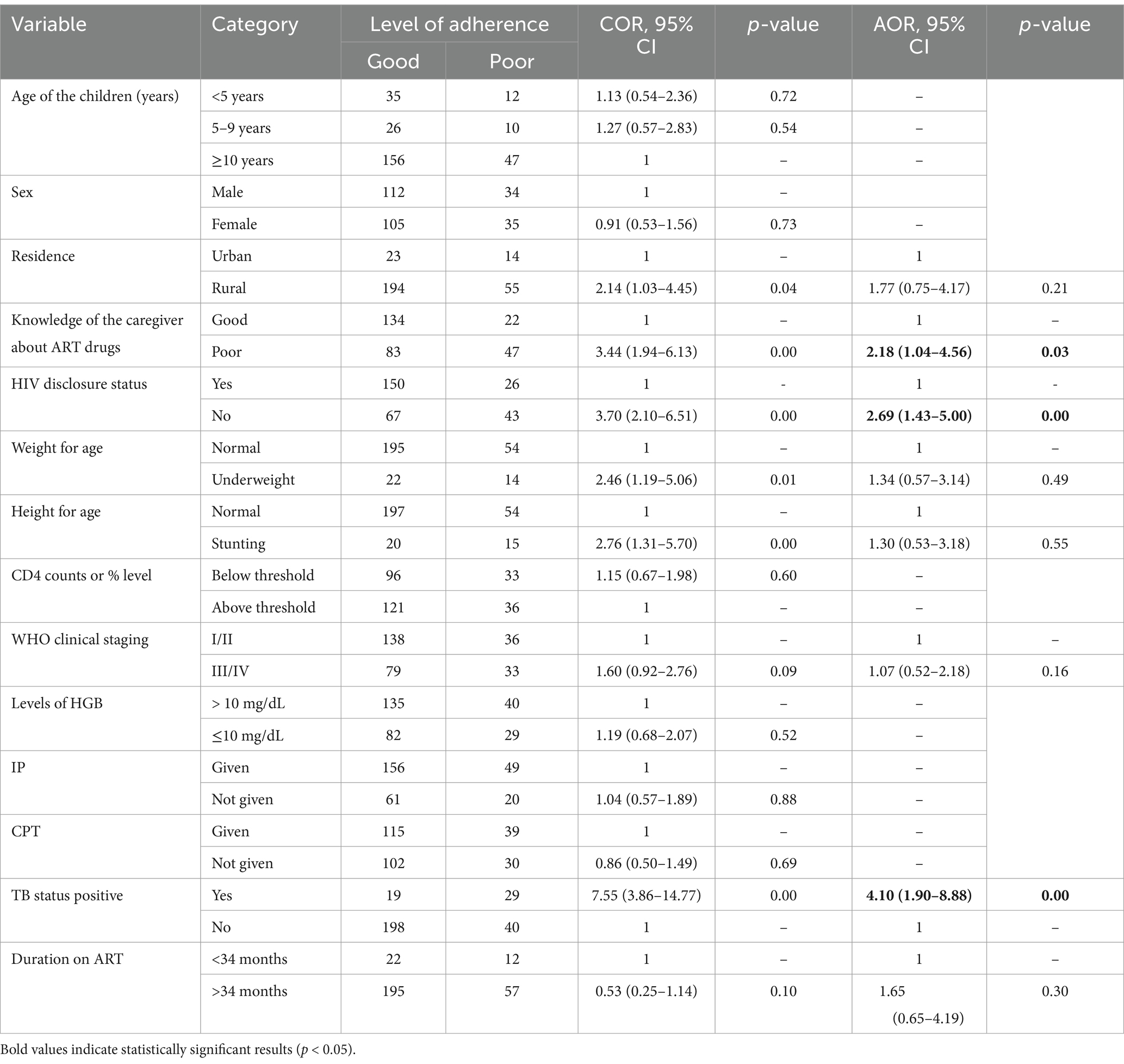
Table 3. Bivariate and multivariate analyses of factors associated with poor adherence to ART at South Wollo Zone Public Hospitals (n = 286).
Adherence is crucial for the success of antiretroviral therapy (ART), yet non-adherence remains a significant challenge, particularly among children receiving ART. This cross-sectional study aimed to assess the level of non-adherence to ART in children with HIV and identify the factors contributing to it.
According to our findings, the total proportion of ART non-adherence among the study participants was 24.1% (95% CI: 19.2 –29.0%). Furthermore, TB co-infection, HIV non-disclosure status, and poor caregiver knowledge were significantly related to poor adherence. This finding is consistent with research conducted in public hospitals in the northeast (21.4%) (11) and northwest (21.1%) (16) Ethiopia, Uganda (21%) (29), Nigeria (23.9%) (30), and Myanmar (23.8%) (31). However, this percentage is less than that of studies conducted in Jimma (36.2%) (32), Fiche Hospital (36%) (33), and Tikur Anbessa Hospital (65.2%) (10). On the other hand, this result is higher than that of research conducted in Ethiopia’s Tigray region (15.2%) (25). This disparity could be explained by the differences in ART-adherence diagnostic techniques. Furthermore, inaccurate reporting is more common in low-income nations than in middle-income countries due to a shortage of skilled healthcare providers and caretakers. This variation considered socioeconomic level, study design, adherence measurement methods, sample size, and setting differences. This variation may reflect differences in local healthcare systems, patient demographics, or methodologies. Higher adherence rates in some studies could be due to better support systems, more effective adherence interventions, or differing standards of care. Conversely, the lower adherence rate could be linked to unique regional challenges or disparities in healthcare resources and support. These differences highlight the need for context-specific strategies to address ART adherence and improve treatment outcomes across diverse settings.
This study result indicates that caregivers with poor knowledge about ART (antiretroviral therapy) drugs are 2.18 times more likely to experience non-adherence compared to those with better knowledge [AOR (: 95% CI) = 2.18 (1.04–4.56)]. This finding is consistent with the results of studies in the Oromia region (24), northeast Ethiopia (34), northern Ethiopia (25) and India (35). This result can be justified by the crucial role caregivers play in managing and supporting ART adherence. Caregivers with limited knowledge may struggle to understand the importance of adherence, proper medication administration, and potential side effects, which can lead to inconsistent or incorrect use of ART. This lack of knowledge can directly impact the effectiveness of the treatment and the patient’s overall health, thereby increasing the likelihood of non-adherence. Improving caregivers’ knowledge about ART is essential for enhancing treatment adherence. Caregivers with a better understanding of ART are more likely to support proper medication use and adherence, which can lead to better health outcomes for the children. Therefore, targeted education and training programs for caregivers should be a priority to reduce non-adherence and improve the effectiveness of ART.
In this study, the odds of non-adherence among children with TB/HIV co-infection were more than four times higher than those in children without TB co-infection [AOR = 4.10, 95% CI (1.90–8.88)]. This finding is similar to those of investigations conducted in northwest Ethiopia (16), Nigeria (30), and Peru (36). The reason for this occurrence could be attributed to the administration of medication for the confection, therapy, and antiretroviral therapy in combination, which may lead to an increase in the number of pills required to be taken, interactions between drugs, and an increasing rise in the occurrence of unfavorable drug reactions. These factors collectively can hinder adherence to antiretroviral drugs (16). The increased risk of non-adherence in children with TB/HIV co-infection underscores the need for specialized support and management strategies. The interaction between treatments and the added burden of co-infection can significantly affect adherence. Targeted interventions and integrated care are crucial for improving adherence and overall treatment outcomes in these children.
This study found that children unaware of their HIV status (AOR = 2.69, 95% CI: 1.43–5.00) were more likely to exhibit poor adherence compared to those who were aware of their status. Similar findings have been reported in other studies (17, 18, 37, 38). Children who do not know their HIV status may lack understanding of the importance of their treatment regimen, which can result in lower motivation to adhere to prescribed ART. Awareness of their condition often encourages better adherence as it fosters a clearer understanding of the necessity of consistent medication for their health. This result aligns with similar studies, reinforcing the need for HIV status disclosure and education to improve adherence outcomes in pediatric patients (39). This result implies that HIV status disclosure is crucial for improving ART adherence in children.
Limitations of the study were we did not account for variables related to household dynamics, such as the quality of the caregiver-child relationship, instances of violence and maltreatment, or health system-related factors. Additionally, factors such as the mode of HIV transmission (vertical or acquired), alcohol abuse, depression, caregiver and patient education levels, the caregiver-patient relationship, age at first diagnosis, and parental HIV status were not considered, which may have influenced the results. Another limitation is that caregivers may inaccurately recall adherence behaviors or knowledge. Since the study was conducted in a specific region, the findings may not apply to other areas with differing healthcare systems, resources, or cultural contexts.
According to the current study, the level of non-adherence to antiretroviral therapy remains high compared to the targets set by the United Nations Joint Program on HIV/AIDS (UNAIDS) Project 95–95-95. TB status-positive (co-infection), diagnostic status not disclosed, and poor caregiver knowledge were discovered to be significantly related to poor adherence. Implement targeted education programs to improve caregiver knowledge about ART and its importance. Develop strategies to encourage and support the disclosure of diagnostic status to ensure better adherence. Strengthen integrated care approaches for children with TB/HIV co-infection to address the unique challenges they face in adhering to treatment. These measures can help improve adherence rates and overall health outcomes for affected children.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Wollo University and a permission letter was taken from South Wollo zone health office. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the minors’ legal guardians/next of kin.
EW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. MA: Formal analysis, Investigation, Methodology, Software, Supervision, Writing – review & editing. SM: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. GB: Conceptualization, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing. BD: Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing – review & editing. MZ: Conceptualization, Data curation, Formal analysis, Project administration, Validation, Writing – review & editing. FA: Conceptualization, Investigation, Methodology, Software, Supervision, Writing – review & editing. EC: Formal analysis, Resources, Supervision, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to express our heartfelt gratitude and admiration to the administrative bodies and healthcare providers in each hospital’s ART clinics, data collectors, and study participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. HIV and AIDS. (2023). Available at: https://www.who.int/news-room/fact-sheets/detail/hiv-aids?gad_source=1&gclid=CjwKCAjw4_K0BhBsEiwAfVVZ_-3ETu8iy6kBBPNHgRquo86fV2EQaei_a08vJdBTOyZeuMeK57Cm5xoCZksQAvD_BwE.
2. WHO. Available at: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment/treatment-and-care-in-children-and-adolescents.
3. UNICEF. Children, HIV and AIDS: The world today and in 2030. (2019). Available at: https://data.unicef.org/resources/children-hiv-and-aids-2030/
4. Lulseged, S. Pediatric HIV epidemic: status and prospects in Ethiopia. Ethiopian journal of pediatrics and child. Health. (2023) 18:1–4. doi: 10.4314/ejpch.v18i1.1
5. Pegurri, E, Konings, E, Crandall, B, Haile-Selassie, H, Matinhure, N, Naamara, W, et al. The missed HIV-positive children of Ethiopia. PLoS One. (2015) 10:e0124041. doi: 10.1371/journal.pone.0124041
6. Unge, C, Södergård, B, Marrone, G, Thorson, A, Lukhwaro, A, Carter, J, et al. Long-term adherence to antiretroviral treatment and program drop-out in a high-risk urban setting in sub-Saharan Africa: a prospective cohort study. PLoS One. (2010) 5:e13613. doi: 10.1371/journal.pone.0013613
7. Starace, F, Massa, A, Amico, KR, and Fisher, JD. Adherence to antiretroviral therapy: an empirical test of the information-motivation-behavioral skills model. Health Psychol. (2006) 25:153–62. doi: 10.1037/0278-6133.25.2.153
8. AIDSinfo. Adherence to antiretroviral therapy in HIV-infected children and adolescents. (2021). Available at: https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv/adherence-antiretroviral-therapy-hiv-infected-children-and-adolescents.
9. CDC. CfDCaP. HIV treatment: How to adhere to antiretroviral therapy. (2021). Available at: https://search.cdc.gov/search/?query=cfdnapt.%20hiv%20treatment%3A%20how%20to%20adverse%20antiretroviral%20therapy.%202021.&dpage=1
10. Biressaw, S, Abegaz, WE, Abebe, M, Taye, WA, and Belay, M. Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers’ report. BMC Pediatr. (2013) 13:1–9. doi: 10.1186/1471-2431-13-132
11. Ketema, AK, and Shewangizaw, WZ. Assessment of adherence to highly active antiretroviral therapy and associated factors among people living with HIV at Debrebrihan referral hospital and health center, Northeast Ethiopia: a cross-sectional study. HIV AIDS (Auckl). (2015) 7:75–81. doi: 10.2147/HIV.S79328
12. Morowatisharifabad, MA, Movahed, E, Farokhzadian, J, Nikooie, R, Hosseinzadeh, M, Askarishahi, M, et al. Antiretroviral therapy adherence and its determinant factors among people living with HIV/AIDS: a case study in Iran. BMC Res Notes. (2019) 12:1–5. doi: 10.1186/s13104-019-4204-5
13. Nozaki, I, Dube, C, Kakimoto, K, Yamada, N, and Simpungwe, JB. Social factors affecting ART adherence in rural settings in Zambia. AIDS Care. (2011) 23:831–8. doi: 10.1080/09540121.2010.542121
14. Prendergast, A, Tudor-Williams, G, Jeena, P, Burchett, S, and Goulder, P. International perspectives, progress, and future challenges of paediatric HIV infection. Lancet. (2007) 370:68–80. doi: 10.1016/S0140-6736(07)61051-4
15. Davies, M-A, Boulle, A, Fakir, T, Nuttall, J, and Eley, B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatr. (2008) 8:1–12. doi: 10.1186/1471-2431-8-34
16. GebreEyesus, F, Mitku, D, Tarekegn, T, Temere, B, Terefe, T, Belete, A, et al. Levels of adherence and associated factors among children on ART over time in northwest, Ethiopia: evidence from a multicenter follow-up study. HIV/AIDS (Auckland, NZ). (2021) 13:829. doi: 10.2147/HIV.S323090
17. Feyera, B, Letta, S, and Kumie, A. Level of adherence and factors associated with antiretroviral therapy among HIV infected children in selected public hospitals, Addis Ababa, Ethiopia. East African J Health Biomed. Sci. (2016) 1:23–30.
18. Mengesha, MM, Embibel, M, Gobena, T, Tunje, A, Jerene, D, and Hallström, IK. Antiretroviral therapy non-adherence among children living with HIV in Dire Dawa, eastern Ethiopia: a case-control study. BMC Pediatr. (2022) 22:653. doi: 10.1186/s12887-022-03697-1
19. HIVinfo.NIH.gov. Guidelines for the use of antiretroviral agents in pediatric HIV infection (2024). Available at: https://www.hiv.gov/.
20. Ethiopia. National CONSOLIDATED guidelines for comprehensive HIV prevention, care and treatment (2018). Available at: https://www.afro.who.int/sites/default/files/2019-04/National%20Comprehensive%20HIV%20Care%20%20Guideline%202018.pdf.
21. Ethiopia. Federal Ministry of Health. National guidelines for comprehensive HIV prevention, care, and treatment (2017). 1226. Available at: https://www.differentiatedservicedelivery.org/wp-content/uploads/POCKET-GUIDE-compressed-1.pdf
22. Mussa, FM, Massawe, HP, Bhalloo, H, Moledina, S, and Assenga, E. Magnitude and associated factors of anti-retroviral therapy adherence among children attending HIV care and treatment clinics in Dar Es Salaam, Tanzania. PLoS One. (2022) 17:e0275420. doi: 10.1371/journal.pone.0275420
23. Alemayehu, B, Etana, B, and Abebe, M. Adherence to antiretroviral therapy and associated factors among children living with HIV in east Wallaga zone public health institutions, Ethiopia. J Int Assoc Providers AIDS Care (JIAPAC). (2023) 22:23259582231215677. doi: 10.1177/23259582231215677
24. Gemechu, GB, Hebo, H, and Kura, Z. Children’s adherence to antiretroviral therapy and associated factors: multicenter cross-sectional study. HIV AIDS (Auckl). (2023) 15:423–34. doi: 10.2147/HIV.S407105
25. Tesfahunegn, TB, Berhe, N, Abraha, TH, Hintsa, S, Yohanes, G, Desta, K, et al. Adherence to antiretroviral therapy and associated factors among HIV-infected children in public health institutions of Adwa, Axum, and Shire towns of Tigray, Northern Ethiopia: A Cross-Sectional Study. HIV AIDS (Auckl). (2023) 15:217–24. doi: 10.2147/HIV.S282938
26. Organization WH. Policy brief: Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Geneva, Switzerland: World Health Organization (2017).
27. FMoH E. Guidelines for management of TB, DR-TB and leprosy in Ethiopia. Ethiopia Federal Ministry of Health Ethiopia: Addis Ababa (2018).
28. Weinberg, JL, and Kovarik, CL. The WHO clinical staging system for HIV/AIDS. AMA J Ethics. (2010) 12:202–6. doi: 10.1001/virtualmentor.2010.12.3.cprl1-1003
29. Wadunde, I, Tuhebwe, D, Ediau, M, Okure, G, Mpimbaza, A, and Wanyenze, RK. Factors associated with adherence to antiretroviral therapy among HIV infected children in Kabale district, Uganda: a cross sectional study. BMC Res Notes. (2018) 11:466. doi: 10.1186/s13104-018-3575-3
30. Ugwu, R, and Eneh, A. Factors influencing adherence to paediatric antiretroviral therapy in Portharcourt, south-South Nigeria. Pan Afr Med J. (2014) 16. doi: 10.11604/pamj.2013.16.30.1877
31. Thandar, M, Mon, AS, Boonyaleepun, S, and Laohasiriwong, W. Antiretroviral treatment adherence and associated factors among people living with HIV in developing country, Myanmar. Int J Comm Med Public Health. (2016) 3:1318–25. doi: 10.18203/2394-6040.ijcmph20161405
32. Abera, A, Fenti, B, Tesfaye, T, and Balcha, F. Factors influencing adherence to antiretroviral therapy among people living with HIV/AIDS at ART Clinic in Jimma University teaching hospital, Southwest Ethiopia. J Pharma Reports. (2015) 1:2.
33. Feyissa, A. Magnitude and associated factors of non-adherence to highly active antiretroviral therapy among children in fiche hospital, north Shewa, Ethiopia 2016. J Pharm Care Health Syst. (2017) 4
34. Arage, G, Tessema, GA, and Kassa, H. Adherence to antiretroviral therapy and its associated factors among children at south Wollo zone hospitals, Northeast Ethiopia: a cross-sectional study. BMC Public Health. (2014) 14:1–7. doi: 10.1186/1471-2458-14-365
35. Kendre, GM, Gabhale, YR, Shah, ND, Jadhav, VM, Nath, K, and Manglani, MV. Adherence to antiretroviral therapy and factors affecting adherence among paediatric HIV patients. Int J Contemp Pediatr Kendre. (2017) 4:1962–8. doi: 10.18203/2349-3291.ijcp20174154
36. Leyva-Moral, JM, Loayza-Enriquez, BK, Palmieri, PA, Guevara-Vasquez, GM, Elias-Bravo, UE, Edwards, JE, et al. Adherence to antiretroviral therapy and the associated factors among people living with HIV/AIDS in northern Peru: a cross-sectional study. AIDS Res Ther. (2019) 16:1–12. doi: 10.1186/s12981-019-0238-y
37. Amankwah-Poku, M, Klutsey, DA, and Asante, KO. Disclosure and health-related outcomes among children living with HIV and their caregivers. AIDS Res Ther. (2021) 18:1–8. doi: 10.1186/s12981-021-00337-z
38. Ammon, N, Mason, S, and Corkery, J. Factors impacting antiretroviral therapy adherence among human immunodeficiency virus–positive adolescents in sub-Saharan Africa: a systematic review. Public Health. (2018) 157:20–31. doi: 10.1016/j.puhe.2017.12.010
Keywords: non-adherence, antiretroviral therapy (ART), children, South Wollo Zone, Ethiopia
Citation: Wondifraw EB, Belege Getaneh F, Amare M, Mihret S, Biset G, Desu Tefera B, Zeleke M, Ahmed F and Chanie ES (2025) Level of non-adherence and associated factors among children on antiretroviral therapy in public hospitals of South Wollo Zone, Northeast Ethiopia. Front. Public Health. 13:1522959. doi: 10.3389/fpubh.2025.1522959
Received: 05 November 2024; Accepted: 20 January 2025;
Published: 28 February 2025.
Edited by:
Lihua Ren, Peking University, ChinaReviewed by:
Xian Zhang, Zhengzhou University, ChinaCopyright © 2025 Wondifraw, Belege Getaneh, Amare, Mihret, Biset, Desu Tefera, Zeleke, Ahmed and Chanie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Endalk Birrie Wondifraw, ZWVhYjIwMDhAZ21haWwuY29t; ZW5kYWxrYmlycmllODVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.