- 1One Health Research Group, Faculty of Health Science, Universidad de Las Americas, Quito, Ecuador
- 2Program in Occupational Safety and Health, The University of Porto, Porto, Portugal
1 Introduction
Snakebites are recognized as one of the 20 neglected tropical diseases and represent a major public health concern in tropical and subtropical regions of Africa, Asia, and Latin America. This issue is particularly acute in remote, underdeveloped, and politically marginalized areas, where access to healthcare and preventive measures is limited (1, 2). According to the World Health Organization, approximately 4.5–5.4 million snakebites occur globally each year, resulting in 2.7 million clinical cases and an estimated 81,000–138,000 fatalities (3). The most vulnerable populations include individuals engaged in agricultural, livestock, fishing, or hunting activities, those living in poorly constructed homes, children involved in labor, and people with limited access to education (3).
Snakebite envenomation can cause a broad spectrum of complications, ranging from localized to systemic effects. These, bleeding disorders, kidney failure, severe tissue destruction, skin infections, compartment syndrome, and serum sickness, among others (4, 5). In severe cases, amputations may be necessary due to extensive tissue damage.
Venomous snakebites are also associated with significant neurological complications, as the venom is a complex mixture of toxins and enzymes, including phospholipase A2, acetylcholinesterase, hyaluronidase, and metalloproteinases. Neurological complications include ophthalmoplegia, ptosis, paralysis of pharyngeal muscles, paralysis of the intercostal muscles and the diaphragm (6–8). Among the most severe neurological complications are cerebrovascular accidents, which can be of the ischemic type (9–15), or also of the hemorrhagic type (16–21). Although stroke is a rare complication of snakebites, it can lead to death or serious sequelae. Little has been said about this complication; most reports come from clinical cases, and there is only limited information about its specific clinical characteristics, the assessment of its severity, and the duration of the follow-up period (22, 23).
In cases where a stroke occurs, whether ischemic or hemorrhagic, a variety of symptoms may appear. In cases of ischemic stroke caused by blockage of blood vessels, the most common symptoms reported include hemiparesis, altered speech (slurring of speech, dysphonia, dysarthria), drowsiness, ptosis, hypertension, and tachycardia (9–15). In cases of hemorrhagic stroke resulting from bleeding within the brain, the most common symptoms include headache, hypertension, hypotension, hematemesis, hematuria, bleeding gums, conjunctival hemorrhage, seizures, and respiratory distress. cardiorespiratory arrest, acute kidney failure and coma have also been reported (16–21). In both ischemic and hemorrhagic strokes, altered state of consciousness, loss of consciousness, miosis, altered pupillary reflex, and decreased muscle strength are present. The most commonly used diagnostic tests include computed tomography and magnetic resonance imaging, which have revealed that strokes affect medium to large vessels, including the middle cerebral artery and anterior cerebral artery (6, 24).
The present article aims to describe the characteristics of this type of complication, as well as the implications and public health interventions needed to address this problem.
2 Diverse components and biological effects of snake venom
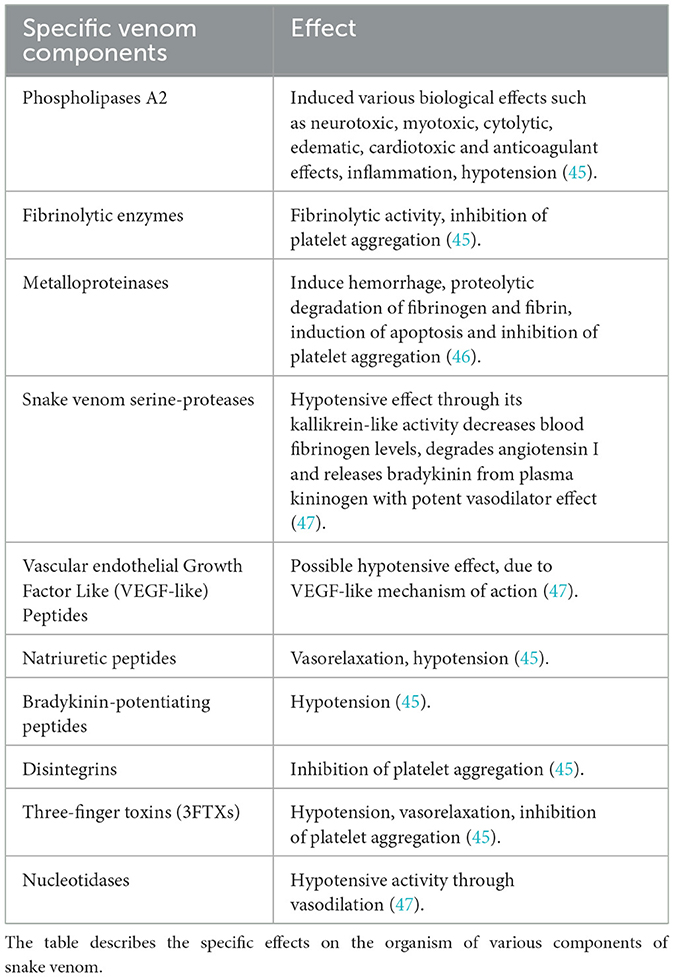
The composition of snake venom is unique to each species and consists of a heterogeneous mixture of proteins and peptides (Table 1). The four dominant protein families in its composition are phospholipase A2, snake venom metalloprotease, three-finger toxins, and snake venom serine protease; these, in turn, are the most important toxins in human envenomation, responsible for causing coagulopathy, neurotoxicity, myotoxicity, and cytotoxicity (25).
Other venom components, such as sapharotoxins and endothelins, induce vasoconstriction of coronary arteries, while certain peptides inhibit angiotensin-converting enzyme (ACE), leading to a reduction in blood pressure (26). Aminopeptidases, on the other hand, contribute to systemic hypotension by altering vascular tone and fluid balance. Meanwhile, toxins that interfere with blood coagulation can cause hemorrhages or thrombosis, depending on the specific biochemical profile of the venom. Non-enzymatic proteins, such as C-type lectins and three-finger toxins, have also been identified. These proteins exhibit anticoagulant or procoagulant activity, functioning as agonists or antagonists of platelet aggregation, further increasing the risk of vascular complications (27–31).
3 Pathophysiological mechanism of cerebrovascular accident development after snake bite
Snake venom is a complex mixture of toxins and enzymes that can cause severe systemic and neurological effects. Among its components are neurotoxic, hemotoxic, and myotoxic substances, which can significantly disrupt the blood vascular system and alter microvascular dynamics, ultimately leading to strokes through various mechanisms following envenomation (26, 30, 32).
The pathophysiology of cerebrovascular events associated with snakebites is multifactorial, involving processes such as thrombus formation, hypotension, and hemorrhage (Figure 1). Each of these mechanisms contributes to the risk of developing ischemic or hemorrhagic strokes. A comprehensive understanding of these processes is crucial for designing targeted interventions and improving clinical outcomes for affected individuals.
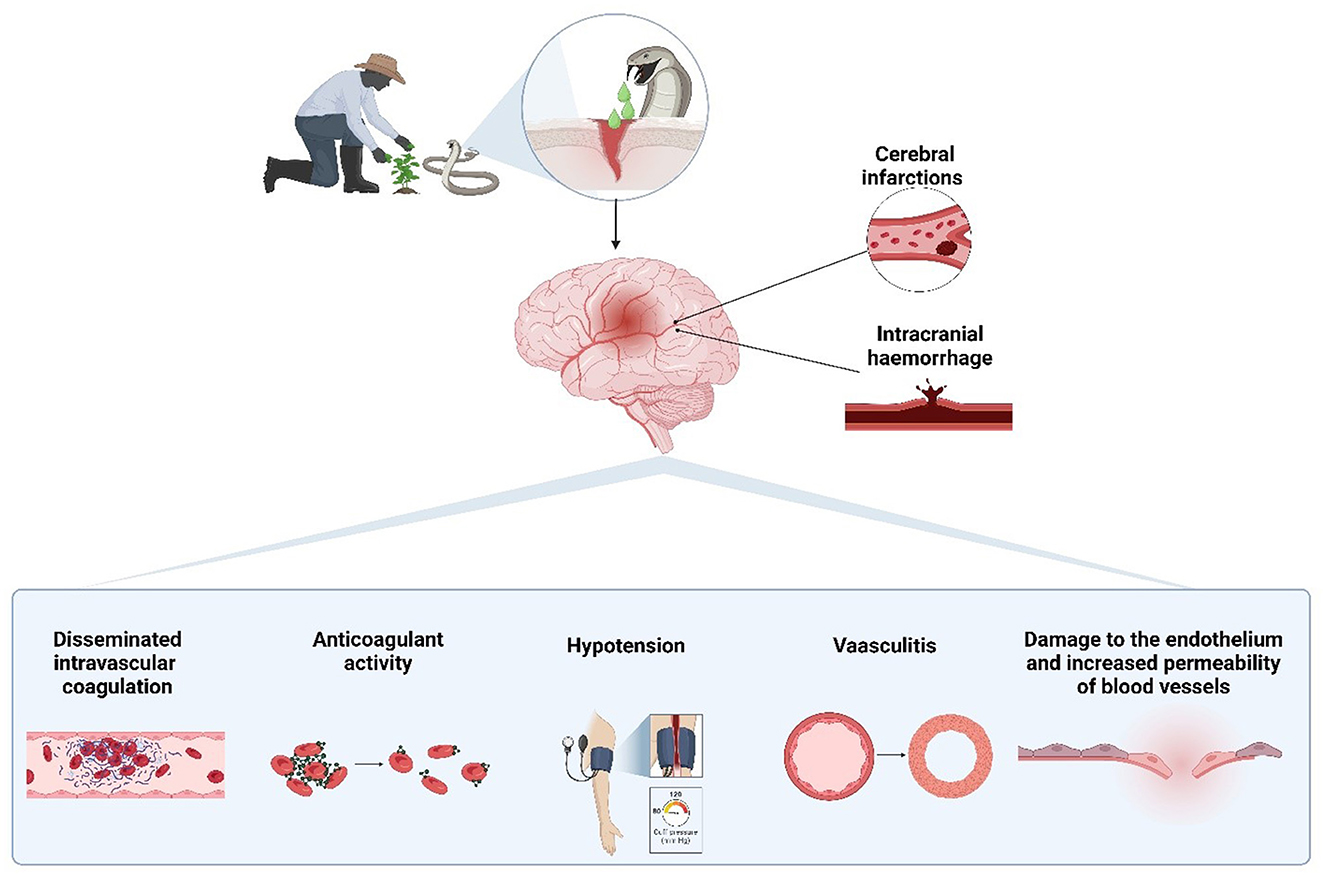
Figure 1. Pathophysiological mechanisms of cerebrovascular events induced by snakebite envenomation. Snake venom can lead to both cerebral infarctions and intracranial hemorrhages through various mechanisms, including disseminated intravascular coagulation, anticoagulant activity, hypotension, vasculitis, and endothelial damage, which increase vascular permeability. Created with BioRender.com.
High concentrations of venom can induce disseminated intravascular coagulation (DIC), leading to the formation of thrombi that can block blood vessels. Additionally, direct damage to the vascular endothelium by venom can cause vasculitis, resulting in thrombosis. Venom's cardiotoxic effects may also promote cardiac thromboembolism, while hyperviscosity due to hypovolemia and hypoperfusion exacerbates the risk of vascular occlusion (33, 34). All these processes lead to the formation of thrombi which can cause an ischemic stroke. For its part phospholipase A2 isoforms present in venom can cause significant vasodilation, leading to hypovolemia from sweating, vomiting, and reduced fluid intake. This can result in hypotension, which in turn may cause watershed infarction, a type of stroke occurring in the regions of the brain that are most susceptible to low blood flow (6, 34).
Hemorrhagins, another venom component, damage endothelial cells and increase blood vessel permeability, potentially leading to spontaneous intracerebral bleeding. The risk of hemorrhage is further heightened by venom-induced thrombocytopenia, as well as the prolongation of prothrombin and partial thromboplastin times, which impair the blood's ability to clot and favor bleeding (4, 35, 36).
4 Coagulopathy and its role in bleeding and vascular rupture
Snake venom-induced coagulopathy is a critical factor contributing to severe bleeding and vascular complications following envenomation. Certain venom components, such as metalloproteinases and C-type lectins, disrupt normal coagulation pathways by interfering with platelet aggregation and degrading fibrinogen, a key protein in blood clot formation (29). This dysregulation can lead to disseminated intravascular coagulation (DIC), characterized by widespread clot formation that depletes clotting factors and platelets, ultimately resulting in a paradoxical increase in bleeding (33, 34). Additionally, venom-induced thrombocytopenia further reduces the blood's clotting ability, while the degradation of endothelial integrity by hemorrhagins weakens blood vessels. These combined effects can culminate in vascular rupture and spontaneous intracerebral bleeding, posing life-threatening risks to affected individuals. Understanding the mechanisms underlying coagulopathy is essential for developing therapeutic strategies to mitigate these complications (37).
5 Epidemiology of snakebite-related strokes
Strokes are a serious but often underreported complication of snakebites. The true incidence of stroke following snakebites remains largely unknown due to significant underreporting and limitations in existing data. Most available information comes from case reports and small case series, making it difficult to accurately assess the true burden of this complication. A 1991 study in Ecuador found that 5.1% of 294 patients who suffered snakebites developed intracranial hemorrhages, although this study was limited by its reliance on clinical diagnosis without imaging studies (38). A more recent Ecuadorian study from 2003 reported that 2.6% of 309 patients bitten by Bothrops snakes developed cerebrovascular events, with CT scans confirming the presence of intracranial hemorrhages and cerebral infarcts (35). In Sri Lanka, a 2007 case series involving 500 patients bitten by Daboia russelii found that nine developed ischemic strokes (6). A scoping review further highlighted that among stroke cases following snakebite, 77.1% were ischemic strokes, 20.5% were intracranial hemorrhages, and 2.4% were infarct-like hemorrhages (24).
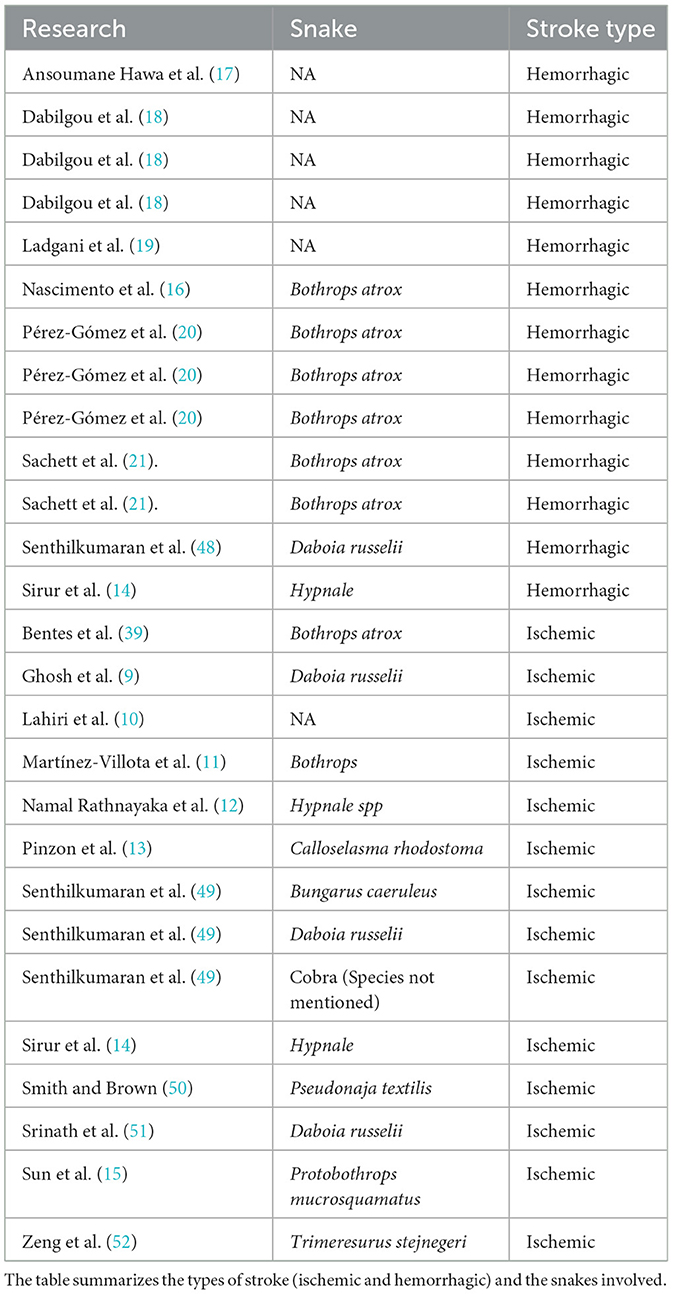
Data from the last 5 years (2019–2024) reveals 23 reported cases of stroke caused by snakebites, including 12 hemorrhagic strokes and 11 ischemic strokes. Most of these cases occurred in the male population, with the majority caused by snakes of the genus Bothrops (Table 2). The lack of studies on this topic is a major problem, as the absence of data makes it difficult to determine the magnitude of the issue, identify the most affected areas, and develop programs or strategies for its control. For this reason, it is crucial to emphasize the importance of conducting large-scale studies to obtain more robust data on all the regions with a high incidence of snake bites.
6 Risk factors for the development of stroke after snake bite
There is no conclusive information on the risk factors that predispose individuals to the development of stroke after sepsis; a case series conducted in 2019, in which 83 cases were reported, mentions that the majority of cases involved patients under 50 years of age who did not present comorbidities or risk factors for hemorrhagic or ischemic stroke. Only 2% of patients had a history of underlying comorbidities that could be potential risk factors, such as diabetes mellitus or hypertension (24). Another case series indicates that the appearance of confusion, mild reduction in the Glasgow coma scale, or hemiparesis should be followed by additional investigation with brain imaging (6).
Regarding the type of snakes, ischemic strokes are more frequent after envenomation by snakes of the Viperidae family. Despite this, it has been observed that Bothrops snakes are more prone to developing hemorrhagic strokes; however, it is important to mention that this also depends on the specific species of snake. In cases of Bothrops atrox envenomation, up to 12.8% report hemorrhagic cerebrovascular alterations. Bothrops lanceolatus and Bothrops caribbaeus are typically associated with cases of ischemic strokes (39). Likewise, poisoning by Daboia russelii snakes is found to be related to the development of ischemic strokes (6).
7 Public health implications and interventions for snakebite-related complications
The public health implications of snakebites extend far beyond the immediate medical consequences. While well-known complications such as amputations are concerning, cerebrovascular events (such as strokes) induced by snake venom can result in long-term disabilities with severe health and socioeconomic repercussions. Stroke, in particular, leaves patients with debilitating sequelae that increase their dependence on the healthcare system. Snakebite victims are often located in remote areas with limited access to healthcare, which exacerbates the challenges of providing timely treatment and rehabilitation. This geographical isolation leads to delayed treatment, resulting in poorer prognoses. Patients with a poor prognosis will likely have diminished ability to work, reduced capacity to care for their families, and increased reliance on medical treatments such as pharmaceuticals, further inflating healthcare costs.
Given the substantial burden that snakebite complications impose on affected individuals and communities, targeted public health interventions are crucial:
- Enhance surveillance systems: Improve the reporting infrastructure to gather detailed data, including the species involved and the location of incidents, ensuring consistency and better preparedness in public health responses (40).
- Implement preventive campaigns: Launch education initiatives in high-risk areas, emphasizing preventive measures like using flashlights at night, avoiding walking barefoot, and wearing protective clothing while engaging in agricultural work. These campaigns should also focus on reducing snake habitats near homes by eliminating rodents and clutter (30).
- Educational outreach in rural areas: Educate communities, particularly in rural and remote areas, on the benefits of prompt medical intervention post-snakebite. In many regions, reliance on traditional medicine due to cultural beliefs or lack of healthcare access delays critical care, worsening outcomes. Educational outreach can help bridge this gap and reduce the time to treatment (41–43).
- Improve access to antivenom and medicines: Address the lack of access to essential treatments, particularly antivenoms. Ensuring a reliable supply of antivenoms is vital, as shortages, distribution inefficiencies, and dependence on imports exacerbate the health risks in regions where snakebites are prevalent. Efforts should focus on promoting local production of antivenom suited to regional snake species and improving the distribution system to reach remote areas (41, 44).
- Evaluate healthcare capabilities: Continuously assess the availability and effectiveness of healthcare services in treating snakebite complications, including stroke. This includes providing adequate diagnostic tools, replacement therapies, and mechanical ventilators. Training healthcare workers on the best practices for treating snakebite-related cerebrovascular events is crucial to improving outcomes (40, 41).
- Public health investment: Encourage investment from both government and private entities to fund the development of affordable and effective antivenoms that are widely accessible. This will help alleviate the financial burden on health systems and ensure that treatments are available in underserved regions (42).
- Research and development: Promote research aimed at understanding the pathophysiological mechanisms that lead to cerebrovascular events following snakebites. This research is critical for developing specific therapeutic interventions that can reduce the long-term impacts of snakebite-related strokes (24).
The combination of long-term health effects, such as reduced productivity and increased healthcare costs, makes snakebites not only a medical issue but a significant public health concern. Addressing the lack of resources, such as antivenoms, medicines, and medical infrastructure, particularly in remote areas, is essential for mitigating the widespread impacts of snakebites. By focusing on prevention, timely access to treatment, and ongoing research, we can significantly reduce the burden of snakebites on individuals and communities.
8 Conclusion
Snakebites represent not only a clinical emergency but also significant public and global health challenges, particularly in remote and underserved regions where access to healthcare is limited. Beyond the immediate physical complications, the long-term effects of cerebrovascular events, such as strokes, lead to severe disabilities, reduced economic productivity, and increased dependence on healthcare systems. These consequences underscore the need for integrated public health strategies, including improving healthcare infrastructure in rural areas, addressing shortages of essential medicines such as antivenoms, and raising public awareness through preventive campaigns. By addressing these issues from a public health perspective, the long-term socioeconomic and health impacts of snakebites can be mitigated, improving the quality of life for affected populations and reducing the global burden of neglected tropical diseases.
The reported incidence of snakebite-induced strokes in Sri Lanka, India, and Ecuador highlights the need for targeted public health interventions in these affected areas. It also emphasizes the necessity of epidemiological studies in regions with a high incidence of snakebites to better understand the occurrence of snakebite-induced strokes and identify risk factors contributing to their development. These interventions should focus on awareness, education, and management, including improved access to antivenoms and better strategies for stroke prevention and management. Finally, further research is essential to understand the specific mechanisms of snake venom-induced strokes and identify the venom components of the primary snake species involved in these events. This will help develop more effective antidotes and treatments tailored to each case.
Author contributions
JV-G: Conceptualization, Investigation, Methodology, Writing – original draft. MN-L: Investigation, Writing – original draft. EO-P: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Universidad de las Américas, Quito, Ecuador, through salary funding and resources provided for the development of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Calvopiña M, Guamán-Charco E, Ramírez K, Dávalos F, Chiliquinga P, Villa-Soxo S, et al. Epidemiología y características clínicas de las mordeduras de serpientes venenosas en el norte de la Amazonía del Ecuador (2017-2021). Biomédica. (2023) 43:93–107. doi: 10.7705/biomedica.6587
2. Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl Trop Dis. (2019) 13:e0007059. doi: 10.1371/journal.pntd.0007059
3. WHO. World Health Organization. Snakebite (2024). Available at: https://www.who.int/health-topics/snakebite (accessed September 19, 2024).
4. Pereda Cardoso O, Peña Atrio GA, Ayala Chinea AP. Mordeduras de serpientes. Rev Cuba Ortop Traumatol. (2007) 21:0–0.
5. Meyers SE, Tadi P. Snake toxicity. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2024). Available from: http://www.ncbi.nlm.nih.gov/books/NBK557565/ (accessed September 19, 2024).
6. Gawarammana I, Mendis S, Jeganathan K. Acute ischemic strokes due to bites by Daboia russelii in Sri Lanka – first authenticated case series. Toxicon. (2009) 54:421–8. doi: 10.1016/j.toxicon.2009.05.006
7. Mehta S, Sashindran V. Clinical features and management of snake bite. Med J Armed Forces India. (2002) 58:247–9. doi: 10.1016/S0377-1237(02)80140-X
8. Vinod KV, Dutta TK. Snakebite, dysautonomia and central nervous system signs. QJM Int J Med. (2013) 106:865–6. doi: 10.1093/qjmed/hct096
9. Ghosh R, León-Ruiz M, Roy D, Naga D, Sardar SS, Benito-León J. Cerebral venous sinus thrombosis following Russell's viper (Daboia russelii) envenomation: a case report and review of the literature. Toxicon. (2022) 218:8–12. doi: 10.1016/j.toxicon.2022.08.014
10. Lahiri D, Sawale VM, Dubey S, Roy BK, Das SK. Status epilepticus and bilateral middle cerebral artery infarction: a rare presentation after viper bite. Ann Afr Med. (2019) 18:111–4. doi: 10.4103/aam.aam_21_18
11. Martínez-Villota VA, Mera-Martínez PF, Portillo-Miño JD, Massive acute ischemic stroke after Bothrops spp. envenomation in southwestern Colombia: case report and literature review. Biomédica. (2022) 42:09–17. doi: 10.7705/biomedica.6114
12. Namal Rathnayaka RMMK, Nishanthi Ranathunga PEA, Kularatne SAM, Jayasinghe S. Acute ischemic stroke: a rare complication of hump-nosed pit viper (Hypnale spp.) bite: a case report. J Med Case Rep. (2022) 16:218. doi: 10.1186/s13256-022-03442-3
13. Pinzon RT, Antonius RA, Veronica V. Ischemic stroke following Calloselasma rhodostoma snakebite: a rare case report. Open Access Emerg Med. (2022) 14:35–9. doi: 10.2147/OAEM.S352865
14. Sirur FM, Balakrishnan JM, Lath V. Hump-nosed pit viper envenomation in Western Coastal India: a case series. Wilderness Environ Med. (2022) 33:399–405. doi: 10.1016/j.wem.2022.08.006
15. Sun I, Lee ST, Chen YG, Mao YC, Chen FC, Chen YH, et al. Thromboembolic events following a pit viper bite from Protobothrops mucrosquamatus (Taiwan Habu): a report of two cases. Toxicon. (2024) 238:107572. doi: 10.1016/j.toxicon.2023.107572
16. Nascimento TP, Gomes TA, Costa BJC, Carvalho E, Cunha AB, Pereira BL, et al. Long-term hospital care needs after Bothrops atrox envenomation with hemorrhagic stroke in the Brazilian Amazon: ‘From social to physical death' – a case report. Toxicon. (2024) 241:107682. doi: 10.1016/j.toxicon.2024.107682
17. Ansoumane Hawa K, Mhaili J, Boutakioute B, Ouali Idrissi M, Idrissi El Ganouni N. Hemorrhagic stroke revealing a snake bite: a case report. Cureus. (2022) 14:e20935. doi: 10.7759/cureus.20935
18. Dabilgou AA, Sondo A, Dravé A, Diallo I, Kyelem JMA, Napon C, et al. Hemorrhagic stroke following snake bite in Burkina Faso (West Africa). A case series. Trop Dis Travel Med Vaccines. (2021) 7:25. doi: 10.1186/s40794-021-00150-6
19. Lagdani M, Hayate E, Manel R, Taoufik AH, Hicham N. Disseminated intravascular coagulation and ischemic stroke due to snake bites: about one case. Int J Innov Res Med Sci. (2024) 9:40–1. doi: 10.23958/ijirms/vol09-i01/1799
20. Pérez-Gómez AS, Monteiro WM, João GAP, Sousa JD de B, Safe IP, Damian MM, et al. Hemorrhagic stroke following viper bites and delayed antivenom administration: three case reports from the Western Brazilian Amazon. Rev Soc Bras Med Trop. (2019) 52:e20190115. doi: 10.1590/0037-8682-0115-2019
21. Sachett J de AG, da Mota da Silva A, Dantas AWCB, Dantas TR, Colombini M, Moura da Silva AM, et al. Cerebrovascular accidents related to snakebites in the amazon—two case reports wilderness. Environ Med. (2020) 31:337–43. doi: 10.1016/j.wem.2020.04.009
22. Hung WH, Sung J, Chen WY, Chiu LT, Yip HT, Wei JCC, et al. Risk of stroke with antivenom usage after venomous snakebite in Taiwan: a population-based cohort study. QJM Int J Med. (2022) 115:587–95. doi: 10.1093/qjmed/hcab259
23. Kim JS, Yang JW, Kim MS, Han ST, Kim BR, Shin MS, et al. Coagulopathy in patients who experience snakebite. Korean J Intern Med. (2008) 23:94–9. doi: 10.3904/kjim.2008.23.2.94
24. Al-Sadawi M, Mohamadpour M, Zhyvotovska A, Ahmed T, Schechter J, Soliman Y, et al. Cerebrovascular accident and snake envenomation: a scoping study. Int J Clin Res Trials. (2019) 2019:7. doi: 10.15344/2456-8007/2019/133
25. Tasoulis T, Isbister GK. A current perspective on snake venom composition and constituent protein families. Arch Toxicol. (2023) 97:133–53. doi: 10.1007/s00204-022-03420-0
26. Gasanov SE, Dagda RK, Rael ED. Snake venom cytotoxins, phospholipase A2s, and Zn2+-dependent metalloproteinases: mechanisms of action and pharmacological relevance. J Clin Toxicol. (2014) 4:1000181. doi: 10.4172/2161-0495.1000181
27. Kochva E, Bdolah A, Wollberg Z. Sarafotoxins and endothelins: evolution, structure and function. Toxicon. (1993) 31:541–68. doi: 10.1016/0041-0101(93)90111-U
28. Vaiyapuri S, Wagstaff SC, Watson KA, Harrison RA, Gibbins JM, Hutchinson EG. Purification and functional characterisation of rhiminopeptidase A, a novel aminopeptidase from the venom of Bitis gabonica rhinoceros. PLoS Negl Trop Dis. (2010) 4:e796. doi: 10.1371/journal.pntd.0000796
29. Doley R, Kini RM. Protein complexes in snake venom. Cell Mol Life Sci. (2009) 66:2851–71. doi: 10.1007/s00018-009-0050-2
30. Del Brutto OH, Del Brutto VJ. Neurological complications of venomous snake bites: a review. Acta Neurol Scand. (2012) 125:363–72. doi: 10.1111/j.1600-0404.2011.01593.x
31. Joseph R, Pahari S, Hodgson WC, Kini RM. Hypotensive agents from snake venoms. Curr Drug Targets Cardiovasc Haematol Disord. (2004) 4:437–59. doi: 10.2174/1568006043335808
32. Osipov A, Utkin Y. What are the neurotoxins in hemotoxic snake venoms? Int J Mol Sci. (2023) 24:2919. doi: 10.3390/ijms24032919
33. Pothukuchi V, Chepuri V, Natta K, Madigani N, Kumar A. A rare case report of Russell's viper snakebite with ischemic stroke. Hong Kong J Emerg Med. (2018) 25:95–7. doi: 10.1177/1024907917735071
34. Paul G, Paul BS, Puri S. Snake bite and stroke: our experience of two cases. Indian J Crit Care Med. (2014) 18:257–8. doi: 10.4103/0972-5229.130585
35. Mosquera A, Idrovo LA, Tafur A, Del Brutto OH. Stroke following Bothrops spp. snakebite. Neurology. (2003) 60:1577–80. doi: 10.1212/01.WNL.0000061614.52580.A1
36. Ethiraj D, Senthilkumar SSV, Bagri N. Intracranial subarachnoid hemorrhage in snakebite. Turk J Neurol. (2024) 30:122–4. doi: 10.4274/tnd.2023.34976
37. Larréché S, Chippaux JP, Chevillard L, Mathé S, Résière D, Siguret V, et al. Bleeding and thrombosis: insights into pathophysiology of Bothrops venom-related hemostasis disorders. Int J Mol Sci. (2021) 22:9643. doi: 10.3390/ijms22179643
38. Kerrigan KR. Venomous snakebite in eastern Ecuador. Am J Trop Med Hyg. (1991) 44:93–9. doi: 10.4269/ajtmh.1991.44.93
39. Bentes KO, de Amorim RLO, Barbosa FBA, Ratis da Silva VCP, Valente J, Almeida-Val F, et al. Long-term disability after cerebral ischemic stroke following a Bothrops atrox snakebite in the Brazilian Amazon. Toxicon. (2024) 247:107793. doi: 10.1016/j.toxicon.2024.107793
40. Ochoa-Avilés A, Heredia-Andino OS, Escandón SA, Celorio-Carvajal CA, Arias-Peláez MC, Zaruma-Torres F, et al. Viperidae snakebites in Ecuador: a review of epidemiological and ecological aspects. Toxicon X. (2020) 7:100051. doi: 10.1016/j.toxcx.2020.100051
41. Chippaux JP, Massougbodji A, Habib AG. The WHO strategy for prevention and control of snakebite envenoming: a sub-Saharan Africa plan. J Venom Anim Toxins Trop Dis. (2019) 25:e20190083. doi: 10.1590/1678-9199-jvatitd-2019-0083
42. Russell JJ, Schoenbrunner A, Janis JE. Snake bite management: a scoping review of the literature. Plast Reconstr Surg Glob Open. (2021) 9:e3506. doi: 10.1097/GOX.0000000000003506
43. Sloan DJ, Dedicoat MJ, Lalloo DG. Healthcare-seeking behaviour and use of traditional healers after snakebite in Hlabisa sub-district, KwaZulu Natal. Trop Med Int Health. (2007) 12:1386–90. doi: 10.1111/j.1365-3156.2007.01924.x
44. Ortiz-Prado E, Yeager J, Andrade F, Schiavi-Guzman C, Abedrabbo-Figueroa P, Terán E, et al. Snake antivenom production in Ecuador: Poor implementation, and an unplanned cessation leads to a call for a renaissance. Toxicon. (2021) 202:90–7. doi: 10.1016/j.toxicon.2021.09.014
45. Frangieh J, Rima M, Fajloun Z, Henrion D, Sabatier JM, Legros C, et al. Snake venom components: tools and cures to target cardiovascular diseases. Molecules. (2021) 26:2223. doi: 10.3390/molecules26082223
46. Olaoba OT, Karina dos Santos P, Selistre-de-Araujo HS, Ferreira de Souza DH. Snake venom metalloproteinases (SVMPs): a structure-function update. Toxicon X. (2020) 7:100052. doi: 10.1016/j.toxcx.2020.100052
47. Péterfi O, Boda F, Szabó Z, Ferencz E, Bába L. hypotensive snake venom components—a mini-review. Molecules. (2019) 24:2778. doi: 10.3390/molecules24152778
48. Senthilkumaran S, Almeida JR, Williams J, Williams HF, Thirumalaikolundusubramanian P, Patel K, et al. Rapid identification of bilateral adrenal and pituitary haemorrhages induced by Russell's viper envenomation results in positive patient outcome. Toxicon. (2023) 225:107068. doi: 10.1016/j.toxicon.2023.107068
49. Senthilkumaran S, Williams J, Almeida JR, Williams HF, Patel K, Thirumalaikolundusubramanian P, et al. Snakebite-induced reversible cerebral vasoconstriction syndrome: Report of three cases. Toxicon. (2024) 251:108161. doi: 10.1016/j.toxicon.2024.108161
50. Smith H, Brown D. Multiple thromboembolic strokes in a toddler associated with Australian Eastern Brown snake envenomation. Radiol Case Rep. (2019) 14:1052–5. doi: 10.1016/j.radcr.2019.05.034
51. Srinath KM, Sethi M, Madhu B, Prasad MC, Sangappa SB. Serpent strikes, sapien sways: a rare case of bilateral cerebellar infarct following viper bite. Ann Afr Med. (2024) 23:104. doi: 10.4103/aam.aam_44_23
Keywords: snakebite, venom-induced stroke, stroke, neurovascular complications, public health
Citation: Vasconez-Gonzalez J, Noboa-Lasso MdL and Ortiz-Prado E (2025) Snake venom and cerebrovascular events: insights and public health implications. Front. Public Health 13:1513453. doi: 10.3389/fpubh.2025.1513453
Received: 18 October 2024; Accepted: 20 January 2025;
Published: 05 February 2025.
Edited by:
José Rafael De Almeida, Regional College Amazon Ikiam, EcuadorReviewed by:
Leticia Gomes De Pontes, Universidade de Minas Gerais, BrazilR. R. Senji Laxme, Indian Institute of Science (IISc), India
Copyright © 2025 Vasconez-Gonzalez, Noboa-Lasso and Ortiz-Prado. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esteban Ortiz-Prado, ZXN0ZWJhbi5vcnRpei5wcmFkb0B1ZGxhLmVkdS5lYw==
 Jorge Vasconez-Gonzalez
Jorge Vasconez-Gonzalez María de Lourdes Noboa-Lasso
María de Lourdes Noboa-Lasso Esteban Ortiz-Prado
Esteban Ortiz-Prado